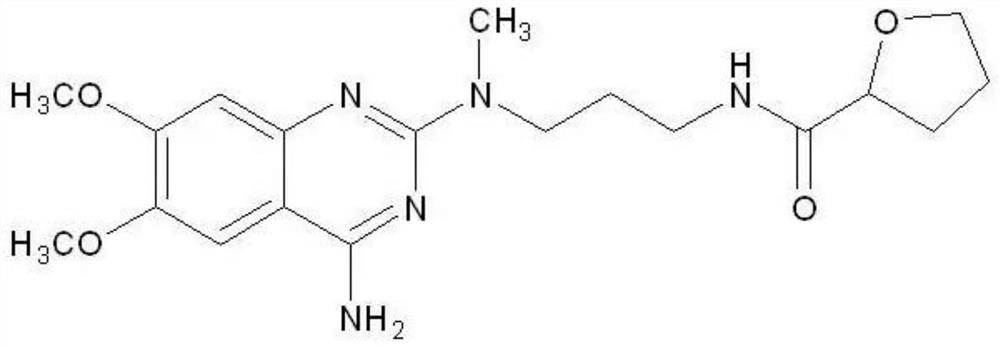
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33 results about "Alfuzosin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Alfuzosin is used by men to treat the symptoms of an enlarged prostate (benign prostatic hyperplasia-BPH).
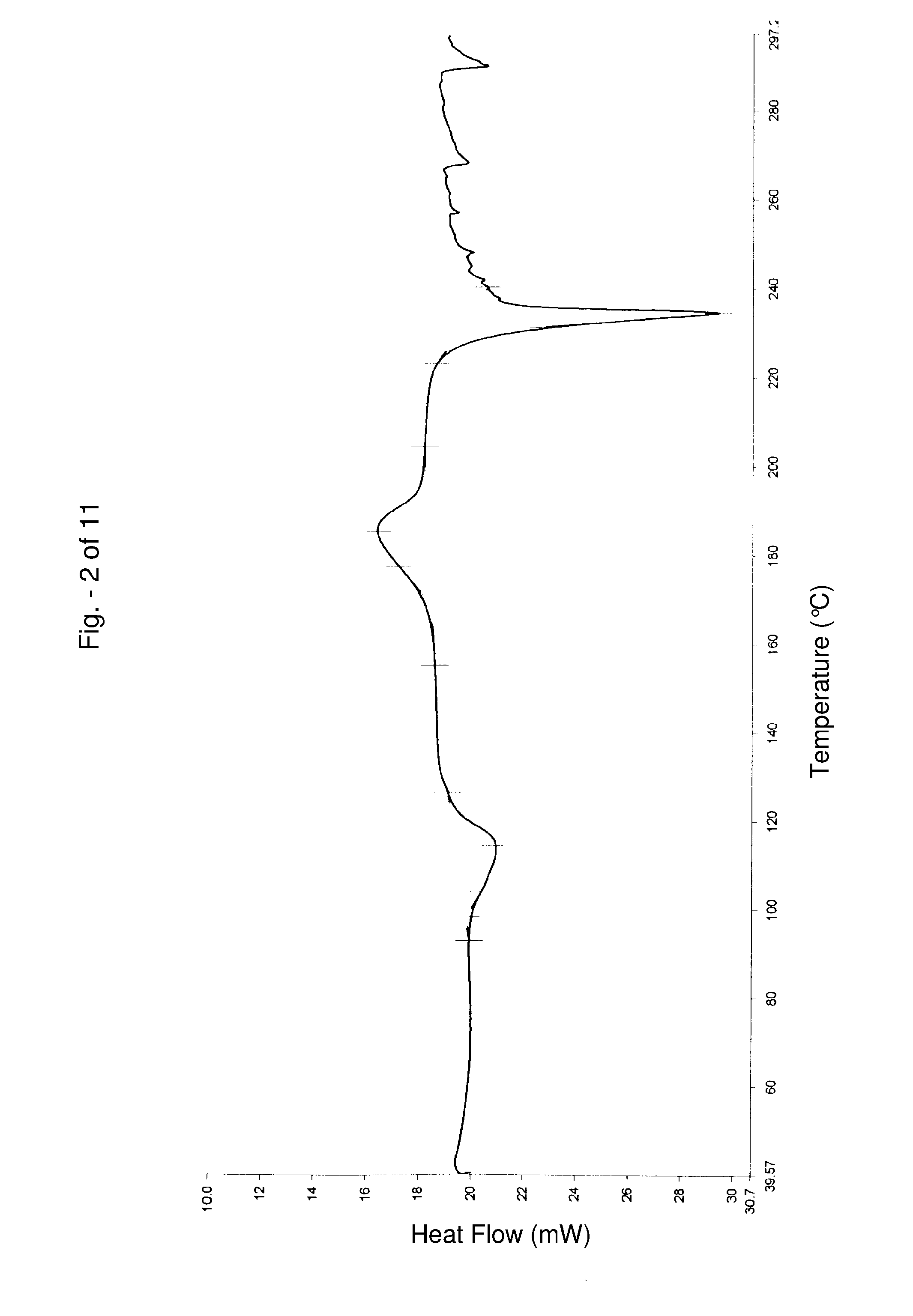
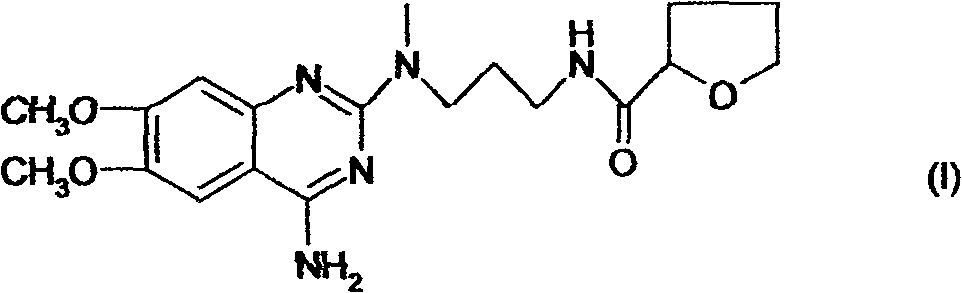
Alfuzosin tablets and synthesis
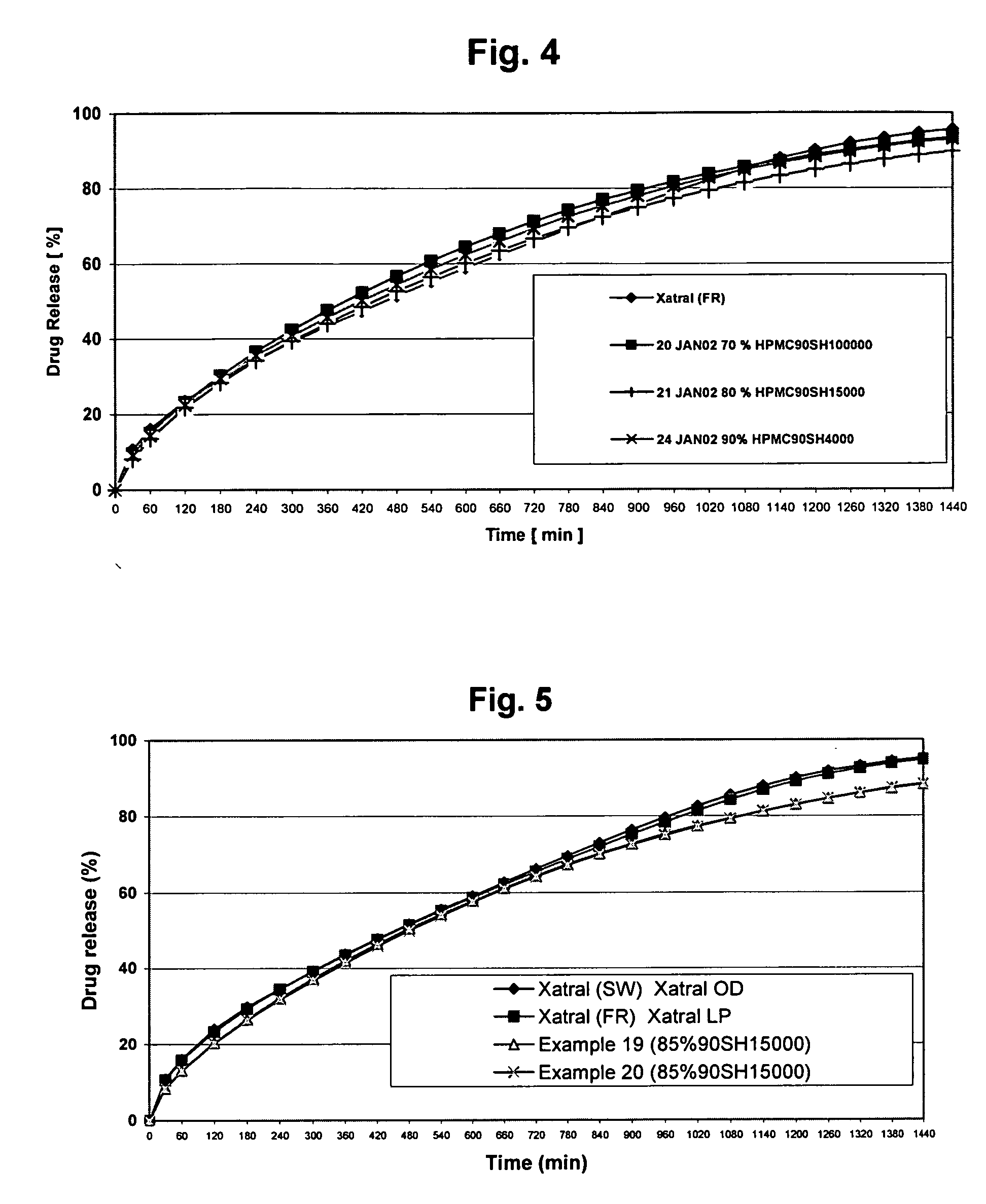
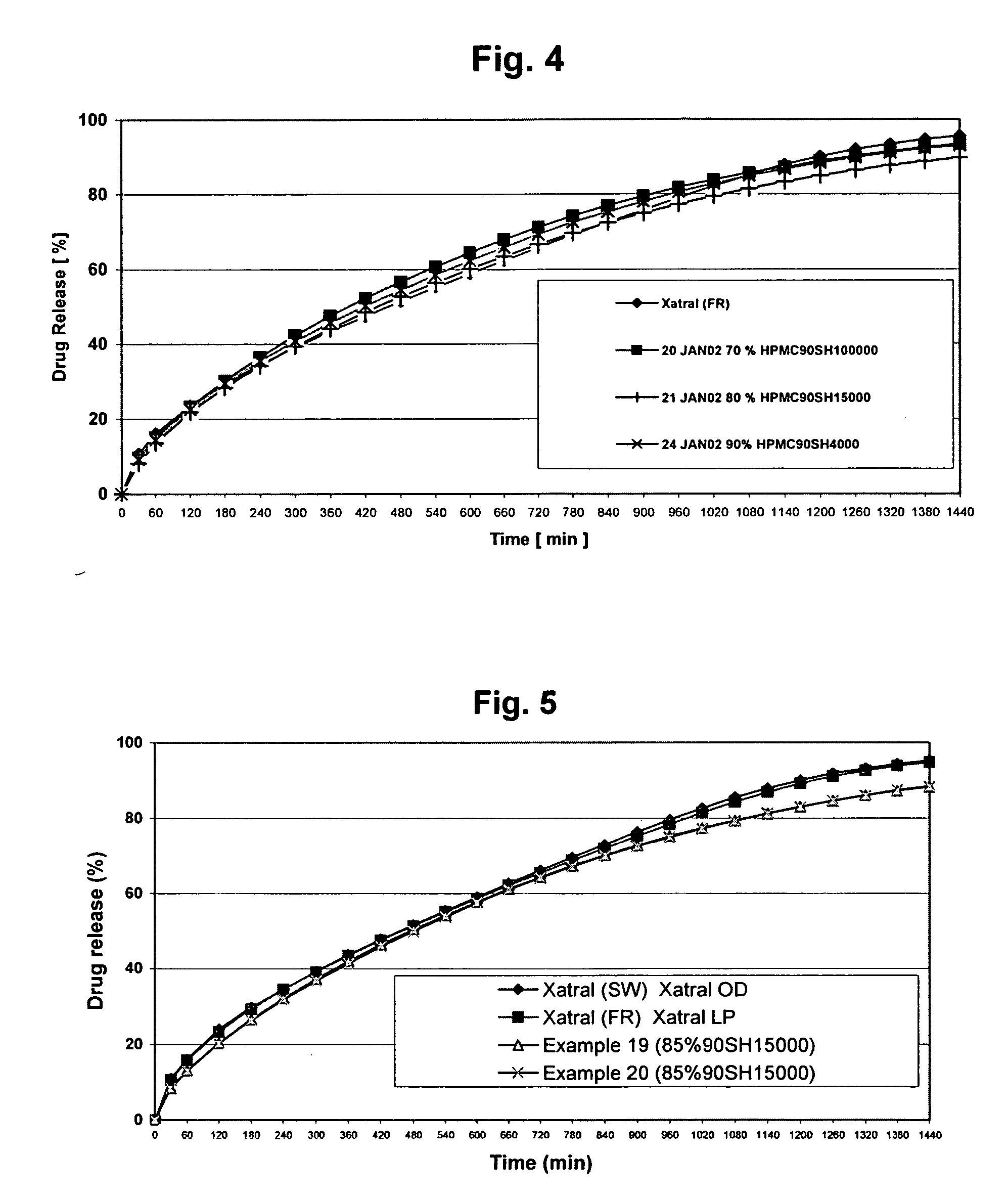
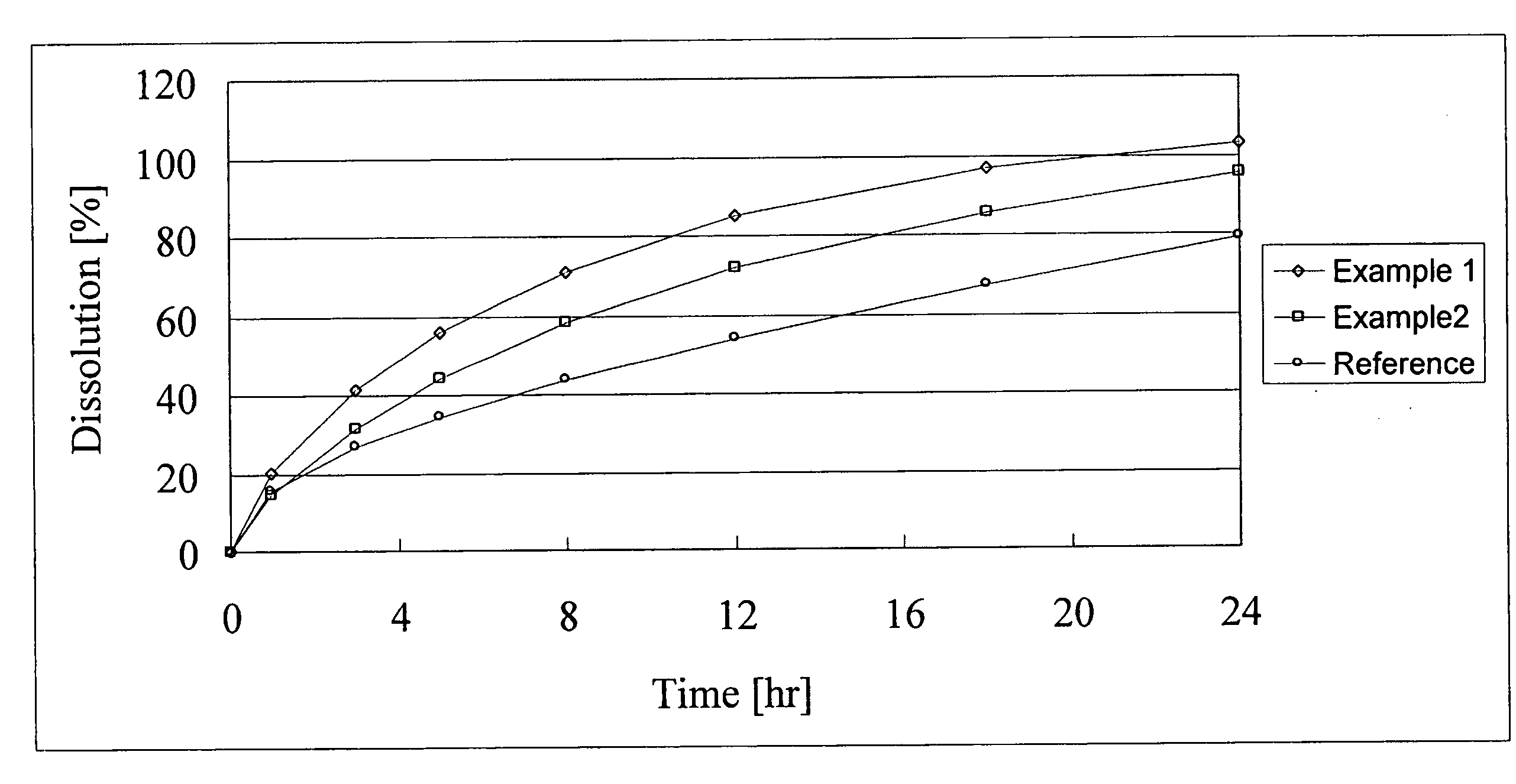
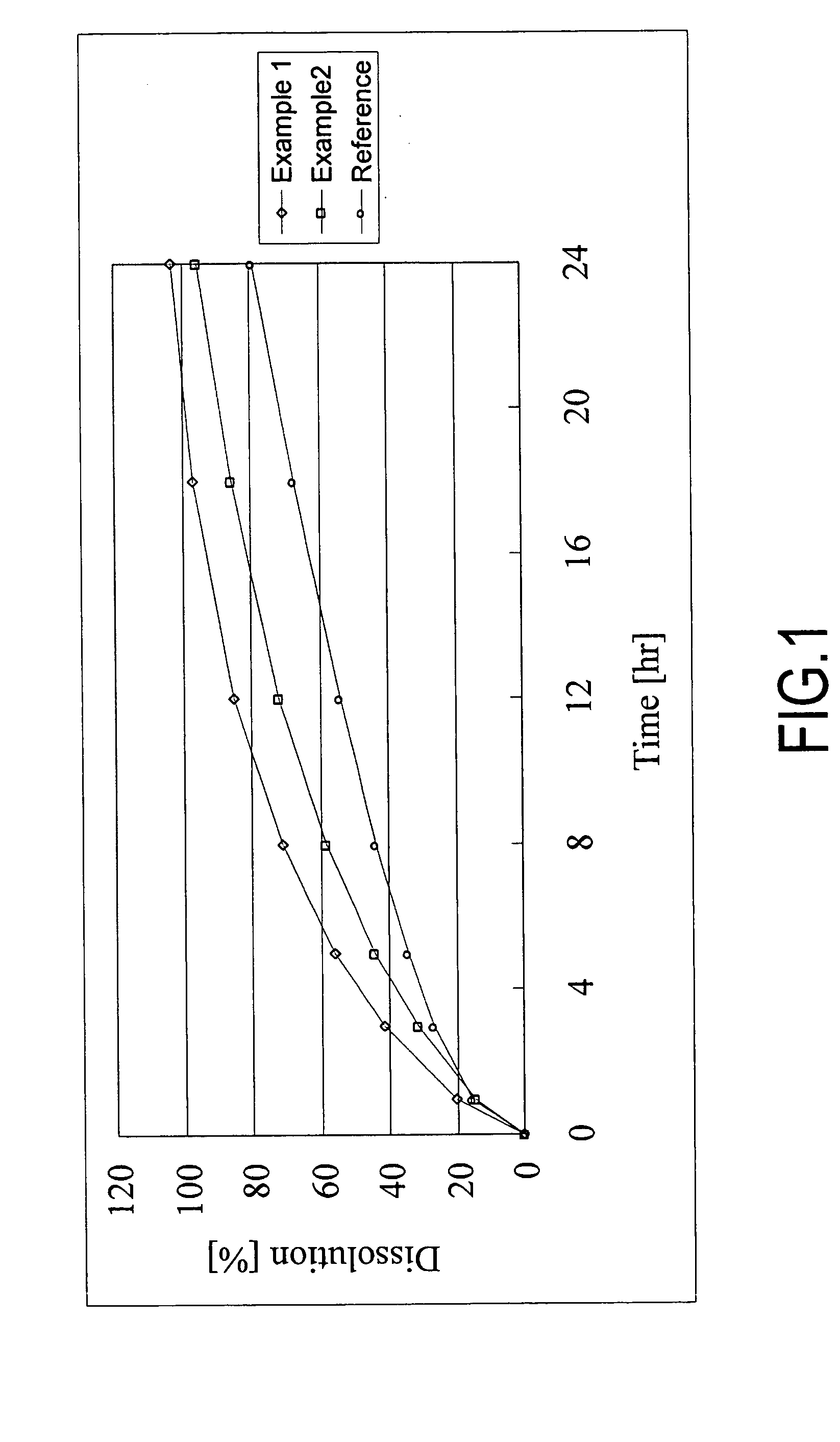
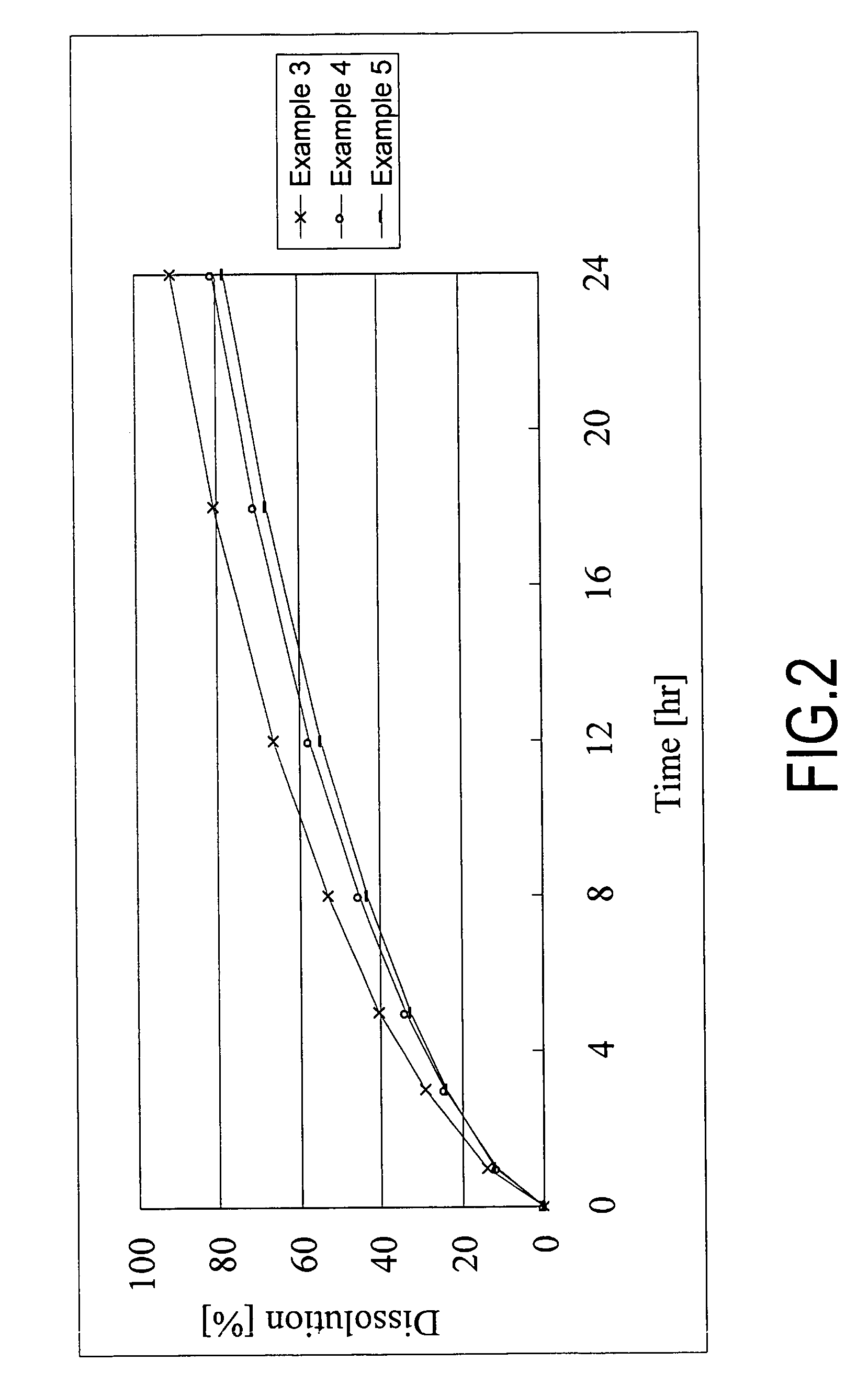
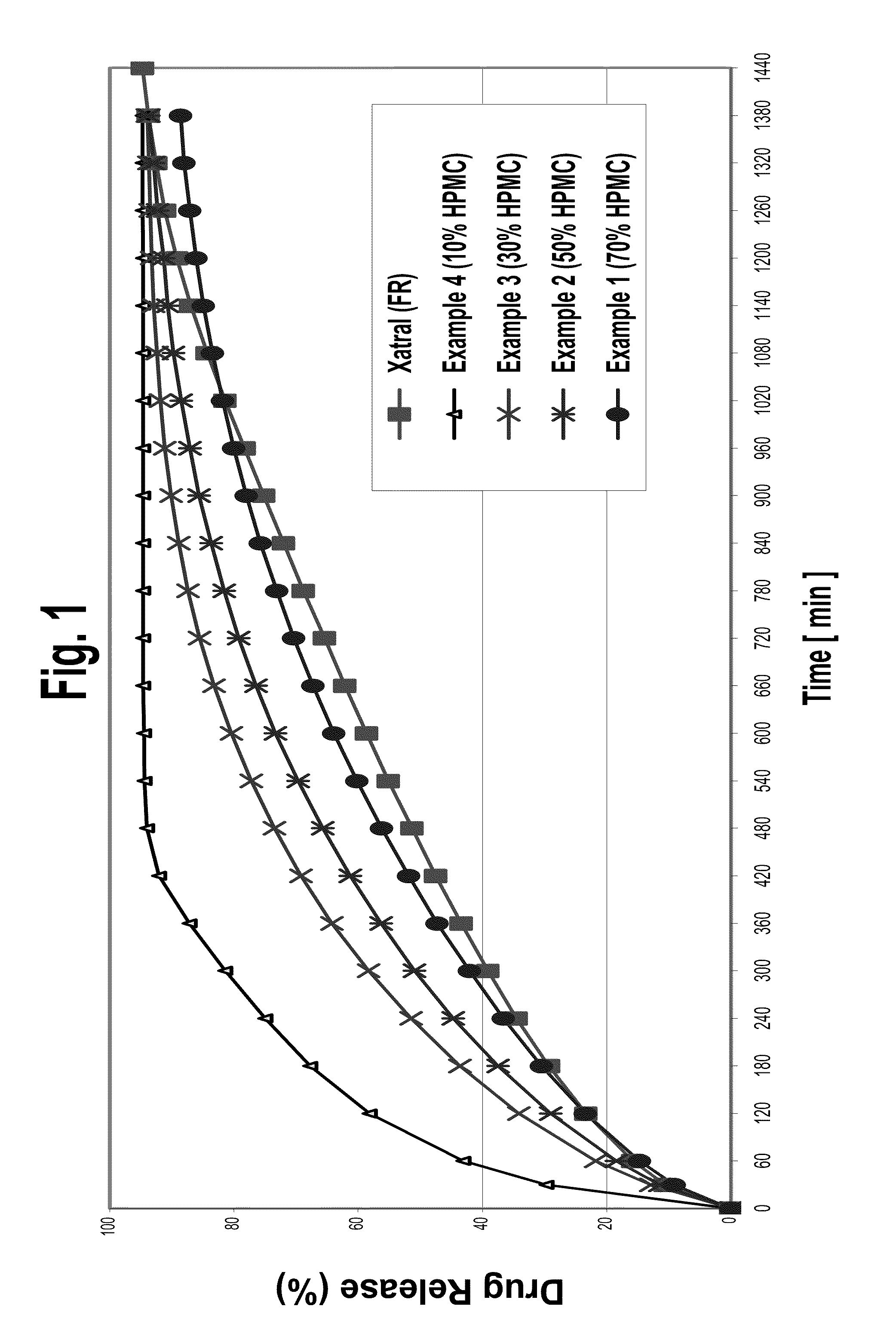
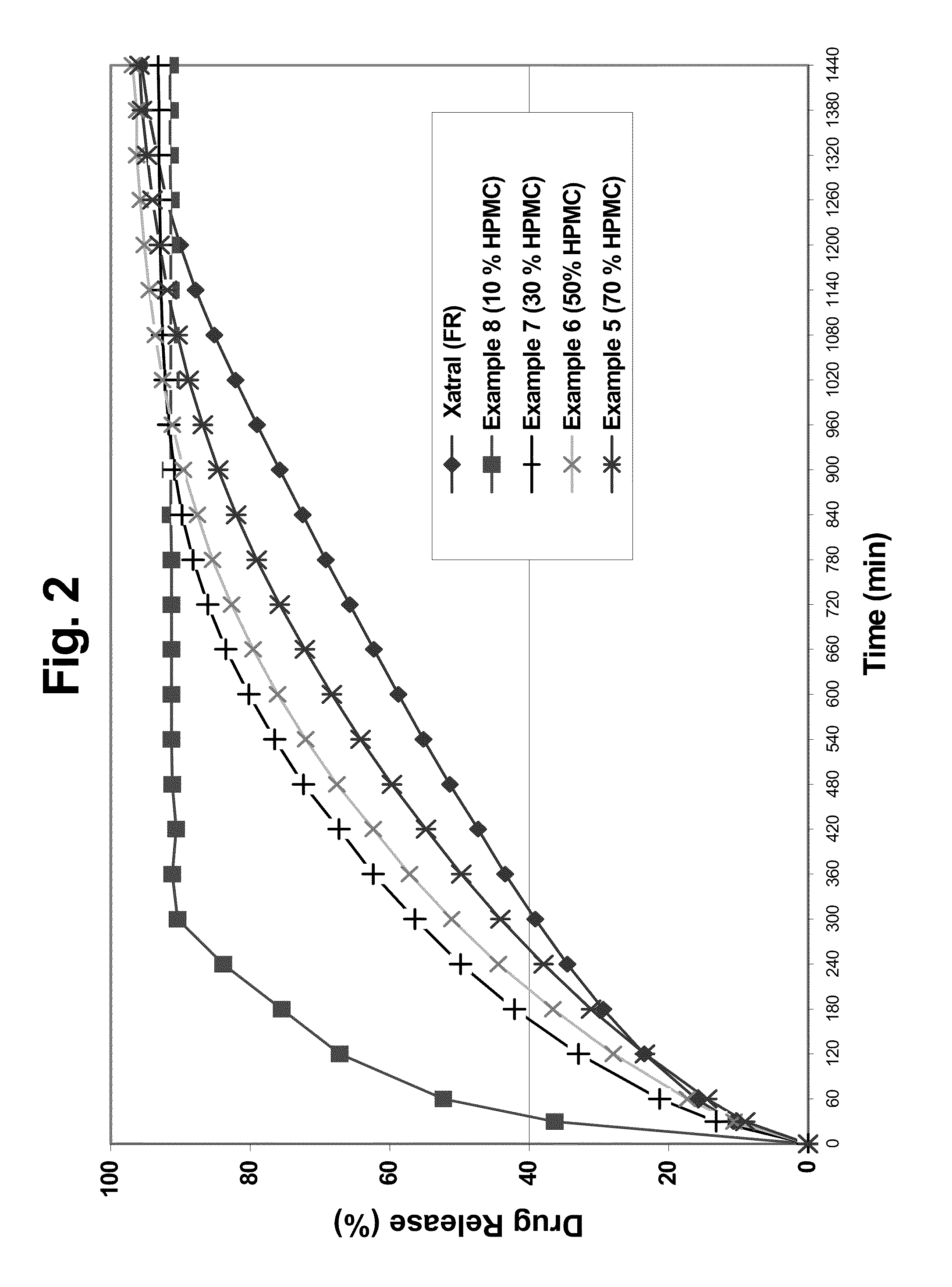
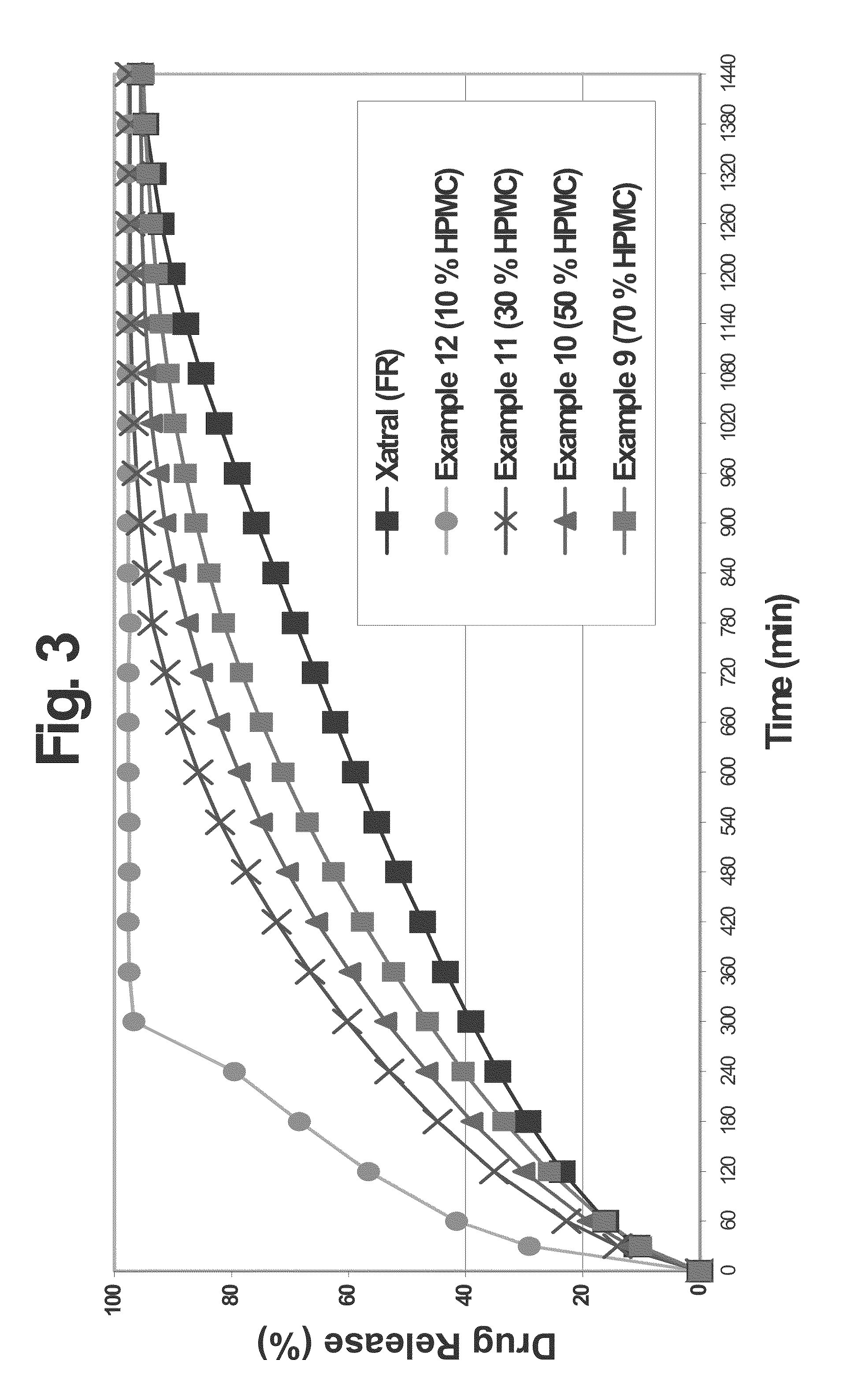
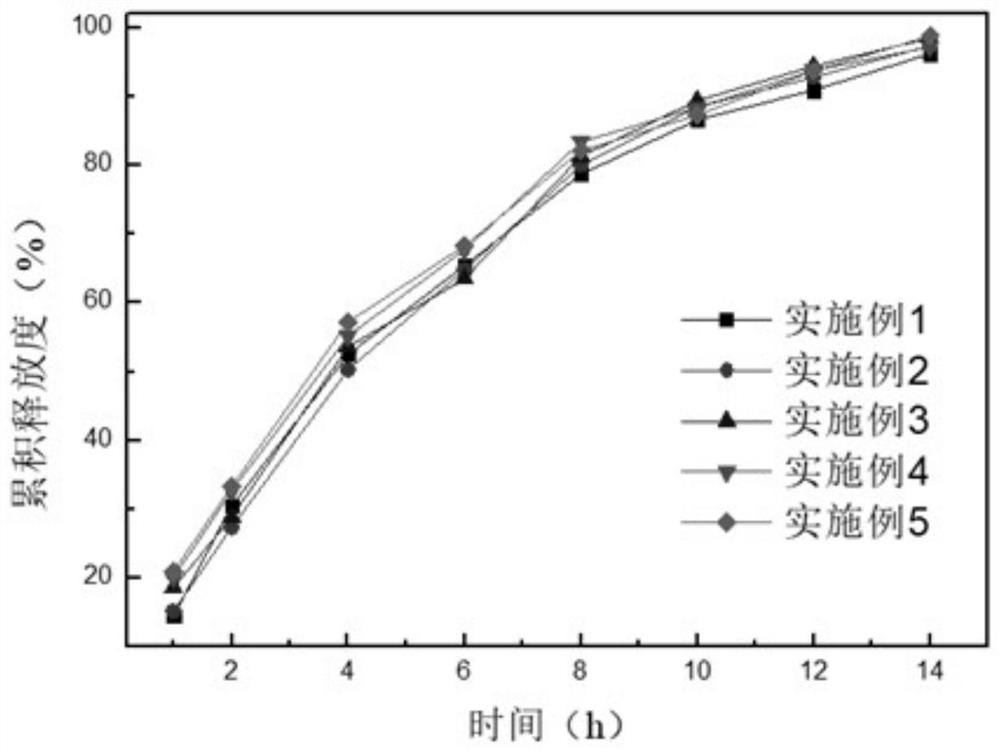
A monolithic composition includes alfuzosin in a polymeric matrix adapted to release 13-33% of the alfuzosin within 2 hours, 40-60% of the alfuzosin within 7 hours, and greater than 80% of the alfuzosin within 20 hours of administration. A unit dosage form includes: a heterogeneous mixture of alfuzosin hydrochloride anhydrate, lactose monohydrate, hydroxypropylmethylcellulose, polyvinylpyrrolidone and magnesium stearate, wherein the heterogeneous mixture is heterogeneously distributed throughout the unit dosage form. A manufacturing process includes: mixing a hydrophilic polymer and alfuzosin to provide a blend; granulating the blend to provide granules; drying the granules on a dryer to provide dried granules; sizing the dried granules to provide sized granules; mixing the sized granules with a lubricant to obtain a mixture; and compressing the mixture to obtain a tablet. A method of treating benign prostatic hyperplasia, includes administering to a patient the composition or unit dosage form once a day.
Owner:CIMEX PHARMA
Sustained release compositions containing alfuzosin
