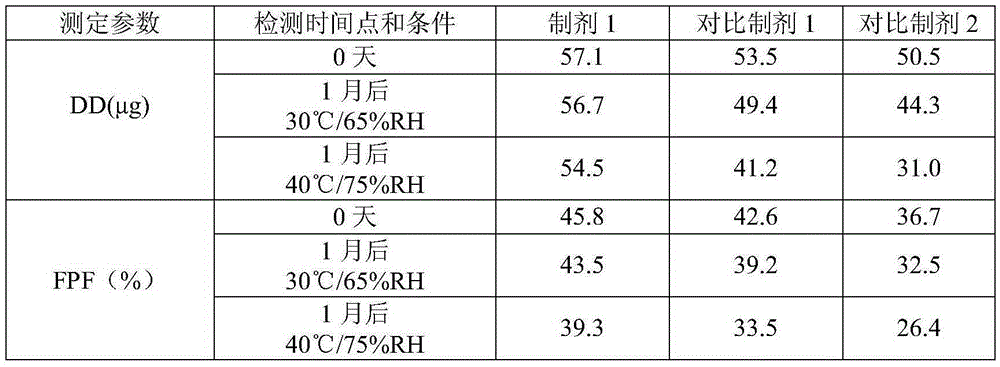
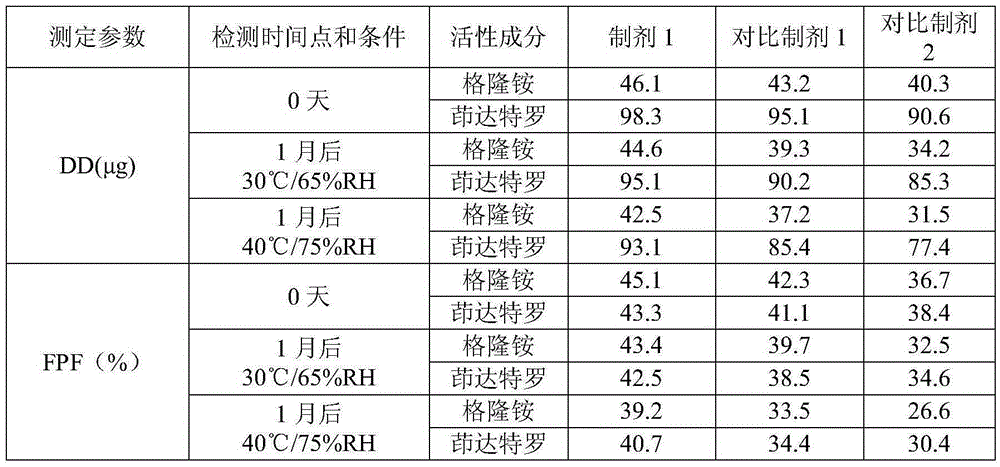
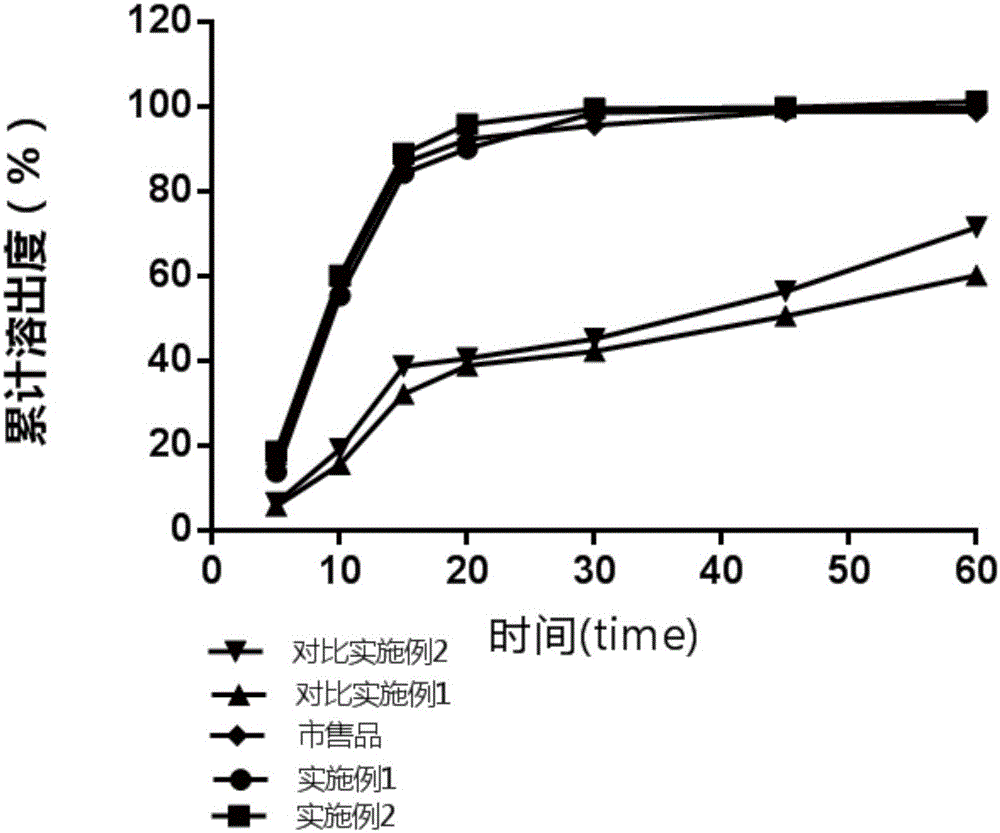
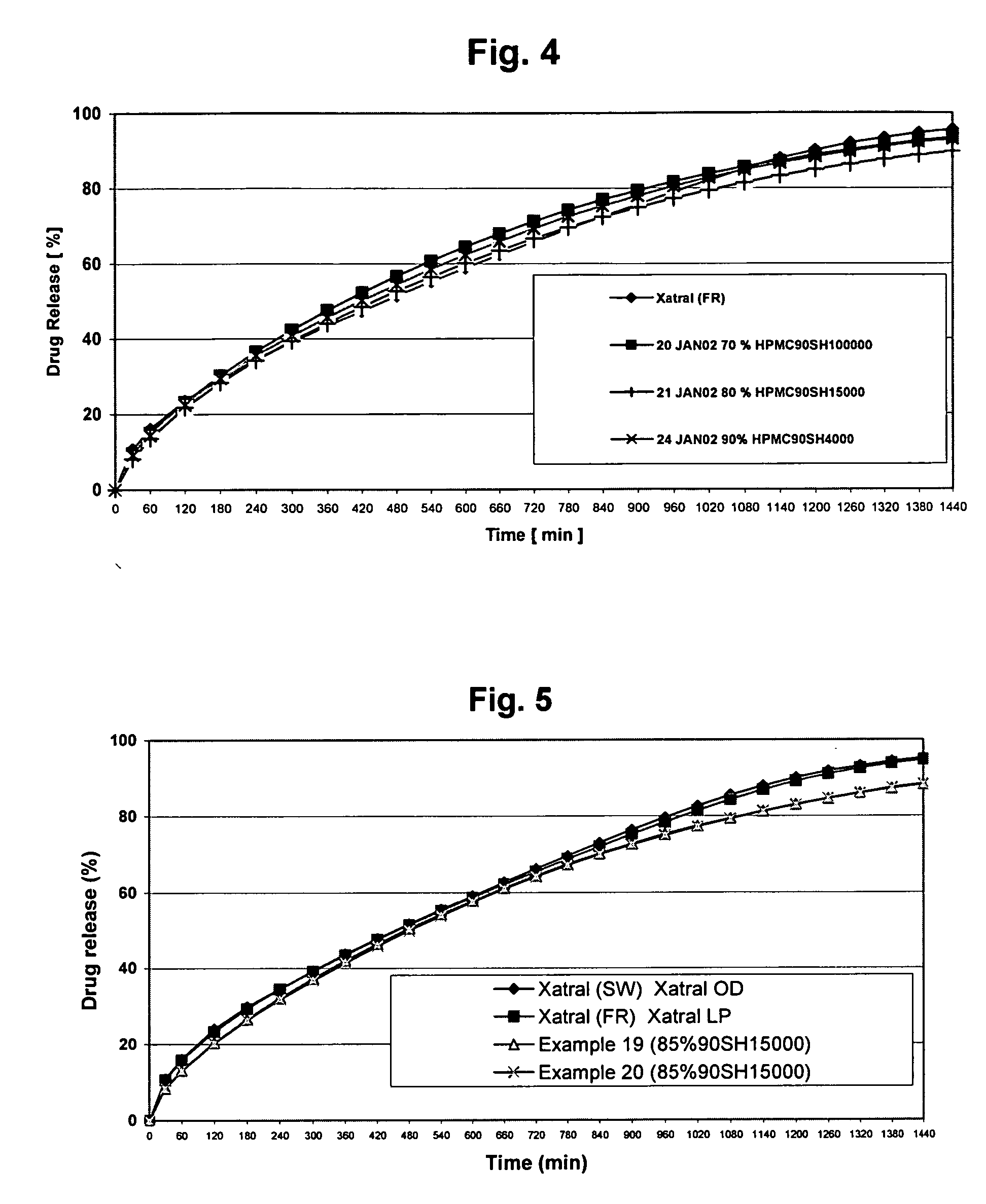
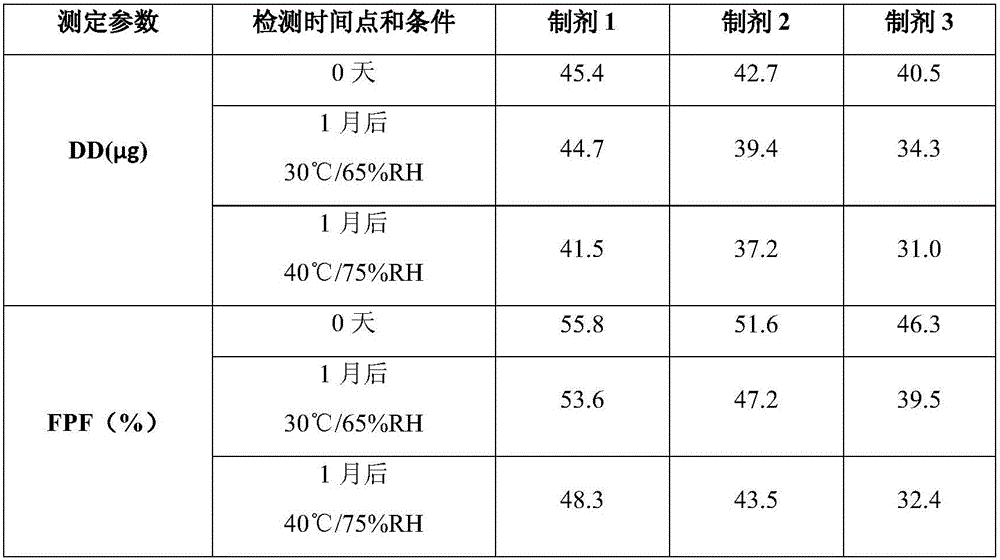
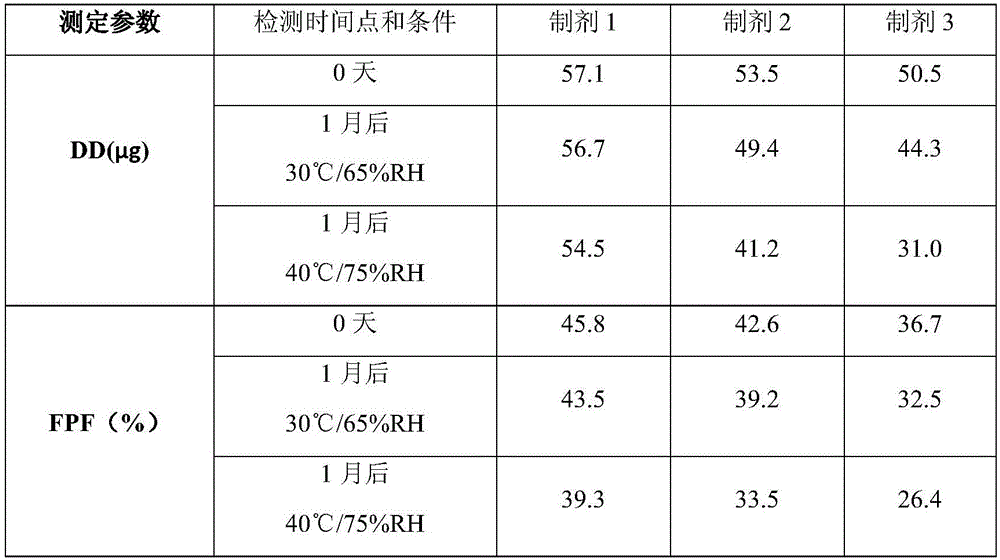
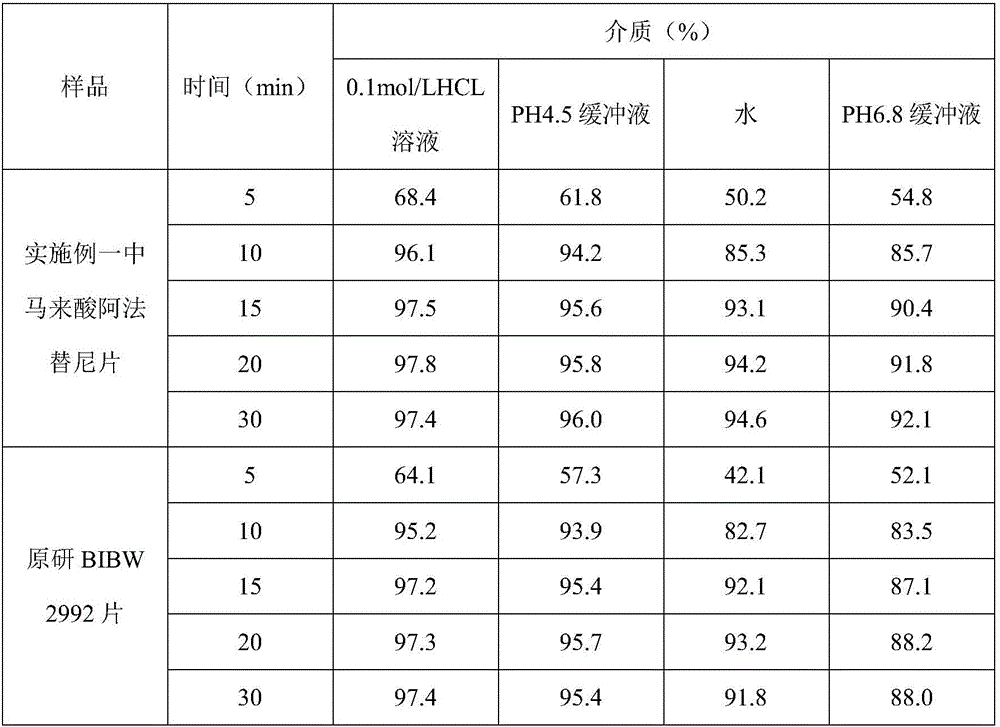
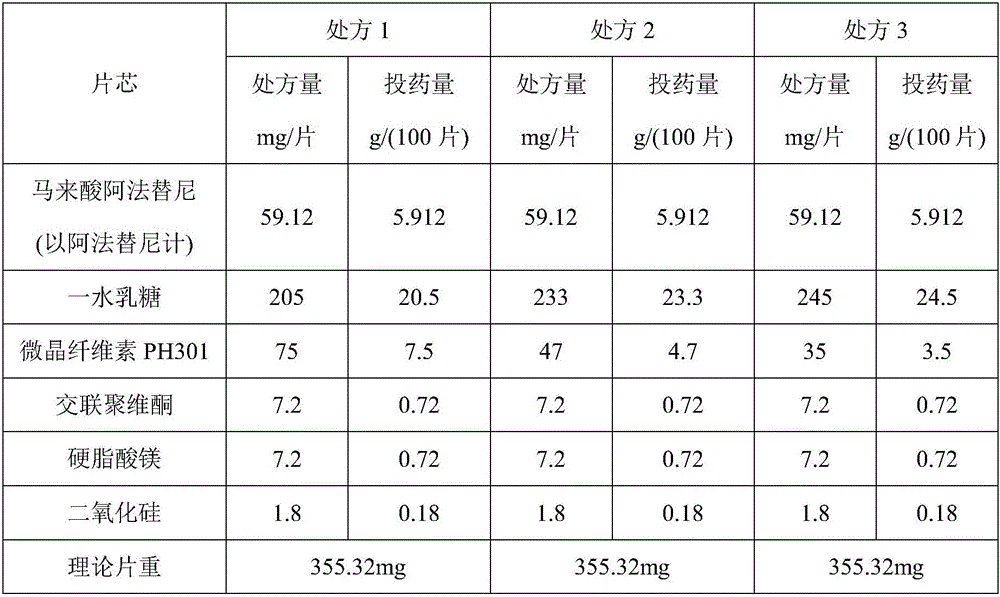
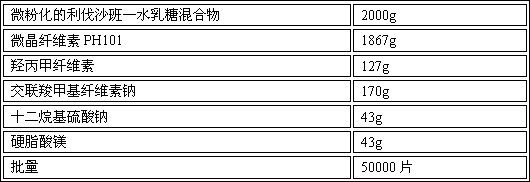
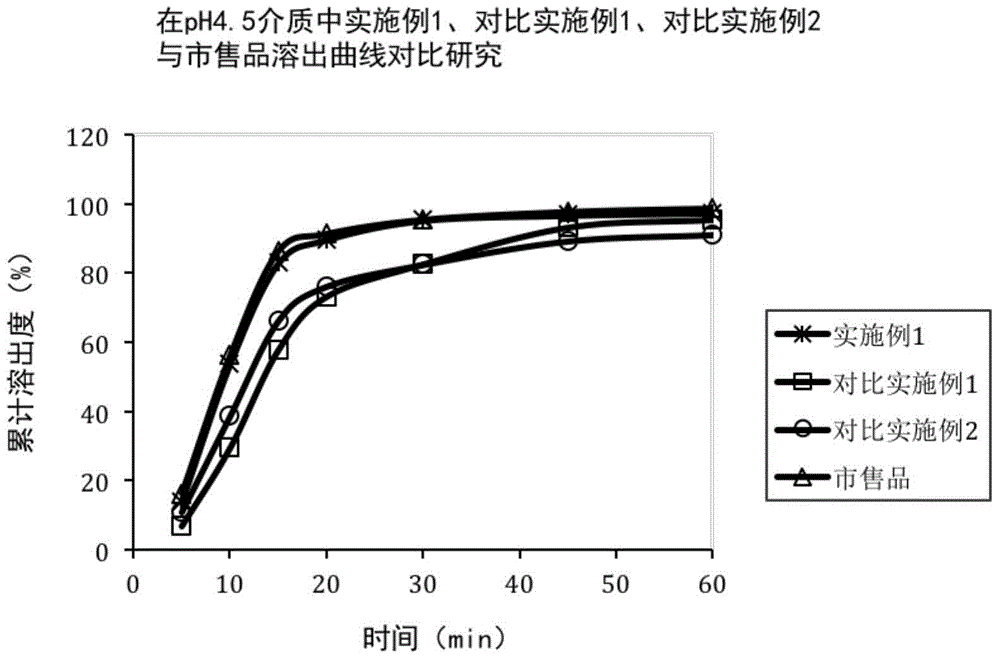
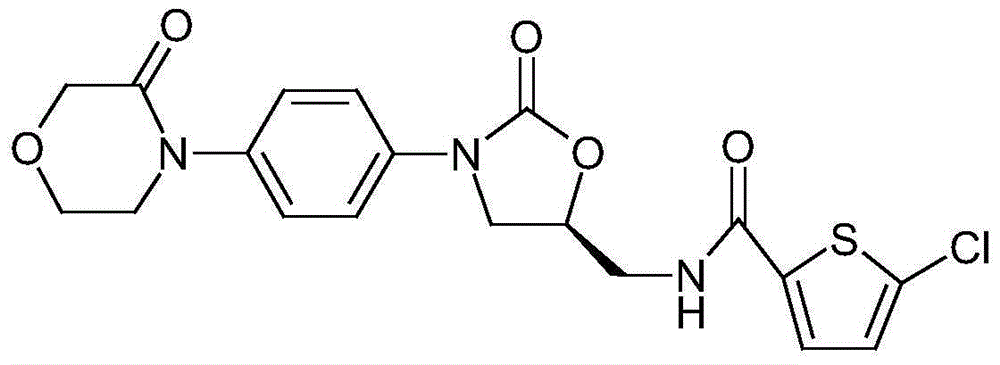
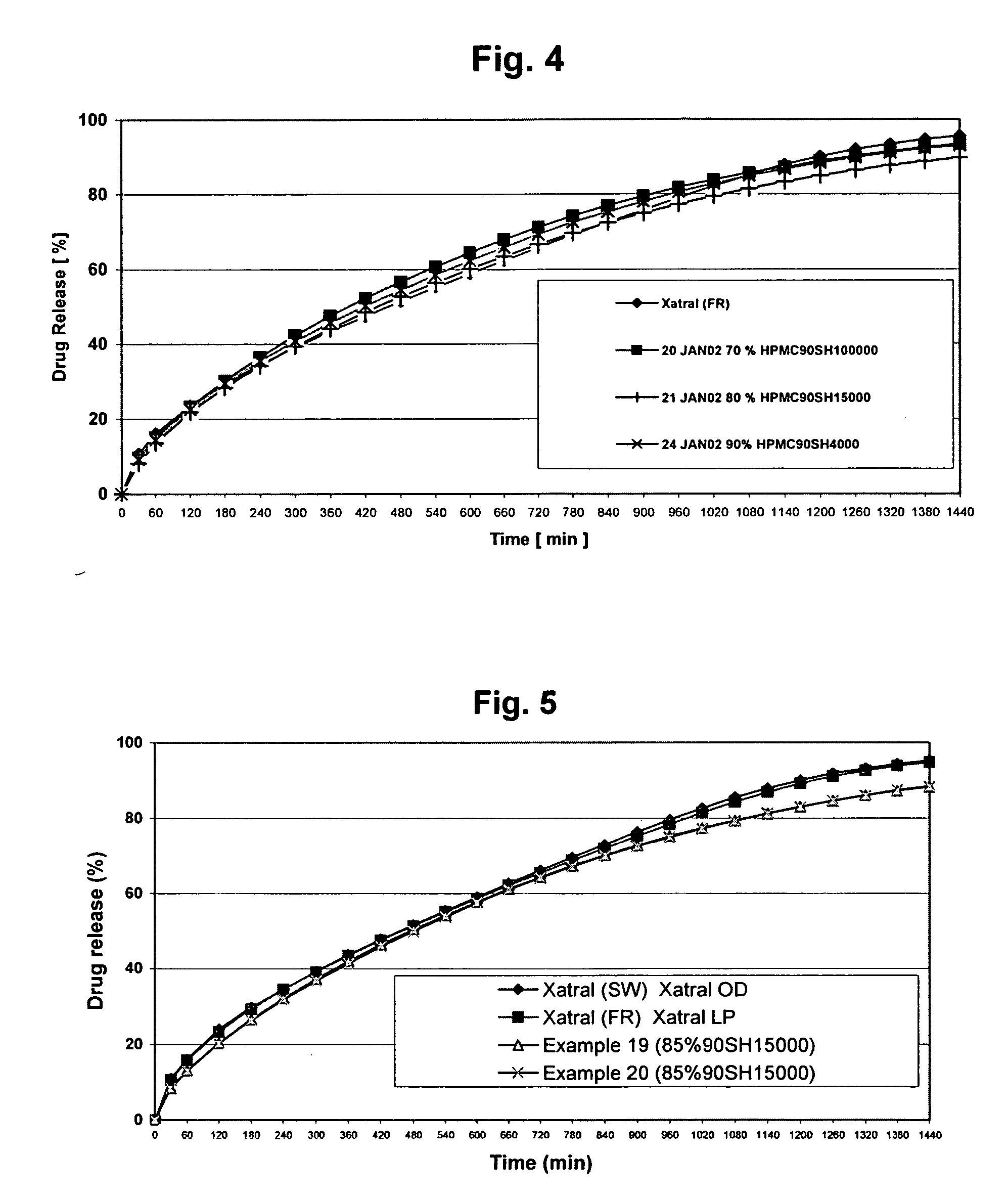
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
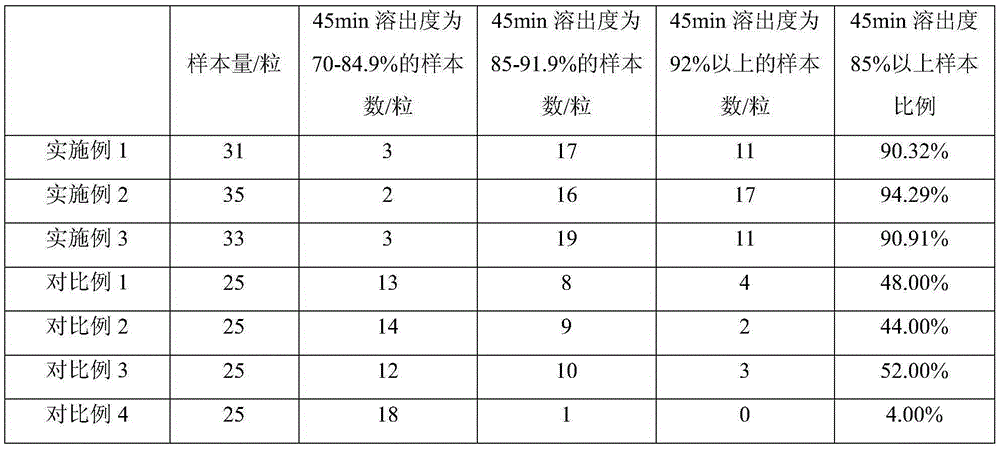
128 results about "LACTOSE MONOHYDRATE" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A: Drugs.com describes lactose monohydrate simply as milk sugar, a disaccharide composed of one galactose and one glucose molecule. The pharmaceutical industry uses lactose monohydrate for its compressibility properties that help in the formation of tablets.
Process and plant for evaporative concentration and crystallization of a viscous lactose-containing aqueous liquid
InactiveUS20030196957A1Crystallization separationSugar crystallisationLiquid crystallineParticulates
Crystalline alpha-lactose monohydrate is recovered frown a viscous lactose-containing aqueous liquid by subjecting said liquid to simultaneous heating, removal of evaporated vapor and mechanical agitation at high shear rate to provide a crystallization promoting decrease of the viscosity of the liquid with crystals formed and suspended therein to progressively concentrate the agitated liquid and simultaneously crystallize lactose therefrom. Subsequent cooling, drying, and disintegration yield particulate alpha-lactose monohydrate.
Owner:NIRO
Irbesartan and hydrochlorothiazide pharmaceutical composition and preparation method thereof
ActiveCN101327213AGood auxiliary effectGood quality and stabilityOrganic active ingredientsPharmaceutical non-active ingredientsLACTITOL MONOHYDRATEAdditive ingredient
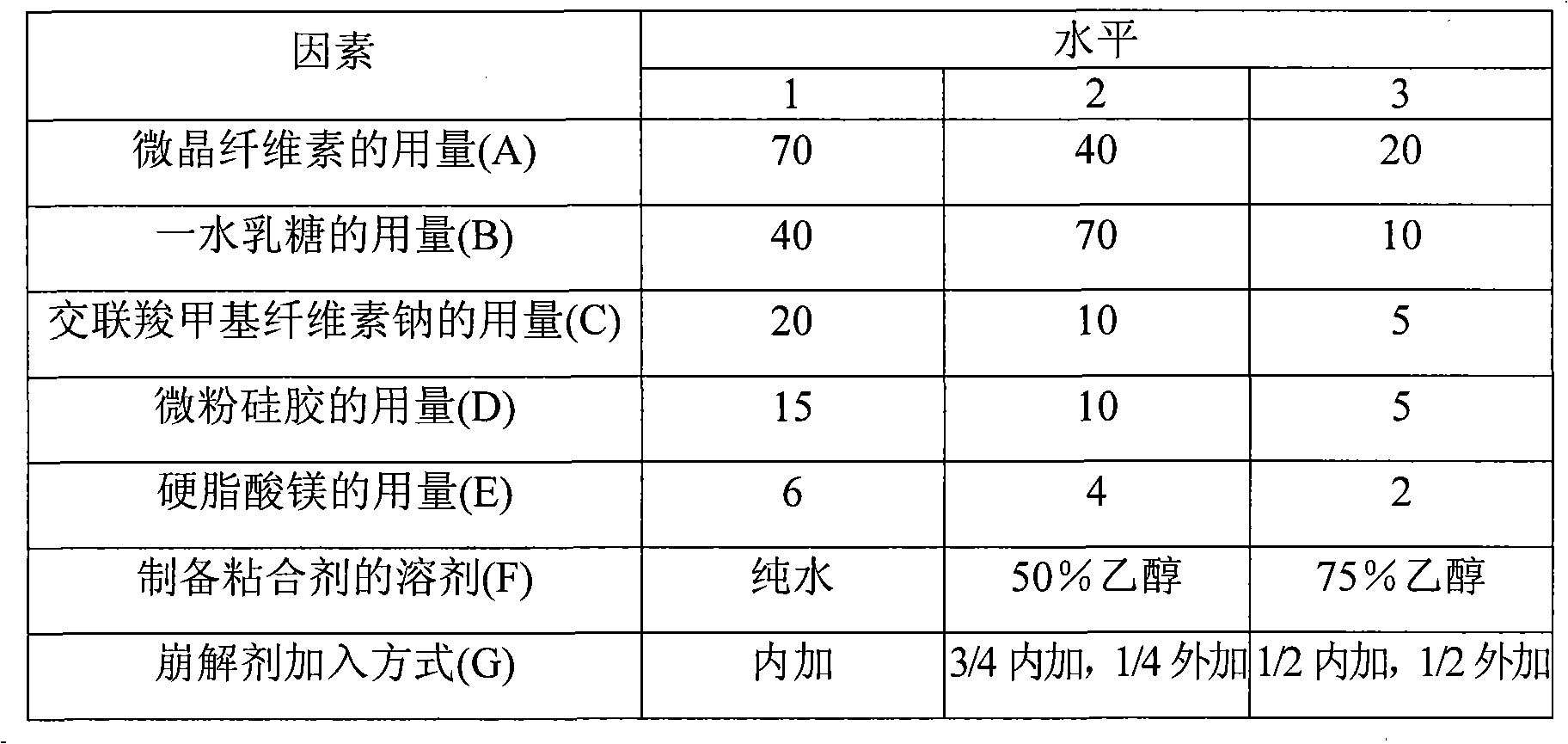
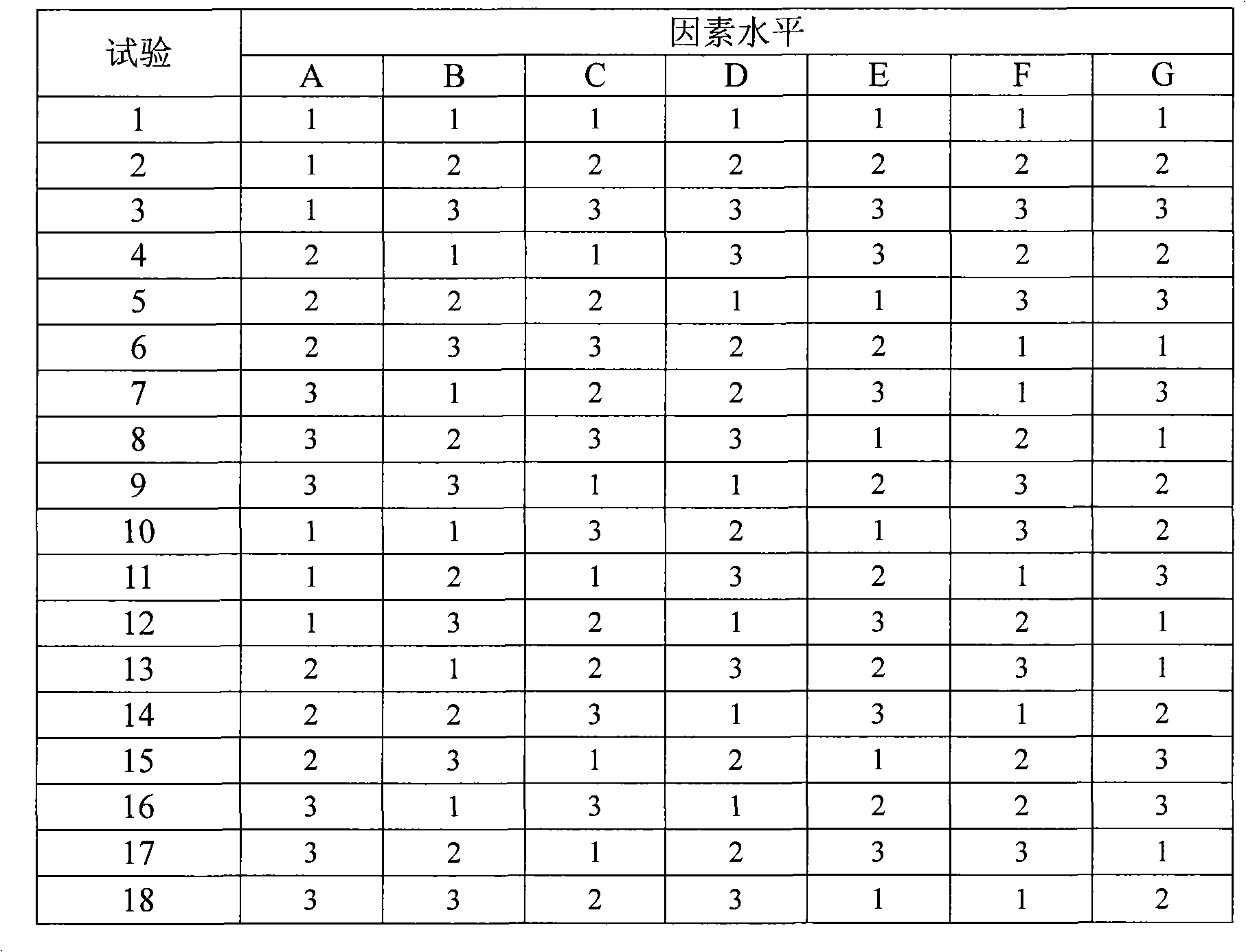
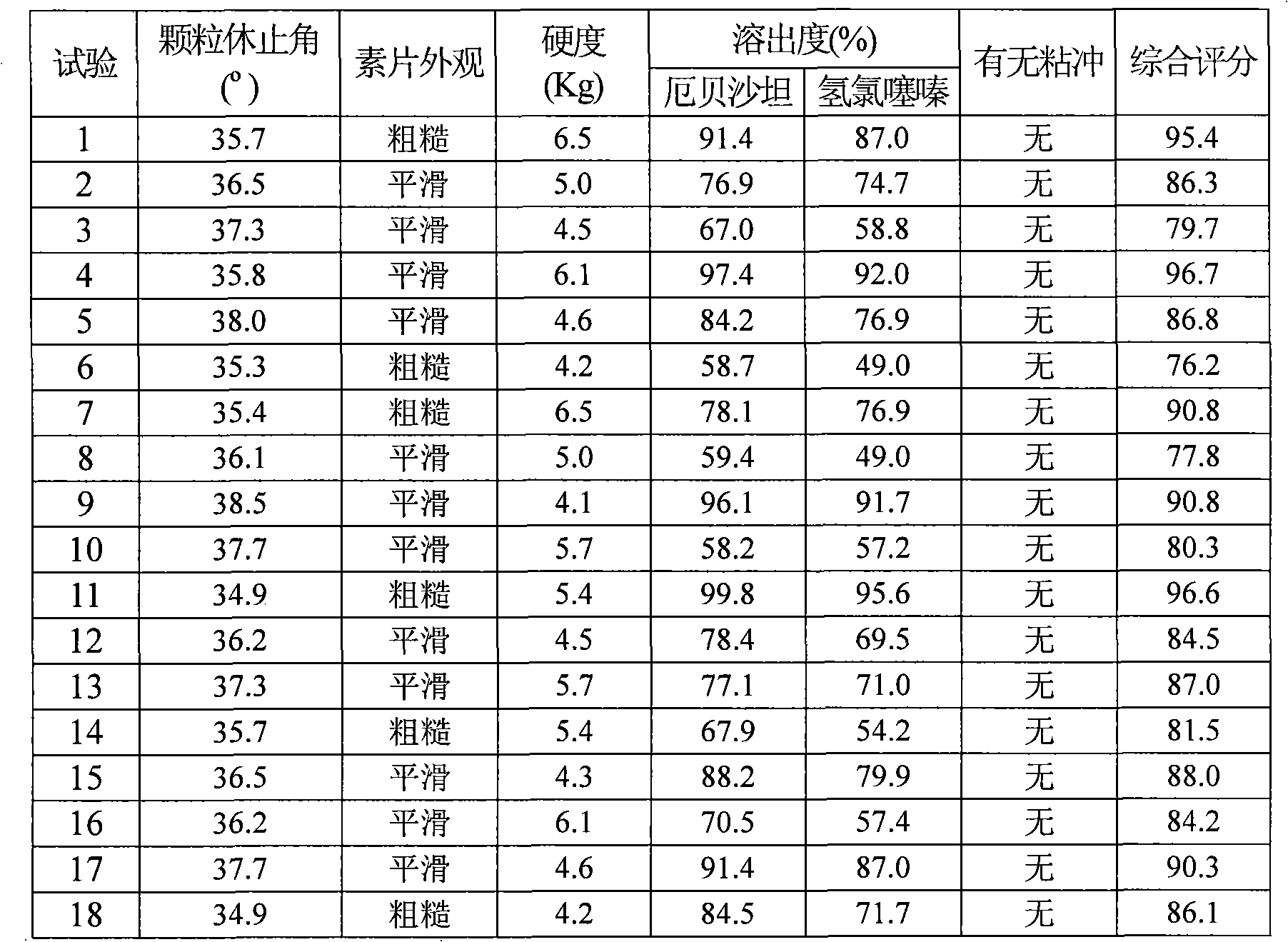
