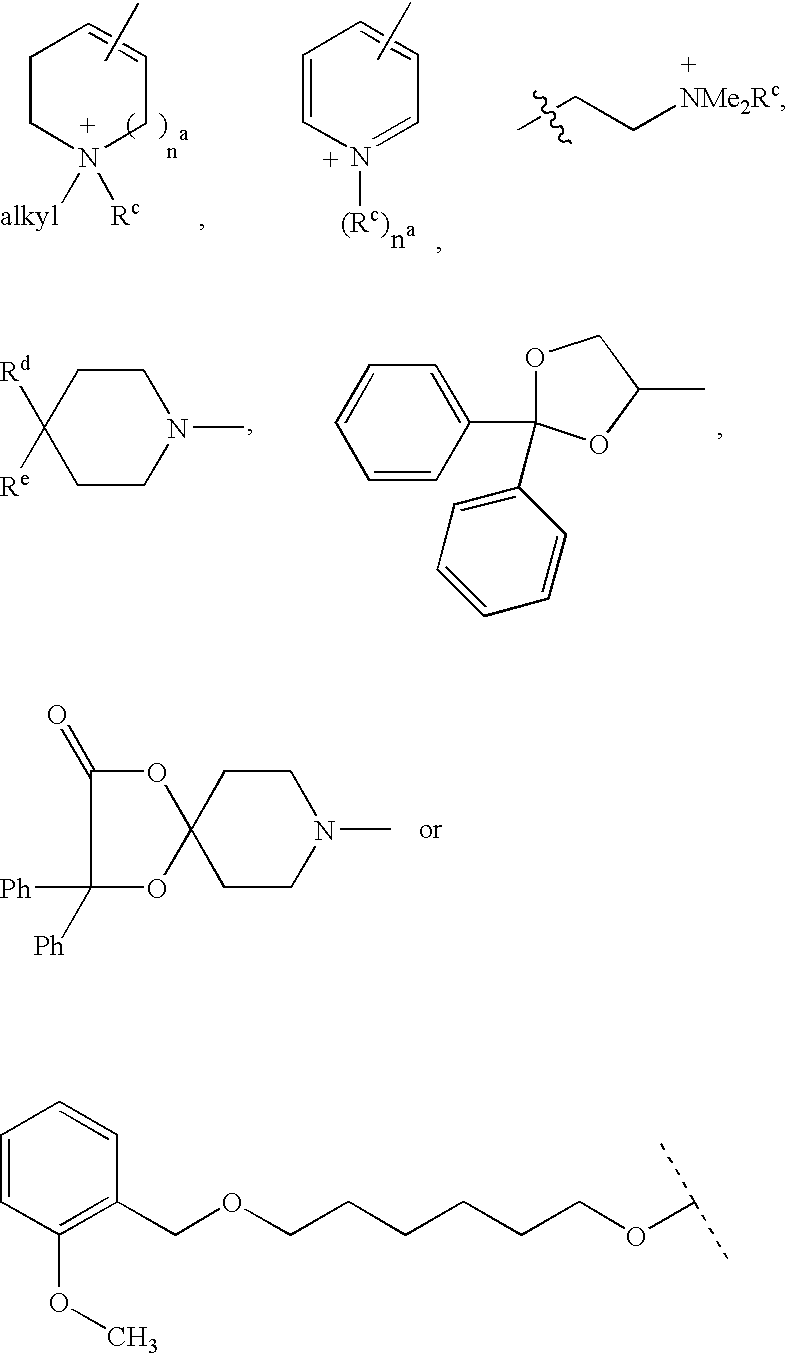
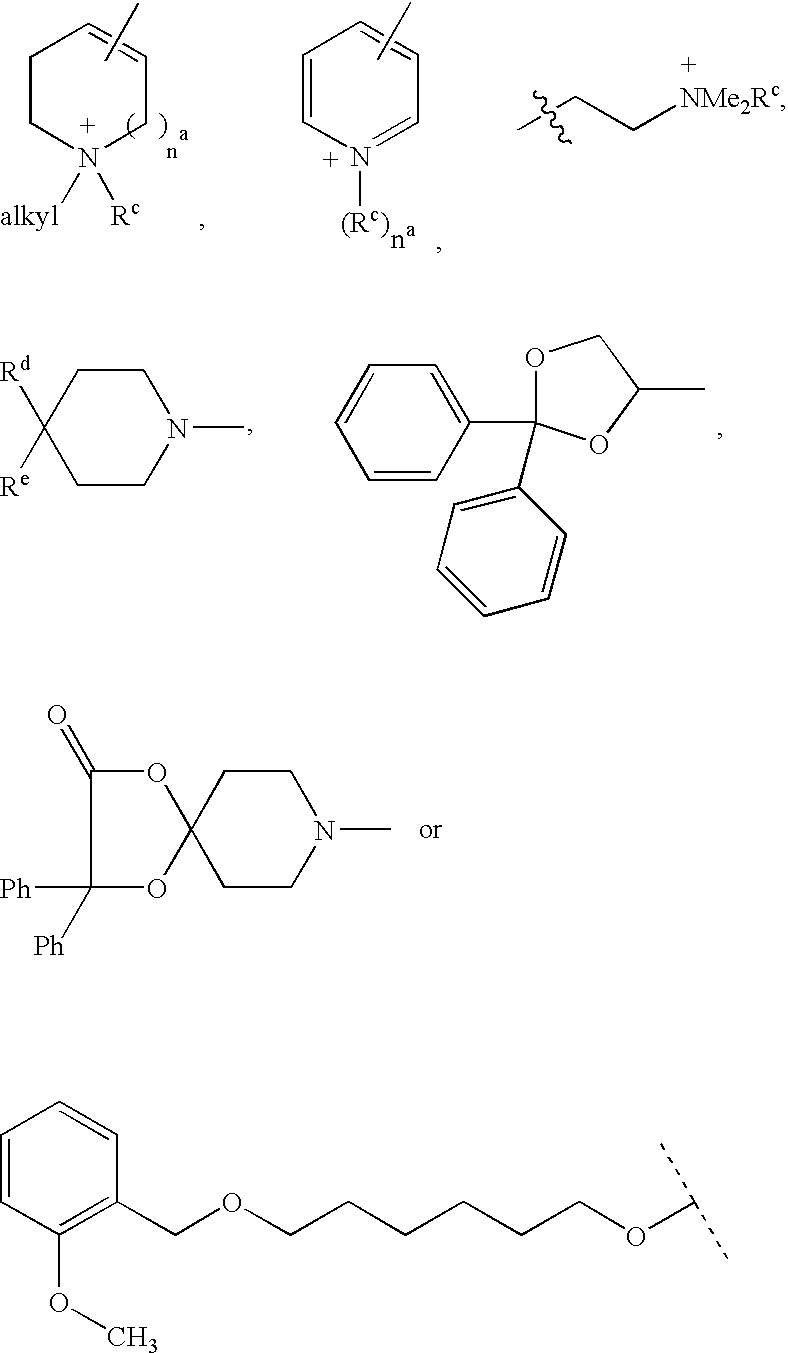
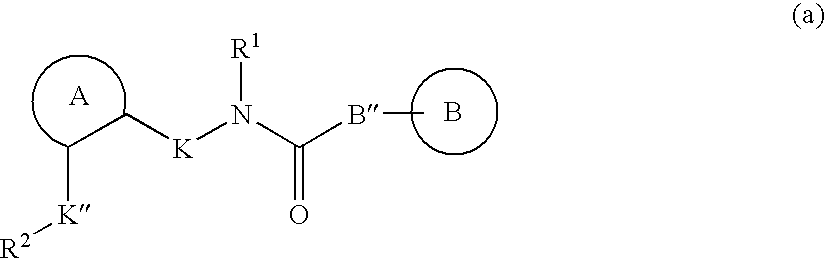
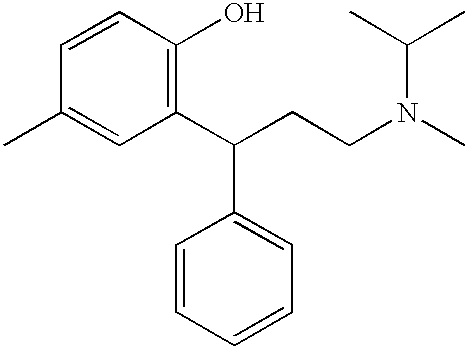
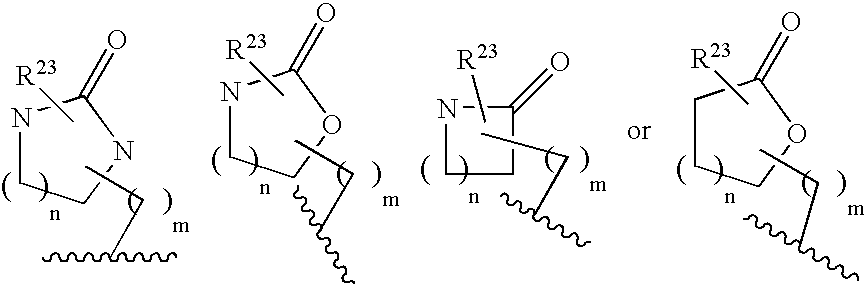
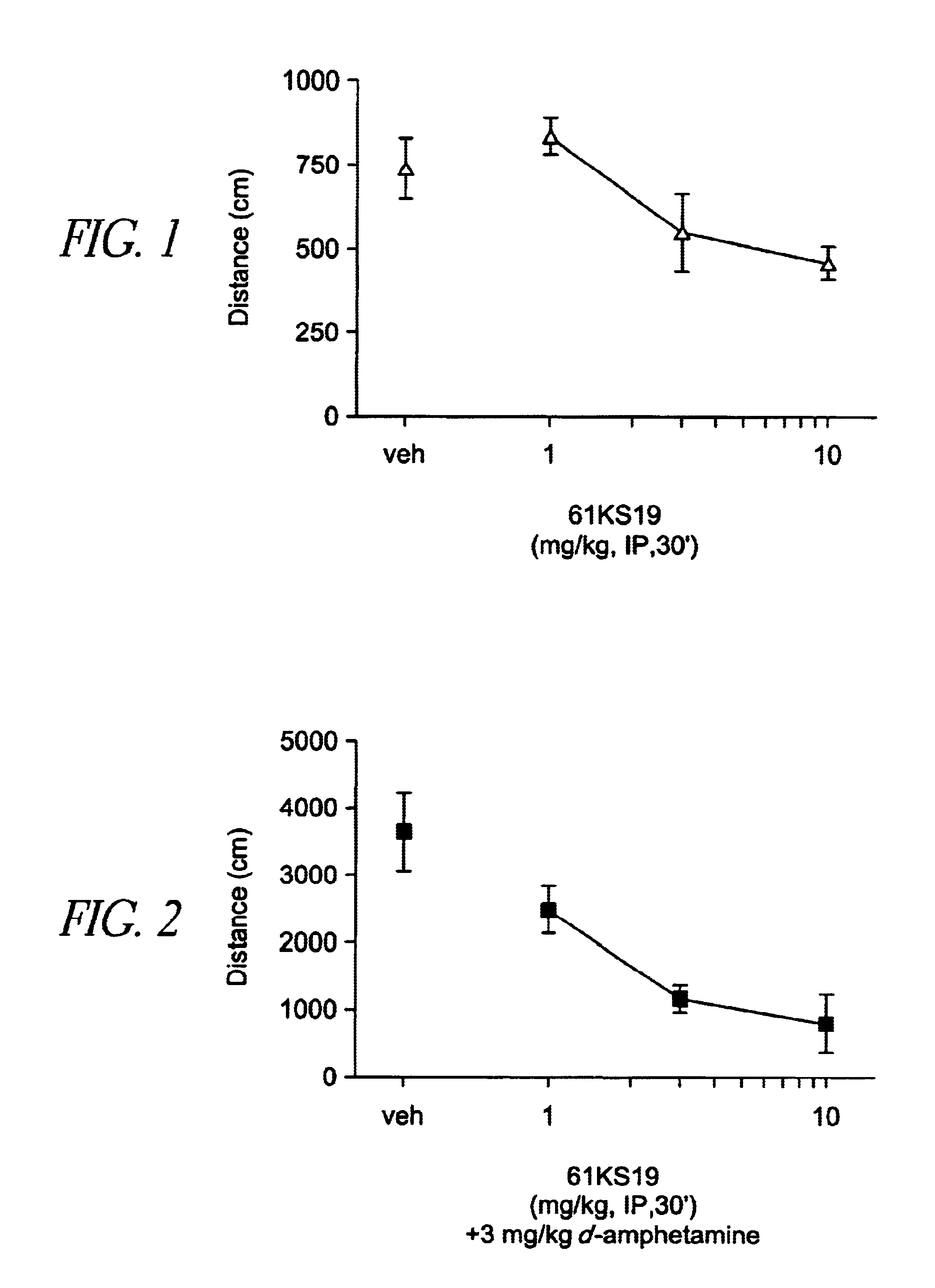
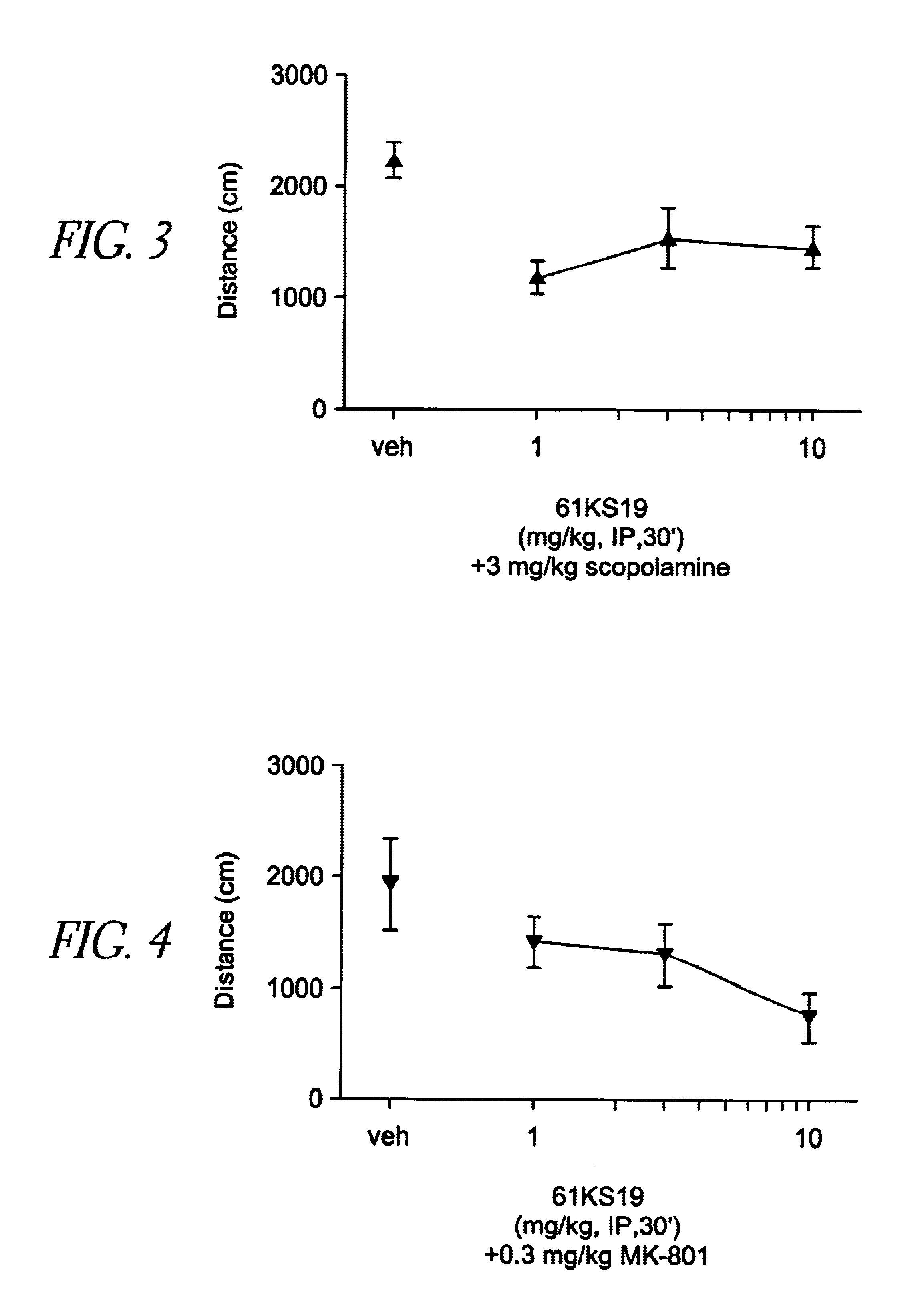
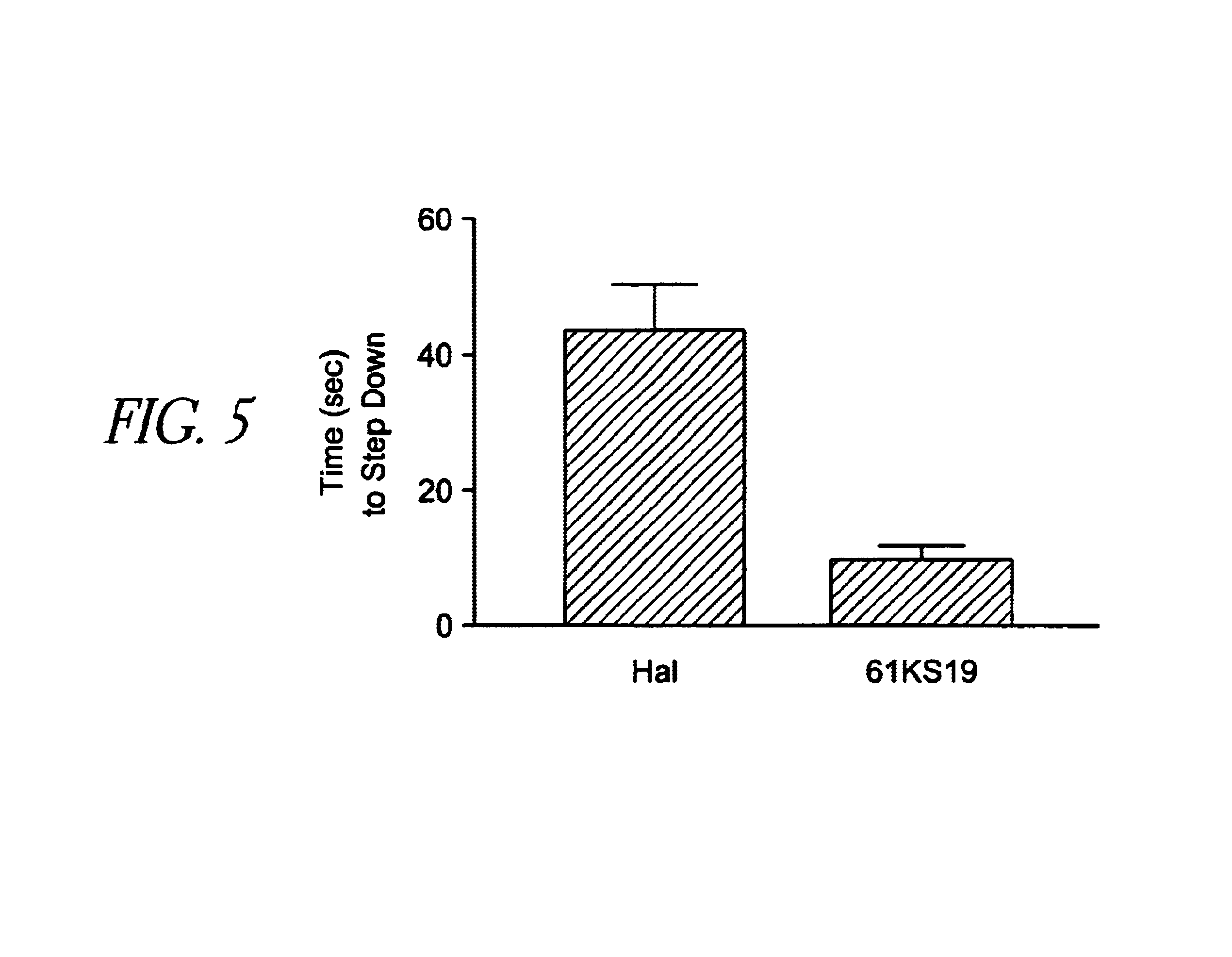
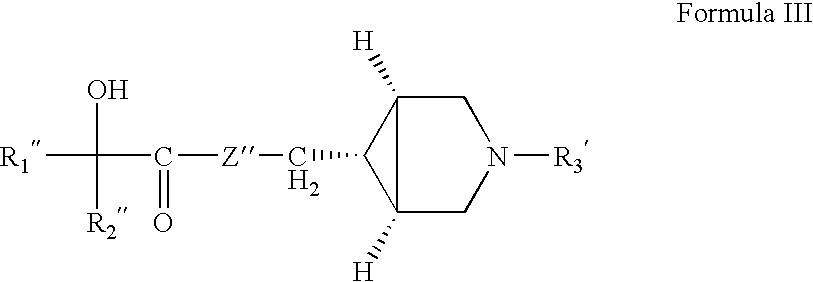
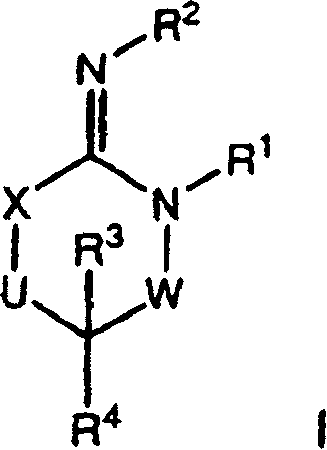
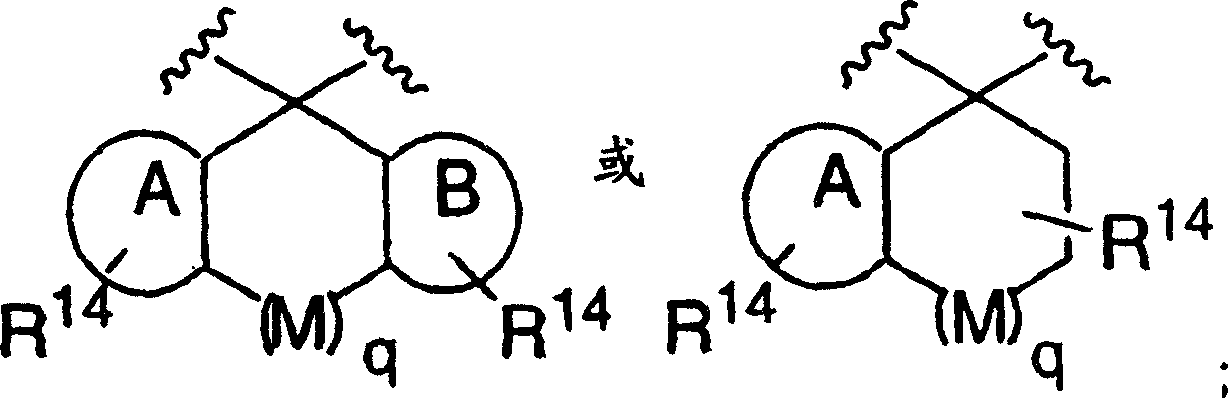
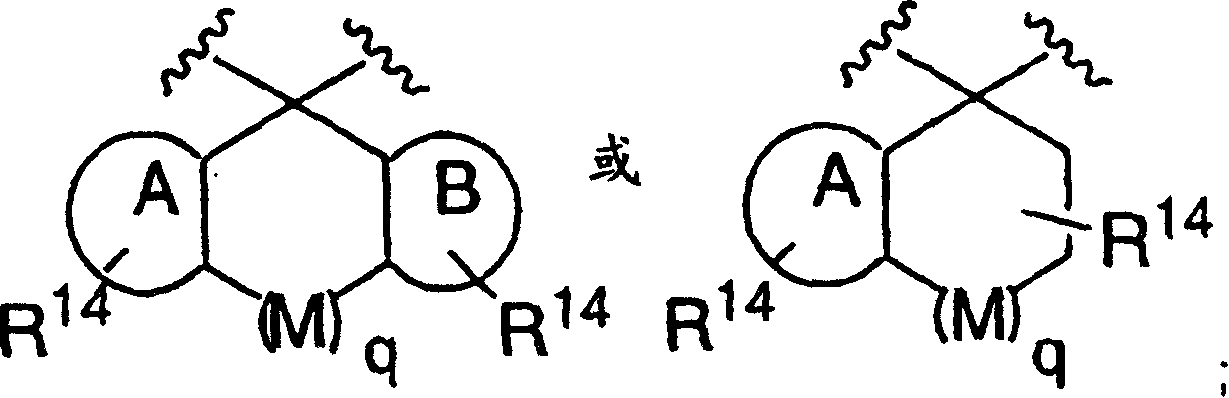
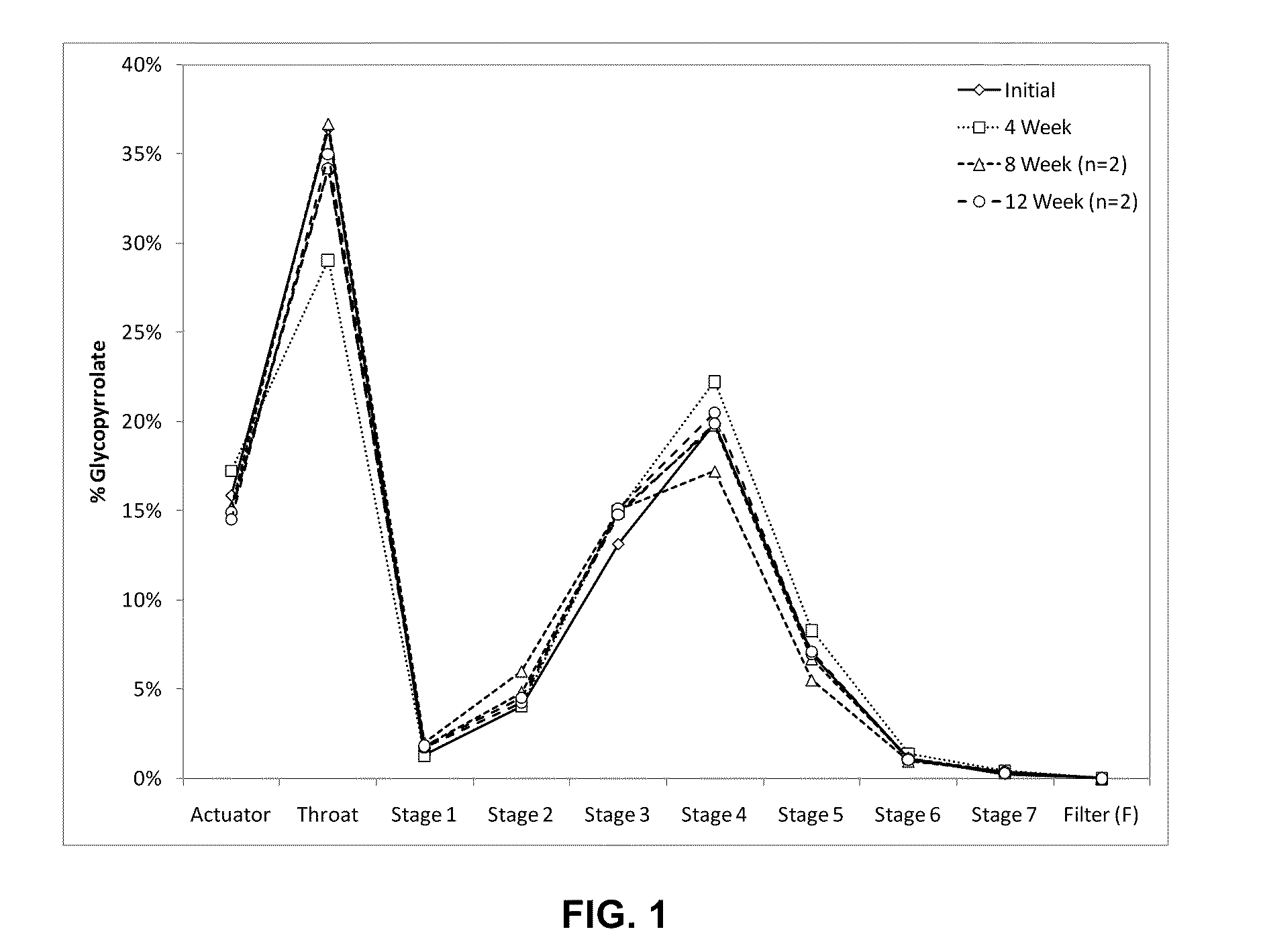
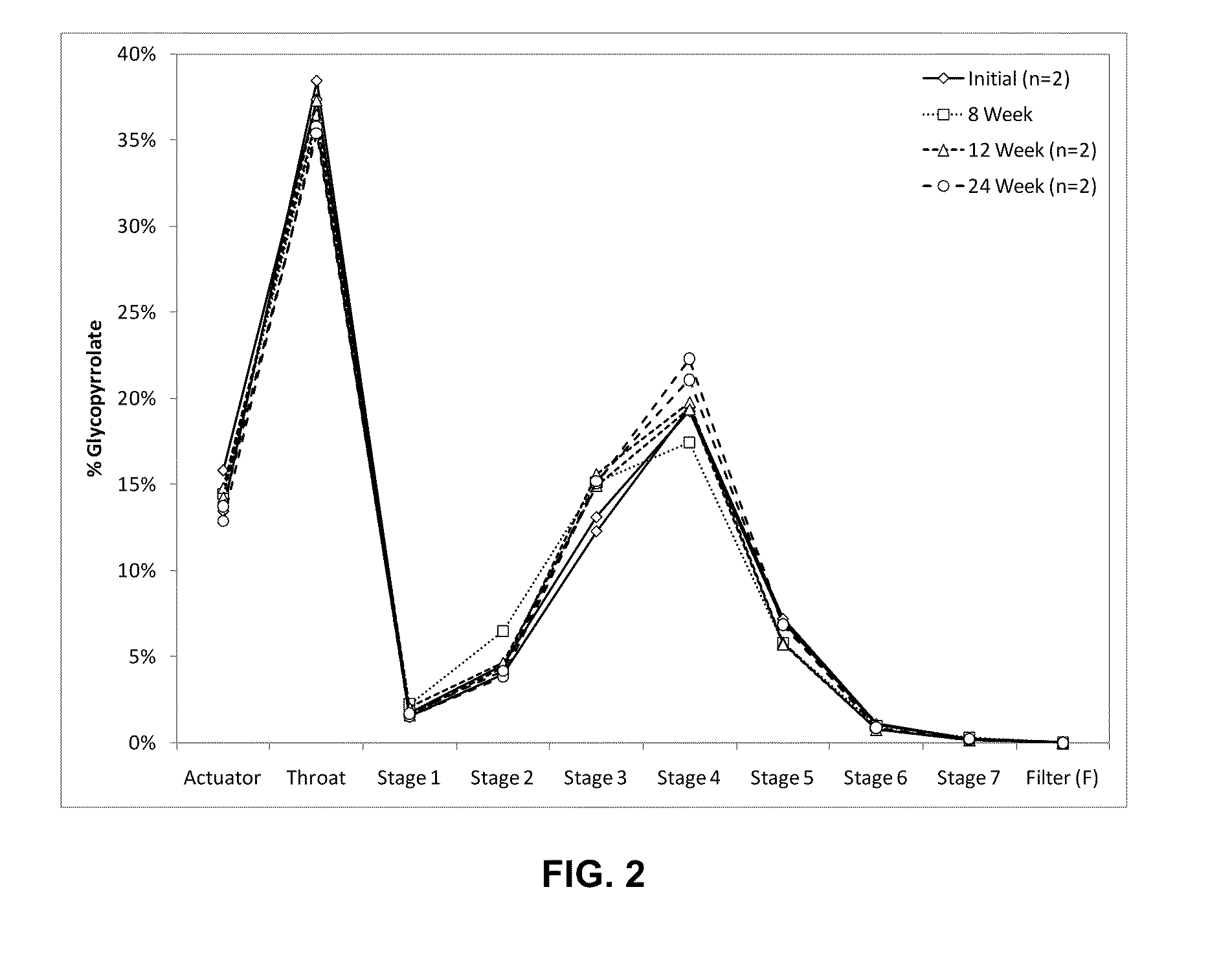
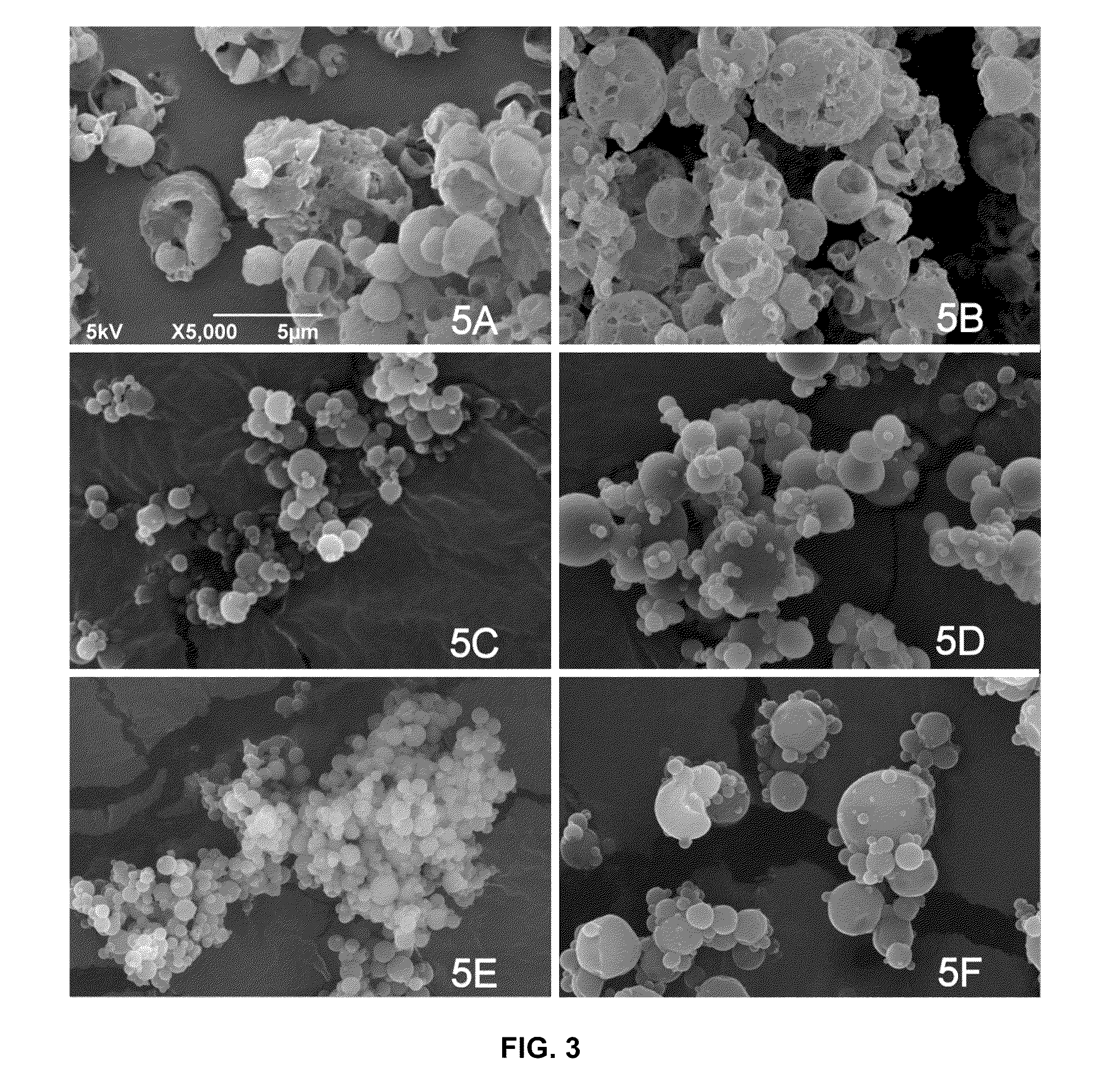
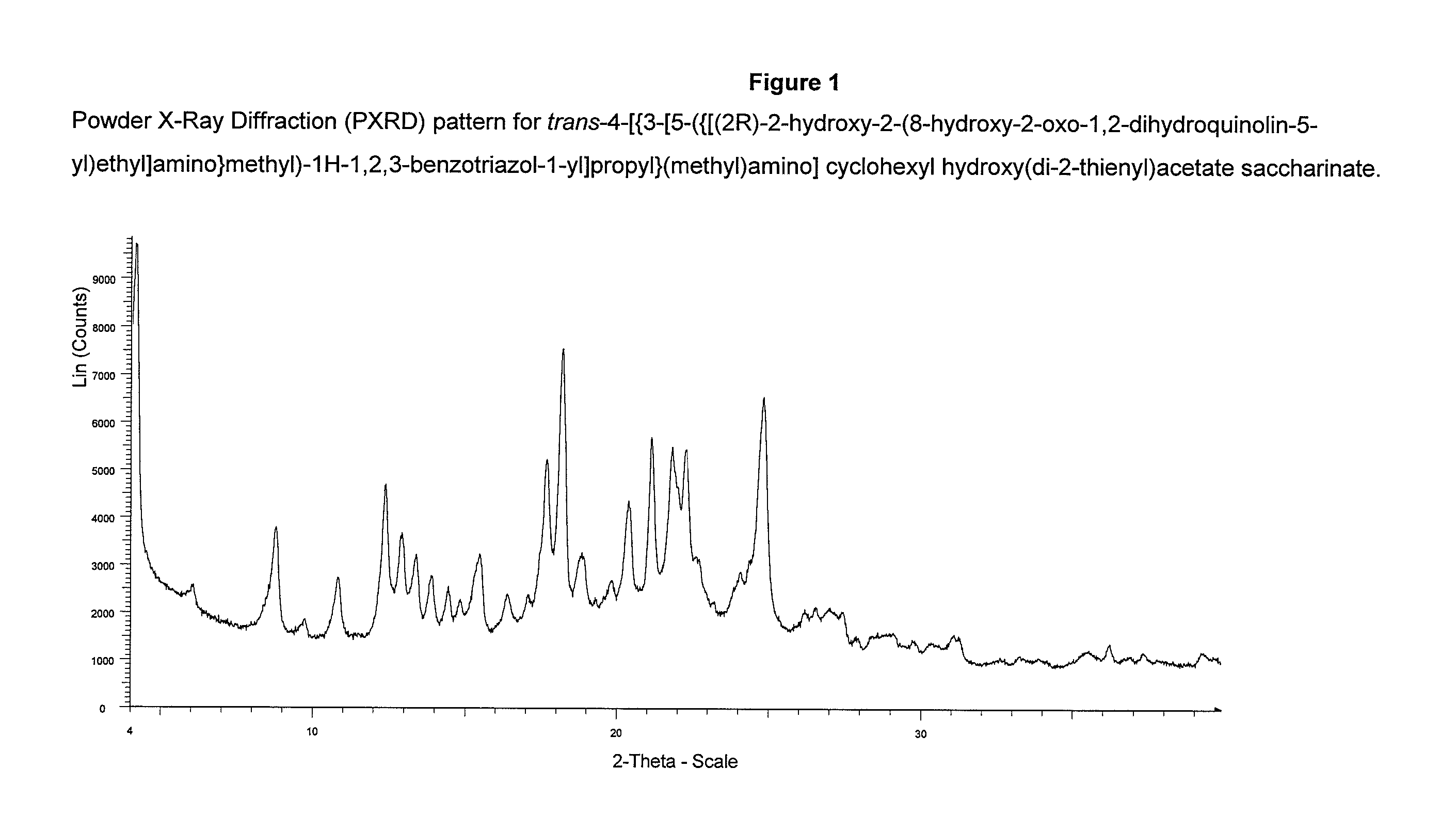
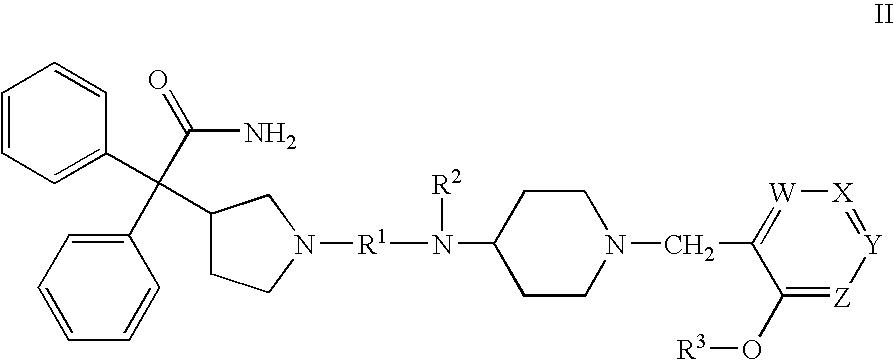
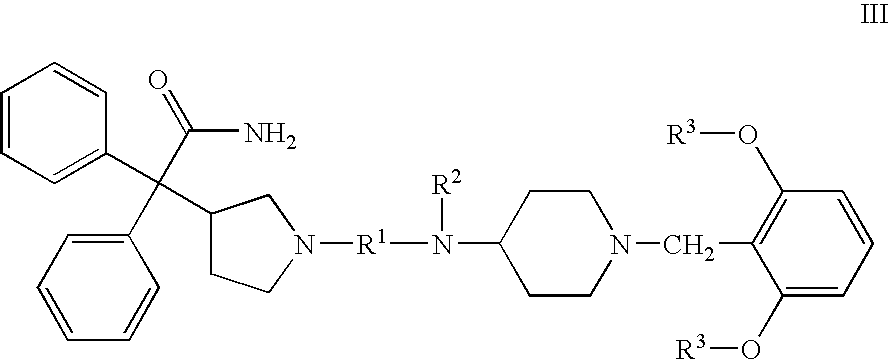
