Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
142 results about "Overactive bladder" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A bladder control problem which leads to a sudden urge to urinate.
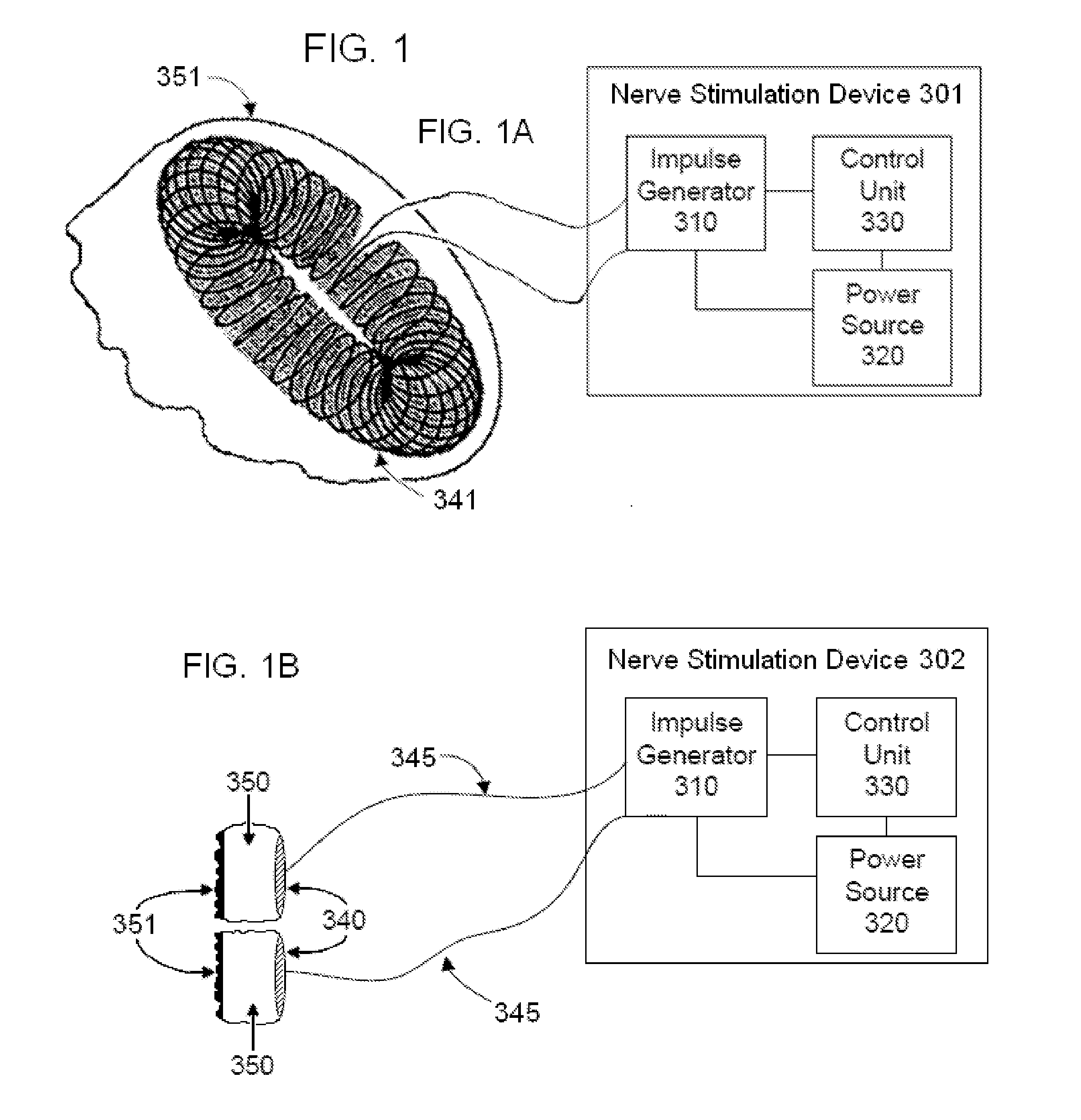
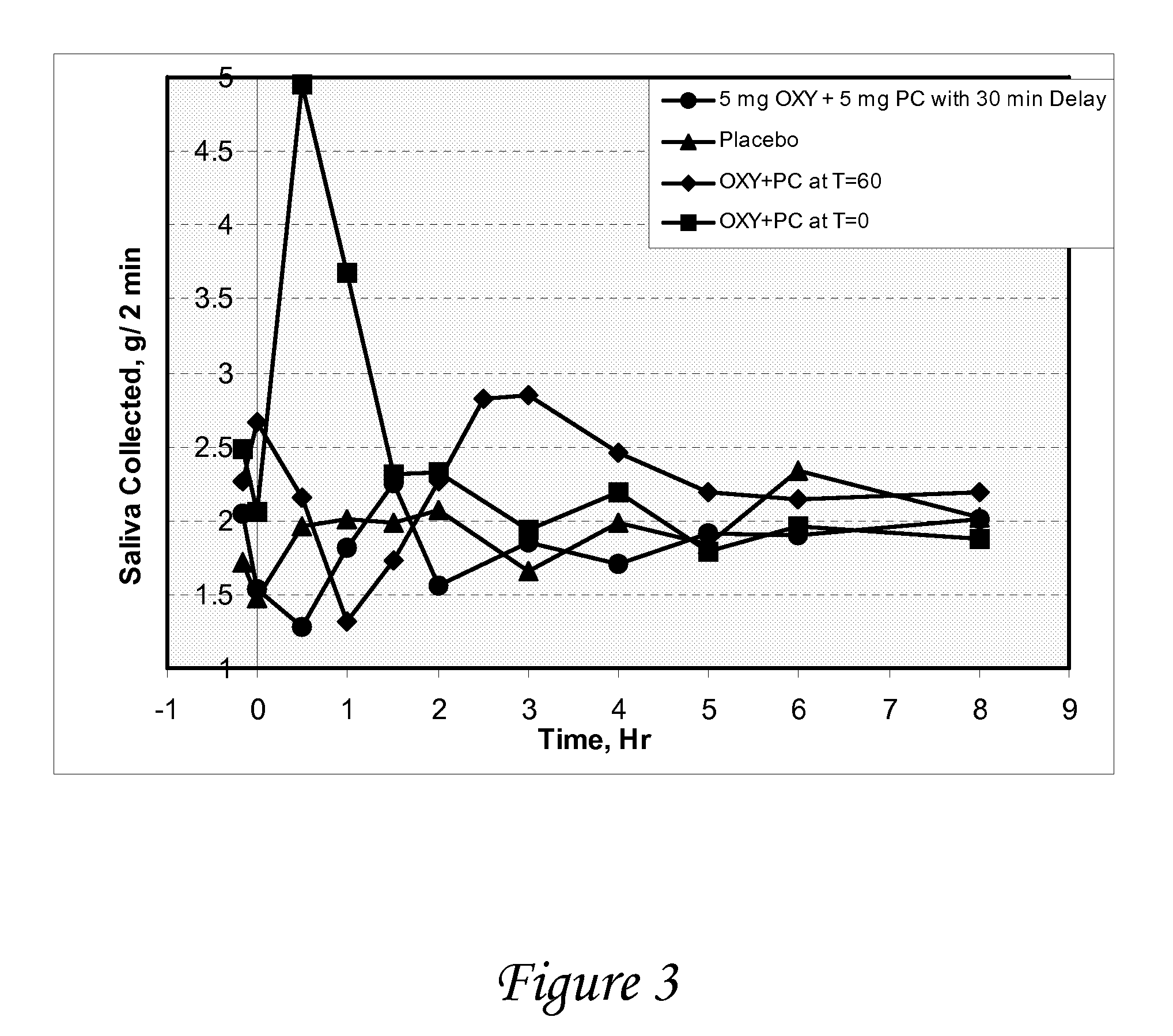
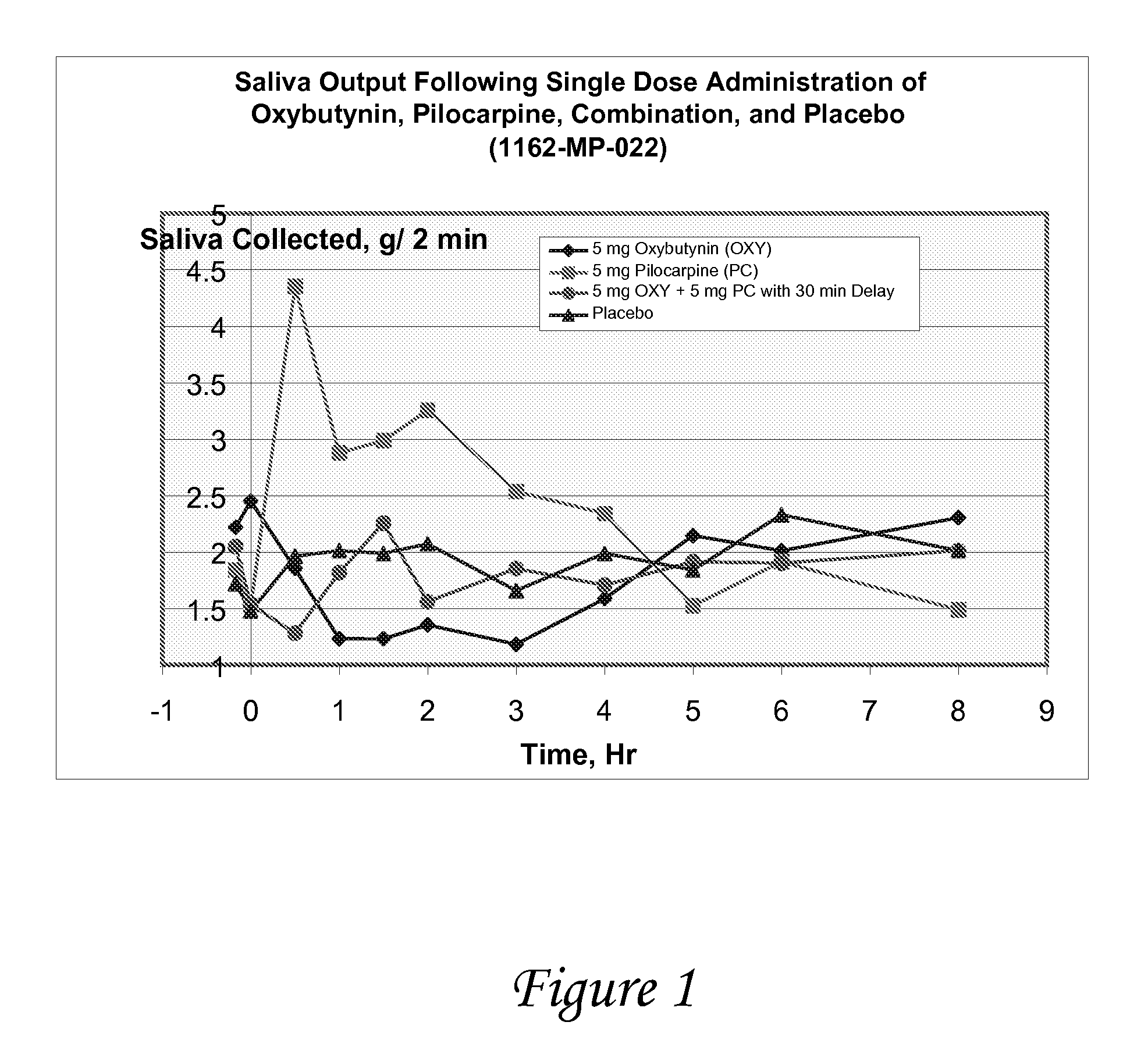
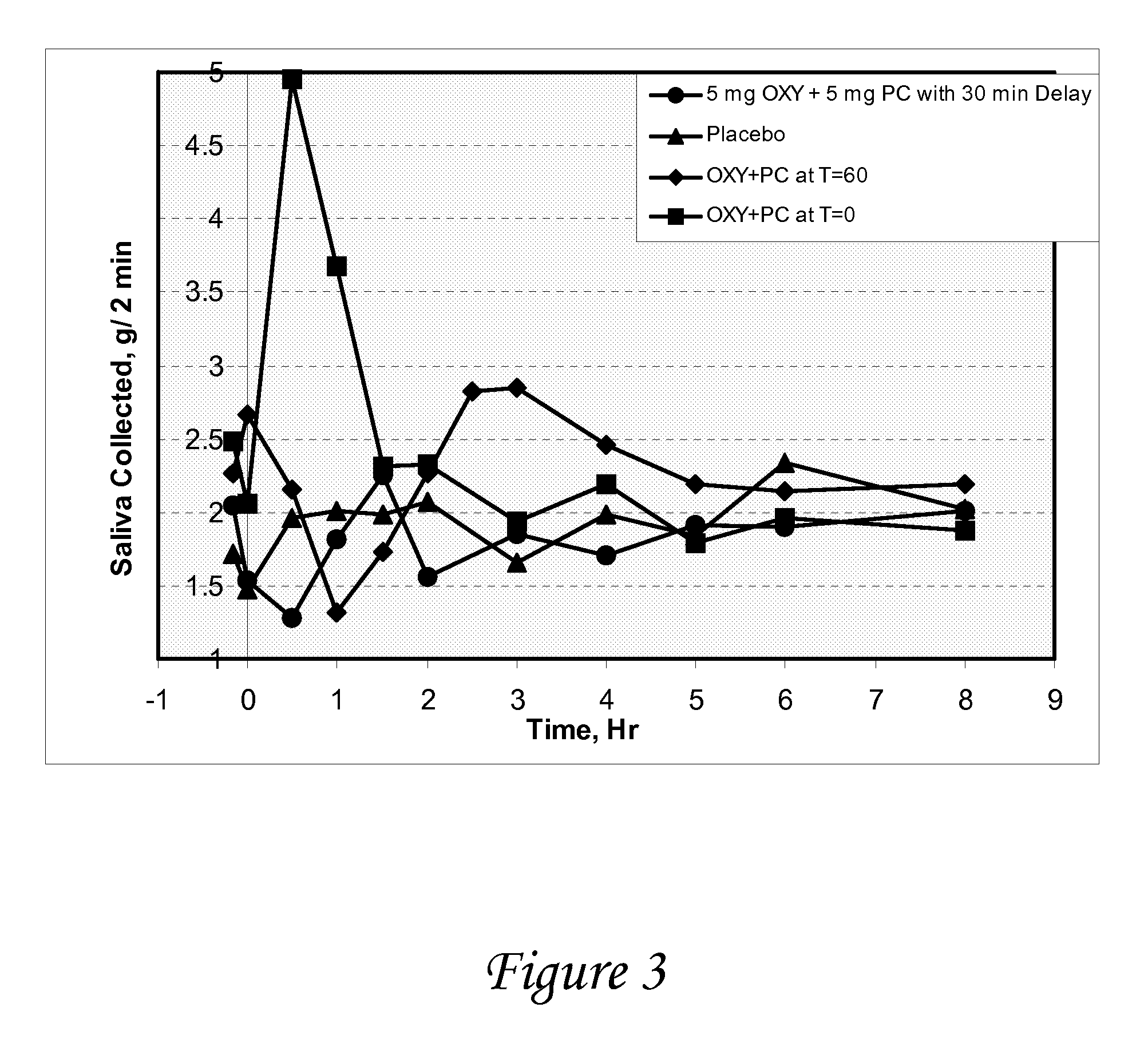
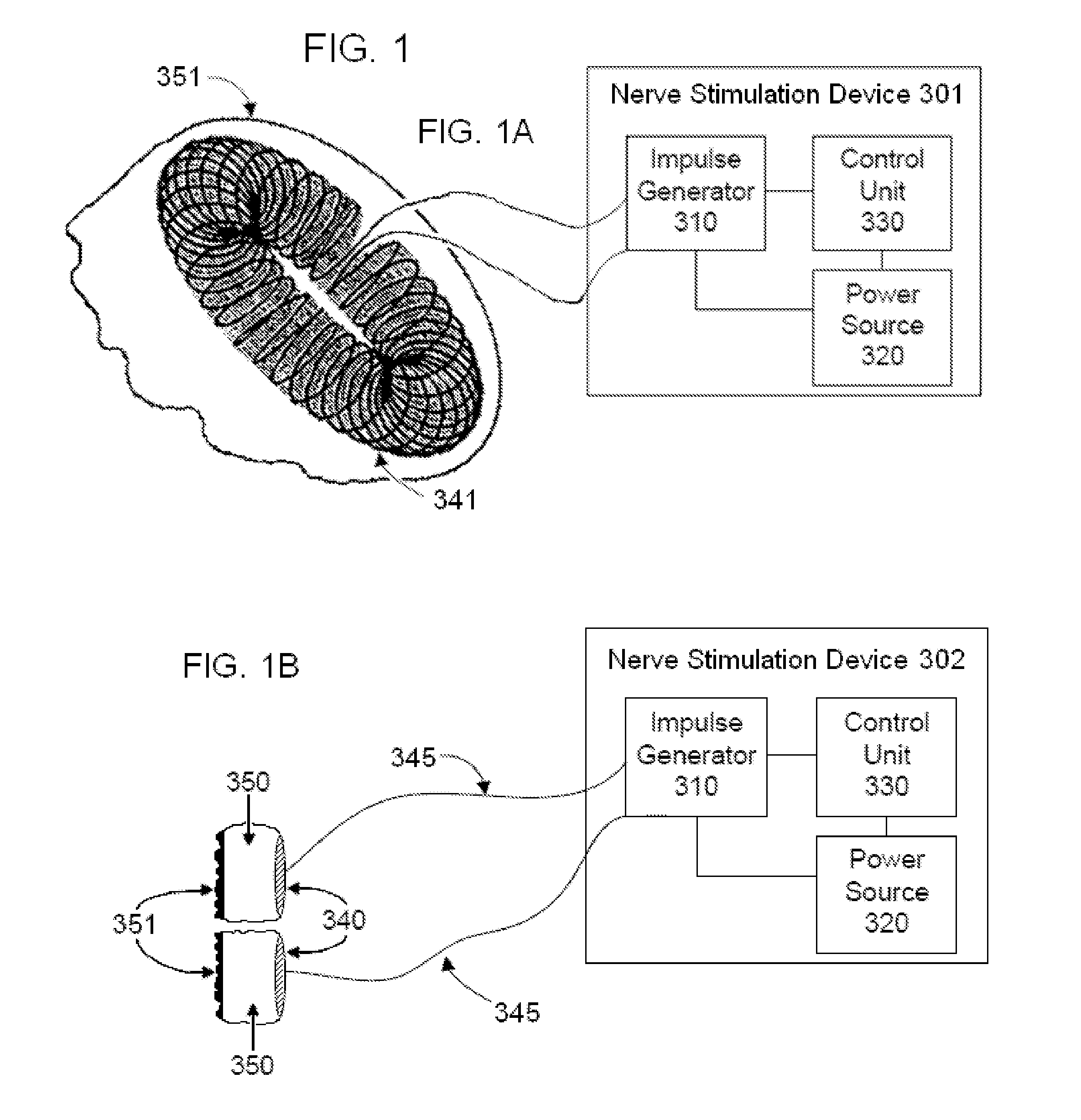
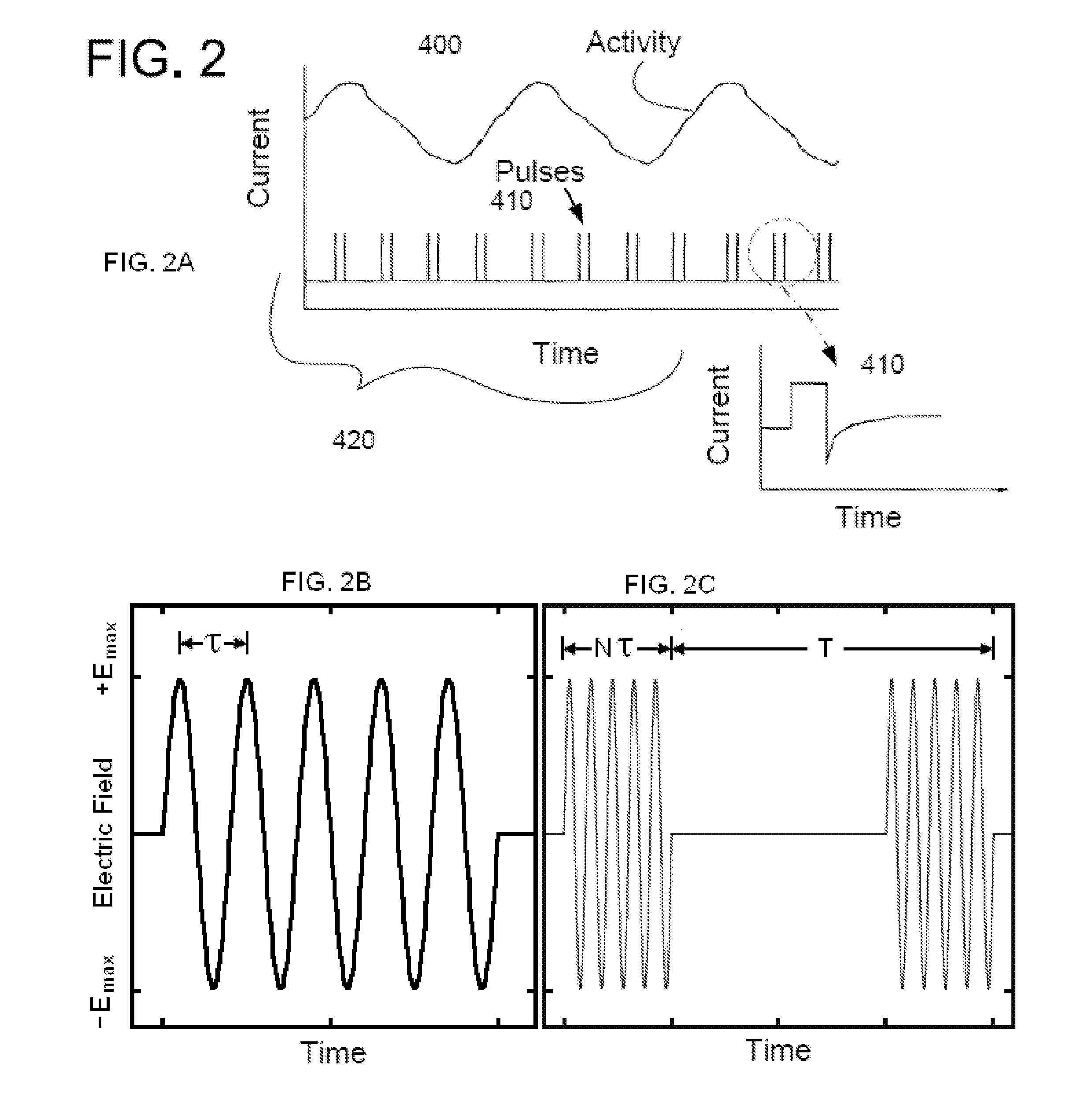
Non-invasive electrical and magnetic nerve stimulators used to treat overactive bladder and urinary incontinence
Transcutaneous electrical and magnetic nerve stimulation devices are disclosed, along with methods of treating lower urinary tract disorders using energy that is delivered noninvasively by the devices. The disorders comprise overactive bladder, urge incontinence, stress, incontinence, urge frequency, non-obstructive urinary retention and interstitial cystitis / painful bladder syndrome. In preferred embodiments of the disclosed methods, a posterior tibial nerve of a patient is stimulated non-invasively. Methods are disclosed for selecting protocol parameters for a nerve stimulation session for treating each individual patient, wherein modules of the bladder of the patient are represented as coupled non-linear oscillators.