The invention relates to a medicinal composite of Irbesartan hydrochlorothiazide. The medicinal composite is composed of 150 portions of Irbesartan, 12.5 portions of hydrochlorothiazide, 20 portions to 60 portions of microcrystalline cellulose, 20 portions to 60 portions of lactose monohydrate, 15 portions to 25 portions of crosslinked sodium carboxymethyl cellulose, 1 portion to 10 portions of hydroxypropylmethyl cellulose, 2 portions to 7 portions of SiliciiDoxydum, 1 portion to 3 portions of magnesium stearate. The major medicinal ingredients are mixed with part of the crosslinked sodium carboxymethyl cellulose and the microcrystalline cellulose, and the mixture is crushed down and then added with the lactose monohydrate for mixing; the mixture obtained from the former step is added with 50 percent of ethanol solvent containing 2 percent of hydroxypropylmethyl cellulose for even mixing, then the mixture is screened, made into integral grains which are then dried; the dry grains are mixed with the SiliciiDoxydum, the magnesium stearate and the residual crosslinked sodium carboxymethyl cellulose, and the mixture then undergoes tabletting and coating so as to obtain the medicinal composite. The prescription of the medicinal composite of the invention is reasonable, the quality is stable and reliable, and the medicinal composite has a satisfactory dissolution rate.
Owner:HAINAN JINRUI PHARMA
Process and plant for evaporative concentration and crystallization of a viscous lactose-containing aqueous liquid
Crystalline alpha-lactose monohydrate is recovered from a viscous lactose-containing aqueous liquid by subjecting said liquid to simultaneous heating, removal of evaporated vapor and mechanical agitation at high shear rate to provide a crystallization promoting decrease of the viscosity of the liquid with crystals formed and suspended therein to progressively concentrate the agitated liquid and simultaneously crystallize lactose therefrom. Subsequent cooling, drying, and disintegration yield particulate alpha-lactose monohydrate.
Owner:NIRO
Pharmaceutical composition for dry powder inhalation and preparation method of composition
ActiveCN105412049AAvoid stimulationReduce surface adsorptionPharmaceutical delivery mechanismPharmaceutical non-active ingredientsLACTOSE MONOHYDRATEMagnesium stearate