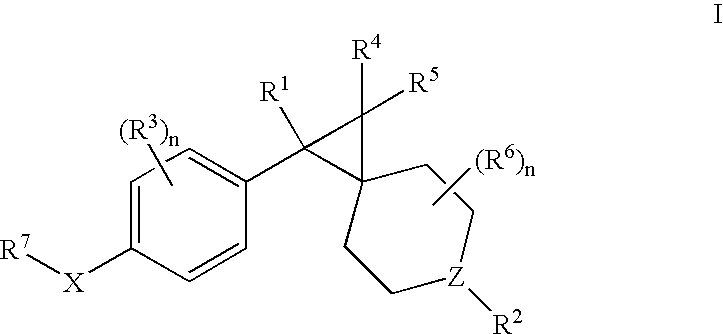
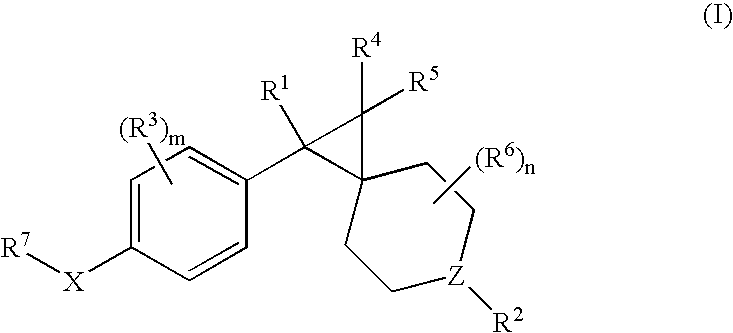
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
87 results about "Muscarinic antagonist" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A muscarinic receptor antagonist (MRA) is a type of anticholinergic agent that blocks the activity of the muscarinic acetylcholine receptor. Acetylcholine (often abbreviated ACh) is a neurotransmitter whose receptor is a protein found in synapses and other cell membranes. Besides responding to their primary neurochemical, neurotransmitter receptors can be sensitive to a variety of other molecules. Acetylcholine receptors are classified into two groups based on this...
Muscarinic receptor antagonists
