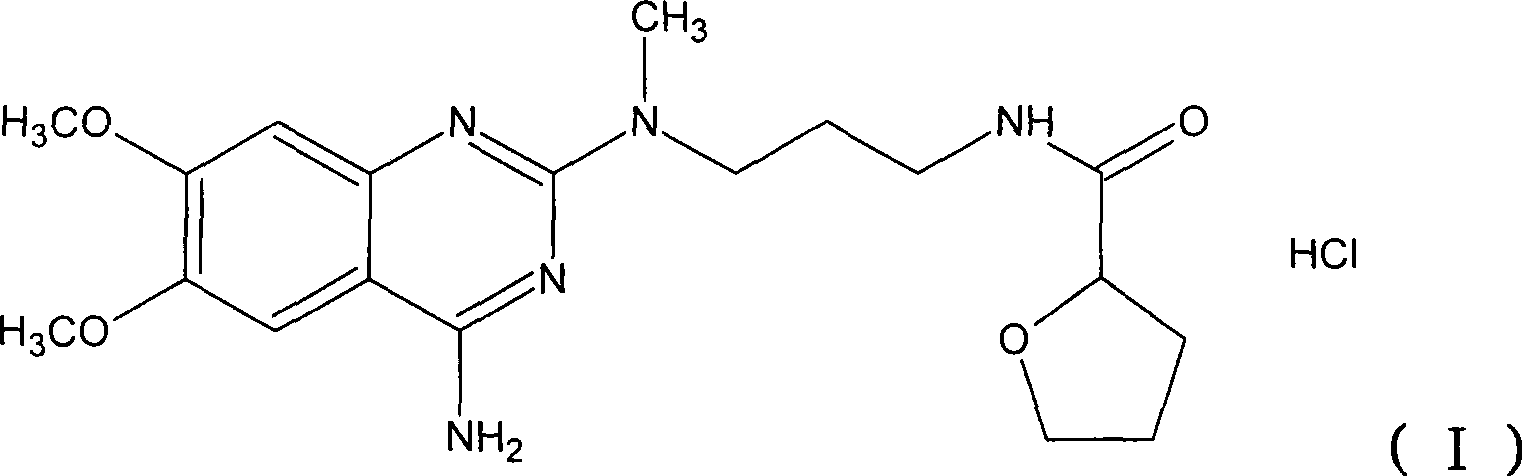
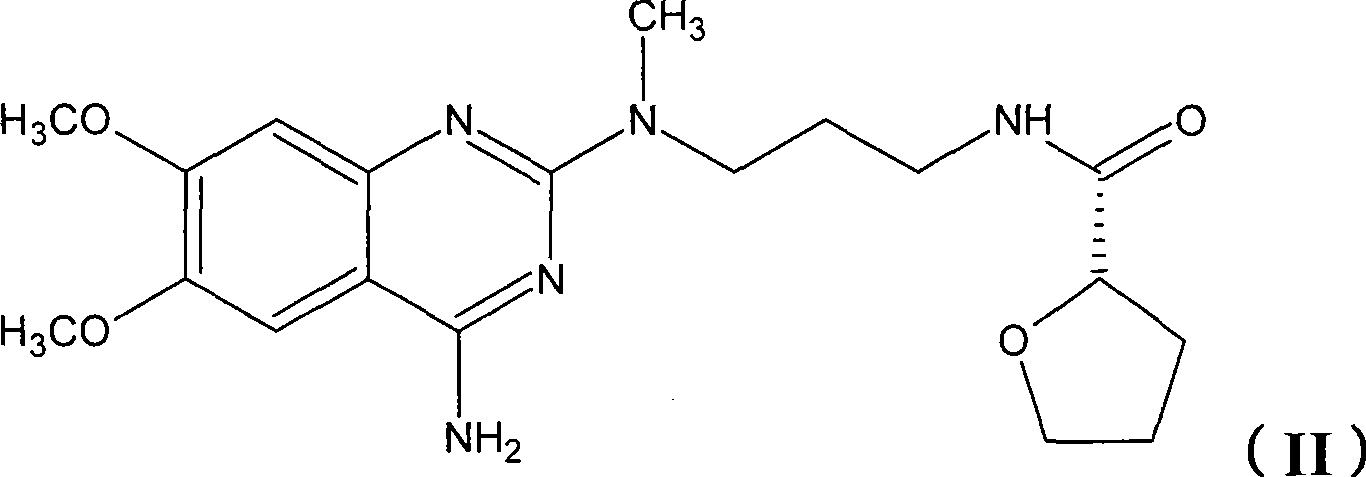
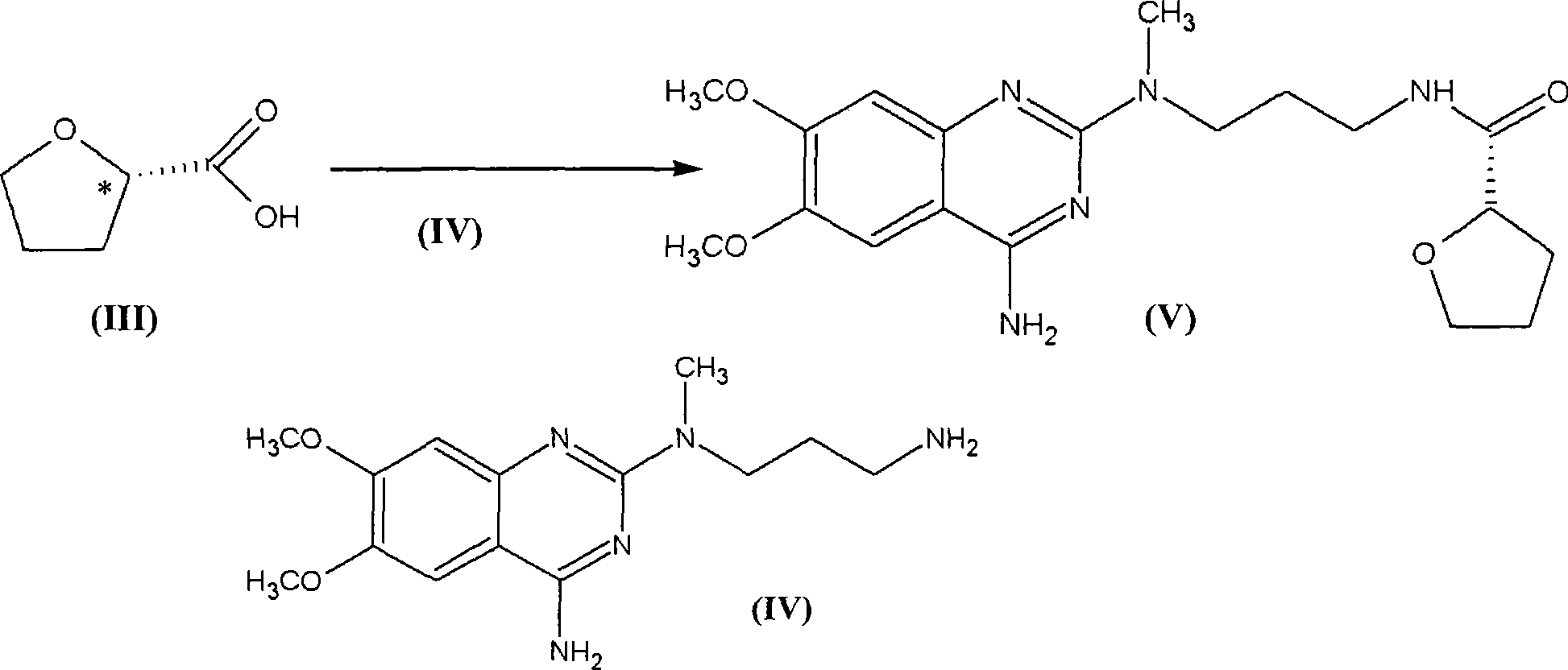
Levalfuzosin, preparation method and use thereof
A technology of dextroalfuzosin and alfuzosin, which is applied in the field of preparation of drugs for the specific treatment of benign prostatic hyperplasia, optically active levofuzosin and its salt and its slow-release preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The preparation method of L-alfuzosin of the present invention is introduced below.
[0028] The levo-alfuzosin of the present invention can be carried out by conventional optical resolution methods. For example, it can be carried out by resolving the commercially available racemic alfuzosin (hereinafter referred to as (±) alfuzosin), first salting the commercially available (±) alfuzosin, and then chromatographically Alternatively, fractional crystallization can be carried out to obtain L-alfuzosin.
[0029] For example, ordinary preparative chromatographic columns can be used, and conventional methods can be used for resolution according to ordinary separation methods, such as adding chiral additives to the mobile phase, and through the interaction between the chiral additives and the racemic alfuzosin to be separated , so that (-) alfuzosin and (+) alfuzosin have different retention times, thereby achieving separation. Afterwards, the chiral additive is removed to ...
preparation example 1
[0052] Preparation Example 1 Preparation of N-(4-amino-6,7-dimethoxyquinazolinyl)-N-methylpropylenediamine (IV)
[0053]