InactiveUS20060147530A1Organic active ingredientsPeptide/protein ingredientsAdditive ingredientEnantiomer
The present invention relates to pharmaceutical compositions of alfuzosin or pharmaceutically acceptable salt, solvate, enantiomers or mixtures thereof, that release the active ingredient over an extended period of time. The pharmaceutical composition can be a sustained release oral dosage form that includes a single functional layer and, optionally, one or more nonfunctional layers adjacent to the single functional layer. The single functional layer includes alfuzosin or pharmaceutically acceptable salt, solvate, enantiomers or mixtures thereof and one or more release retarding ingredients.
Owner:RANBAXY LAB LTD
Alfuzosin tablets and synthesis
A monolithic composition includes alfuzosin in a polymeric matrix adapted to release 13-33% of the alfuzosin within 2 hours, 40-60% of the alfuzosin within 7 hours, and greater than 80% of the alfuzosin within 20 hours of administration. A unit dosage form includes: a heterogeneous mixture of alfuzosin hydrochloride, lactose monohydrate, hydroxypropylmethylcellulose, polyvinylpyrrolidone and magnesium stearate, wherein the heterogeneous mixture is heterogeneously distributed throughout the unit dosage form. A manufacturing process includes: mixing a hydrophilic polymer and alfuzosin to provide a blend; granulating the blend to provide granules; drying the granules on a dryer to provide dried granules; sizing the dried granules to provide sized granules; mixing the sized granules with a lubricant to obtain a mixture; and compressing the mixture to obtain a tablet. A method of treating benign prostatic hyperplasia, includes administering to a patient the composition or unit dosage form once a day.
Owner:ACINO PHARMA
Use of alpha-adrenergic blockers for the treatment of dysmenorrhea
