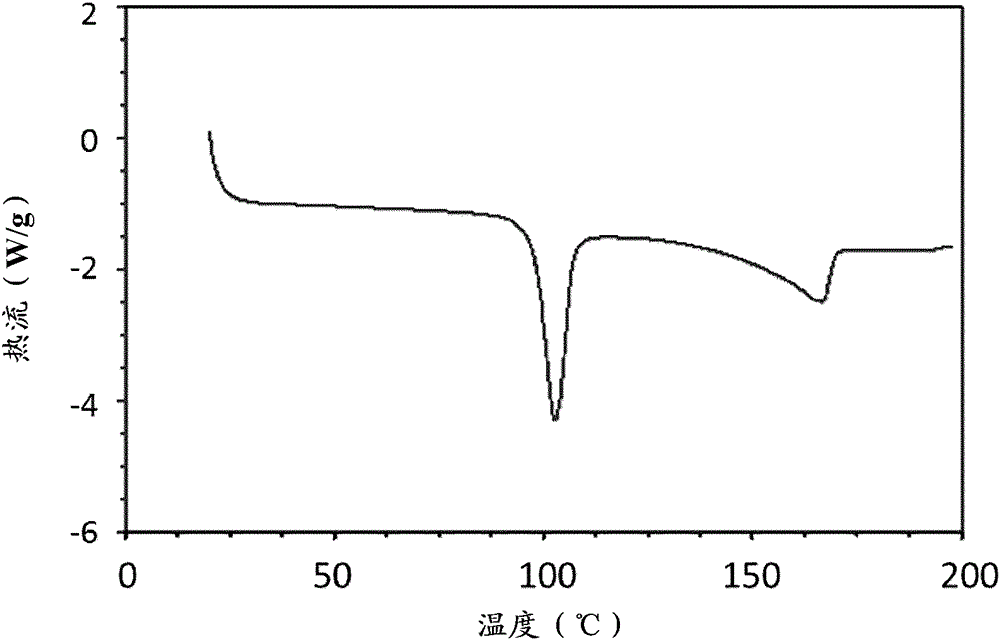
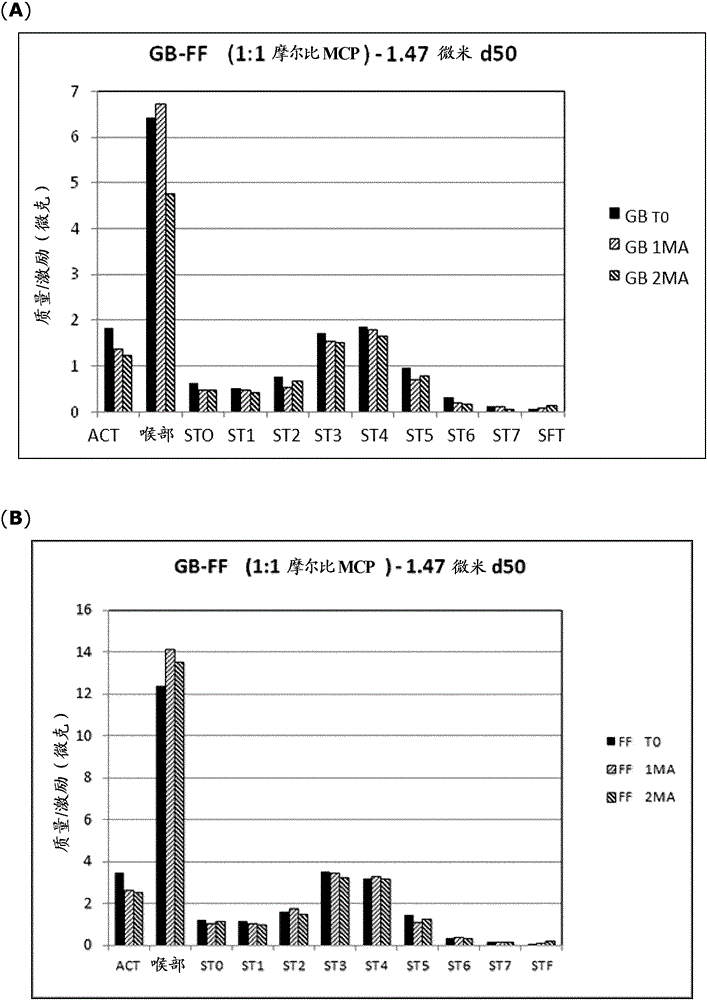
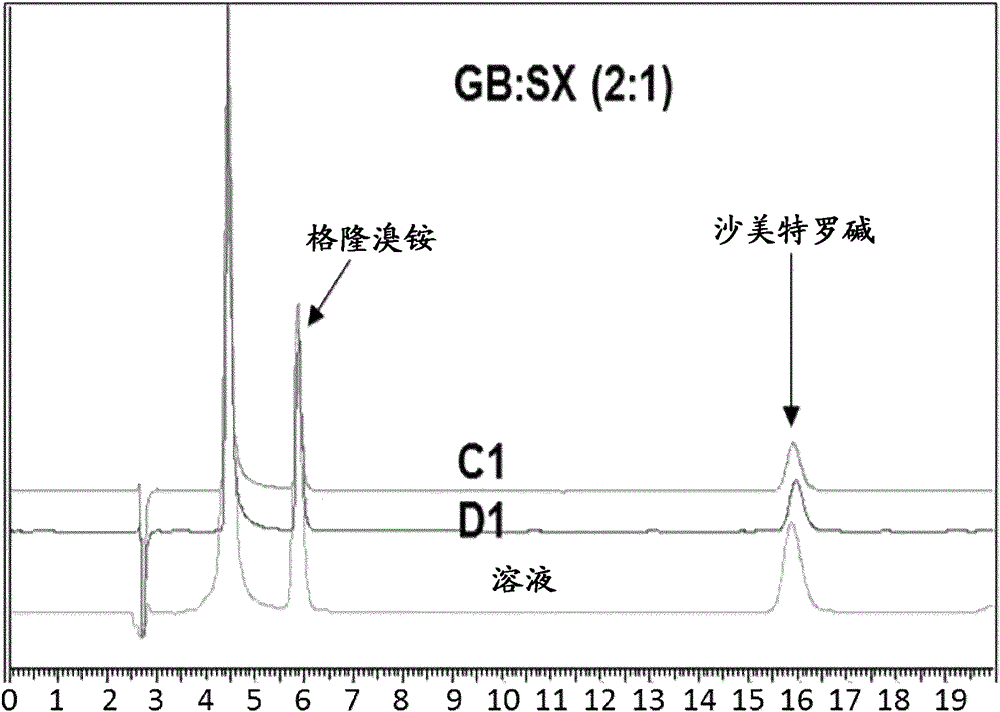
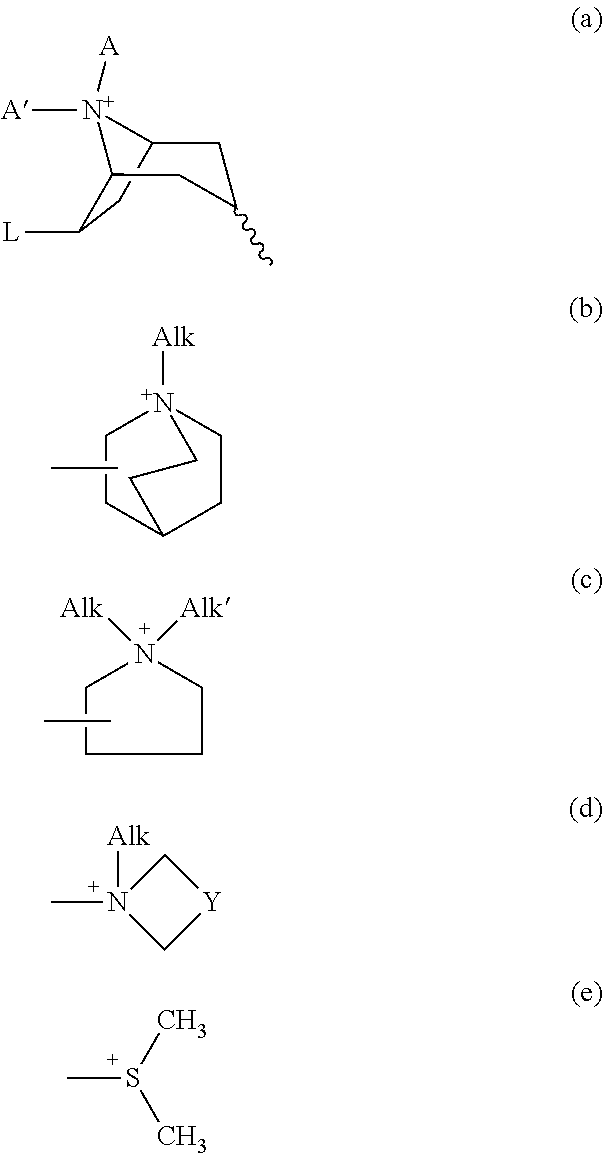
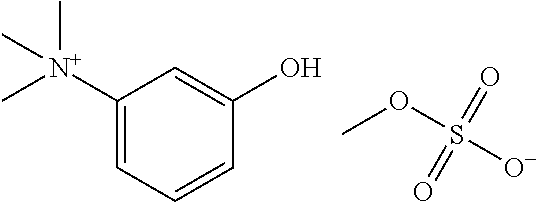
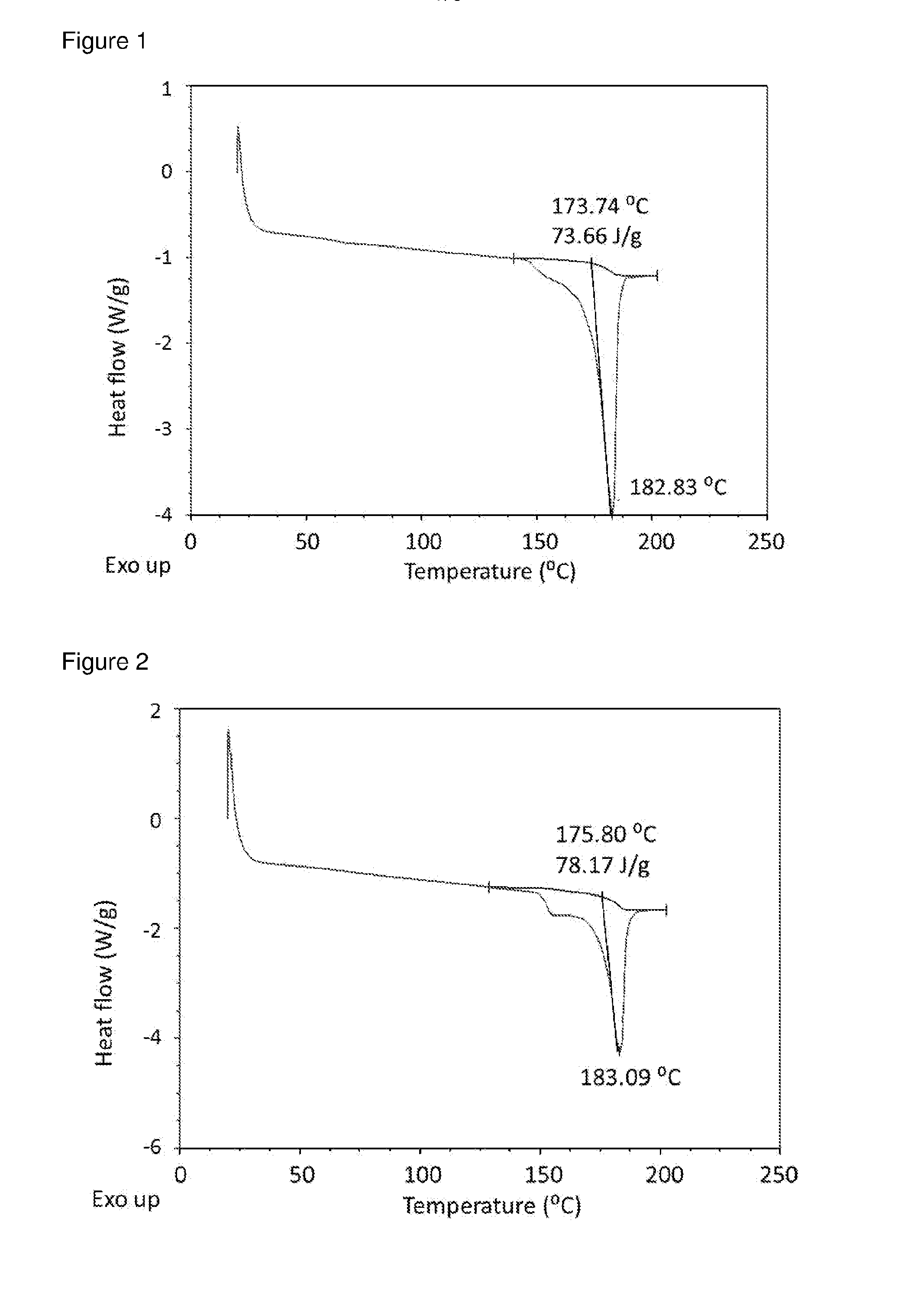
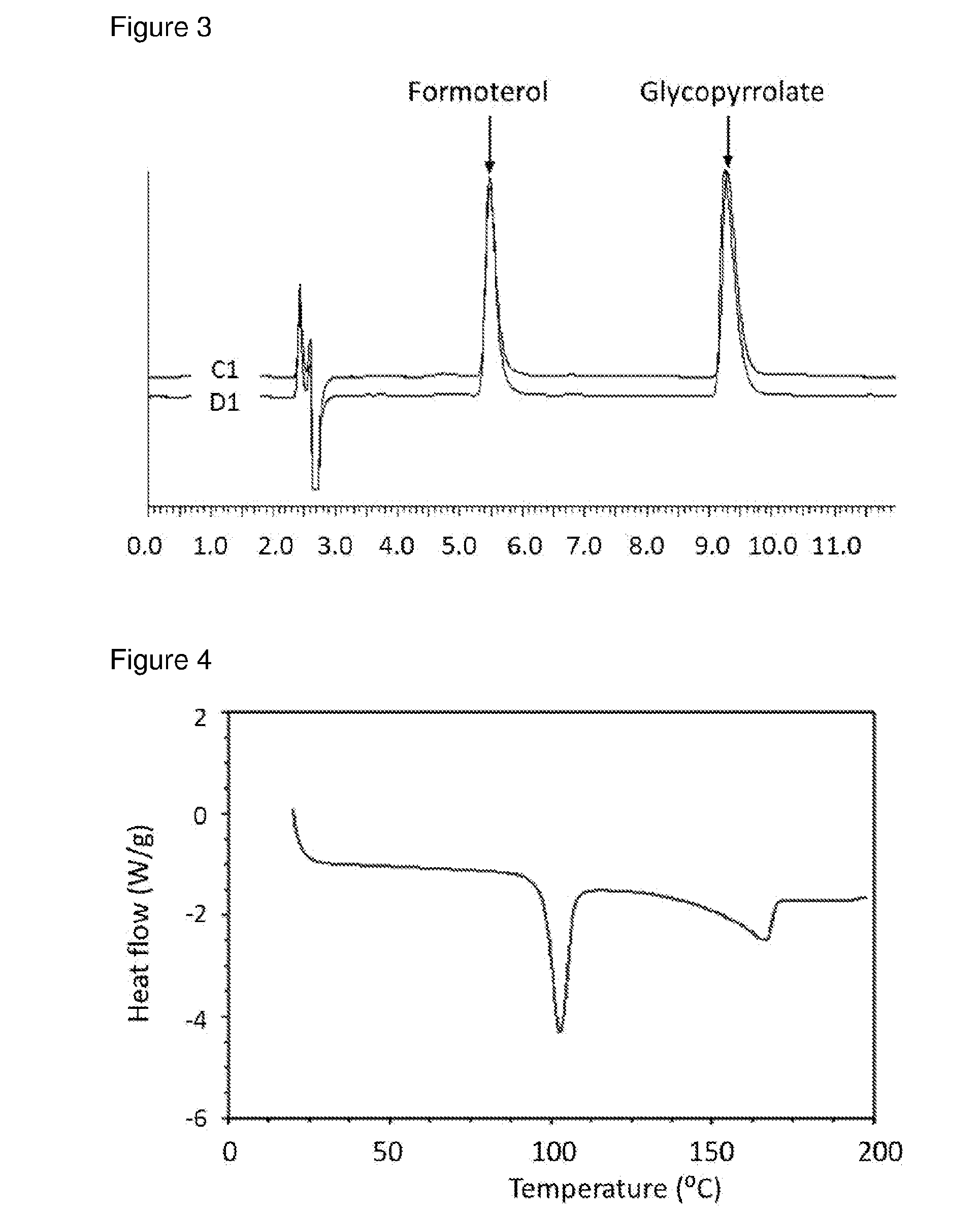
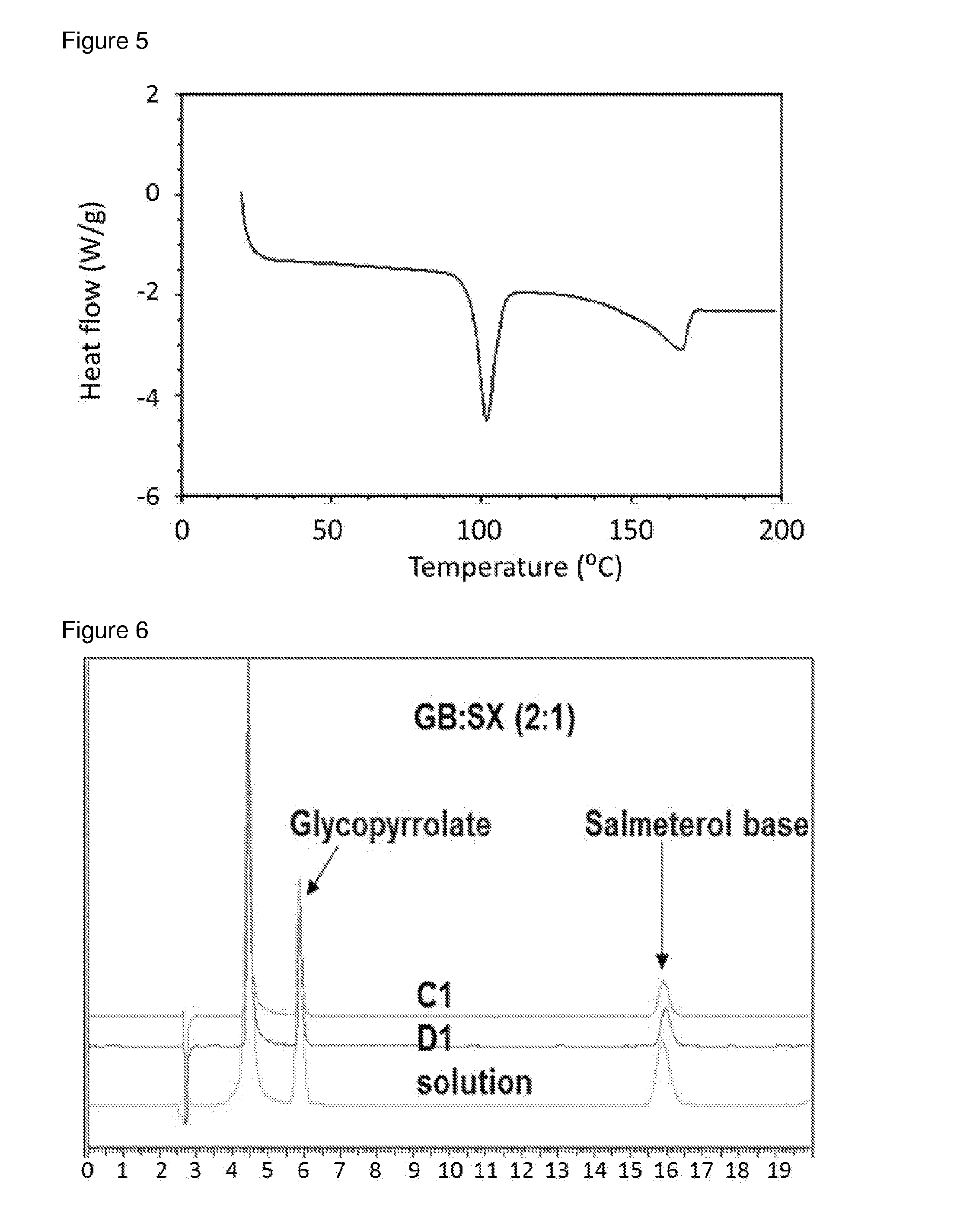
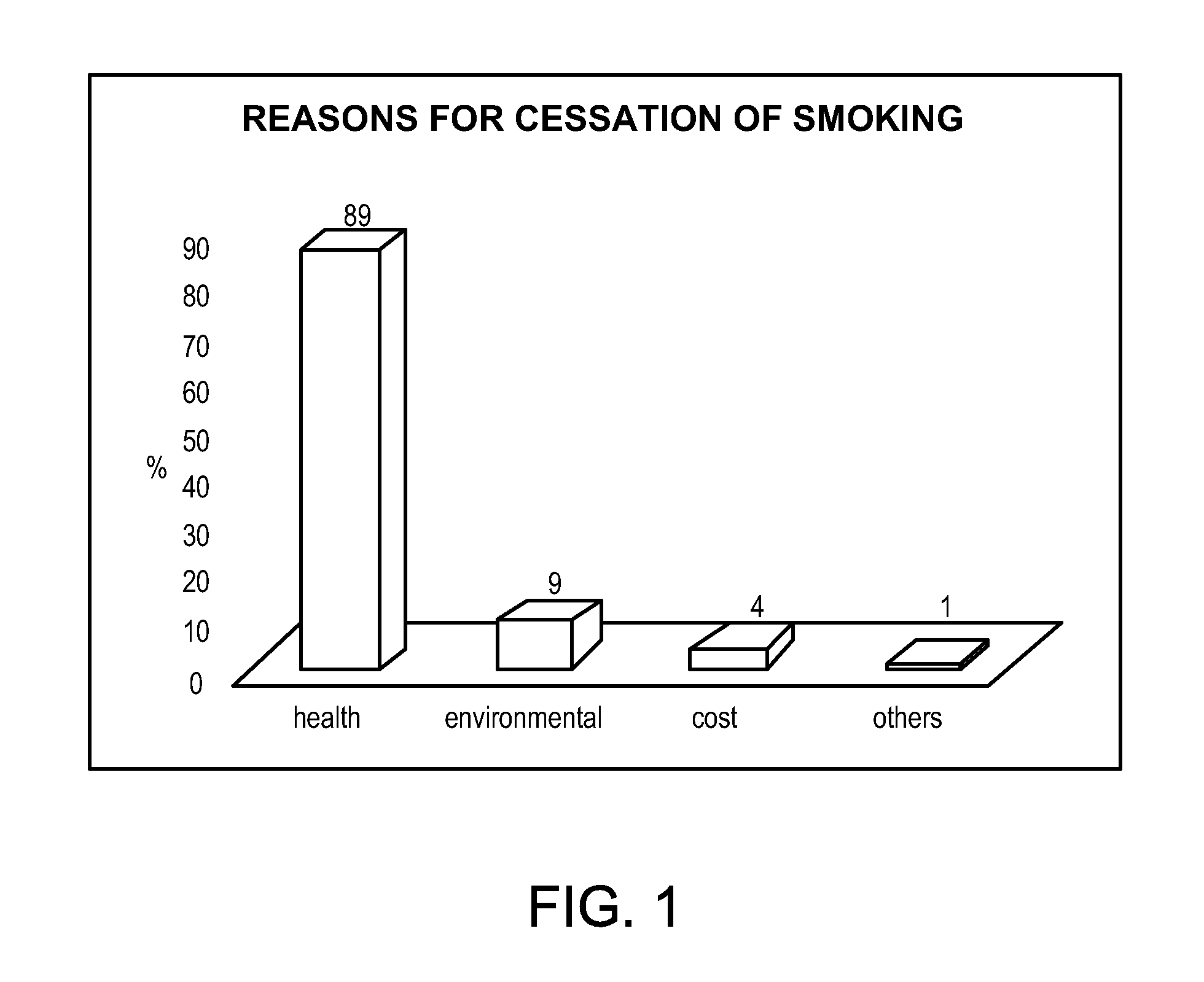
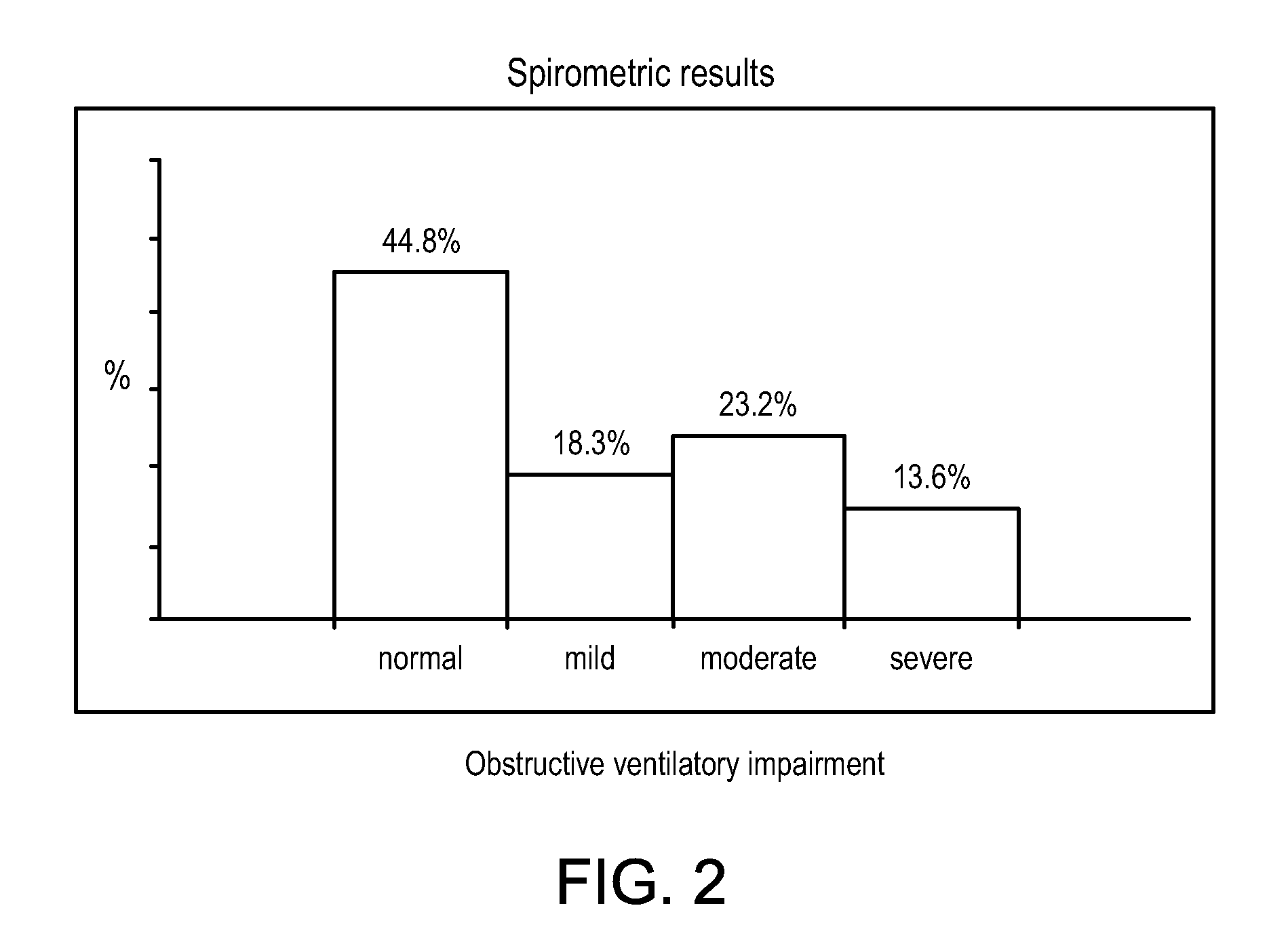
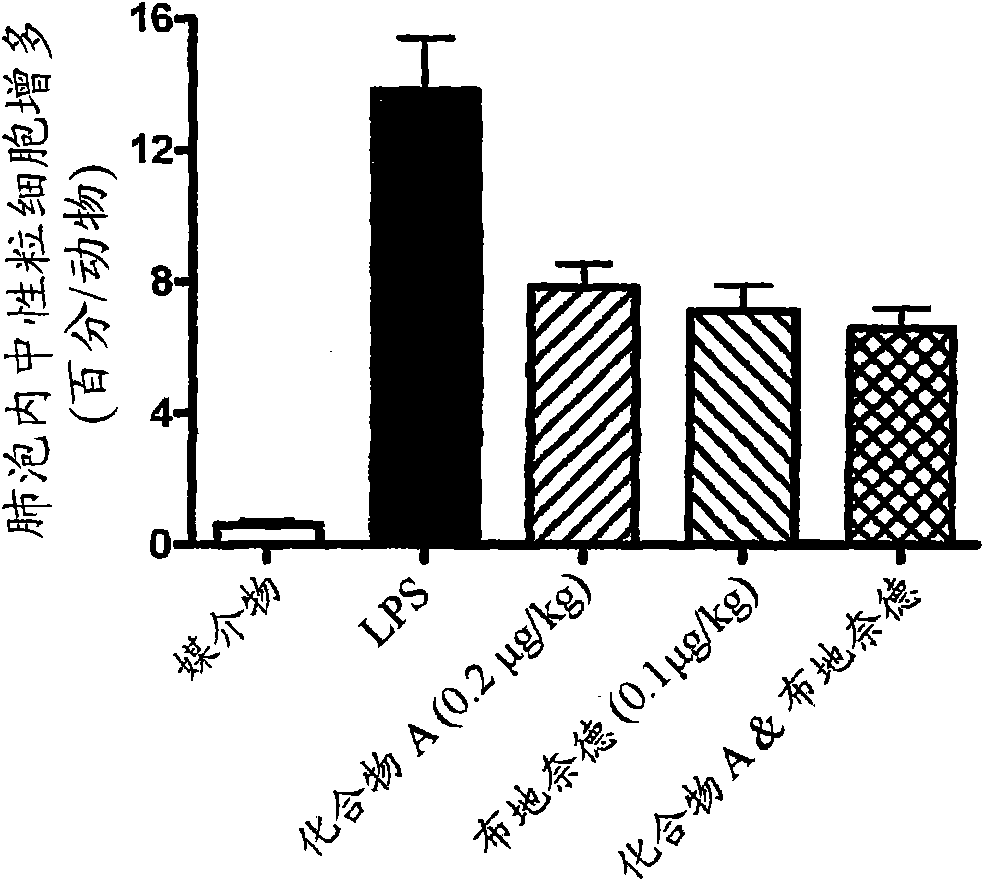
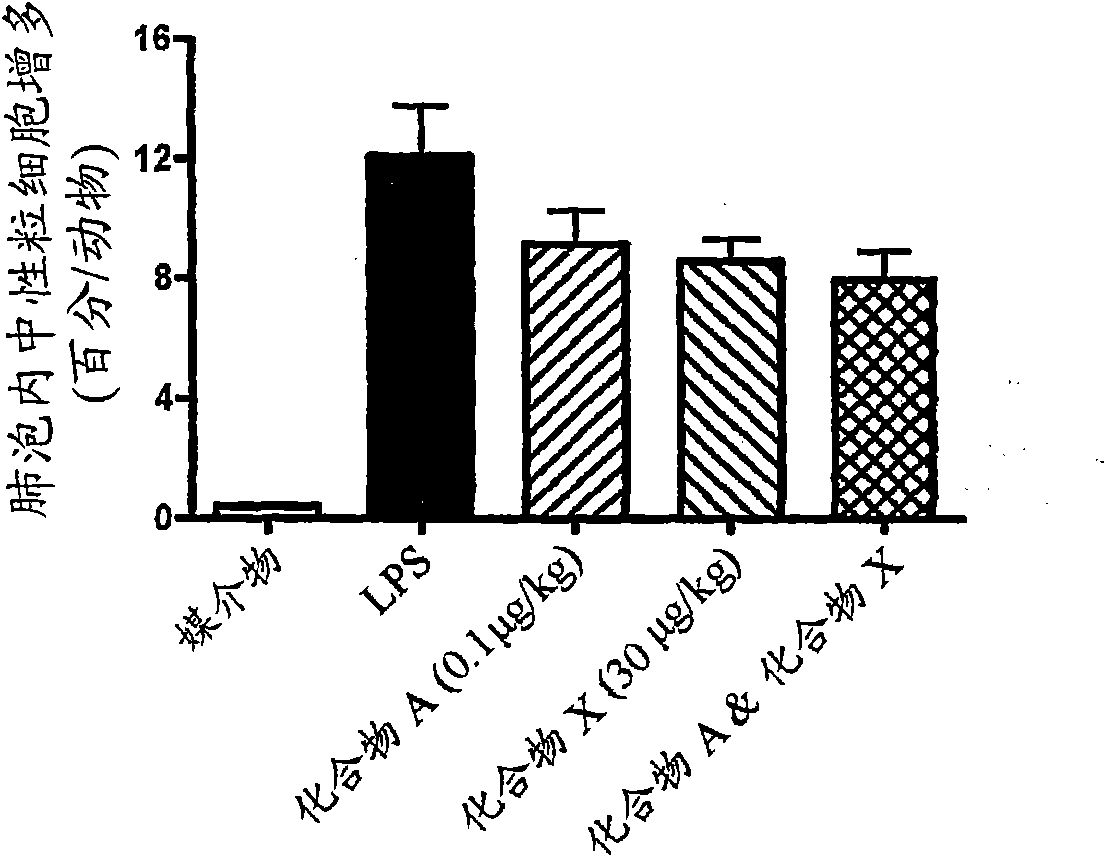Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
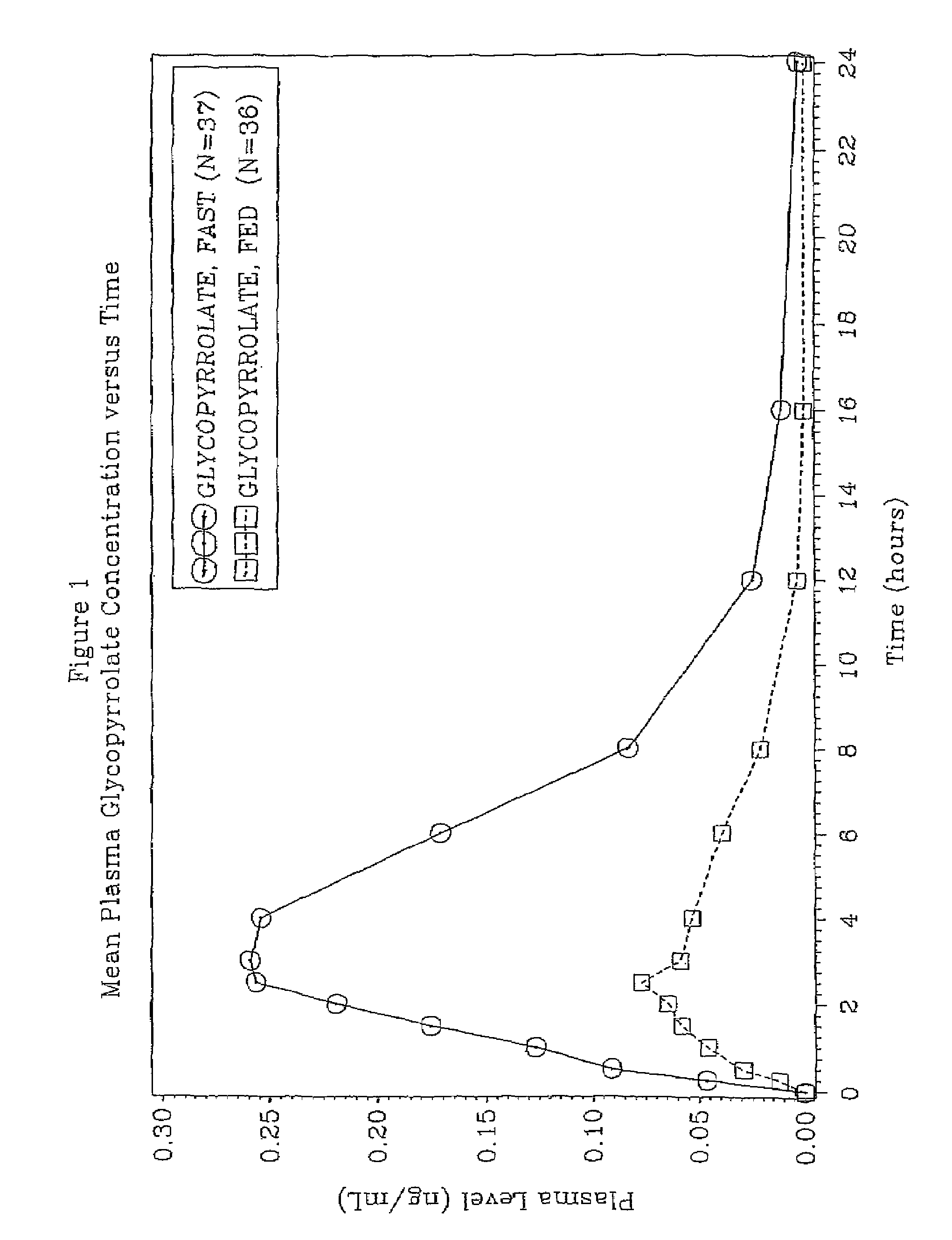
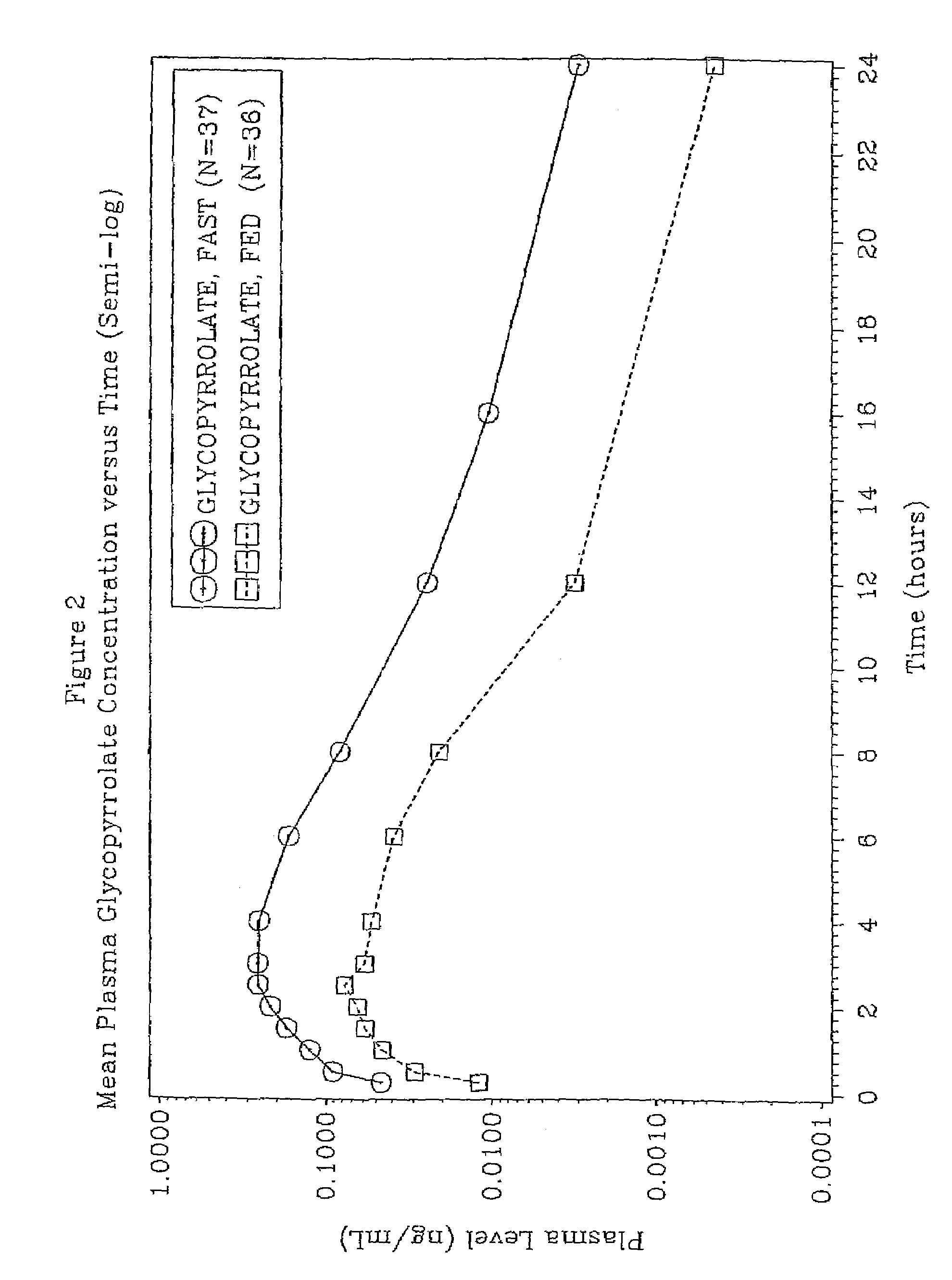
46 results about "Glycopyrrolate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A muscarinic antagonist used as an antispasmodic, in some disorders of the gastrointestinal tract, and to reduce salivation with some anesthetics.
Topical glycopyrrolate formulations