Owner:ELECTROCORE
Benzamide derivative or salt thereof
InactiveUS20070167444A1Strong inhibitory activityBiocideNervous disorderNeurogenic painOveractive bladder
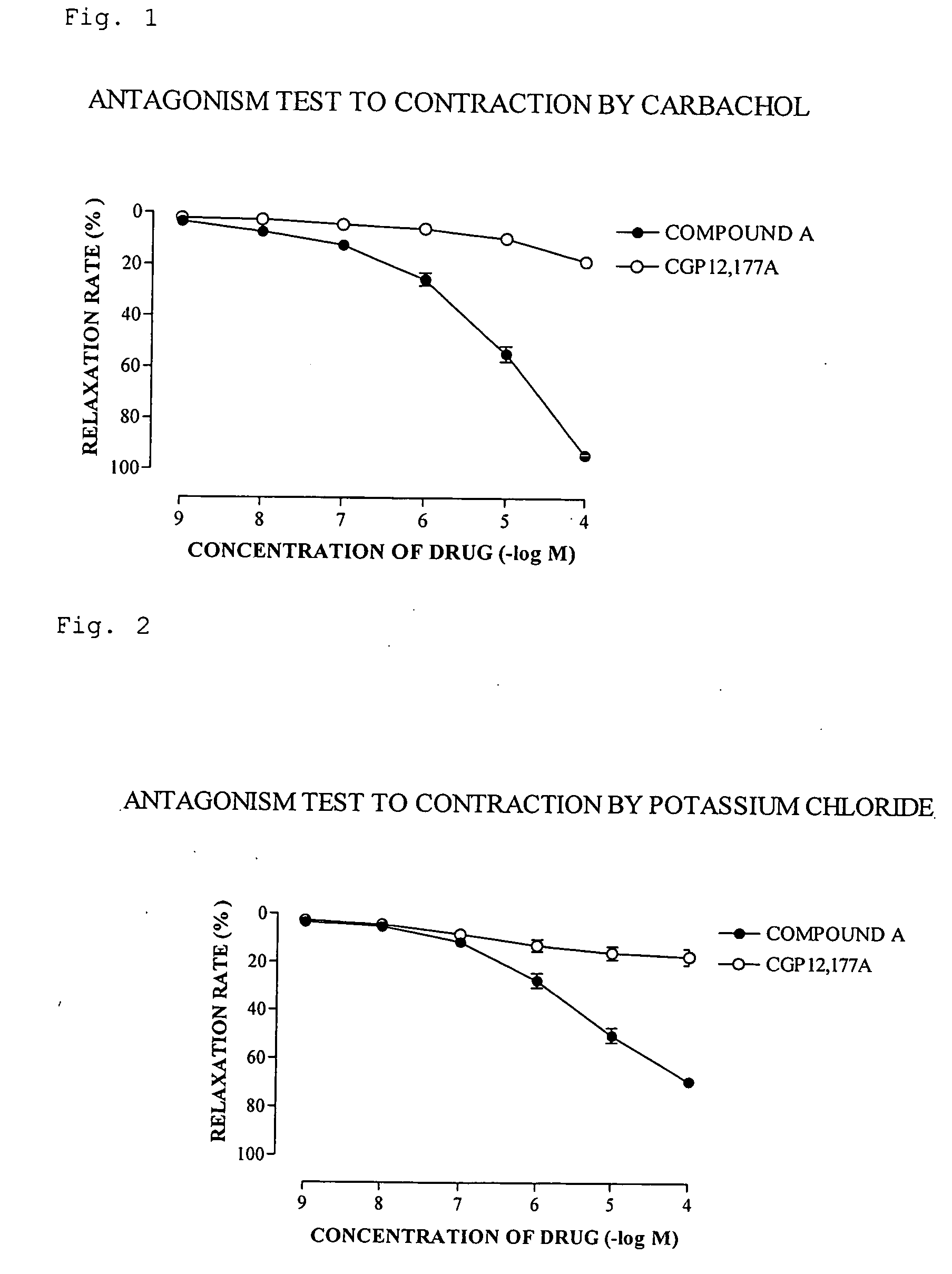
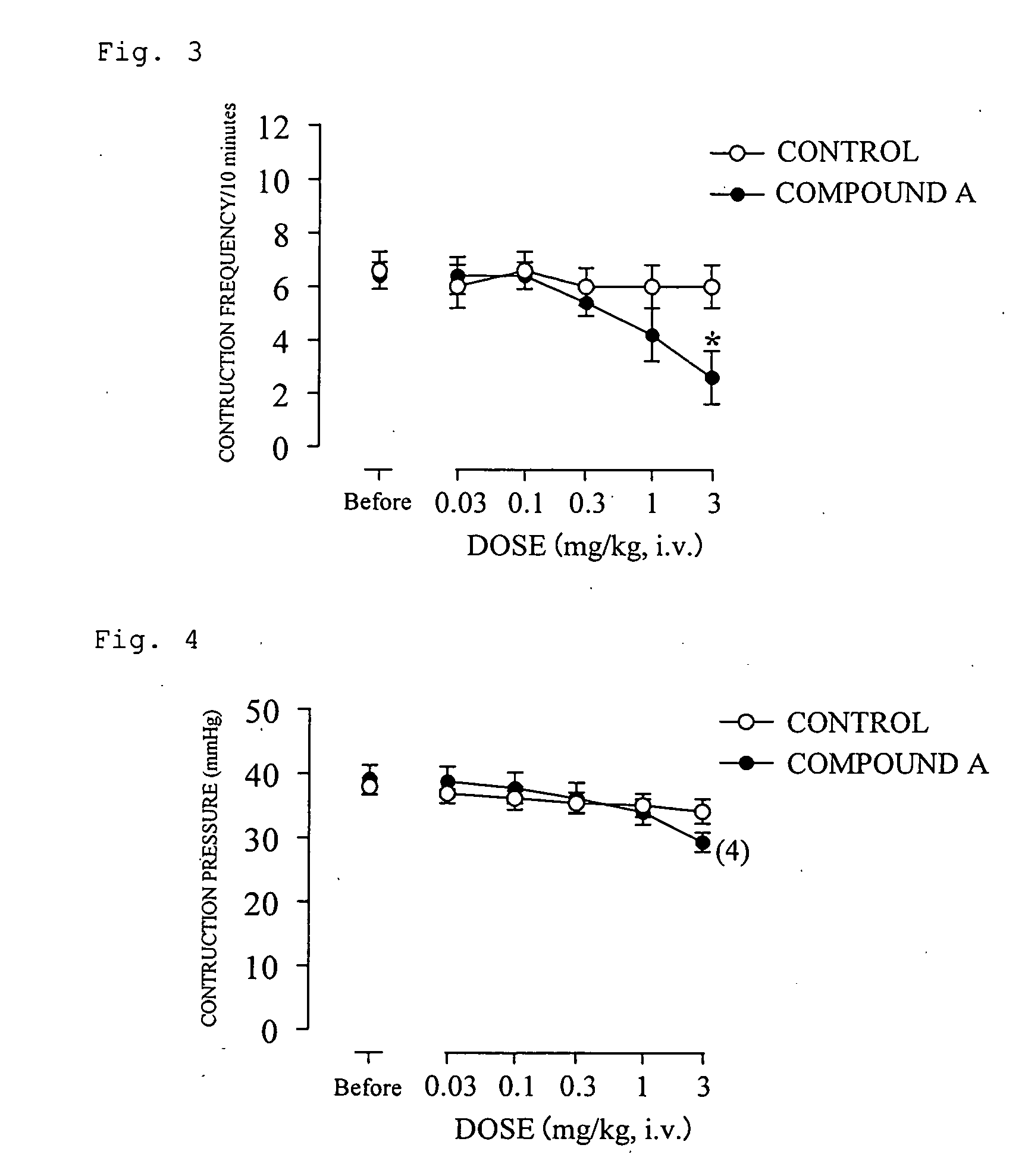
There is provided a compound having a capsaicin receptor VR1 inhibitory activity and useful as a therapeutic agent for various pains including inflammatory pain and neurogenic pain, migraine, cluster headache, bladder diseases including overactive bladder, and the like. A benzamide derivative or a salt thereof wherein a benzene ring is attached to a D ring (a monocyclic or bicyclic hydrocarbon ring or a monocyclic or bicyclic heteroaromatic ring) through an amide bond, the benzene ring is directly bonded to an E ring (a monocyclic or bicyclic hydrocarbon ring or a monocyclic or bicyclic heteroaromatic ring), and the benzene ring is further bonded to A (an amino moiety, a monocyclic or bicyclic heterocycle) through L (a lower alkylene).
Owner:ASTELLAS PHARMA INC
Therapy for the treatment of disease
ActiveUS20070053995A1Relieve constipationBiocideNervous disorderAnticholinergic agentsCompound (substance)
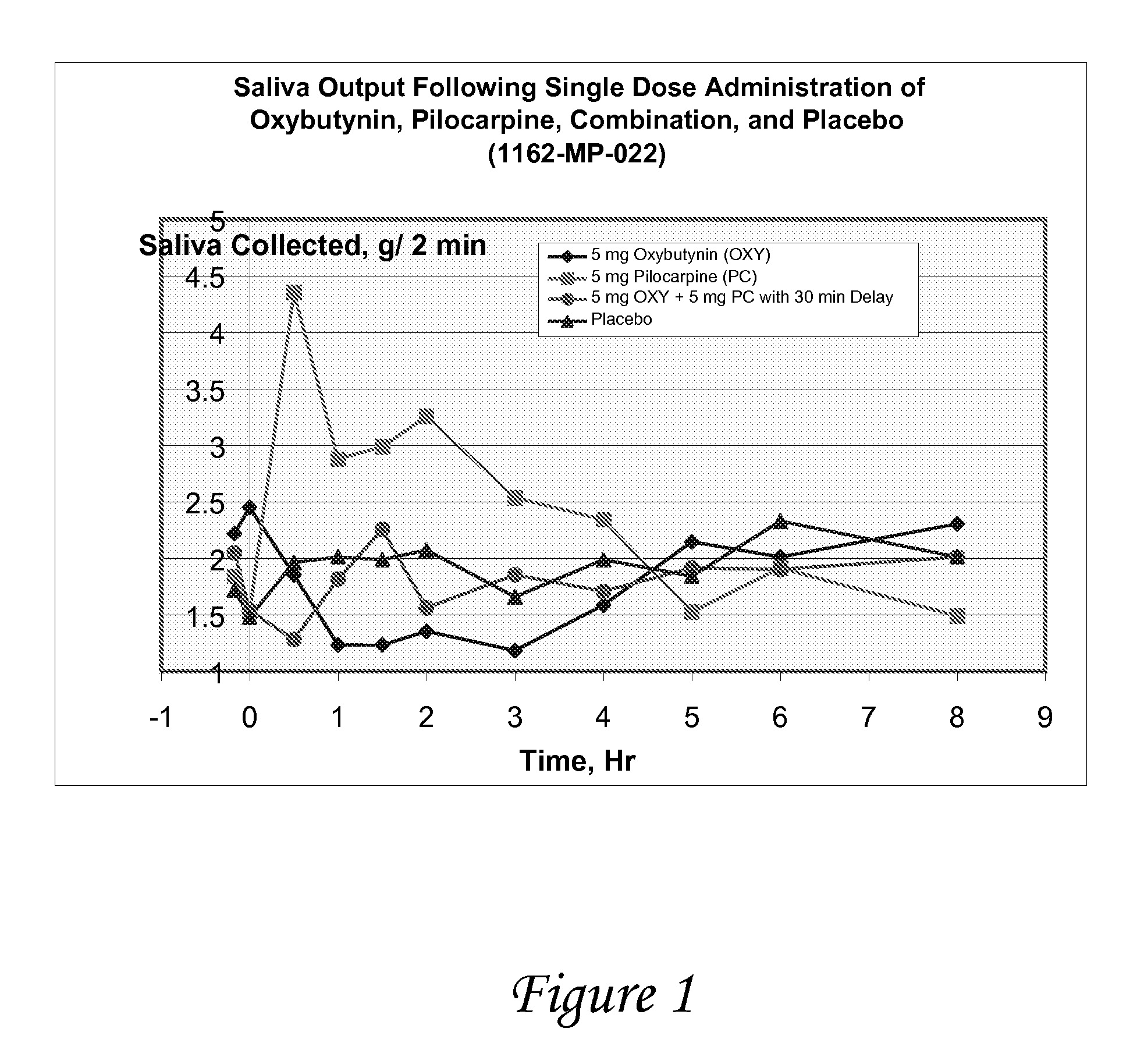
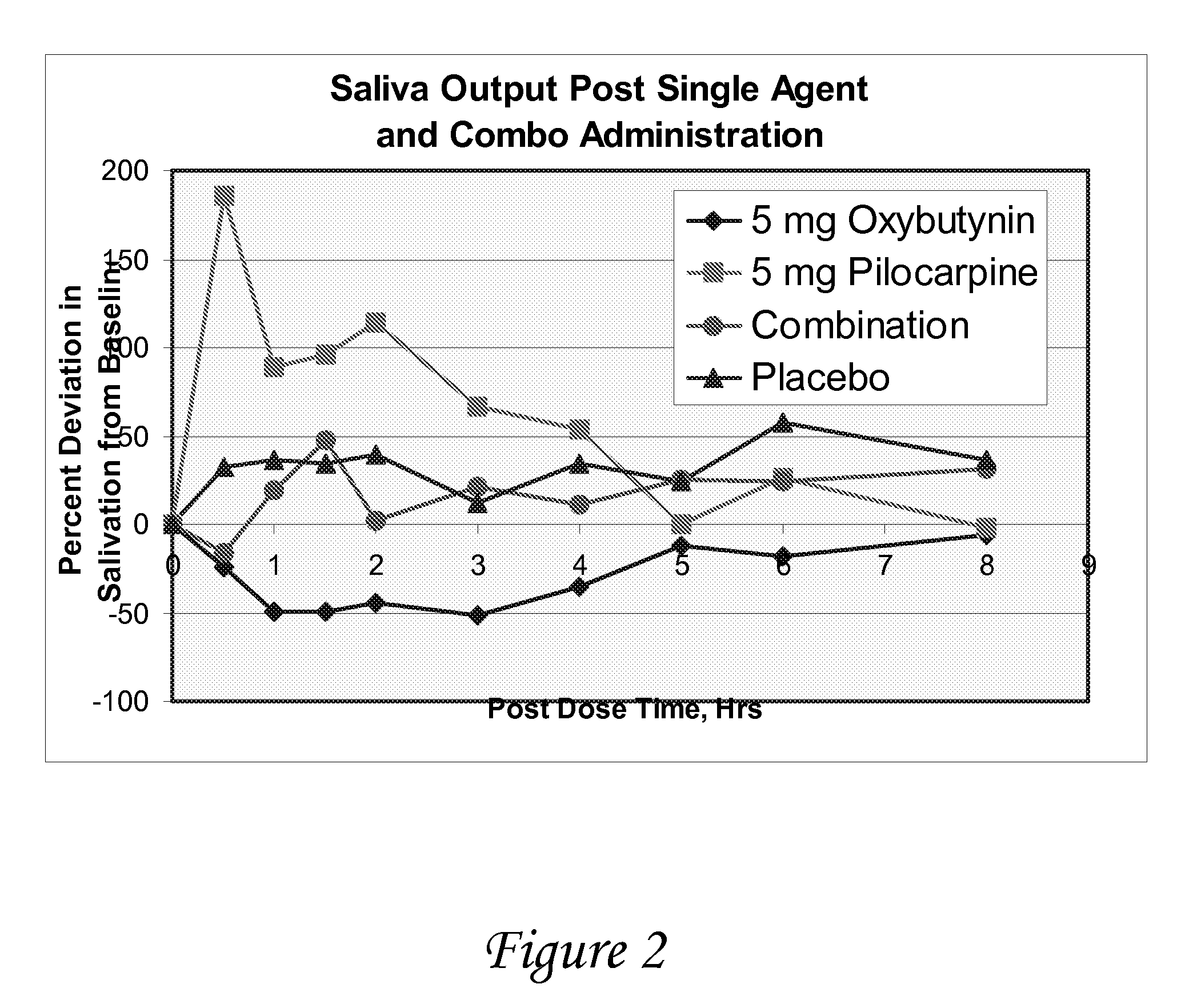
Disclosed herein are pharmaceutical compositions comprising various combinations of an antimuscarinic or an anticholinergic agent, a compound that causes stimulation of salivary glands, and a compound that relieves constipation. Also disclosed are methods of treating a patient suffering from overactive bladder comprising administering to the patient the above pharmaceutical composition.
Owner:THERAVIDA INC
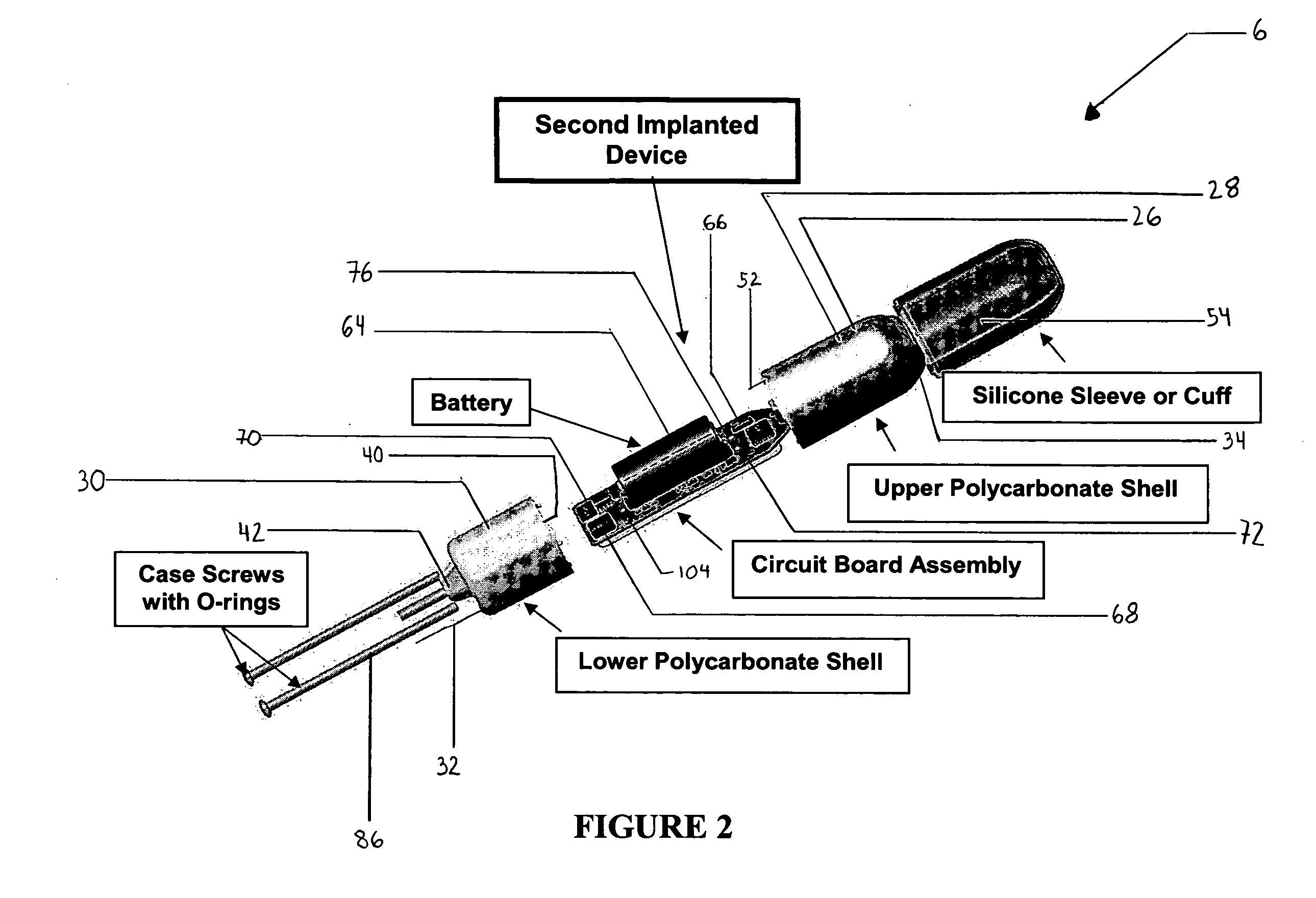
System and method for urodynamic evaluation utilizing micro electro-mechanical system technology
ActiveUS20080139875A1Negative buoyancyMinimizes and eliminates possibilitySurgeryDiagnostic recording/measuringBladder SpasmData acquisition
An implantable urodynamic system includes an implantable first device deployable in a patient's bladder, an implantable second device deployable in a patient's vaginal canal, and a data acquisition and analysis module or processing unit external to the body of the patient. The first device includes a magnet and an inductive coil, and the second device includes a magnet, an inductive coil and a battery. When deployed in the patient's body, attraction between the magnets maintains the two devices in close proximity to one another to effect an inductive coupling between the coils so that the first device may be powered by the battery of the second device. The urodynamic system is intended to facilitate measurement, collection, and wireless transmission of real-time, or near real-time, data (bladder pressure, abdominal pressure, and temperature) from an ambulatory patient. This data is of value in diagnosing a number of abnormal bladder conditions, such as infection, overactive bladder, bladder spasms, and the like.