The invention provides a pharmaceutical composition for a dry powder inhalation and a preparation method of the composition. The composition comprises a coating agent, lactose monohydrate for a carrier and micronized active pharmaceutical ingredients, wherein the coating agent is inhaled magnesium stearate or a combination of the inhaled magnesium stearate and micronized lactose monohydrate. The pharmaceutical composition for the dry powder inhalation and the preparation method of the composition have the advantages that the inhaled magnesium stearate with specific particle size characteristics or the combination of the inhaled magnesium stearate and the micronized lactose monohydrate serves as the coating agent to be applied to the composition for the dry powder inhalation, stimulus to the lung caused by deposition of magnesium stearate on the lung or other side effects can be avoided, in-vitro determining parameters of the active ingredients can be increased, fine particle proportion of the active ingredients can be increased, and the hydrothermal stability of the product can be improved.
Owner:SICHUAN HAISCO PHARMA CO LTD
Pharmaceutical Compositions of Sevelamer
InactiveUS20120219626A1Urinary disorderSynthetic polymeric active ingredientsCoated tabletsLACTOSE MONOHYDRATE
The invention relates to a pharmaceutical immediate release tablet comprising a core comprising 70-85 weight percent of sevelamer carbonate, calculated as an anhydrous compound, 10-25 weight percent of lactose monohydrate and, optionally, a water soluble film coat surrounding the to a process of making such tablets, to their use in medicine, and to the use of polyvinyl alcohol-polyethylene glycol graft copolymer for making such coated tablets.
Owner:SYNTHON BV
Preparation method of Azilsartan tablets
InactiveCN105030711ADissolution rate is fastSimple processOrganic active ingredientsPill deliveryLACTOSE MONOHYDRATEMicrometer
The invention discloses a preparation method of Azilsartan tablets and belongs to the technical field of medicine. The preparation method comprises the following steps: 1, micronizing the Azilsartan, controlling D90 less than 15 micrometers, and conducting weighing according to formula dosage; 2, screening lactose monohydrate with a 60-mesh