Heterocyclic aspartyl protease inhibitors
A heteroaryl and solvate technology, applied in the field of heterocyclic aspartyl protease inhibitors, can solve the problems of not being able to stop the disease process and not meeting medical requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
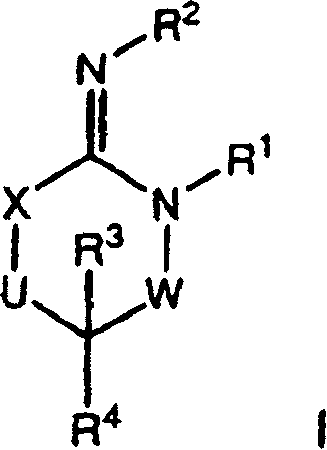
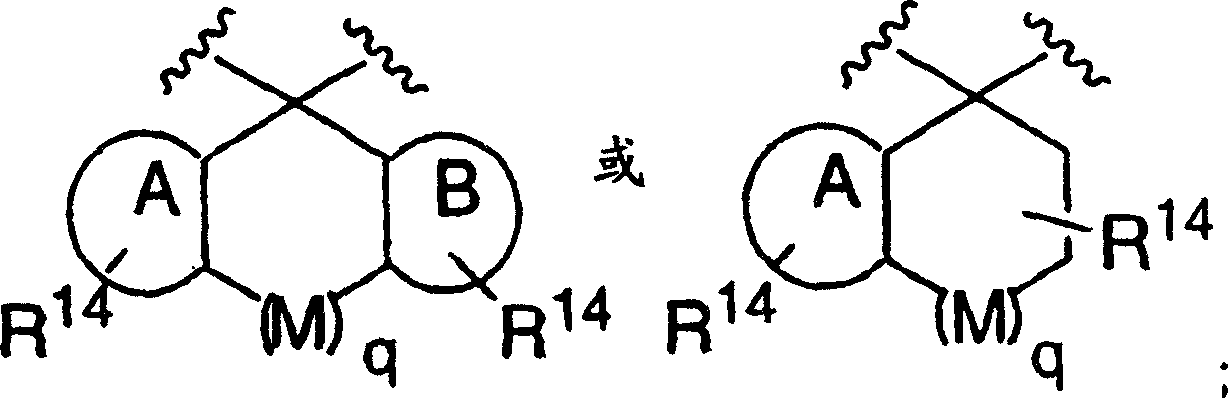
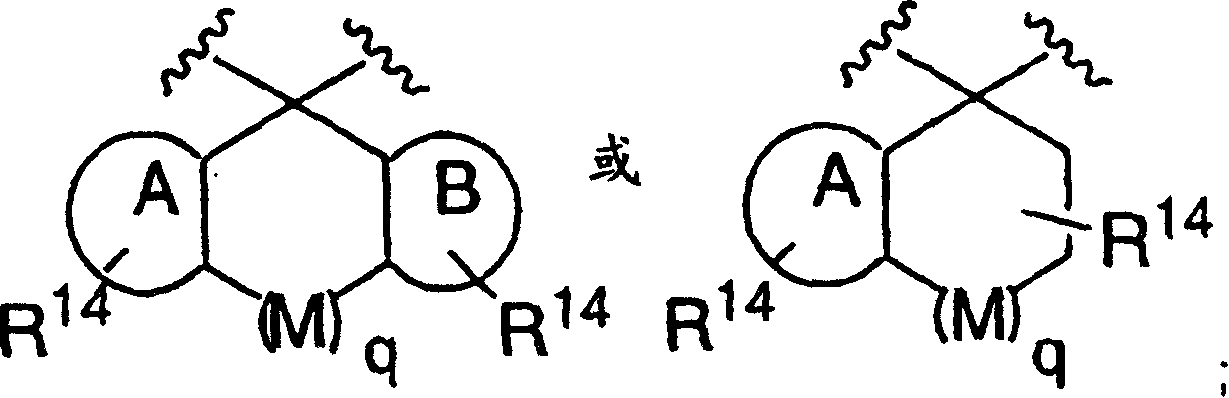
[0072] Compounds of formula I (wherein X, W and U are as defined above) include each of the following preferred structures:
[0073]
[0074] In compounds of formulas IA to IF, U is preferably a bond or -C(R 6 )(R 7 )-. In compounds of formula IG and IH, U is preferably -C(O)-.
[0075] It should be understood that since R 1 The definition of R 5 The definition of is the same, so when X is -N(R 5 )-, then W is a chemical bond and U is a chemical bond, -S(O)-, -S(O) 2 -, -C(O)-, -O-, -C(R 6 )(R 7 )-or-N(R 5 )-The compound of formula I is equivalent to U being a chemical bond and W being a chemical bond, -S(O)-, -S(O) 2 -, -C(O)-, -O-, -C(R 6 )(R 7 )-or-N(R 5 )- compound of formula I.
[0076] Compounds of the present invention are more preferably compounds of formula IB in which U is a chemical bond or U is -C(R 6 )(R 7 )- compound of formula IB.
[0077] Another preferred group of compounds of formula I are R 2 A compound of formula I which is H.
[0078] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com