Owner:ETHICON INC
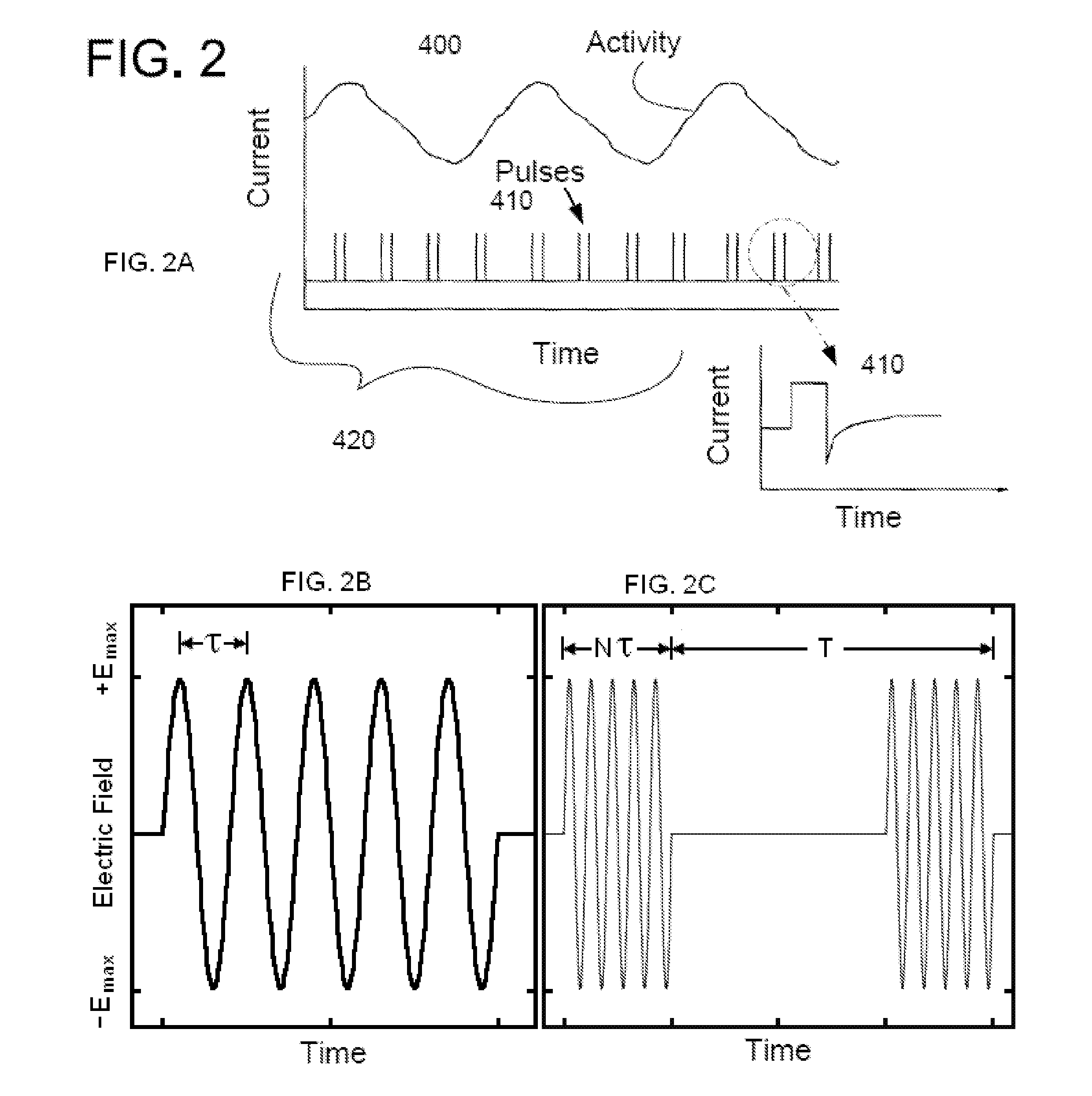
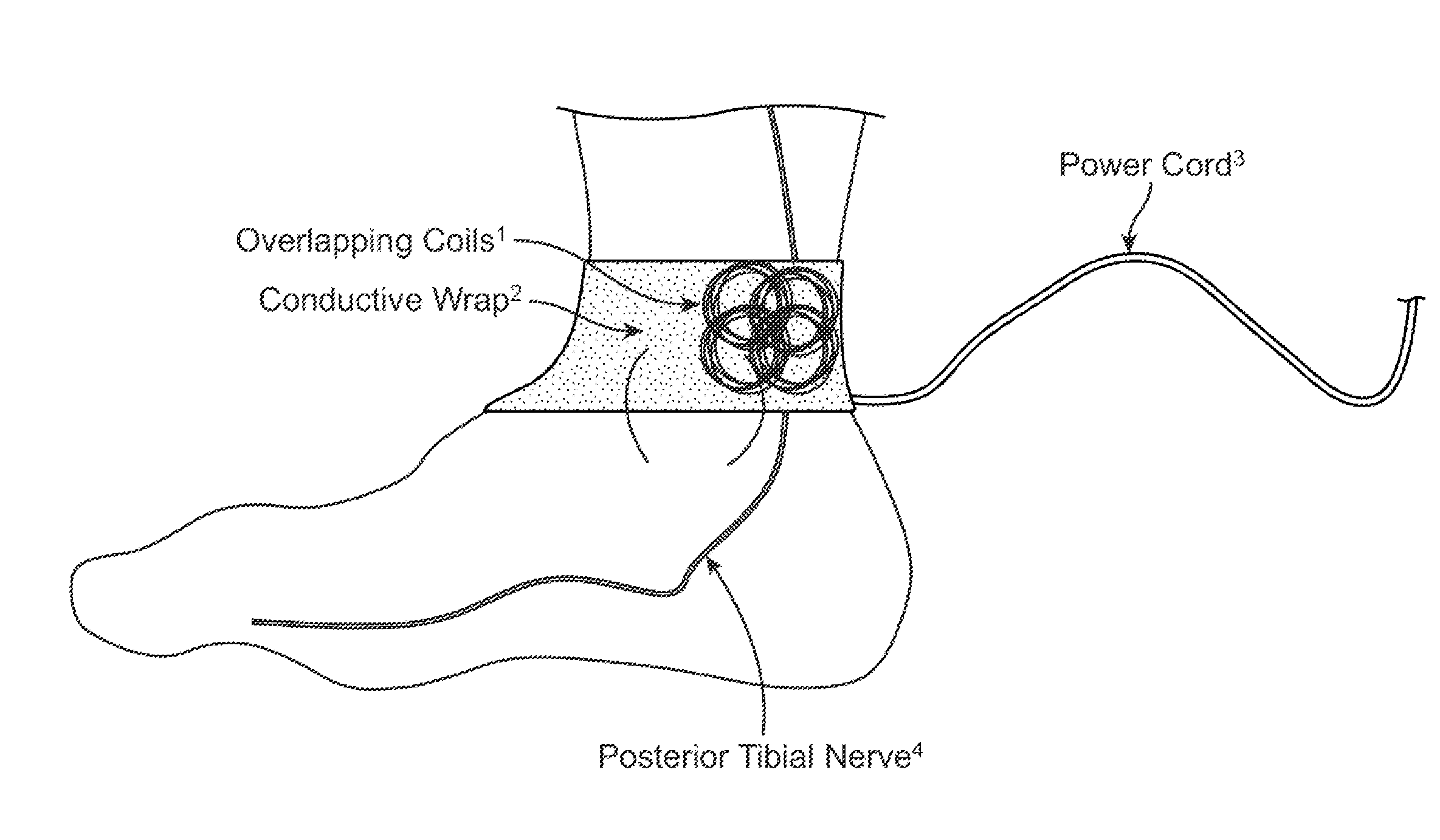
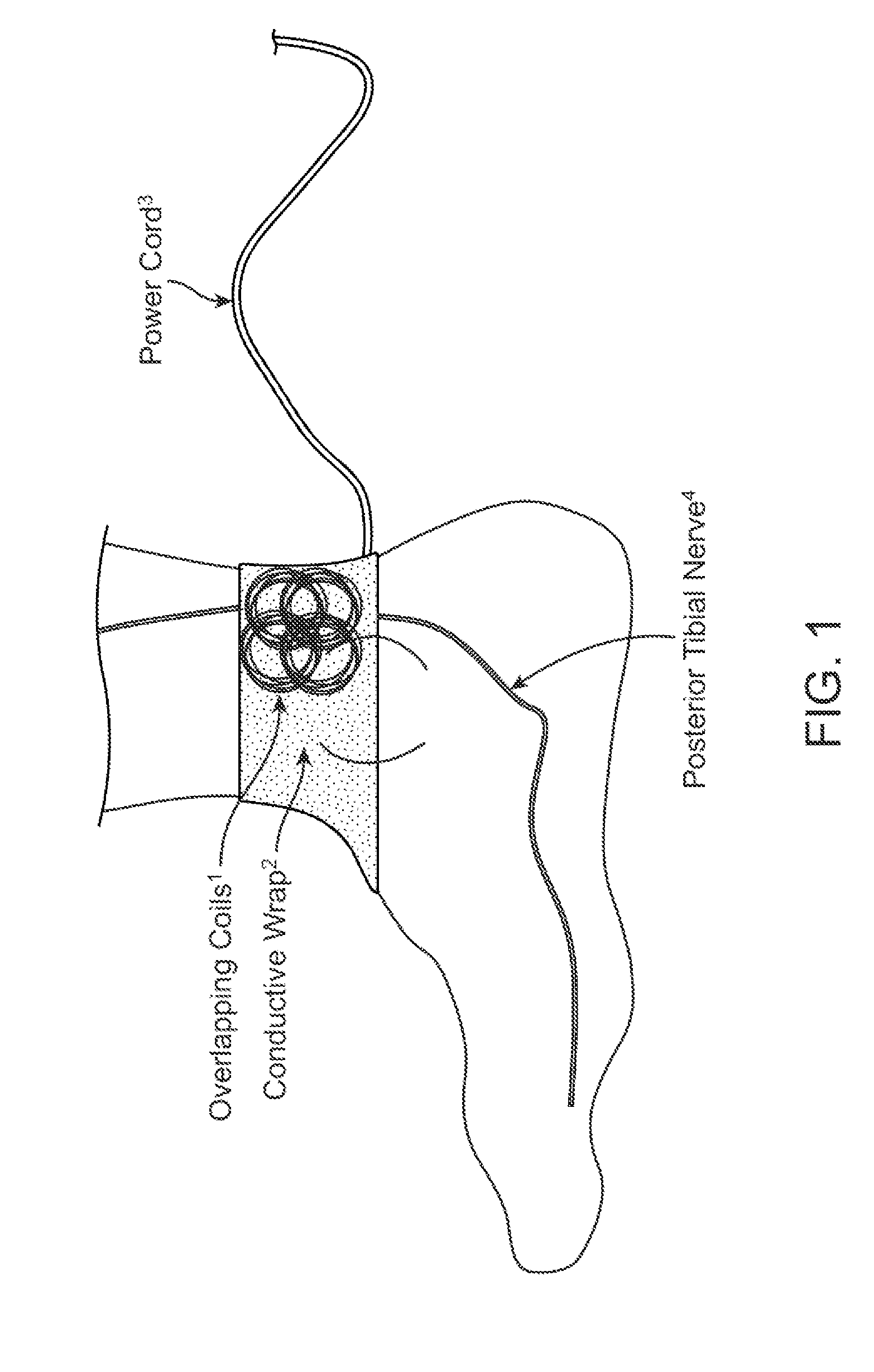
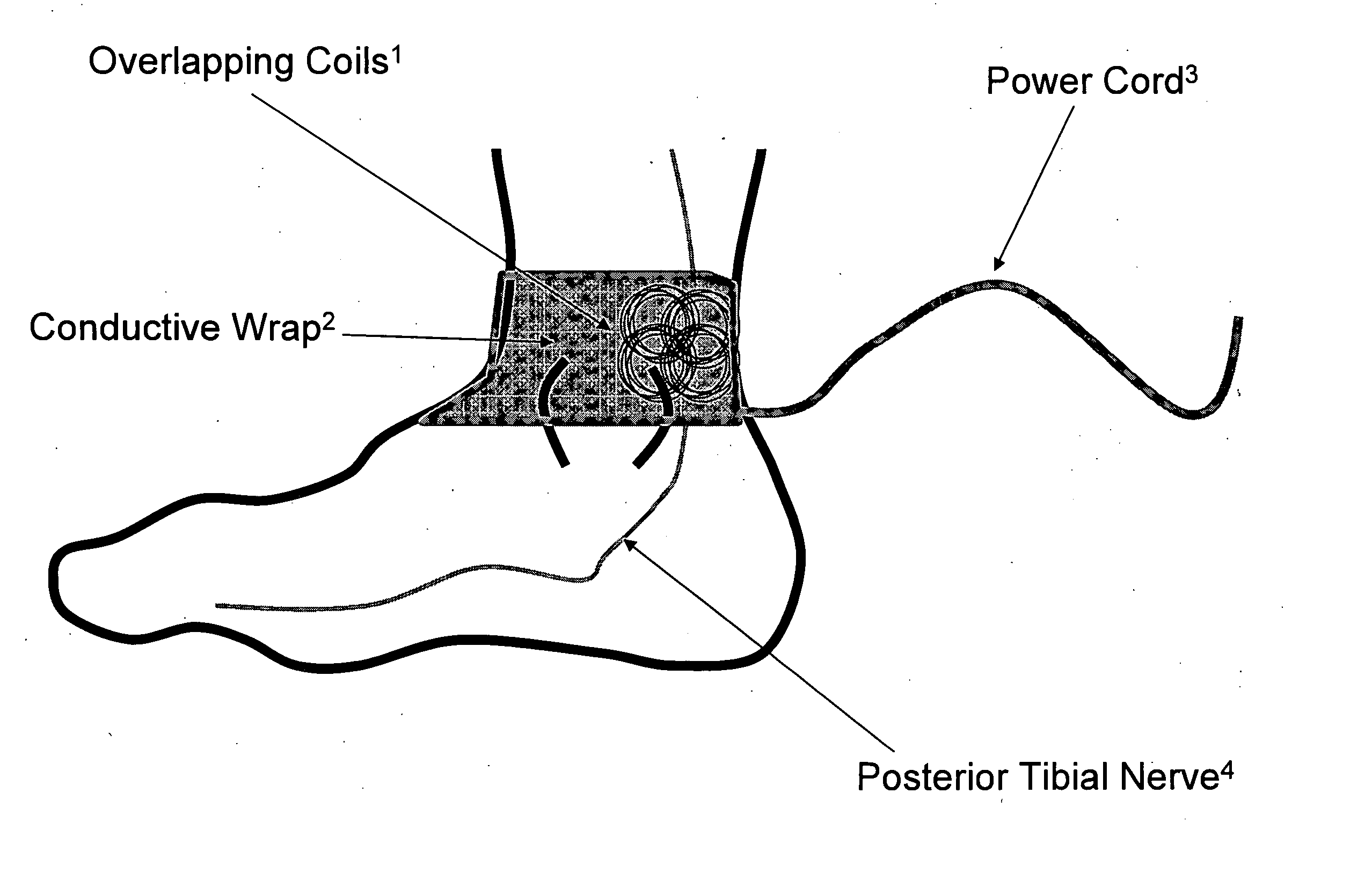
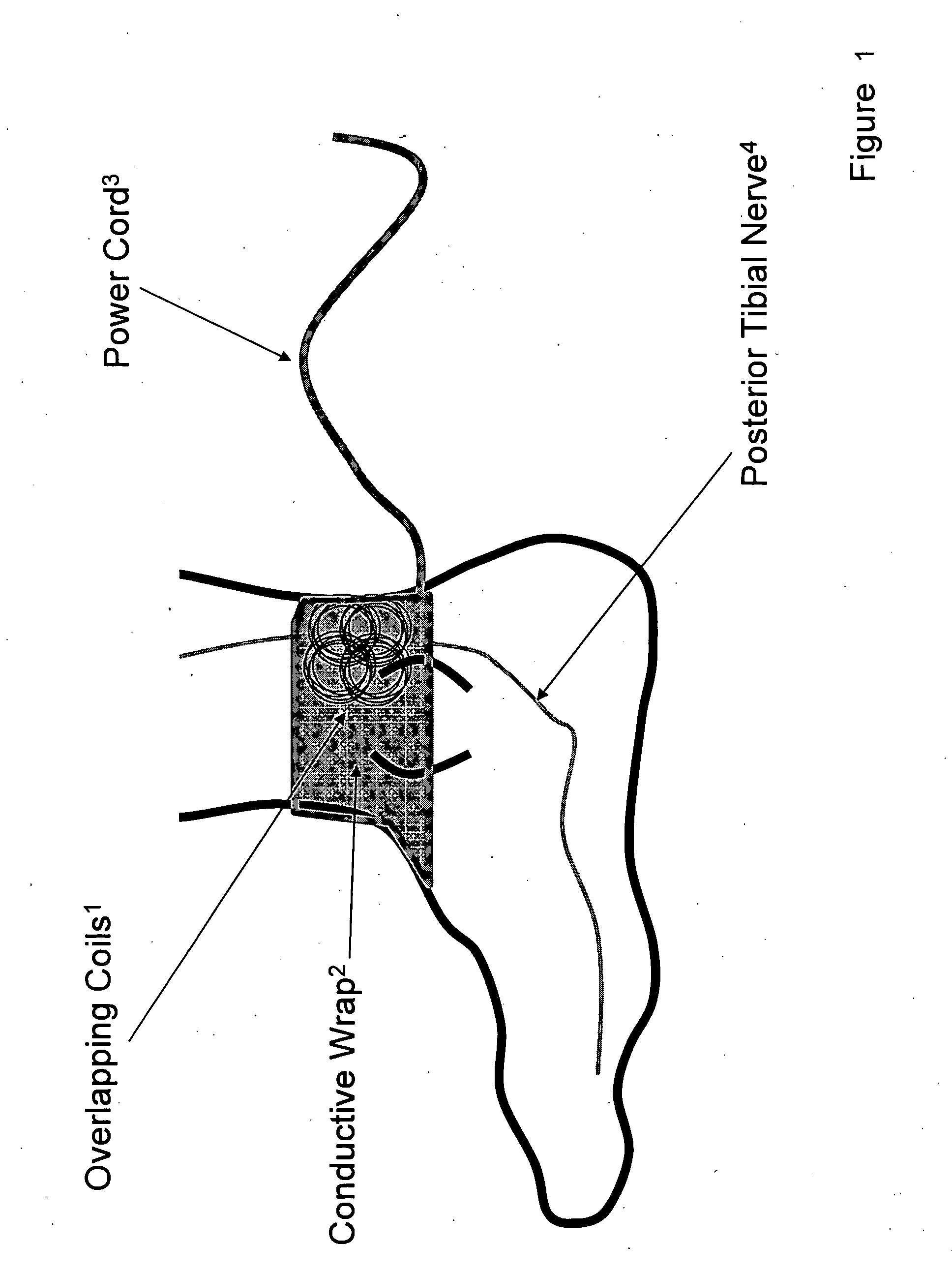
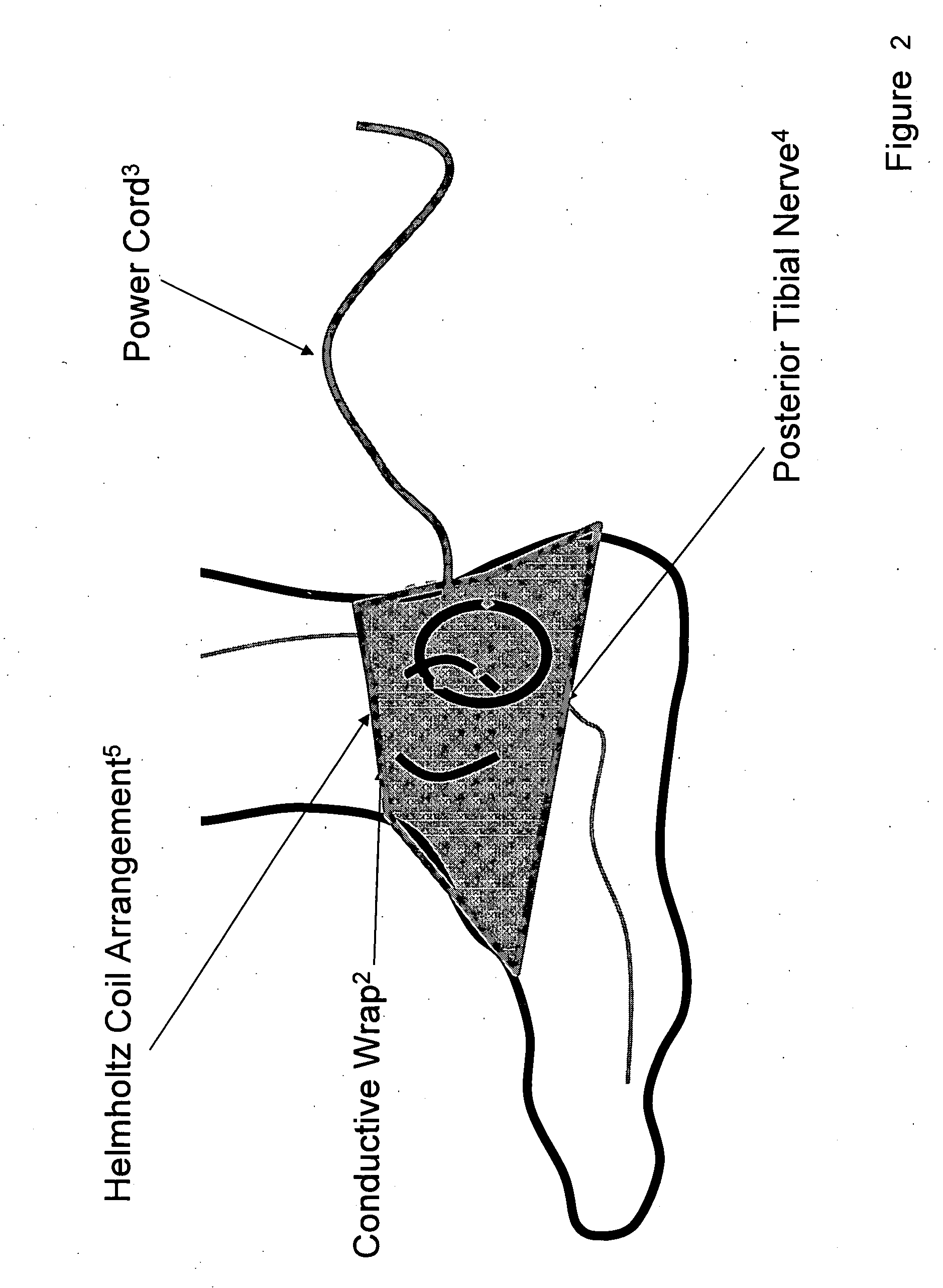
Method and apparatus for low frequency induction therapy for the treatment of urinary incontinence and overactive bladder
InactiveUS20100222630A1Easy to useEasy to placeElectrotherapyMagnetotherapy using coils/electromagnetsTreatment choicesTreatment options
Owner:EMKINETICS
Method and apparatus for low frequency induction therapy for the treatment of urinary incontinence and overactive bladder
Current treatment options for Overactive Bladder and Urinary Incontinence include exercise and behavioral modifications, pharmacological therapies, surgical intervention, and neuromodulation. Although each of these treatments is used in the treatment of individuals with these conditions, each has severe limitations. Building on the limitations of existing therapies, and with the distillation of lessons learned from the field of pulsed electric stimulation, the present invention employs Low Frequency Induction Therapy for the delivery of an effective, cost efficient, noninvasive alternative to available treatment options. The device of the present application allows for consistent, user-friendly modulation of the pudendal nerve and the sacral plexus, via pulsed electromagnetic stimulation of the posterior tibial nerve, on an outpatient basis. The device has two primary components: a programmable Logic Controller (LC), which generates the required current, and a Conductive Wrap (CW), through which the current is channeled in generating the pulsed electromagnetic fields.
Owner:EMKINETICS
Pharmaceutical combination
ActiveUS20130172277A1High potencyImprove efficacyBiocidePharmaceutical delivery mechanismMuscarinic antagonistAdrenergic
Pharmaceutical combinations comprising a beta-3 adrenergic receptor agonist and a muscarinic receptor antagonist, and methods for their use are disclosed. Methods of using the pharmaceutical combinations for the treatment of one or more symptoms associated with overactive bladder, for example, frequency of urgency, nocturia, and urinary incontinence, are also disclosed.