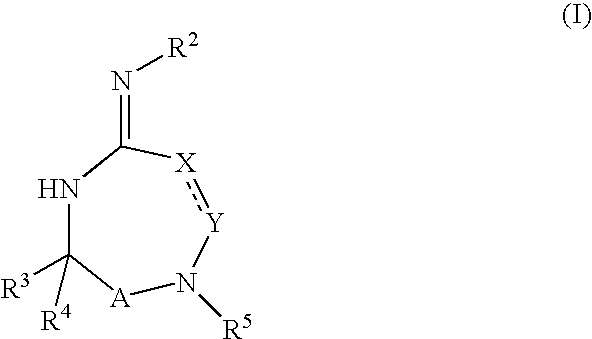
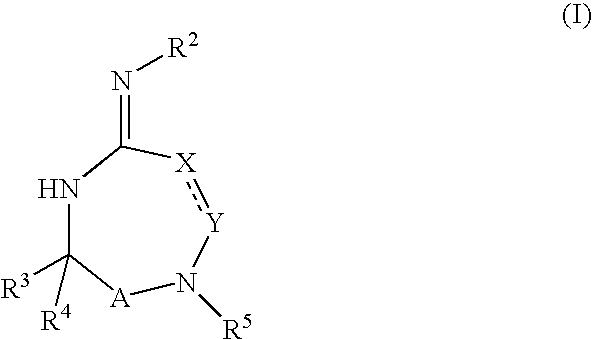
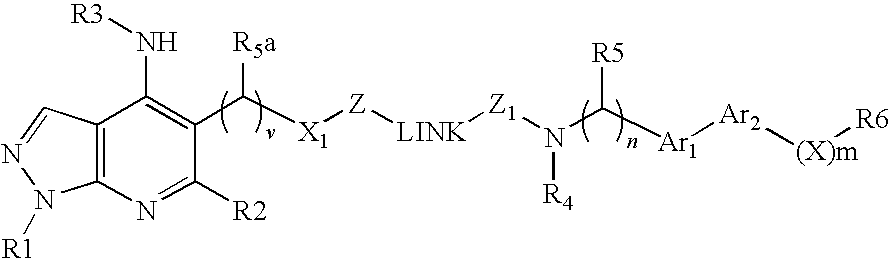
Disclosed are multibinding compounds which are muscarinic receptor antagonists. The multibinding compounds of this invention containing from 2 to 10 ligands covalently attached to one or more linkers. Each ligand is, independently of each other, a muscarinic receptor antagonist or an allosteric modulator provided that at least one of said ligand is a muscarinic receptor antagonist. The multibinding compounds of this invention are useful in the treatment and prevention of diseases such as chronic obstructive pulmonary disease, chronic bronchitis, irritable bowel syndrome, urinary incontinence, and the like.
