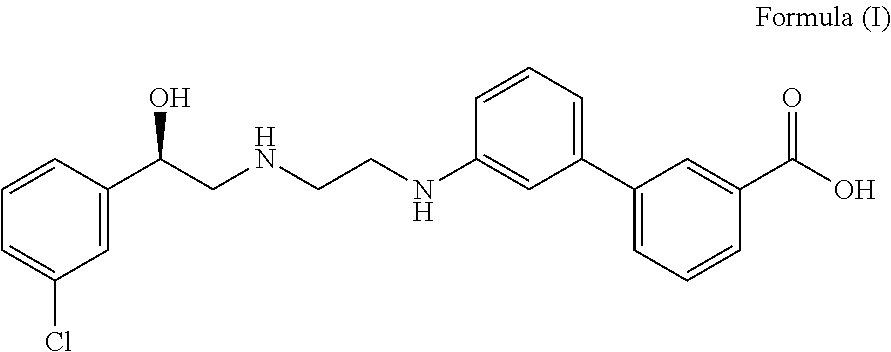
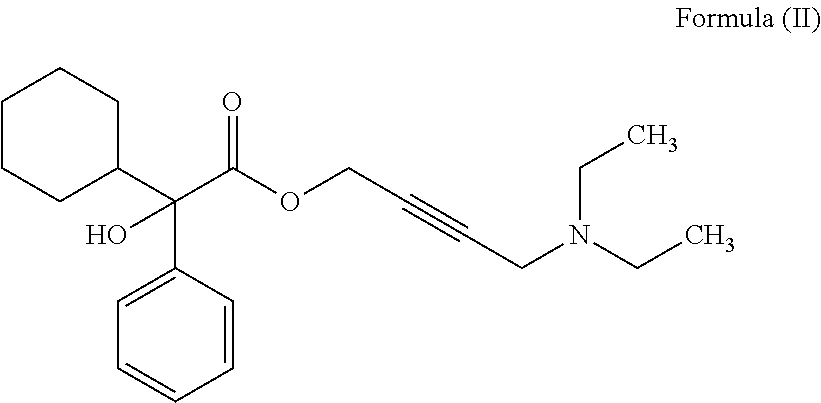
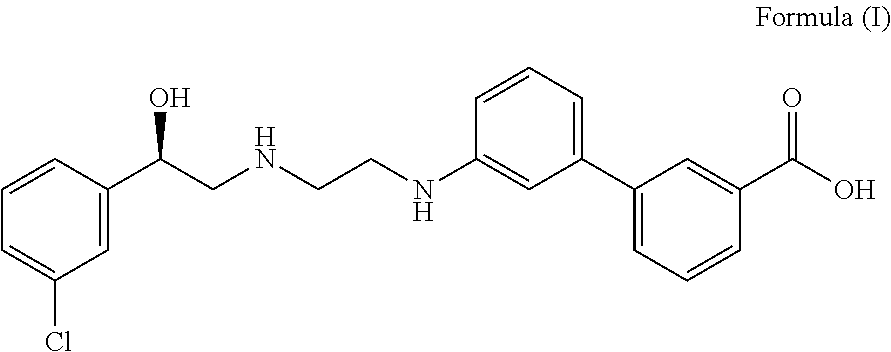
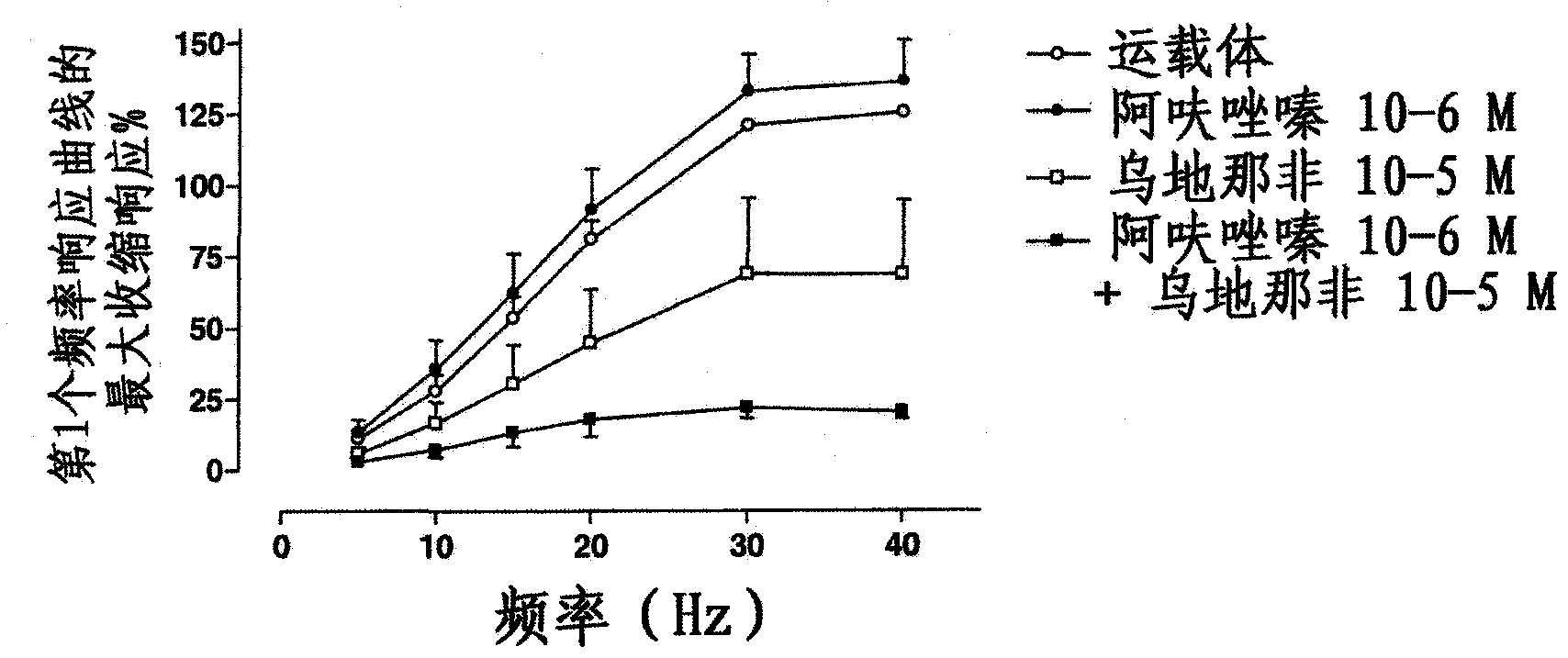
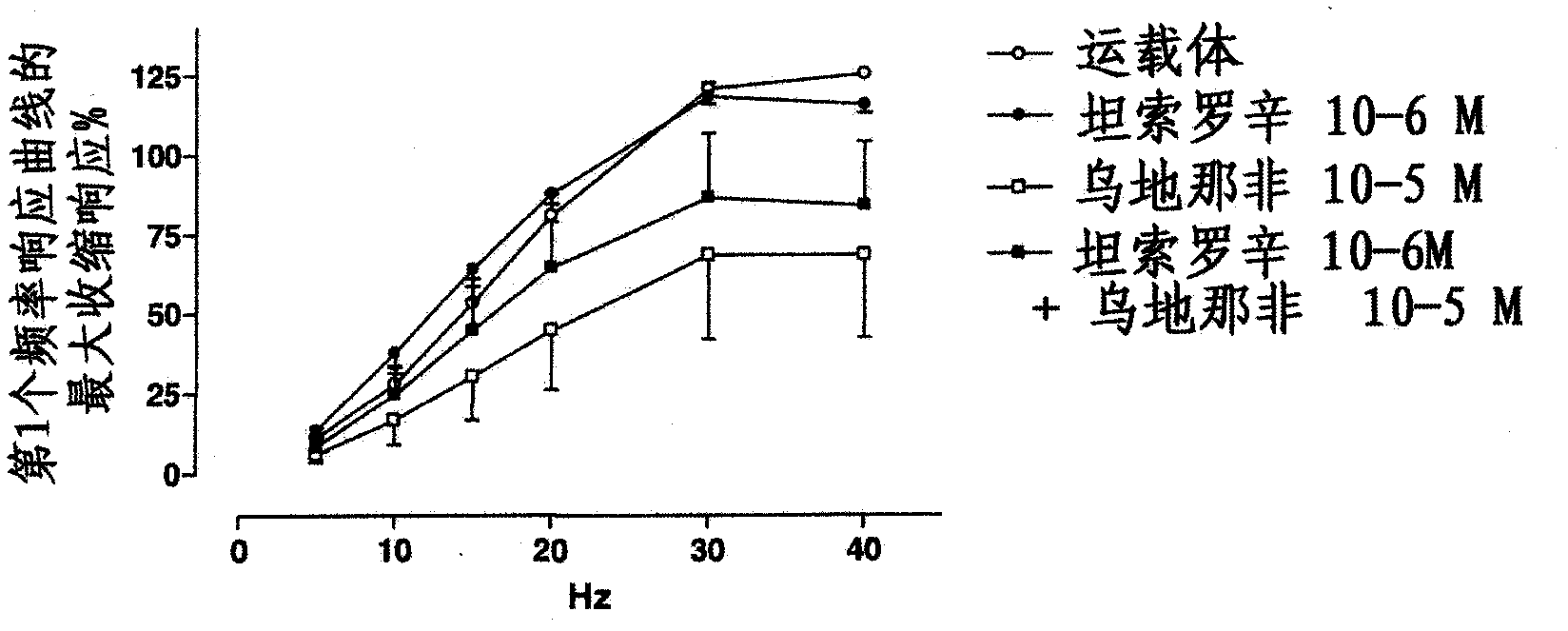
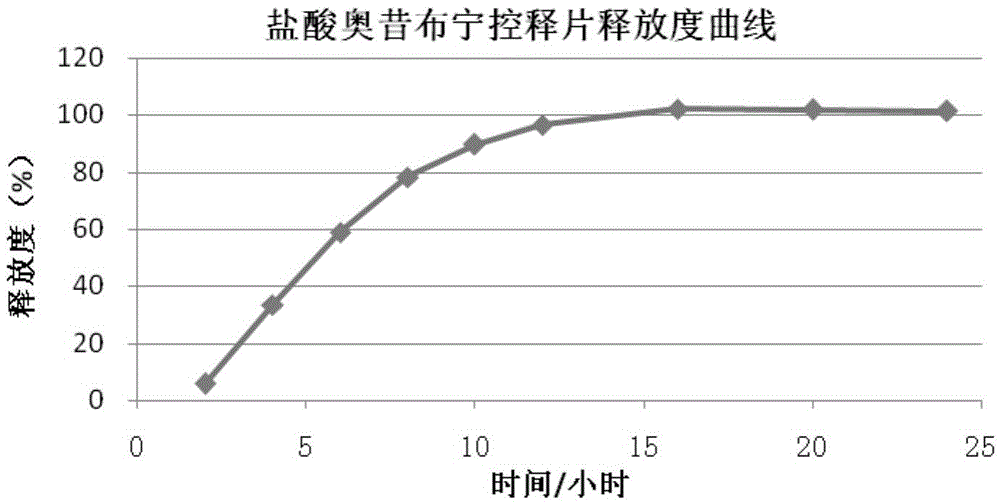
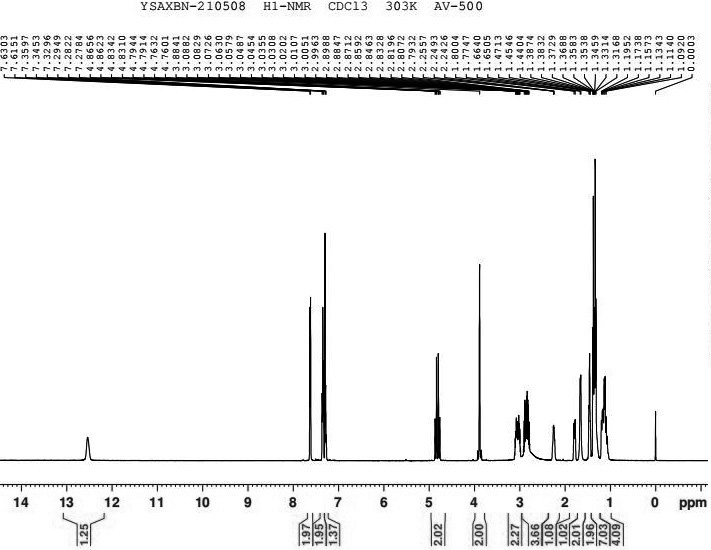
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
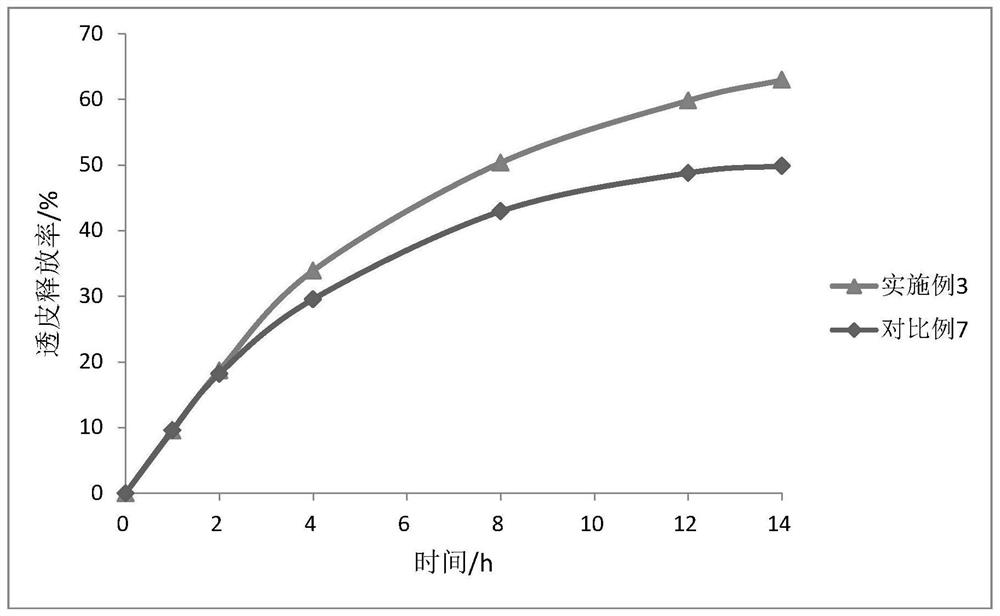
31 results about "Oxybutynine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Multi-tablet oxybutynin system for treating incontinence
The present invention provides a simple multi-tablet system for the treatment of urinary incontinence with oxybutynin. Particular embodiments of the invention provide a first tablet that releases oxybutynin over a short period of time, e.g. less than six hours, and a second tablet that releases oxybutynin over an extended period of time, e.g., eighteen to twenty-four hours, to maintain therapeutically effective levels oxybutynin in the mammal for a period of about twenty four hours. Unlike other systems, this system is easily adaptable to compensate for patient to patient variability in response to oxybutynin therapy. The invention also provides a method of treating urinary incontinence with the above system and a kit comprising various first and second tablets to rapidly develop a patient's preferred dosing regimen, i.e., the dosing regimen which provides the greatest therapeutic benefit and / or least amount or severity of side effects.