A method of treating primary dysmenorrhea which comprises administering to a human female suffering from the same a therapeutically effective amount of alpha-adrenergic blocker. Exemplary alpha-adrenergic blockers are phenoxybenzamine, alfuzosin, doxazosin, terazosin, prazosin, and tamsulosin, or a pharmaceutically acceptable salt or ester thereof. Tamsulosin HCl is preferred.
Owner:BOEHRINGER INGELHEIM PHARMA INC
Application of alfuzosin compound in prevention or treatment of Alzheimer's disease and related diseases
PendingCN111035642AImprove mitochondrial metabolismFunction increaseOrganic active ingredientsNervous disorderHuntingtons choreaAmytrophic lateral sclerosis
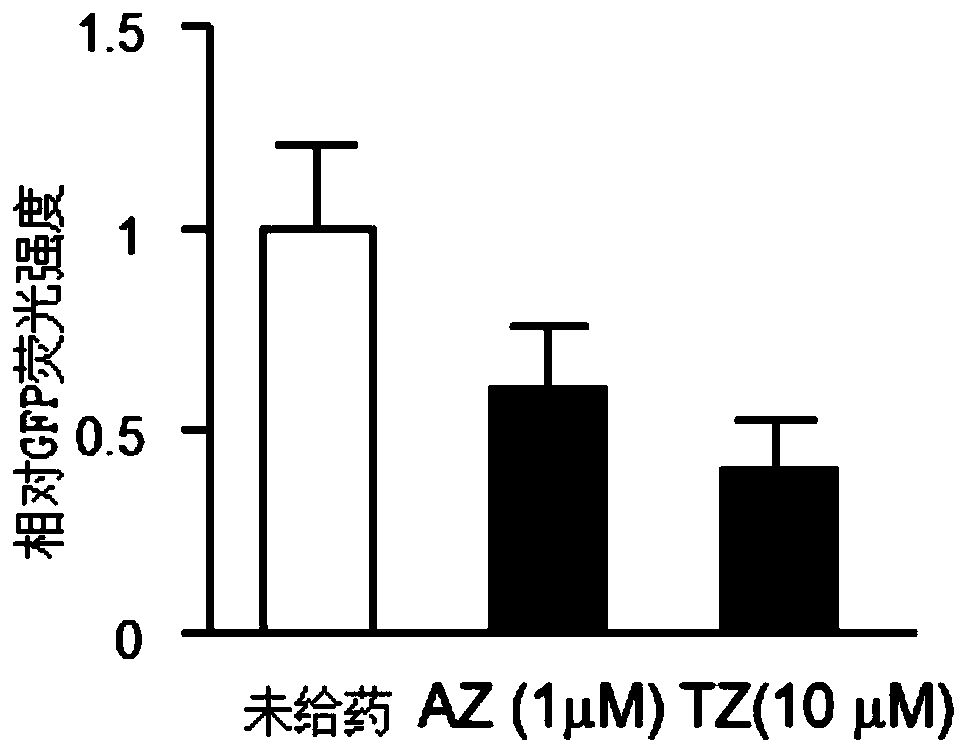
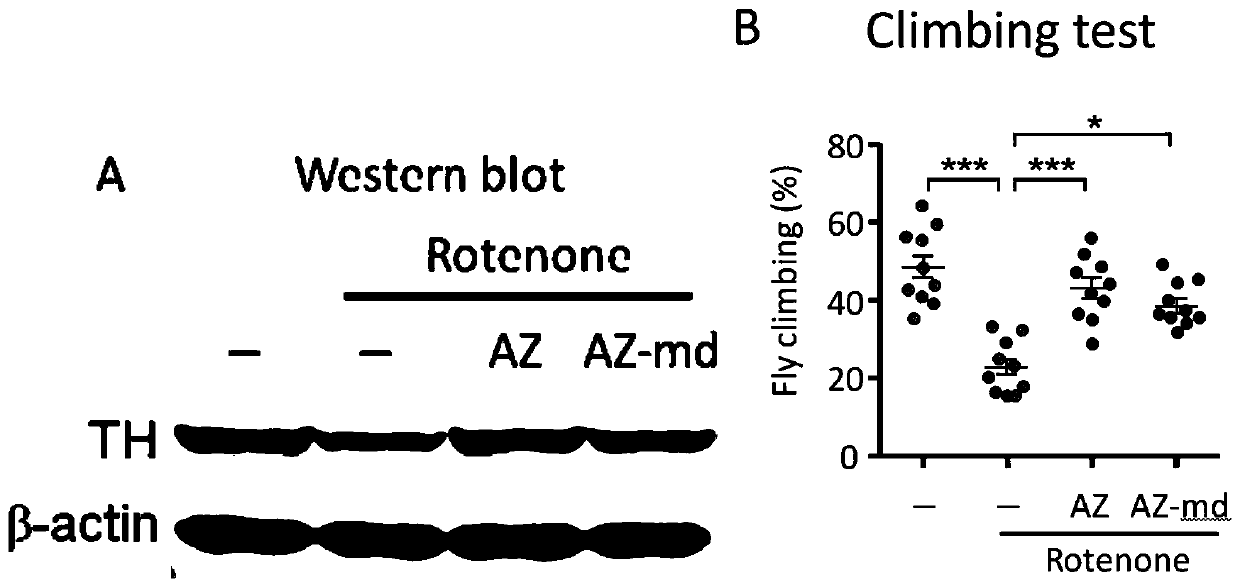
The invention relates to an application of an alfuzosin compound in prevention or treatment of Alzheimer's disease and related diseases, and belongs to the technical field of medicines. The alfuzosincompound has the effects of improving mitochondrial metabolism, degrading accumulation of various pathological proteins and improving vascular endothelial cell functions. The invention provides a newpharmaceutical application of the alfuzosin compound. The new pharmaceutical application can provide a new choice for treatment of clinical related diseases. Alfuzosin, an alfuzosin isomers, TZ-md andAZ-md can be beneficially applied to Alzheimer's disease and related complications, such as seeing and hearing impairment, anemia, blood pressure instability, insomnia, depression, limb dysfunction,malnutrition, weakness, dizziness, gait instability and the like, and diseases related to protein accumulation and metabolic disorder, such as Huntington's disease, amyotrophic lateral sclerosis, dementia with Lewis bodies, multi-system atrophy and the like.