Owner:THERAVANCE BIOPHARMA R&D IP LLC
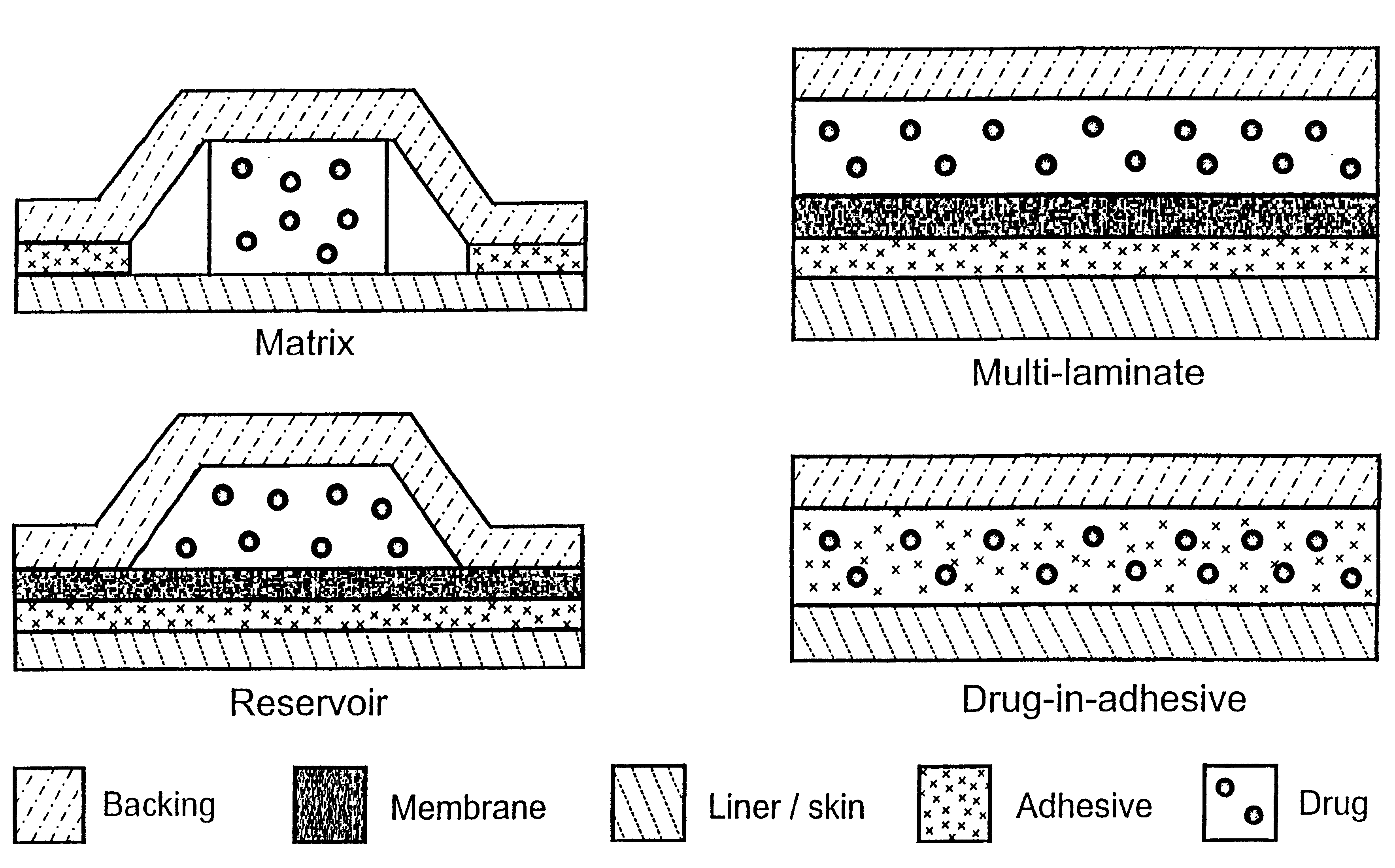
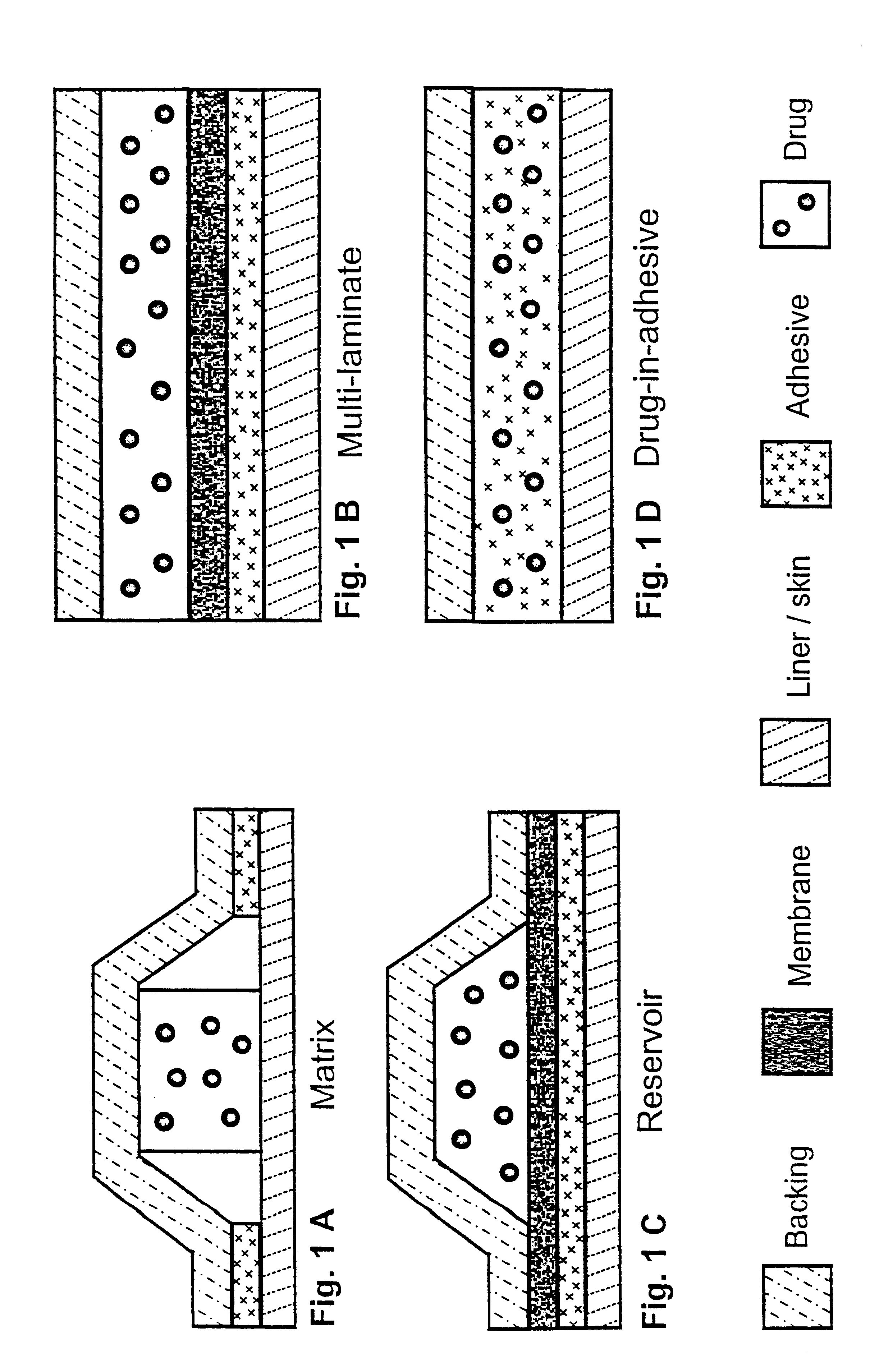
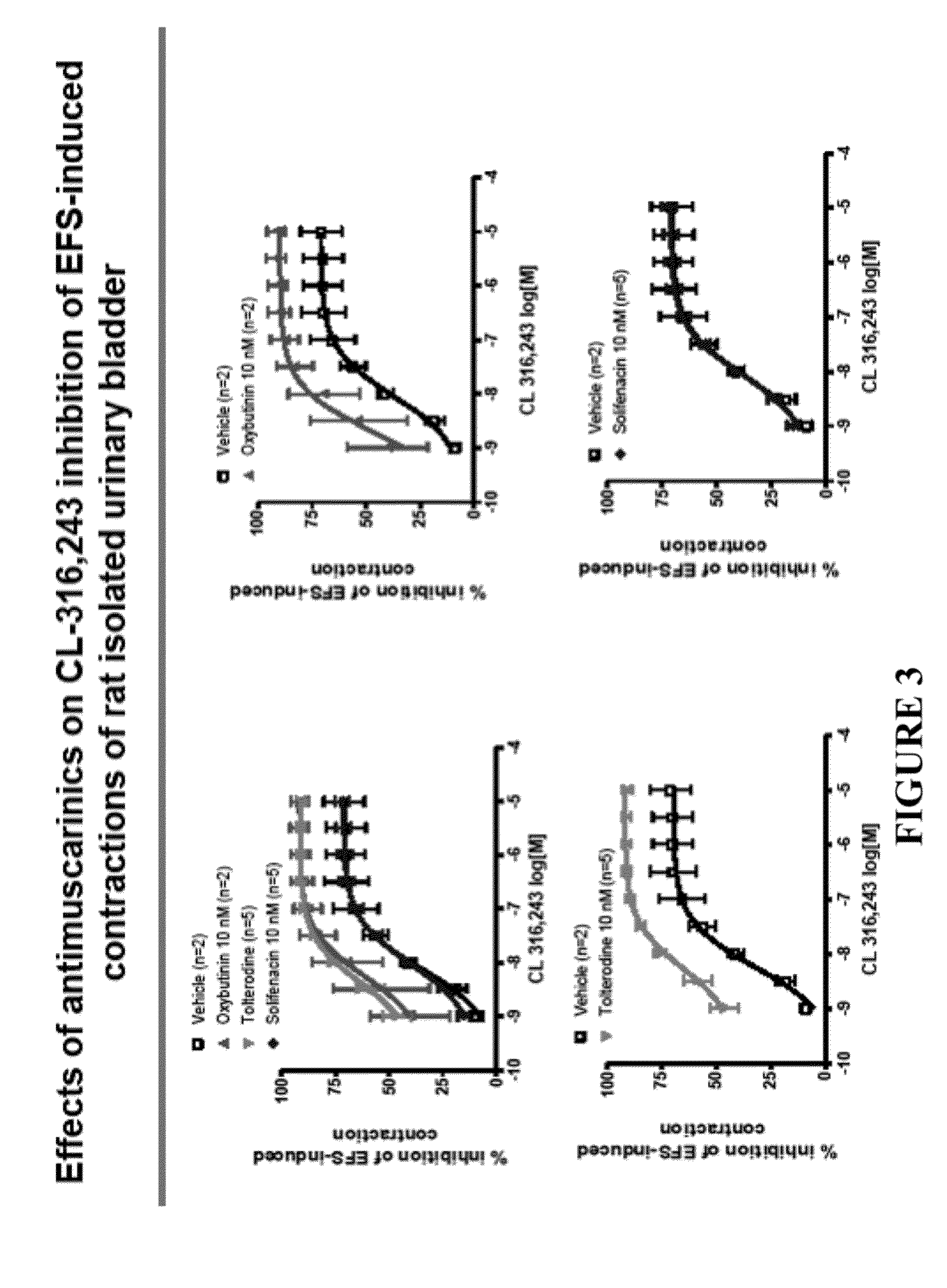
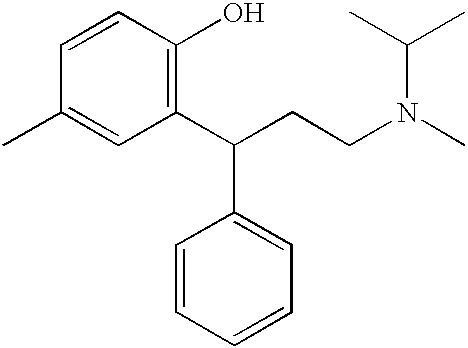
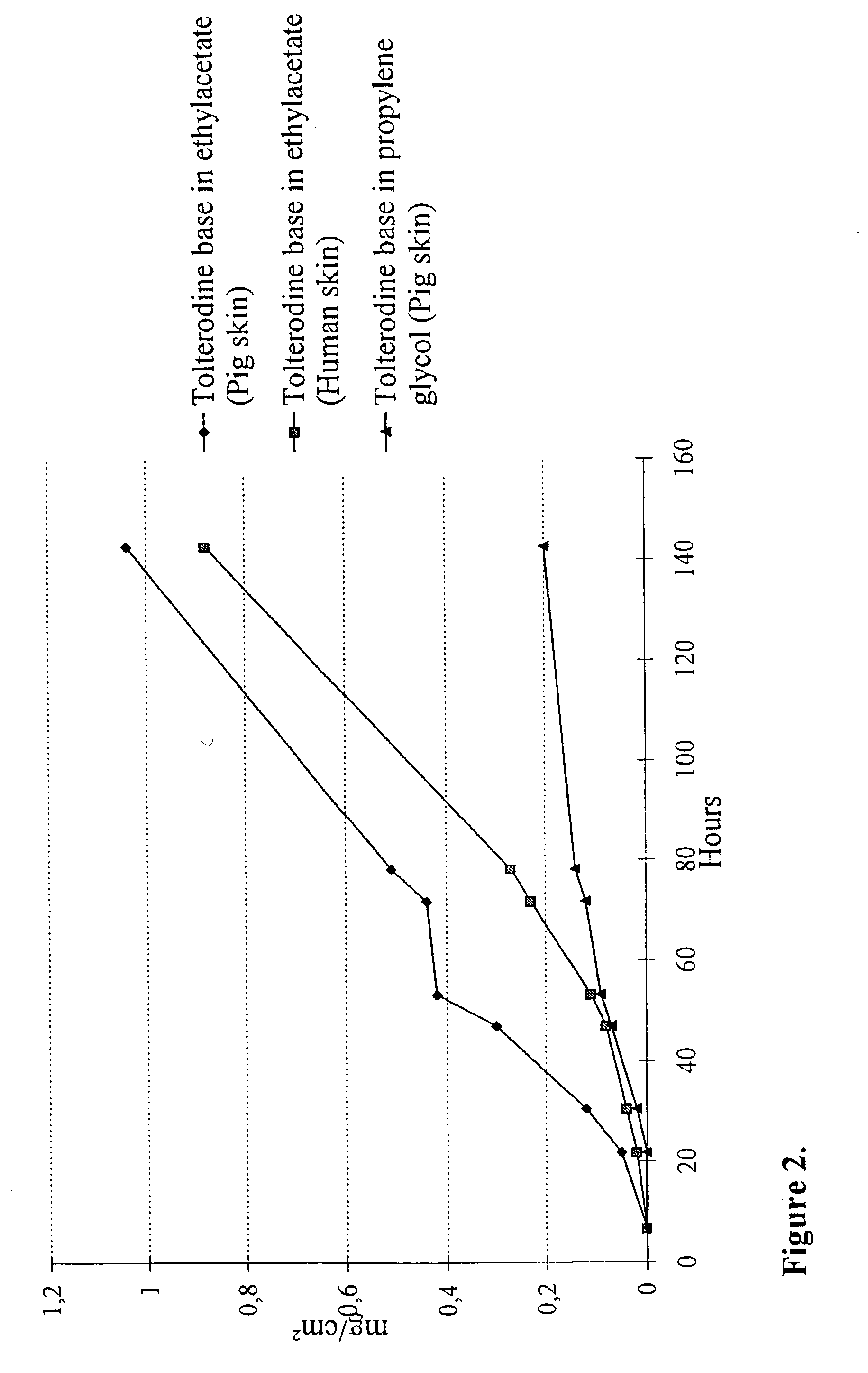
Transdermally administered tolterodine as anti-muscarinic agent for the treatment of overactive bladder
InactiveUS6517864B1Achieve effectClinical efficacyOrganic active ingredientsAerosol deliveryMuscarinic antagonistMetabolite
Device for transdermal administration of tolterodine, optionally encompassing salts, prodrugs and metabolites thereof, optionally together with pharmaceutically acceptable carrier(s) to a human being or an animal in order to achieve an effect against overactive bladder. Use of a compound having an effect against overactive bladder comprising tolterodine, optionally encompassing salts, prodrugs and metabolites thereof, and optionally together with pharmaceutically acceptable carrier(s), for the manufacture of a composition to be administered transdermally for achieving an effect against overactive bladder. Method for achieving an effect against overactive bladder in a living body by transdermal administration of a compound comprising tolterodine, optionally encompassing salts, prodrugs and metabolites thereof, and optionally together with pharmaceutically acceptable carrier(s).
Owner:MCNEIL AB +1
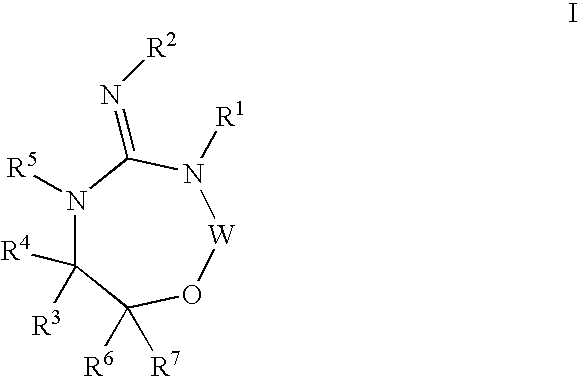
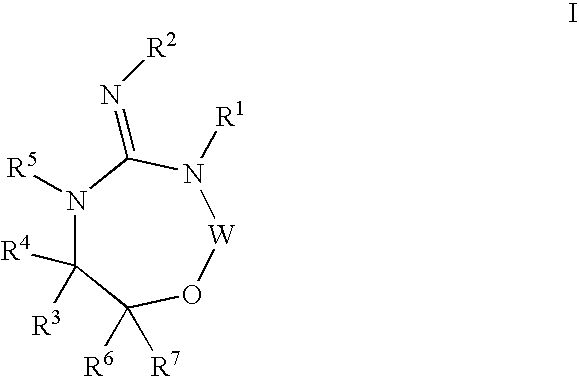
Aspartyl protease inhibitors
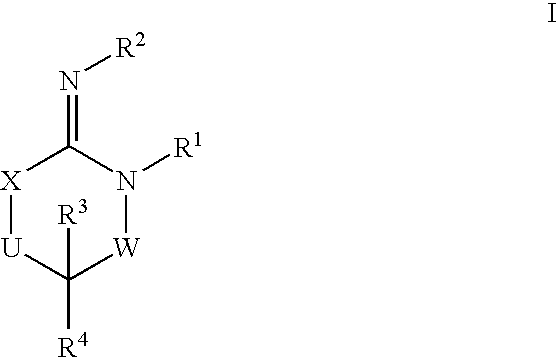
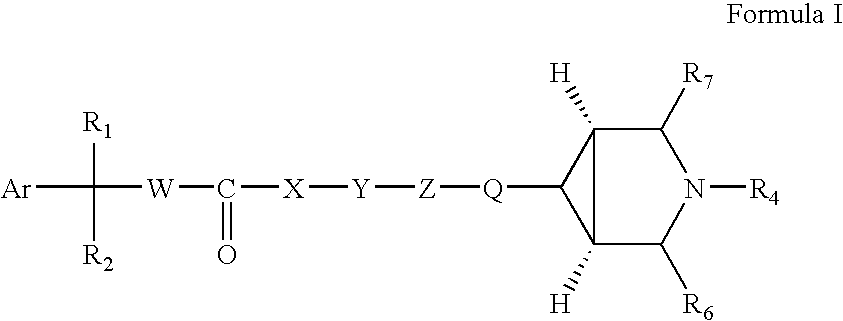
Disclosed are compounds of the formula I or a stereoisomer, tautomer, or pharmaceutically acceptable salt or solvate thereof, wherein W, R1, R2, R3, R4, R5, R6, and R7 are as defined in the specification; and pharmaceutical compositions comprising the compounds of formula I. Also disclosed is the method of inhibiting aspartyl protease, and in particular, the methods of treating cardiovascular diseases, cognitive and neurodegenerative diseases, and the methods of inhibiting of Human Immunodeficiency Virus, plasmepins, cathepsin D and protozoal enzymes. Also disclosed are methods of treating cognitive or neurodegenerative diseases using the compounds of formula I in combination with a cholinesterase inhibitor or a muscarinic antagonist.
