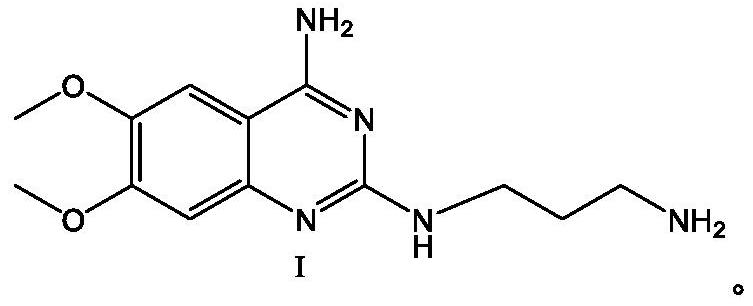
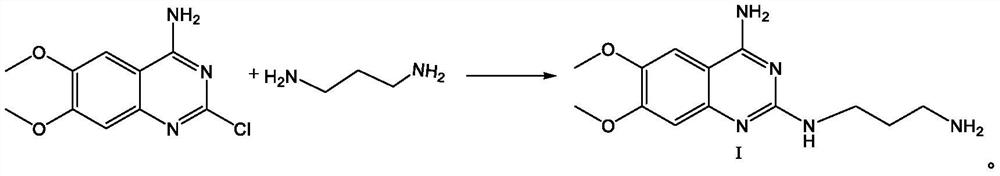
Alfuzosin hydrochloride intermediate compound
A technology of alfuzosin hydrochloride and intermediates, which is applied in the field of pharmaceutical synthesis, can solve the problems of unsuitable industrial production, high risk, and high equipment requirements, and achieve the effects of avoiding high temperature and high pressure reactions, low cost, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Add N,N-dimethyl In methyl formamide (120ml), the temperature was raised to 100°C for reaction, TLC detected that the reaction was complete, cooled to room temperature, and suction filtered to obtain the solid intermediate compound I hydrochloride; then the intermediate compound I hydrochloride was transferred to the reaction flask , add purified water (100ml), heat up to 70-80°C, keep stirring until the solids are completely dissolved, then add NaOH solution with a concentration of 30% dropwise, adjust the pH value to 12-14, gradually turn off the heating after solids are precipitated, and cool down to Stir and crystallize at 20-30°C for 3 hours, start suction filtration, rinse the filter cake with purified water until the pH value of the filtrate is equal to 7, and dry the filter cake to obtain 26.60 g of white solid intermediate compound Ⅰ with a yield of 95.91 %, purity 99.63%.
Embodiment 2
[0060] Add 2-chloro-4-amino-6,7-dimethoxyquinazoline (24.00g, 0.10mol) and 1,3-propanediamine (8.15g, 0.11mol) to isopropanol (120ml) in sequence During the reaction, the temperature was raised to 80°C for reaction, TLC detected that the reaction was complete, and the reaction was completed after cooling down to room temperature and suction filtration to obtain the solid intermediate compound I hydrochloride; then the intermediate compound I hydrochloride was transferred to the reaction bottle, and purified water (100ml ), heat up to 70-80°C, heat and stir until all the solids are dissolved, then add NaOH solution with a concentration of 30% dropwise, adjust the pH value to 12-14, gradually turn off the heating after solids are precipitated, cool down to 20-30°C, and stir After crystallization for 3 hours, suction filtration was started, and the filter cake was rinsed with purified water to pH 7. After suction filtration, the filter cake was dried to obtain 25.72 g of white sol...
Embodiment 3
[0062] Add 2-chloro-4-amino-6,7-dimethoxyquinazoline (24.00g, 0.10mol) and 1,3-propylenediamine (11.12g, 0.15mol) to diglyme in sequence (120ml), heat up to 120°C for reaction, TLC detects that the reaction is complete, cool down to room temperature, and filter with suction to obtain a solid intermediate compound I hydrochloride; then transfer the intermediate compound I hydrochloride into the reaction flask, add purification Water (100ml), heat up to 70-80°C, heat and stir until the solids are completely dissolved, then add NaOH solution with a concentration of 30% dropwise, adjust the pH value to 12-14, turn off the heating after solids are gradually precipitated, and cool down to 20-30 ℃, stirred and crystallized for 3 hours, started suction filtration, rinsed the filter cake with purified water to a pH value of 7, completed the suction filtration, and dried the filter cake to obtain 25.49 g of white solid intermediate compound Ⅰ with a yield of 91.91% and a purity of 99.12%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com