Owner:北京安塞迩生物科技有限公司
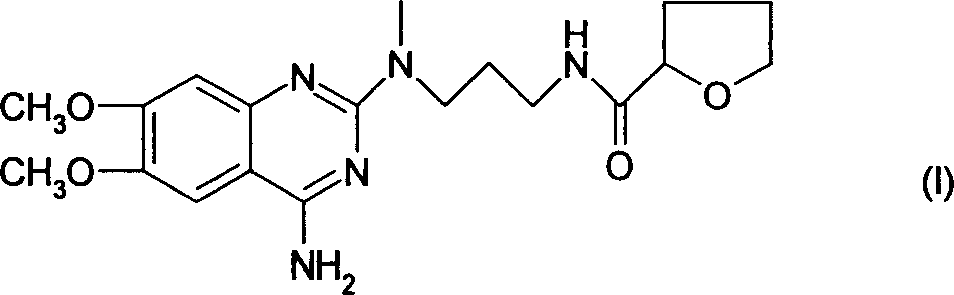
Process for preparing alfuzosin hydrochloride
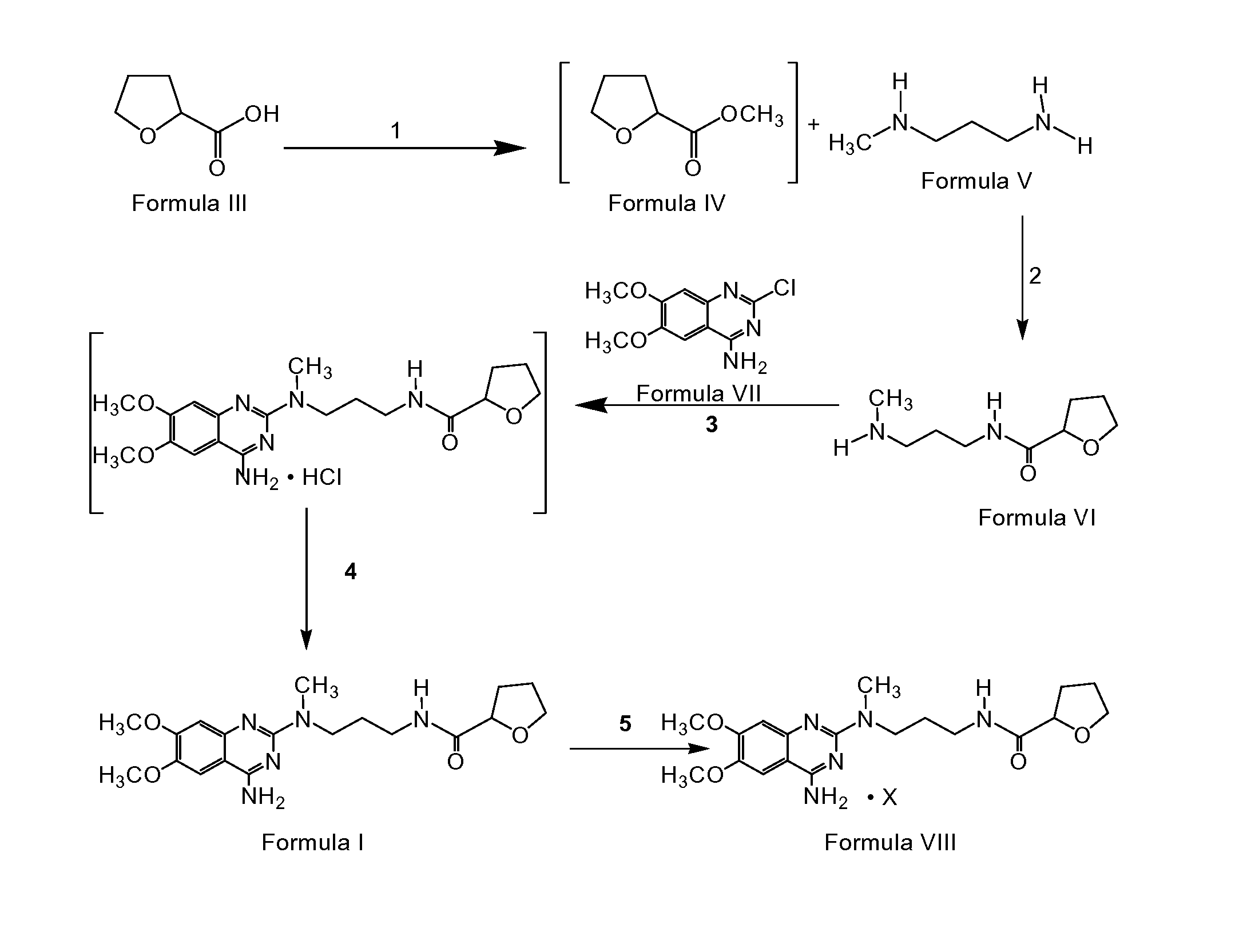
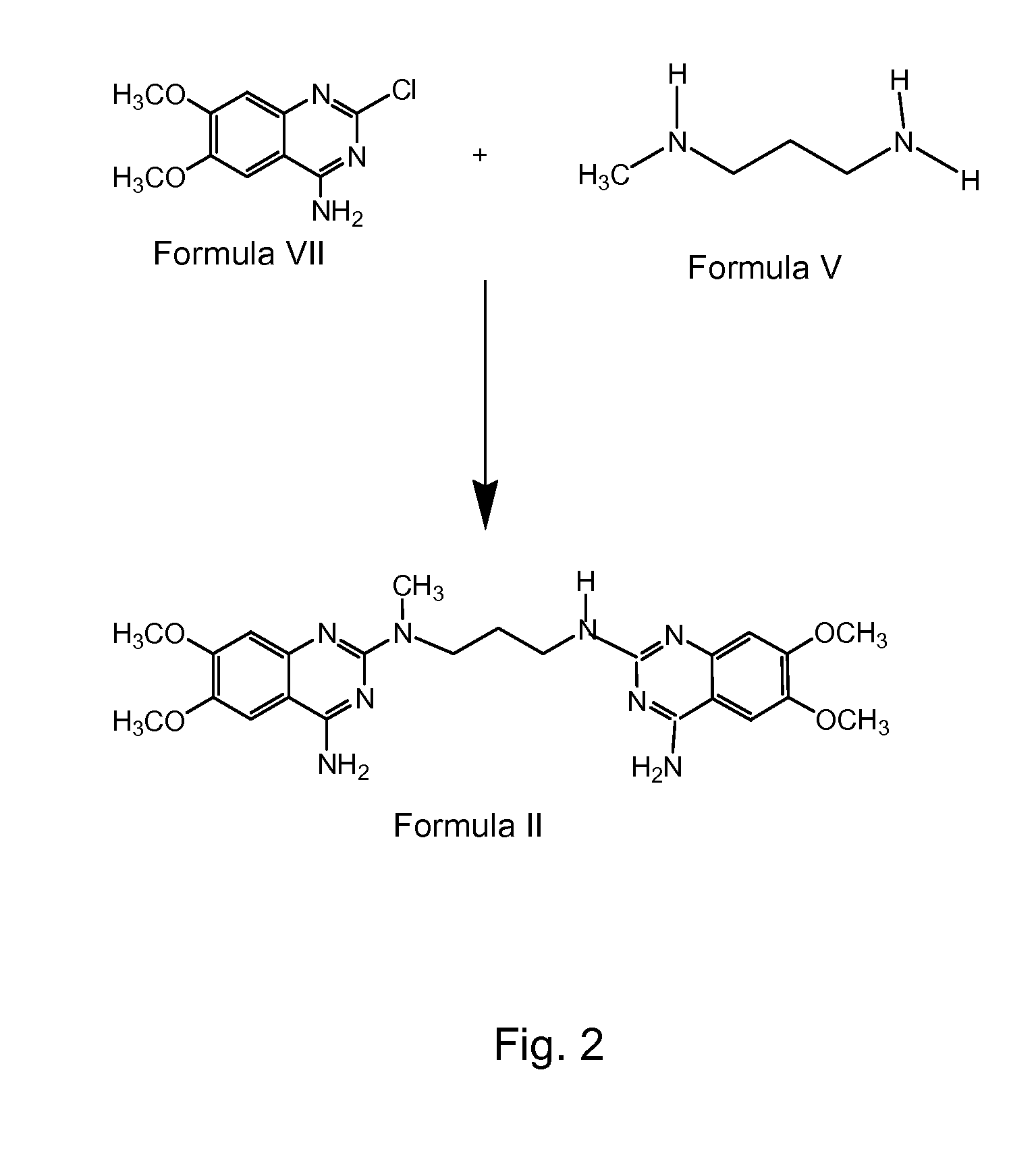
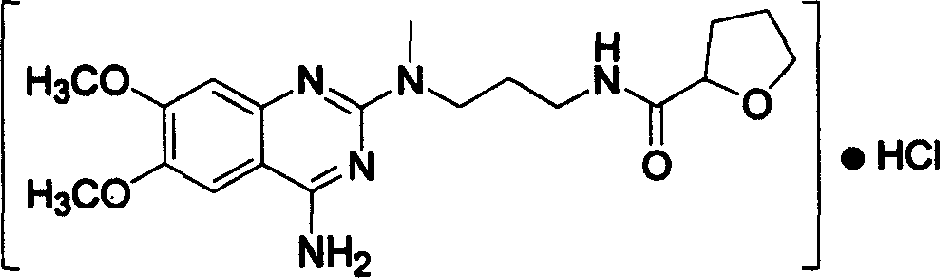
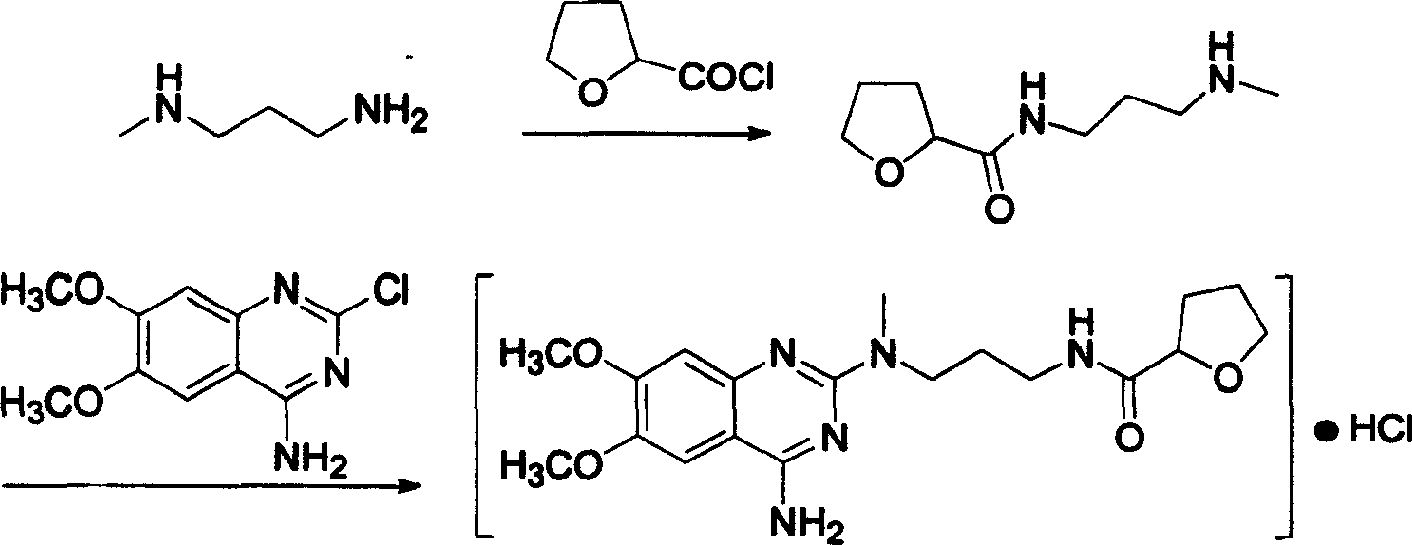
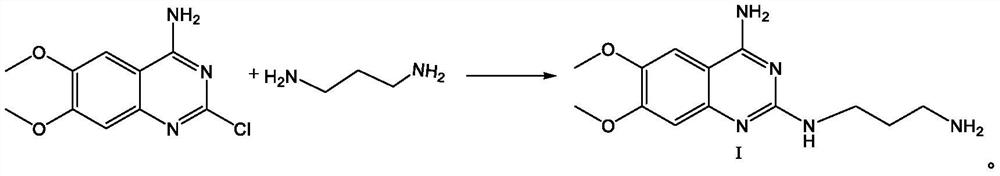
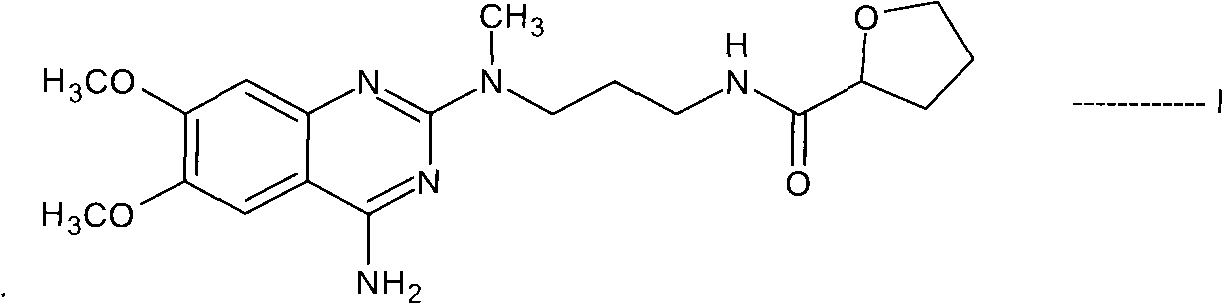
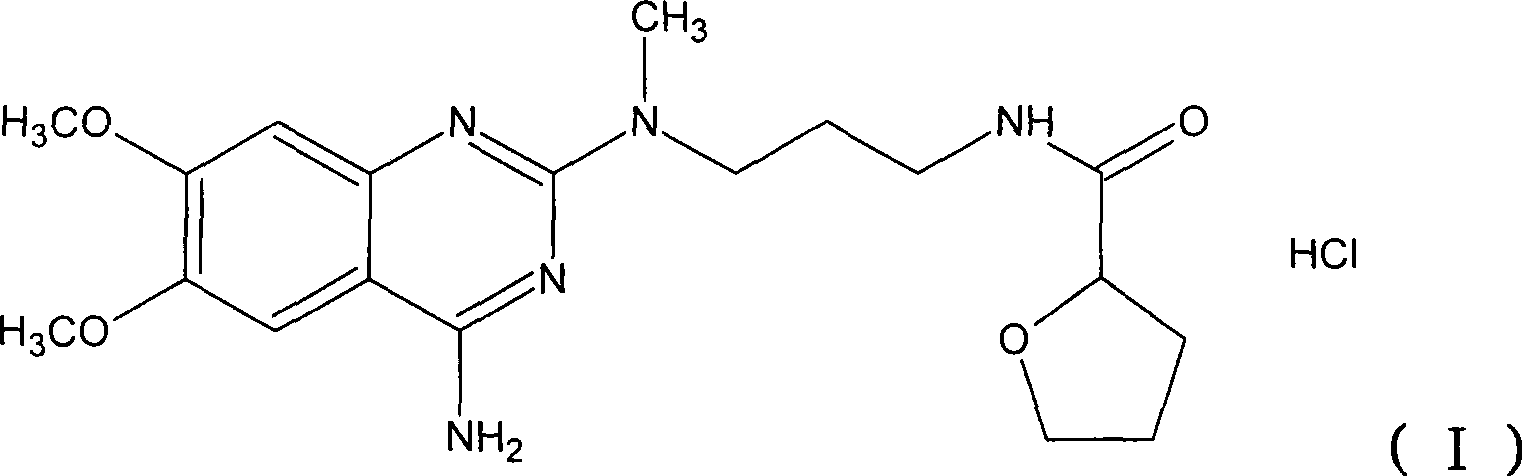
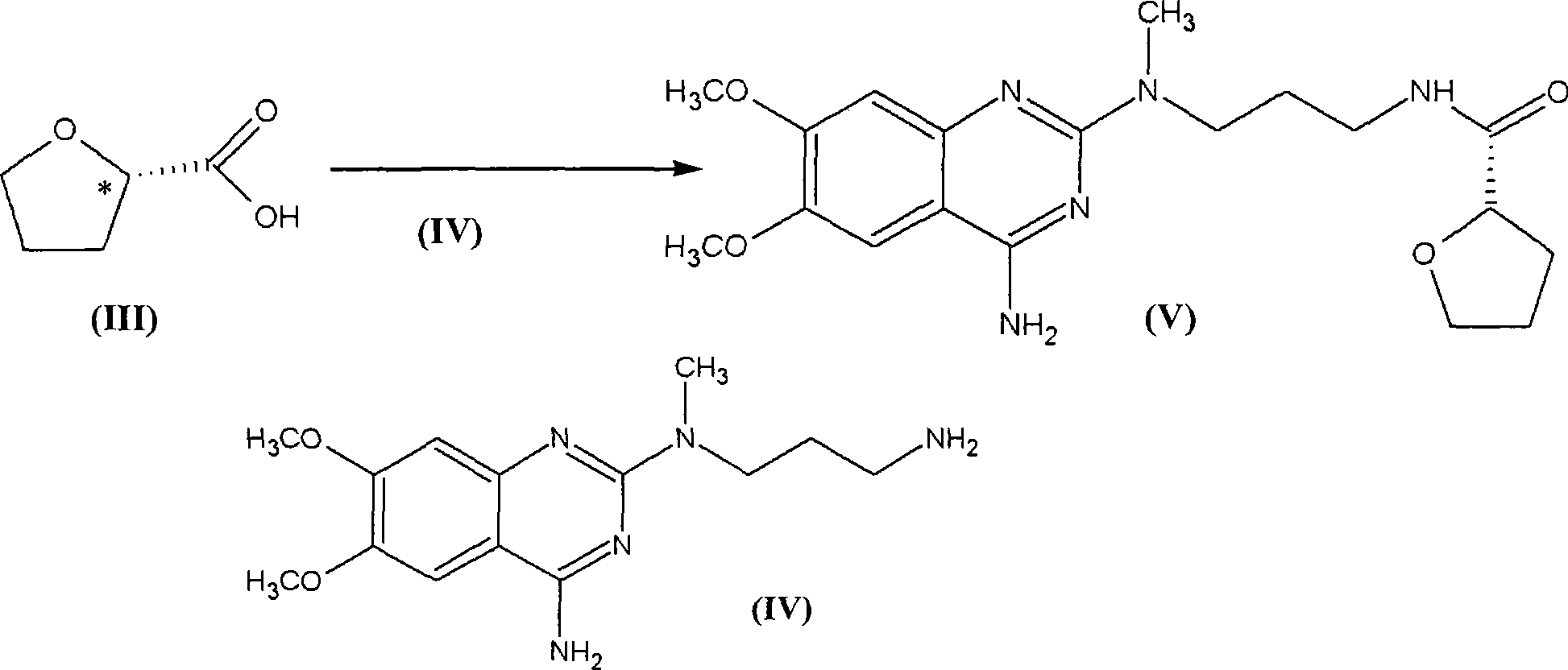
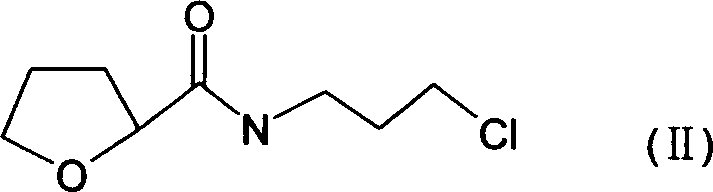
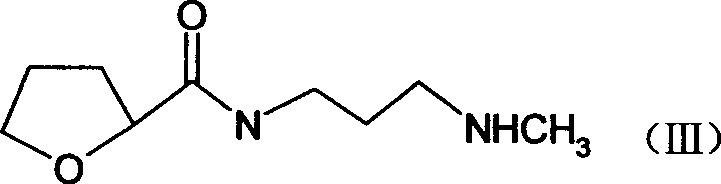
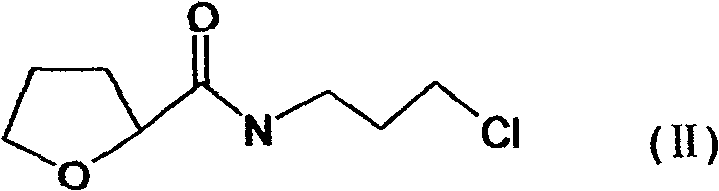
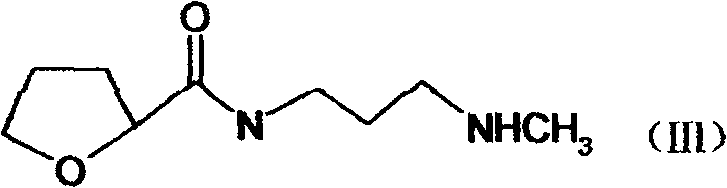
The present invention discloses the preparation process of alfuzosin hydrochloride. N-methyl propylenediamine as material is condensed with 2-tetrahydrofuran formyl chloride or 2-tetrahydrofuran formic acid to obtain condensate; the condensate without being purified is then condensed with 2-chloro-4-amino-6, 7-dimethoxy quinazoline to obtain alfuzosin; and alfuzosin is finally reacted with hydrochloric acid to form alfuzosin hydrochloride. The said process has simple operation, low cost, high yield and easy-to-realize reaction conditions, and is suitable for industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
Sustained release alfuzosin hydrochl formulation and method for their production
A sustained release alfuzosin hydrochloride formulation contains alfuzosin hydrochloride as about 1% to about 5% by weight of the formulation, hydrophilic polymers as about 35% to about 75% by weight of the formulation, hydrophobic polymers as about 10% to about 30% by weight