screen, and conducting weighing according to formula dosage; 3, screening a disintegrating agent and diluent with a 60-mesh screen respectively, and conducting weighing according to formula dosage; 4, mixing the auxiliary ingredients in the step 1, 2 and 3, adding solubilizer, conducting mixing, and screen the mixture with a 60-mesh screen; mixing the auxiliary ingredients uniformly, and adding adhesion agents for soft materials; conducting sieving, granulating, drying and arranging, adding lubricating agents, mixing the mixture uniformly, and tabletting. According to the preparation method, by controlling the particle diameter of the Azilsartan and the weight ratio between the Azilsartan and the lactose monohydrate and the varieties and adjusting the proportion of the disintegrating agents, the lubricating agents, the diluent, the solubilizer and the binding agents, the problem of dissolution rate can be solved, and the problem of external consistent dissolution is solved as well.
Owner:JIANGSU ZHONGBANG PHARMA
Ivabradine hydrochloride tablet and preparation method thereof
InactiveCN106265582AImprove compliancePharmaceutical non-active ingredientsDrageesLACTOSE MONOHYDRATEIVABRADINE HYDROCHLORIDE
The invention belongs to the technical field of medicine preparation, and relates to an ivabradine hydrochloride tablet and a preparation process thereof. A mark is formed in the ivabradine hydrochloride tablet, and two cracked tablets fulfill the dosage of 2.5+ / -0.1mg after the tablet is torn according to the mark. The ivabradine hydrochloride tablet is prepared from ivabradine hydrochloride, lactose monohydrate, starch, PVPK30, aerosil and magnesium stearate. When a patient takes the tablet, the patient takes half tablet by tearing the tablet according to the mark so as to fulfill the dosage of 2.5mg, or takes the whole tablet to fulfill the dosage of 5mg, or takes one and a half tablets to fulfill the dosage of 7.5mg. Compared with the prior art, the ivabradine hydrochloride tablet and the preparation process have the advantages that a ivabradine hydrochloride tablet with small specification can meet the requirement of multiple usages and dosages, so that the patient compliance can be improved.
