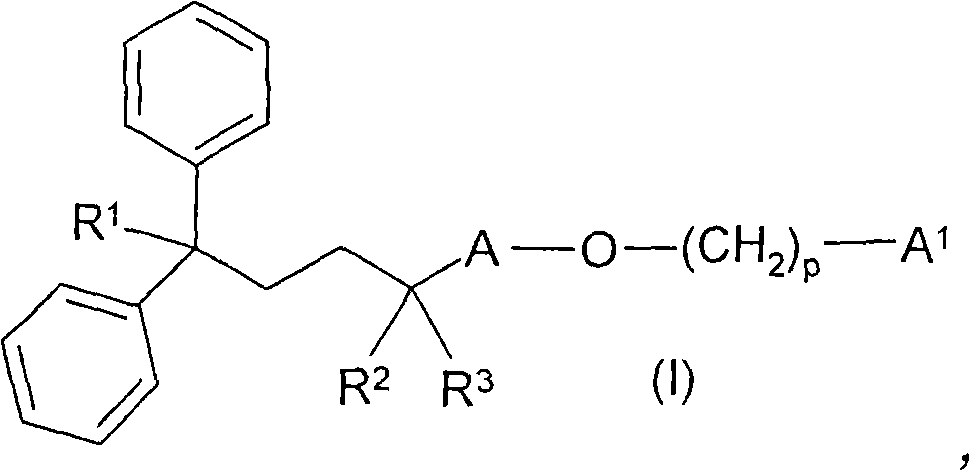
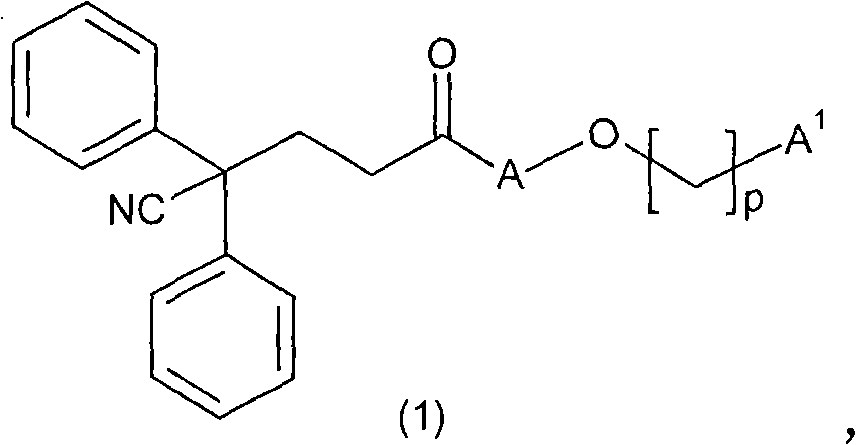
Carboxamide derivatives as muscarinic receptor antagonists
A technology of solvates and diphenylhexanamide, applied in the field of compounds of general formula: can solve problems such as limiting the dose of inhaled drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0938] 5-Methyl-5-[(3S)-3-phenoxypyrrolidin-1-yl]-2,2-diphenylcapronitrile
[0939]
[0940] A solution of the product of Preparation 11 (3.31 g, 8.07 mmol) in THF (90 mL) was cooled to -20°C. Zirconium chloride (3.76 g, 16.15 mmol) was added and the reaction mixture was stirred at -20°C for 1 hour. Then, methylmagnesium chloride (3M in tetrahydrofuran, 24 mL, 72 mmol) was added dropwise and the mixture was stirred for 2 hours keeping the temperature below -10°C. The reaction was quenched with 1M aqueous sodium hydroxide solution (25 mL), then, via Celite Filter and wash well with ethyl acetate (2 x 50 mL). The filtrate was washed with brine (70 mL), concentrated in vacuo, and the residue was recrystallized from hexane / ethyl acetate to afford the title compound as a pale orange crystalline solid, 59% yield, 2 g.
[0941] 1 HNMR (400MHz, CD3OD) δ: 0.99(s, 3H), 1.04(s, 3H), 1.23-1.27(m, 2H), 1.85-1.93(m, 1H), 2.07-2.16(m, 1H), 2.40 -2.45(m, 2H), 2.58-2.67(m, 2H), 2.72...
Embodiment 2
[0943] 5-Methyl-5-[(3S)-3-phenoxypyrrolidin-1-yl]-2,2-diphenylhexanamide
[0944]
[0945] Potassium hydroxide (5.10 g, 91.98 mmol) was added to a solution of the product of Example 1 (1.95 g, 4.60 mmol) in 3-methyl-3-pentanol (40 ml), and the mixture was heated under reflux for 24 hours . The reaction mixture was then cooled to room temperature, concentrated in vacuo, and the residue partitioned between ethyl acetate (70 mL) and water (40 mL). The aqueous layer was separated, extracted with ethyl acetate (50 mL), and the combined organic solutions were dried over sodium sulfate and concentrated in vacuo. The residue was then recrystallized from hexane / ethyl acetate and dried in vacuo for 18 hours to afford the title compound as a white solid in 82% yield, 1.66 g.
[0946] 1 HNMR (400MHz, CD 3 OD)δ: 0.97(s, 3H), 1.02(s, 3H), 1.19-1.33(m, 2H), 1.82-1.91(m, 1H), 2.02-2.17(m, 1H), 2.37-2.47(m , 2H), 2.48-2.64(m, 2H), 2.65-2.75(m, 1H), 2.81-2.89(m, 1H), 4.75(m, 1H), 6.76...
Embodiment 3
[0948] 5-Methyl-5-[(3R)-3-phenoxypyrrolidin-1-yl]-2,2-diphenylcapronitrile
[0949]
[0950] A solution of the product of Preparation 12 (0.84 g, 2.05 mmol) in THF (15 mL) was cooled to -10°C. Titanium(IV) chloride (0.23 mL, 2.05 mmol) was added and the reaction mixture was stirred at -10°C for 15 minutes. Then, methylmagnesium bromide (3M in diethyl ether, 4.1 mL, 12.3 mmol) was added dropwise and the mixture was stirred below -5°C for 10 minutes and at room temperature for 18 hours. The reaction mixture was quenched slowly with water (4 mL), diluted with ethyl acetate (20 mL), and decanted. The residual solid was extracted with ethyl acetate (3 x 20 mL), and the combined organic solutions were dried over sodium sulfate and concentrated in vacuo. The residue was purified by column chromatography on silica gel (eluting with 60:40 ethyl acetate:hexanes) to afford the title compound in 54% yield, 0.47 g.
[0951] LRMS APCI m / z 425[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com