[0054] In 200 milliliters of n-butanol, add 24 grams (0.1mol) of 4-amino-2-chloro-6,7-dimethoxyquinazoline and 16.6 grams (0.2mol) of 3-methylaminopropionitrile and reflux 5 hours, cooled and filtered, washed with ethanol, and dried to a weight of 30.7 grams, with a yield of 95%.
[0055] In a 500 ml autoclave, add 250 ml of 15% ethanol ammonia solution, 11.5 g of the above product, add 0.5 g of Raney nickel, 80 kg of pressure, react at 80 ° C for 6 hours, filter the catalyst, evaporate the solvent to dryness, add concentrated hydrochloric acid for 15 After 1 ml, evaporate to dryness and recrystallize with isopropanol to obtain 6.7 g of N-(4-amino-6,7-dimethoxyquinazolinyl)-N-methylpropanediamine hydrochloride, melting point 270°C .
preparation example 2
[0056] Preparation Example 2 Preparation of Levo-Alfuzosin Hydrochloride
[0057] At room temperature, stir dropwise 40 ml of thionyl chloride to 25 ml of S-(-)-tetrahydrofuran-2-carboxylic acid [α] D 20 =+19 (pure product), after reacting for 4 hours, reflux for 30 minutes, and distill under reduced pressure to obtain 28 g of S-(-)-tetrahydrofuran-2-formyl chloride (intermediate 1), with a yield of 93.3%.
[0058] Add 6.0 g of N-(4-amino-6,7-dimethoxyquinazolinyl)-N-methylpropylenediamine hydrochloride into 100 ml of DMF, and add 5 ml of triethylamine to dissolve it. Add 2.46 g of S-(-)-tetrahydrofuran-2-formyl chloride (intermediate 1) and react at room temperature for 3 hours, add 500 ml of water, precipitate a solid, filter and wash with water, then dry to obtain 7.0 g of the product base, with a yield of 98%.
[0059] After adding 8 ml of hydrochloric acid to the base product above, evaporate to dryness and dissolve with 20 ml of absolute ethanol, add 30 ml of acetone, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com