Owner:CISEN PHARMA
Alfuzosin tablets and synthesis
A monolithic composition includes alfuzosin in a polymeric matrix adapted to release 13-33% of the alfuzosin within 2 hours, 40-60% of the alfuzosin within 7 hours, and greater than 80% of the alfuzosin within 20 hours of administration. A unit dosage form includes: a heterogeneous mixture of alfuzosin hydrochloride anhydrate, lactose monohydrate, hydroxypropylmethylcellulose, polyvinylpyrrolidone and magnesium stearate, wherein the heterogeneous mixture is heterogeneously distributed throughout the unit dosage form. A manufacturing process includes: mixing a hydrophilic polymer and alfuzosin to provide a blend; granulating the blend to provide granules; drying the granules on a dryer to provide dried granules; sizing the dried granules to provide sized granules; mixing the sized granules with a lubricant to obtain a mixture; and compressing the mixture to obtain a tablet. A method of treating benign prostatic hyperplasia, includes administering to a patient the composition or unit dosage form once a day.
Owner:CIMEX PHARMA
Dry powder inhalation medicine composition and preparation method thereof
InactiveCN105982880AAvoid stimulationImproving In Vitro Assay ParametersPowder deliveryPharmaceutical non-active ingredientsIndacaterolAdditive ingredient
The invention provides a dry powder inhalation medicine composition and a preparation method thereof. The composition is prepared from a coating agent with a specific particle size characteristic, lactose monohydrate with a specific particle size characteristic for a carrier and a micronized medicinal active ingredient, wherein the coating agent is an inhaling magnesium stearate or a mixture of the inhaling magnesium stearate and micronized lactose monohydrate; and the medicinal active ingredient is selected from at least one of glycopyrronium bromide, umeclidinium, indacaterol, formoterol, vilanterol, fluticasone and pharmaceutically available salt of the active ingredients. The preparation method comprises the following steps of sufficiently mixing and coating the coating agent and the lactose monohydrate, and uniformly mixing with the micronized medicinal active ingredients.
Owner:SICHUAN HAISCO PHARMA CO LTD
Afatinib dimaleate tablet and preparation method thereof
InactiveCN106074427AInhibition of segregationAvoid unevennessOrganic active ingredientsPharmaceutical non-active ingredientsCrospovidonesLACTOSE MONOHYDRATE
The invention provides an afatinib dimaleate tablet and a preparation method thereof. The afatinib dimaleate tablet is composed of a table core and a film coating wrapping the exterior of the table core and prepared by taking lactose monohydrate and microcrystalline cellulose PH301 as filling agents, taking crospovidone as a disintegrating agent, taking silicon dioxide as a flow aid and taking magnesium stearate as a lubricating agent. The preparation method has the advantages that the tablet is prepared by adopting a dry granulation technique, the fluidity can be improved, separation of all the components can be prevented to avoid the condition that the tablet components are not uniform, the bulk density is regulated, and the dissolving property and uniform pressure transmission in tablet production are improved.
Owner:NANJING CHENGONG PHARM CO LTD
Stable enalapril maleate capsules and preparation method thereof
InactiveCN105125514AHigh hardnessImprove the disintegration effectPill deliveryPharmaceutical non-active ingredientsLACTOSE MONOHYDRATEArginine
The invention relates to stable enalapril maleate capsules and a preparation method thereof, and belongs to the technical field of medicine. Enalapril maleate, lactose monohydrate, microcrystalline cellulose, startch, hydroxypropyl methyl cellulose, sodium alga acid, poloxamer 407, polyethylene glycol 4000 and L-arginine are preferably used as medical components of the stable enalapril maleate capsules, the degradation speed of the enalapril maleate is remarkably decreased through the mutual synergistic effect, the stability of the enalapril maleate capsules is greatly improved, and it is ensured that clinical medicine is safe, effective and capable of being stored for a long time.
Owner:CSPC OUYI PHARM CO LTD
Solid composition containing rivaroxaban and preparation method thereof
InactiveCN107773548AImprove crushing efficiencyHigh yieldOrganic active ingredientsPharmaceutical non-active ingredientsLACTITOL MONOHYDRATELACTOSE MONOHYDRATE
The invention discloses a solid composition containing rivaroxaban. The solid composition is prepared from the following components in percentage by weight: 20 percent to 80 percent of a micronized rivaroxaban lactose monohydrate mixture, wherein in the micronized rivaroxaban lactose monohydrate mixture, the weight percent ratio of rivaroxaban to lactose monohydrate ranges from (1 to 2) to (1 to 10); the solid composition is prepared from the following components: 20 percent to 80 percent of a diluting agent, 1 percent to 10 percent of a bonding agent, 2 percent to 8 percent of a disintegrating agent, 0.5 percent to 8 percent of a surfactant and 0.5 percent to 5 percent of a lubricant. Furthermore, the invention further provides a preparation method of the solid composition containing therivaroxaban; the preparation method comprises the following steps: crushing crude drugs, mixing, granulating, drying, totally blending, tabletting and coating. Compared with the prior art, a crushingmethod of the crude drugs has high yield and the crude drugs have small granularity and are uniformly micronized; rivaroxaban tablets disclosed by the invention have a high dissolution rate, the content of a single impurity and total impurities is lower and the stability is high; the production quality is ensured.
Owner:CHONGQING ZEN PHARMACEUTICAL CO LTD
Azilsartan tablets and preparation method thereof
InactiveCN105853384ASolve the hardnessSolve the defect of poor friabilityOrganic active ingredientsPill deliveryLACTOSE MONOHYDRATESpray Granulation
The invention discloses azilsartan tablets and a preparation method thereof, and belongs to the field of pharmacy. For the azilsartan tablets, each azilsartan tablet is prepared from, by mass, 7.80%-16.30% of azilsartan, 59.78%-70.18% of lactose monohydrate, 15.00%-18.00% of microcrystalline cellulose, 1.15%-1.40% of hydroxypropyl methylcellulose (5cps), 2.20%-2.80% of low-substituted hydroxypropyl cellulose, 2.20%-2.80% of polyethylene glycol 6000 and 0.55%-0.85% of magnesium stearate. By means of a fluidized bed top-spraying granulation method, three processes of mixing, granulation and drying in a traditional technology are completed in one step, the production efficiency is improved, and the prepared granules are uniform in granule size and good in fluidity and compression formability; meanwhile, due to the fact that a disintegrating agent is added internally and externally, the problem that the azilsartan tablets disintegrate and dissolve slowly is solved.
Owner:JIANGSU ZHONGBANG PHARMA
Rivaroxaban tablets and preparation method for same
InactiveCN105078915ASolve the problem of slow disintegration and dissolutionUniform particle sizeOrganic active ingredientsPharmaceutical product form changeLACTITOL MONOHYDRATEDissolution
The invention provides rivaroxaban tablets and a preparation method for the same, and belongs to the field of pharmacy. Each rivaroxaban tablet is prepared from the following components in percentage by mass: 8.16 to 16.63 percent of rivaroxaban, 30.00 to 35.00 percent of lactose monohydrate, 41.56 to 48.94 percent of microcrystalline cellulose (ph101), 1.63 to 2.49 percent of sodium dodecyl sulfate, 2.08 to 3.26 percent of hydroxypropyl methyl cellulose, 3.32 to 4.9 percent of croscarmellose sodium and 0.49 to 0.67 percent of magnesium stearate. According to the novel rivaroxaban tablets and the internal and external disintegrant addition mixed-tabletting preparation method, croscarmellose sodium is internally and externally added, so that the problem of slow disintegration and dissolution of azilsartan tablets is solved; with adoption of a fluidized bed top-spraying granulation method, three procedures of mixing, granulation and drying in a conventional process are implemented by one step, so that production efficiency is improved, prepared granules are uniform in granularity and high in flowability and compression formability, and in addition, the problems of sticking, loosening and the like in a tabletting process are also solved.
Owner:JIANGSU ZHONGBANG PHARMA
Alfuzosin tablets and synthesis
A monolithic composition includes alfuzosin in a polymeric matrix adapted to release 13-33% of the alfuzosin within 2 hours, 40-60% of the alfuzosin within 7 hours, and greater than 80% of the alfuzosin within 20 hours of administration. A unit dosage form includes: a heterogeneous mixture of alfuzosin hydrochloride, lactose monohydrate, hydroxypropylmethylcellulose, polyvinylpyrrolidone and magnesium stearate, wherein the heterogeneous mixture is heterogeneously distributed throughout the unit dosage form. A manufacturing process includes: mixing a hydrophilic polymer and alfuzosin to provide a blend; granulating the blend to provide granules; drying the granules on a dryer to provide dried granules; sizing the dried granules to provide sized granules; mixing the sized granules with a lubricant to obtain a mixture; and compressing the mixture to obtain a tablet. A method of treating benign prostatic hyperplasia, includes administering to a patient the composition or unit dosage form once a day.
Owner:ACINO PHARMA
Medicine composition containing Enalapril maleate, folic acid and acid stabilizer
ActiveCN107007838AImprove stabilityStable than commercially availableOrganic active ingredientsPill deliveryLACTOSE MONOHYDRATEDissolution
The invention provides a new Enalapril maleate and folic acid composite preparation. An acid stabilizer with weight ratio of 1%-5% is added in the preparation to improve the stability of the preparation at high temperature and long-term standing condition; and meanwhile, a mixture of microcrystalline cellulose and lactose monohydrate is selected asfiller, the dissolution of the folic acid in the 0.1M hydrochloric acid medium is improved, and a finally prepared tablet is basically consistent with the market tablet. The composition disclosed by the invention is good in stability, capable of guaranteeing the clinical safe use of the medicine, simple in process operation, and suitable for industrial production.
Owner:NANJING YOKO PHARMA +2
Frozen semen diluent of equus animals and preparation method thereof
The invention discloses a frozen semen diluent of equus animals and a preparation method thereof. The diluent at least comprises a basic solution prepared from the following raw materials: D-lactose monohydrate, prostaglandin, EDTA (Ethylene Diamine Tetraacetic Acid), a bee pollen aqueous solution, hyaluronic acid and squalane. The diluent also comprises egg yolk liquid, glycerin, penicillin and streptomycin. The preparation method comprises the following steps: (1) preparing the bee pollen aqueous solution; (2) preparing the basic solution; (3) adding the egg yolk liquid, the glycerin, the penicillin and / or the streptomycin. By adopting the technical scheme, the preparation method disclosed by the invention has the benefits that the bee pollen aqueous solution, the hyaluronic acid, the squalane and the like are creatively combined for preparation of the frozen semen diluent of the equus animals to ensure that the forming of large ice crystals in semen freezing process is prevented to the maximum limit, so that the damage of ice crystallization to semen is avoided, the survival rate and the acrosome intact rate of frozen semen are remarkably increased, the quality of the frozen semen is further improved and the freezing tolerance of spermatid is increased.