of the formulation, disintegrating agents as 10% to 30% by weight of the formulation and a binder as about 2% to about 12% by weight of the formulation. The hydrophilic and hydrophobic polymers are used as the release-modulating agent to control the dissolution profile of the alfuzosin hydrochloride formulation so that the formulation releases alfuzosin hydrochloride slowly and continuously as the formulation passed through the gastrointestinal tract. The present invention also relates to a method for preparing the above formulation.
Owner:STANDARD CHEM & PHARMA
Application of Alfuzosin to treating or preventing Parkinson's disease and related diseases
InactiveCN110354131APharmaceutically activeOrganic active ingredientsNervous disorderDiseaseAlfuzosin
The invention relates to application of Alfuzosin to treating or preventing the Parkinson's disease and related diseases. The invention belongs to the technical field of medicines, and relates to theapplication of Alfuzosin newly-prepared medicines, in particular to application of the Alfuzosin to treating or preventing the Parkinson's disease and related diseases.
Owner:北京安塞迩生物科技有限公司
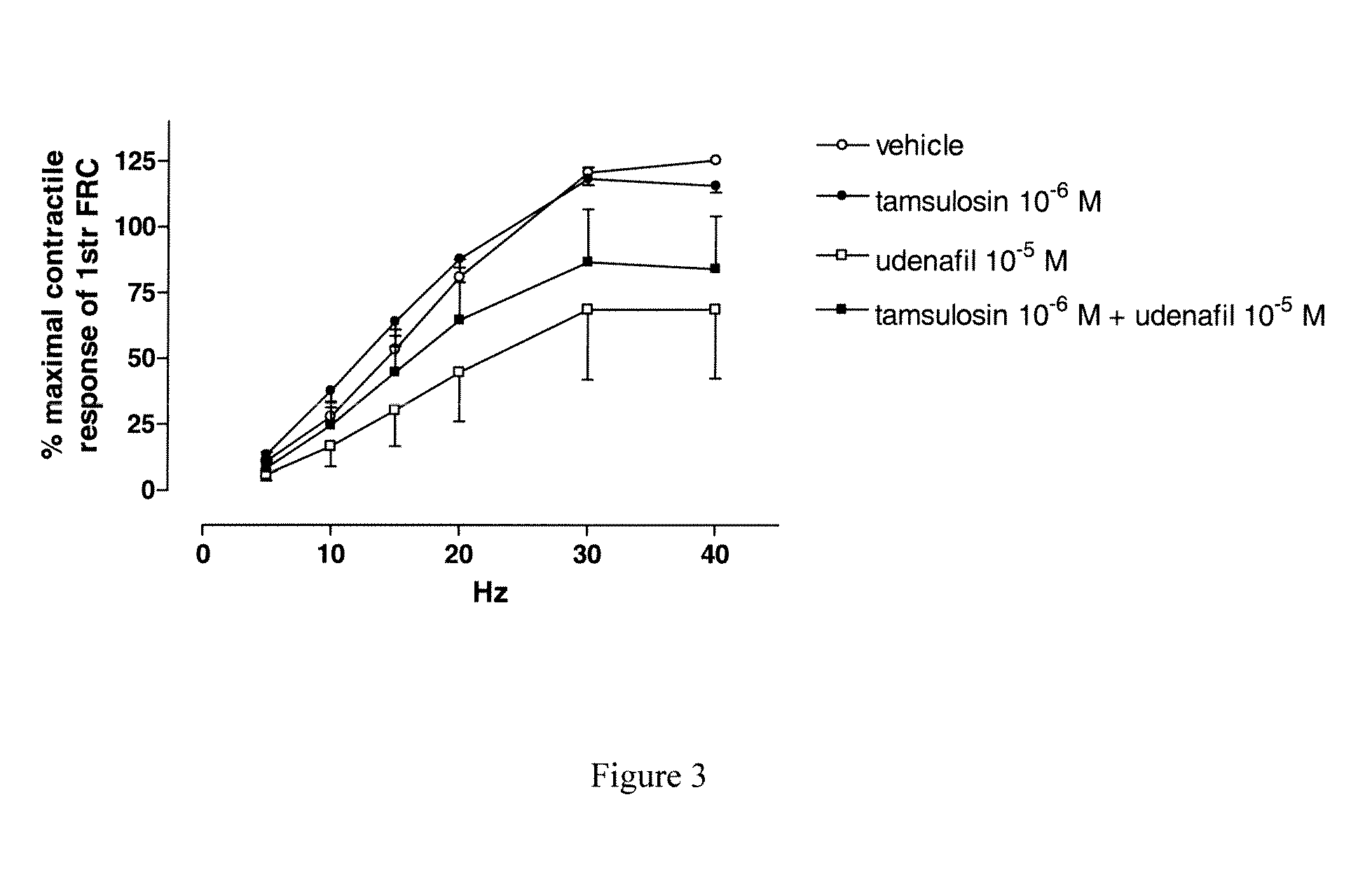
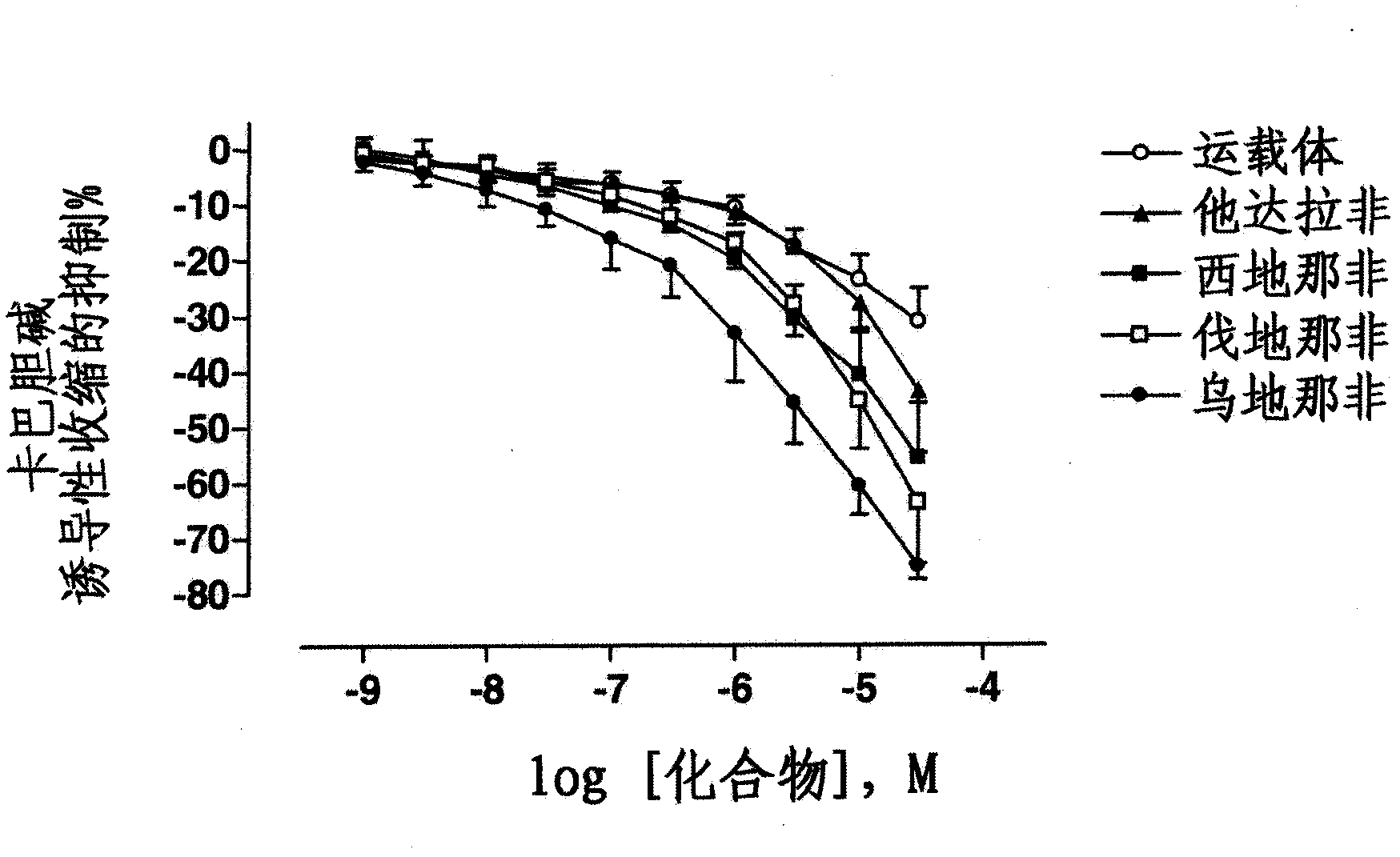
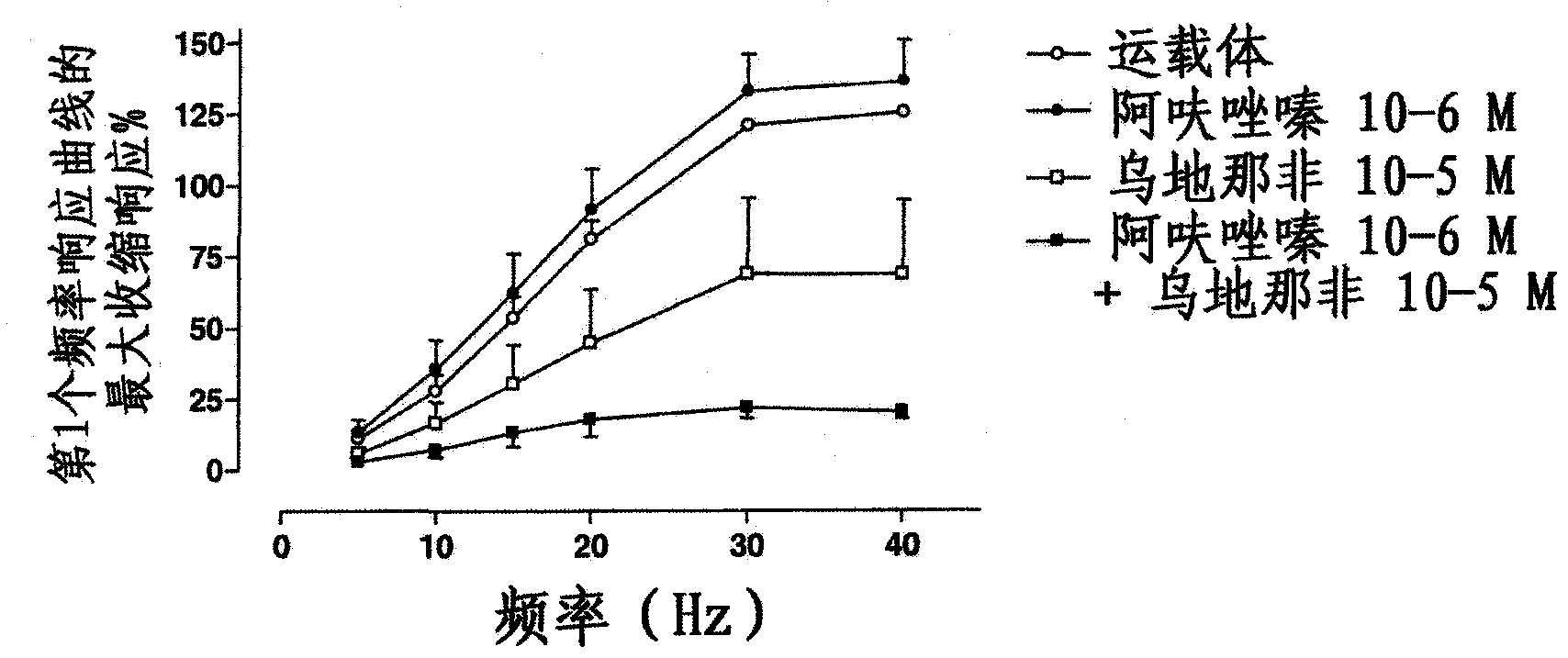
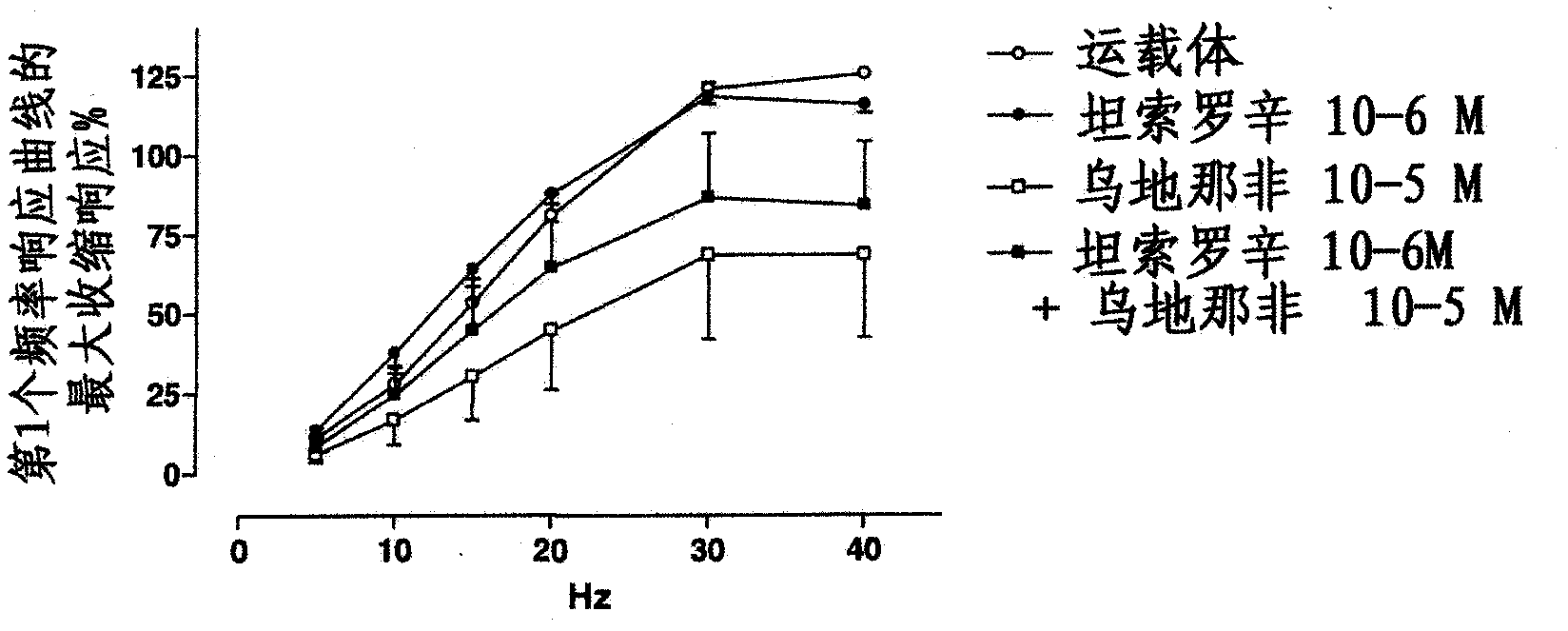
Use of a Combination of Udenafil and Alfuzosin or Oxybutynin for the Treatment of Overactive Bladder
The invention relates to a specific combination of two active agents: udenafil and one of alfuzosin and oxybutynin and its use for the treatment of overactive bladder.