Owner:SCHERING CORP
Benzimidazolidinone derivatives as muscarinic agents
InactiveUS6951849B2Inhibition formationInhibit progressBiocideNervous disorderMuscarinic antagonistReceptor subtype
Benzimidazolidinone derivative compounds, which increase acetylcholine signaling or effect in the brain, and highly selective muscarinic agonists, particularly for the M1 and / or M4 receptor subtypes, pharmaceutical compositions comprising the same, as well as methods of treating psychosis using these compounds are disclosed.
Owner:ACADIA PHARMA INC
Preparation and use of compounds as aspartyl protease inhibitors
ActiveUS20070010667A1Nervous disorderMetabolism disorderImmunodeficiency virusNeuro-degenerative disease
Disclosed are compounds of the formula I or a stereoisomer, tautomer, or pharmaceutically acceptable salt or solvate thereof, wherein A is a bond, —C(O)—, or —C(R3′)(R4′)—; X is —N(R1)— or —C(R6)(R7)—; Y is —S(O)2—, —C(═O)—, —PO(OR9) or —C(R6′R7′)—; is a single or double bond and R, R1, R2, R3, R4, R3′, R4′, R5, R6, R6′, R7 and R7′ are as defined in the specification; and pharmaceutical compositions comprising the compounds of formula I. Also disclosed is the method of inhibiting aspartyl protease, and in particular, the methods of treating cardiovascular diseases, cognitive and neurodegenerative diseases, and the methods of inhibiting of Human Immunodeficiency Virus, plasmepins, cathepsin D and protozoal enzymes. Also disclosed are methods of treating cognitive or neurodegenerative diseases using the compounds of formula I in combination with a cholinesterase inhibitor or a muscarinic antagonist.
Owner:MERCK SHARP & DOHME LLC
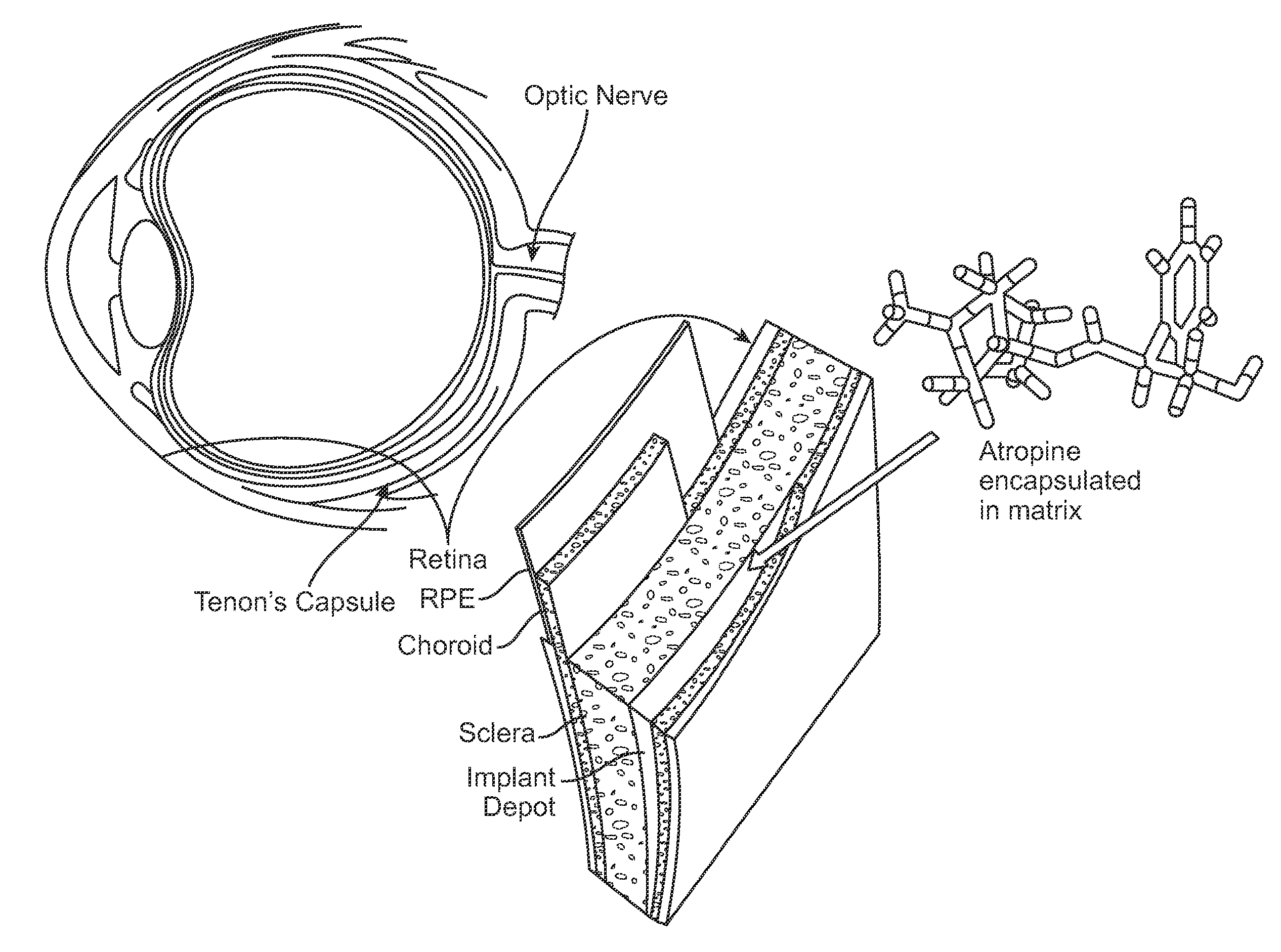
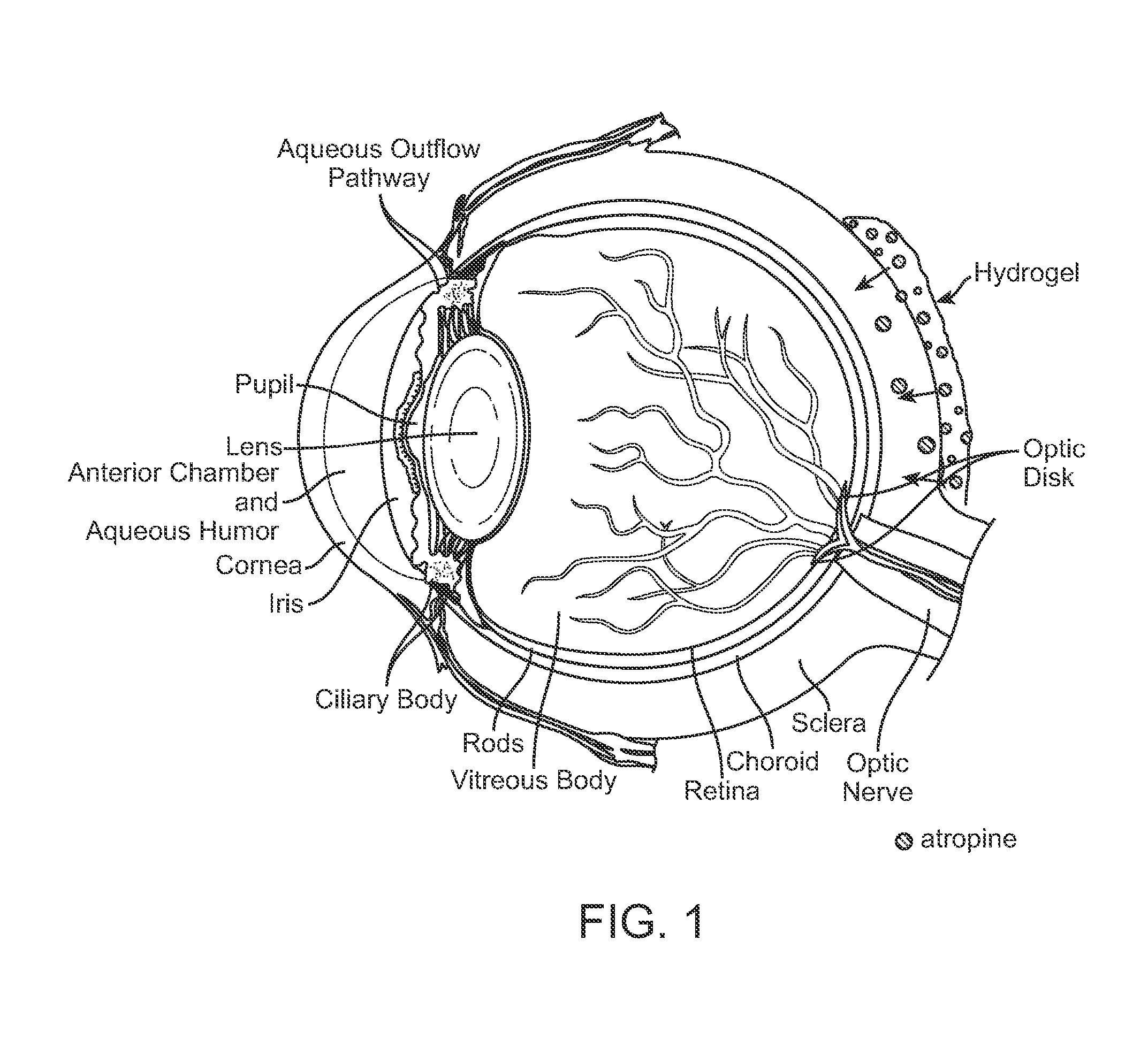
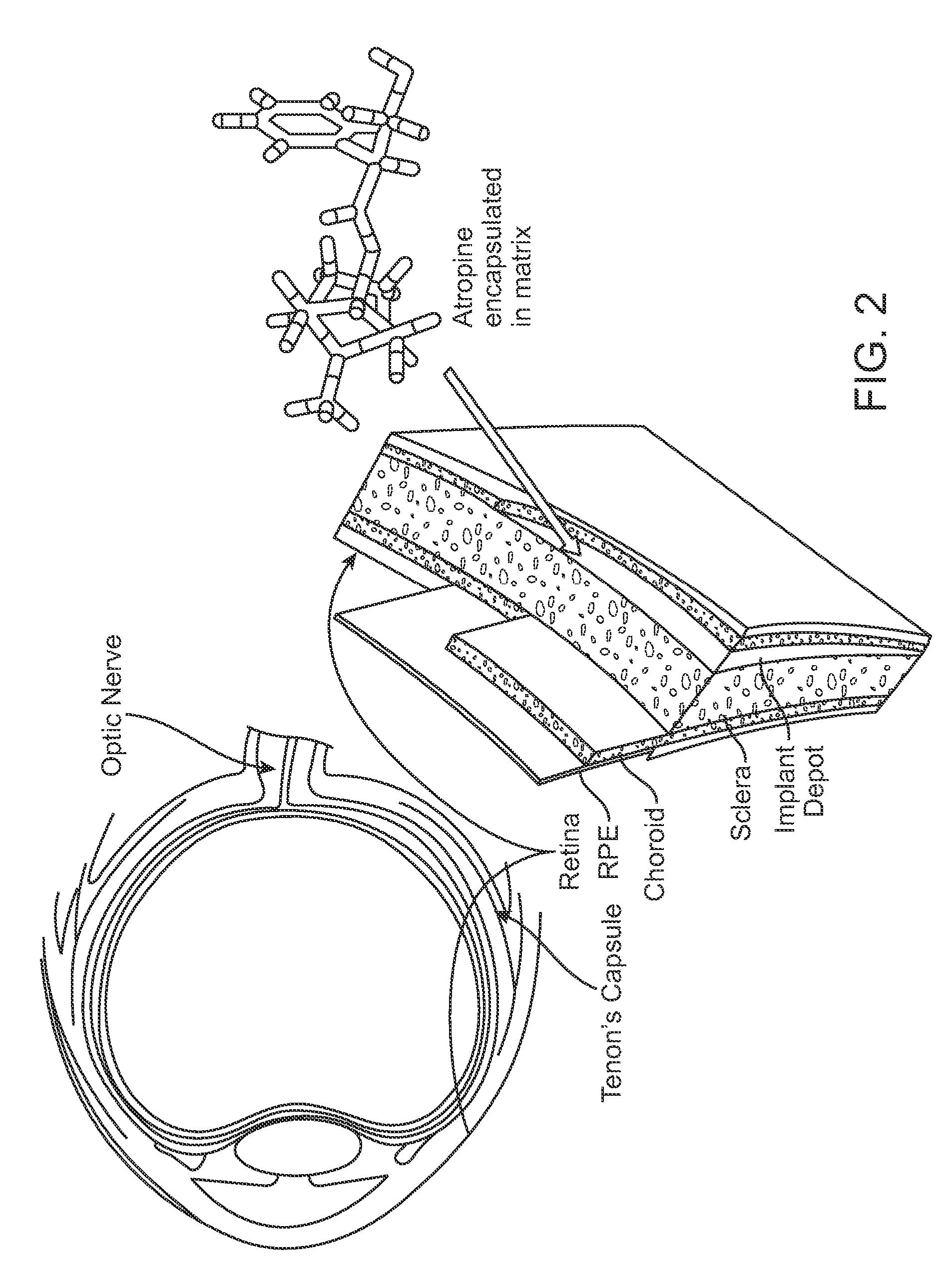
Implantable Delivery Vehicle for Ocular Delivery of Muscarinic Antagonists
Owner:RGT UNIV OF CALIFORNIA
Pharmaceutical combination
ActiveUS20130172277A1High potencyImprove efficacyBiocidePharmaceutical delivery mechanismMuscarinic antagonistAdrenergic
Pharmaceutical combinations comprising a beta-3 adrenergic receptor agonist and a muscarinic receptor antagonist, and methods for their use are disclosed. Methods of using the pharmaceutical combinations for the treatment of one or more symptoms associated with overactive bladder, for example, frequency of urgency, nocturia, and urinary incontinence, are also disclosed.
Owner:B3AR THERAPEUTICS INC
Transdermally administered tolterodine as anti-muscarinic agent for the treatment of overactive bladder
InactiveUS20030124179A1Clinical efficacyReduce riskBiocideOrganic active ingredientsMuscarinic antagonistNasal cavity
The present invention is drawn to set of formulations of at least one compound selected from tolterodine, salts thereof, prodrugs thereof and / or metabolites thereof, wherein in the set of formulations contains at least one device for transdermal administration and at least one formulation for oral, sublingual, buccal, nasal, pulmonary, rectal and / or other transmucosal administration, in order to achieve an effect against overactive bladder and / or symptoms associated with this condition. The present invention is further drawn to methods of treating an overactive bladder with the formulations.