Owner:ILI RUIDE JUNFA BIOTECH +2
Rivaroxaban tablet and preparation method thereof
InactiveCN105267169AImprove liquiditySuitable for useOrganic active ingredientsOrganic chemistryLACTOSE MONOHYDRATEMedicine
The invention relates to a Rivaroxaban tablet and a preparation method thereof, and belongs to the technical field of medicinal preparations. The Rivaroxaban tablet is prepared from, by weight, 10-20 parts of Rivaroxaban, 4 parts of internally-added superfine silica powder, 21-25 parts of internally-added microcrystalline cellulose, 22.9-24.9 parts of lactose-monohydrate, 2 parts of internally-added croscarmellose sodium, 10-14 parts of externally-added microcrystalline cellulose, 1 part of externally-added croscarmellose sodium, 3 parts of hydroxypropyl methyl cellulose 5cp, 0.5 part of lauryl sodium sulfate and 0.6 part of externally-added superfine silica powder. According to the Rivaroxaban tablet, the mutual synergistic effect is achieved at the specific matching, and the stability of tablet products is greatly improved; in addition, the dissolution rate is high, the quality problem existing in existing products all the time is solved, the safety, effectiveness and long-term storage of clinical medication can be better guaranteed.
Owner:SHIJIAZHUANG KANGHEWEI PHARMA
Abiraterone acetate tablet and preparing method thereof
InactiveCN104069075AReduce pollutionReduce processing operationsOrganic active ingredientsPill deliveryLACTOSE MONOHYDRATELactose
A stable abiraterone acetate tablet and a preparing method thereof are disclosed. The abiraterone acetate tablet is free of aerosil and is prepared from following components: abiraterone acetate, lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, povidone, sodium dodecylsulfate and magnesium stearate. The abiraterone acetate tablet has characteristics of stability, simple preparation process and controllability.
Owner:NANJING CAVENDISH BIO ENG TECH +1
Method for the treatment of acne and certain dosage forms thereof
ActiveUS20100035846A1Less side effectsEffective treatmentBiocideTetracycline active ingredientsLACTOSE MONOHYDRATELactose
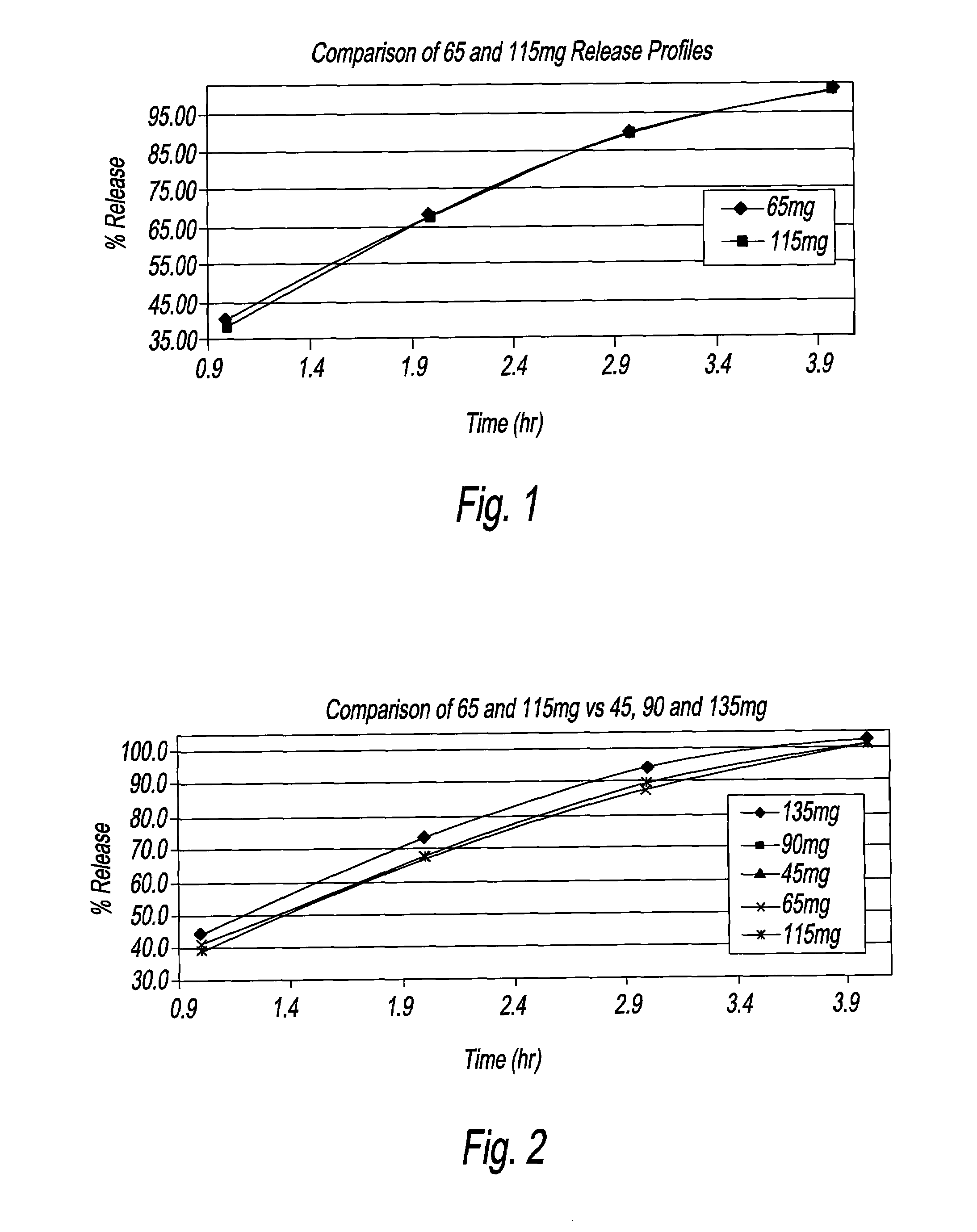
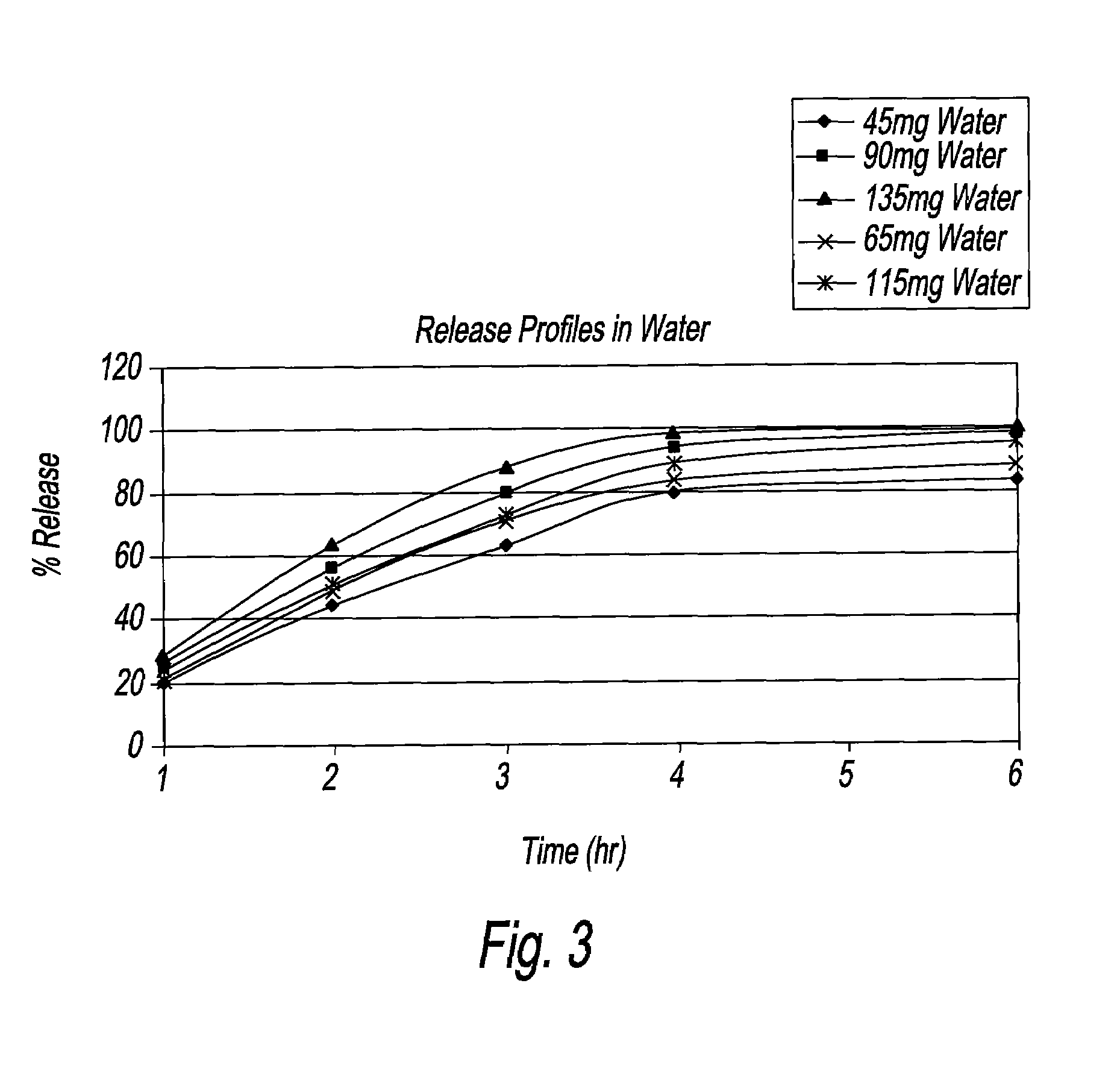
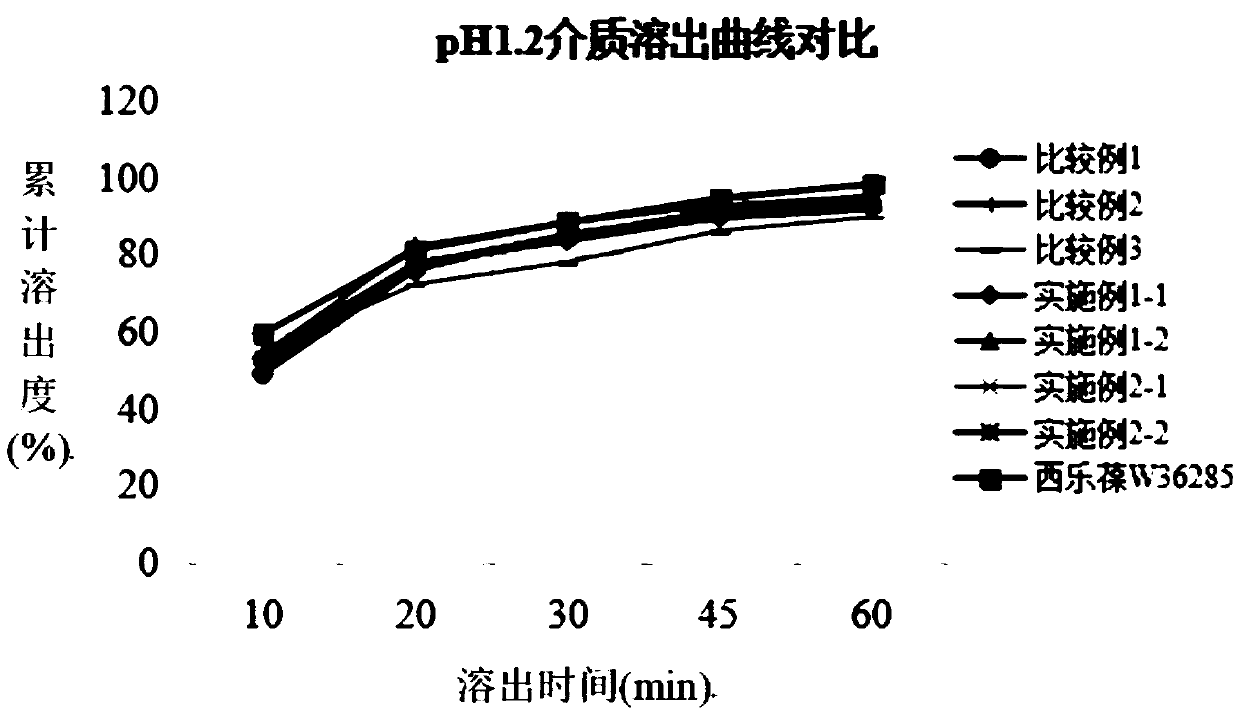
An oral dosage form, including 65 mg of minocycline, an amount of lactose monohydrate, and an amount of hydroxypropylmethylcellulose. The hydroxypropylmethylcellulose is hydroxypropylmethylcellulose that is about 8.9±0.2% hydroxypropoxylated. An oral dosage form, including 115 mg of minocycline, an amount of lactose monohydrate, and an amount of hydroxypropylmethylcellulose. The hydroxypropylmethylcellulose is hydroxypropylmethylcellulose that is about 8.9±0.2% hydroxypropoxylated.