Owner:PELVIPHARM
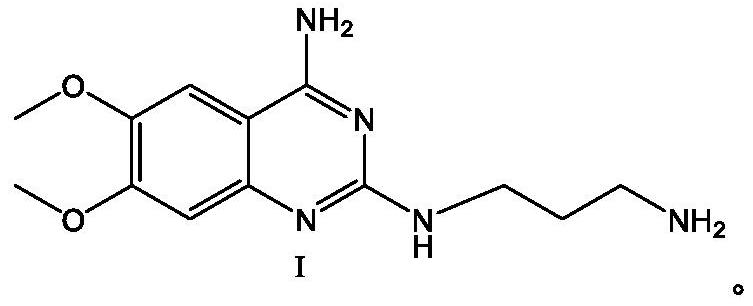
Alfuzosin hydrochloride intermediate compound
PendingCN113801069ASimple and fast operationOperational securityOrganic chemistryPropionitrileCombinatorial chemistry
The invention belongs to the technical field of medicine synthesis, and particularly relates to an alfuzosin hydrochloride intermediate compound I and a preparation method thereof. 3-methylaminopropionitrile commonly used in the alfuzosin hydrochloride synthesis process is replaced with 1, 3-propane diamine. As the activities of two amino groups are consistent, the selectivity problem does not exist, and side reactions are few. Meanwhile, a high-temperature and high-pressure cyano reduction reaction is avoided. A method for preparing alfuzosin hydrochloride by using the intermediate is simple and convenient to operate, low in cost, high in yield and purity and suitable for large-scale industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
Alfuzosin hydrochloride sustained release tablets and preparation method thereof
InactiveCN105287422AReduce absorption rateSmall toxicityOrganic active ingredientsPharmaceutical delivery mechanismAlfuzosinBlood drug concentration
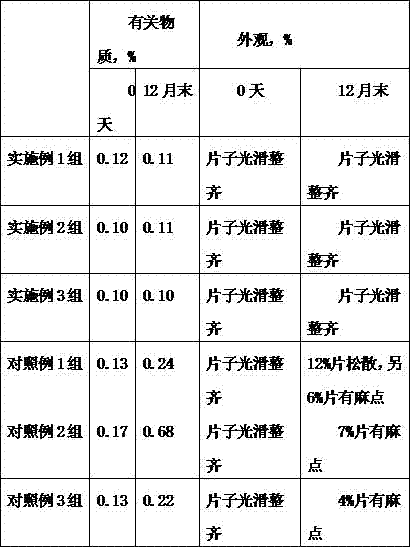
The invention discloses alfuzosin hydrochloride sustained release tablets and a preparation method thereof. The sustained release tablets are prepared by adopting the following raw materials: alfuzosin hydrochloride, hydroxypropyl methylcellulose and lubricating agents, and adopting the processing steps of material preparation, mixing, granulation, total blending, tabletting, aluminium-plastic packaging, and the like. The alfuzosin hydrochloride sustained release tablets have the beneficial effects that the alfuzosin hydrochloride sustained release tablets are mainly used for treating functional symptoms of benign prostatic hyperplasia; the more novel sustained release preparation is adopted; sustained release refers to that the rate of absorption of the medicines into bodies is reduced by reducing the rate of release of the medicines from the dosage form, thus achieving the more stable treatment effects; the effective blood concentration can be maintained in a longer time, the toxic and side effects of the medicines can be also reduced and the medicine safety is improved; the alfuzosin hydrochloride sustained release tablets are convenient to use, are especially suitable for chronic disease patients who take medicines for a long term, and have the effect of improving the compliance of the patients; alfuzosin hydrochloride sustained release tablets have the advantages of good medicine stability, convenience in packaging, transportation and storage, and the like; and the preparation method is simple and practicable and is suitable for industrial production.
Owner:HEILONGJIANG ZHICHENG MEDICAL TECH
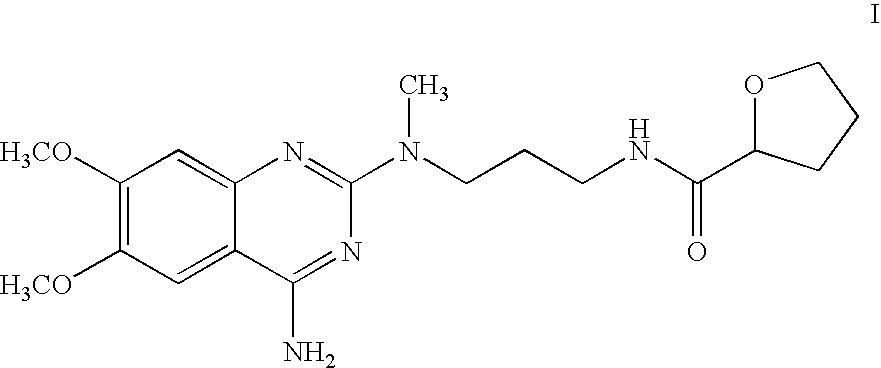
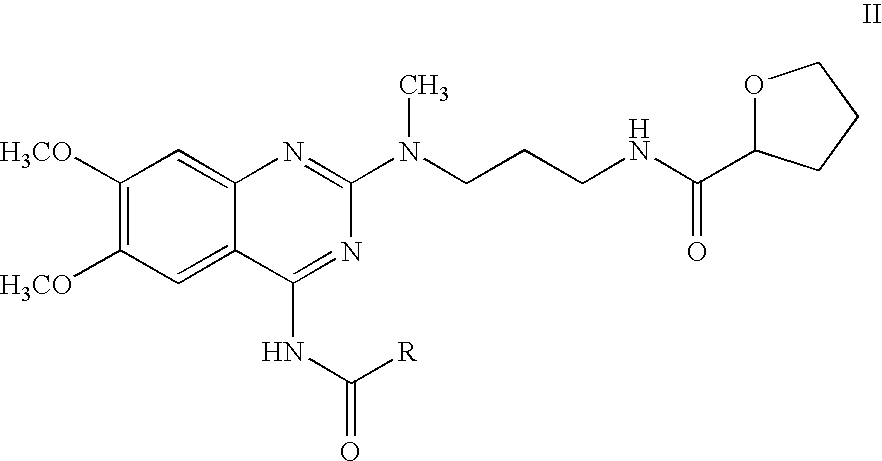
Process for the preparation of alfuzosin and salts thereof
InactiveCN101687859ASimple preparation processEfficient manufacturing processOrganic chemistryAlfuzosinStereochemistry
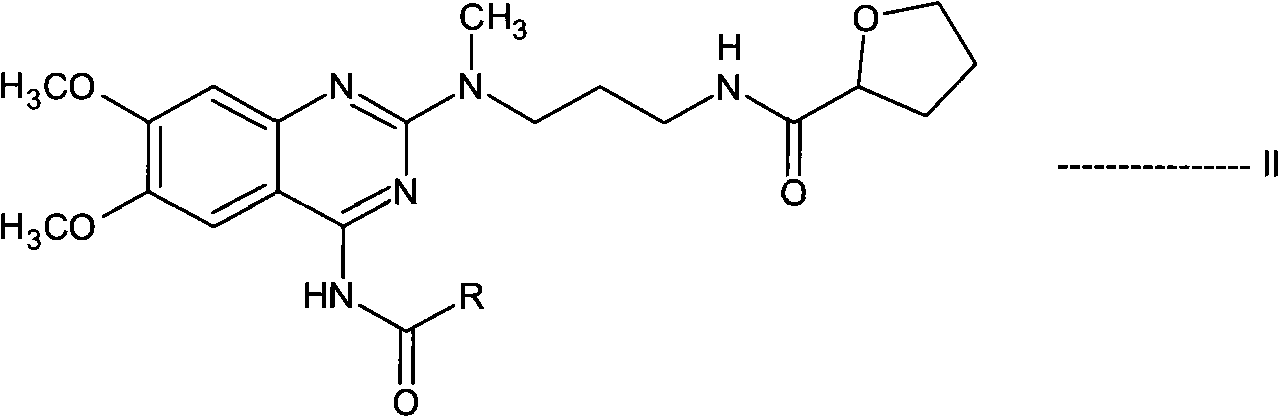
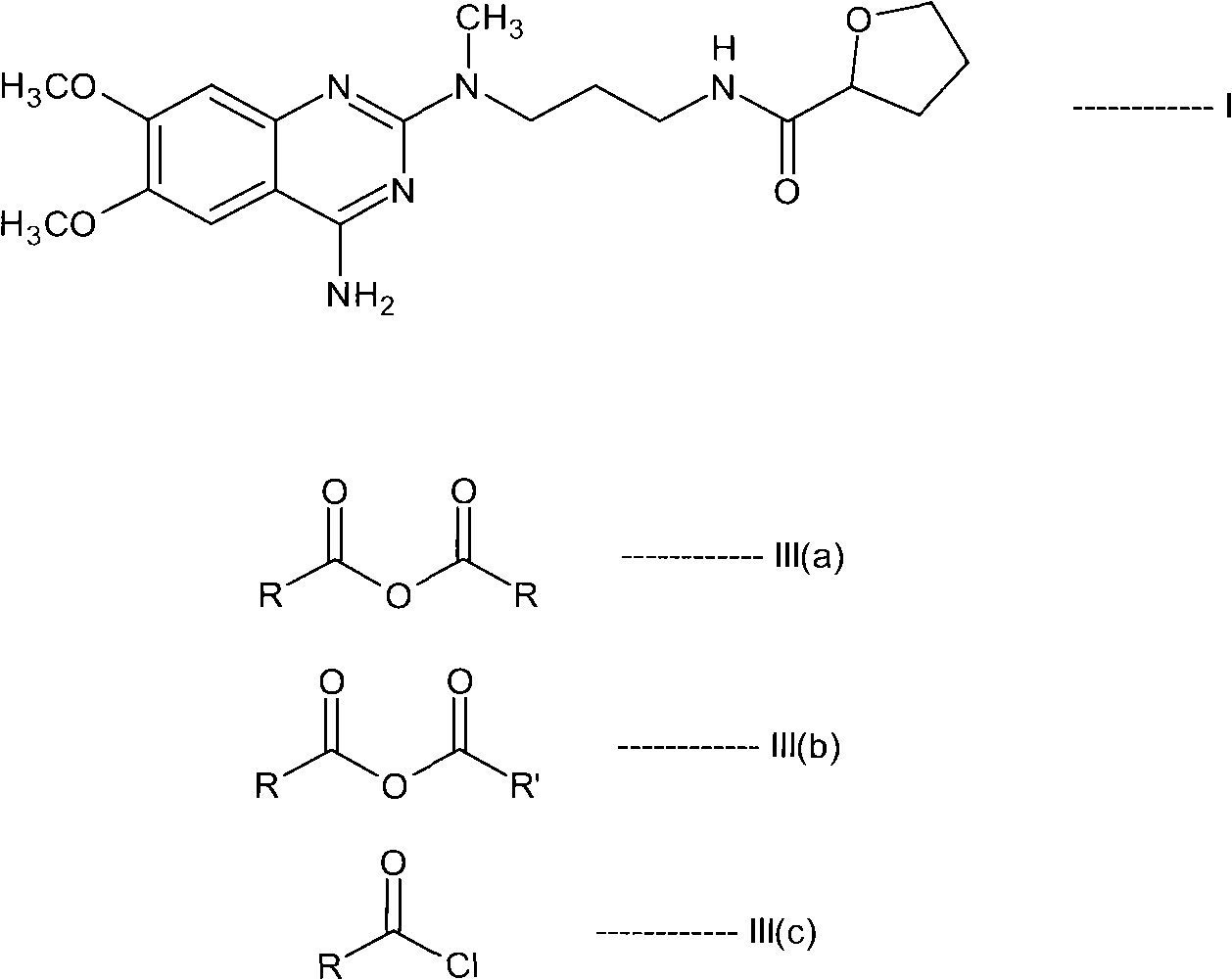
The present invention relates to novel N-[3-[(4-acyl- / aroyl-substituted amino-6,7-dimethoxy-2-quinazolinyl)methylamino]propyl]tetrahydro-2-furancarboxamide derivatives, and a process for the preparation thereof. The novel compounds are useful for preparing alfuzosin or a pharmaceutically acceptable salt thereof in high yield and purity.