Owner:B3AR THERAPEUTICS INC
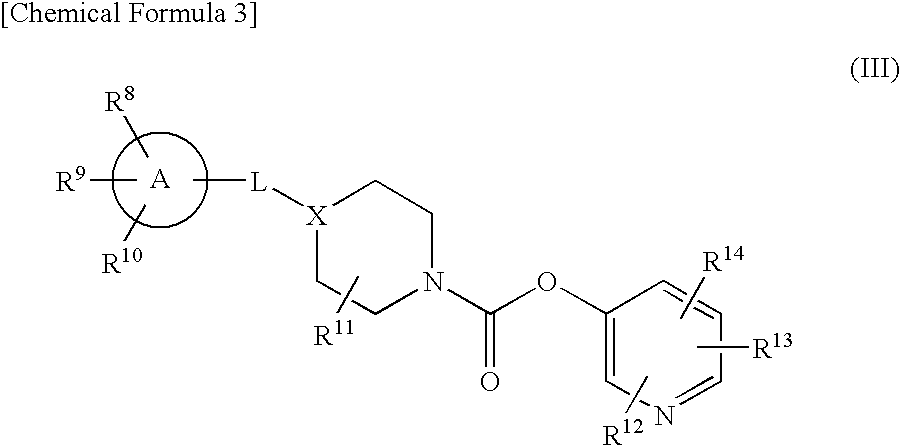
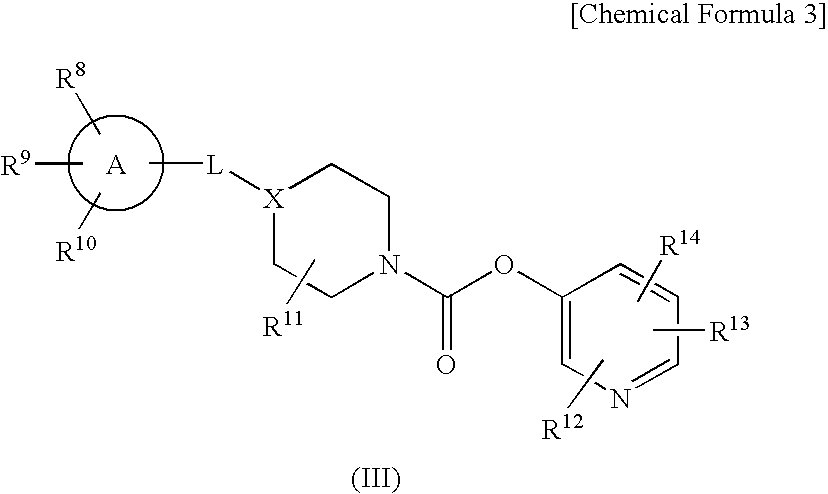
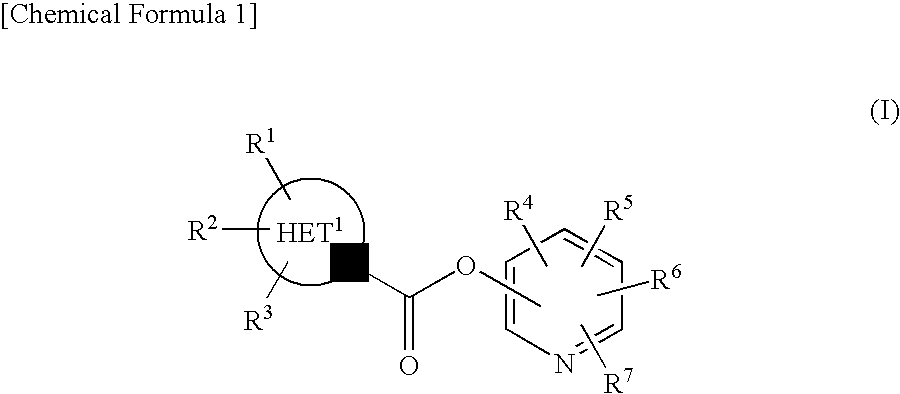
Pyridyl Non-Aromatic Nitrogen-Containing Heterocyclic-1-Carboxylate Compound
ActiveUS20100009971A1Excellent therapeutical effectIncrease capacityBiocideSenses disorderCompound aNitrogen
A novel pyridyl non-aromatic nitrogen-containing heterocyclic-1-carboxylate compound or its pharmaceutically acceptable salt has a potent FAAH-inhibitory activity. Further, the pyridyl non-aromatic nitrogen-containing heterocyclic-1-carboxylate compound of the present disclosure is also useful in the treatment of urinary frequency and urinary incontinence, overactive bladder and / or pain.
Owner:AUTOBAHN THERAPEUTICS INC
Therapy for the treatment of disease
Disclosed herein are pharmaceutical compositions comprising various combinations of an antimuscarinic or an anticholinergic agent, a compound that causes stimulation of salivary glands, and a compound that relieves constipation. Also disclosed are methods of treating a patient suffering from overactive bladder comprising administering to the patient the above pharmaceutical composition.
Owner:THERAVIDA INC
Non-invasive electrical and magnetic nerve stimulators used to treat overactive bladder and urinary incontinence
Transcutaneous electrical and magnetic nerve stimulation devices and methods generate energy that is delivered noninvasively to selected nerve fibers within the patient to treat lower urinary tract disorders. The disorders comprise overactive bladder, urge incontinence, stress, incontinence, urge frequency, non-obstructive urinary retention and interstitial cystitis / painful bladder syndrome. In preferred embodiments, a posterior tibial nerve of a patient is stimulated non-invasively to treat bladder disorders.
Owner:ELECTROCORE
Pyridyl Non-Aromatic Nitrogen-Containing Heterocyclic-1-Carboxylate Derivative
ActiveUS20080306046A1Excellent therapeutical effectIncrease capacityBiocideSenses disorderDiseaseBladder capacity
[Problem] To provide a compound usable for treatment of diseases associated with fatty acid amide hydrolase (FAAH), especially for treatment of urinary frequency and urinary incontinence, overactive bladder and / or pain.[Means for Solution] We have found that a novel pyridyl non-aromatic nitrogen-containing heterocyclic-1-carboxylate derivative and its pharmaceutically acceptable salt has a potent FAAH-inhibitory activity. Further, the pyridyl non-aromatic nitrogen-containing heterocyclic-1-carboxylate derivative of the present invention has an excellent effect for increasing an effective bladder capacity, an excellent effect for relieving urinary frequency and an excellent anti-allodynia effect, and is therefore usable for treatment of urinary frequency and urinary incontinence, overactive bladder and / or pain.
Owner:AUTOBAHN THERAPEUTICS INC
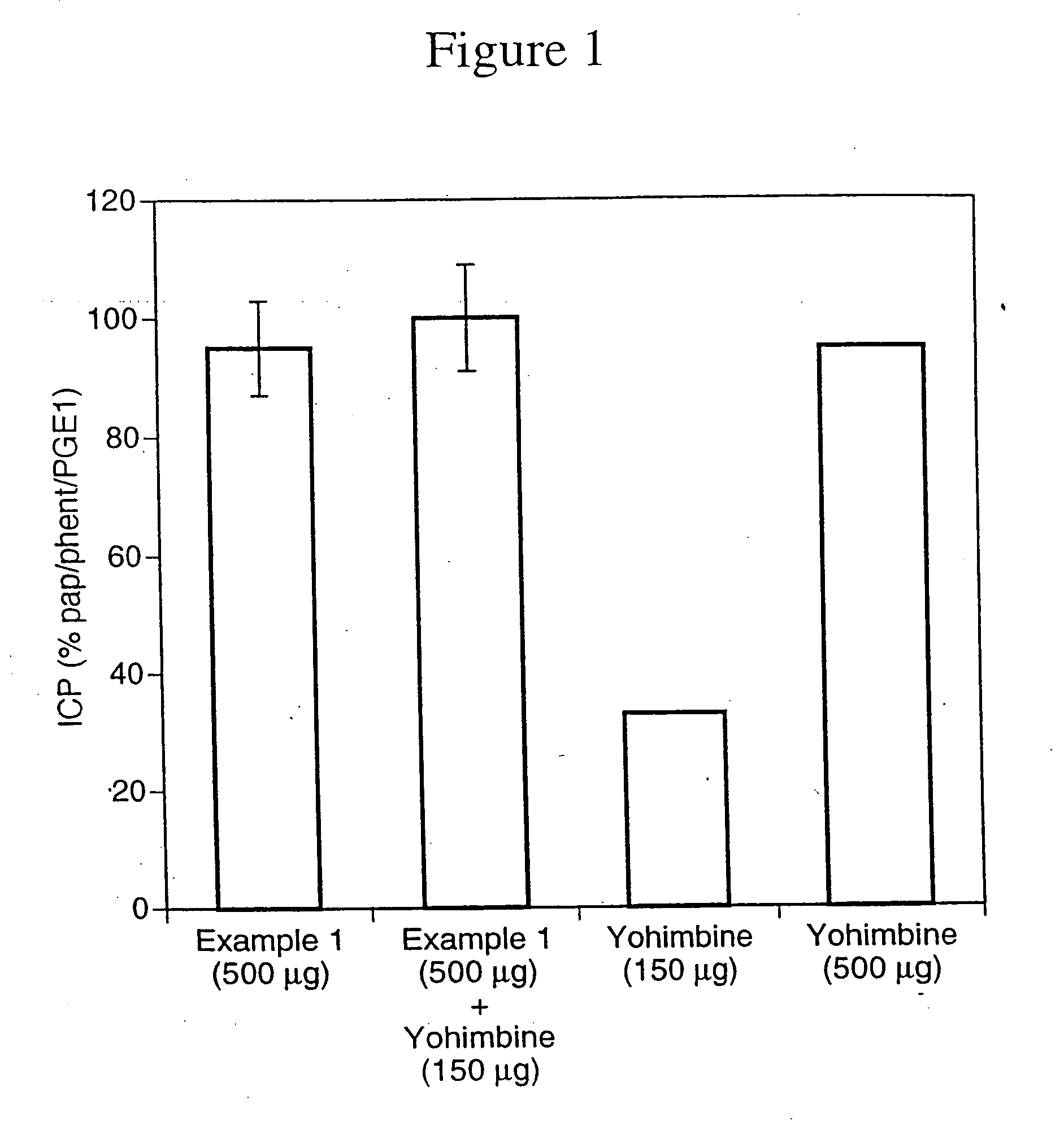
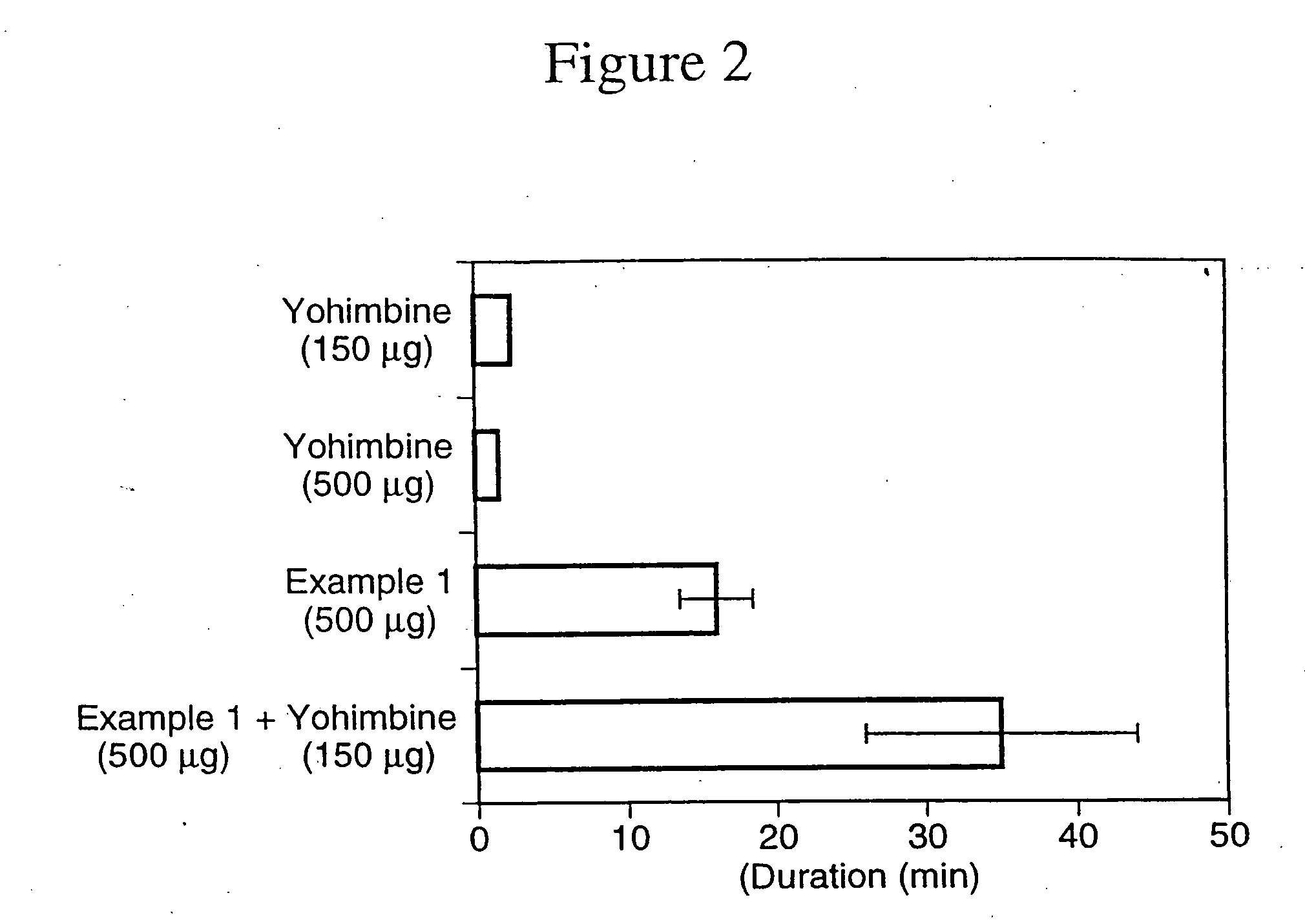
Nitrosated and nitrosylated alpha-adrenergic receptor antagonist compounds
The present invention describes novel nitrosated and / or nitrosylated α-adrenergic receptor antagonists, and novel compositions containing at least one nitrosated and / or nitrosylated α-adrenergic receptor antagonist, and, optionally, one or more compounds that donate, transfer or release nitric oxide, elevate endogenous levels of endothelium-derived relaxing factor, stimulate endogenous synthesis of nitric oxide or are a substrate for nitric oxide synthase, and / or one or more vasoactive agents. The present invention also provides novel compositions containing at least one α-adrenergic receptor antagonist, and one or more compounds that donate, transfer or release nitric oxide, elevate endogenous levels of endothelium-derived relaxing factor, stimulate endogenous synthesis of nitric oxide or is a substrate for nitric oxide synthase and / or one or more vasoactive agents. The present invention also provides methods for treating or preventing sexual dysfunctions in males and females, for enhancing sexual responses in males and females, and for treating or preventing benign prostatic hyperplasia, hypertension, congestive heart failure, variant (Printzmetal) angina, glaucoma, neurodegenerative disorders, vasospastic diseases, cognitive disorders, urge incontinence, or overactive bladder, and for reversing the state of anesthesia.
Owner:NITROMED
Bladder tissue modification for overactive bladder disorders
ActiveUS20150157389A1Reduce generationDecrease their propagationUltrasound therapyBiocideElectricityUrethra
Regions of tissue having reduced electrical propagation are created in a bladder to affect its electrical or mechanical properties. To create these tissue regions, a tubular device is advanced through the urethra leading to the interior of the bladder, a distal expandable structure of the device is expanded to contact the inner wall of the bladder, and electrodes or other active energy delivery elements of the device are activated to deliver ablation energy. The electrodes or other active energy delivery elements are disposed over the expandable structure which is shaped to conform to the interior of the bladder. The inner wall of the organ is ablated in a predetermined pattern. The same or other electrodes disposed over the expandable structure can used to electrically map the bladder. This map of electrical activity can be used to create the predetermined pattern.
Owner:NEWURO
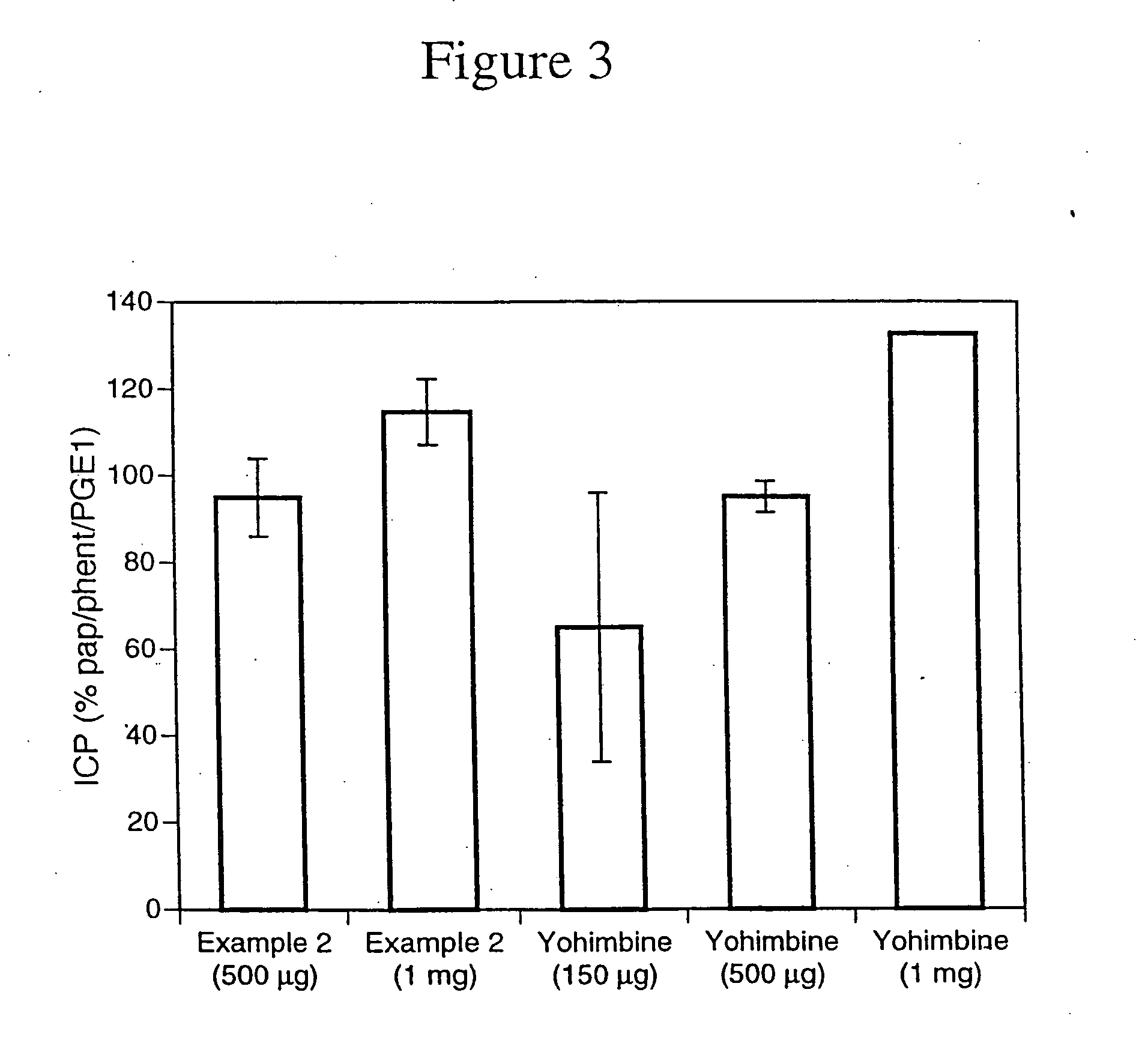
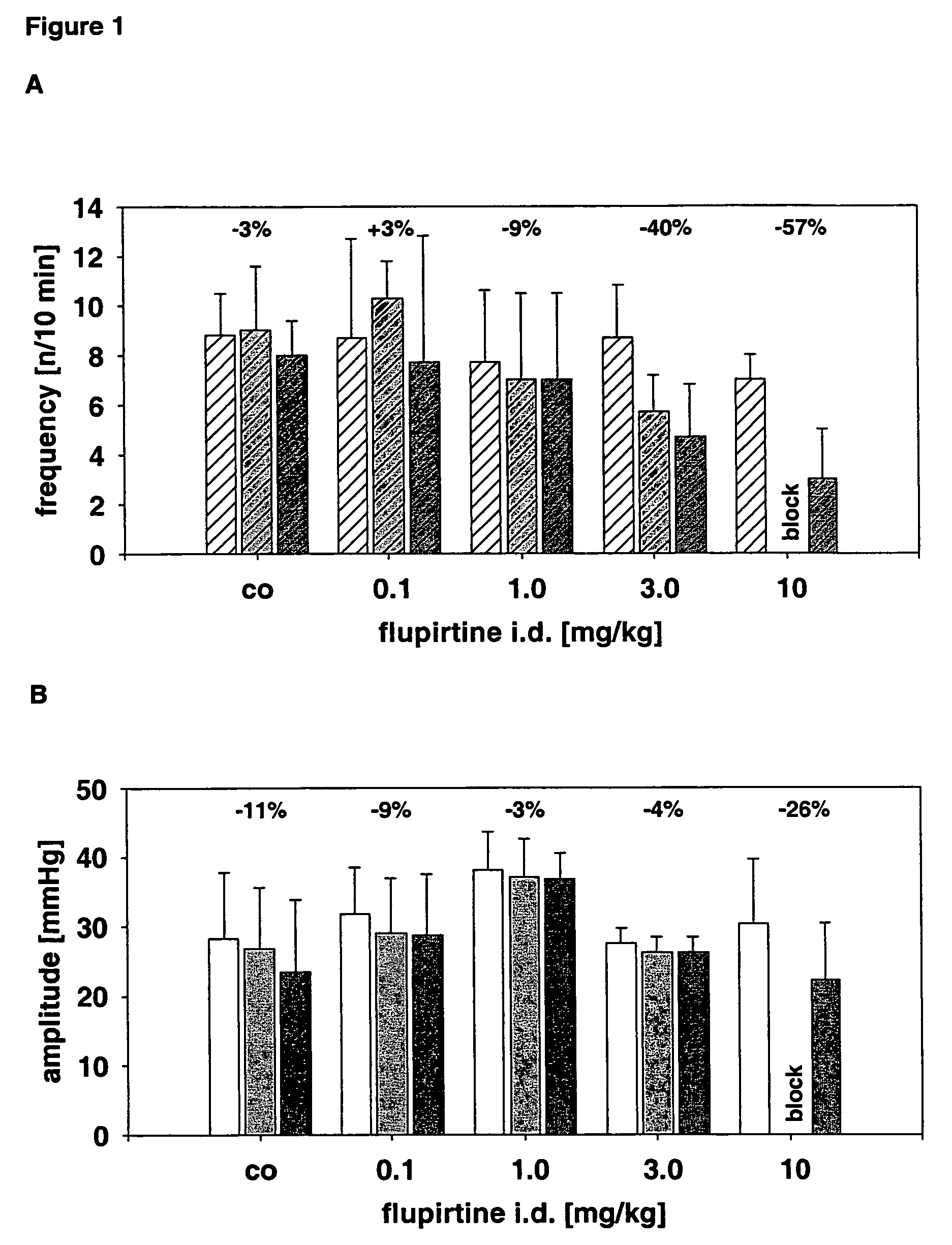
Use of the non-opiate analgesic drug flupirtine for the treatment of overactive bladder and associated diseases including urge incontinence, urinary flow problems as a result of prostate hyperplasia and irritable bowel syndrome
InactiveUS7309713B2Reduce concentrationReduce maintenanceBiocideDigestive systemAnalgesics drugsFecal incontinence
The present invention is directed to the prevention, reversal and medical treatment of lower urinary tract dysfunction including bladder instability and other related diseases as described below including urinary flow problems, urgency and incontinence as a result of prostate hyperplasia (BPH) and to the prevention, reversal and medical treatment of irritable bowl syndrome (IBS) with special focus on the diarrhea-predominant and mixed diarrhea-constipation type IBS, both in human beings and animals.