Owner:MEDICIS PHARMA CORP
Solid dispersion method of celecoxib and preparation method of celecoxib capsules
ActiveCN110604722AEffective dispersionHigh densityOrganic active ingredientsPowder deliveryLACTOSE MONOHYDRATEMechanical milling
The invention provides a solid dispersion method of celecoxib and a preparation method of celecoxib capsules, and the solid dispersion method comprises the following steps: mixing the celecoxib with pharmaceutical adjuvants to obtain raw and auxiliary material mixed powder, and carrying out ultramicro jet milling and / or mechanical milling on the raw and auxiliary material mixed powder, wherein thepharmaceutical adjuvants at least comprise lactose monohydrate and lauryl sodium sulfate. The solid dispersion method disclosed by the invention not only overcomes the characteristic of poor preparation of a celecoxib preparation, but also solves the problem of slow dissolution of the celecoxib, and the preparation is good in stability, so that the preparation is consistent with an original developed preparation (Celebrex) in prescription and dissolution, and the quality consistency and bioequivalence of the celecoxib preparation and the original developed preparation (Celebrex) are ensured.
Owner:SHANDONG CHUANGXIN PHARMA RES & DEV
Celecoxib capsules and production technology thereof
InactiveCN105232494ALow costSimple processOrganic active ingredientsAntipyreticLACTITOL MONOHYDRATELACTOSE MONOHYDRATE
The invention discloses celecoxib capsules. The celecoxib capsules are prepared from celecoxib, lactose monohydrate, povidone, crosslinking sodium carboxymethylcellulose, lauryl sodium sulfate and magnesium stearate. The invention also discloses a production technology of the celecoxib capsules. The production technology comprises the steps of material distribution, preparation of a granulation solution, premixing, granulation, wet size stabilization, drying, dry size stabilization, final mixing, granule discharging, capsule filling and packaging. The celecoxib capsules and the production technology thereof have the advantages that formulation raw materials are low in cost, and the technology is simple; the dissolution rate of celecoxib is high, and celecoxib can be easily absorbed by the human body.
Owner:青岛百洋制药有限公司
Lercanidipine hydrochloride and losartan potassium compound preparation and preparation method thereof
ActiveCN102600146AReduce adverse reactionsImprove protectionOrganic active ingredientsMetabolism disorderTolerabilitySodium starch
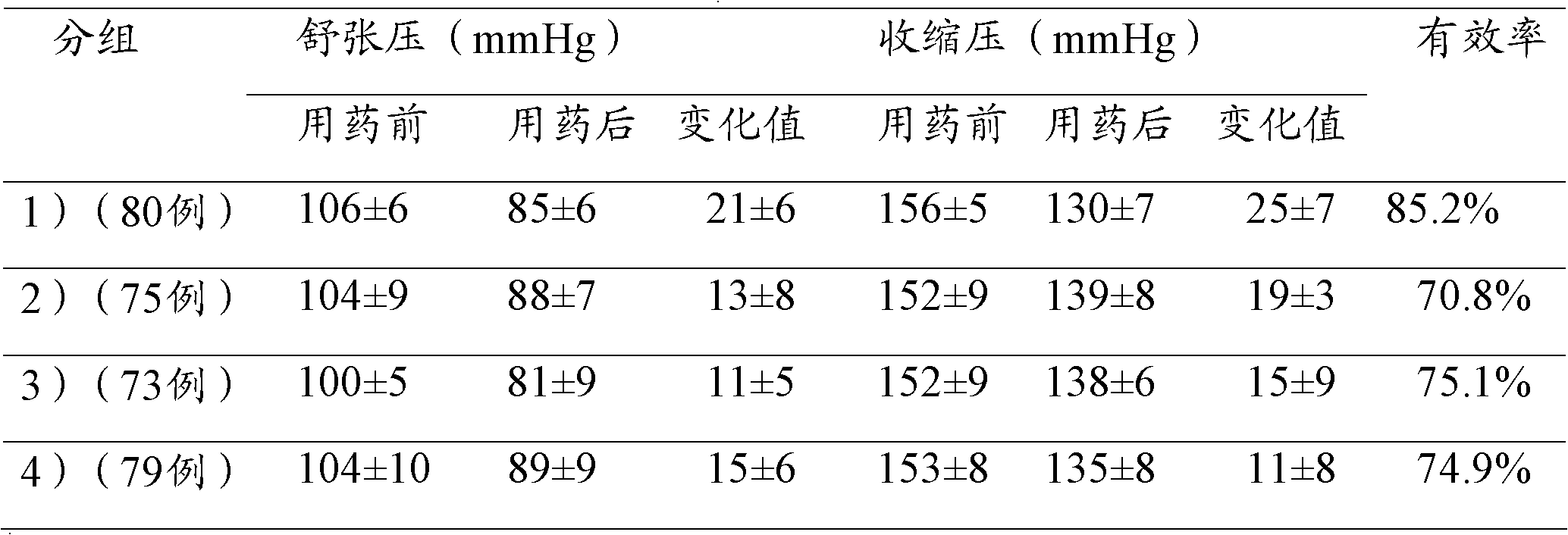
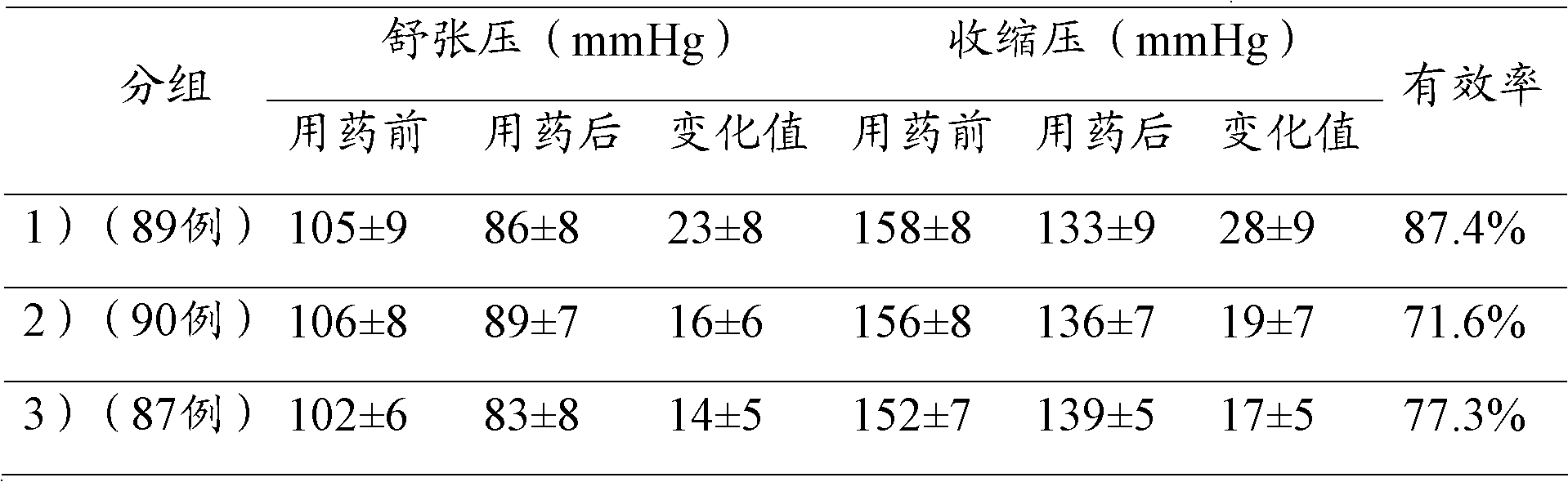
The invention relates to the field of medicines, and in particular discloses a lercanidipine hydrochloride and losartan potassium compound preparation. Particularly, the invention also provides a compound preparation which takes lercanidipine hydrochloride and losartan potassium as basic remedy, takes lactose monohydrate, microcrystalline cellulose, A-type sodium starch glycollate, povidone K30, magnesium stearate, pregelatinized starch and colloidal silicon dioxide as excipients, and takes white Opadry as a coating to prepare a tablet. Clinical tests prove that compared with the single-component preparation, the lercanidipine hydrochloride and losartan potassium compound preparation provided by the invention is remarkably increased in effective rate in light and moderate blood pressures, is remarkably reduced in occurrence rate of adverse effects, and has better clinical application prospect because patients have good tolerance.
Owner:ZHAOKE PHARMA HEFEI
Isosorbide mononitrate tablet and quality detection method thereof
InactiveCN110559269AReasonable compositionSimple preparation processComponent separationPill deliveryLACTOSE MONOHYDRATEMicrosphere
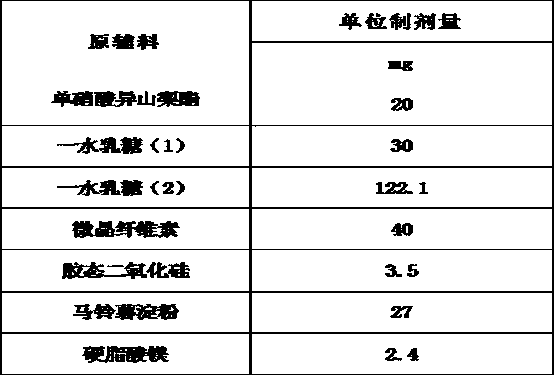
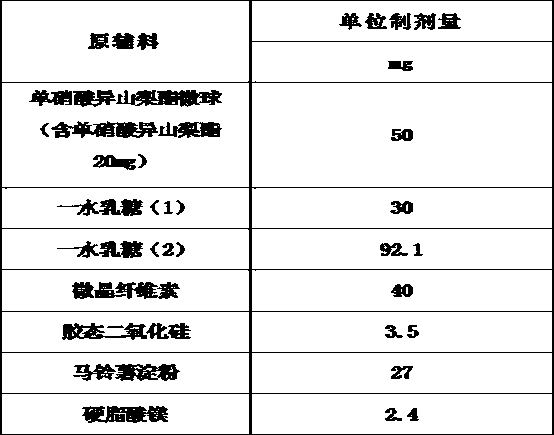
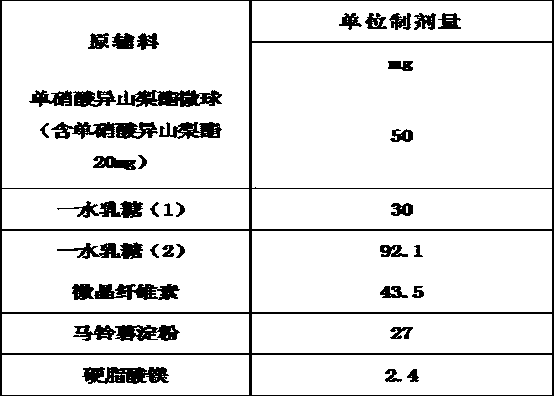
The invention belongs to the technical field of medicines, and discloses an isosorbide mononitrate tablet, which comprises 50 mg of isosorbide mononitrate microspheres, 30 mg of lactose monohydrate (1), 92.1 mg of lactose monohydrate (2), 40 mg of microcrystalline cellulose, 3.5 mg of colloidal silicon dioxide, 27 mg of potato starch and 2.4 mg of magnesium stearate. The tablet is reasonable in components and high in stability.
