Oxybutynin nanosuspension, composition containing oxybutynin nanosuspension and preparation method of oxybutynin nanosuspension
A kind of nano-suspension, nano-suspension technology, applied in the composition containing oxybutynin nano-suspension, the field of preparation of oxybutynin nano-suspension, to improve solubility and dissolution rate, improve transdermal Good absorption and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
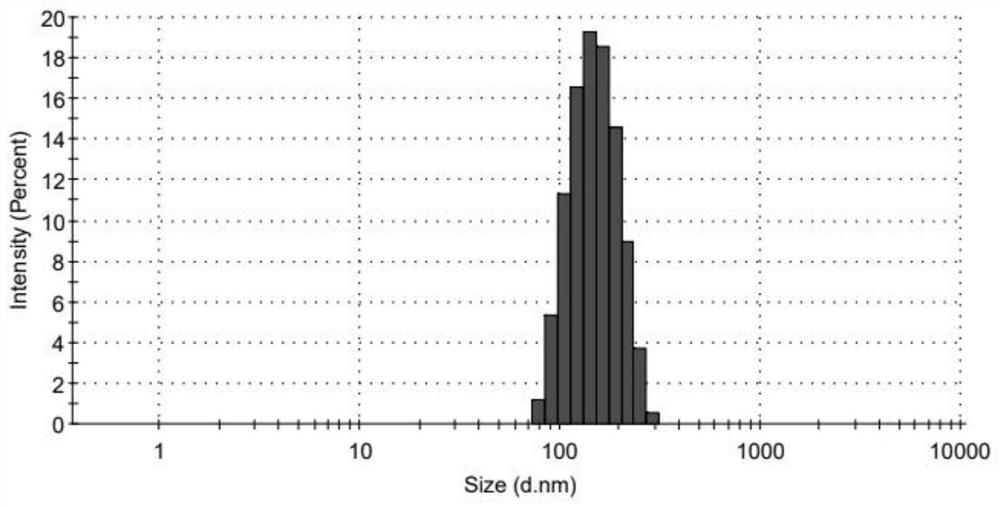
[0031] Weigh oxybutynin, vitamin E succinate and poloxamer 188 (mass ratio is 2:2:1), dissolve in an appropriate amount of absolute ethanol as the organic phase; take 100mL purified water as the water phase; The organic phase was slowly dropped into the water phase under low pressure; then the ethanol was removed by rotary evaporation at 45°C; 800bar high-pressure homogenization was performed 20 times to obtain the oxybutynin nanosuspension. The measured average particle diameter is 179.73±1.28nm, and the PDI is 0.193±0.009.
Embodiment 2
[0033] Weigh oxybutynin, vitamin E succinate and poloxamer 188 (mass ratio is 1:1:1), dissolve in an appropriate amount of absolute ethanol as the organic phase; take 100mL purified water as the water phase; The organic phase was slowly dropped into the water phase under low pressure; then the ethanol was removed by rotary evaporation at 45°C; 800bar high-pressure homogenization was performed 20 times to obtain the oxybutynin nanosuspension. The measured average particle diameter is 190.74±1.59nm, and the PDI is 0.221±0.017.
Embodiment 3
[0035] Weigh oxybutynin, vitamin E succinate and poloxamer 188 (mass ratio 1:1:2), dissolve in an appropriate amount of absolute ethanol as the organic phase; take 100mL purified water as the water phase; The organic phase was slowly dropped into the water phase under low pressure; then the ethanol was removed by rotary evaporation at 45°C; 800bar high-pressure homogenization was performed 20 times to obtain the oxybutynin nanosuspension. The measured average particle diameter is 163.47±1.09nm, and the PDI is 0.114±0.025.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com