A kind of oxybutynin hydrochloride osmotic pump controlled-release tablet and preparation method thereof
A technology of oxybutynin hydrochloride and osmotic pump controlled release, which is applied in the direction of pharmaceutical formulations, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc. Obvious differences and other issues to achieve the effect of ensuring stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
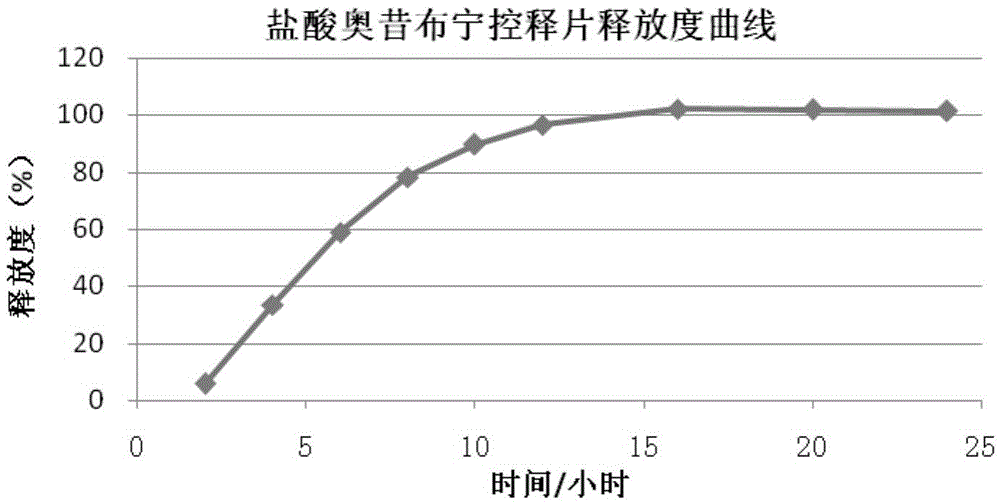
Image
Examples
Embodiment 1
[0082] The tablet core is composed of: 5g oxybutynin hydrochloride, 11g sodium chloride, 11g lactose, 124g polyoxyethylene (molecular weight: 100000), 6g hypromellose, 1.0g magnesium stearate;
[0083] The composition of the semipermeable film coating layer with drug release channels is: 96g cellulose acetate, 4g polyethylene glycol 6000;
[0084] The moisture-proof clothing layer is: Opadry 295W680000.
[0085] Preparation method:
[0086]Compression of single-layer tablet core: Weigh oxybutynin hydrochloride, polyoxyethylene (molecular weight: 100,000), hypromellose, sodium chloride, and lactose in the formula, pass through 80-mesh sieve, mix evenly, and 90% The soft material is prepared from ethanol solution, sieved and granulated, dried at 40-50°C, sieved and granulated, added with the prescribed amount of magnesium stearate, mixed evenly, pressed into tablets, and the drug tablet core is obtained, ready for use;
[0087] Coating layer with semi-permeable membrane: Disso...
Embodiment 2
[0091] The tablet core is composed of: 5.5g oxybutynin hydrochloride, 11g sodium chloride, 11g lactose, 124g polyoxyethylene (molecular weight: 100000), 6g hypromellose, 1.0g magnesium stearate;
[0092] The composition of the semi-permeable film coating layer with drug release channels is: 96g cellulose acetate, 4g polyethylene glycol 6000;
[0093] The moisture-proof clothing layer is: Opadry 295W680000.
[0094] Preparation method:
[0095] Compression of single-layer tablet cores: Weigh oxybutynin hydrochloride, polyoxyethylene, hypromellose, sodium chloride, and lactose in the prescribed amount, pass through 80 mesh sieves, mix evenly, and make a soft material with 90% ethanol solution , sieve and granulate, dry at 40-50°C, sieve and granulate, add the prescribed amount of magnesium stearate, mix evenly, and press into tablets to obtain drug tablet cores for future use;
[0096] Coating layer with semi-permeable membrane: Dissolve the formulated amount of cellulose acet...
Embodiment 3
[0100] The tablet core is composed of: 5.5g oxybutynin hydrochloride, 16g sodium chloride, 16g lactose, 121.5g polyoxyethylene (molecular weight: 100000), 0.8g magnesium stearate;
[0101] The composition of the semi-permeable film coating layer with drug release channels is: 96g cellulose acetate, 4g polyethylene glycol 6000;
[0102] The moisture-proof clothing layer is: Opadry 295W680000.
[0103] Preparation method:
[0104] Compression of single-layer tablet core: Weigh oxybutynin hydrochloride, polyoxyethylene (molecular weight: 100,000), hypromellose, sodium chloride, and lactose in the formula, pass through 80-mesh sieve, mix evenly, and 90% The soft material is prepared from ethanol solution, sieved and granulated, dried at 40-50°C, sieved and granulated, added with the prescribed amount of magnesium stearate, mixed evenly, pressed into tablets, and the drug tablet core is obtained, ready for use;
[0105] Coating layer with semi-permeable membrane: Dissolve the for...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com