Owner:RUNDFELDT DR CHRISTIAN
Remedy for overactive bladder comprising acetic acid anilide derivative as the active ingredient
ActiveUS20060115540A1Symptoms improvedRelieve symptomsOrganic active ingredientsBiocideMeasurement testBULK ACTIVE INGREDIENT
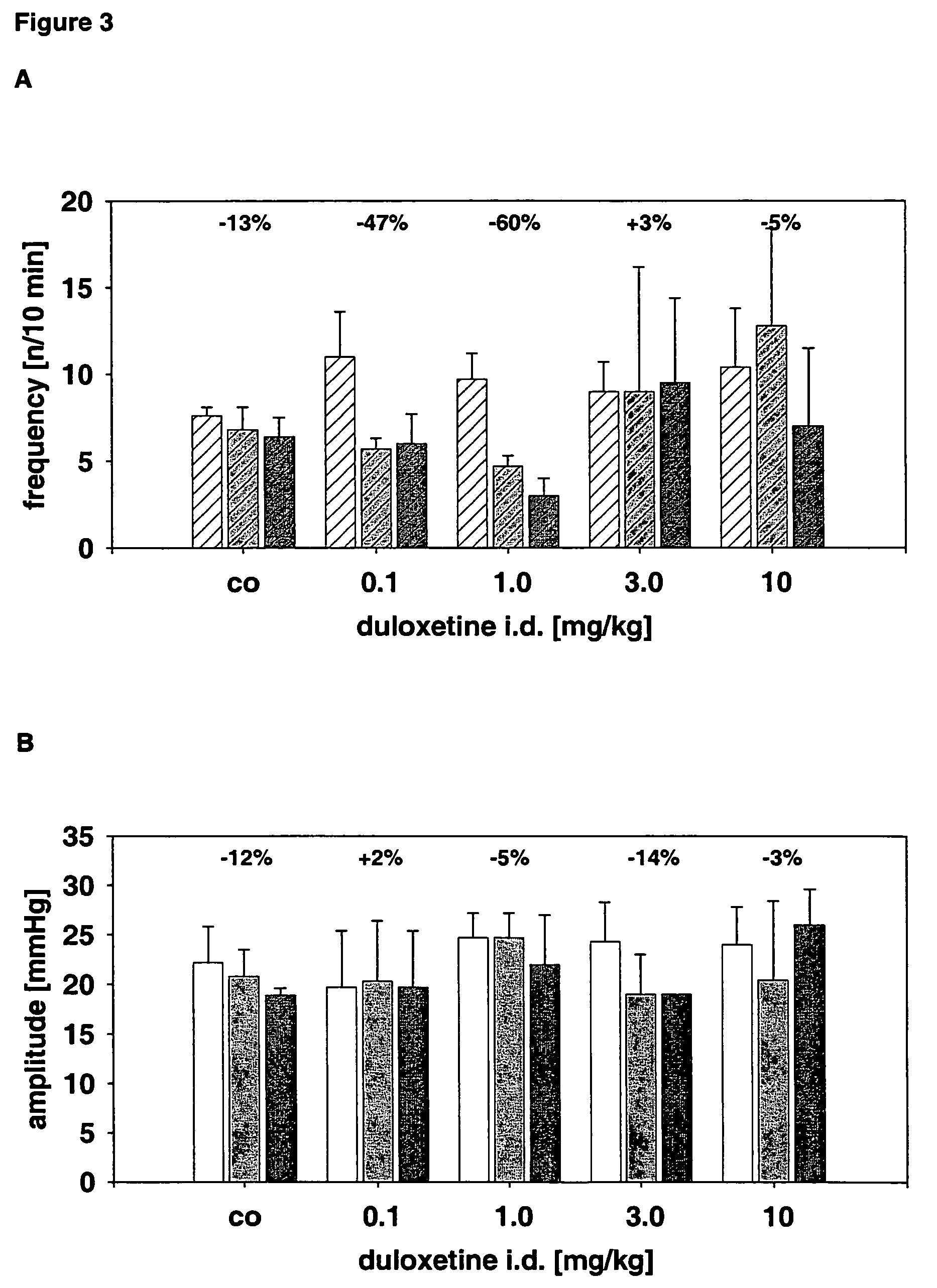
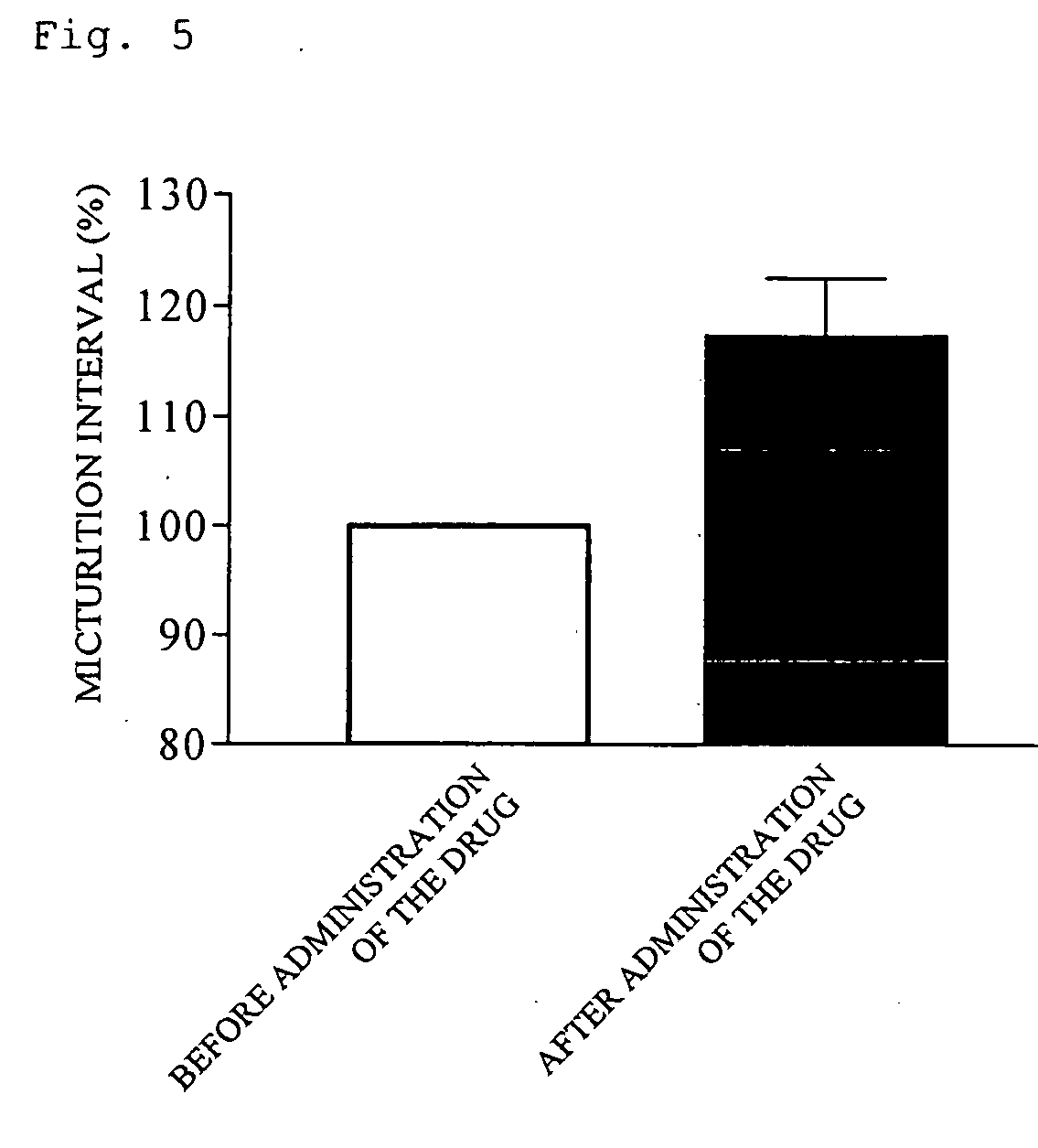
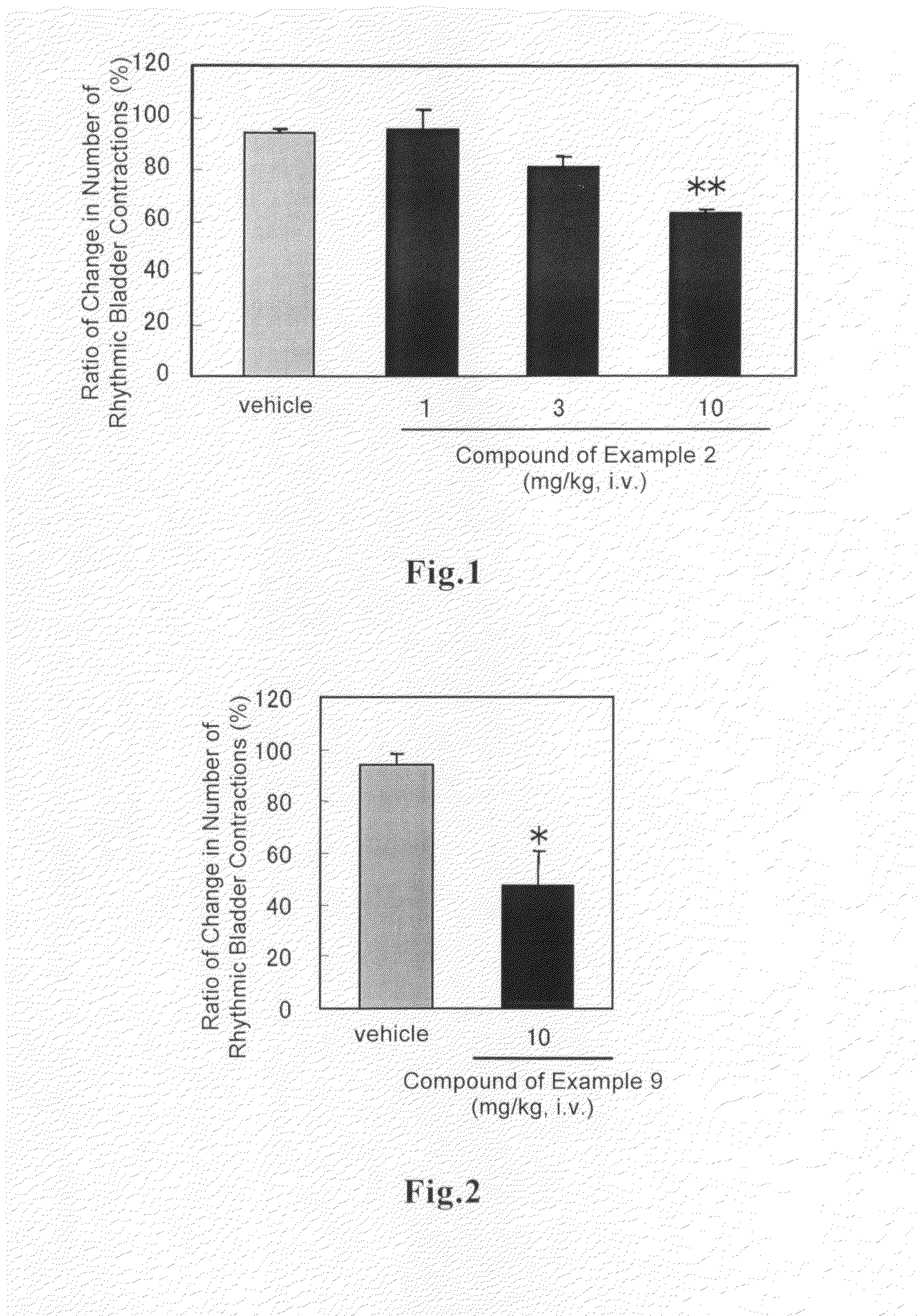
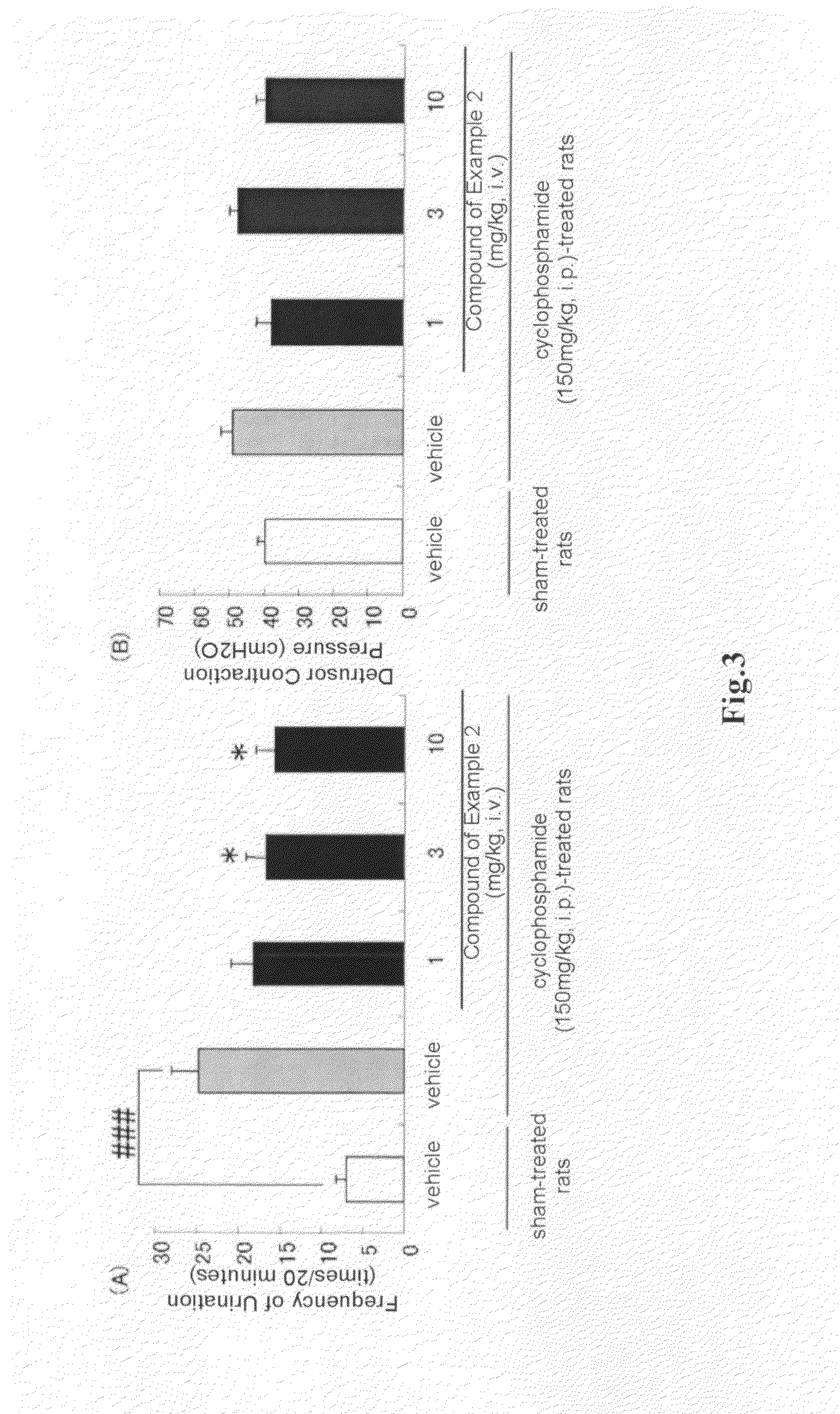
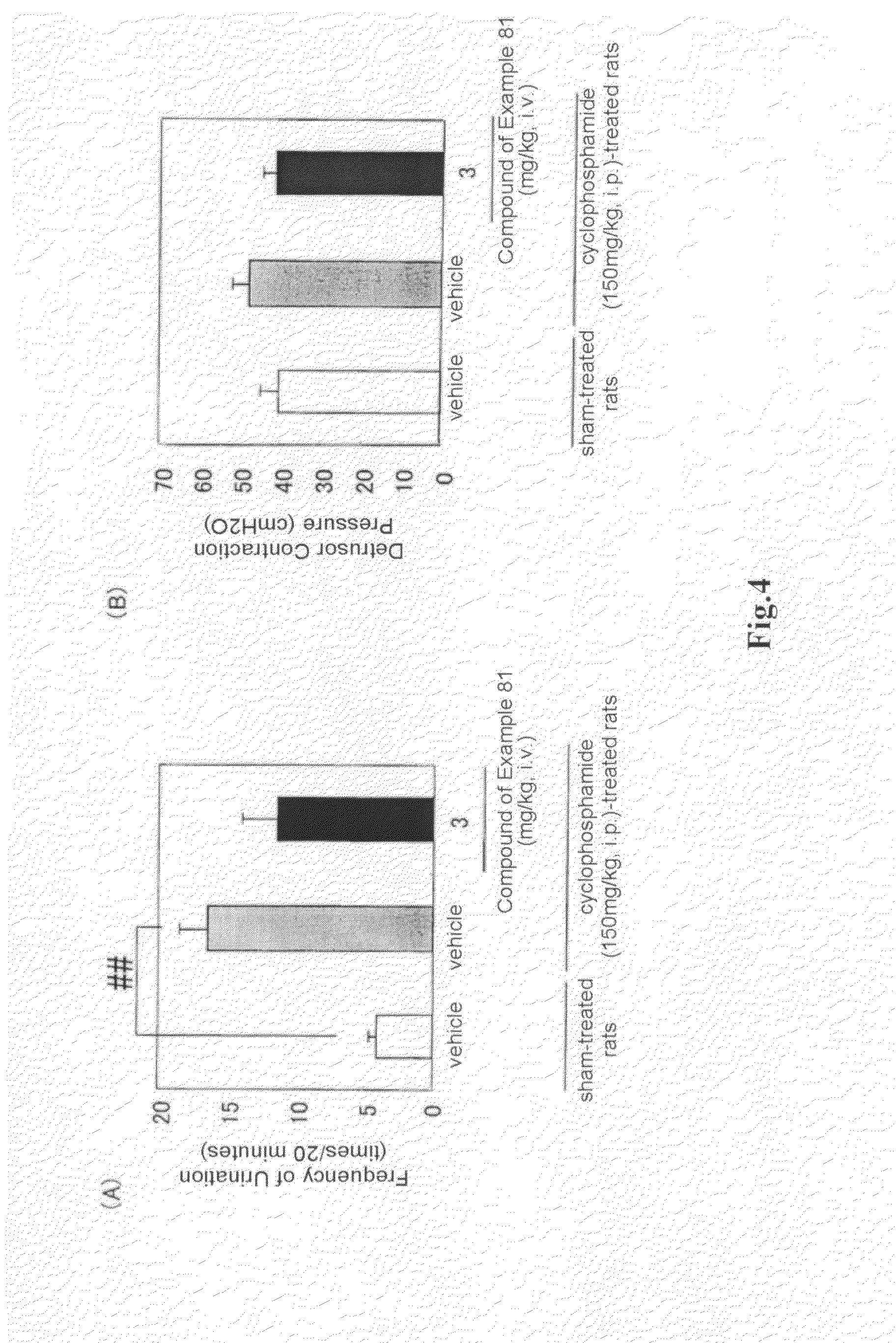
(R)-2-(2-aminothiazol-4-yl)-4′-[2-[(2-hydroxy-2-phenylethyl)amino]ethyl]acetic acid anilide or its salt shows a potent bladder relaxation effect in “isolated rat bladder smooth muscle relaxation test”, dose-dependently lowers the contraction frequency of rhythmic bladder contractions in “rat rhythmic bladder contraction measurement test” and, moreover, prolongs the urination intervals in “urination functions measurement test on cyclophosphamide-induced overactive bladder model rat”. Owing to these effects, the above compound is useful as a remedy for ovaractive bladder.
Owner:ASTELLAS PHARMA INC
Pyridyl Non-Aromatic Nitrogen-Containing Heterocyclic-1-Carboxylate Compound
ActiveUS20100009972A1Excellent therapeutical effectIncrease capacityBiocideSenses disorderCompound aNitrogen
Owner:AUTOBAHN THERAPEUTICS INC
Overactive bladder treating drug
A therapeutic agent for overactive bladder containing tamsulosin or a pharmaceutically acceptable salt thereof as an effective ingredient.
Owner:ASTELLAS PHARMA INC
Methods of improving quality of sleep
InactiveUS20110245294A1Improve sleep qualityBiocideNervous disorderAnticholinergic agentsOveractive bladder
Disclosed herein are methods of treating a patient suffering from overactive bladder (OAB) comprising administering to the patient a combination of antimuscarinic or anticholinergic agent and muscarinic agonist for the treatment of poor quality of sleep in the OAB patient.
Owner:THERAVIDA INC
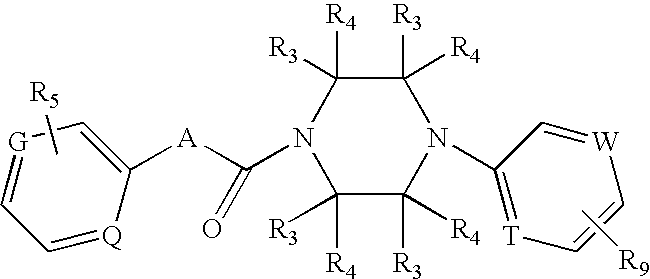
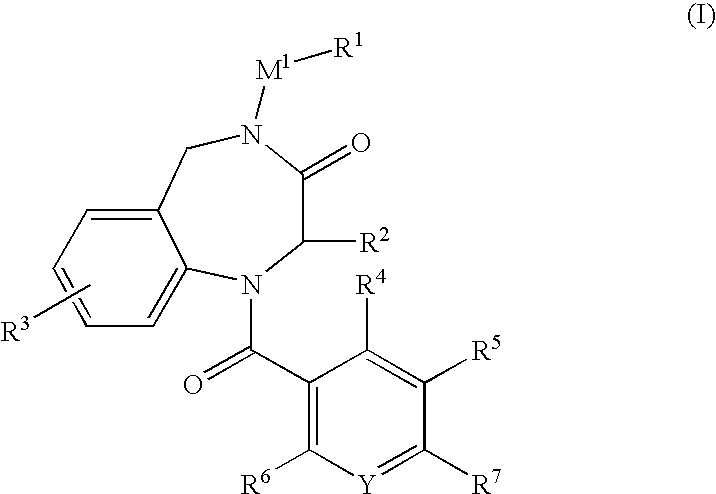
Aromatic amide derivatives, medicinal compositions containing the same, medical uses of both
A present invention provides aromatic amide derivatives which have an agonism of V2 receptor, are useful as agents for the treatment or prevention of diabetes insipidus, nocturia, nocturnal enuresis, overactive bladder or the like, and are represented by the general formula (I): wherein R1 represents a hydrogen atom or a C1-6 alkyl group which may have a substituent, R2 is a hydrogen atom or a C1-6 alkyl group, R3 is a hydrogen atom, a C1-6 alkyl group or the like, R4, R5 and R6 are independently a hydrogen atom, a halogen atom or the like, R7 is a hydrogen atom, a heteroaryl group which may have a substituent, a C3-8 cycloalkyl group, an amino group which may have a substituent or a C1-6 alkoxy group which may have a substituted group; M1 is a single bond, a C1-4 alkylene group or the like Y is N or CRF (in the formula, RF represents a hydrogen atom, a C1-6 alkyl group or the like or a pharmaceutically acceptable salt thereof, or a prodrug thereof, or pharmaceutical compositions comprising the same and pharmaceutical uses thereof.