ActiveUS20100276329A1Hyperhidrosis is substantially reducedEasy to carryBiocideCosmetic preparationsHydrophilic polymersHydrophobic polymer
Individually packaged topical formulations comprising about 0.25 to about 6% w / w of glycopyrrolate for the treatment of hyperhidrosis, wherein said wipe is contained within a pouch resistant to leakage. The formulations may further comprise ethanol, a buffering agent and water. In addition, the formulations may further comprise a polymer system comprising a hydrophobic polymer in combination with a hydrophilic polymer.
Owner:ROSE U
New uses for quaternary ammonium anticholinergic muscarinic receptor antagonists in patients being treated for cognitive impairment or acute delirium
ActiveUS20080114014A1Maximizing beneficial effectMaximize the effectBiocideNervous disorderSolubilityFecal incontinence
A method for treating the adverse effects of acetyl-cholinesterase inhibitors used in the treatment of cognitive disorders such as acute delirium and cognitive impairment in elderly human patients. The administration of a clinically effective amount of a quaternary ammonium anti-cholinergic muscarinic receptor antagonist having very low lipid solubility substantially eliminates the adverse effects of urinary and / or fecal incontinence, nausea, bradycardia, bronchorrhea or brochospasm caused by the acetyl-cholinesterase inhibitors, without affecting the beneficial activity of the acetyl-cholinesterase inhibitors. This permits the administration of the optimum effective dosing of acetyl-cholinesterase inhibitors to provide maximum benefit to the patient with the added benefit of reducing or eliminating the unwanted side effects of fecal and urinary incontinence. Further, the combination of rivastigmine and glycopyrrolate has been effective in significantly improving cognitive function in patients suffering from acute dementia or cognitive impairment.
