Glycopyrrolate for treating childhood asthma
A technology of glycopyrronium bromide and children's asthma, which is applied in the field of children's asthma treatment and can solve problems such as systemic side effects and insufficient dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0061] A 75:25 mass ratio mixture of micronized glycopyrronium bromide and magnesium stearate (total mass approximately 1 g) was placed on top of 100 g of 2 mm diameter stainless steel balls in a ball mill. Grind volume was about 58.8ml. Add 5 ml of cyclohexane to wet the above mixture. The ball mill was sealed and secured in a Retsch S100 centrifuge. It was then centrifuged at 500 rpm for a total of 240 minutes. A small sample (approximately 5-10 mg) of the wet powder was removed from the ball mill every 60 minutes. The samples were vacuum dried in an oven at 37°C.
[0062] The resulting formulations were assayed. The method and results are documented below:
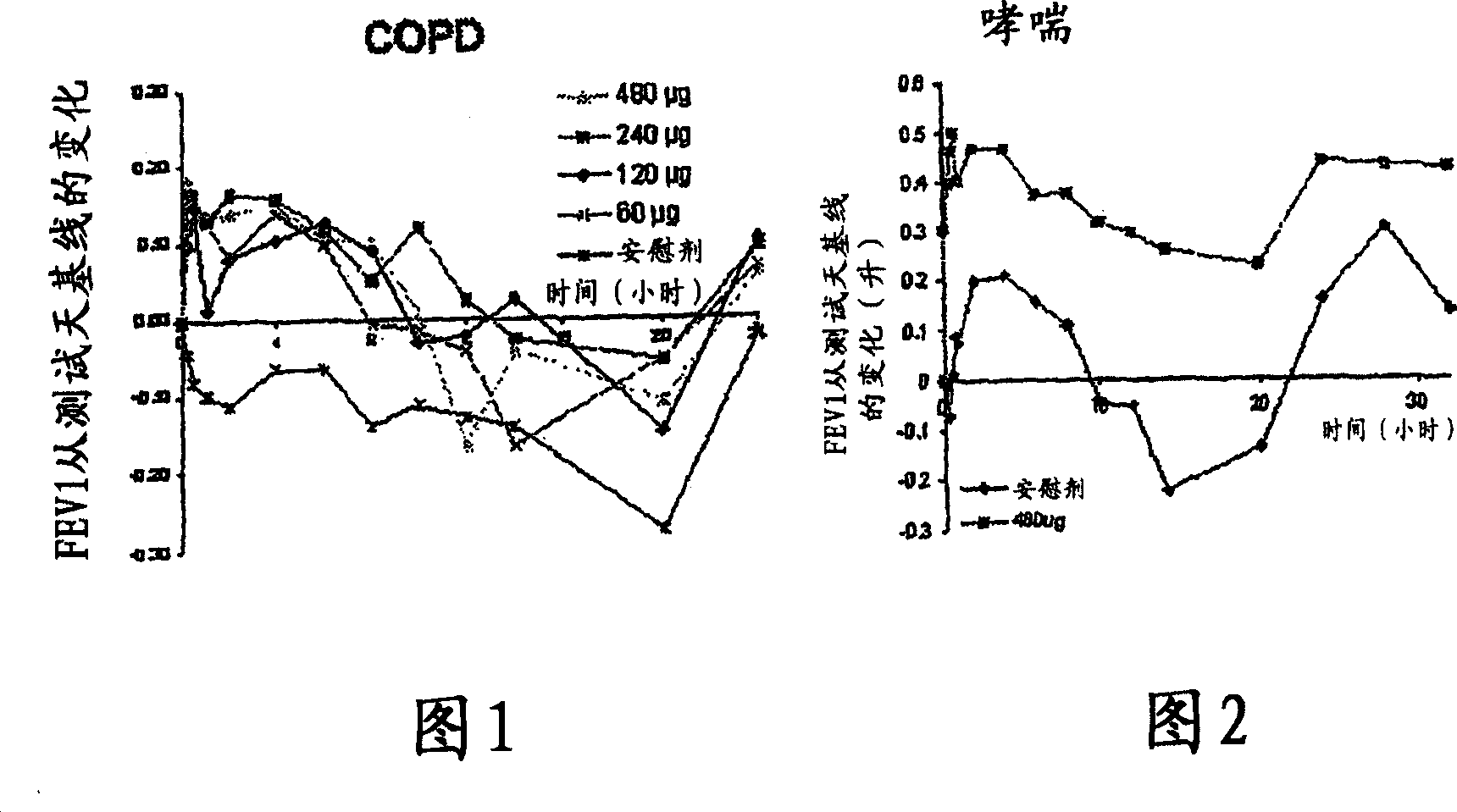
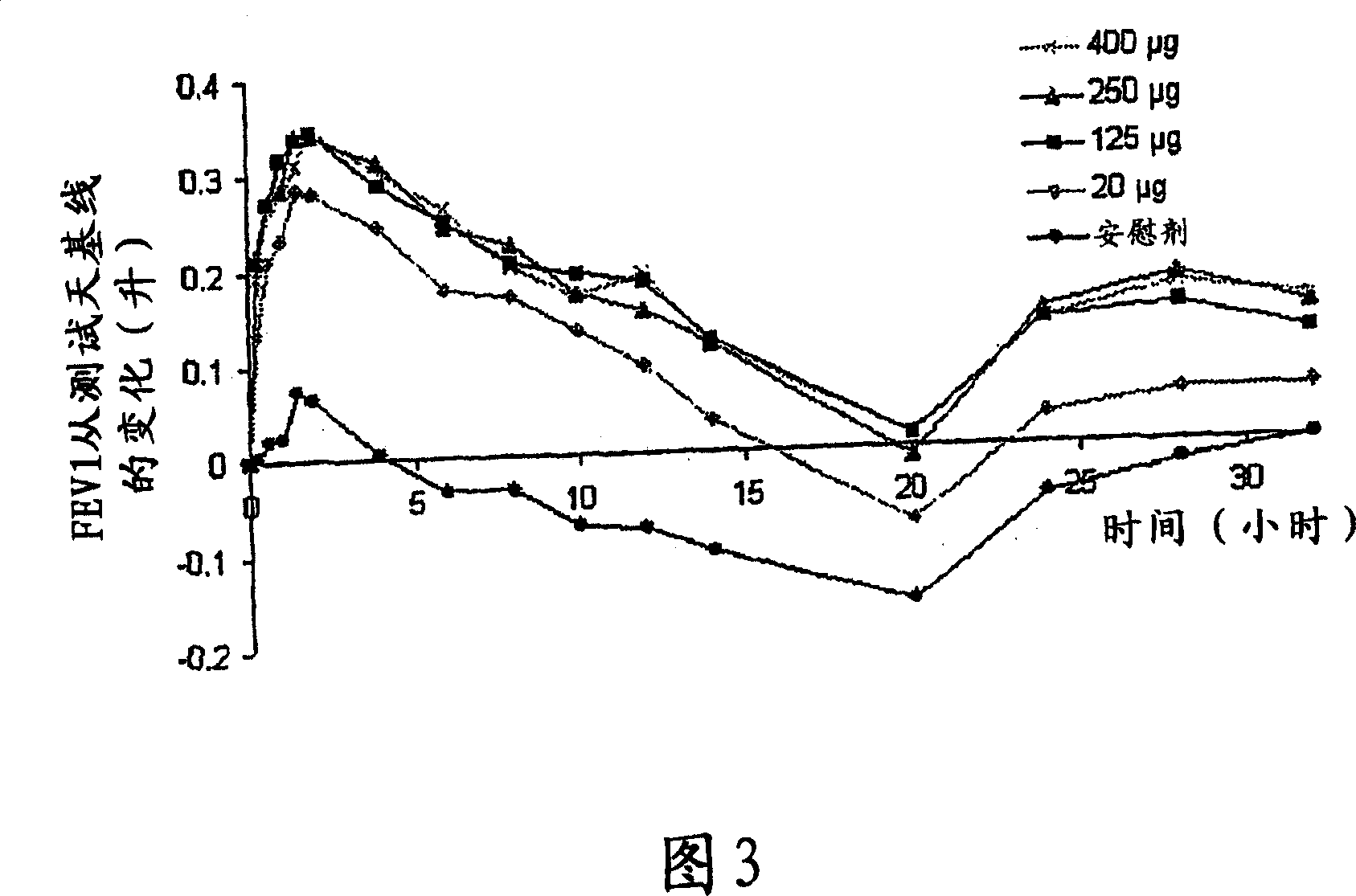
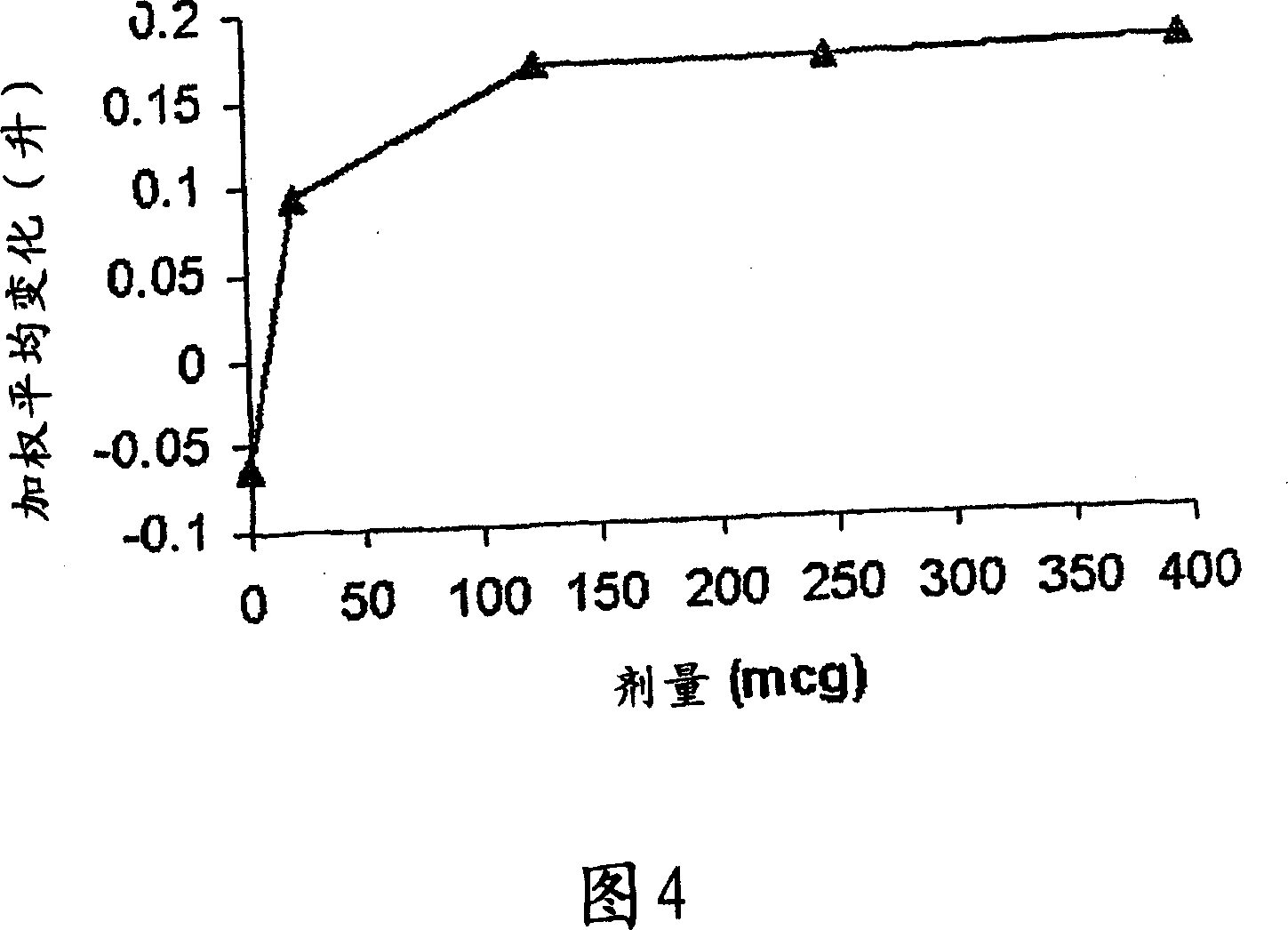
[0063] Primary Research in COPD - Research Criteria
[0064] Single-dose, double-blind, placebo-controlled ascending-dose study
[0065] 4 treatment days: randomly dosed in sequence of 60→120→240→480 μg with placebo
[0066] A total of 8 patients (1 withdrew midway)
[0067] COPD (FEV1; FVC1 <70%)
[0068] to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com