Owner:KISSEI PHARMA
Methods for decreasing detrusor
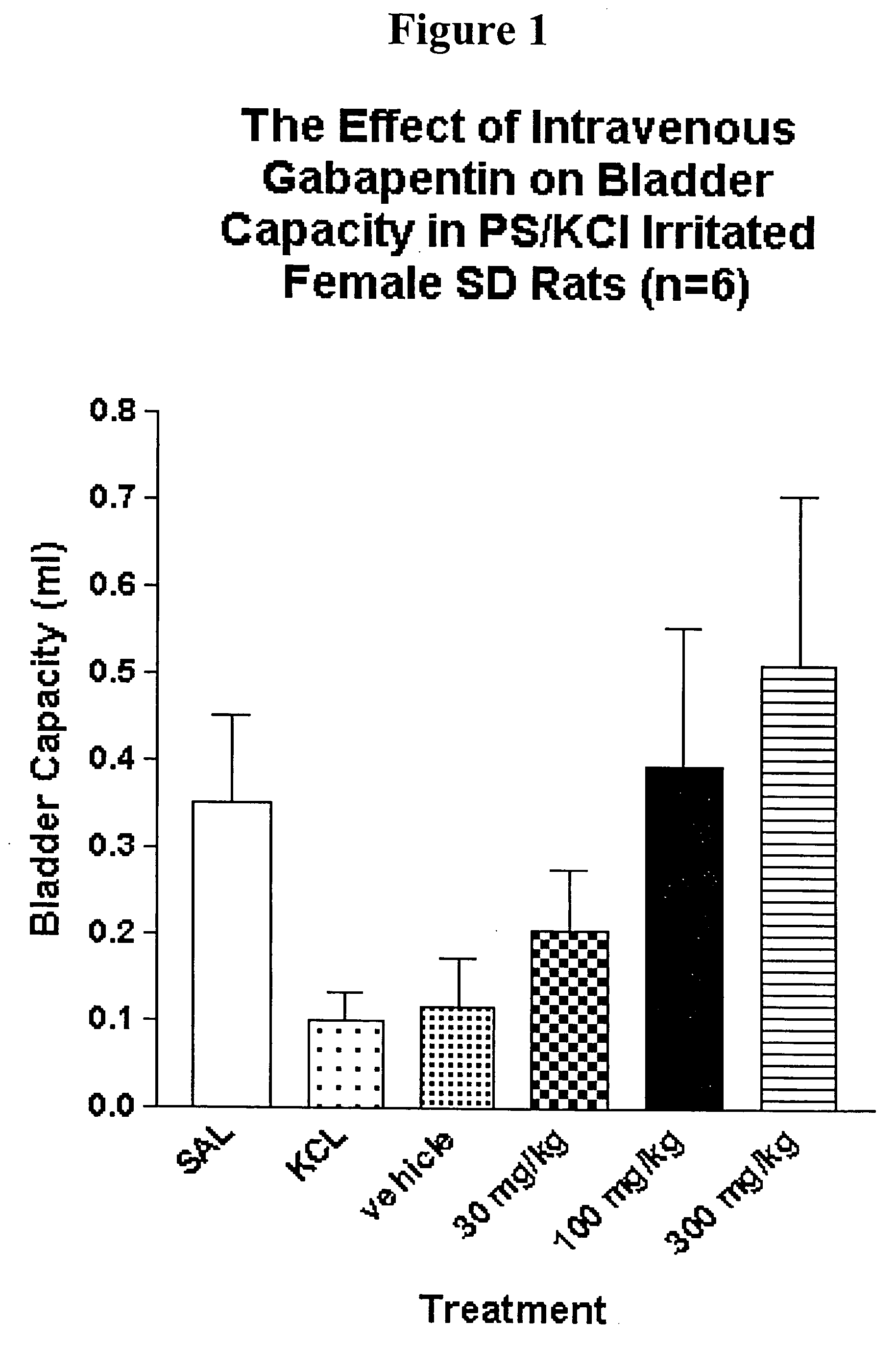
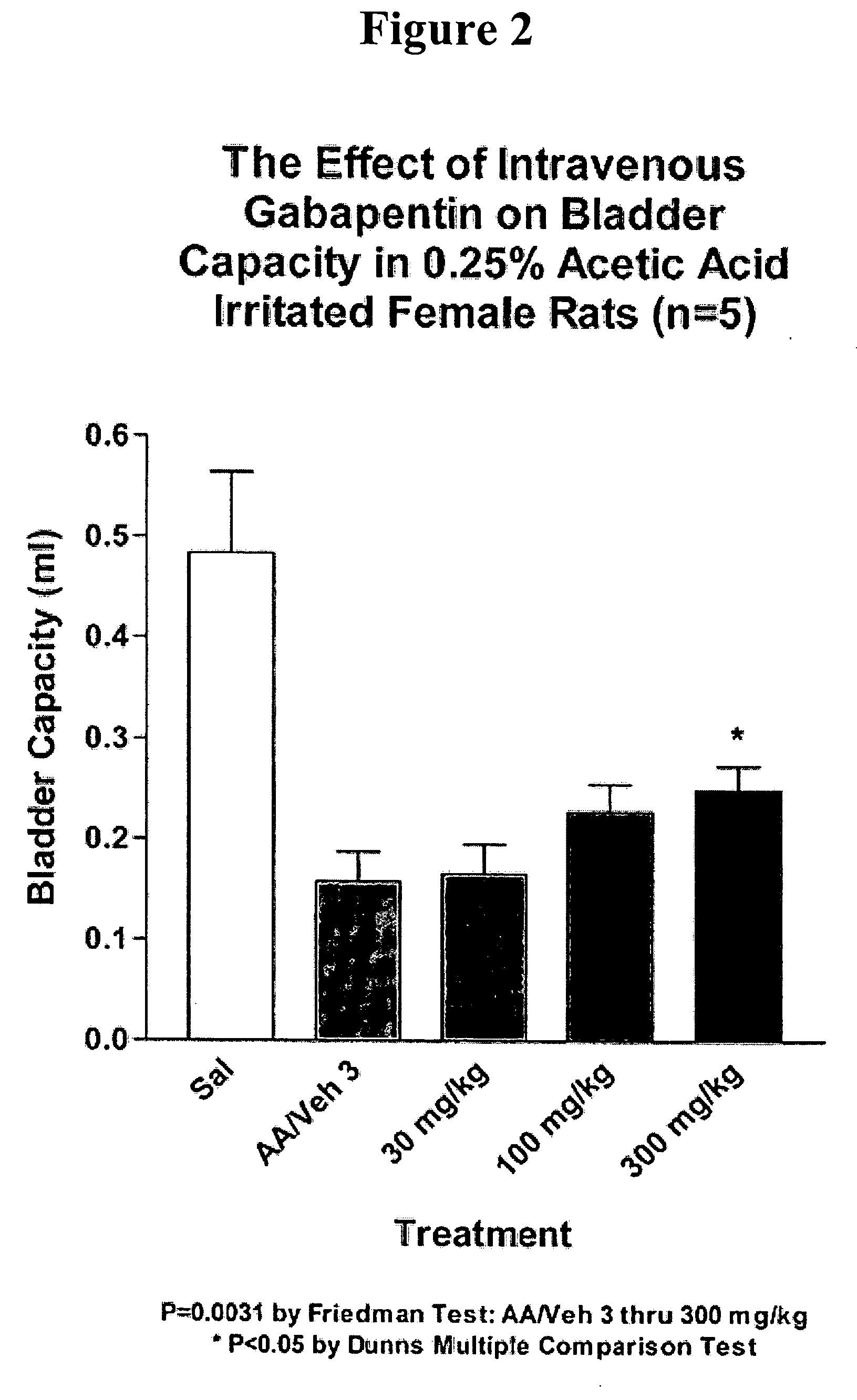
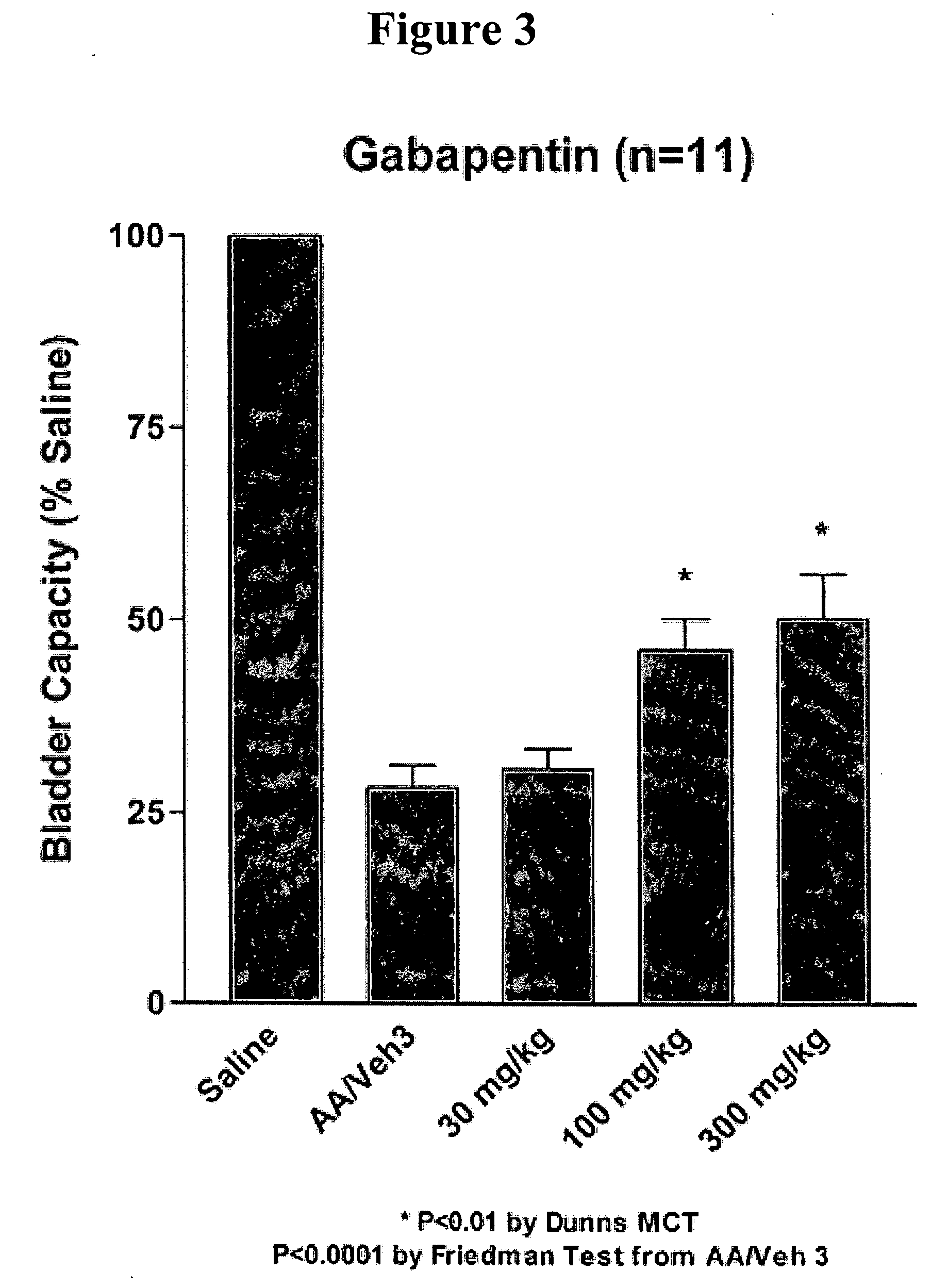
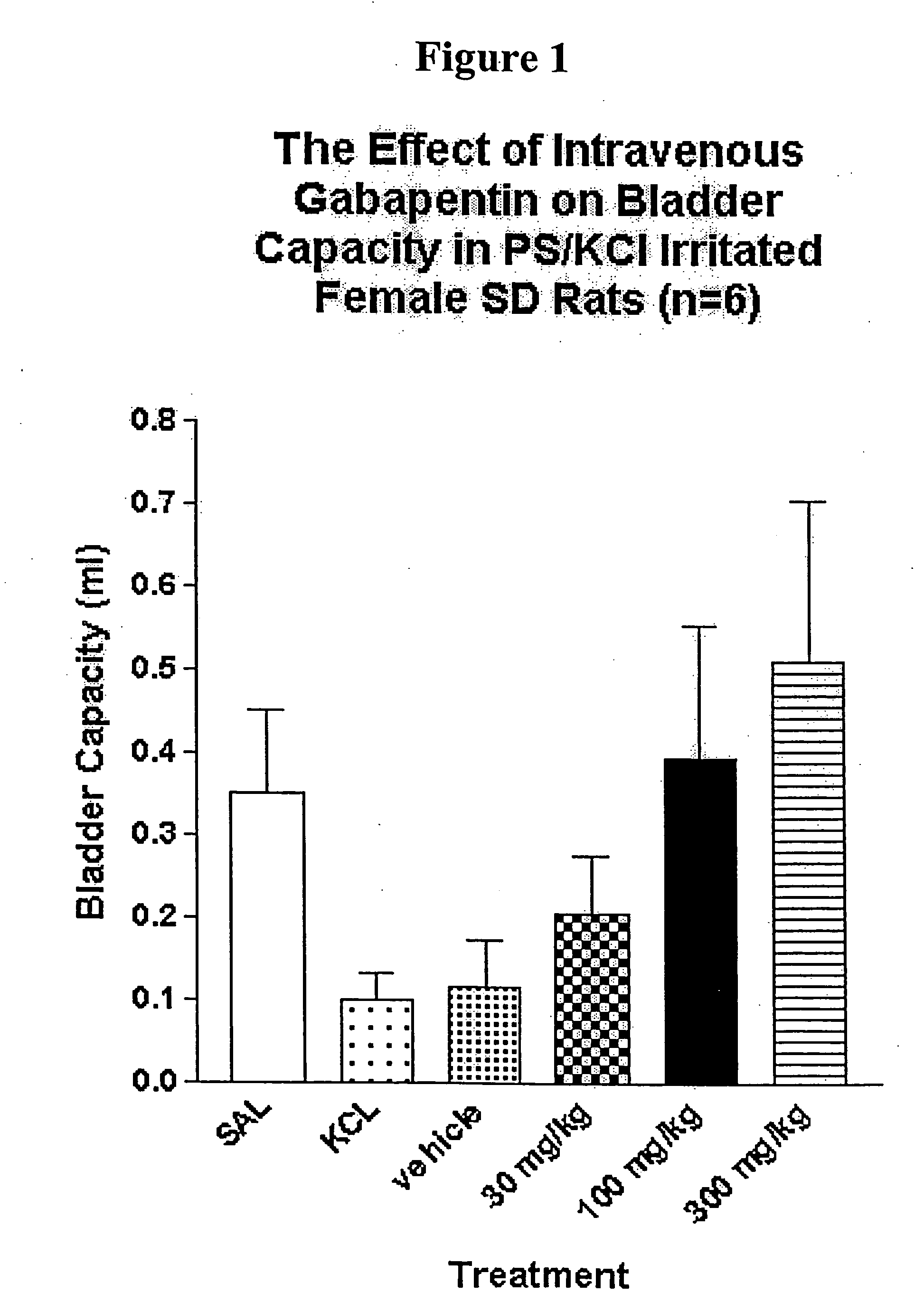
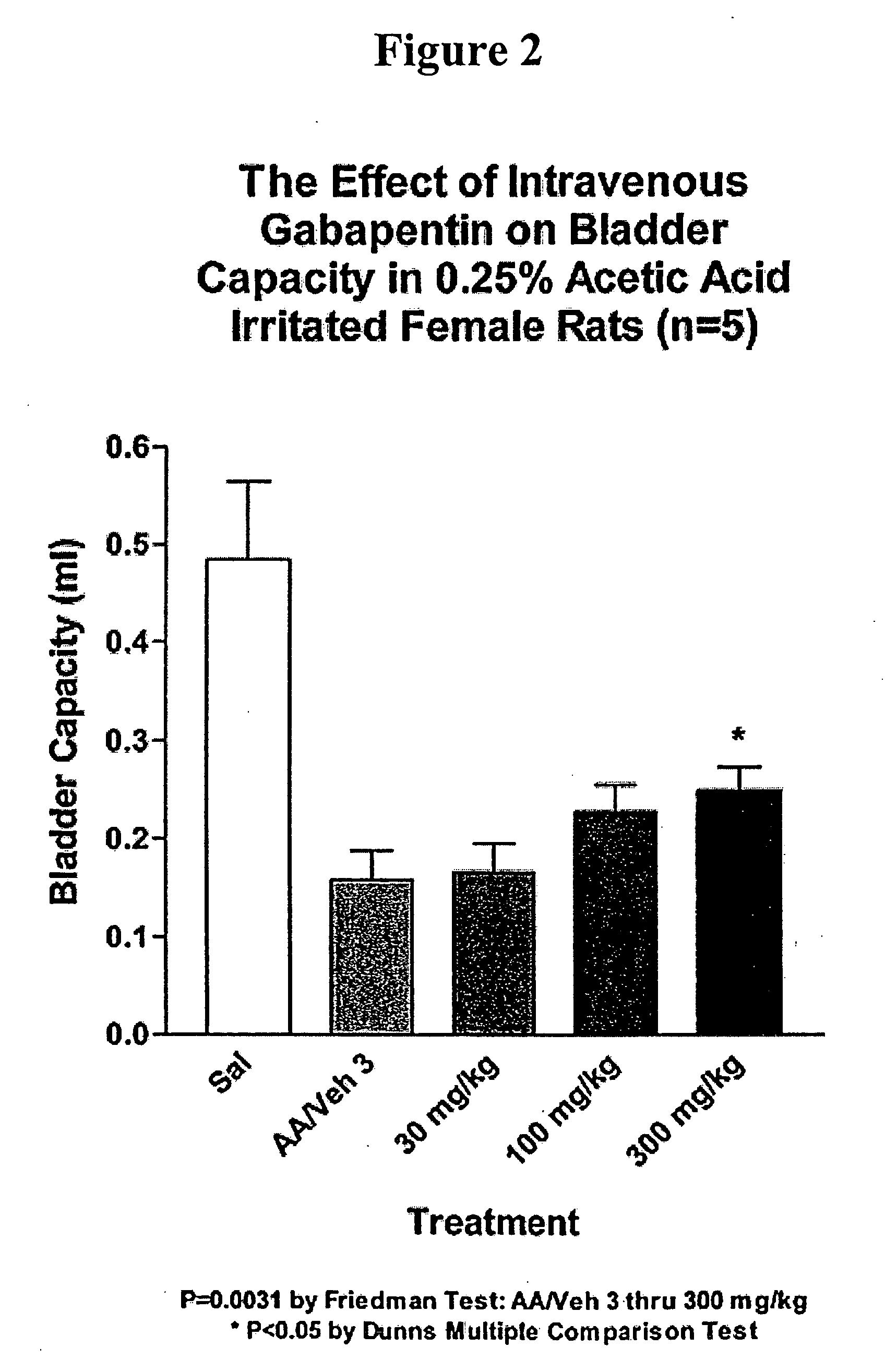
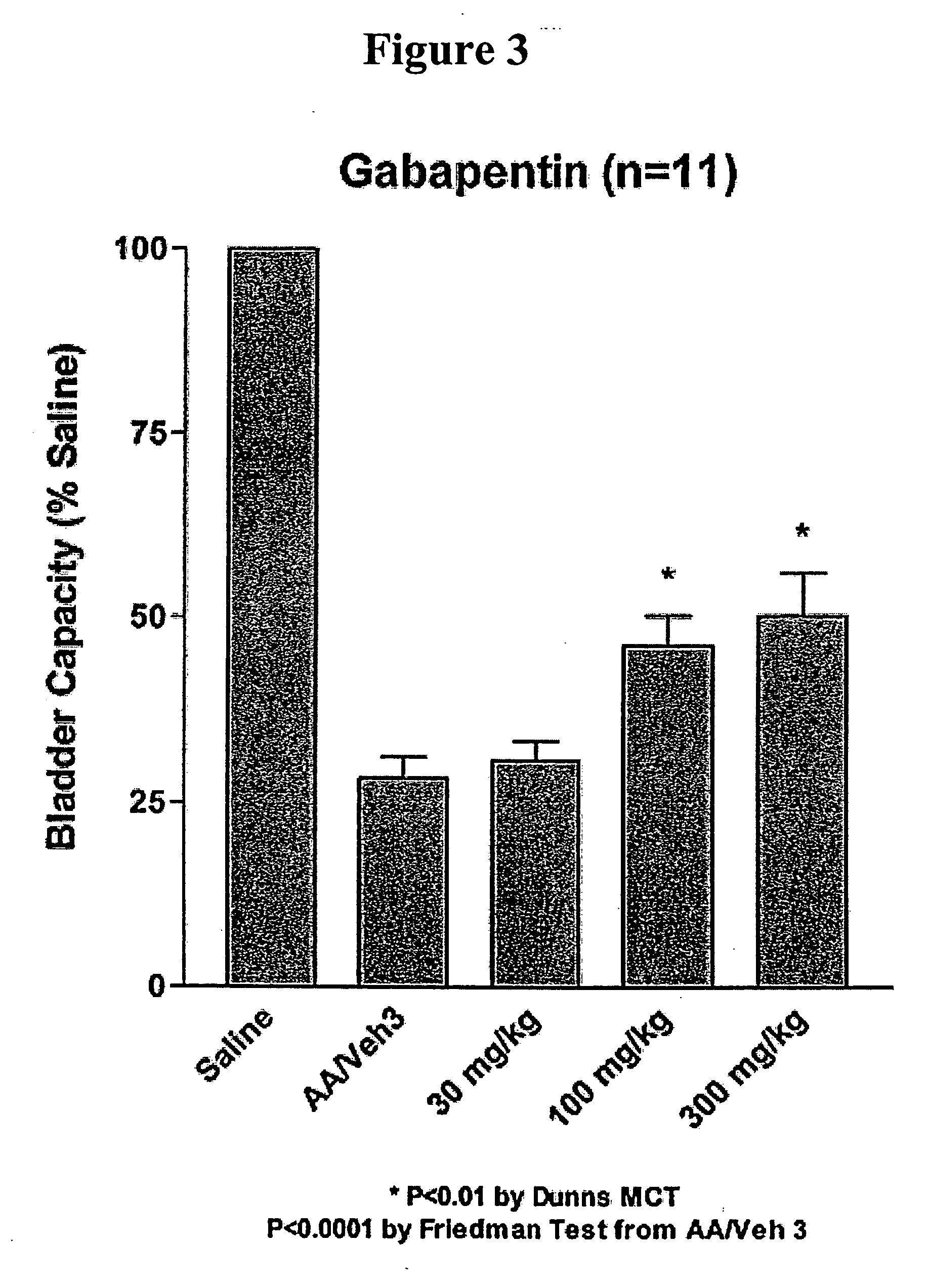
A method is provided for treatment of non-painful bladder disorders, particularly non-painful overactive bladder without loss of urine. The method comprises administration of an α2δ subunit calcium channel modulator, including gabapentin, pregabalin, GABA analogs, fused bicyclic or tricyclic amino acid analogs of gabapentin, amino acid compounds, and other compounds that interact with the α2δ calcium channel subunit.