Owner:OSMOTICA CORP
Transdermal compositions for anticholinergic agents
InactiveUS20100216880A1Avoiding undesirable peak in drug concentrationReduce morbidityAntibacterial agentsBiocideOxybutyninLong chain fatty acid
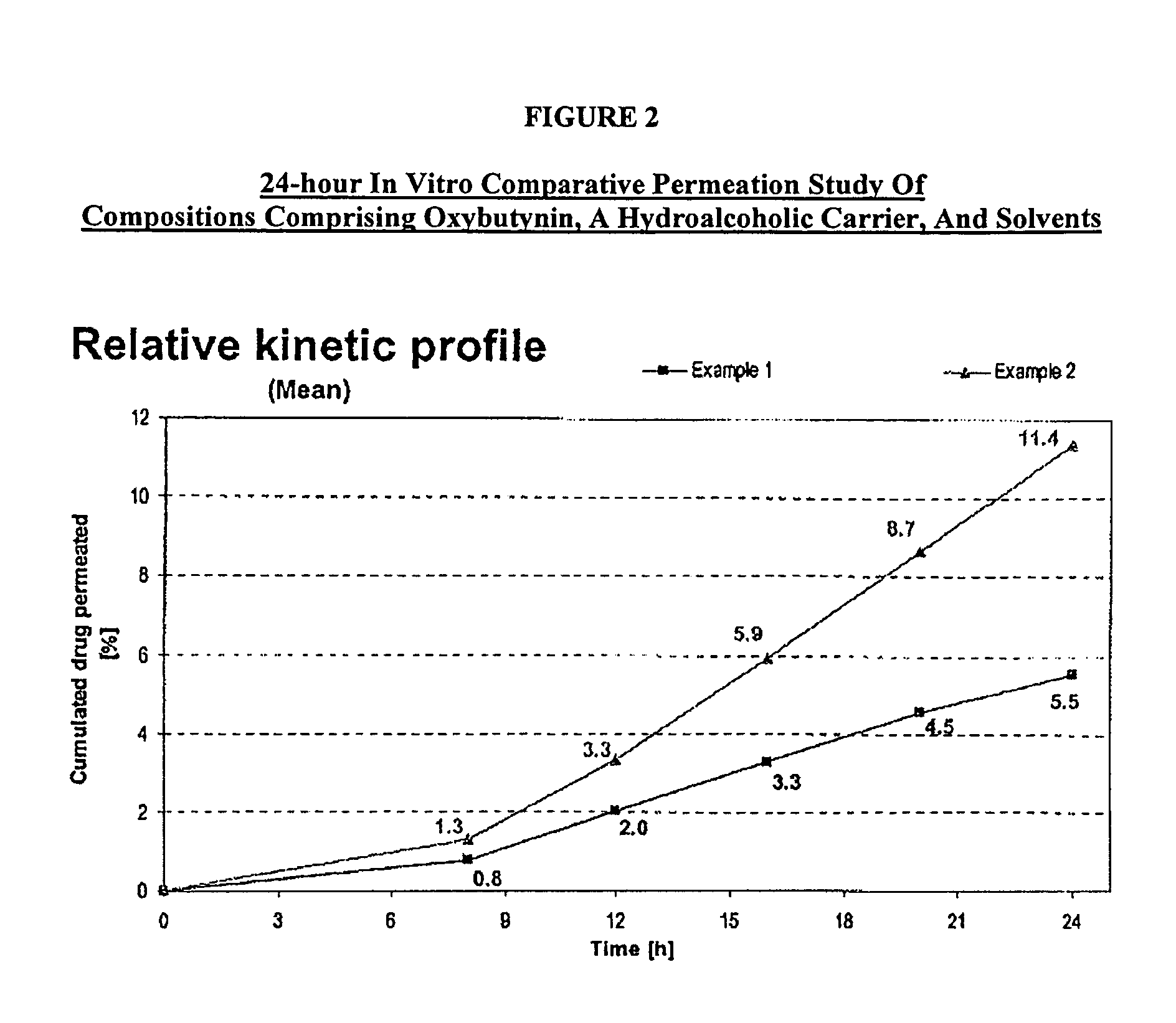
The present invention relates generally to compositions or formulations for transdermal or transmucosal administration of anticholinergic agents such as oxybutynin. The invention utilizes a novel delivery vehicle and is a substantially malodorous-free and irritation free transdermal formulation which is substantially free of long chain fatty alcohols, long-chain fatty acids, and long-chain fatty esters. A method is disclosed for treating a subject for urinary incontinence with these formulations while reducing the incidences of peak concentrations of drug and undesirable side effects associated with oral anticholinergics.
Owner:ANTARES PHARMA IPL
Pharmaceutical combinations
Pharmaceutical combinations comprising a beta-3 adrenergic receptor agonist and a muscarinic receptor antagonist, and methods for their use are disclosed. Disclosed combinations include solabegron and oxybutynin. Methods of using the pharmaceutical combinations for the treatment of one or more symptoms associated with overactive bladder, for example, frequency of urgency, nocturia, and urinary incontinence, are also disclosed.