Owner:MCNEIL AB
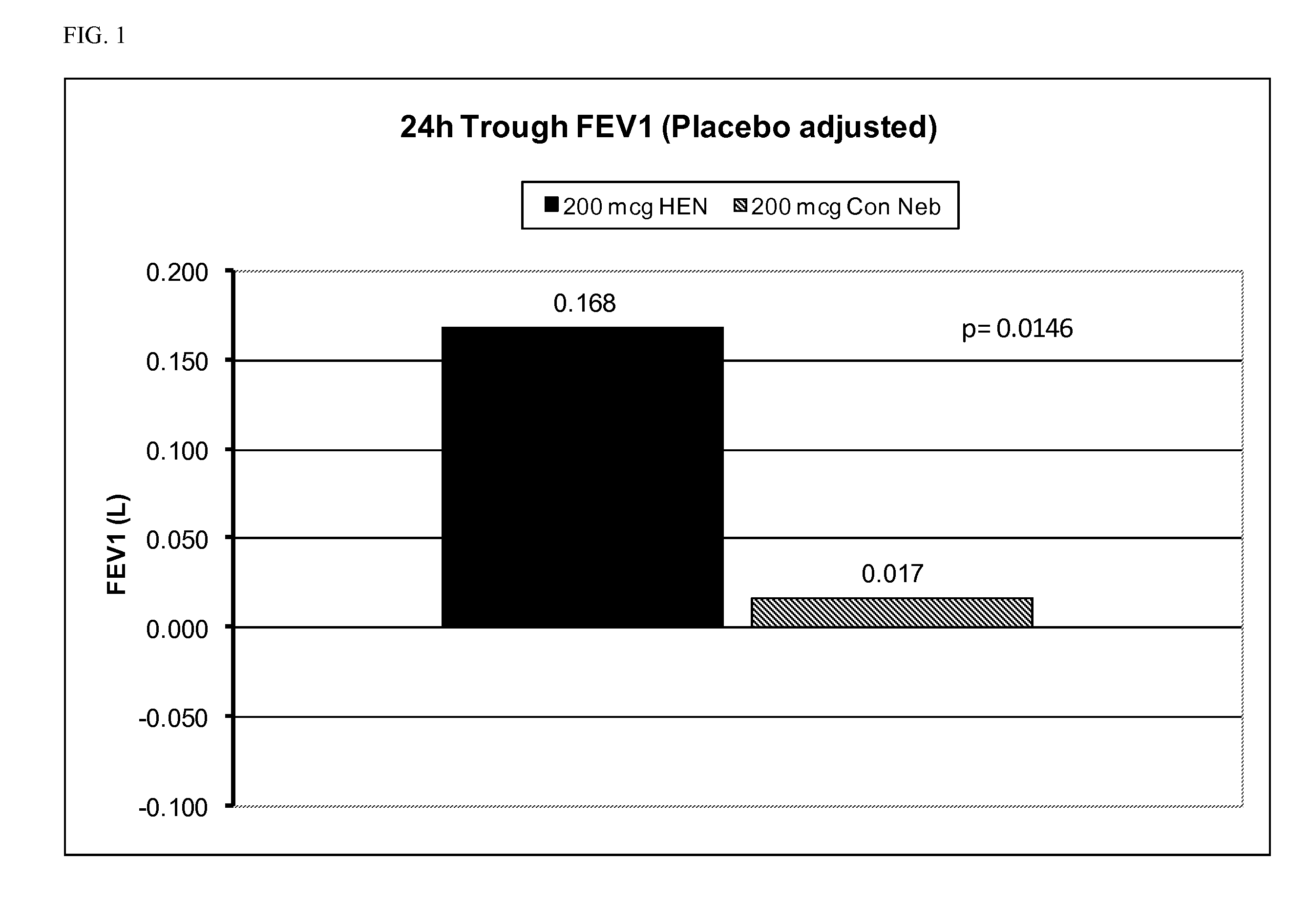
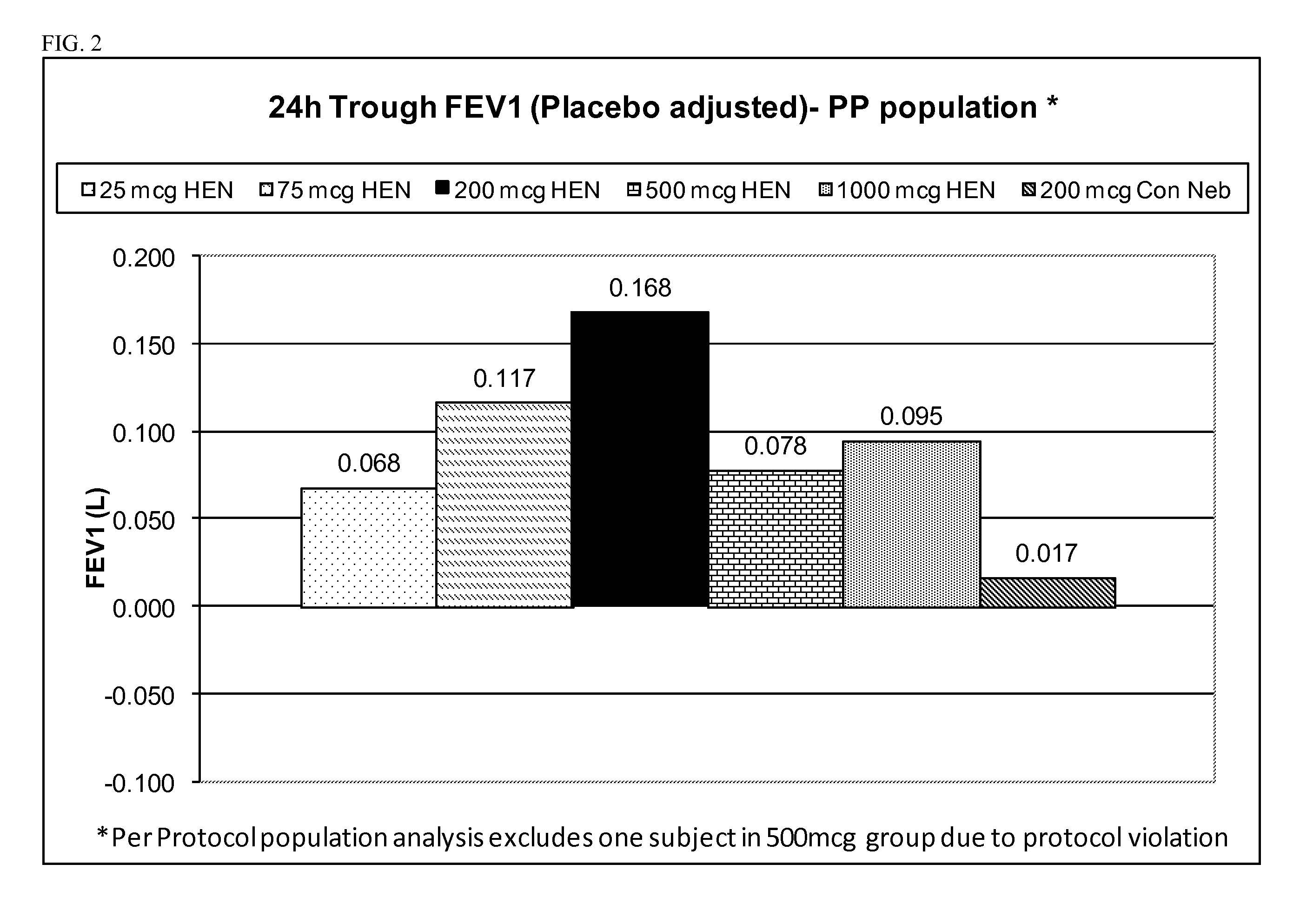
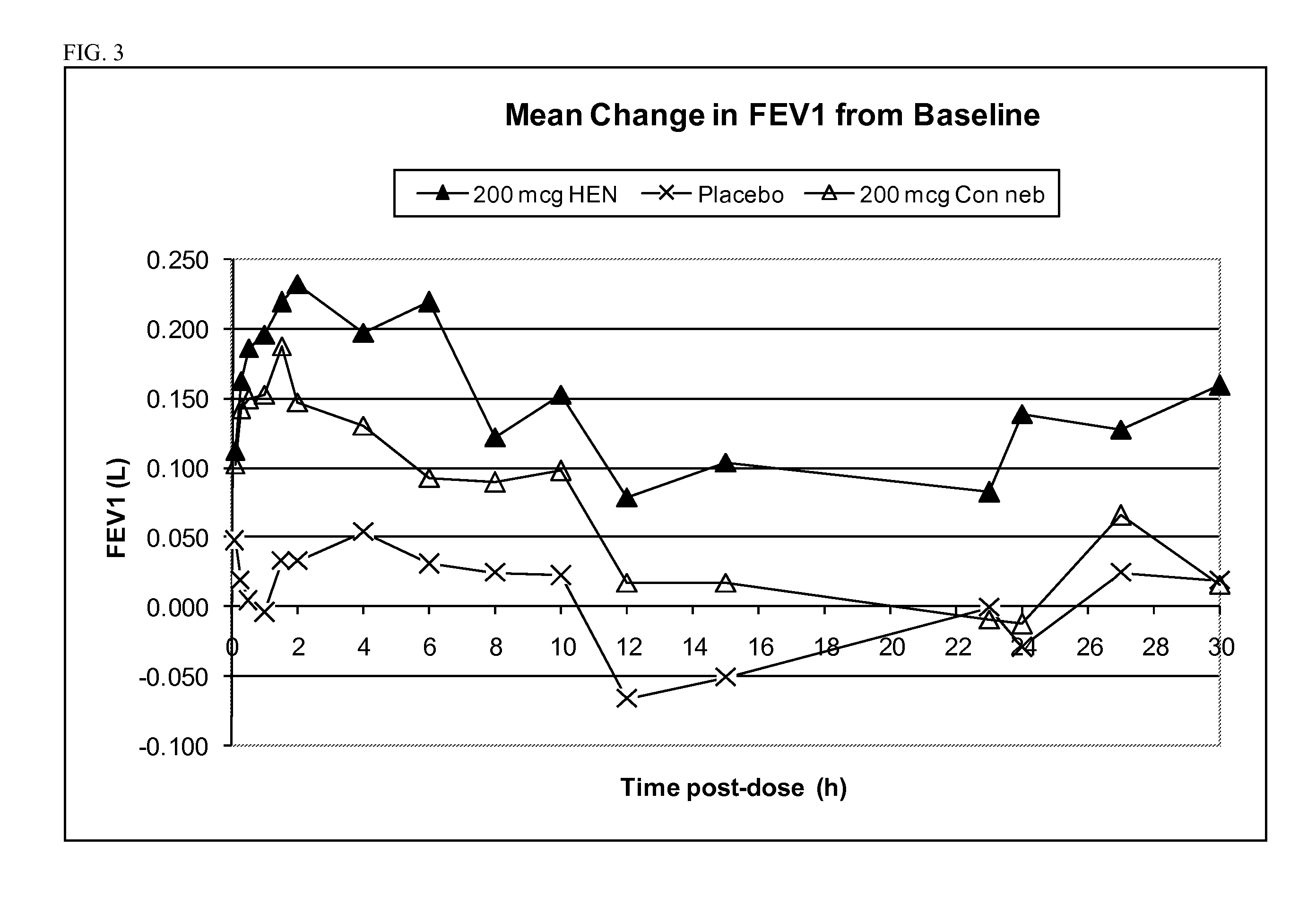
Treatment of chronic obstructive pulmonary disease with nebulized beta 2-agonist or combined nebulized beta 2-agonist and anticholinergic administration
InactiveUS20110132355A1Good curative effectExtended durationDispersion deliverySolution deliveryMuscarinic antagonistAnticholinergic agents
Inhalation solutions for administration of beta 2-agonists or combinations of muscarinic antagonists and beta 2-agonists for the treatment of breathing disorders, such as COPD, are provided. The inhalation solutions are administered by nebulization, particularly with a high efficiency nebulizer.
Owner:SUNOVION RESPIRATORY DEV
Method and system for the treatment of chronic obstructive pulmonary disease with nebulized anticholinergic administrations
Inhalation solutions for administration of muscarinic antagonists for the treatment of breathing disorders, such as COPD, are provided.
Owner:SUNOVION RESPIRATORY DEV
Dual Pharmacophores - PDE4-Muscarinic Antagonistics
InactiveUS20090203657A1Efficient deliveryGreat potentialBiocideOrganic chemistryMuscarinic antagonistDrug
The present invention is directed to novel compounds of Formula (I) and pharmaceutically acceptable salts thereof,pharmaceutical compositions and their use as dual chromaphores having inhibitory activity against PDE4 and muscarinic acetylcholine receptors (mAChRs), and thus being useful for treating respiratory diseases.
Owner:GLAXO GROUP LTD
Heterocyclic aspartyl protease inhibitors
Disclosed are compounds of the formula Ior a stereoisomer, tautomer, or pharmaceutically acceptable salt or solvate thereof, whereinW is a bond, —C(═S)—, —S(O)—, —S(O)2—, —C(═O)—, —O—, —C(R6)(R7)—, —N(R5)— or —C(═N(R5))—;X is —O—, —N(R5)— or —C(R6)(R7)—; provided that when X is —O—, U is not —O—, —S(O)—, —S(O)2—, —C(═O)— or —C(═NR5)—;U is a bond, —S(O)—, —S(O)2—, —C(O)—, —O—, —P(O)(OR15)—, —C(═NR5)—, —(C(R6)(R7))b— or —N(R5)—; wherein b is 1 or 2; provided that when W is —S(O)—, —S(O)2—, —O—, or —N(R5)—, U is not —S(O)—, —S(O)2—, —O—, or —N(R5)—; provided that when X is —N(R5)— and W is —S(O)—, —S(O)2—, —O—, or —N(R5)—, then U is not a bond;and R1, R2, R3, R4, R5, R6, and R7 are as defined in the specification;and pharmaceutical compositions comprising the compounds of formula I.Also disclosed is the method of inhibiting aspartyl protease, and in particular, the methods of treating cardiovascular diseases, cognitive and neurodegenerative diseases, and the methods of inhibiting of Human Immunodeficiency Virus, plasmepins, cathepsin D and protozoal enzymes.Also disclosed are methods of treating cognitive or neurodegenerative diseases using the compounds of formula I in combination with a cholinesterase inhibitor or a muscarinic antagonist.
Owner:MERCK SHARP & DOHME LLC +1
Method and system for the treatment of chronic obstructive pulmonary disease with nebulized anticholinergic administrations
InactiveUS20100055045A1Reduced and acceptable side effectImprove efficiencyDispersion deliveryAerosol deliveryMuscarinic antagonistNebulizer
A method is provided for improving lung function in COPD by administering a muscarinic antagonist with a high efficiency nebulizer.