Owner:ACTAVIS GRP PTC EHF
Alfuzosin dripping pills and its preparation
InactiveCN1546031ADisintegration and dissolution fastQuality improvementOrganic active ingredientsPill deliveryAlfuzosinBiomedical engineering
The invention relates to an Alfuzosin drop pill prepared by utilizing ultramicro disintegration and drop pill manufacturing process, which has the advantages of improving collapse and dissolving speed, quick effect, increased medicament stability, reduced adjuvant consumption, lowered production costs, and easiness in carrying and use. It has good compliance, thus is especially suitable for children, the elderly, bedridden patients and dysphagia patients.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Sustained release pharmaceutical compositions of alfuzosin and process for preparation thereof
InactiveUS20080095844A1Organic active ingredientsBiocideCombinatorial chemistryPharmaceutical medicine
Owner:TORRENT PHARMA LTD
Medicinal composition for treating benign prostatic hyperplasia
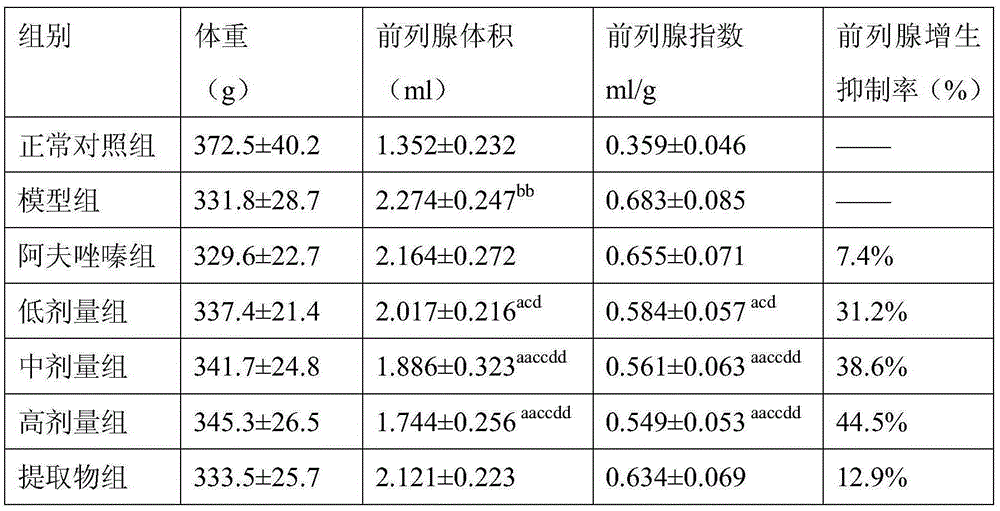
InactiveCN103989753AInhibit benign prostatic hyperplasiaGood synergyOrganic active ingredientsUrinary disorderAlcoholTreatment effect
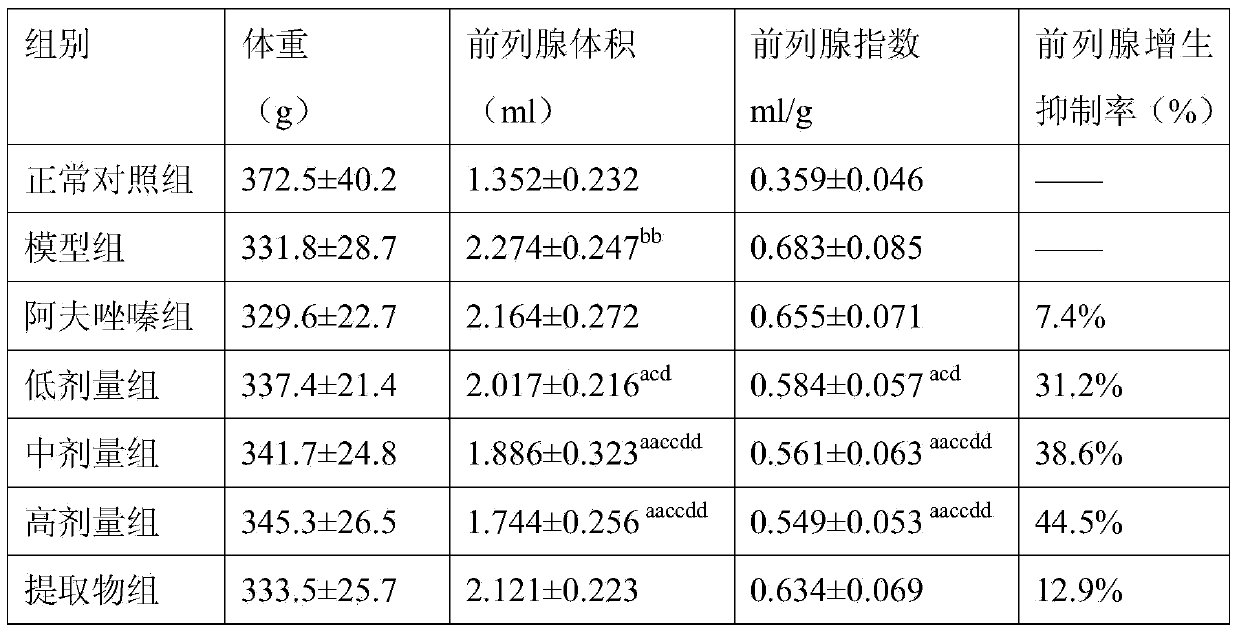
The invention relates to a medicinal composition for treating benign prostatic hyperplasia. The medicinal can be tablets, capsules, granules and the like prepared from alfuzosin or pharmaceutically acceptable salt thereof with effective dosage and plantain seed alcohol extract in a mass ratio of 1:(1-10000) by the amount of alfuzosin. Pharmacological researches on the medicinal composition discover that the medicinal composition for treating benign prostatic hyperplasia has perfect treatment effect, and can remarkably inhibit benign prostatic hyperplasia of a rat subjected to orchiectomy. The alfuzosin or pharmaceutically acceptable salt and the plantain seed alcohol extract have a synergetic effect, and achieve an unexpected technical effect.
Owner:重庆三峡云海药业股份有限公司
Levalfuzosin, preparation method and use thereof
The invention relates to optical-active levo-alfuzosin ((-) alfuzosin) or the salt thereof and the medicines of the levo-alfuzosin or the salt thereof containing effective curing quantity and the medical carrier and used for treating the benign prostatic hyperplasia. The invention also relates to the optical-active (plus) alfuzosin or the salt thereof and the application of the (plus) alfuzosin or the salt thereof for treating the hypertension.
Owner:RENHE YIKANG MEDICINE TECH TIANJIN
Alfuzosin tablet composition
InactiveCN107998090AGood content uniformityImprove moisture resistanceOrganic active ingredientsUrinary disorderCarboxymethyl starchCITRATE ESTER
The invention relates to an alfuzosin tablet composition, and belongs to the technical field of pharmacy. The alfuzosin tablet composition is characterized in that the alfuzosin tablet composition ofa unit dose is prepared from 2.5 mg of alfuzosin, 20 to 40mg of lactose, 8 to 16mg of sodium carboxymethyl starch, 30 to 50mg of microcrystalline cellulose, 12 to 20mg of calcium citrate, 3 to 8mg ofpolyethylene glycol 4000 and 1.2 to 1.7mg of magnesium stearate. The invention provides the stable alfuzosin tablet composition.
Owner:WEIHAI GUANBIAO INFORMATION TECH
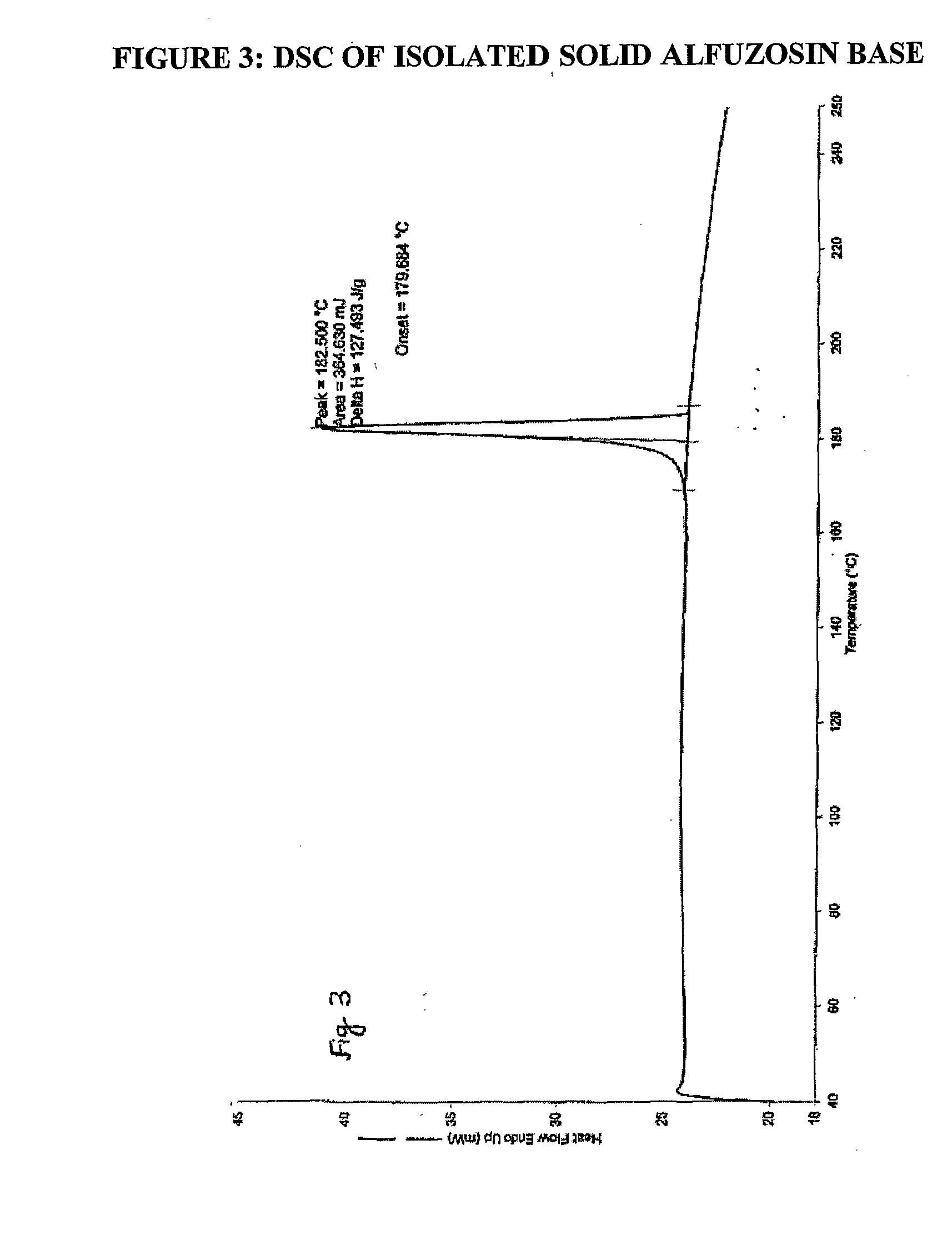
Processes for the Preparation of Alfuzosin
The invention relates to processes for the preparation of alfuzosin or pharmaceutically acceptable salts thereof in high purity. More particularly, it relates to the preparation of pure crystalline alfuzosin base. The invention also relates to pharmaceutical compositions that include the pure alfuzosin or a pharmaceutically acceptable salt thereof.
Owner:WOCKHARDT LTD
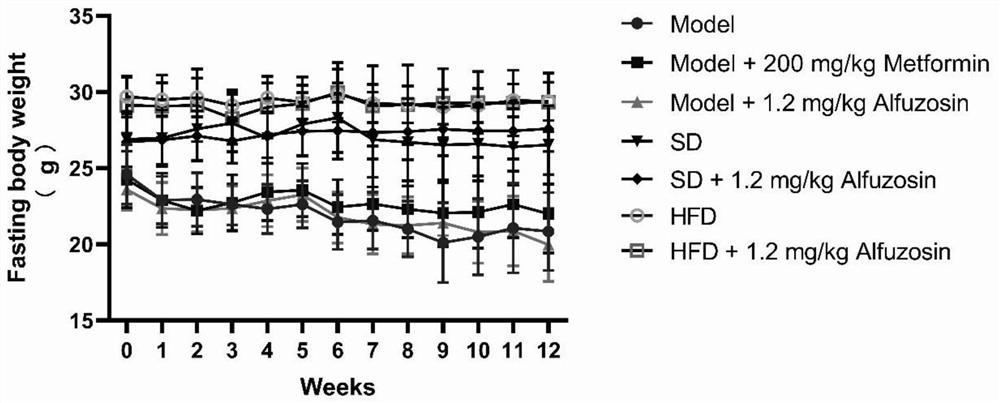
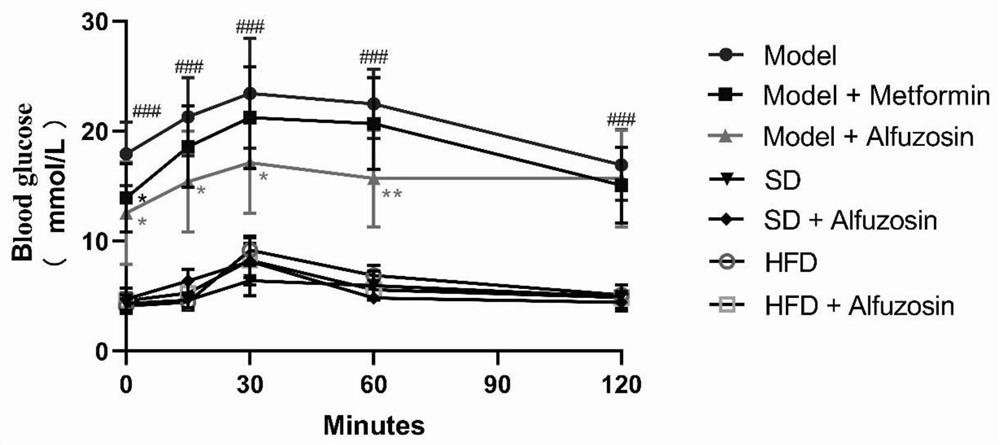
Application of Alfuzosin in preparation of medicine for treating diabetes and complications thereof
ActiveCN113599385AGood treatment effectSuppress deathOrganic active ingredientsNervous disorderA lipoproteinAlfuzosin
The invention belongs to the technical field of medicines, and particularly relates to a novel purpose of Alfuzosin in preparation of a medicine for treating diabetes and complications thereof. The invention finds that the Alfuzosin participates in a saccharification and oxidation process of an organism, inhibits cell death, enhances the renal function, can effectively reduce the blood sugar of a diabetic model mouse, improves the renal function and reduces cardiovascular and cerebrovascular complication related indexes, including triglyceride, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol and the like, the diabetes and the complications thereof can be effectively prevented or treated, the effect of the Alfuzosin is 165 times of the effect of a clinical medicine metformin, a new choice is provided for the preparation of the medicine for clinical treatment of the diabetes and the complications thereof, and the Alfuzosin has a good clinical application prospect.
Owner:LANZHOU UNIVERSITY
Method for preparing alfuzosin
InactiveCN1935806AReduction reaction to avoidAvoid preparationOrganic chemistrySynthesis methodsChloride
The invention discloses an Alfuzosin preparing method, adopting 3- halogenated propylamine as raw material, making condensation reaction with 2-tetrahydrofurioic acid or 2-tetrahydro furoyl chloride and obtaining intermediate N-3-halogenated propyl-2-tetrahydro furoyl amine, which then condenses with methylamine to obtain intermediate N-3-methylamino propyl-2-tetrahydro furoyl amine, which then condenses with 2-chloro-4-amino-6, 7-dimethoxy-quinazoline to obtain Alfuzosin. And the mentioned synthesis method is simple, low-cost, and has reacting conditions easy to control, high product yield and relatively improved quality of product, suitable for industrialized production.