Owner:B3AR THERAPEUTICS INC
Pharmaceutical combinations of beta-3 adrenergic receptor agonists and muscarinic receptor antagonists
ActiveUS8642661B2BiocidePeptide/protein ingredientsHyperactivity bladderAdrenergic receptor agonists
Owner:B3AR THERAPEUTICS INC
Compositions and methods for transdermal oxybutynin therapy
InactiveUS7179483B2Minimizing adverse side effectOrganic active ingredientsPlastersMetaboliteOxybutynine
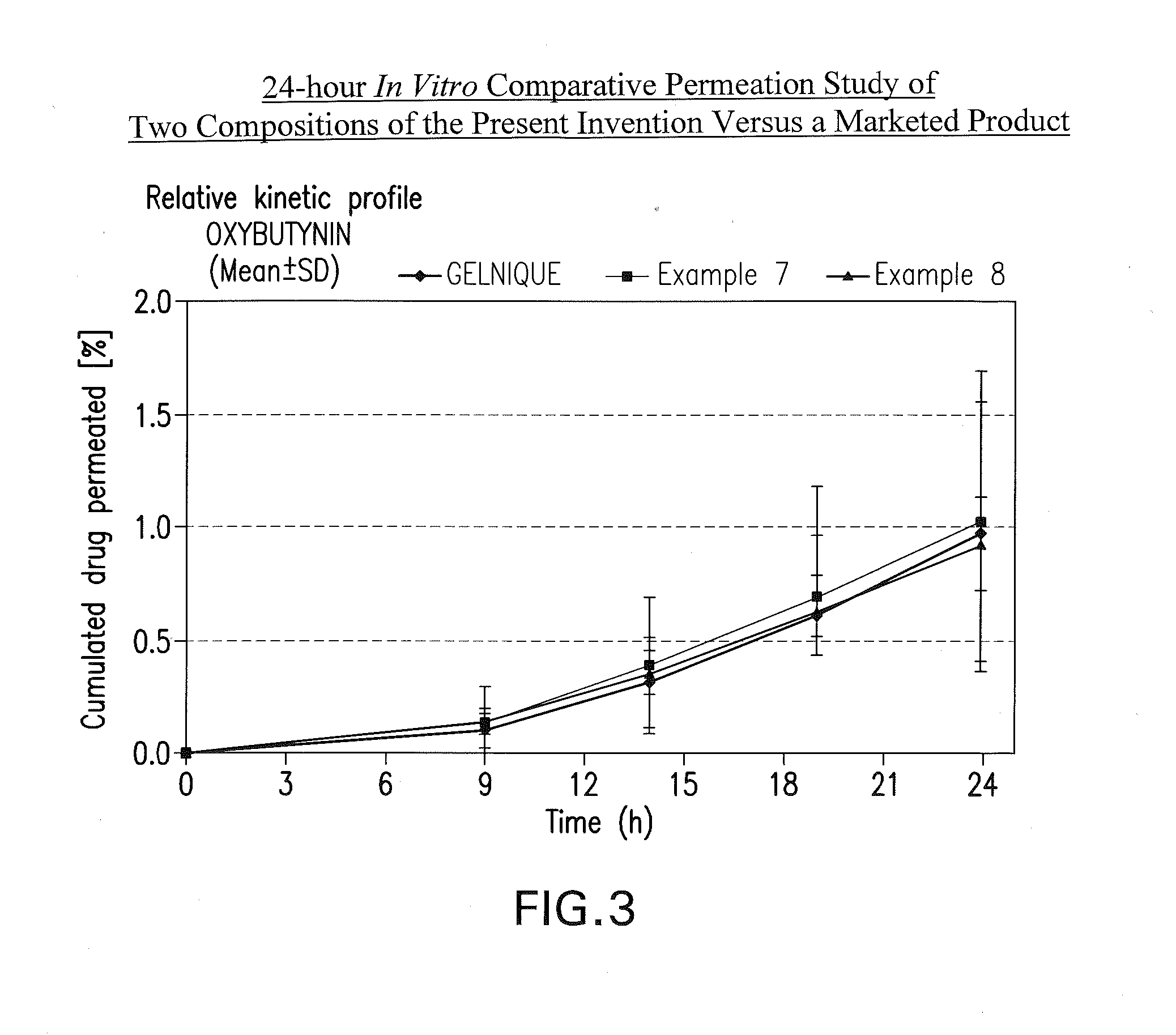
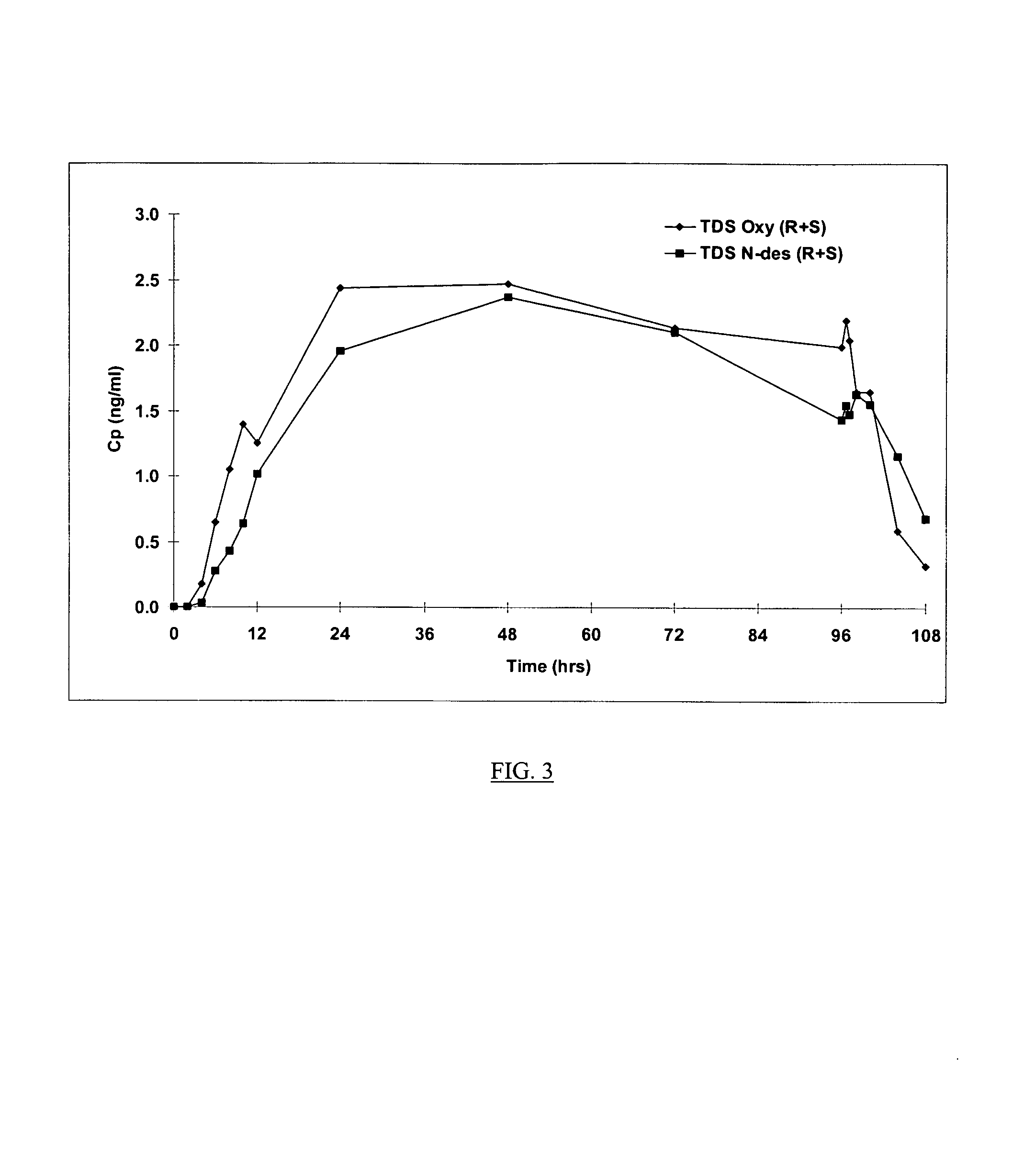
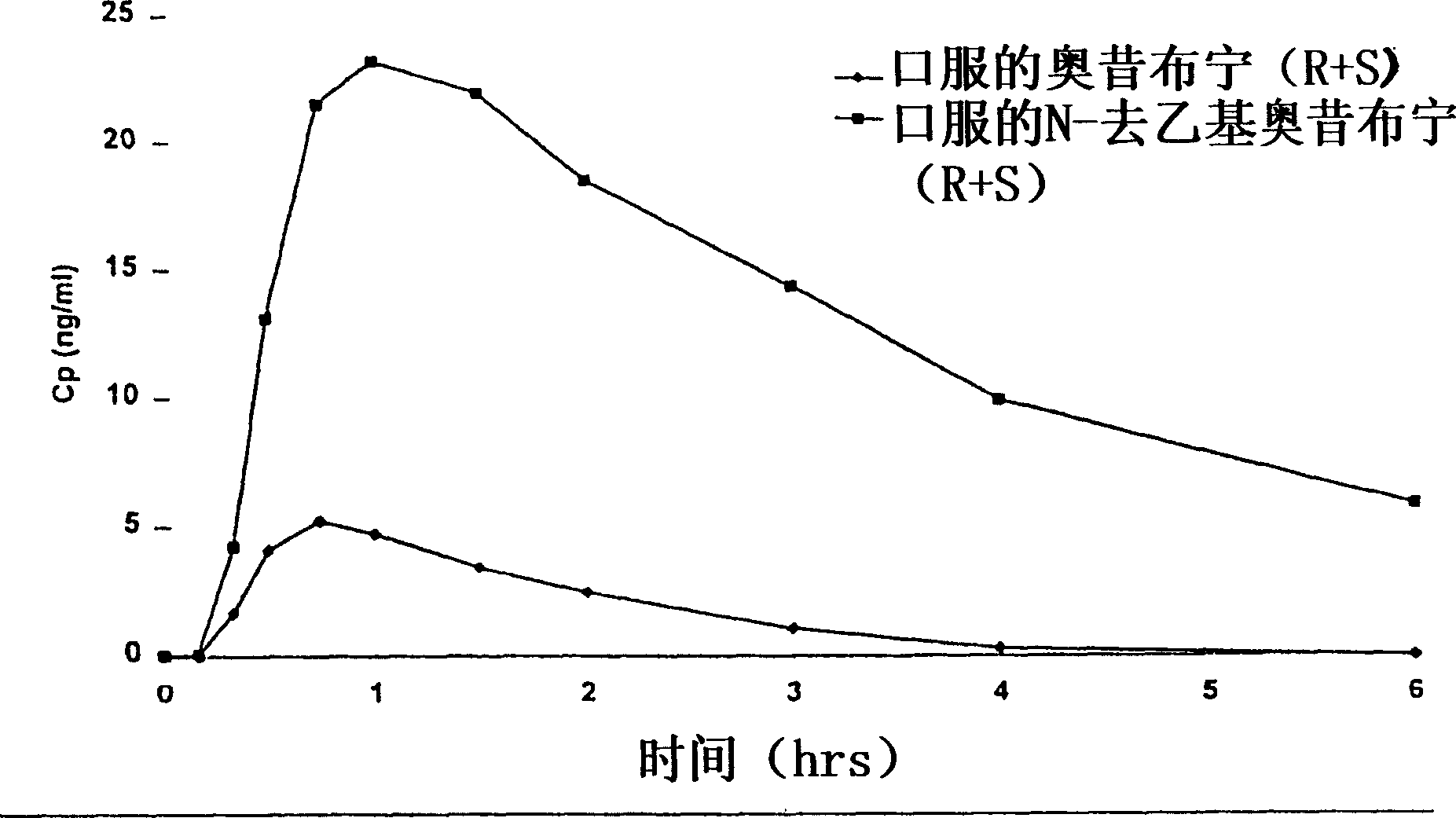
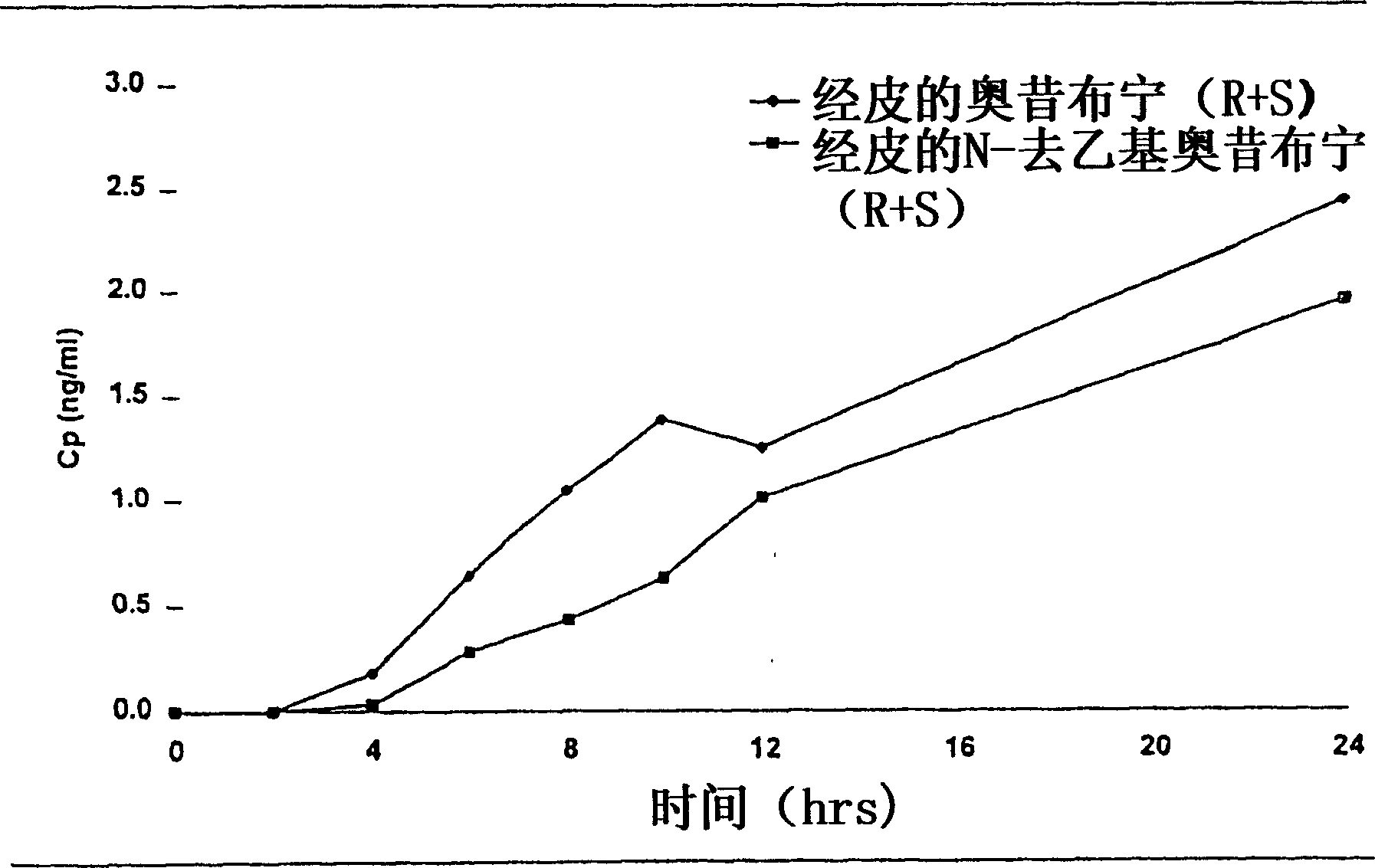
The present invention provides compositions and methods for administering oxybutynin while minimizing the incidence and or severity of adverse drug experiences associated with oxybutynin therapy. In one aspect, these compositions and methods provide a lower plasma concentration of oxybutynin metabolites, such as N-desethyloxybutynin, which is presumed to be contributing at least in part to some of the adverse drug experiences, while maintaining sufficient oxybutynin plasma concentration to benefit a subject with oxybutynin therapy. The invention also provides isomers of oxybutynin and its metabolites that meet these characteristics of minimized incidence and / or severity of adverse drug experiences, and maintenance of beneficial and effective therapy for overactive bladder. In some aspects, the composition may be presented in the form of an unoccluded or free form topically administered gel.
Owner:ALLERGAN SALES LLC +1
Implantable or insertable medical devices for controlled drug delivery
Implantable or insertable medical devices are provided, which comprises: (a) a biocompatible polymer; and (b) at least one therapeutic agent selected from an anti-inflammatory agent, an analgesic agent, an anesthetic agent, and an antispasmodic agent. The medical devices are adapted for implantation or insertion at a site associated with pain or discomfort upon implantation or insertion. In many embodiments, the therapeutic will be selected from at least one of (i) ketorolac and pharmaceutically acceptable salts thereof (e.g., ketorolac tromethamine) and (ii) 4-diethylamino-2-butynylphenylcyclohexyl glycolate and pharmaceutically acceptable salts thereof (e.g., oxybutynin chloride). Also provided are uses for the implantable or insertable medical devices, which uses comprise reducing pain or discomfort accompanying the implantation or insertion of such devices. Further uses may comprise reducing microbial buildup along the device. Methods for manufacturing implantable or insertable medical devices are also provided.
Owner:BOSTON SCI SCIMED INC
Methods and compositions for administration of oxybutynin