Owner:SUNOVION RESPIRATORY DEV
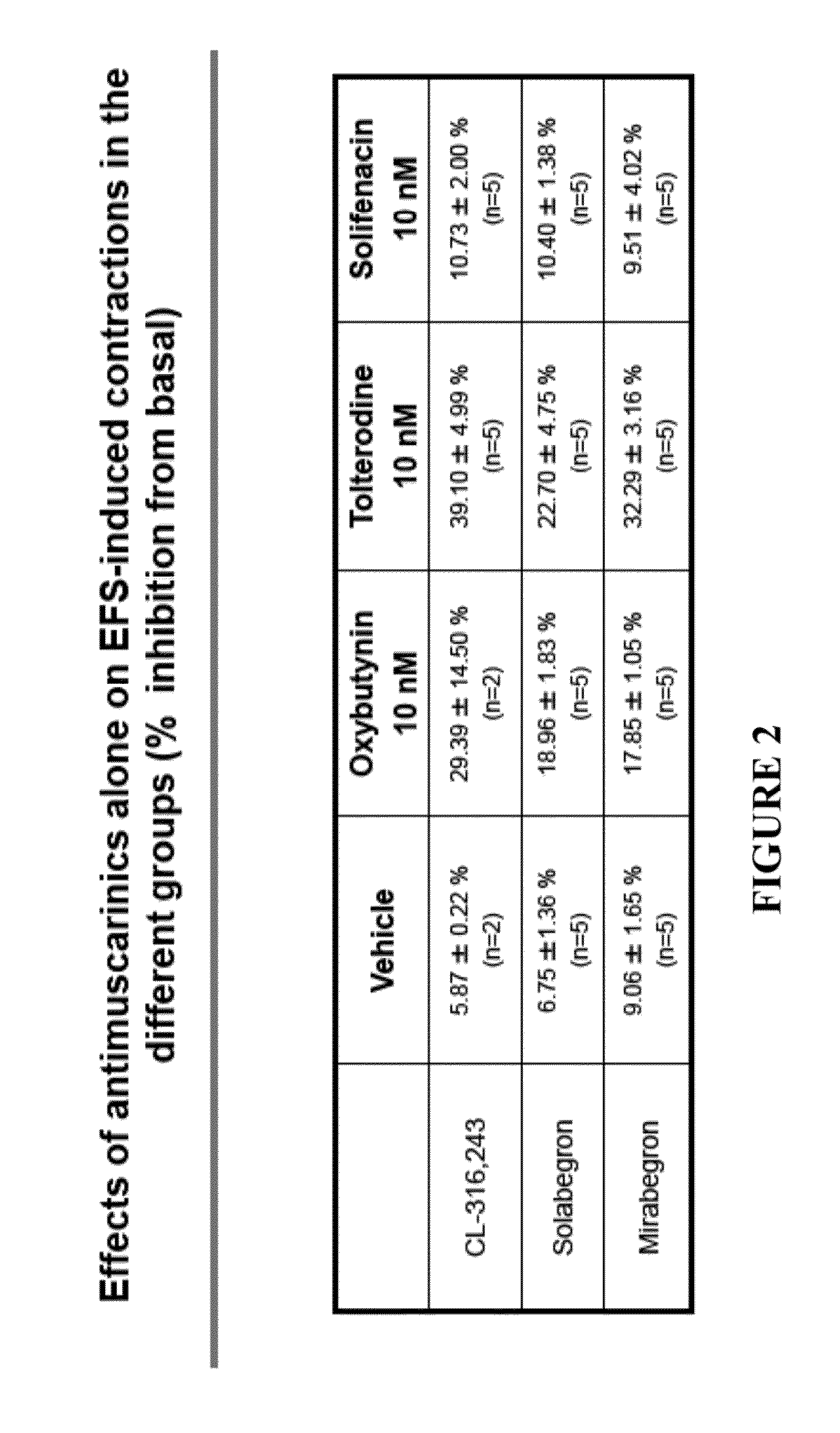
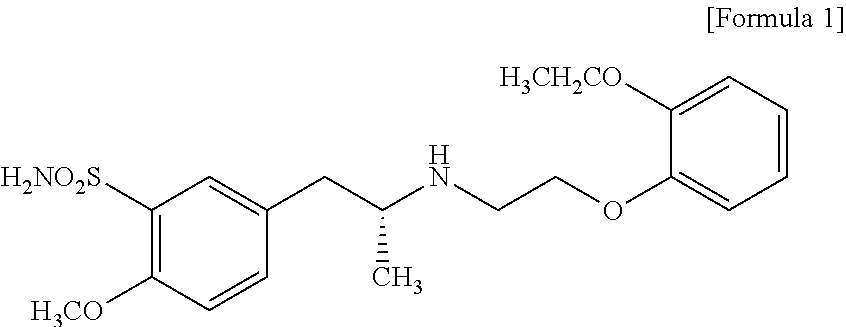
Combined Use of an Alpha-Adrenergic Receptor Antagonist and an Anti-Muscarinic Agent
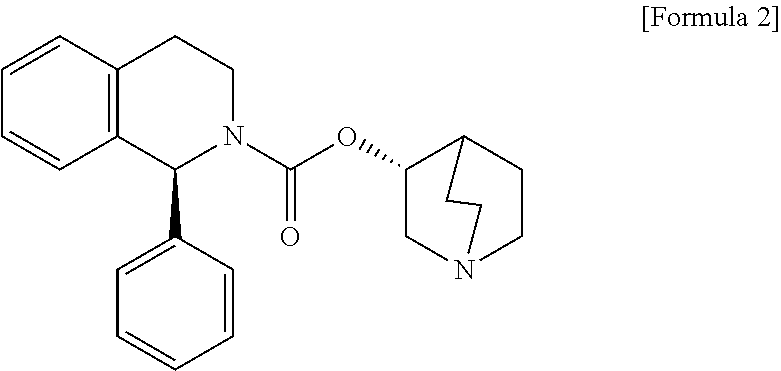
The combined use of (R)-5-(2-{[2-(2-ethoxyphenoxy)ethyl]amino}propyl)-2-methoxybenzene-1-sulfonamide (tamsulosin), or its pharmaceutically acceptable salt, and (1S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline-2-carboxylic acid (3R)-quinuclidin-3-yl ester (solifenacin), or its pharmaceutically acceptable salt, for the preparation Of a medicament for the improvement of lower urinary tract symptoms associated with benign prostatic hyperplasia (LUTS / BPH) with a substantial storage component is provided.
Owner:ASTELLAS IRELAND
Pharmaceutical Combination
Pharmaceutical combinations comprising a beta-3 adrenergic receptor agonist and a muscarinic receptor antagonist, and methods for their use are disclosed. Methods of using the pharmaceutical combinations for the treatment of one or more symptoms associated with overactive bladder, for example, frequency of urgency, nocturia, and urinary incontinence, are also disclosed.
Owner:B3AR THERAPEUTICS INC
Heterocyclic aspartyl protease inhibitors
Disclosed are compounds of the formula Ior a stereoisomer, tautomer, or pharmaceutically acceptable salt or solvate thereof,whereinW is a bond, —C(═S)—, —S(O)—, —S(O)2—, —C(═O)—, —O—, —C(R6)(R7)—, —N(R5)— or —C(═N(R5))—;X is —O—, —N(R5)— or —C(R6)(R7)—; provided that when X is —O—, U is not —O—, —S(O)—, —S(O)2—, —C(═O)— or —C(═NR5)—;U is a bond, —S(O)—, —S(O)2—, —C(O)—, —O—, —P(O)(OR15)—, —C(═NR5)—, —(C(R6)(R7))b— or —N(R5)—; wherein b is 1 or 2; provided that when W is —S(O)—, —S(O)2—, —O—, or —N(R5)—, U is not —S(O)—, —S(O)2—, —O—, or —N(R5)—; provided that when X is —N(R5)— and W is —S(O)—, —S(O)2—, —O—, or —N(R5)—, then U is not a bond;and R1, R2, R3, R4, R5, R6, and R7 are as defined in the specification; and pharmaceutical compositions comprising the compounds of formula I.Also disclosed is the method of inhibiting aspartyl protease, and in particular, the methods of treating cardiovascular diseases, cognitive and neurodegenerative diseases, and the methods of inhibiting of Human Immunodeficiency Virus, plasmepins, cathepsin D and protozoal enzymes.Also disclosed are methods of treating cognitive or neurodegenerative diseases using the compounds of formula I in combination with a cholinesterase inhibitor or a muscarinic antagonist.
Owner:MERCK SHARP & DOHME LLC
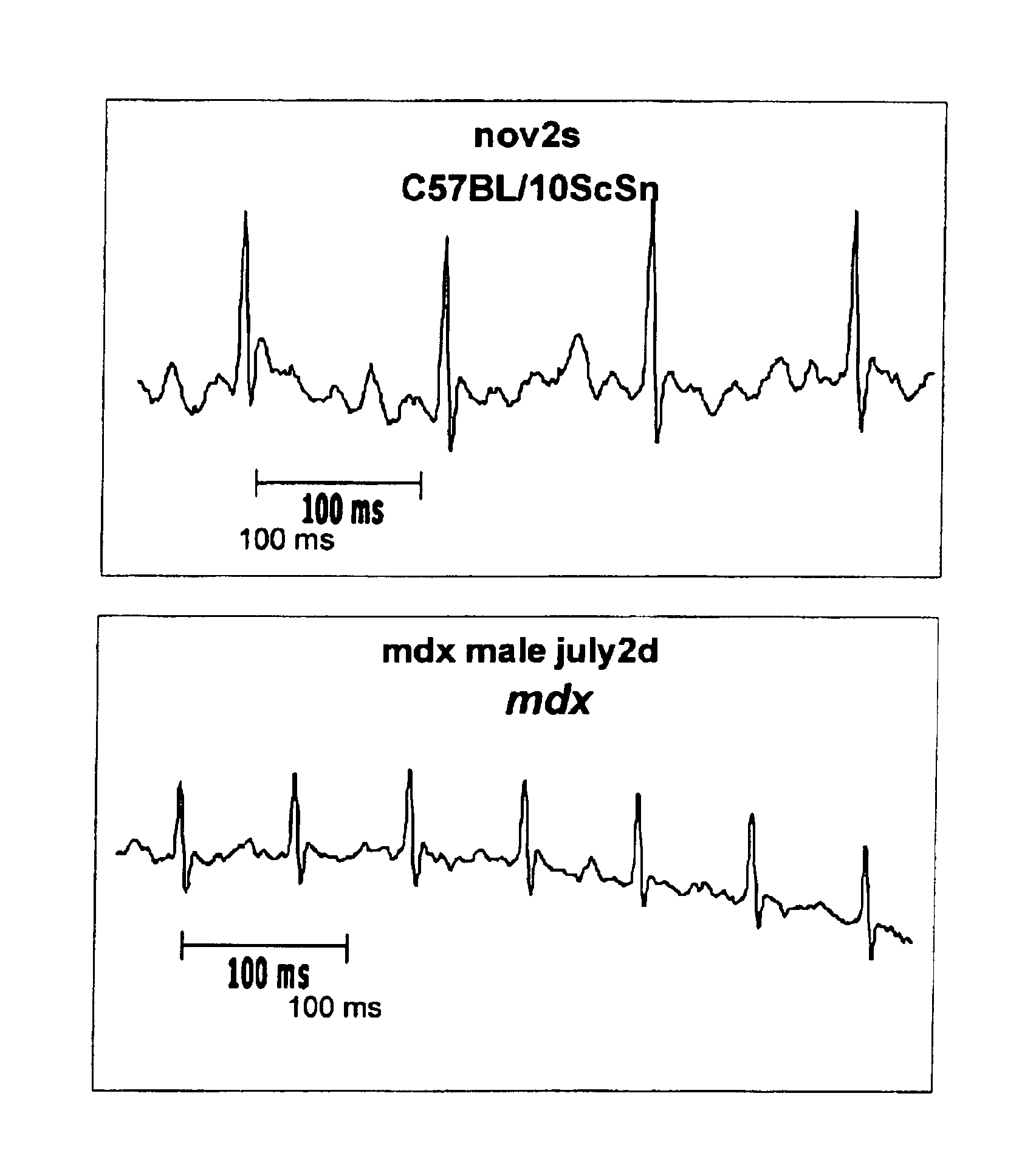
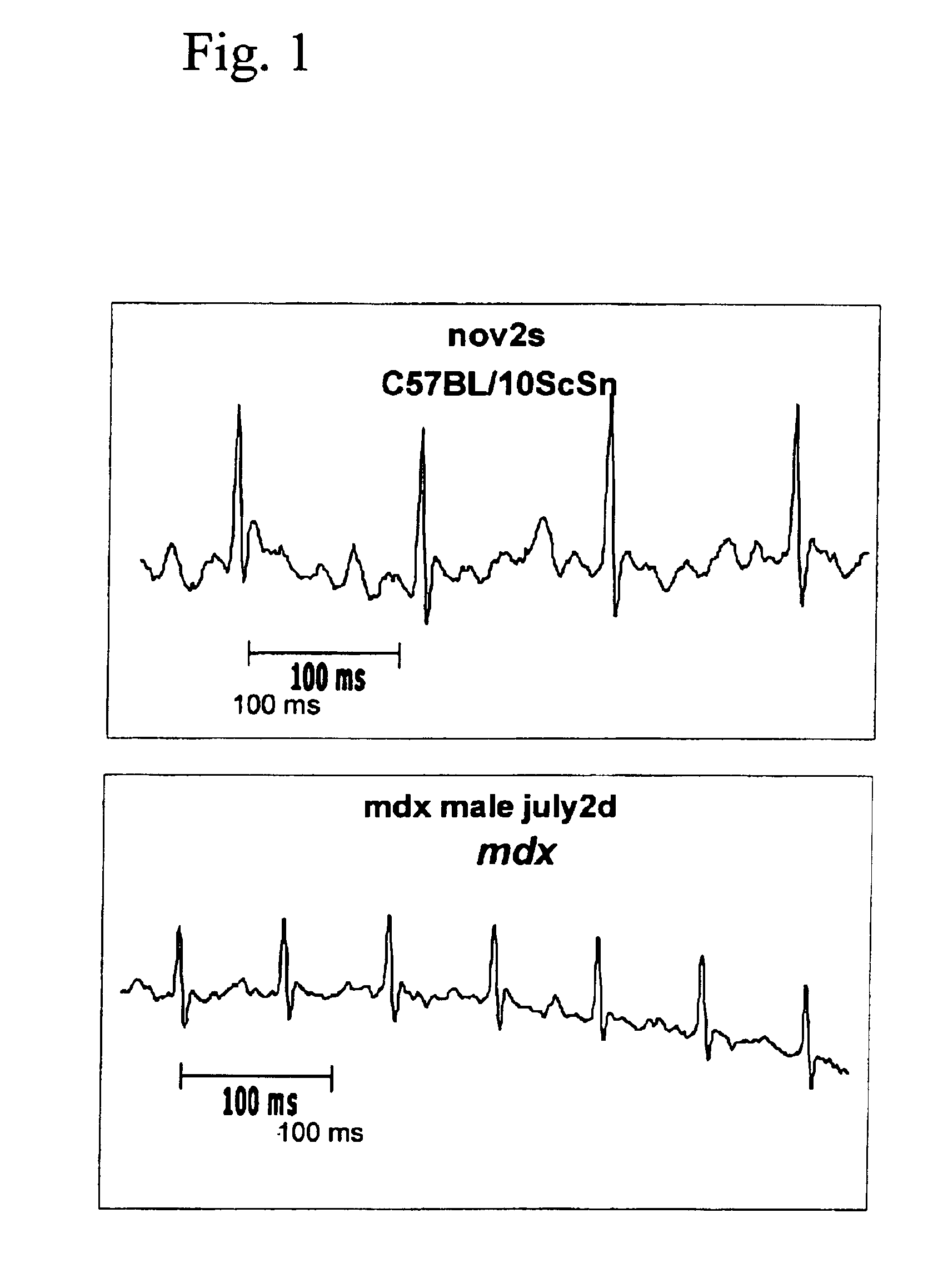
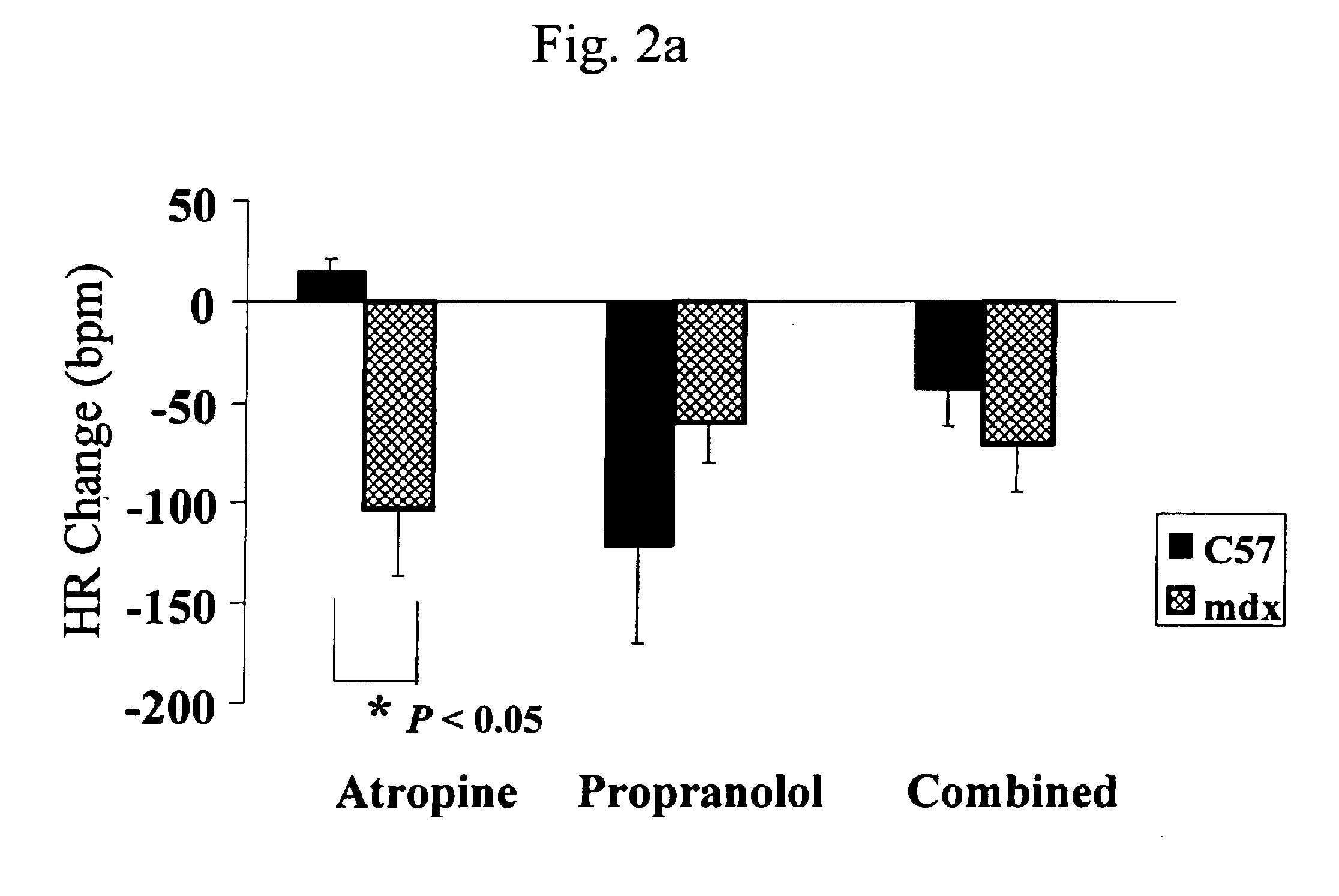
Method of early detection of Duchenne muscular dystrophy and other neuromuscular disease
InactiveUS6875418B2Compounds screening/testingDiagnostic recording/measuringDiseaseDecreasing heart rate
The mdx mouse is a model of Duchenne muscular dystrophy. The present invention describes that mdx mice exhibited clinically relevant cardiac phenotypes. A non-invasive method of recording electrocardiograms (ECGs) was used to a study mdx mice (n=15) and control mice (n=15). The mdx mice had significant tachycardia, consistent with observations in patients with muscular dystrophy. Heart-rate was nearly 15% faster in mdx mice than control mice (P<0.01). ECGs revealed significant shortening of the rate-corrected QT interval duration (QTc) in mdx mice compared to control mice (P<0.05). PR interval duration were shorter at baseline in mdx compared to control mice (P<0.05). The muscarinic antagonist atropine significantly increased heart-rate and decreased PR interval duration in C57 mice. Paradoxically, atropine significantly decreased heart-rate and increased PR interval duration in all mdx mice. Pharmacological autonomic blockade and baroreflex sensitivity testing demonstrated an imbalance in autonomic nervous system modulation of heart-rate, with decreased parasympathetic activity and increased sympathetic activity in mdx mice. These electrocardiographic findings in dystrophin-deficient mice provide new bases for diagnosing, understanding, and treating patients with Duchenne muscular dystrophy.