Owner:DYNOGEN PHARM INC
System and method for urodynamic evaluation utilizing micro electro-mechanical system technology
ActiveUS8128576B2Minimizes and eliminates possibilityNegative buoyancySurgeryDiagnostic recording/measuringBladder SpasmData acquisition
Owner:ETHICON INC
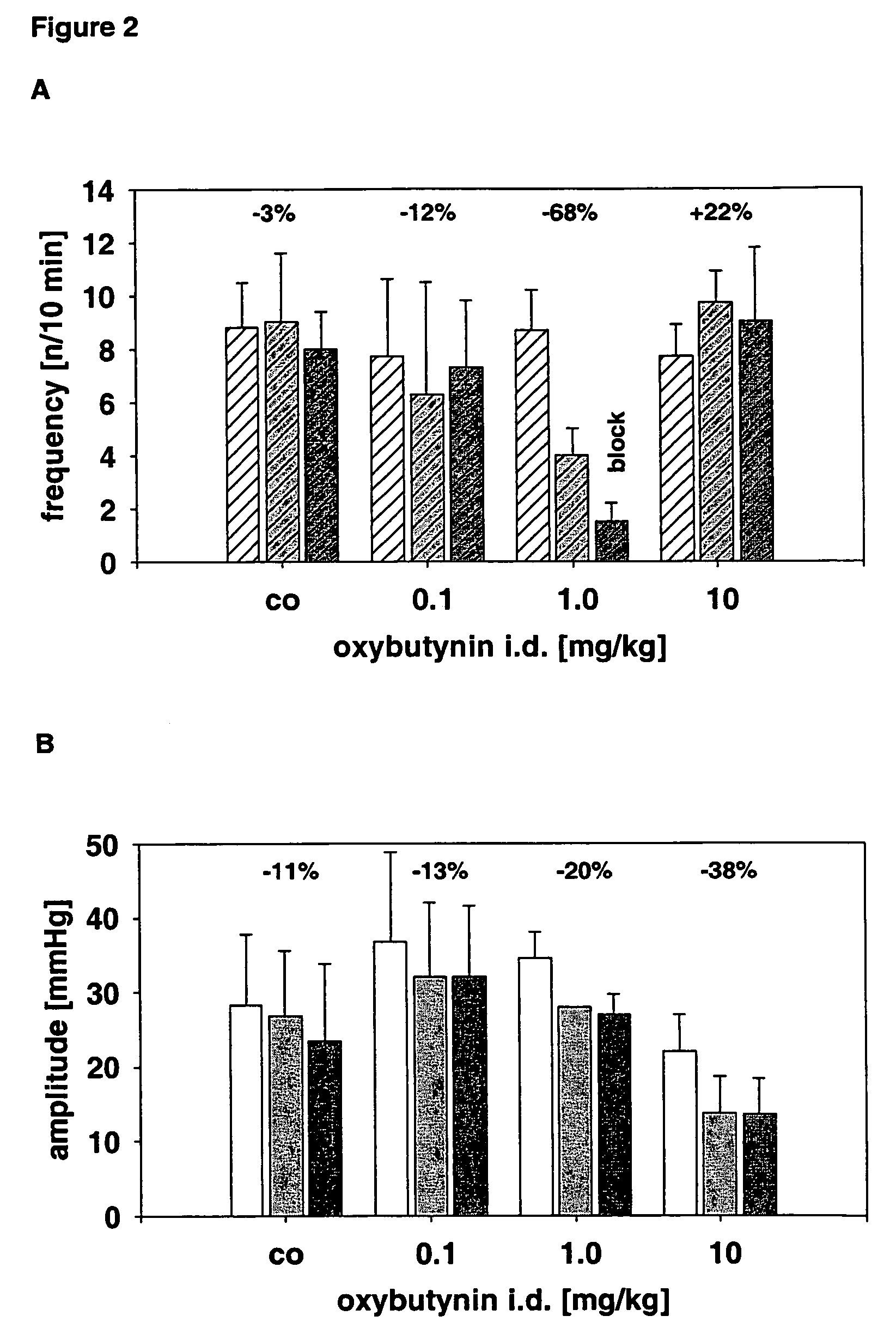
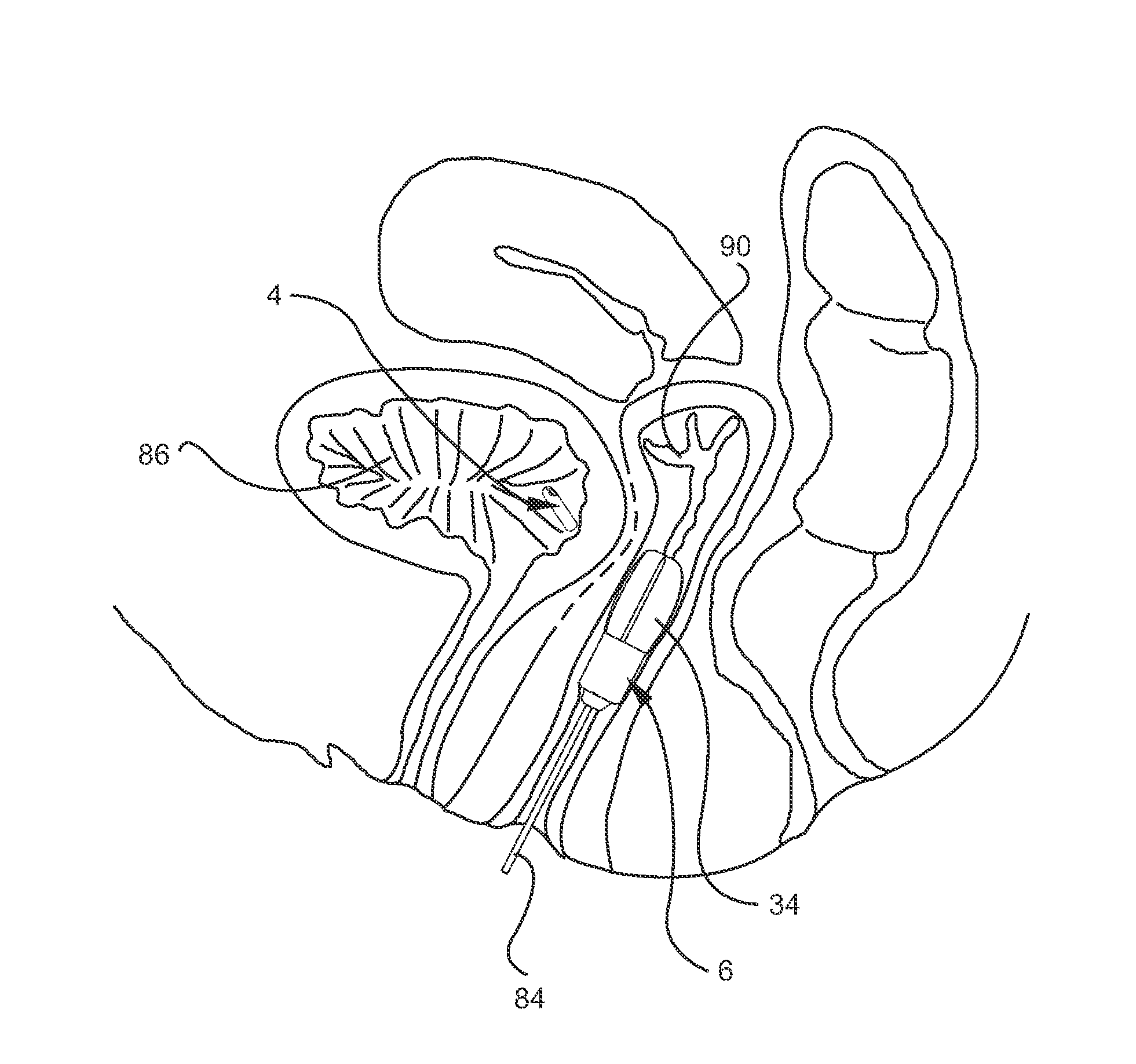
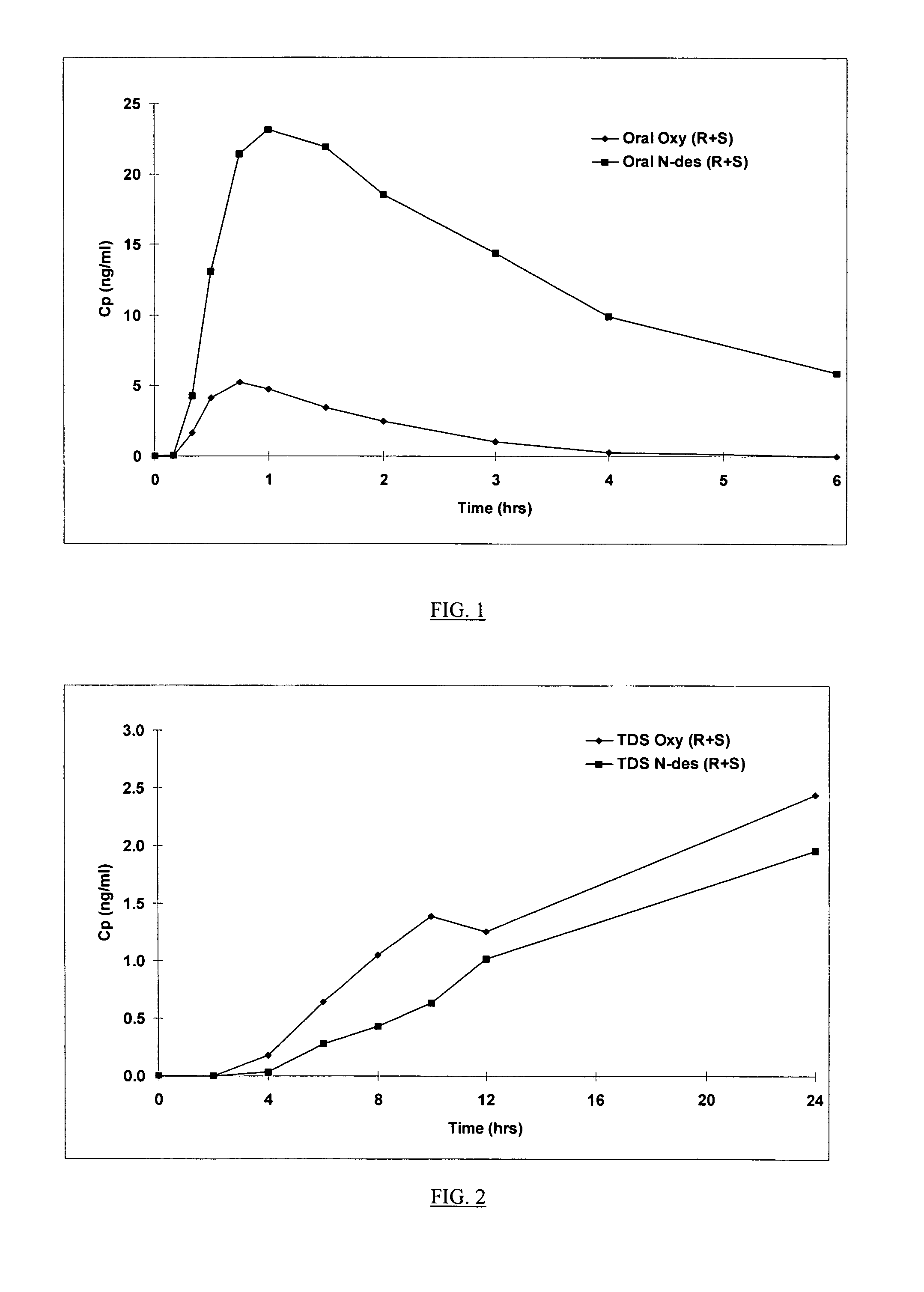
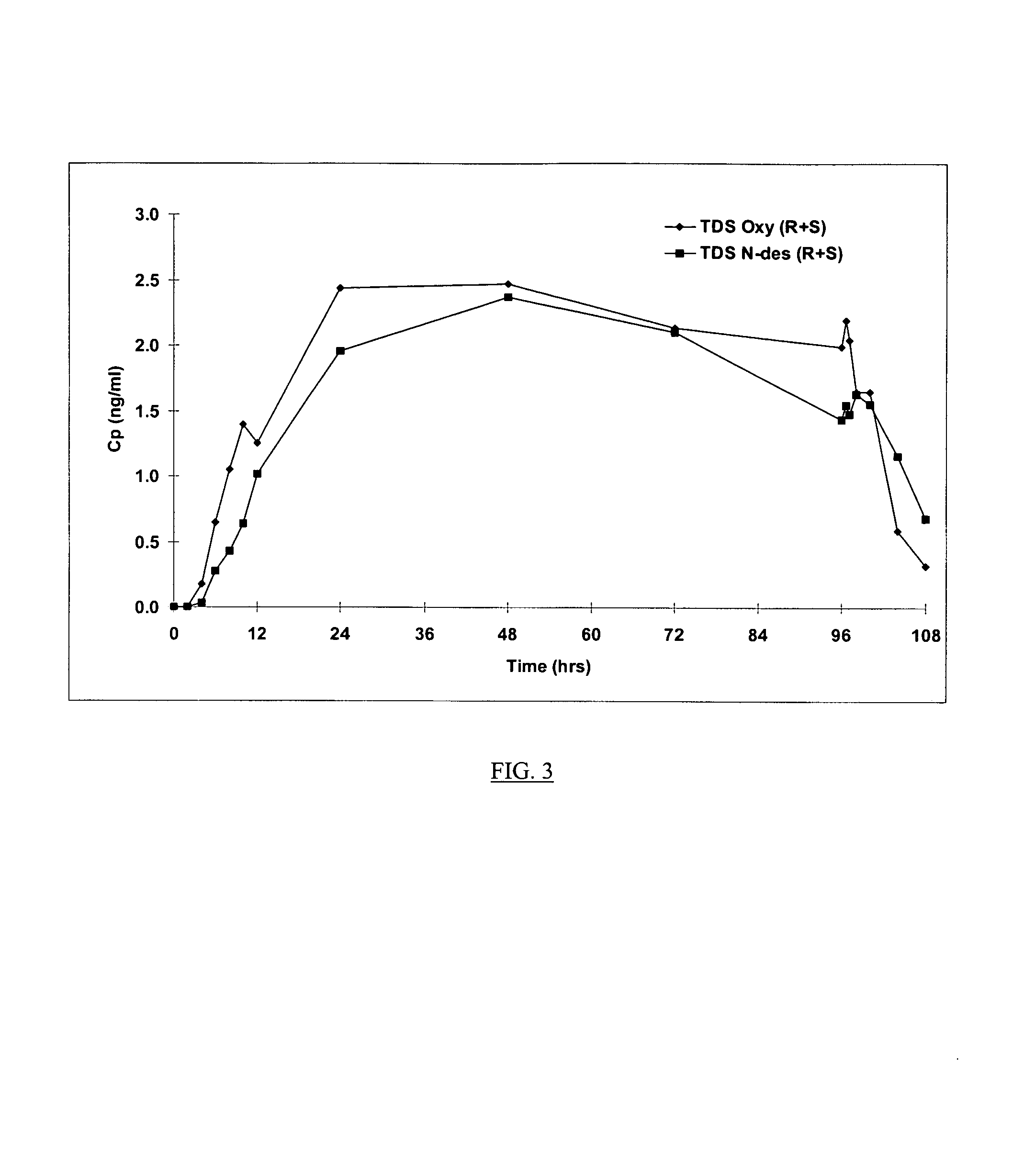
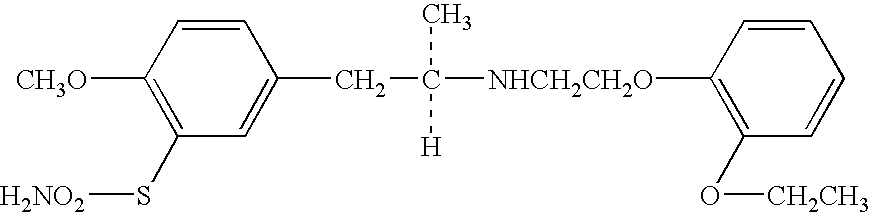
Compositions and methods for transdermal oxybutynin therapy
InactiveUS7179483B2Minimizing adverse side effectOrganic active ingredientsPlastersMetaboliteOxybutynine
The present invention provides compositions and methods for administering oxybutynin while minimizing the incidence and or severity of adverse drug experiences associated with oxybutynin therapy. In one aspect, these compositions and methods provide a lower plasma concentration of oxybutynin metabolites, such as N-desethyloxybutynin, which is presumed to be contributing at least in part to some of the adverse drug experiences, while maintaining sufficient oxybutynin plasma concentration to benefit a subject with oxybutynin therapy. The invention also provides isomers of oxybutynin and its metabolites that meet these characteristics of minimized incidence and / or severity of adverse drug experiences, and maintenance of beneficial and effective therapy for overactive bladder. In some aspects, the composition may be presented in the form of an unoccluded or free form topically administered gel.
Owner:ALLERGAN SALES LLC +1
Overactive bladder treating drug
A therapeutic agent for overactive bladder containing tamsulosin or a pharmaceutically acceptable salt thereof as an effective ingredient.
Owner:ASTELLAS PHARMA INC
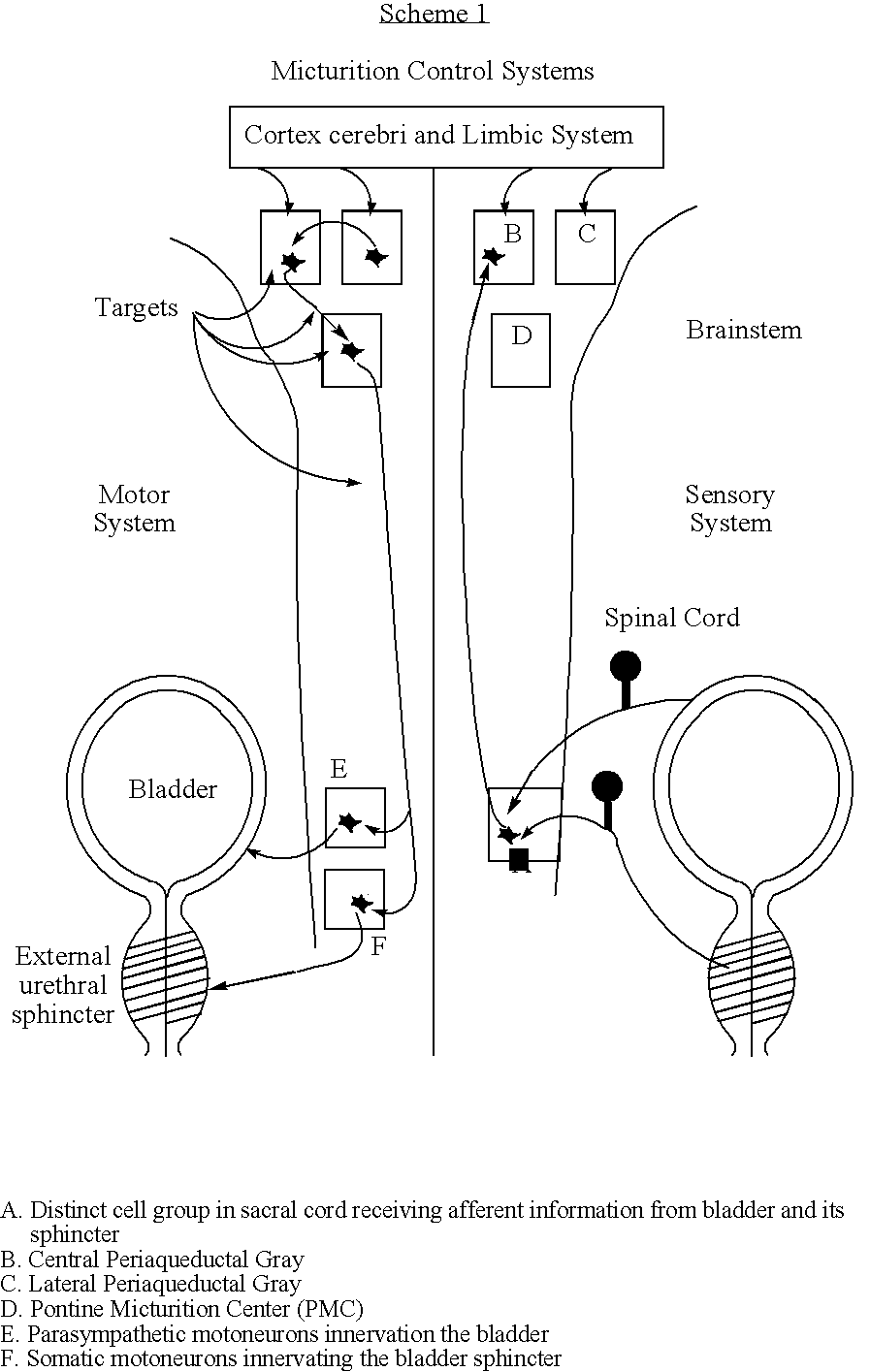
Method for controlling micturition
InactiveUS20070191903A1Restore self-confidenceImprove the quality of lifeMedical devicesImplantable neurostimulatorsBacteriuriaQuality of life
The present invention concerns the stimulation of neural tissue, in particular brain tissue for the control of micturition. By activation of the pontine micturition centre (PMC) to induce micturition and deactivation of the pontine micturition centre to inhibit micturition, patients suffering from overactive bladder (OAB) and urge incontinence regain control of the disposal of urine. This treatment will greatly improve these patients' quality of life and helps them to restore their self-confidence.