InactiveUS20080299207A1Fast absorptionImprove bioavailabilityBiocidePowder deliveryDiseaseOxybutynin
Administration of Oxybutynin in nebulized dry powder form directly to a patient's lungs for treating urinary incontinence or respiratory disease.
Owner:MICRODOSE THERAPEUTX INC
Compositions and methods for transdermal oxybutynin therapy
The present invention provides compositions and methods for administering oxybutynin while minimizing the incidence and or severity of adverse drug experiences associated with oxybutynin therapy. In one aspect, these compositions and methods provide a lower plasma concentration of oxybutynin metabolites, such as N-desethyloxybutynin, which is presumed to be contributing at least in part to some of the adverse drug experiences, while maintaining sufficient oxybutynin plasma concentration to benefit a subject with oxybutynin therapy. The invention also provides isomers of oxybutynin and its metabolites that meet these characteristics of minimized incidence and / or severity of adverse drug experiences, and maintenance of beneficial and effective therapy for overactive bladder. In some aspects, the composition may be presented in the form of an unoccluded or free form topically administered gel.
Owner:WATSON LAB INC
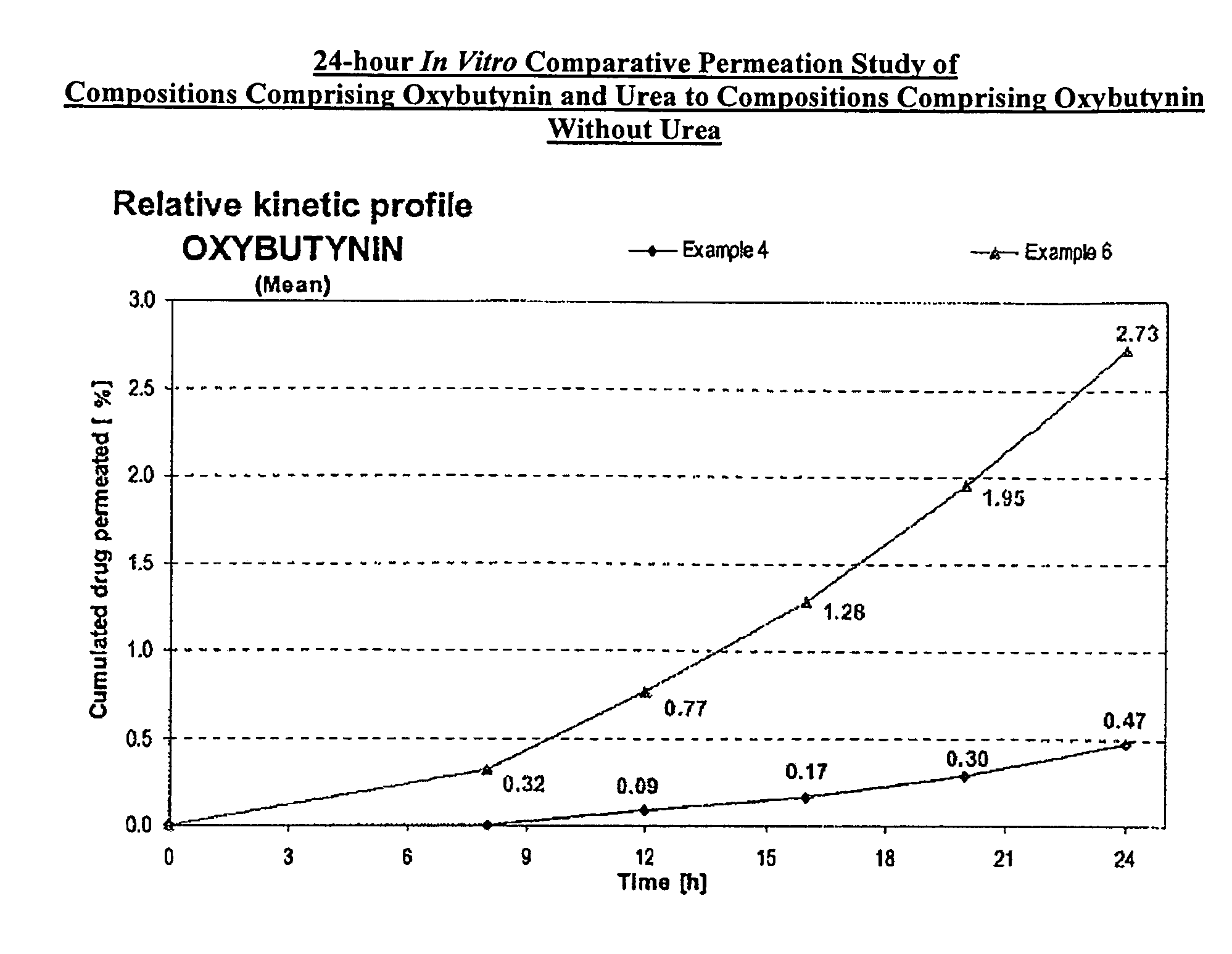
Permeation enhancing compositions for anticholinergic agents
InactiveUS20080260842A1Avoiding undesirable peak in drug concentrationReduce morbidityBiocideAerosol deliveryOxybutyninAnticholinergic agents
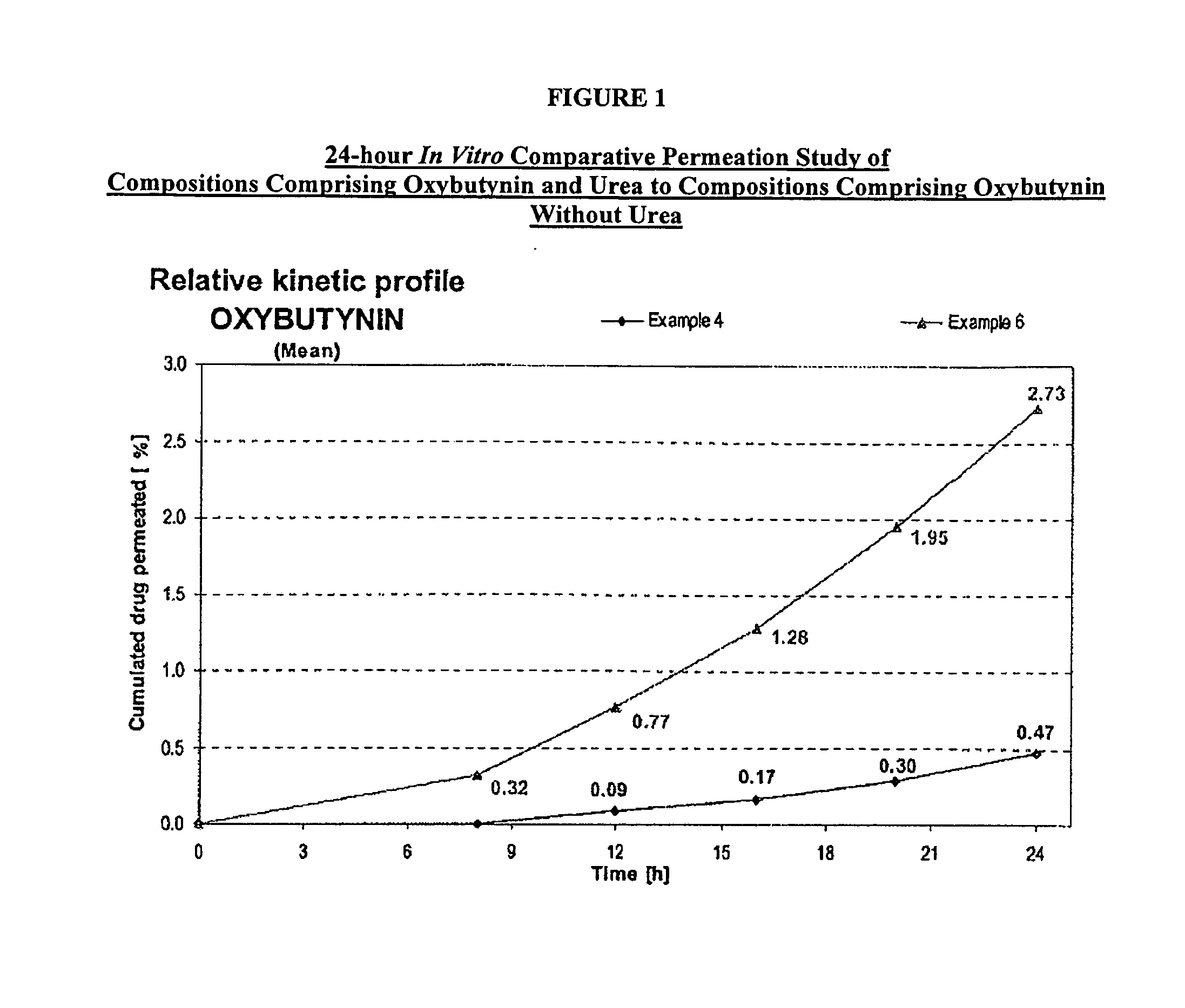
A transdermal or topical skin-friendly composition including anticholinergic agents, such as oxybutynin, a urea-containing compound and a carrier system. A method is disclosed for treating a subject for urinary incontinence while reducing the incidences of peak concentrations of drug and undesirable side effects associated with oral anticholinergics.
Owner:ANTARES PHARMA IPL
Combinations of beta -3 adrenergic receptor agonists and muscarinic receptor antagonists for treating overactive bladder
ActiveCN103269692AOrganic active ingredientsUrinary disorderHyperactivity bladderAdrenergic receptor agonists
Pharmaceutical combinations comprising a beta-3 adrenergic receptor agonist and a muscarinic receptor antagonist, and methods for their use are disclosed. Disclosed combinations include solabegron and oxybutynin. Methods of using the pharmaceutical combinations for the treatment of one or more symptoms associated with overactive bladder, for example, frequency of urgency, nocturia, and urinary incontinence, are also disclosed.
Owner:B3AR THERAPEUTICS INC
Oxybutynin transdermal gel and preparation method thereof
InactiveCN103156804AOrganic active ingredientsPharmaceutical delivery mechanismHyperactivity bladderPharmaceutical medicine