Owner:QAAM PHARMA LLC
Compositions and methods for bowel care in individuals with chronic intestinal pseudo-obstruction
ActiveUS7635709B2Shorten the construction periodDifficult to administerBiocideAmine active ingredientsBowel careSide effect
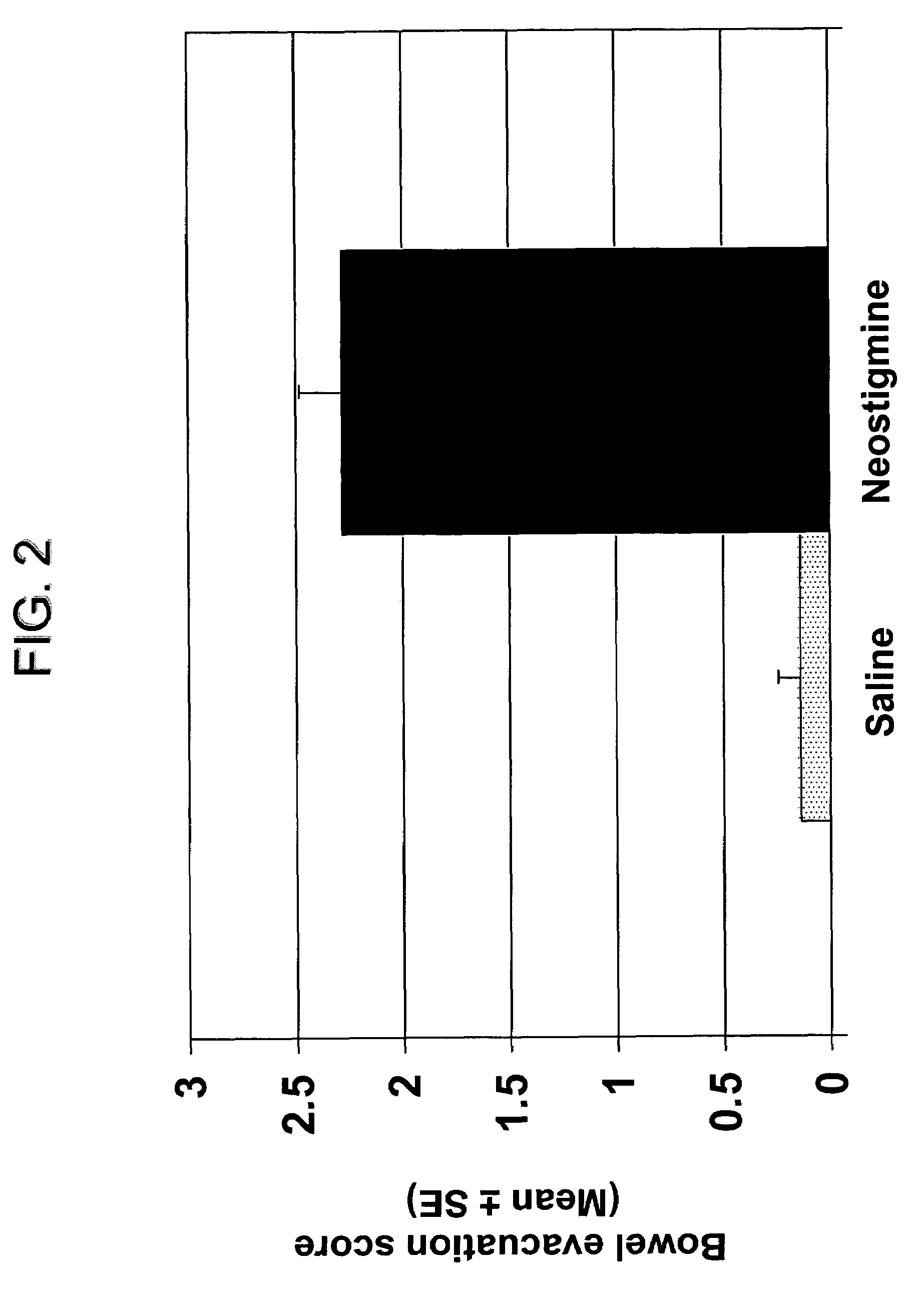
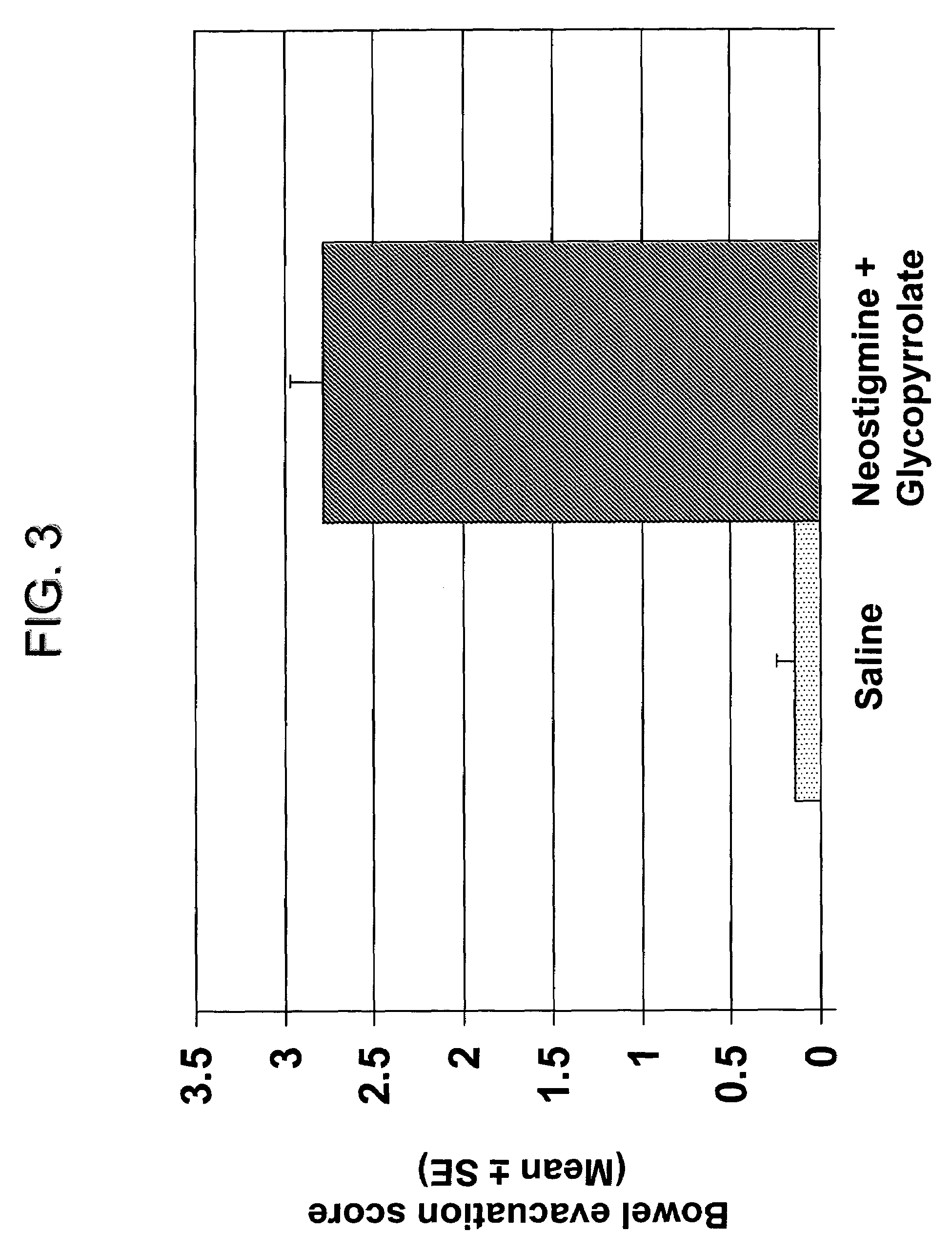
The present disclosure provides compositions and methods for on-going bowel care for persons with chronic intestinal pseudo-obstruction. The compositions and methods can be administered in a non-clinical setting. The compositions comprise acetylcholinesterase inhibitors for stimulating motility of the bowel in combination with anti-cholinergic agents to counteract the potentially dangerous cardiac side effects of the acetylcholinesterase inhibitor. In some examples, the acetylcholinesterase inhibitor, neostigmine, and the anti-cholinergic agent, glycopyrrolate, are combined in a pharmaceutical composition. Certain examples also provide the frequency and duration of administration of the disclosed drug combinations.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
Topical glycopyrrolate formulations
ActiveUS8618160B2Easy to carryEasy to useCosmetic preparationsBiocideHydrophilic polymersHydrophobic polymer
Individually packaged topical formulations comprising about 0.25 to about 6% w / w of glycopyrrolate for the treatment of hyperhidrosis, wherein said wipe is contained within a pouch resistant to leakage. The formulations may further comprise ethanol, a buffering agent and water. In addition, the formulations may further comprise a polymer system comprising a hydrophobic polymer in combination with a hydrophilic polymer.
Owner:ROSE U
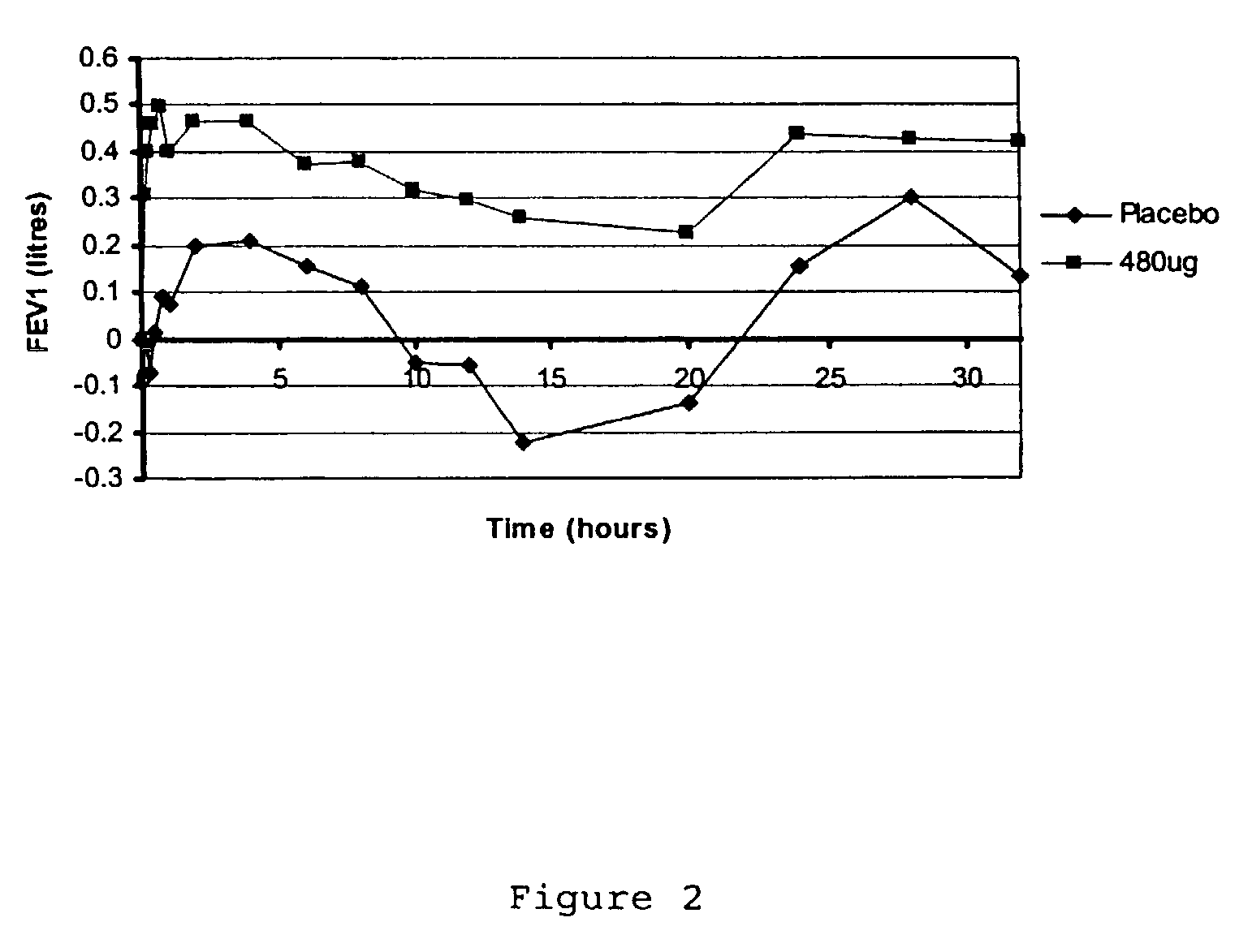
Treatment of respiratory disease
InactiveUS7229607B2Improve complianceReduced activityPowder deliveryPeptide/protein ingredientsDiseaseRespiratory tract disease
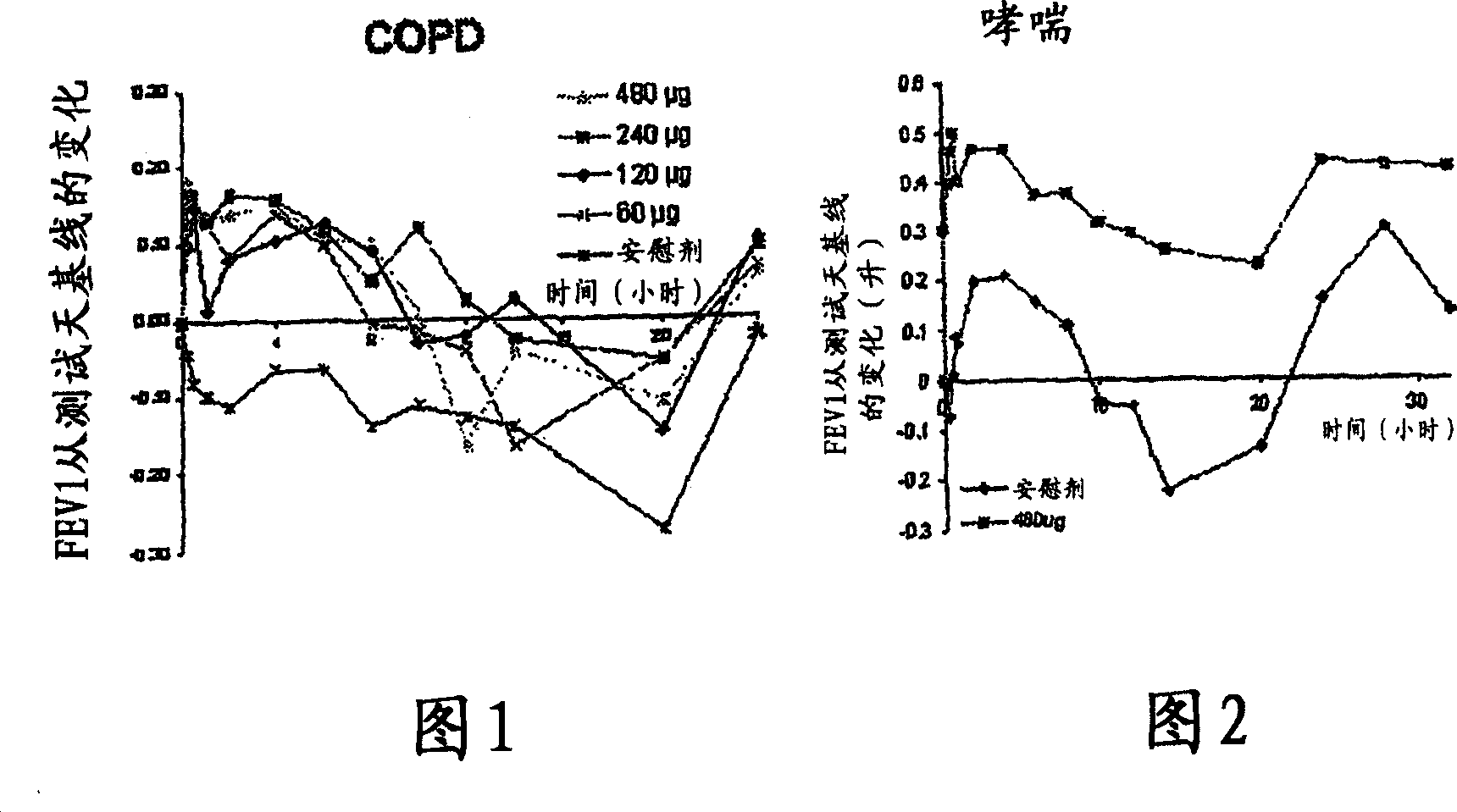
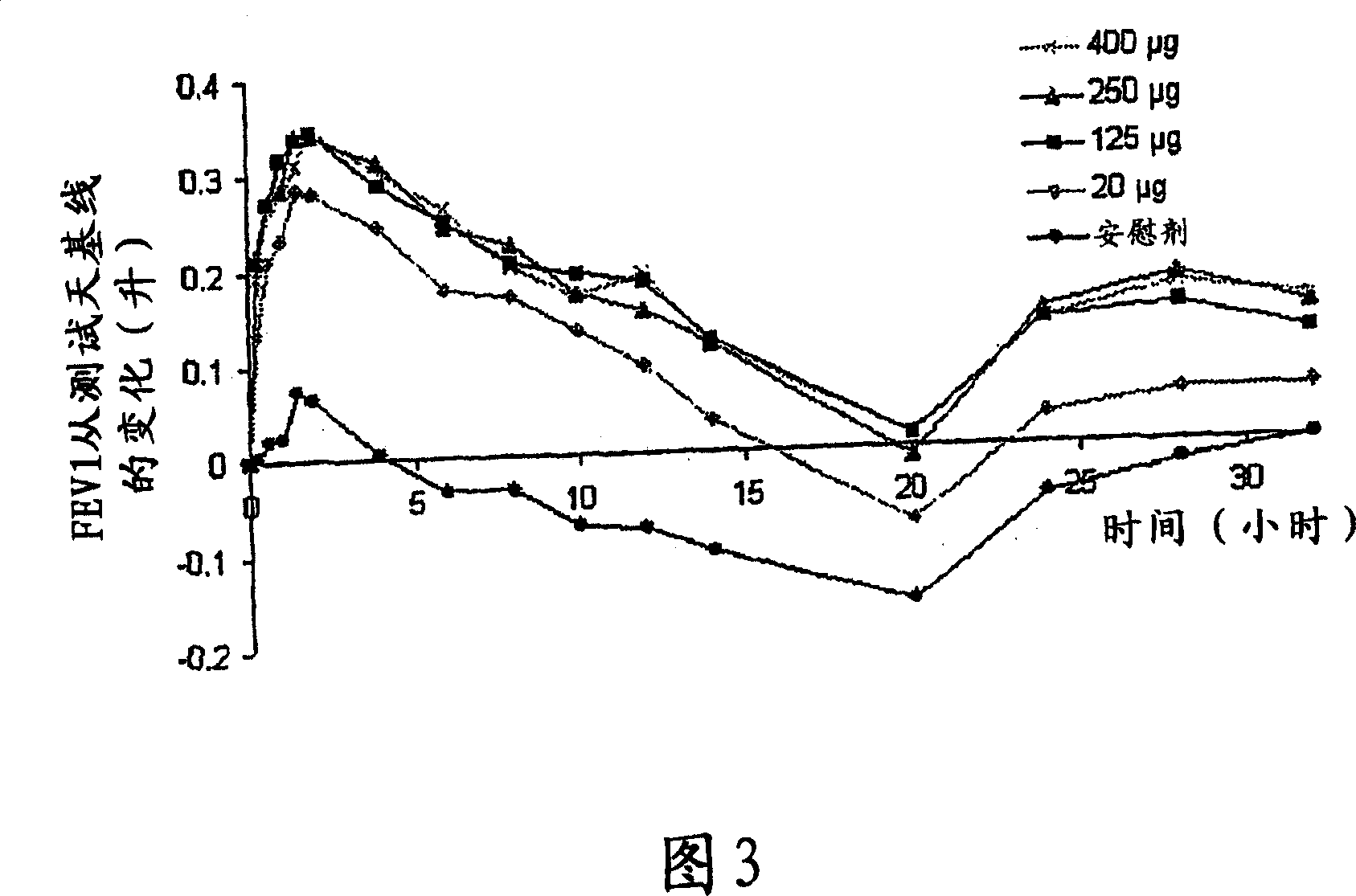
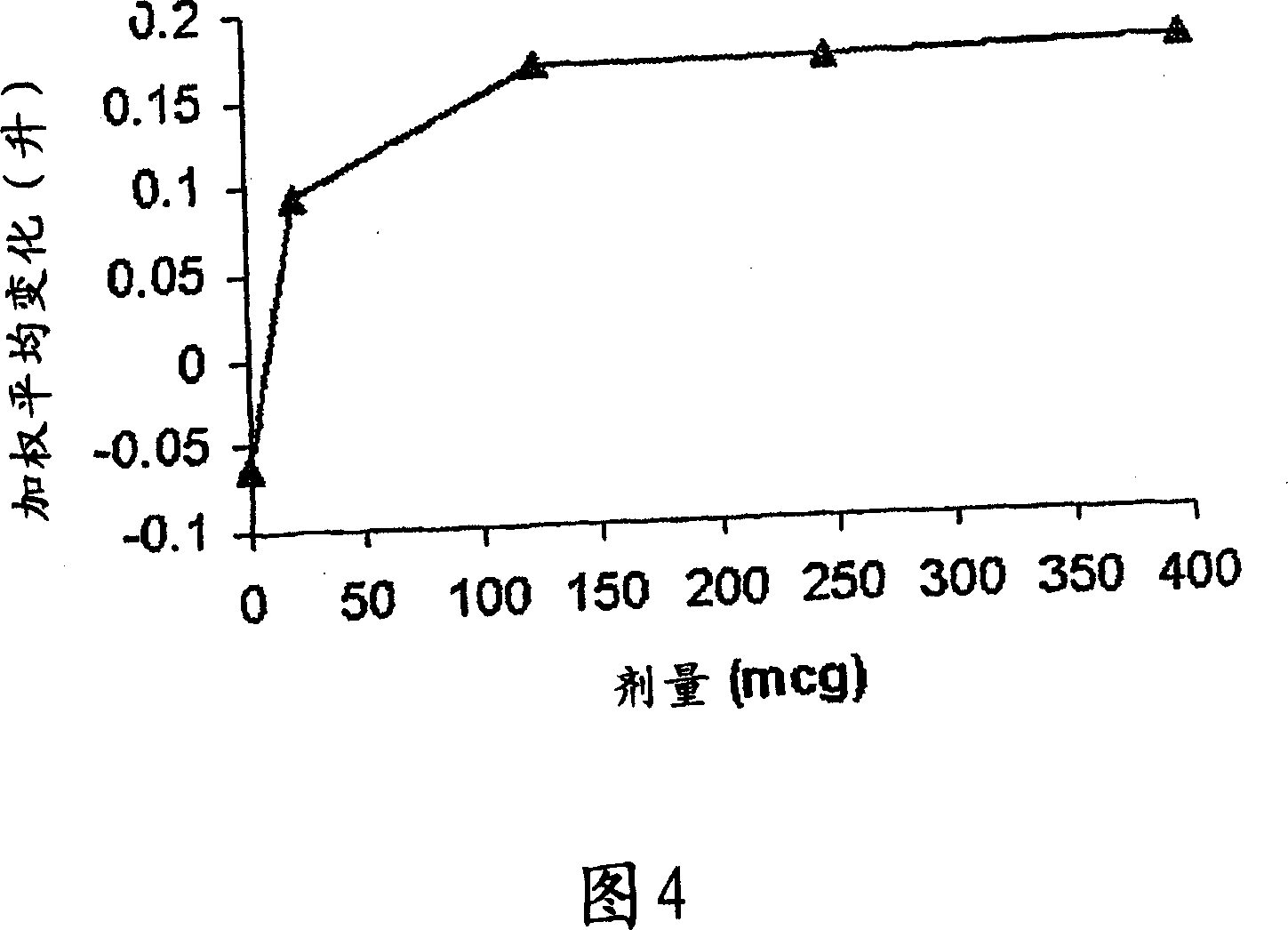
A pharmaceutical composition for pulmonary delivery comprises glycopyrrolate in a controlled release formulation, wherein, on administration, the glycopyrrolate exerts its pharmacological effect over a period greater than 12 hours.
Owner:HEPTARES THERAPEUTICS
Topical glycopyrrolate product
InactiveUS7060289B2Easy to closeEasy to openOrganic active ingredientsCosmetic preparationsSide effectEndoscopic thoracic sympathectomy
This invention relates to a convenient and safe product and method of applying glycopyrrolate topically in order to reduce excessive sweating in localized areas for those who suffer from this condition. This invention also relates to combining oral and topical delivery of glycopyrrolate to reduce excessive sweating and minimize side effects. This invention also relates to a convenient and safe product and method of applying glycopyrrolate topically to areas of compensatory sweating after endoscopic thoracic sympathectomy.
Owner:PUREPHARM
Glycopyrrolate salts
Salts of glycopyrrolate, including solid forms and formulations such as topicals thereof, are disclosed. Methods of making glycopyrrolate salts, including formulations such as topicals thereof, and methods of treating hyperhidrosis with salts of glycopyrrolate, and formulations such as topicals thereof, are disclosed.
Owner:JOURNEY MEDICAL CORP
Pharmaceutical Composition
The present invention relates to pharmaceutical compositions for inhalation comprising glycopyrrolate, a beta2-agonist, and optionally an inhaled corticosteroid; to a process for preparing such compositions and to the use of such compositions for the prevention and / or treatment of respiratory, inflammatory or obstructive airway disease.
Owner:CIPLA LTD
Composition of glycopyrrolate and a beta2-agonist
The present invention relates to pharmaceutical compositions for inhalation comprising glycopyrrolate, a beta2-agonist, and optionally an inhaled corticosteroid; 103501776to a process for preparing such compositions and to the use of such compositions for the prevention and / or treatment of respiratory, inflammatory or obstructive airway disease.
Owner:CIPLA LTD
Use of glycopyrrolate for treating tachycardia
InactiveUS20140080890A1Treatment or prophylaxisBiocidePowder deliveryObstructive Pulmonary DiseasesGlycopyrrolate
The invention relates to a novel use of the antimuscarinic agent glycopyrrolate, for example the salt glycopyrronium bromide. In particular, the invention relates to glycopyrrolate for use as a heart rate lowering agent and more particularly, but not exclusively, for use in patients suffering from respiratory conditions such as chronic obstructive pulmonary disease.
Owner:SOSEI R&D LIMITED
Transdermal delivery system comprising glycopyrrolate to treat sialorrhea