Owner:BRUINSTROOP JAN KEES PIET
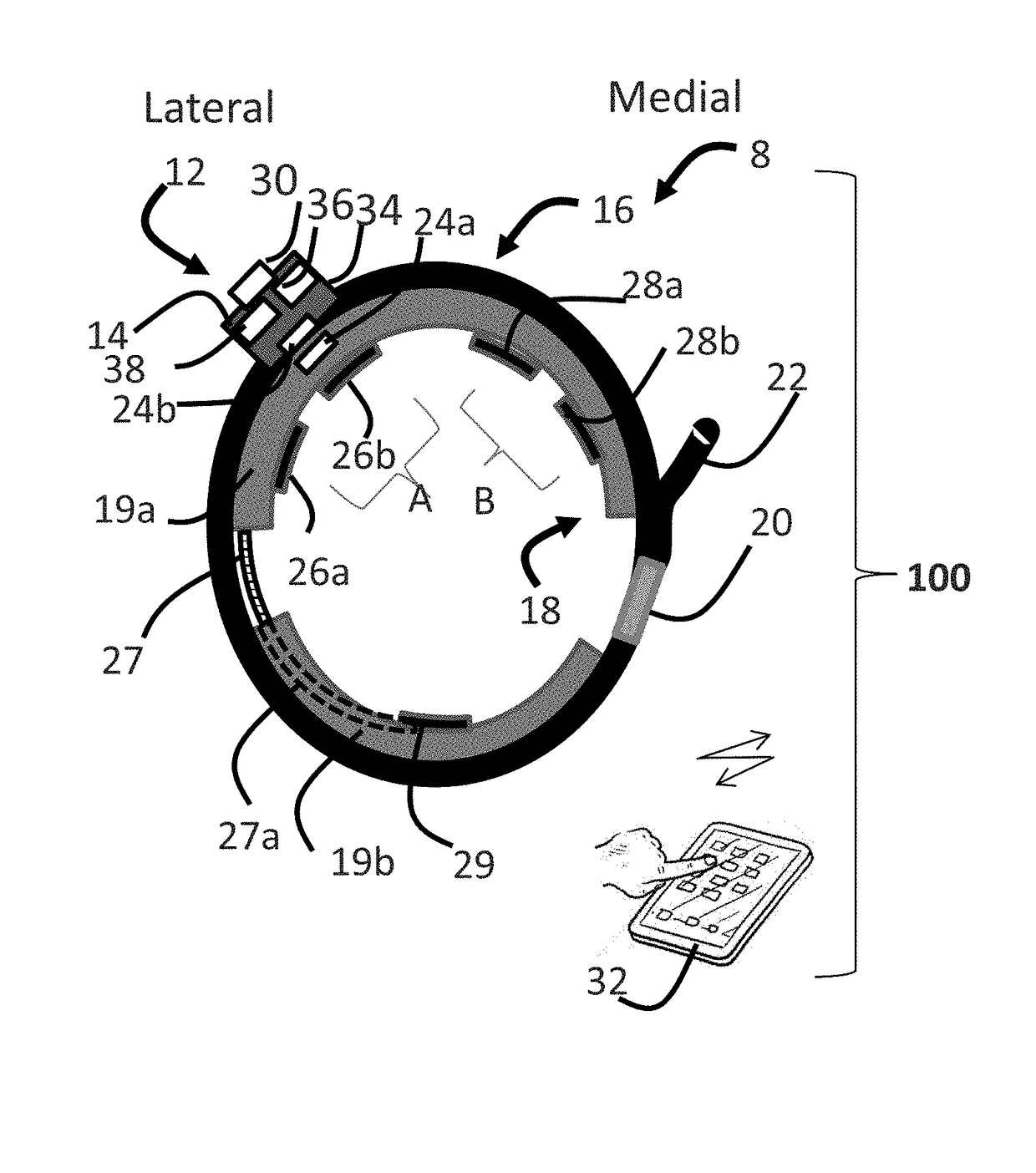
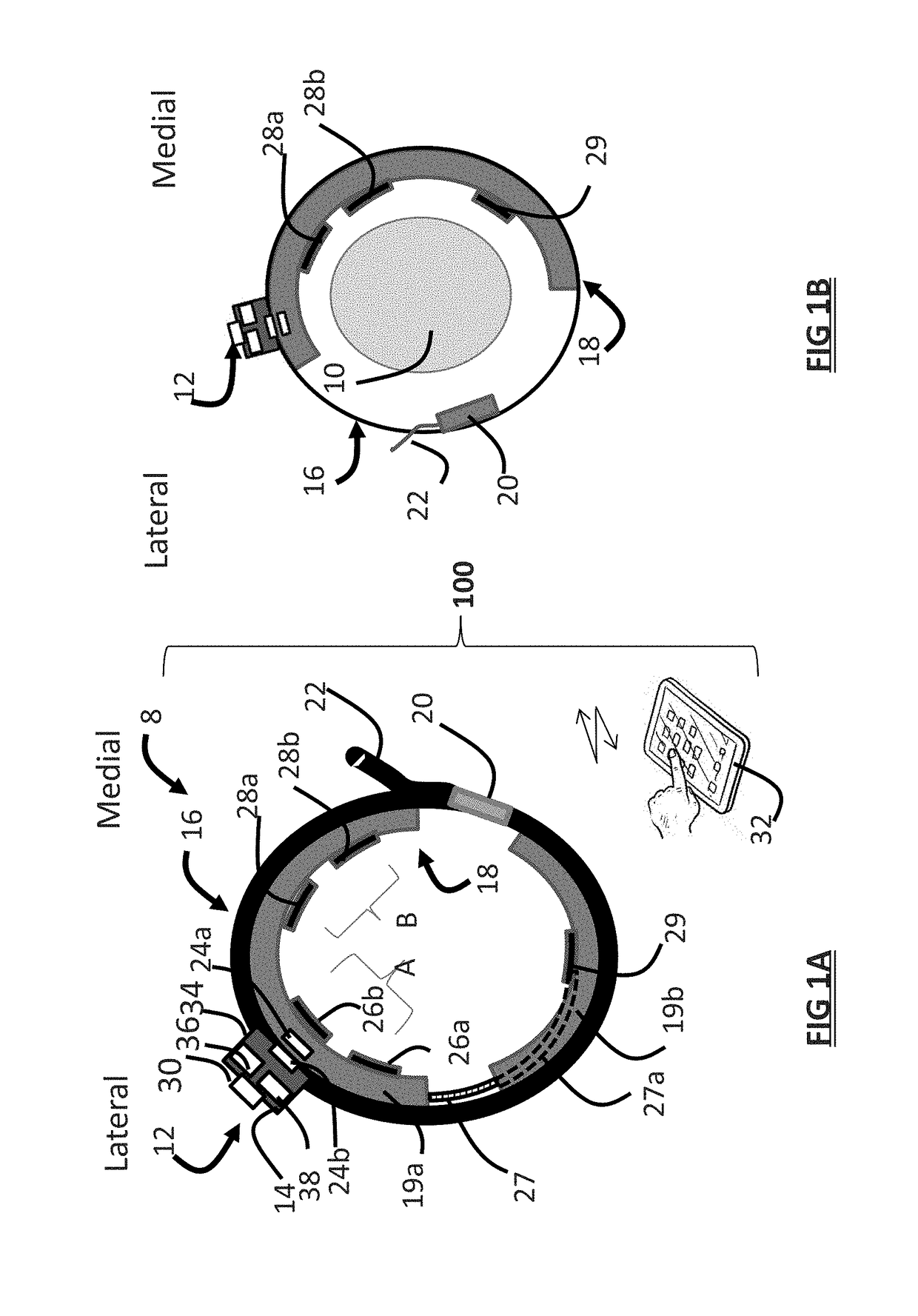
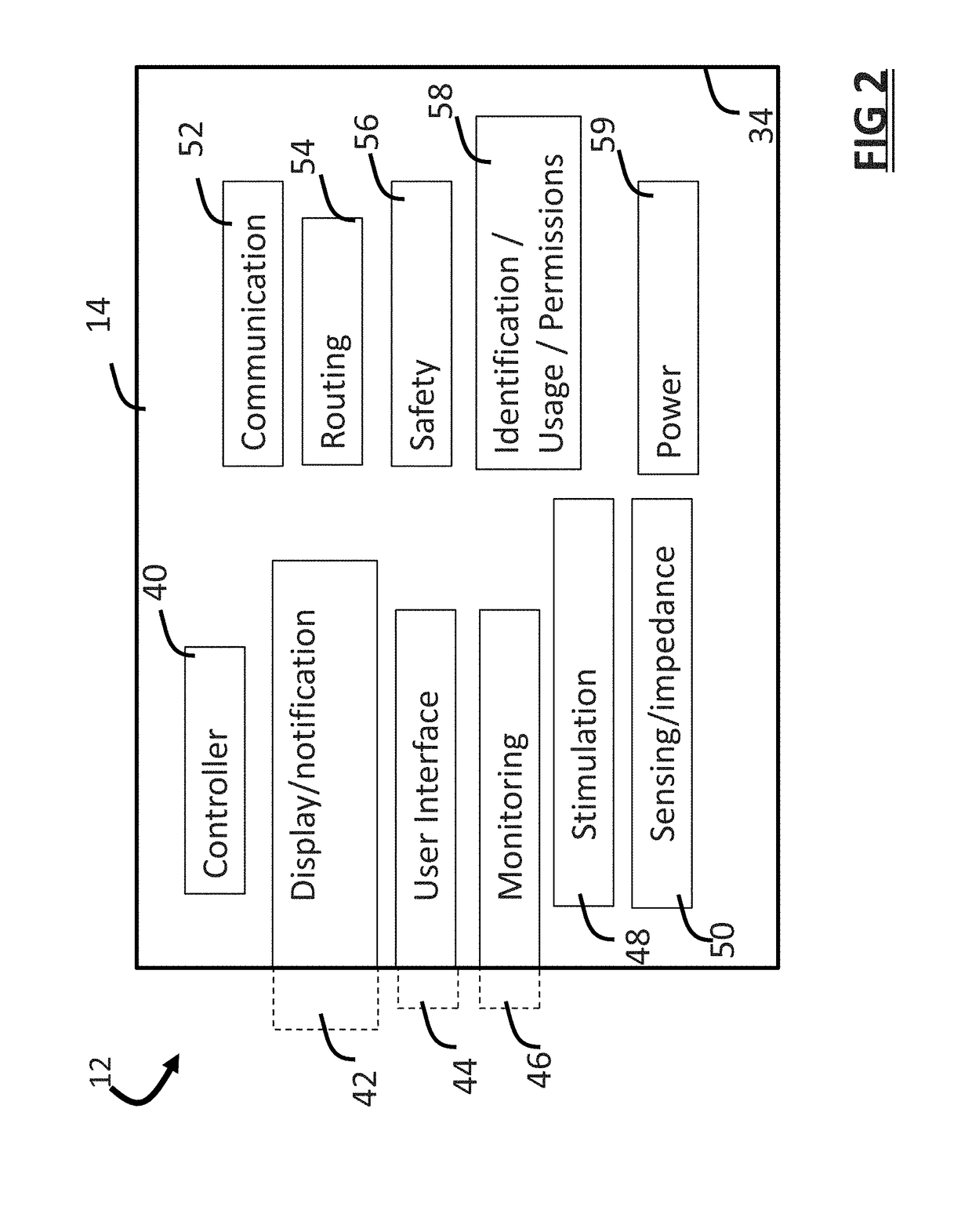
Systems and methods for assessing pelvic floor disorder therapy
ActiveUS20180296834A1Easy and rapid and clinically useful placementIncrease the number ofSensorsExternal electrodesDiseasePhysical therapy
Systems and methods provide stimulation of peripheral targets such as targets in the lower limbs. Electrode arrays realized in circumferential or longitudinal embodiments have pads with horizontal and / or vertical offsets. Electrode array geometries are customizable and adaptable to individual users and treatment of different disorders. Novel systems of customization include software and hardware implemented solutions. A single device can provide treatment of two or more disorders or unwanted states using selected electrode geometries and stimulation protocols. Systems and methods for assessment of candidate stimulation sites and protocols use subjective or objective measures or both to determine which meet stimulation success criteria. Simulation is provided using transcutaneous, percutaneous, or implantable stimulators. A main advantage is the improved treatment of pelvic floor disorders, and especially overactive bladder (OAB).
Owner:EBT MEDICAL INC
Methods of treating non-painful bladder disorders using alpha2delta subunit calcium channel modulators
A method is provided for treatment of non-painful bladder disorders, particularly non-painful overactive bladder without loss of urine. The method comprises administration of an α2δ subunit calcium channel modulator, including gabapentin, pregabalin, GABA analogs, fused bicyclic or tricyclic amino acid analogs of gabapentin, amino acid compounds, and other compounds that interact with the α2δ calcium channel subunit.
Owner:DYNOGEN PHARM INC
Arylmethylene urea derivative and use thereof
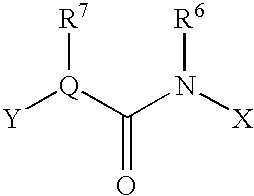
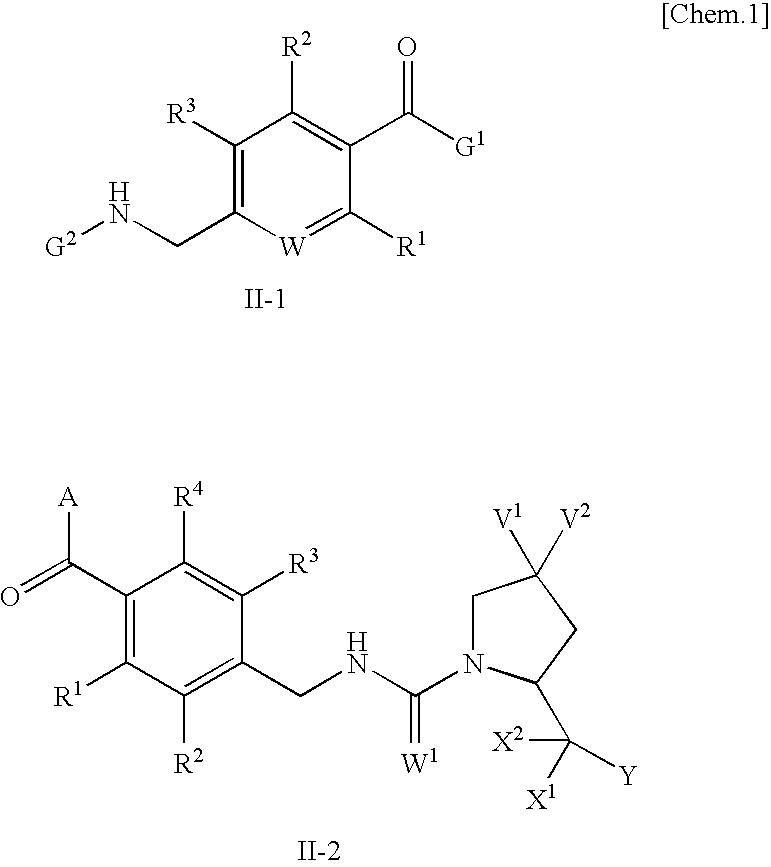
This invention relates to a pharmaceutical comprising as an effective ingredient an arylmethylene urea exemplified by the following formula:or a pharmaceutically acceptable salt thereof. The arylmethylene urea and the pharmaceutically acceptable salts thereof are useful for therapy or prophylaxis of inflammatory bowel disease and overactive bladder.
Owner:TORAY IND INC
Herbal compositions for the prevention or treatment of urinary incontinence and overactive bladder
InactiveUS7378115B2BiocidePteridophyta/filicophyta medical ingredientsDiseaseUpper urinary tract infection
The present invention relates to herbal compositions for the prevention or treatment of disorders of the urogenital system, e.g., urinary incontinence, enuresis (e.g., bed-wetting), benign prostatic hyperplasia, urinary calculi, cystitis, urinary tract infection, and overactive bladder. Specifically, the invention provides compositions that contain C. nurvala and E. arvense and methods of use thereof.
Owner:BIOLOGIC HEALTH SOLUTIONS PTY LTD (AU)
Therapeutic agent for urinary tract disease
Disclosed is a therapeutic agent for difficulty in urination associated with overactive bladder, frequent urination, urinary incontinence or prostatomegaly or urinary calculus, which comprises a compound having a PDE9-inhibiting activity as an active ingredient.
Owner:ASKA PHARMACEUTICAL CO LTD
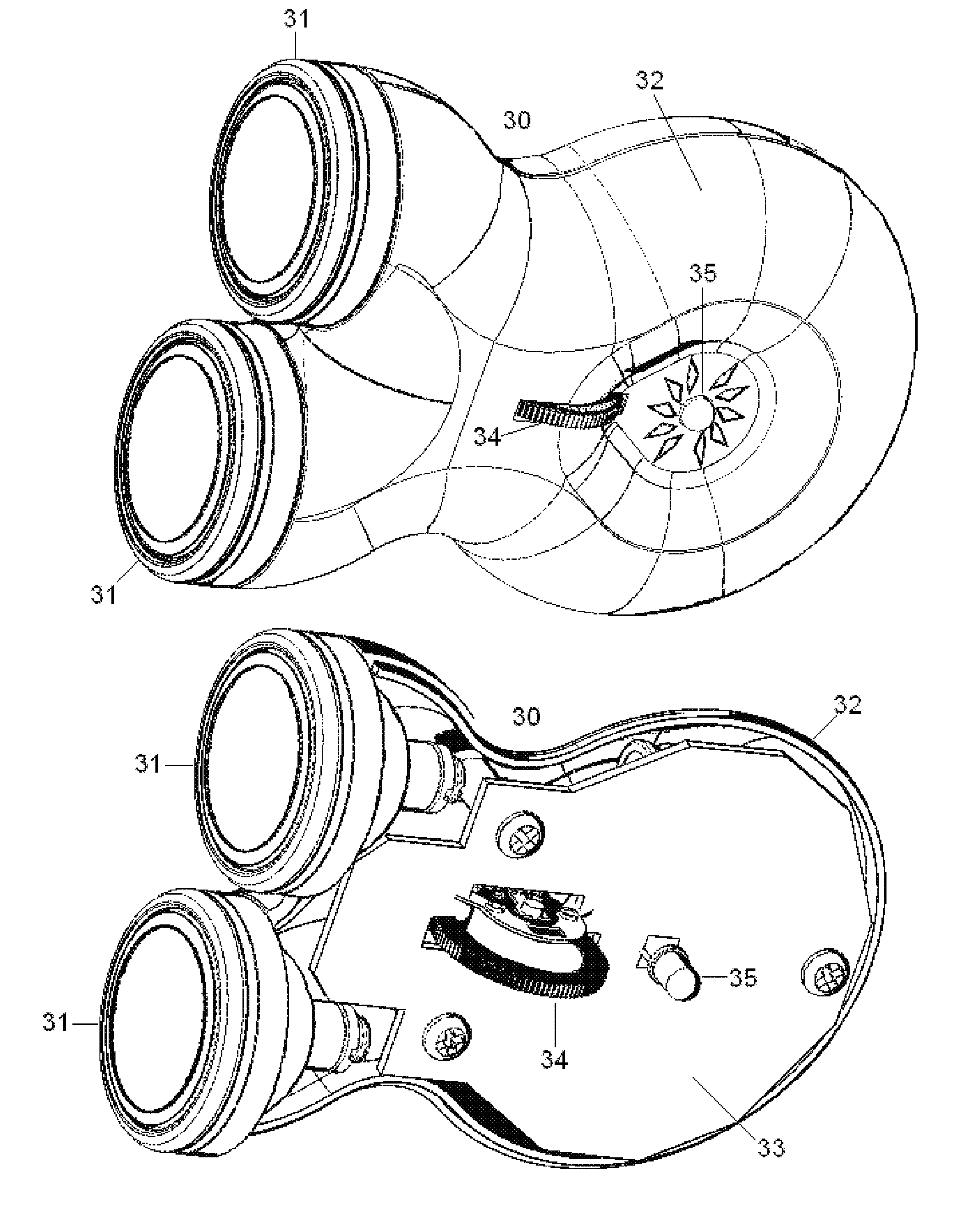
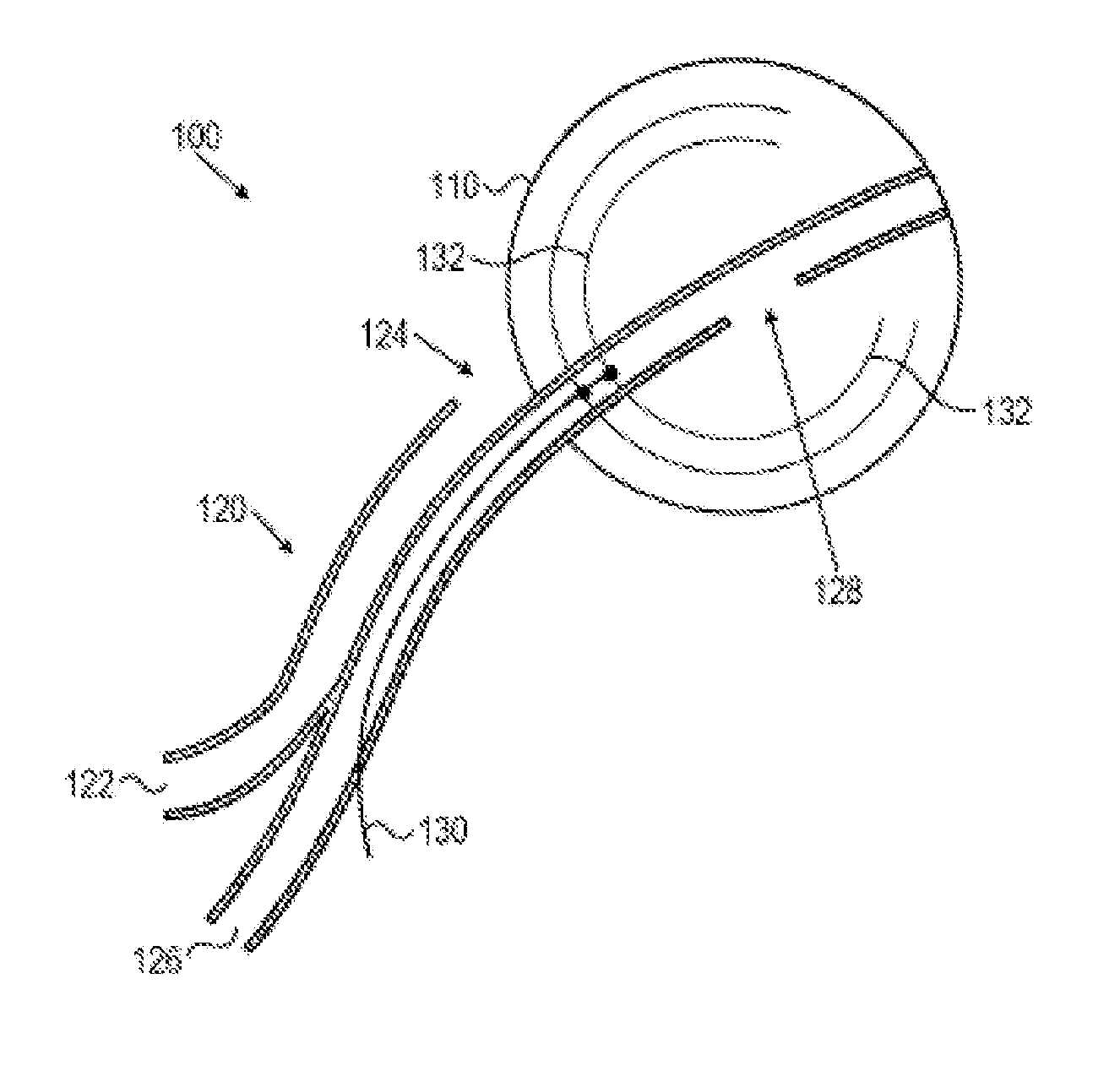
Topical nerve stimulation device
PendingUS20190117974A1External electrodesArtificial respirationPosterior tibial nervePhysical therapy
The present disclosure is directed to a transcutaneous nerve stimulation device and system for treating overactive bladder (OAB) and its symptoms. The devices and systems described herein are intuitively shaped to enable proper placement by the user to stimulate the user's tibial nerve.
Owner:THE PROCTER & GAMBLE COMPANY +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com