The invention relates to a transdermal gel of oxybutynin or a pharmaceutically acceptable salt thereof used for treating overactive bladder with the symptoms of frequent urination, urgent urination and urinary incontinence, and a preparation method of the transdermal gel. The transdermal gel comprises oxybutynin or the pharmaceutically acceptable salt thereof, a gel matrix hydroxypropyl methylcellulose, a pH value regulator, a humectants and a solvent of anhydrous ethyl alcohol and water.
Owner:CHONGQING PHARMA RES INST
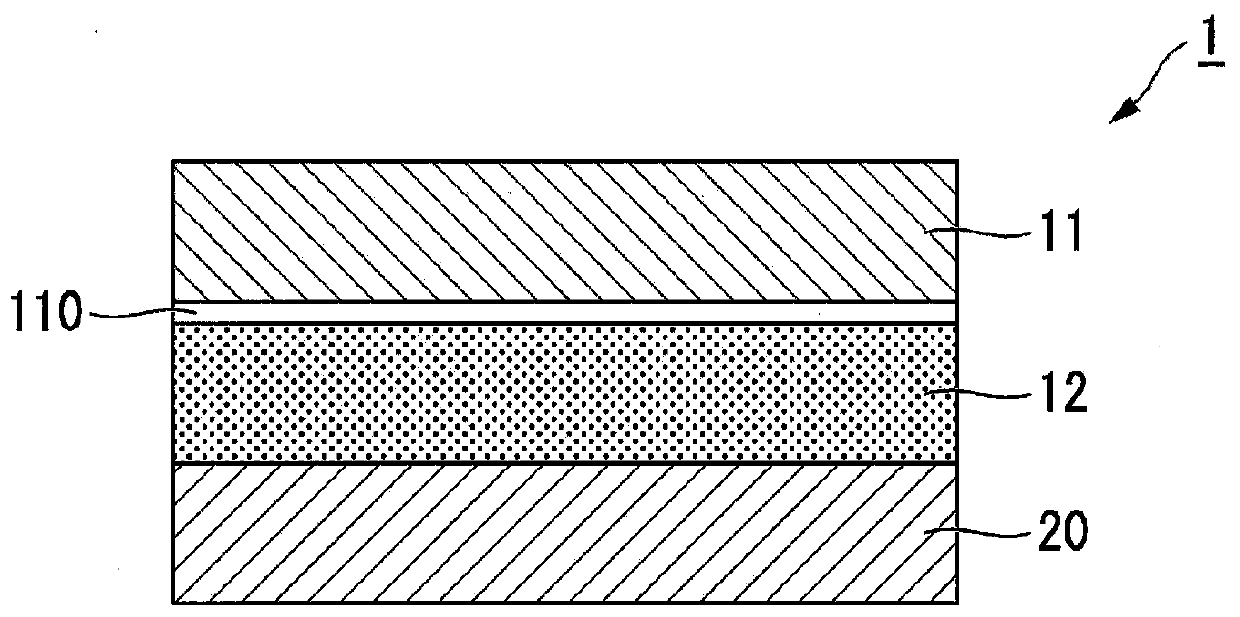
Patch and method for producing the same
ActiveUS8877235B2High levelImprove penetrabilityOrganic active ingredientsAdhesive dressingsOrganic acidPolymer science
A method for producing a patch comprising a support layer and an adhesive agent layer arranged on at least one surface of the support layer, the method comprising:a step A of obtaining an adhesive agent layer composition comprising oxybutynin hydrochloride as a drug, an acrylic-based polymer and / or a rubber-based polymer as an adhesive base agent, liquid paraffin, a sterol, an organic acid, and a tackifier;a step B of heating the adhesive agent layer composition at a temperature in a range from 55 to 70° C. for 1 to 24 hours; anda step C of cooling the heated adhesive agent layer composition to a temperature lower than room temperature at an average rate of temperature drop of 1 to 20° C. / hour, thereby obtaining the adhesive agent layer comprising the drug at a supersaturated concentration in a dissolved form.
Owner:HISAMITSU PHARM CO INC
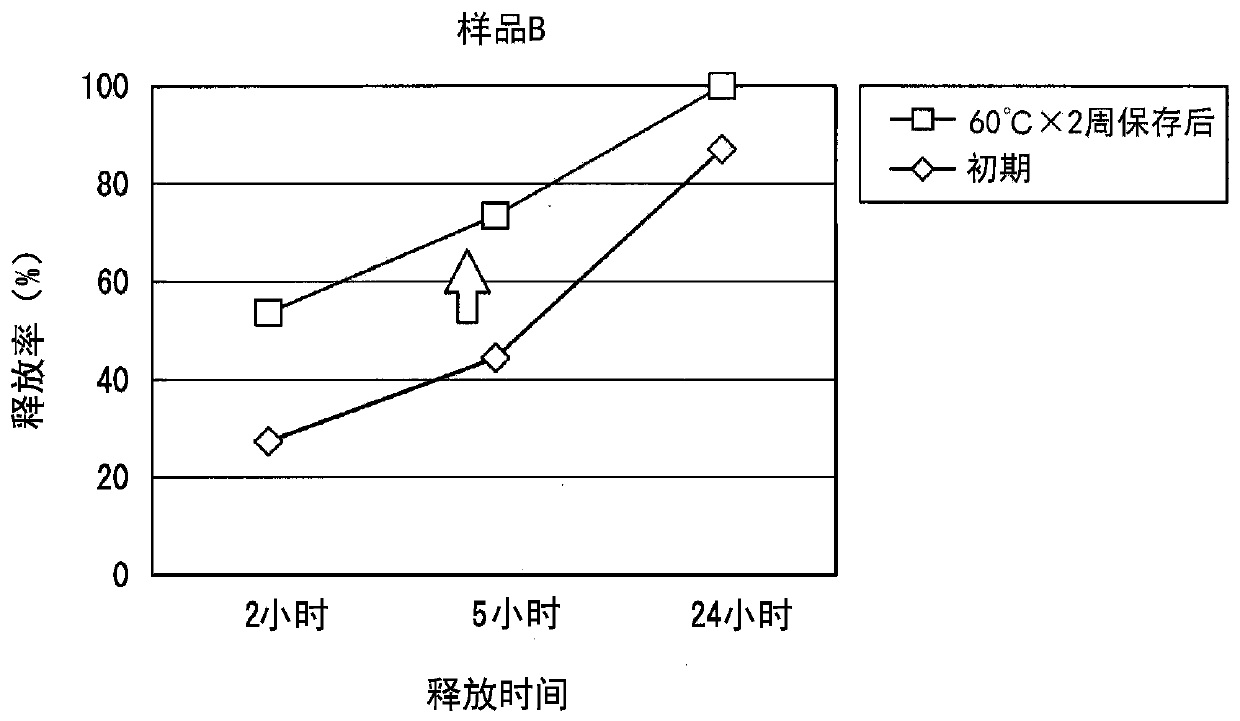
Oxybutynin hydrochloride sustained-release capsule and preparation method thereof
ActiveCN105213350AImprove medication complianceEffective plasma concentrationPharmaceutical delivery mechanismUrinary disorderSide effectTreatment effect
The invention discloses an oxybutynin hydrochloride sustained-release capsule and a preparation method thereof. The oxybutynin hydrochloride sustained-release capsule is prepared from, by weight, 6.8% of oxybutynin hydrochloride, 86.8% of sugared pills, 1.3% of hydroxypropyl methylcellulose, 0.3% of 35% dimethicone emulsion, 3.7% of aqueous ethylcellulose dispersion, 0.8% of sebacic acid dibutyl ester and 0.3% of talcum powder. By means of the oxybutynin hydrochloride sustained-release capsule, effective blood concentration can be kept in vivo, no peak valley phenomenon is caused, side effects caused when the blood concentration is too high are relatively reduced, and meanwhile the non-treatment effect caused when the blood concentration is too low is reduced; the number of medicine taking times in each day is decreased, and the medicine taking compliance of patients is improved.