InactiveUS20080317832A1Alleviate sialorrheaInhibit metabolismBiocideNervous disorderSialorrheaDrug in adhesive
In one aspect, the invention includes a method for treating sialorrhea, comprising the steps of identifying a patient afflicted with sialorrhea and administering a therapeutically effective amount of glycopyrrolate to the patient using a transdermal route of administration. In another aspect, the invention is a transdermal drug delivery system for treating a patient exhibiting sialorrhea, including a transdermal patch, a therapeutically effective amount of glycopyrrolate contained in the transdermal patch to alleviate sialorrhea, and a pharmaceutically acceptable carrier. The transdermal patch can be a single layer drug-in-adhesive patch, a multi-layer drug-in-adhesive patch, a matrix patch, or a reservoir patch.
Owner:SHIONOGI INK
Anticholinergic neuroprotective composition and methods
The present invention relates to a pharmaceutical composition comprising propiverine, trospium or glycopyrrolate; and a non-anticholinergic antiemetic agent. It is also related to a pharmaceutical composition comprising a high dose of solifenacin or a pharmaceutically acceptable salts thereof; and a non-anticholinergic antiemetic agent. Pharmaceutical compositions containing high dose of nsPAChA for use for increasing the AChEI blood concentrations and for combating neurodegeneration are also described. The invention also relates to a method for inducing neuroprotection and combating neurodegeneration in a patient suffering from Alzheimer type dementia as well as to a method for increasing the blood levels of an acetyl choline esterase inhibitor (AChEI) in a human subject treated with an AChEI dose.
Owner:CHASE PHARMA CORP
Topical glycopyrrolate product
InactiveUS20030211134A1Reduce sweatingReduced strengthOrganic active ingredientsCosmetic preparationsSide effectEndoscopic thoracic sympathectomy
This invention relates to a convenient and safe product and method of applying glycopyrrolate topically in order to reduce excessive sweating in localized areas for those who suffer from this condition. This invention also relates to combining oral and topical delivery of glycopyrrolate to reduce excessive sweating and minimize side effects. This invention also relates to a convenient and safe product and method of applying glycopyrrolate topically to areas of compensatory sweating after endoscopic thoracic sympathectomy.
Owner:PUREPHARM
Glycopyrrolate for treating childhood asthma
Owner:SOSEI R&D LTD
Treatment of respiratory diseases
InactiveUS20050019271A1Eliminate the effects ofImprove compliancePowder deliveryBiocideDiseaseRespiratory disease
A pharmaceutical composition for pulmonary delivery comprises glycopyrrolate in a controlled release formulation, wherein, on administration, the glycopyrrolate exerts its pharmacological effect over a period greater than 12 hours.
Owner:HEPTARES THERAPEUTICS
Treatment of Childhood Asthma
InactiveUS20070243260A1High and immediate onsetSuitable for treatmentPowder deliveryBiocideGlycopyrrolateChildhood asthma
Owner:SOSEI R&D LIMITED
Multi-component crystalline particles for inhalation therapy
InactiveCN104955449AImprove co-locationIncrease the likelihood of synergyPowder deliveryDispersion deliveryActive agentInhalation
Pharmaceutical Preparations Multi-component crystalline particles and compositions, methods for their preparation, their uses in inhalation therapy and inhaler devices containing said particles are provided, in particular particles comprising glycopyrrolate. The particles can be prepared substantially free of excipients and agents other than active agents or their precursors in the presence of ultrasonic irradiation in a process comprising contacting a solution in a first flowing stream with an anti-solvent in a re-circulating second flowing stream, causing the mixing thereof and collecting crystals that are generated.
Owner:CIRCASSIA
Anticholinergic neuroprotective composition and methods
The present invention relates to a pharmaceutical composition comprising propiverine, trospium or glycopyrrolate; and a non-anticholinergic antiemetic agent. It is also related to a pharmaceutical composition comprising a high dose of solifenacin or a pharmaceutically acceptable salts thereof; and a non-anticholinergic antiemetic agent. Pharmaceutical compositions containing high dose of nsPAChA for use for increasing the AChEI blood concentrations and for combating neurodegeneration are also described. The invention also relates to a method for inducing neuroprotection and combating neurodegeneration in a patient suffering from Alzheimer type dementia as well as to a method for increasing the blood levels of an acetyl choline esterase inhibitor (AChEI) in a human subject treated with an AChEI dose.