Owner:MOUSE SPECIFICS
Pharmaceutical compositions of muscarinic receptor antagonists
Provided herein are pharmaceutical compositions comprising one or more muscarinic receptor antagonists (“MRA”), and at least one additional active ingredients selected from one or more β2-agonists, p38 MAP kinase inhibitors, PDE-IV inhibitors, corticosteroids or a mixture thereof and optionally one or more pharmaceutically acceptable carriers, excipients or diluents. In addition, methods of treating autoimmune, inflammatory or allergic diseases or disorders are provided.
Owner:RANBAXY LAB LTD
Heterocyclic aspartyl protease inhibitors
Compounds of the formula I or a stereoisomer, tautomer, or pharmaceutically acceptable salt or solvate thereof, and pharmaceutical compositions comprising the compounds of formula I. Also disclosed is the method of inhibiting aspartyl protease, and in particular, the methods of treating cardiovascular diseases, cognitive and neurodegenerative diseases, and the methods of inhibiting of Human Immunodeficiency Virus, plasmepins, cathepsin D and protozoal enzymes. Also disclosed are methods of treating cognitive or neurodegenerative diseases using the compounds of formula I in combination with a cholinesterase inhibitor or a muscarinic antagonist.
Owner:MERCK & CO INC +1
Compositions for pulmonary delivery of long-acting β2 adrenergic receptor agonists and associated methods and systems
Compositions, methods and systems are provided for pulmonary delivery of long-acting muscarinic antagonists and long-acting β2 adrenergic receptor agonists via a metered dose inhaler. In particular embodiments, the compositions include a suspension medium, active agent particles, and suspending particles, in which the active agent particles and suspending particles form a co-suspension within the suspension medium.
Owner:PEARL THERAPEUTICS
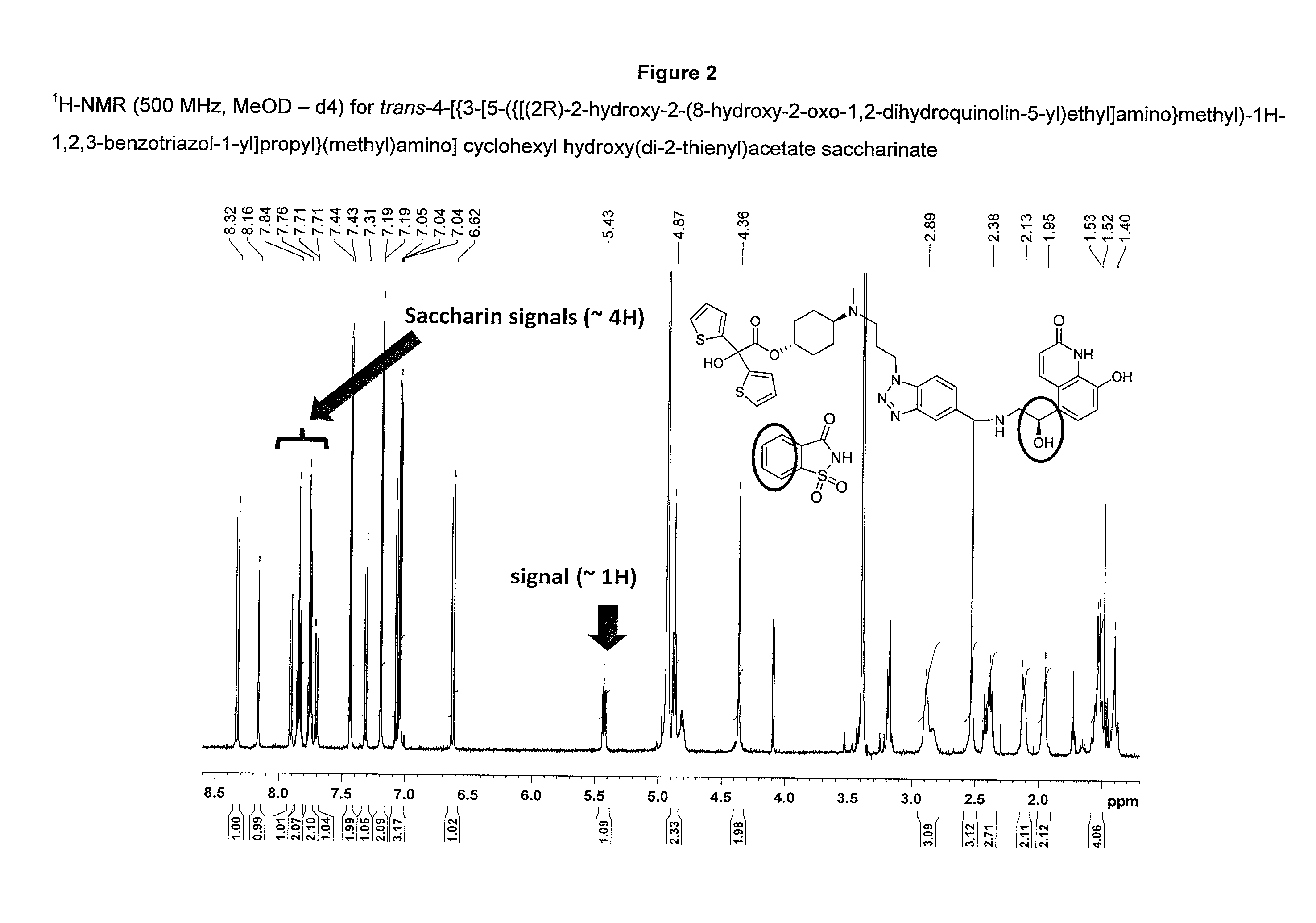
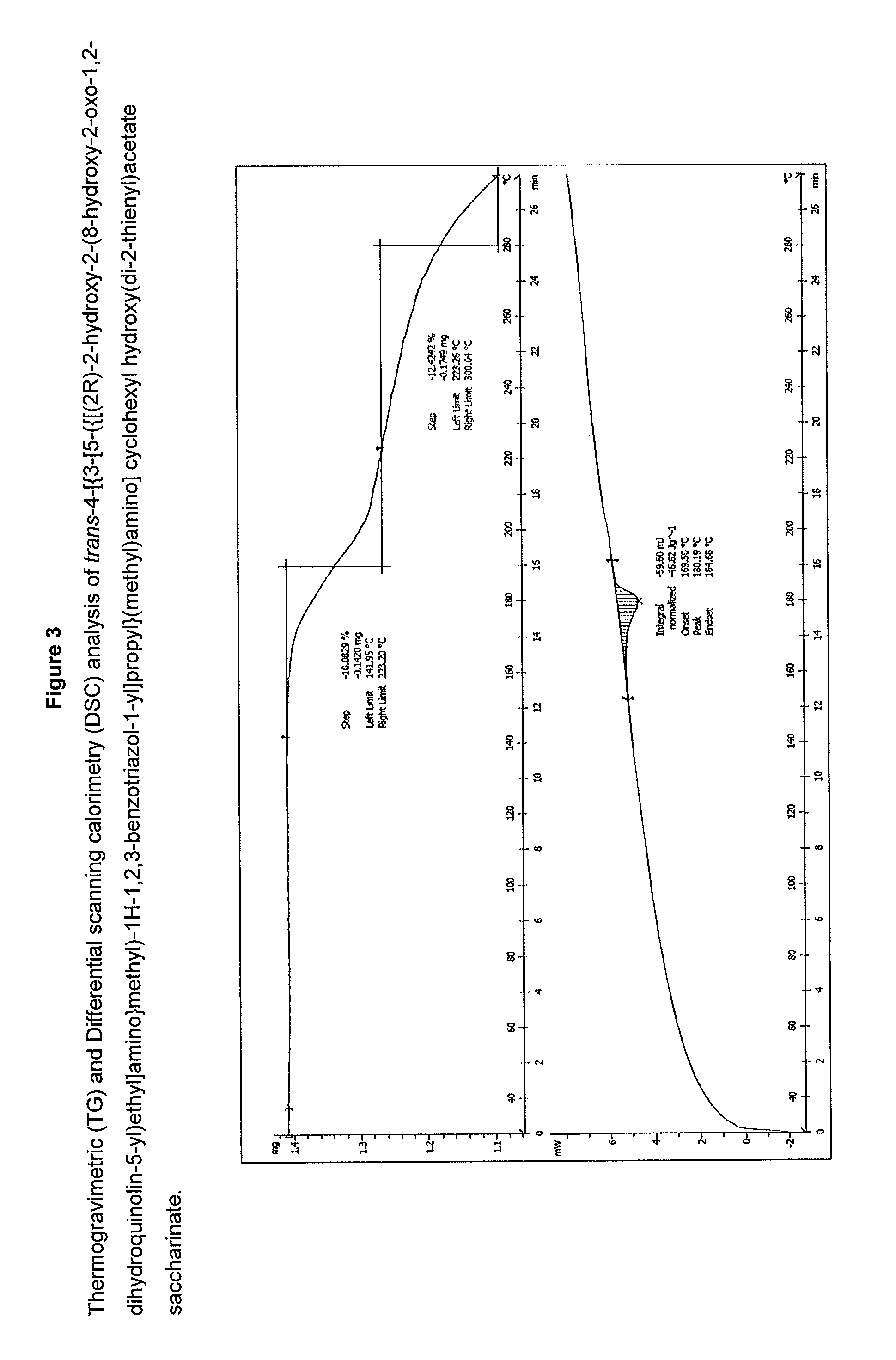
Salts of 2-amino-1-hydroxyethyl-8-hydroxyquinolin-2(1H)-one derivatives having both muscarinic receptor antagonist and beta2 adrenergic receptor agonist activities
The present invention is directed to crystalline addition salts of (ii) 8-hydroxyquinolin-2(1H)-one derivatives and (ii) a dicarboxylic acid or a sulfimide, or a pharmaceutically acceptable solvates thereof.
Owner:ALMIRALL
Dry powder inhalers comprising a carrier other than lactose
This invention relates to novel pharmaceutical compositions for inhalation comprising separately, sequentially or together, drugs having amine in the form of a dry powder in admixture with a pharmaceutically acceptable carrier, other than lactose, and its use in the treatment of respiratory condition selected from asthma and chronic obstructive pulmonary disease (COPD) and other obstructive airways diseases. In addition, the present invention relates to novel pharmaceutical composition for inhalation based on combinations of long acting muscarinic antagonists, long acting beta agonists, short acting beta-2 agonists, corticosteroids or a combination of two or more of them.
Owner:ARVEN ILAC SANAYI VE TICARET
Dual Pharmacophores - PDE4-Muscarinic Antagonistics
The present invention is directed to novel compounds of Formula (I), pharmaceutical compositions and their use in therapy, for example as inhibitors of phosphodiesterase type IV (PDE4) and as antagonists of muscarinic acetylcholine receptors (mAChRs), in the treatment of / and or prophylaxis of respiratory diseases, including antiinflammatory and / or allergic diseases such as chronic obstructive pulmonary disease (COPD), asthma, rhinitis (e.g. allergic rhinitis), atopic dermatitis or psoriasis.
Owner:GLAXO GROUP LTD
Dry powder inhalers comprising a carrier other than lactose and a ternary component
This invention relates to novel pharmaceutical compositions for inhalation comprising separately, sequentially or together, drugs having amine in the form of a dry powder in admixture with a pharmaceutically acceptable carrier, other than lactose, and its use in the treatment of respiratory condition selected from asthma and chronic obstructive pulmonary disease (COPD) and other obstructive airways diseases. More particularly, the invention relates to pharmaceutical composition for inhalation further comprising a ternary component. In addition, the present invention relates to novel pharmaceutical composition for inhalation based on combinations of long acting muscarinic antagonists, long acting beta agonists, short acting beta-2 agonists, corticosteroids or a combination of two or more of them.
Owner:ARVEN ILAC SANAYI VE TICARET
Muscarinic antagonists and combinations thereof for the treatment of airway disease in horses
ActiveUS20150366855A1Reduce partial pressureStart fastPowder deliveryBiocideDiseaseMuscarinic antagonist
The invention relates to the field of medicine, in particular to the field of veterinary medicine. The invention specifically relates to muscarinic antagonists (including long acting muscarinic antagonists (LAMAs)) for the treatment of airway disease, such as pulmonary disease, preferably recurrent airway obstruction (RAO), summer pasture associated obstructive pulmonary disease (SPAOPD), and inflammatory airway disease (IAD) in animals, preferably equines such as horses.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Carboxamide derivatives as muscarinic receptor antagonists
Owner:PFIZER INC
Substituted 4-amino-1-benzylpiperidine compounds
Owner:THERAVANCE BIOPHARMA R&D IP LLC
Muscarinic antagonists
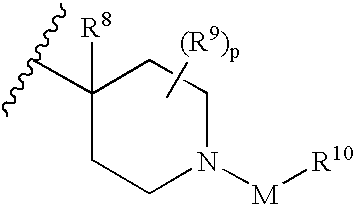
The invention described herein are compounds in accordance with formula I: wherein R1, R2, R3, R4, R5, R6, R2, R8, R9, n, X, and Z are as defined herein, pharmaceutical compositions containing at least one compound of formula I, methods of preparation thereof, and methods of treating disorders with at least one compound of formula I or at least one compound of formula I in association with at least one acetylcholinesterase inhibitor.
Owner:MERCK SHARP & DOHME CORP
Methods and compositions for treating hyperhidrosis
ActiveUS10328057B2Speed up heat lossHyperhidrosis is substantially reducedOrganic active ingredientsNervous disorderMuscarinic antagonistSide effect
Aspects of the disclosure include methods for treating hyperhidrosis in a subject with a composition including a muscarinic antagonist and a muscarinic agonist. In practicing methods according to certain embodiments, a therapeutically effective amount of a composition including a muscarinic antagonist or a pharmaceutically acceptable salt thereof and a muscarinic agonist or a pharmaceutically acceptable salt thereof is administered to a subject and is sufficient to reduce hyperhidrosis in the subject and to reduce a dry mouth side effect of the muscarinic antagonist. Compositions for practicing the subject methods are also described as well as dose units containing one or more of the subject compositions.
Owner:THERAVIDA INC
Aerosol containing muscarine receptor antagonist and beta 2 receptor stimulant, and preparation method thereof
InactiveCN106943350AImprove delivery efficiencyStable dose deliveredDispersion deliveryAerosol deliveryVasopressin AntagonistsStimulant
The invention discloses an aerosol containing muscarine receptor antagonist and beta 2 receptor stimulant, and a preparation method thereof. The aerosol contains a propulsive agent, long-acting beta 2 receptor stimulant particles, and long-acting muscarine antagonist particles; in LABA particles, by volume, the geometric particle size of 90% particles is smaller than 10<mu>m, and the geometric particle size of 50% particles is smaller than 4<mu>m or even smaller; the middle particle size of LAMA particles ranges from 500nm to 10<mu>m; the LAMA particles are particles composed of LAMA, calcium chloride, and a surfactant; or the LAMA particles are particles composed of LAMA and an excipient. 1 to 100<mu>g of the aerosol is sprayed in each time of pressing of a MDI (metered dose inhaler), and the amount of LABA or LAMA in the aerosol ranges from 0.02 to 2mg / mL. The advantages of the aerosol are that: delivery efficiency is relatively high, and the delivery dosage is stable in long term of storing.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com