Owner:上海爱的发制药有限公司
Compositions and methods for mucosal delivery
The present invention relates to a dosage unit comprising a water-soluble hydrocolloid and a mucosal surface-coat-forming film, such film including an effective dose of active agent. In the dosage unit slidenafil citrate, nicotine, hydromorphone, oxybutynine or estradiol are used as active agents.
Owner:LAVIPHARM LABORATORIES INC
Oxybutynin transdermal therapeutic system combination
ActiveUS10149828B2Large doseMaximum efficacyNervous disorderEster active ingredientsOxybutyninCholinesterase inhibition
There is described a pharmaceutical combination comprising oxybutynin or a pharmaceutically acceptable addition salt thereof, in a transdermal therapeutic system, and an acetylcholinesterase inhibitor, useful for safely treating hypocholinergic disorders of the central nervous system such as Alzheimer type dementia. In this combination, the acetylcholinesterase inhibitor (AChEI) is present at a dose that is higher than the maximal recommended dose, per unit form. In particular, the transdermal therapeutic system comprising oxybutynin is in combination with rivastigmine in a transdermal formulation or oral form.
Owner:CHASE PHARMA CORP
Combination of selected opioids with muscarine antagonists for treating urinary incontinence
Active compound combinations of compounds of group A, particularly opioids such as (+)-(2R,3R)-1-dimethylamino-3-(3-methoxy-phenyl)-2-methyl-pentan-3-ol or a salt thereof with a physiologically tolerated acid, and compounds of group B, particularly anti-muscarine agents such as oxybutynin or a salt thereof with a physiologically tolerated acid suitable for treatment of an increased urge to urinate or urinary incontinence. Related pharmaceutical formulations and methods of treatment of an increased urge to urinate or urinary incontinence are also provided.
Owner:GRUNENTHAL GMBH
Method For Treating Overactive Bladders And A Device For Storage And Administration Of Topical Oxybutynin Compositions
ActiveUS20100286630A1Safe and durable and stableEasily open deviceOrganic active ingredientsPowder deliveryOxybutyninUrinary bladder
The invention relates to a method for treating overactive bladders and a device for storing and administering non-occluded oxybutynin topical compositions.
Owner:ALLERGAN SALES LLC
Treating smooth muscle hyperactivity with (R)-oxybutynin and (R)-desethyloxybutynin
The optically pure R(-)-isomer of oxybutynin and the optically pure R(-)-isomer of desethyl-oxybutynin, which are substantially free of the corresponding S(+)-isomers, are potent anticholinergic spasmolytics, useful for relief of symptoms associated with urinary bladder smooth muscle hyperactivity, such as Urinary Incontinence and gastrointestinal smooth muscle hyperactivity, such as Irritable Bowel Syndrome. Methods are disclosed utilizing the optically pure R(-)-isomers of oxybutynin and desethyloxybutynin for treating cholinergically mediated smooth muscle hyperactivity while minimizing the side effects associated with administration of racemic oxybutynin or racemic desethyloxybutynin.
Owner:WATSON PHARMA INC
Methods and compositions for treatment of peripheral neuropathies
A composition for therapy of a peripheral neuropathy disorder in a subject in need thereof. The composition comprises an effective amount of an agent selected from a group consisting of pirenzepine, oxybutynin, muscarinic toxin 7, a muscarinic receptor antagonist, and combinations thereof, and a pharmacologically acceptable carrier and / or an excipient. The composition is useful for therapy of peripheral neuropathies exemplified by peripheral neuropathies induced by systemic diseases, peripheral neuropathies induced by metabolic diseases, chemotherapy-induced peripheral neuropathies, compression-induced peripheral neuropathies, peripheral neuropathies induced by exposure to dichloroacetate, immune- mediated peripheral neuropathies, peripheral neuropathies induced by infections, and genetically acquired peripheral neuropathies.
Owner:UNIVERSITY OF MANITOBA
Oxybutynin transdermal plaster and preparation method thereof
ActiveCN113143891AHigh porosityImprove breathabilityOrganic active ingredientsAntipyreticPectinaseCellulose
The invention relates to the technical field of pharmaceutical preparations, and discloses an oxybutynin transdermal plaster and a preparation method thereof. The oxybutynin transdermal plaster comprises a backing layer and an ointment-containing body, wherein the ointment-containing body comprises the following raw materials in percentage by mass: 3-5% of oxybutynin and / or pharmaceutically acceptable salt thereof, 10-15% of sodium polyacrylate, 5-8% of pre-crosslinked pectin, 1-2% of hydroxypropyl methylcellulose, 0.5-1% of dihydroxyaluminium aminoacetate, 1.5-3.5% of pectinase @ ethyl cellulose / chitosan microcapsule, 10-15% of sorbitol, 1-2% of laurinol azone, 10-15% of ethanol and the balance water; and the pH value of the ointment-containing body is controlled to be 5.0-6.0 by a pH regulator. The oxybutynin transdermal plaster disclosed by the invention has relatively high air permeability, and can promote the release of oxybutynin by slowly releasing pectinase in the later period of application, thereby prolonging the drug effect duration and application time of the plaster.
Owner:杭州仁德药业股份有限公司
Oxybutynin transdermal patch and preparation method thereof
ActiveCN113143891BHigh porosityImprove breathabilityOrganic active ingredientsAntipyreticTransdermal patchPectinase
Owner:杭州仁德药业股份有限公司
Oxybutynin-containing transdermal absorption preparation
ActiveCN109803649AReduce releaseReduce adhesionOrganic active ingredientsPharmaceutical non-active ingredientsMedicineCholesterol
This oxybutynin-containing transdermal absorption preparation is provided with: a support layer which comprises a base fabric and at least a part of which has water-repellent properties; and an adhesive layer which is layered on one surface of the support layer, wherein the adhesive layer contains an adhesive composition that includes oxybutynin or a pharmaceutically acceptable salt thereof, a cholesterol, and a rubber-based adhesive base material. The oxybutynin may be oxybutynin hydrochloride, and the part having water-repellent properties may be formed from a fluorine-containing compound, and the base fabric may be a knitted fabric.
Owner:HISAMITSU PHARM CO INC
Patch
ActiveUS20200069603A1Reduce skin irritationReduce contentOrganic active ingredientsAntimycoticsCortisoneOxybutynine
Owner:HISAMITSU PHARM CO INC
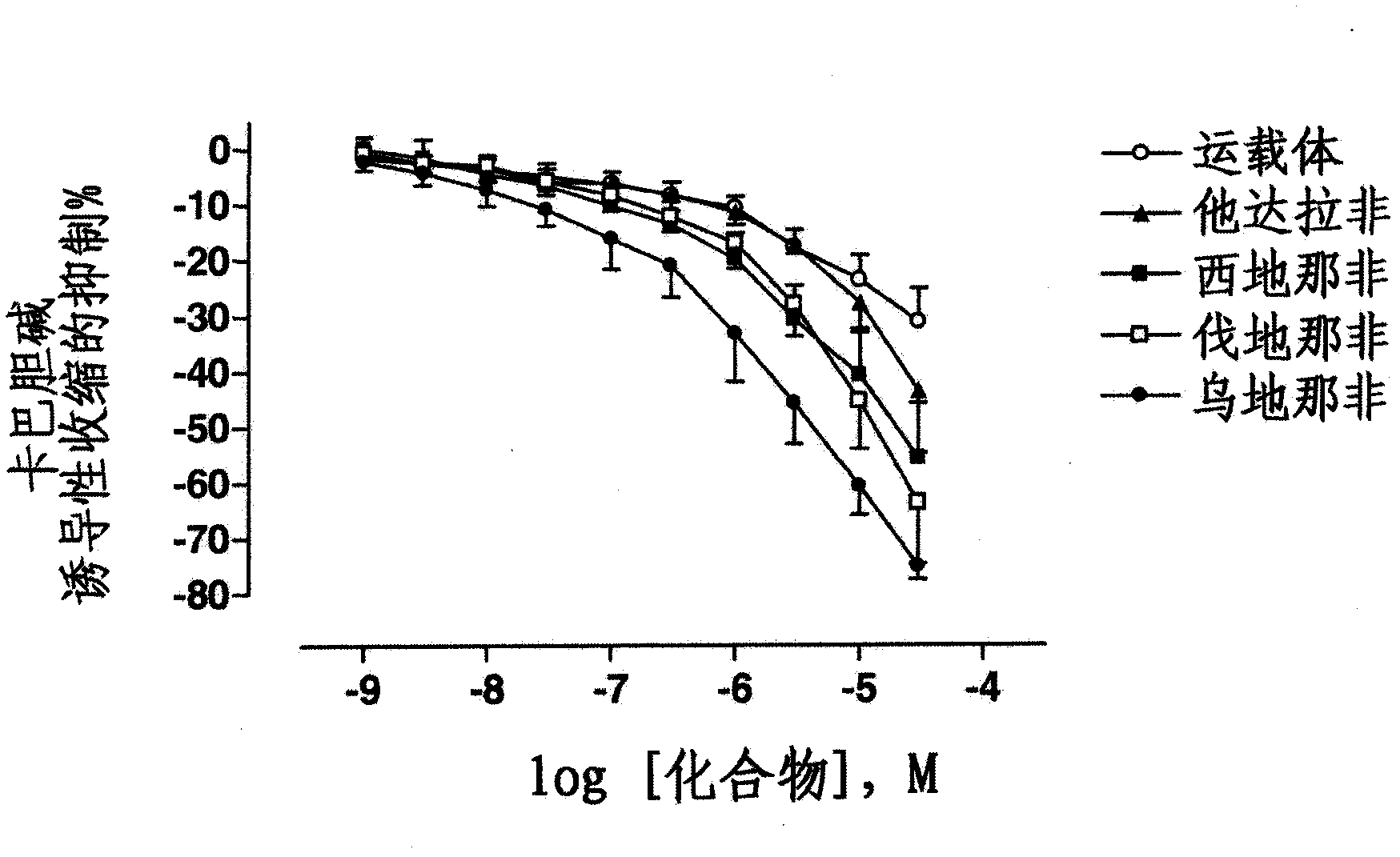
Use of a combination of udenafil and alfuzosin or oxybutynin for the treatment of overactive bladder.