Owner:CHASE PHARMA CORP
Uses for quaternary ammonium anticholinergic muscarinic receptor antagonists in patients being treated for cognitive impairment or acute delirium
A method for treating the adverse effects of acetyl-cholinesterase inhibitors used in the treatment of cognitive disorders such as acute delirium and cognitive impairment in elderly human patients. The administration of a clinically effective amount of a quaternary ammonium anti-cholinergic muscarinic receptor antagonist having very low lipid solubility substantially eliminates the adverse effects of urinary and / or fecal incontinence, nausea, bradycardia, bronchorrhea or brochospasm caused by the acetyl-cholinesterase inhibitors, without affecting the beneficial activity of the acetyl-cholinesterase inhibitors. This permits the administration of the optimum effective dosing of acetyl-cholinesterase inhibitors to provide maximum benefit to the patient with the added benefit of reducing or eliminating the unwanted side effects of fecal and urinary incontinence. Further, the combination of rivastigmine and glycopyrrolate has been effective in significantly improving cognitive function in patients suffering from acute dementia or cognitive impairment.
Owner:QAAM PHARMA LLC
Pharmaceutical Composition
Owner:CIPLA LTD
Glycopyrrolate salts
ActiveUS9610278B2Organic chemistry methodsPharmaceutical non-active ingredientsMedicineGlycopyrrolate
Salts of glycopyrrolate, including solid forms and formulations such as topicals thereof, are disclosed. Methods of making glycopyrrolate salts, including formulations such as topicals thereof, and methods of treating hyperhidrosis with salts of glycopyrrolate, and formulations such as topicals thereof, are disclosed.
Owner:JOURNEY MEDICAL CORP
Ready-to-use injectable pharmaceutical compositions comprising neostigmine and glycopyrrolate
ActiveUS20190374462A1Pharmaceutical delivery mechanismPharmaceutical non-active ingredientsReady to useGlycopyrrolate
The present invention provides stable, ready-to-use injectable pharmaceutical compositions, comprising the combination of neostigmine, glycopyrrolate, a stabilizing amount of one or more aminopolycarboxylic acids, and a pharmaceutically acceptable liquid vehicle. Other aspects of the invention relate to methods for making such compositions and methods of using such compositions for reversing the effects of non-depolarizing neuromuscular blocking agents. Preferably, the composition comprises neostigmine methylsulfate, glycopyrronium bromide, ethylenediaminetetraacetic acid (EDTA) and a pharmaceutically acceptable liquid vehicle, and is provided in a pre-filled, ready-to-use sealed container, such as a pre-filled syringe, suitable for intravenous administration.
Owner:SLAYBACK PHARMA LLC
Multi-Component Crystalline Particles for Inhalation Therapy
InactiveUS20150352077A1Increased co-locationRapid onsetBiocidePowder deliveryAnti solventActive agent
Pharmaceutical Preparations Multi-component crystalline particles and compositions, methods for their preparation, their uses in inhalation therapy and inhaler devices containing said particles are provided, in particular particles comprising glycopyrrolate. The particles can be prepared substantially free of excipients and agents other than active agents or their precursors in the presence of ultrasonic irradiation in a process comprising contacting a solution in a first flowing stream with an anti-solvent in a re-circulating second flowing stream, causing the mixing thereof and collecting crystals that are generated.
Owner:CIRCASSIA
Anti-Nicotine Treatment
InactiveUS20070287727A1Reduce initial withdrawal symptomMinimize long-term relapse rateBiocideAnimal repellantsAnticholinergic DrugsAnticholinergic agents
A method is described for treatment of nicotine dependency and cessation or reduction of tobacco use in humans which combines pharmacotherapy designed to ameliorate the initial withdrawal symptoms followed by behavioral modification intended to minimize the relapse rate. Pharmacotherapy consists of the use of anticholinergic agents, which facilitate a smooth withdrawal from nicotine. A composition is described comprising scopolamine, glycopyrrolate and benztropine which is injected parenterally. In addition the individuals participate in weekly counseling sessions for at least 4 weeks after treatment.
Owner:HYTHIAM
Combinations of beta- 2 -adrenoceptor agonistic benzothiazolone
The invention provides a pharmaceutical product comprising a first active ingredient which is N-[2-(Diethylamino)ethyl]-N-(2-{[2-(4-hydroxy-2-oxo-2,3-dihydro-1,3-benzothiazol-7-yl)ethyl]amino}ethyl)-3-[2-(1-naphthyl)ethoxy]propan amide or a salt thereof, and a second active ingredient selected from: a non-steroidal Glucocorticoid Receptor (GR Receptor) Agonist; an antioxidant; a CCR1 antagonist; achemokine antagonist (not CCR1); a corticosteroid; a CRTh2 antagonist; a DP1 antagonist; an Histone Deacetylase Inducer; an IKK2 inhibitor; a COX inhibitor; a lipoxygenase inhibitor; a leukotriene receptor antagonist; an MPO inhibitor; a muscarinic antagonist which is Aclidinium bromide, Glycopyrrolate, Oxitropium bromide, Pirenzepine, telenzepine, Tiotropium bromide,3(R)-(2-hydroxy-2,2-dithien-2-ylacetoxy)-1-(3-phenoxypropyl)-1-azoniabicyclo[2.2.2]octane bromide,3(R)-1-phenethyl-3-(9H-xanthene-9-carbonyloxy)-1-azoniabicyclo[2.2.2]octane bromide or (3R)-3-[(2S)-2-cyclopentyl-2-hydroxy-2-thien-2-ylacetoxy]-1-(2-phenoxyethyl)-1-azoniabicyclo[2.2.2]actane bromide; a p38 inhibitor; a PDE inhibitor; a PPARy agonist; a protease inhibitor; a Statin; a thromboxane antagonist; a vasodilator; or, an ENAC blocker (Epithelial Sodium-channel blocker); and its use in the treatment of respiratory disease.
Owner:ASTRAZENECA AB
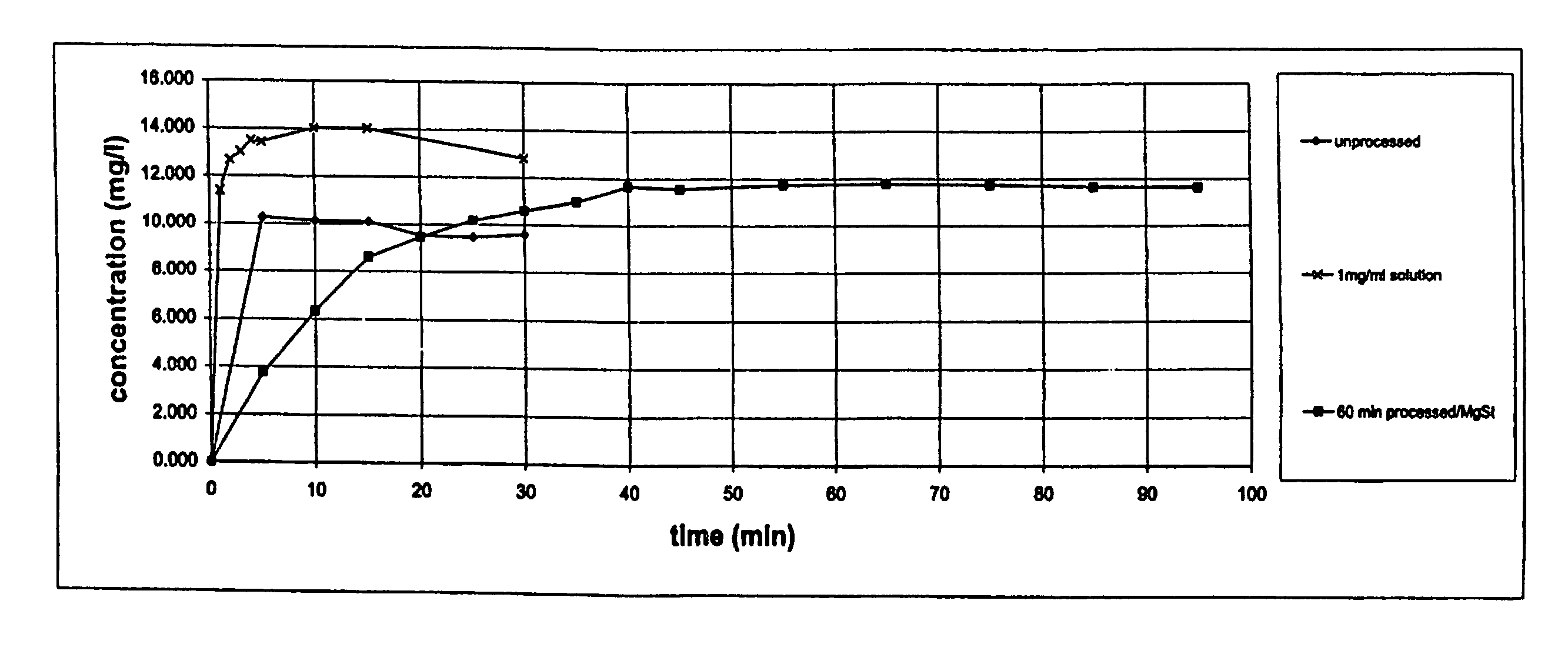
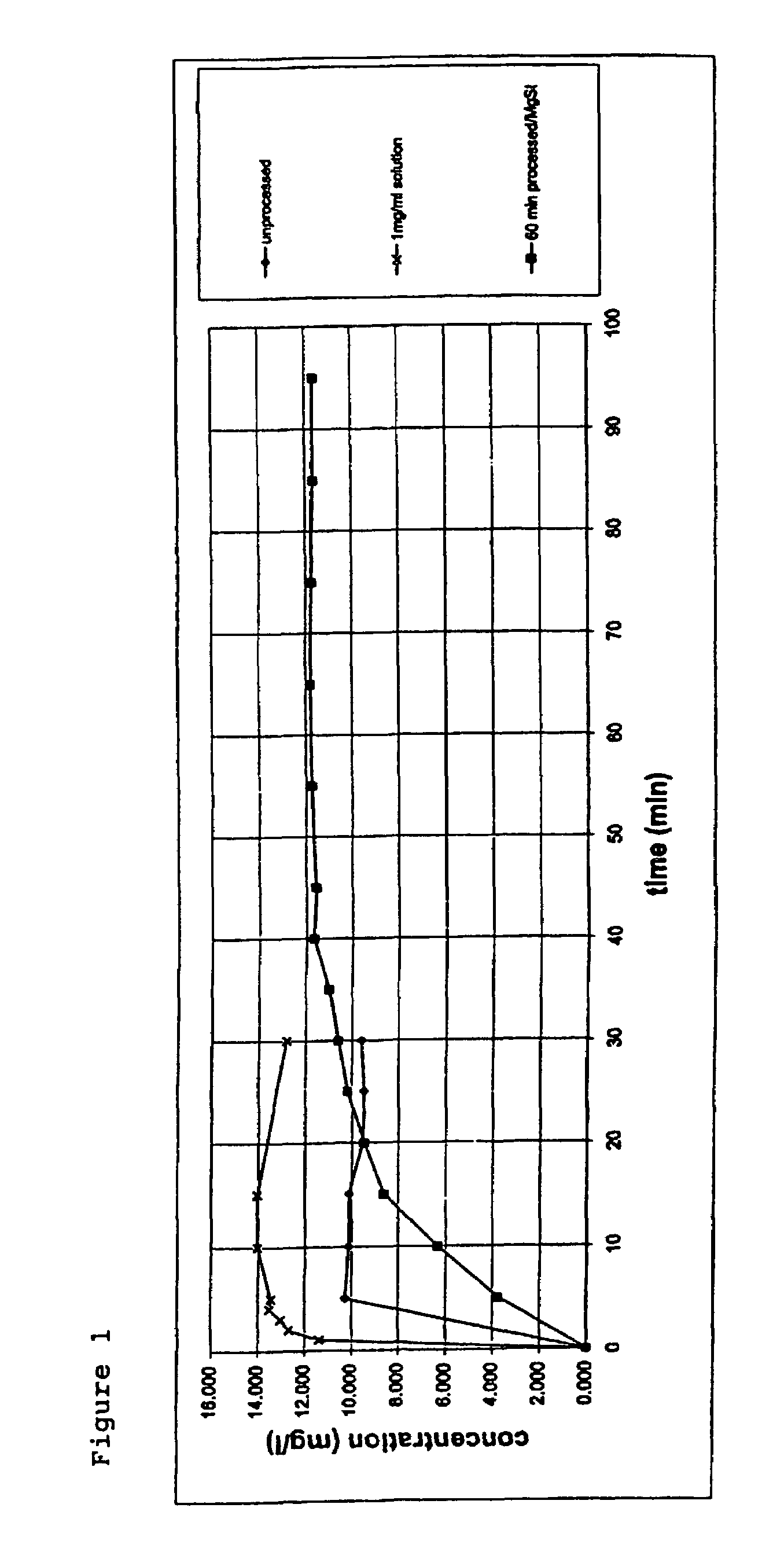
Formulation comprising glycopyrrolate, method and apparatus
ActiveUS20170258762A1Improved Fine Particle FractionSmall particle sizePowder deliveryGranular deliveryMagnesium stearateGlycopyrrolate
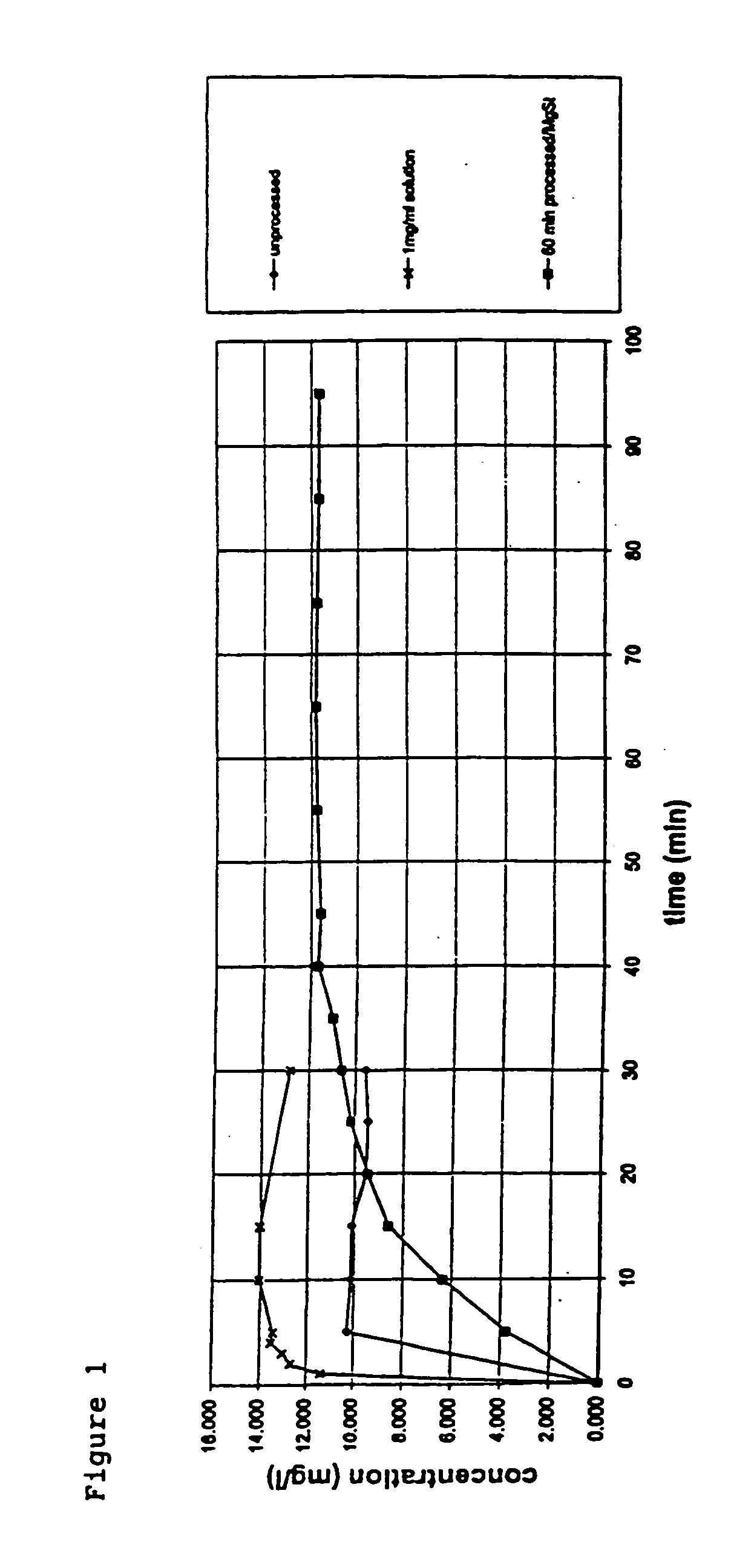
A method is disclosed for making a pharmaceutical composition for pulmonary administration comprising co-jet milling glycopyrrolate and magnesium stearate, wherein the co-jet milled glycopyrrolate and magnesium stearate is then subjected to a conditioning step which includes exposure of the co-jet milled glycopyrrolate and magnesium stearate to humidity. A composition made by this method is also disclosed.
