Alfuzosin hydrochloride sustained release preparation and preparation method thereof
A technology of alfuzosin hydrochloride and alfuzosin hydrochloride, which is applied in the field of alfuzosin hydrochloride sustained-release preparations and its preparation, can solve the problems of difficult medication for patients, poor release effect, low uniformity of stability and content, etc. Achieve the effect of improving the uniformity of drug content, improving drug stability, and wide application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
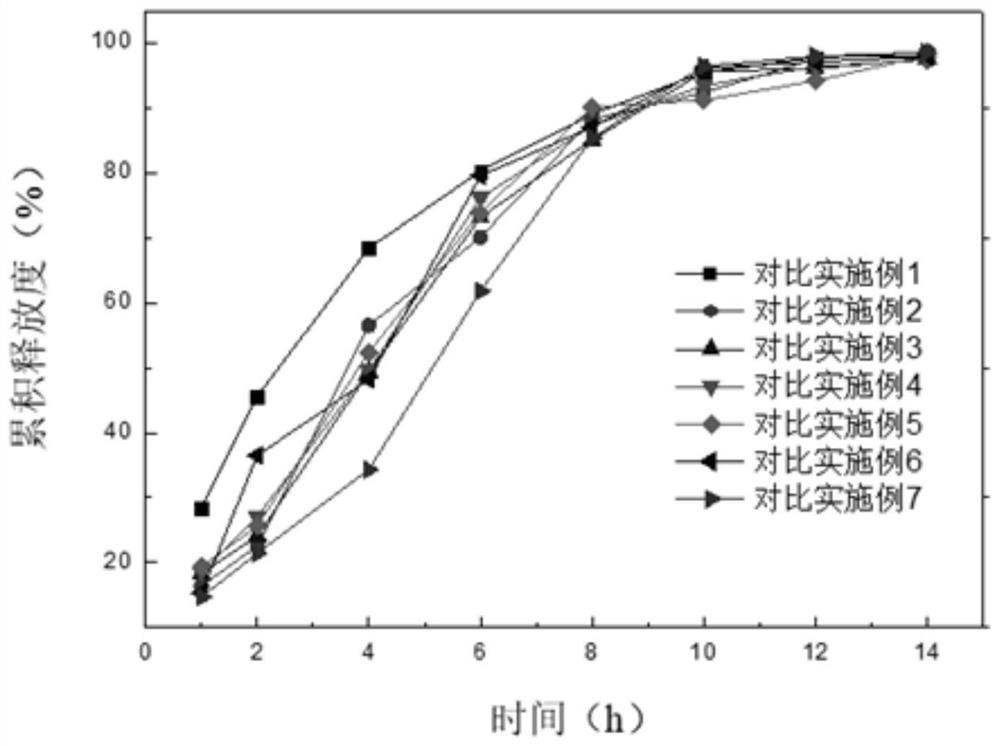
Examples
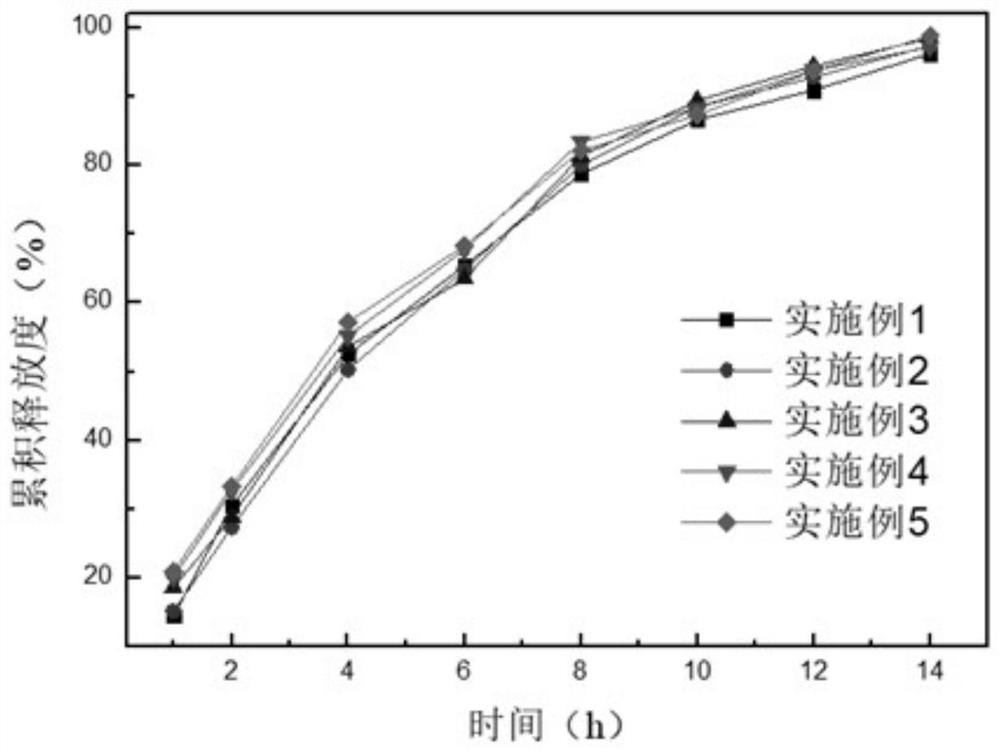
Embodiment 1
[0029] Embodiment 1: 20000 tablets
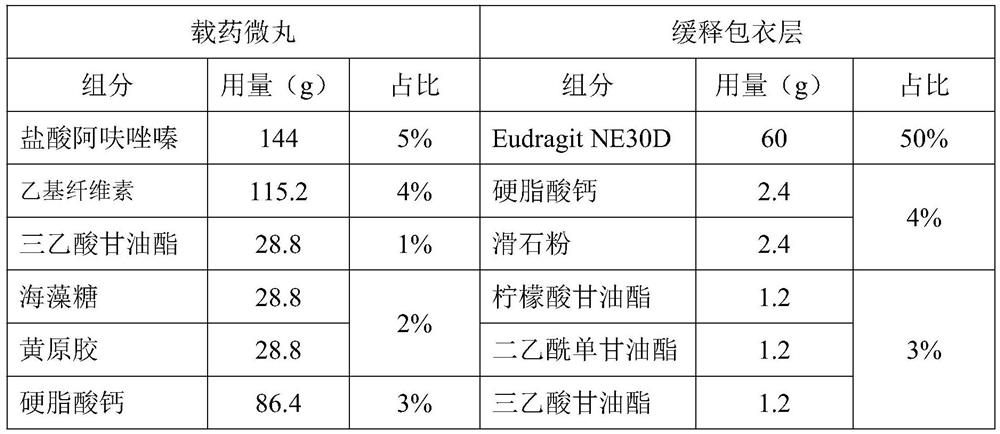
[0030] Prescription of pellets
[0031]
[0032]
[0033] Micropellet preparation method:
[0034] (1) Soft material: Pre-dissolve alfuzosin hydrochloride in purified water, mix the filler, lubricant, and stabilizer in a granulator first, mix, then add the solution containing the raw materials, and then add the slow-release Skeleton material, plasticizer, appropriate amount of purified water, and granulation to obtain a suitable soft material;
[0035] (2) extrude: pour the soft material obtained in the step (1) into an extruder and extrude to obtain broken particles;
[0036] (3) Spheronization: Pour the granules obtained in step (2) into a spheronizer for spheronization, the rotating speed of the turntable is 100rmp, 10min;
[0037] (4) Drying: drying the wet pellets obtained in step (3) in a drying oven to obtain dry pellets;
[0038] (5) Coating: prepare coating liquid coating, coating weight gain is 4%;
[0039] (6) Pellet c...
Embodiment 2
[0043] Embodiment 2: 50000 pieces
[0044]
[0045]
[0046] Pellet prescription and preparation process are the same as embodiment 1
[0047] Pellet compression:
[0048] components Content (g) Pellets 152 Microcrystalline Cellulose 102 82 Sodium carboxymethyl starch 28 colloidal silica 4 Calcium stearate 9 sucrose 25
[0049] Preparation method: with the preparation method of embodiment 1.
Embodiment 3
[0050] Embodiment 3: 20000 pieces
[0051] Prescription of pellets
[0052]
[0053] Preparation process is with embodiment 1
[0054] Pellet compression:
[0055]
[0056]
[0057] Preparation method: with the preparation method of embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com