The invention relates to a specific combination of two active agents: udenafil and one of alfuzosin and oxybutynin and its use for the treatment of overactive bladder.
Owner:PELVIPHARM
A kind of oxybutynin hydrochloride osmotic pump controlled-release tablet and preparation method thereof
ActiveCN103860514BTo achieve the purpose of controlled releaseOrganic active ingredientsInorganic non-active ingredientsBlood concentrationOxybutynine
The invention provides oxybutynin hydrochloride osmotic pump controlled release tablets and a preparation method thereof. The controlled release tablets are stable in drug release speed in release media of different pH values, and the oxybutynin hydrochloride osmotic pump controlled release tablets are released in a gastrointestinal environment at a basically constant rate, so that the blood concentration of oxybutynin hydrochloride is constantly maintained in an effective concentration range for a long time, a peak valley phenomenon is avoided, the toxic and side effects are reduced, and the bioavailability can be improved.
Owner:HEFEI HUAFANG PHARMA SCI & TECH
Preparation method of oxybutynin hydrochloride
PendingCN114805100AFew stepsMild reaction conditionsOrganic compound preparationUrinary disorderPtru catalystChemical reaction
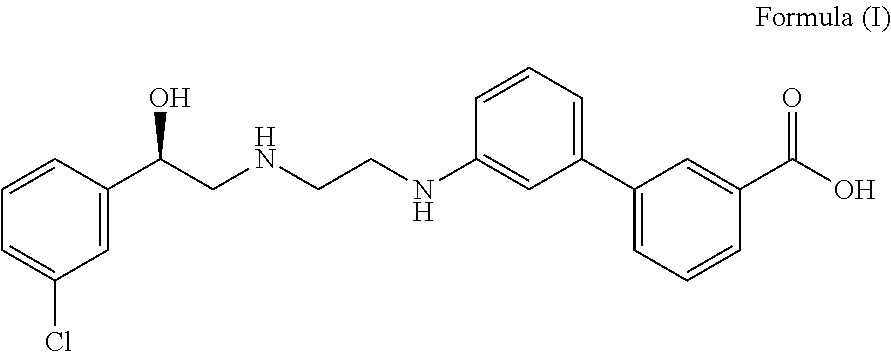
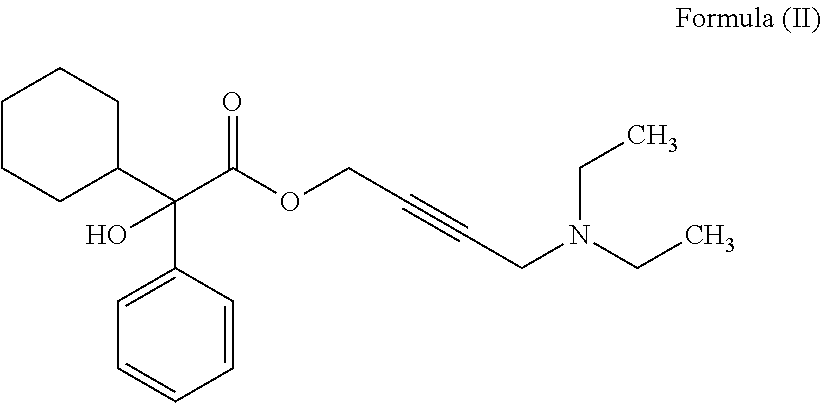
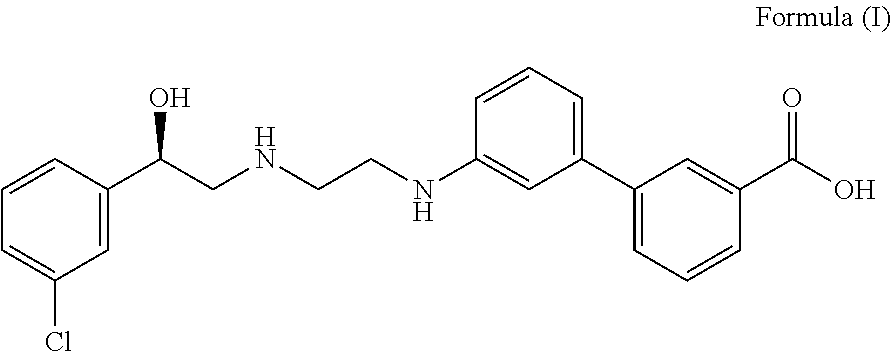
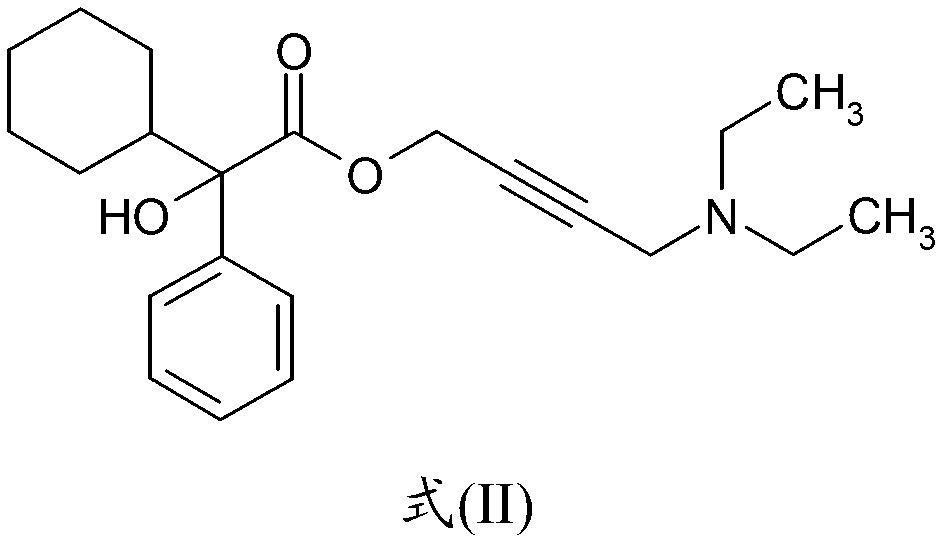
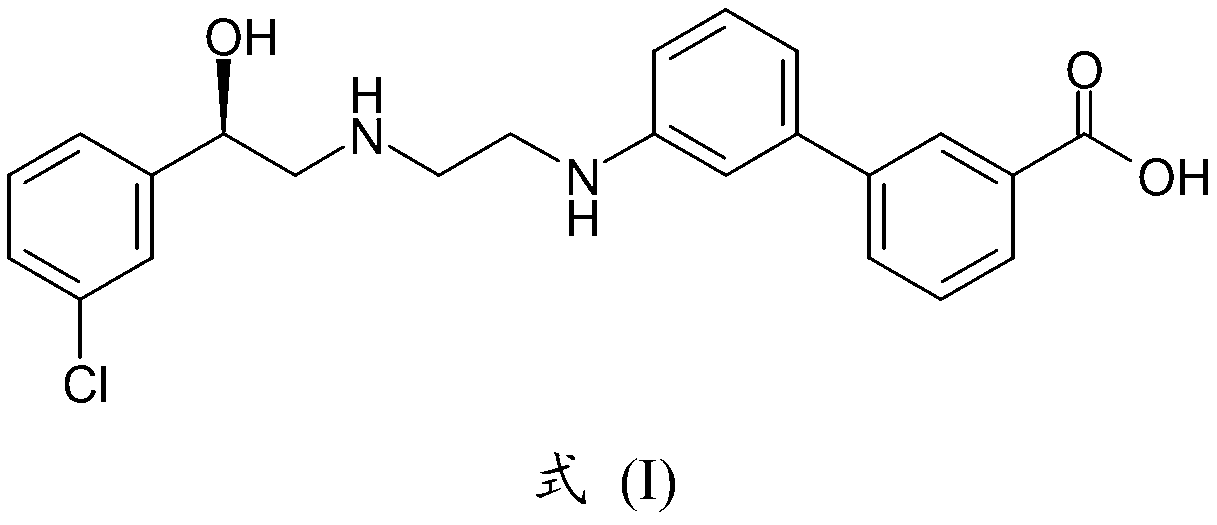
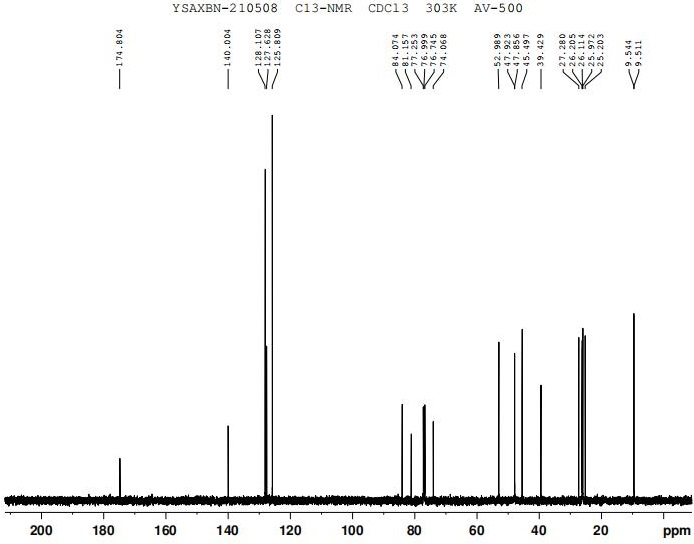
The invention discloses a preparation method of oxybutynin hydrochloride (the chemical name of the oxybutynin hydrochloride is alpha-cyclohexyl-alpha-hydroxy-phenylacetic acid-4-diethylamino-2-butyne ester hydrochloride). Comprising the following steps: carrying out esterification reaction on alpha-cyclohexyl-alpha-hydroxyphenylacetic acid and 3-propargyl chloride in the presence of a proper solvent and a basic catalyst to generate an intermediate, namely alpha-cyclohexyl-alpha-hydroxyphenylacetic acid propargyl ester, and then carrying out Mannich reaction on the alpha-cyclohexyl-alpha-hydroxyphenylacetic acid propargyl ester, a 36% formaldehyde solution and diethylamine to generate oxybutynin, and finally salifying with hydrochloric acid to generate oxybutynin hydrochloride. The method has simple reaction routes, does not involve reactions under harsh chemical reaction conditions, does not involve a high-risk chemical reaction process, and is suitable for industrial production.