Owner:VECTURA LTD
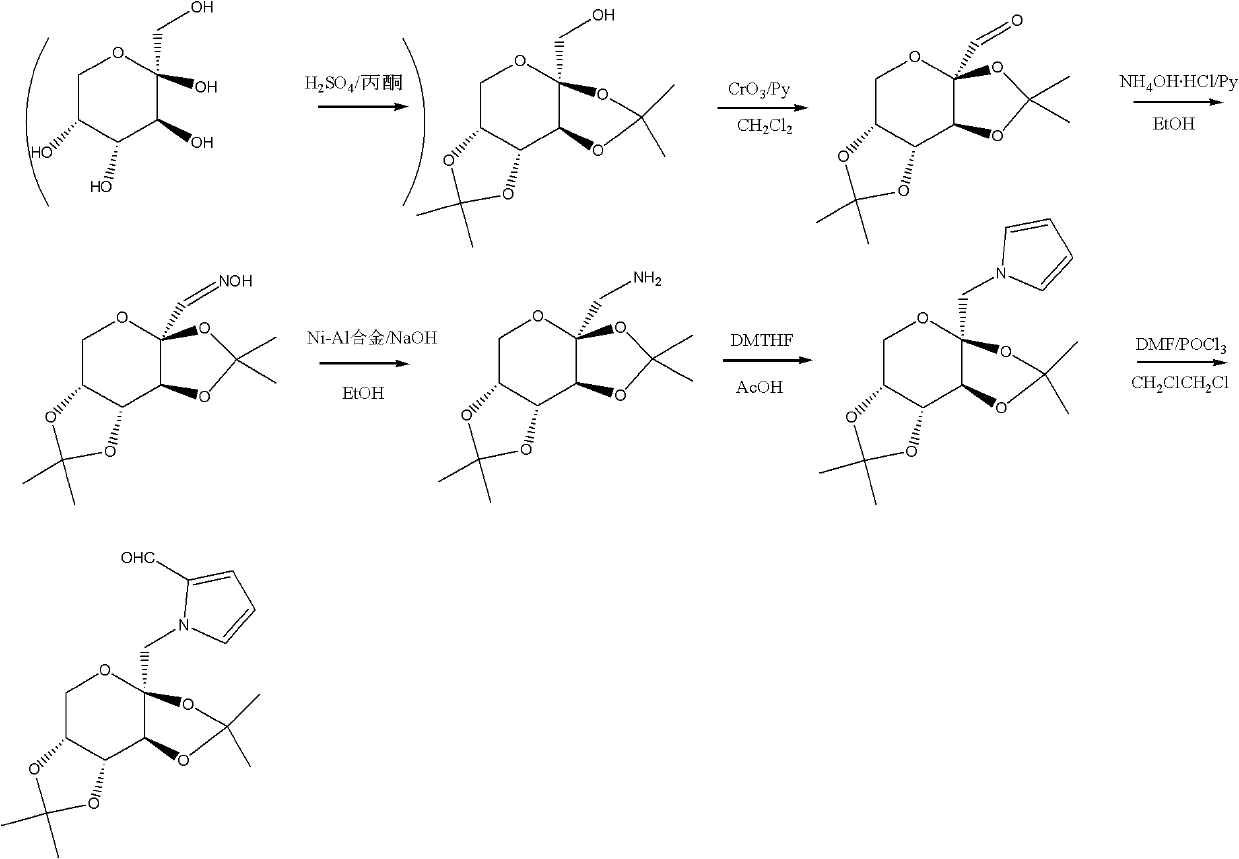
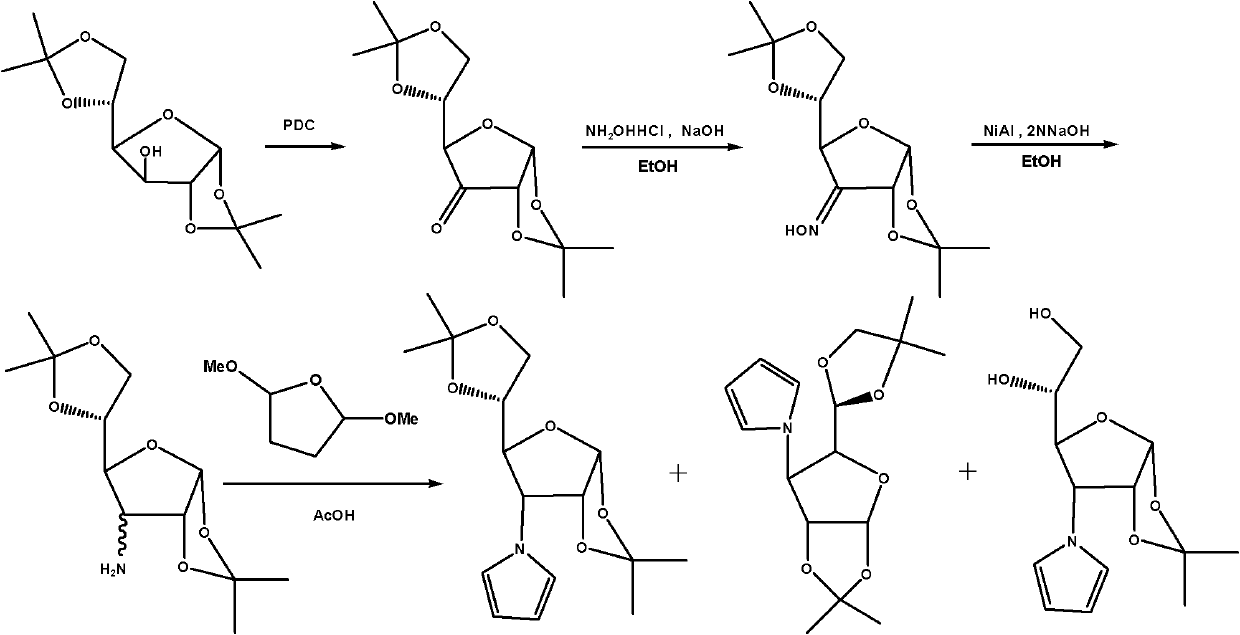
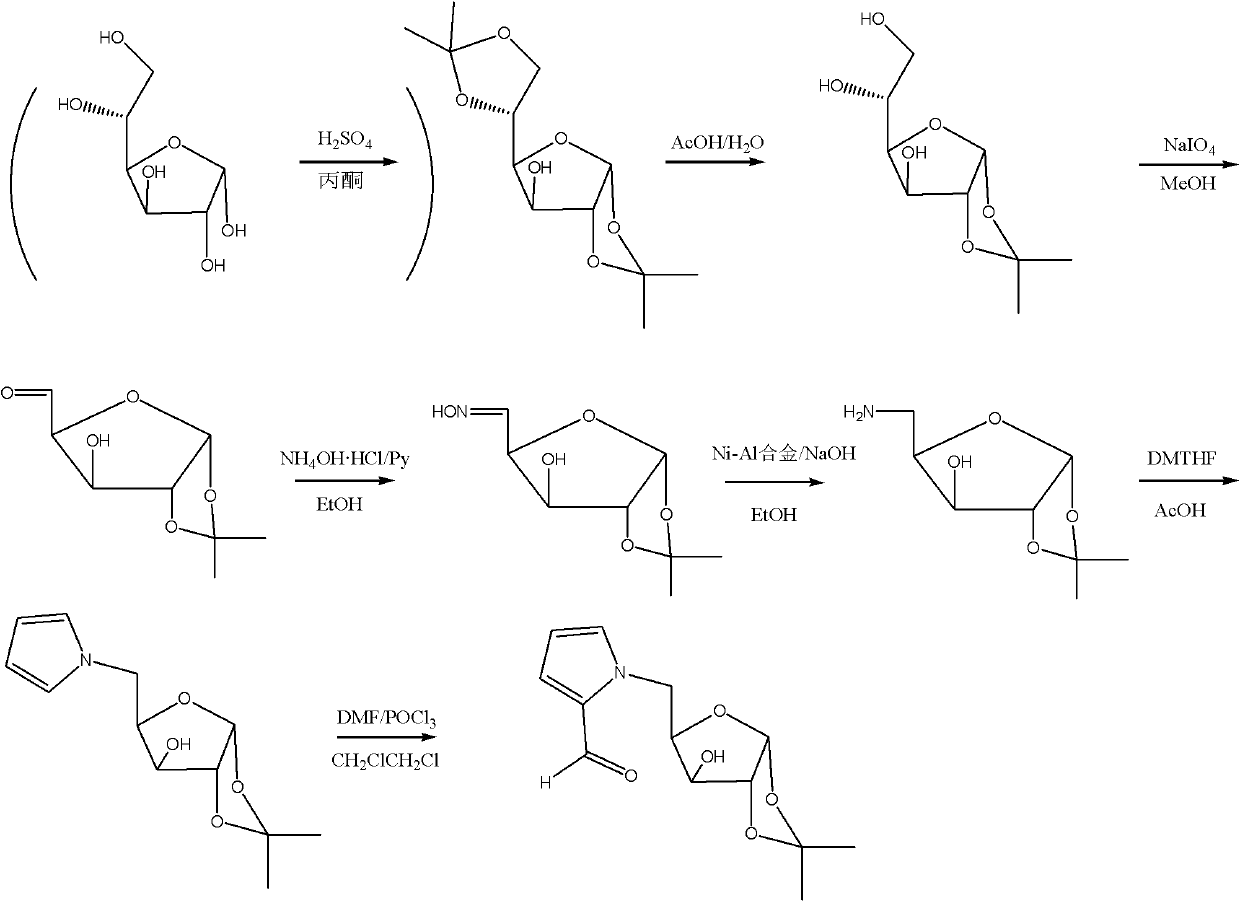
Glycopyrrolate compounds and synthesis method thereof
InactiveCN102241719ASolve the problem of insufficient biological sourcesSimple processSugar derivativesSugar derivatives preparationSynthesis methodsOxygen
The invention discloses glycopyrrolate compounds, which are compounds formed by connecting deoxidized saccharide hydroxyl and a pyrrole ring, and have a structure shown as a general formula I or II, wherein R1, R6, R10, R15 and R16 are independently selected from -H, -OH and monosaccharide hydroxyl protective group; and n1 and n2 are independent integers ranging from 1 to 6. Saccharide ring and pyrrole connected compounds designed and synthesized in the technical scheme are important intermediates for synthesizing natural saccharide quinazoline alkaloid by a chemical method. A problem that biological sources of the compounds are insufficient can be solved; and the technological process is concise, and the compounds are easy to synthesize.