Owner:仁和堂药业有限公司
Extended-release minocycline dosage forms
An oral dosage form has the following: an amount of minocycline selected from the group consisting of 55 mg, 80 mg, and 105 mg; an amount of lactose monohydrate; an amount of hydroxypropylmethylcellulose. The hydroxypropylmethylcellulose is at least 8.3 to about 9.8% hydroxypropoxylated. The minocycline in the oral dosage form has a dissolution profile or release rates about 35% to about 50% in 1 hour, about 60% to about 75% in 2 hours, and at least about 90% in 4 hours. There is also provided a method of treating acne in a human and a method of assisting a physician in prescribing a dose of minocycline for the treatment of acne.
Owner:MEDICIS PHARMA CORP
Flower-shaped lactose loaded curcumin nano dry powder inhalant and preparation method thereof
InactiveCN111956631ALarge specific surface areaImprove liquidityPowder deliveryKetone active ingredientsLACTOSE MONOHYDRATEDrug carrier
The invention relates to a flower-shaped lactose loaded curcumin nano dry powder inhalant and a preparation method thereof, and belongs to the technical field of medical treatment. The flower-shaped lactose loaded curcumin nano dry powder inhalant consists of a drug carrier and curcumin solid lipid nanoparticles; the drug carrier is flower-shaped lactose; the flower-shaped lactose is prepared by performing a reaction on lactose monohydrate and citric acid; the flower-shaped lactose has a pore surface area of 30 plus and minus 7m2 / g, a pore volume of 0.7 plus and minus 0.3cm3 / g, a diameter peakvalue of 3.4, 5.6 and 12.4nm and an aerodynamic particle size of 2 to 4mum, and is adjustable in crude powder size; the curcumin solid lipid nanoparticles have an entrapment rate of 87.73%, a drug-loading rate of 7.72%, a particle size of 156.9 plus and minus 2.2nm, a polydispersity coefficient of 0.480 and an average Zeta potential of minus 24.8mV; and the dry powder inhalant is administrated toa respiratory system by a dry powder inhaler.
Owner:常州道宁医药科技有限公司 +1
Indapamide tablet and preparation method thereof
InactiveCN110538160ASolve the speed problemQuality improvementOrganic active ingredientsDrageesAdhesivePolyvinyl alcohol
The invention belongs to the technical field of pharmaceutical preparations, and particularly relates to an indapamide tablet and a preparation method thereof. The indapamide tablet is composed of a tablet core and a coating layer, and the tablet core is prepared from the following components in parts by weight: 0.5-2 parts of indapamide, 10-40 parts of a filler, 0.5-5 parts of an adhesive, and 0.5-10 parts of a lubricant, wherein the filler is any one or a combination of two or more of lactose anhydrous, lactose monohydrate, corn starch, microcrystalline cellulose lactose and starch lactose,the adhesive is any one or a composition of two or more of povidone K30, hydroxypropyl methylcellulose, polyvinyl alcohol and carboxymethylcellulose, and the lubricant is any one or a composition of two or more of talcum powder, magnesium stearate and silicon dioxide. The obtained indapamide tablet is a stable drug preparation which is lower in impurity content than a reference listed drug reference preparation, and has the bioequivalence.
Owner:TIANJIN PHARMA GROUP XINZHENG
Tenofovir alafenamide fumarate raw material medicine and production process thereof
InactiveCN108299500AExcellent anti-hepatitis B effectStrengthen the anti-hepatitis B effectGroup 5/15 element organic compoundsCarboxylic acid salt preparationTenofovir alafenamideEthyl acetate
The invention discloses a tenofovir alafenamide fumarate raw material medicine and a production process thereof. The tenofovir alafenamide fumarate raw material medicine includes anhydrous tenofovir,triphenyl phosphite, 4-dimethylaminopyridine, triethylamine, acetonitrile, ethyl acetate, methanol, concentrated hydrochloric acid, thionyl chloride, toluene, dichloromethane, L-alanine isopropyl ester hydrochloride, potassium hydrogencarbonate, fumaric acid and isopropanol. Production raw material excipients for the tenofovir alafenamide fumarate raw material medicine include lactose monohydrate,microcrystalline cellulose, croscarmellose sodium and magnesium stearate. The new manufacturing raw materials and the production raw material excipients are selected, so that the anti-hepatitis B effect of the medicine is more excellent, mass production can be performed through the novel production method, and the anti-hepatitis B effect is strengthened to some extent.
Owner:安徽安科恒益药业有限公司
Medicinal composition containing candesartan cilexetil and hydrochlorothiazide and preparation method thereof
InactiveCN102138920APromote dissolutionImprove stabilityOrganic active ingredientsPill deliveryLACTOSE MONOHYDRATEHydrochlorothiazide
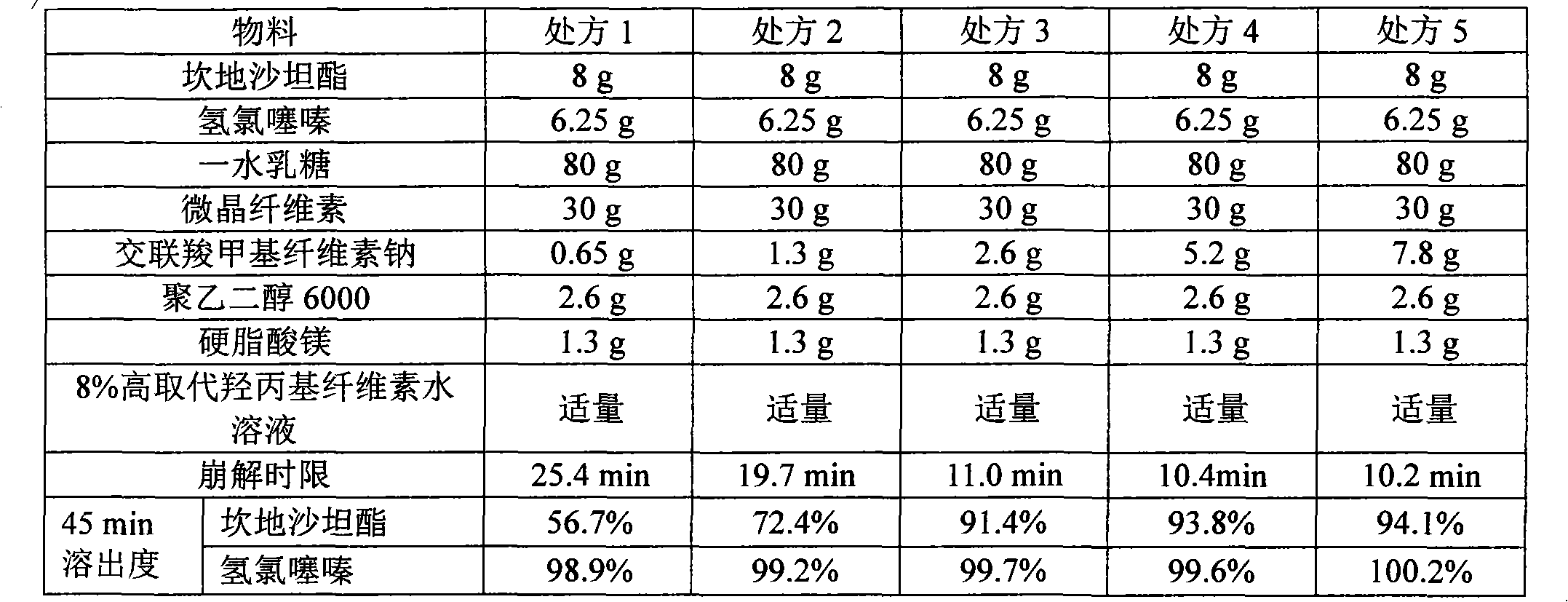
The invention relates to a medicinal composition containing candesartan cilexetil and hydrochlorothiazide and a preparation method thereof. The medicinal composition comprises the following components in parts by weight: 4 or 8 parts of candesartan cilexetil, 6.25 parts of hydrochlorothiazide, 60 to 100 parts of lactose monohydrate, 15 to 40 parts of microcrystalline cellulose, 2.6 parts of croscarmellose sodium, 2.6 parts of polyglycol 6000, and 1.3 parts of magnesium stearate. After the medicinal composition is uniformly mixed, an 8 percent high-substitution hydroxypropyl cellulose aqueous solution is used as a binder to granulate by a wet method and to tablet. In the method, by adopting a certain amount of the polyglycol 6000 as a stabilizer, the preparation stability is remarkably improved; and over 90 percent of dissolution rate can be achieved in 45 minutes by adopting less disintegrant and binder; the preparation method has a simple production process without adding extra equipment; the tablets produced according to the method has good stability and high disintegration speed, so that the dissolution of the medicament is remarkably improved, and measurement proves that: the dissolution of the tablets prepared by adopting the method reaches over 90 percent in 45 minutes.
Owner:HAINAN ZHONGJI MEDICAL TECH
Cefpiramide composite
ActiveCN101966152AEasy to operateRaw materials are easy to getAntibacterial agentsPowder deliveryDextrose MonohydrateMannitol
The invention relates to a cefpiramide composite which is used for the cefpiramide powder-injection in the medicinal manufacture field. The invention discloses the following characteristic of the cefpiramide composite: the composite for the cefpiramide powder-injection which is prepare by the conventional production technology of powder-injection contains two or more of the following components: (a) sterile cefpiramide, (b) sterile sodium carbonate; and (c) sterile anhydrous glucose, dextrose monohydrate, sodium chloride, mannitol, sorbitol, anhydrous lactose or lactose monohydrate, wherein the weight ratio of the component (a) to the component (b) and the component (c) is 1:0.15-0.4:0.03-4.0. The invention has the advantages that the problem that the cefpiramide for injection on the market is difficult to dissolve, especially at a low temperature is solved; the operation of the nurse is easy; and the raw materials are accessible and the drug stability is good.
Owner:GUANGZHOU BAIYUSN TIANXIN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com