Owner:南京亿华药业有限公司
Oxybutynine ethosomal composition and preparation method thereof
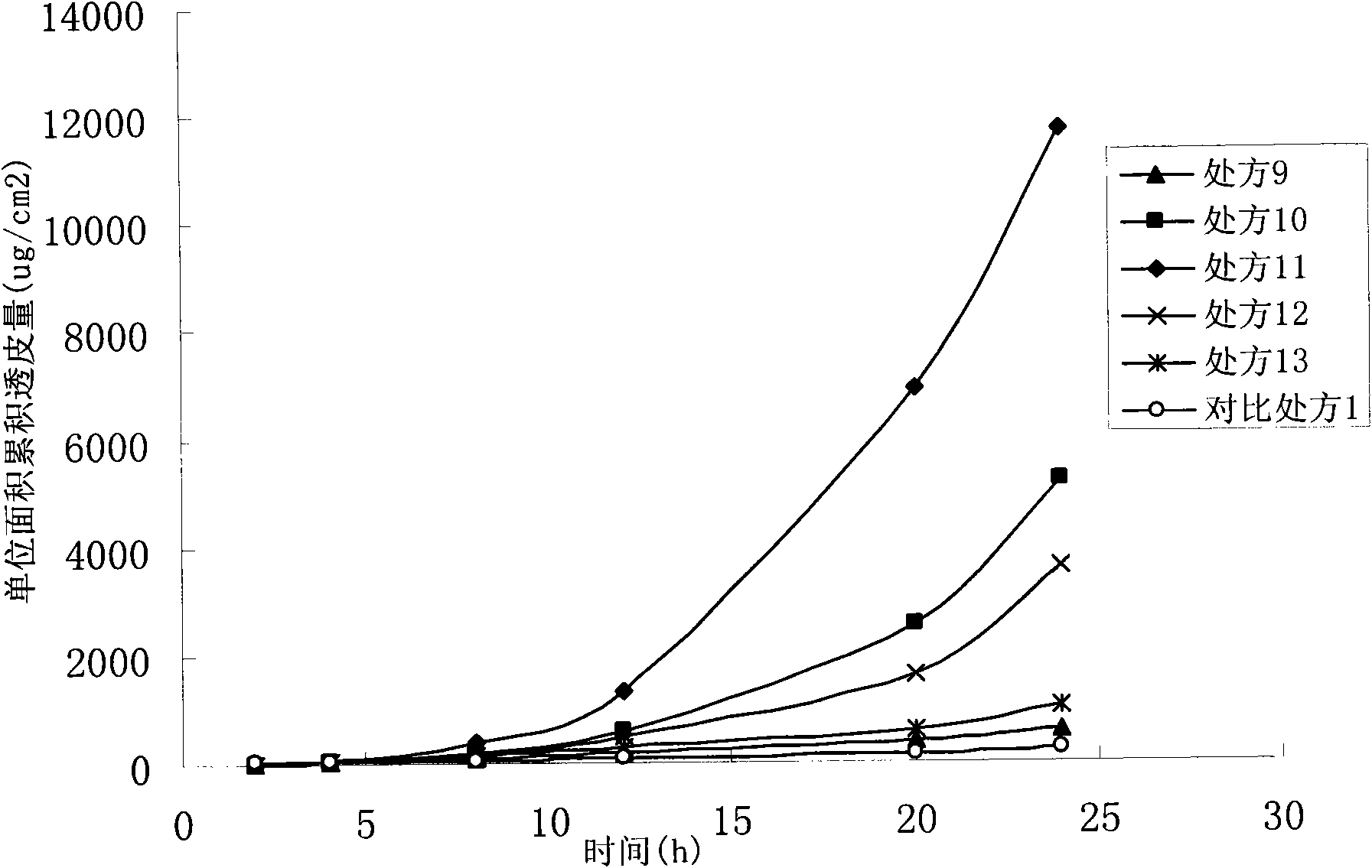
InactiveCN102258469BImprove bioavailabilityImprove skin penetration rateOrganic active ingredientsAerosol deliveryAlcoholOxybutynine
The invention relates to an oxybutynine ethosomal composition and a preparation method thereof. The oxybutynine ethosomal composition mainly comprises oxybutynine or a pharmaceutically acceptable salt, phospholipid, short-chain alcohol and water. The composition is prepared into a proper preparation form, such as gel and the like. The oxybutynine ethosomal composition is used for transdermal drug delivery and has higher permeation rate and bioavailability than those of an oxybutynine transdermal drug delivery preparation, such as gel and the like.
Owner:CHONGQING PHARMA RES INST
Oxybutynin transdermal therapeutic system muscarinic agonist combination
ActiveUS20180050008A1Safety managementIncrease awarenessNervous disorderAerosol deliveryNervous systemHigh doses
Pharmaceutical compositions and combinations containing a muscarinic receptor antagonist, such as oxybutynin in a transdermal therapeutic system, and a muscarinic receptor agonist, optionally with an acetyl cholinesterase inhibitor, and methods of using the same for treatment of hypocholinergic disorders of the central nervous system such as Alzheimer type dementia. The respective pharmaceutical compositions and combinations of the present invention allow for safe administration of high doses of muscarinic receptor agonist, and improved efficacy of the muscarinic receptor agonist for treatment of hypocholinergic disorders of the central nervous system. The pharmaceutical compositions and combinations also allow for a maximum supply of acetylcholine to the central nervous system, when an acetyl cholinesterase inhibitor is used in combination with a muscarinic receptor antagonist and a muscarinic receptor agonist.
Owner:CHASE PHARMA CORP
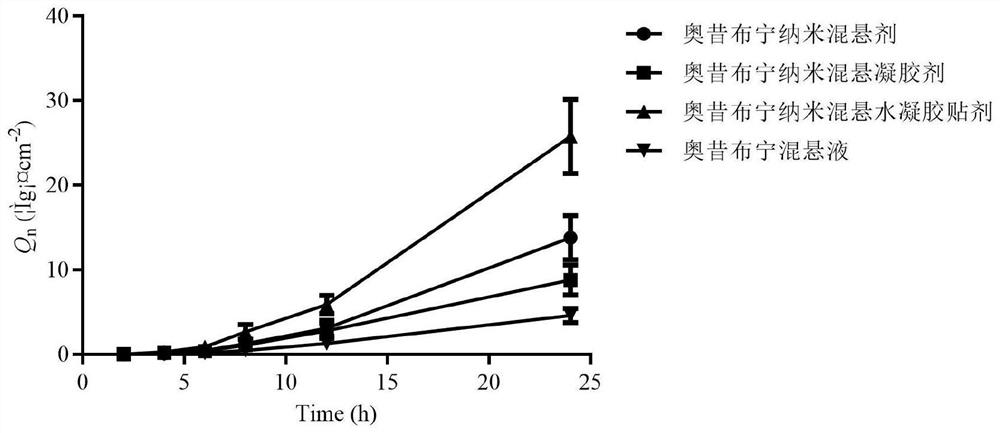
Oxybutynin nanosuspension, composition containing oxybutynin nanosuspension and preparation method of oxybutynin nanosuspension
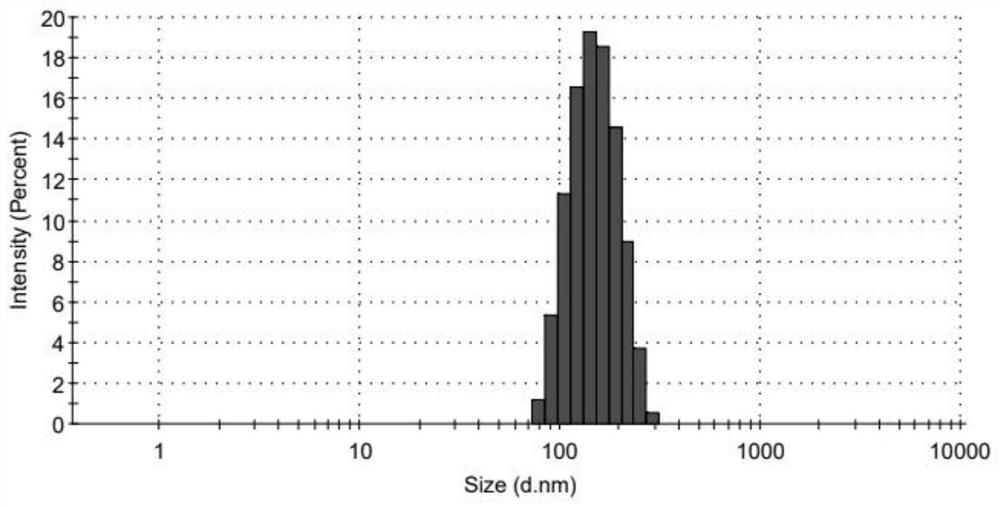
ActiveCN113797166APromote transdermal absorptionImprove transdermal drug delivery efficiencyPowder deliveryOrganic active ingredientsDrug reservoirDrugs preparations
The invention belongs to the field of pharmaceutical preparations, particularly relates to an oxybutynin nanosuspension, a composition containing the oxybutynin nanosuspension and a preparation method of the oxybutynin nanosuspension, and relates to the composition containing the oxybutynin nanosuspension. The oxybutynin nanosuspension is composed of oxybutynin, a stabilizer and a co-stabilizer, the preparation method is simple, and industrial production is facilitated. The composition containing the oxybutynin nanosuspension is oxybutynin nanosuspension freeze-dried powder, oxybutynin nanosuspension gel and oxybutynin nanosuspension hydrogel patch, the stability is better, and the administration is flexible and convenient. After the oxybutynin nanosuspension and the composition containing the oxybutynin nanosuspension are applied to the skin, the transdermal absorption of the oxybutynin can be remarkably improved, and a drug reservoir can be formed in the skin to slowly release drugs.
Owner:CHINA PHARM UNIV
Oxybutynin-xanomeline transdermal therapeutic system combinations
Transdermal therapeutic system and method of using the same for safely treating hypocholinergic disorders of the central nervous system such as Alzheimer type dementia. The transdermal therapeutic system comprises oxybutynin in combination with a cholinergic receptor agonist (CRA) such as xanomeline.
Owner:CHASE PHARMA CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com