Owner:DALIAN UNIV OF TECH
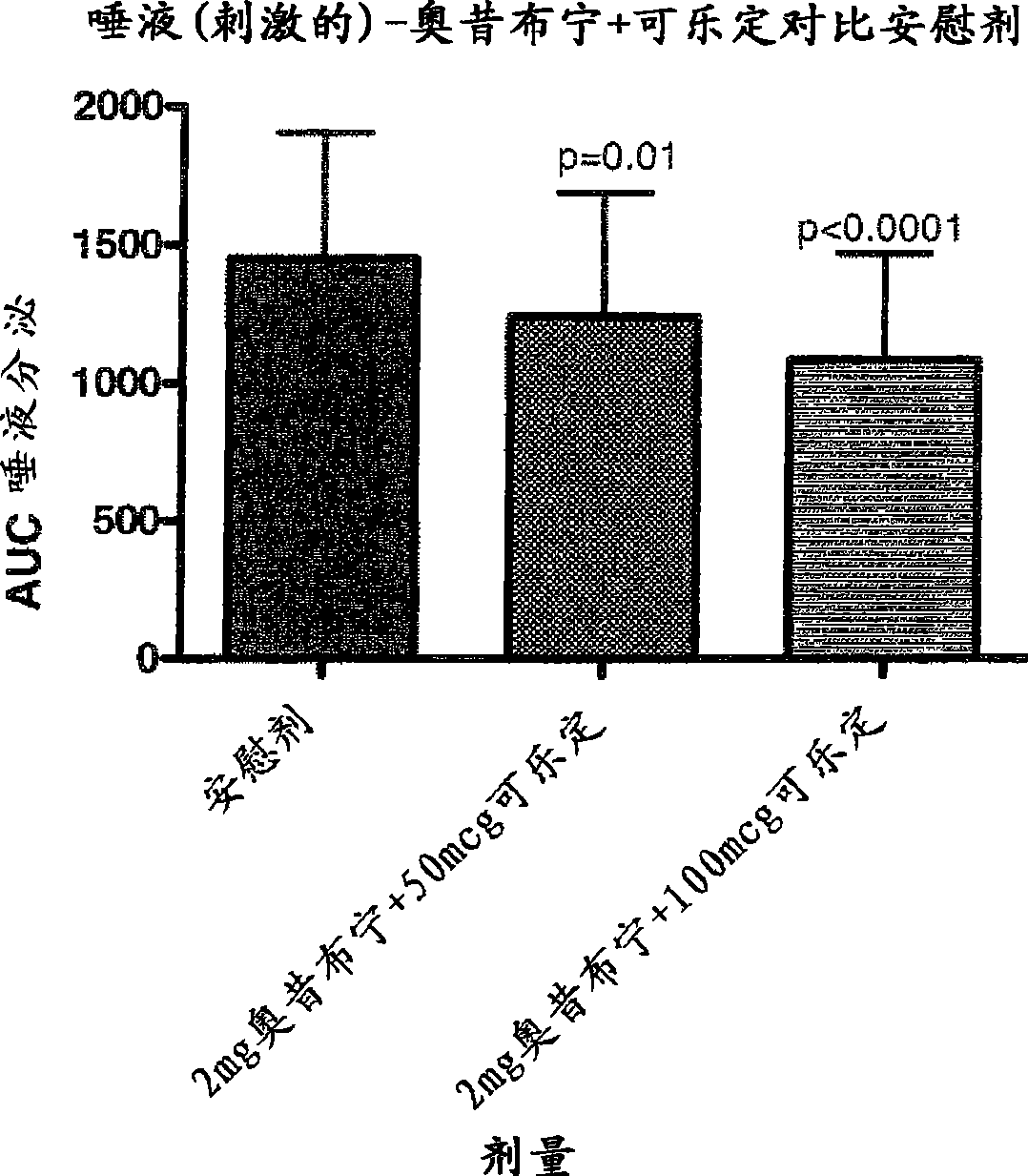
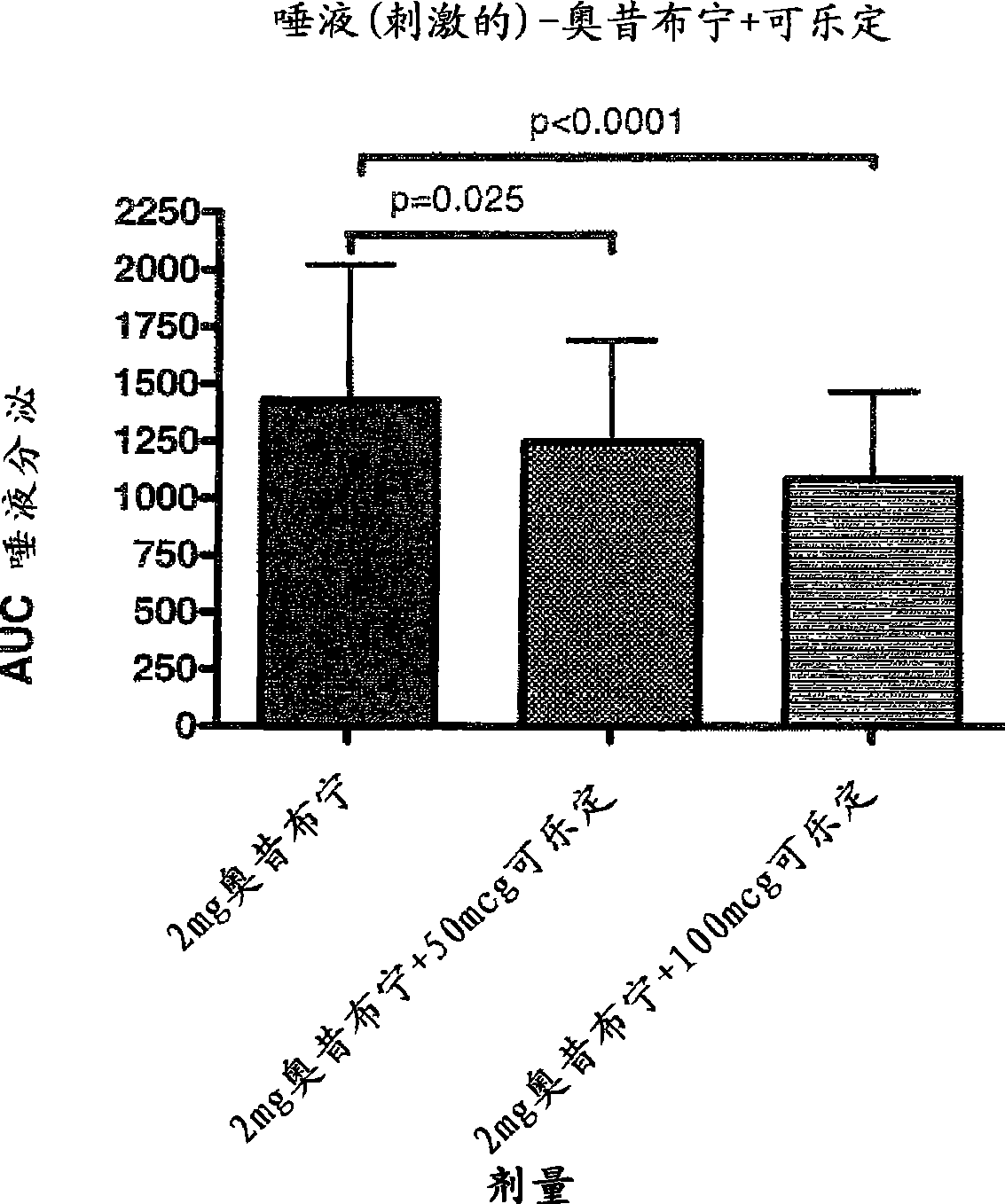
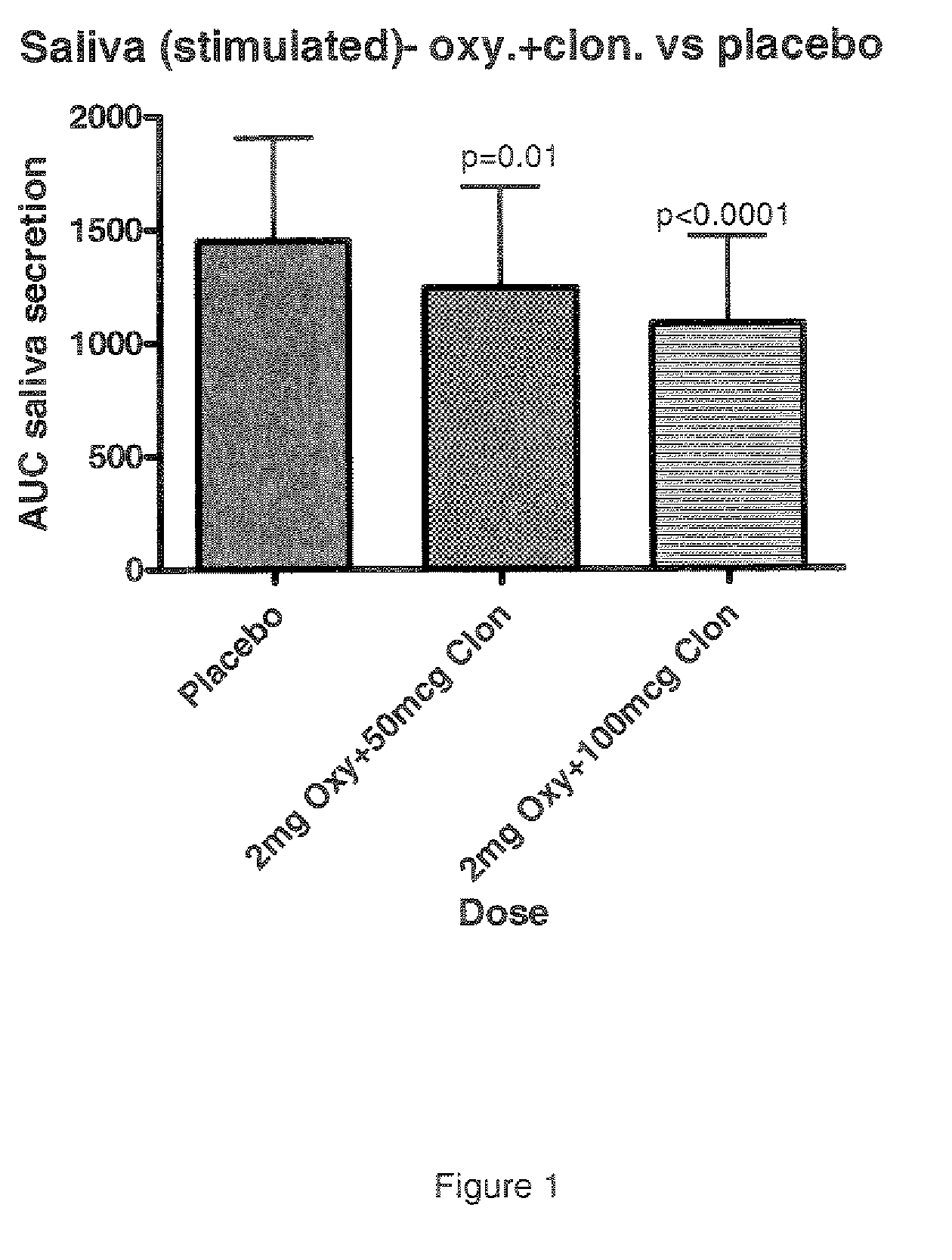
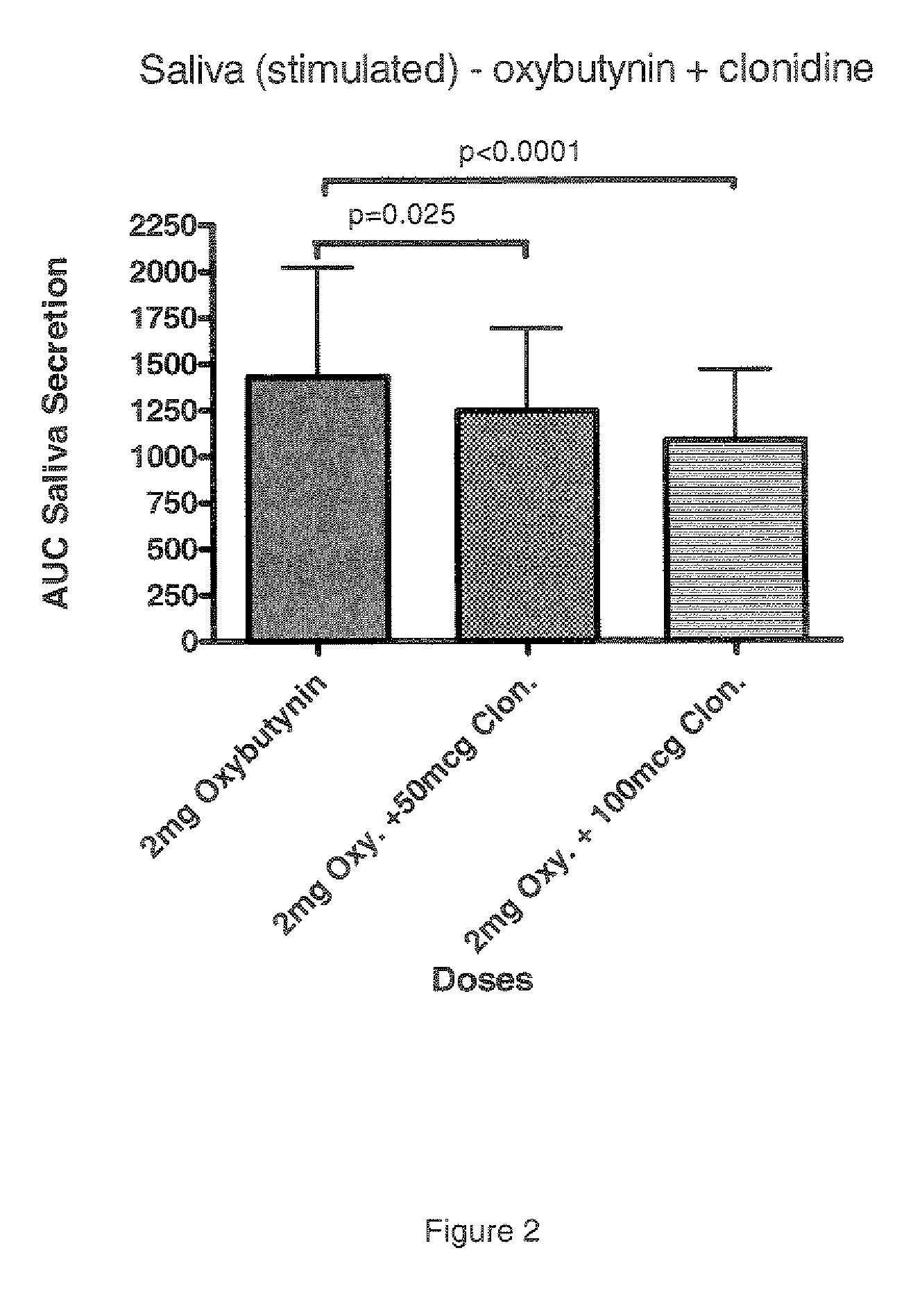
Combination of alpha-2 receptor agonist (clonidin) and an anti-muscarinic agent (oxybutynin) for the treatment of sialorrhoea
InactiveCN101400347AReduce trafficNo side effectsOrganic active ingredientsDrug compositionsOxybutyninMuscarinic antagonist
An alpha2 adrenoreceptor agonist eg. clonidine, brimonidine, monoxidine, lofexidine is useful for the treatment of siaiorrhoea, administered by the paraungual, sublingual or buccal route. The patient to be treated is also given an anti-muscarinic agent eg. oxybutynin, glycopyrrolate, ipratropium.
Owner:东方药业(萨摩亚)有限公司
Method for increasing the bioavailability of glycopyrrolate
InactiveUS7638552B1Promote absorptionImprove bioavailabilityBiocideAnimal repellantsMedical prescriptionGlycopyrrolate
Owner:MERZ PHARMA LLC
Combination of alpha-2 receptor agonist (clonidin) and Anti-muscarinic agent (oxybutynin) for the treatment of sialorrhoea
ActiveUS20090221659A1Good effectEliminate side effectsOrganic active ingredientsBiocideOxybutyninGlycopyrrolate
An alpha2 adrenoreceptor agonist eg. clonidine, brimonidine, monoxidine, lofexidine is useful for the treatment of siaiorrhoea, administered by the paraungual, sublingual or buccal route. The patient to be treated is also given an anti-muscarinic agent eg. oxybutynin, glycopyrrolate, ipratropium.
Owner:ORIENT PHARMA SAMOA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com