Owner:浙江华纳药业有限公司
Alfuzosin tablets and synthesis
A monolithic composition includes alfuzosin in a polymeric matrix adapted to release 13-33% of the alfuzosin within 2 hours, 40-60% of the alfuzosin within 7 hours, and greater than 80% of the alfuzosin within 20 hours of administration. A unit dosage form includes: a heterogeneous mixture of alfuzosin hydrochloride, lactose monohydrate, hydroxypropylmethylcellulose, polyvinylpyrrolidone and magnesium stearate, wherein the heterogeneous mixture is heterogeneously distributed throughout the unit dosage form. A manufacturing process includes: mixing a hydrophilic polymer and alfuzosin to provide a blend; granulating the blend to provide granules; drying the granules on a dryer to provide dried granules; sizing the dried granules to provide sized granules; mixing the sized granules with a lubricant to obtain a mixture; and compressing the mixture to obtain a tablet. A method of treating benign prostatic hyperplasia, includes administering to a patient the composition or unit dosage form once a day.
Owner:ACINO PHARMA
Processes for the preparation of alfuzosin
The invention relates to processes for the preparation of alfuzosin or pharmaceutically acceptable salts thereof in high purity. More particularly, it relates to the preparation of pure crystalline alfuzosin base. The invention also relates to pharmaceutical compositions that include the pure alfuzosin or a pharmaceutically acceptable salt thereof.
Owner:WOCKHARDT LTD
Use of a combination of udenafil and alfuzosin or oxybutynin for the treatment of overactive bladder.
The invention relates to a specific combination of two active agents: udenafil and one of alfuzosin and oxybutynin and its use for the treatment of overactive bladder.
Owner:PELVIPHARM
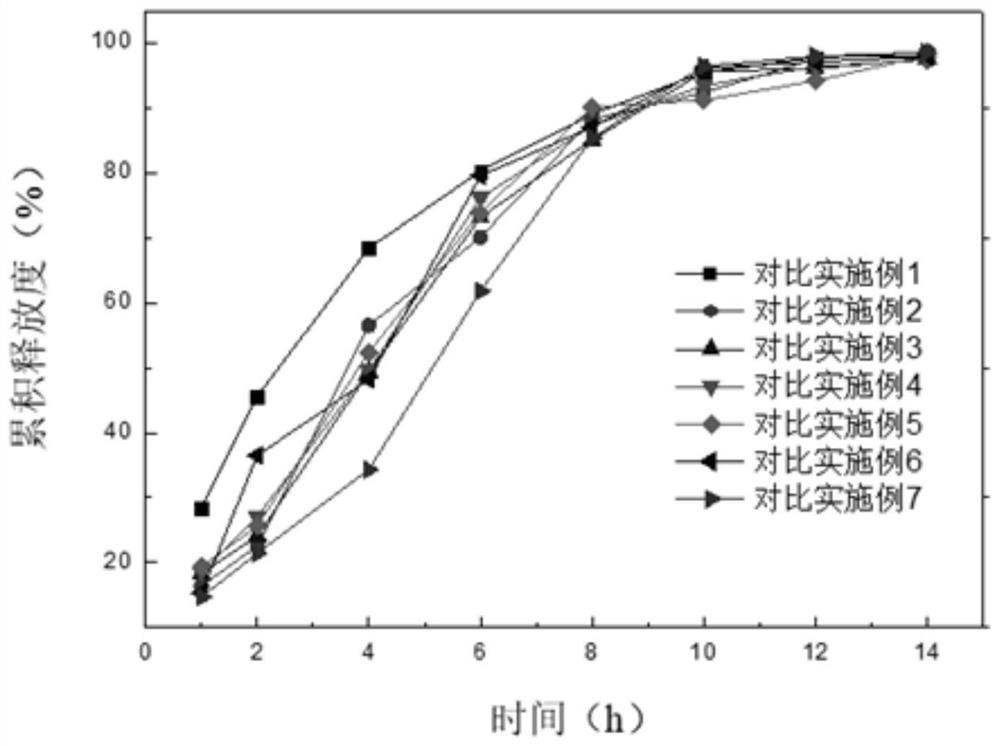
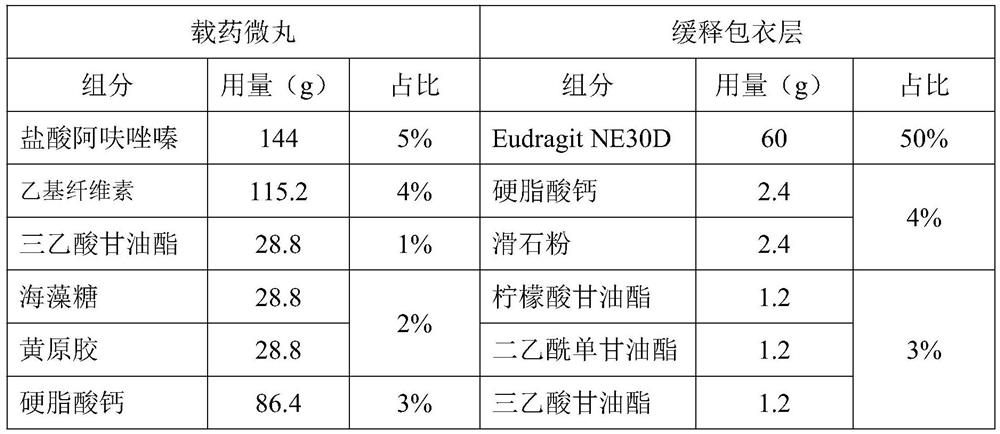
Alfuzosin hydrochloride sustained release preparation and preparation method thereof
ActiveCN114209668AFor controlled releaseImprove efficacyOrganic active ingredientsPharmaceutical non-active ingredientsOrally disintegrating tabletPharmaceutical Aids
The invention belongs to the field of sustained release preparations, and particularly relates to an alfuzosin hydrochloride sustained release preparation and a preparation method thereof. The alfuzosin hydrochloride sustained-release preparation provided by the invention comprises a drug-loaded pellet, a sustained-release coating layer and a pharmaceutic adjuvant layer, and the drug-loaded pellet comprises alfuzosin hydrochloride, a filler, a plasticizer, a sustained-release framework material, a lubricant and a stabilizer; the sustained-release coating layer comprises a film-forming material, a plasticizer, a lubricant and purified water. The pharmaceutic adjuvant layer comprises a filling agent, a disintegrating agent, a flavoring agent, a lubricating agent and a flow aid. The preparation of the alfuzosin hydrochloride orally disintegrating tablet has the characteristics that the alfuzosin hydrochloride orally disintegrating tablet can be disintegrated or dissolved without water in the oral cavity, has the characteristics of convenience in taking, quick absorption and high bioavailability, and can improve the medication compliance of patients and increase the clinical medication choices of doctors for different patients.
Owner:SHANDONG NEWTIME PHARMA
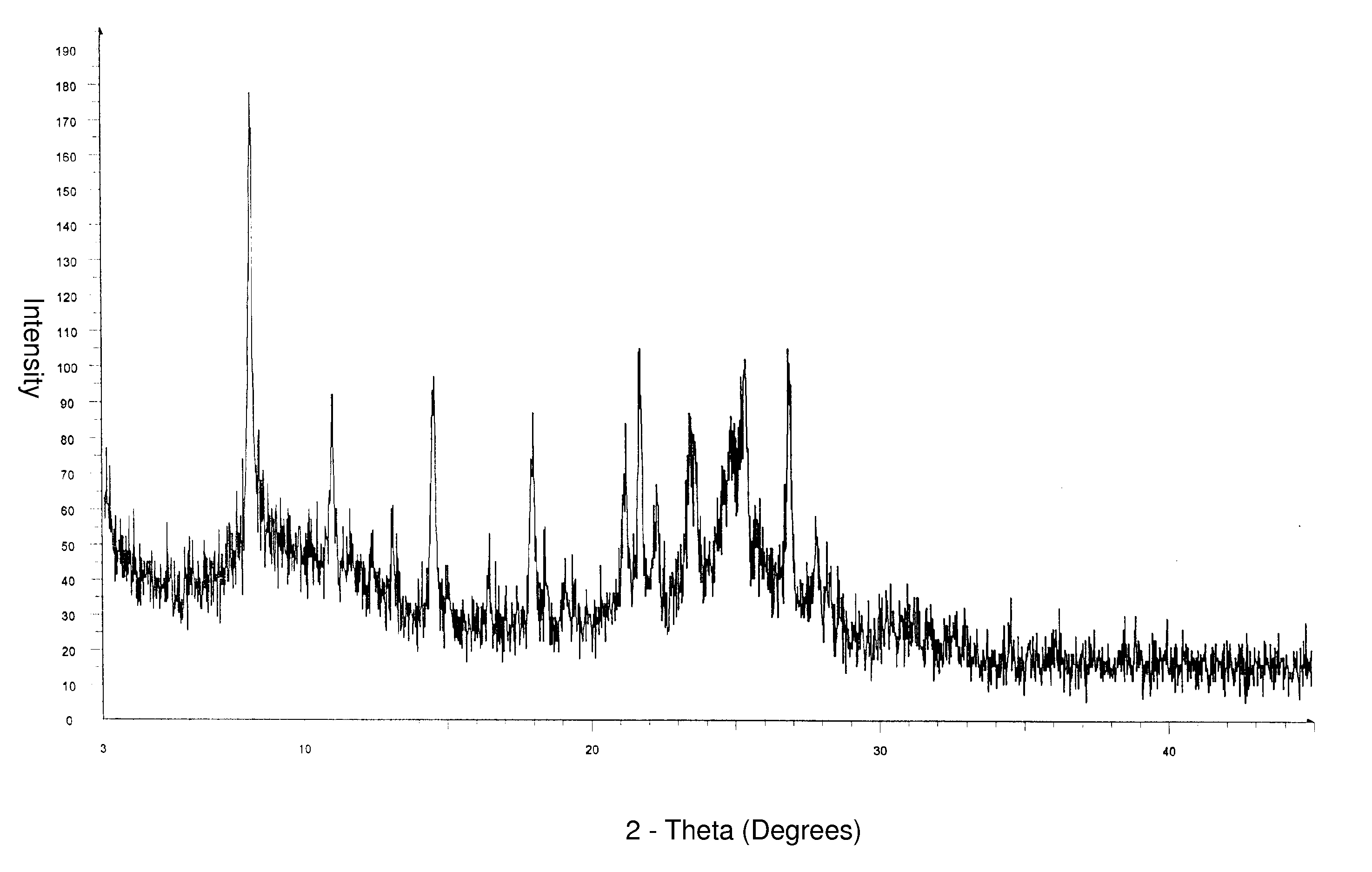
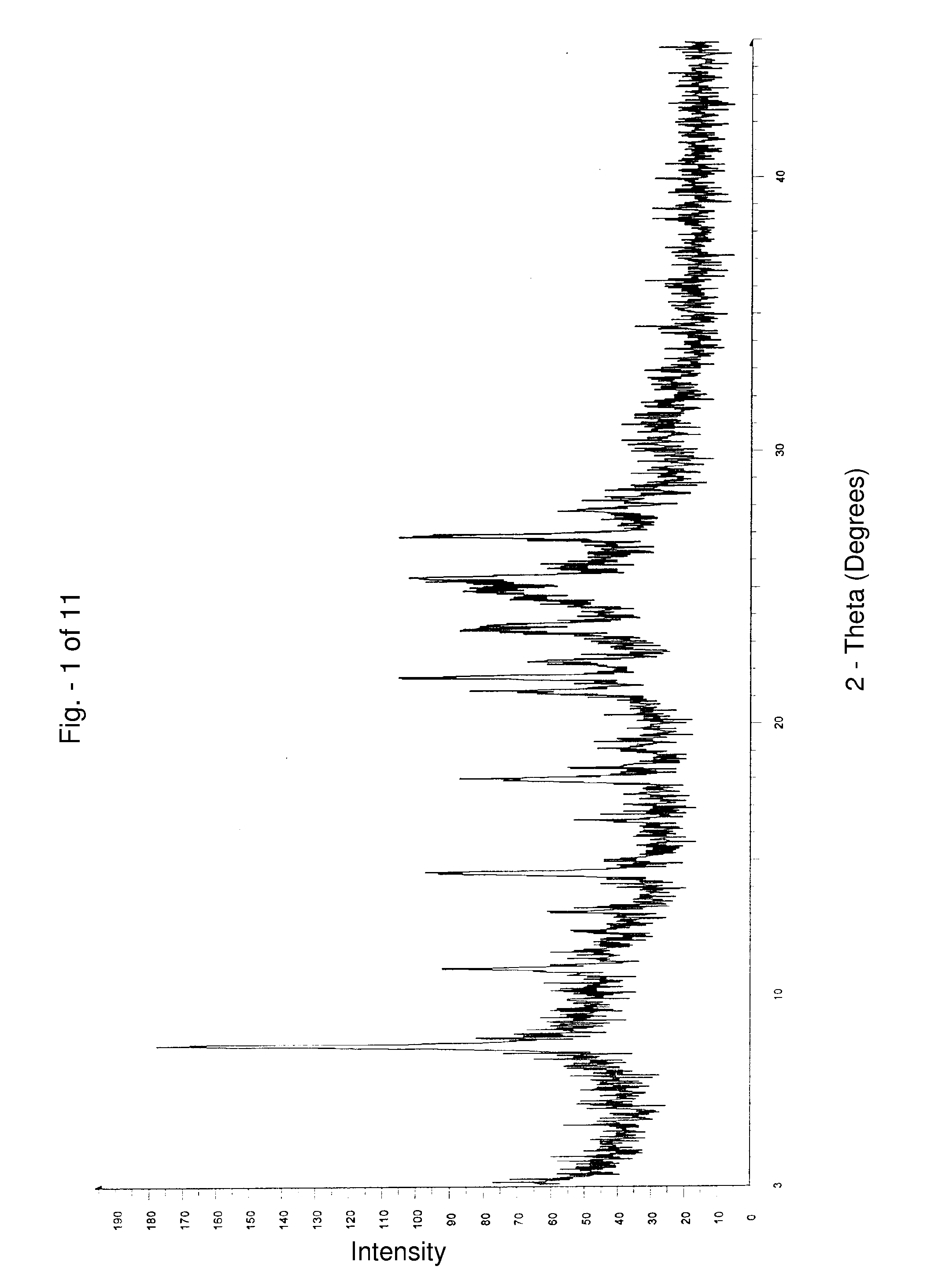
Alfuzosin hydrochloride polymorphs
Alfuzosin hydrochloride crystalline and amorphous polymorphic forms and processes for preparing them.
Owner:DR REDDYS LAB LTD +1
Method for preparing alfuzosin
InactiveCN100564376CReduction reaction to avoidAvoid preparationOrganic chemistrySynthesis methodsChloride
Owner:浙江华纳药业有限公司
A kind of pharmaceutical composition for treating benign prostatic hyperplasia
InactiveCN103989753BInhibit benign prostatic hyperplasiaGood synergyOrganic active ingredientsUrinary disorderTesticleTherapeutic effect
Owner:重庆三峡云海药业股份有限公司
Process for the preparation of alfuzosin and salts thereof
InactiveUS20100174073A1Less hazardousEasy to commercial scaleOrganic chemistryCompound (substance)Alfuzosin
The present invention relates to novel N-[3-[(4-acyl- / aroyl-substituted amino-6,7-dimethoxy-2-quinazolinyl)methylamino]propyl]tetrahydro-2-furancarboxamide derivatives, and a process for the preparation thereof. The novel compounds are useful for preparing alfuzosin or a pharmaceutically acceptable salt thereof in high yield and purity.
Owner:ACTAVIS GRP PTC EHF
Combined tablet for treating erectile dysfunction and premature ejaculation
PendingCN112791090AGood effectSmall doseOrganic active ingredientsPill deliverySexual impotenceFood grade
The invention belongs to the technical field of medical drugs, and particularly relates to a combined tablet for treating erectile dysfunction and premature ejaculation, and the combined tablet is prepared from the following raw materials in parts by weight: tadalafil, dapoxetine hydrochloride, alfuzosin, a food-grade binder, a disintegrating agent and a food-grade filler. According to the invention, dapoxetine hydrochloride is filled into the capsule, tadalafil and alfuzosin are compressed around the nucleus to be prepared into tablets, and the tablets are used for treating patients with erectile dysfunction, premature ejaculation and coexistence of the erectile dysfunction and the premature ejaculation, have the advantages of being good in effect, small in dosage, high in tolerance, few in side effect and the like and have good practicability.
Owner:河北雄安日出海上生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com