Rivaroxaban tablet and preparation method thereof
A technology for rivaroxaban and tablets, which is applied in the directions of pill delivery, pharmaceutical formulations, and non-active ingredients medical preparations, etc., can solve the problems of limited wide application, safety problems, large changes in substances, etc., and achieves good fluidity. , The effect of improving stability and high dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 Preparation of rivaroxaban according to the present invention
[0031] Weigh 10 g of crude rivaroxaban, add it into 50 mL of dioxane: acetonitrile: water = 1:2.5:1 mixed solvent, stir, heat to reflux, dissolve completely, reflux for 20 minutes, and then Lower the temperature of the reaction solution to 10°C at a cooling rate of 10°C, stir at this temperature for 1 hour at a stirring speed of 50 rpm, filter, and dry the obtained solid at 35°C overnight to obtain 9.85 g of rivaroxaban, Yield 98.5%.
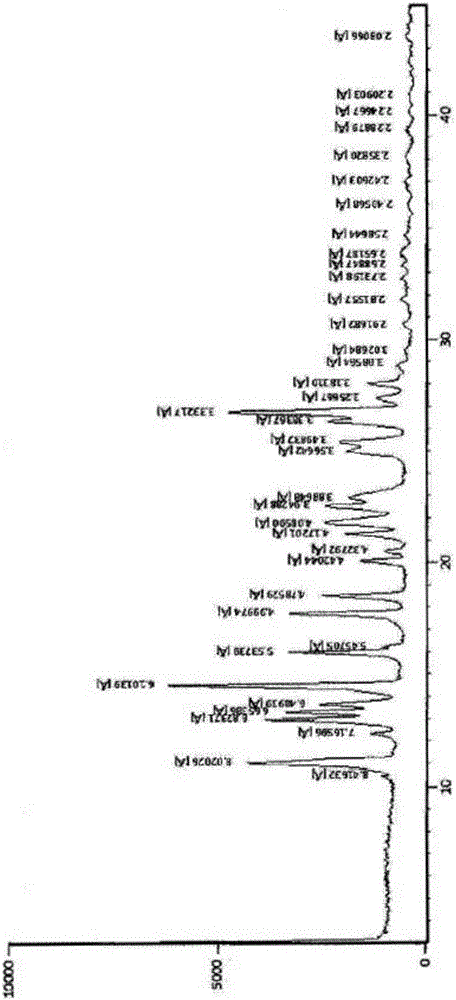
[0032] In the X-ray powder diffraction pattern of rivaroxaban prepared in this example, the 2θ is 11.0±0.2, 12.9±0.2, 13.3±0.2, 13.6±0.2, 14.5±0.2, 16.0±0.2, 17.7±0.2 , 18.5±0.2, 21.3±0.2, 21.7±0.2, 22.5±0.2, 22.9±0.2, 25.5±0.2, 26.3±0.2, 26.75±0.2 have characteristic absorption peaks, such as figure 1 shown.
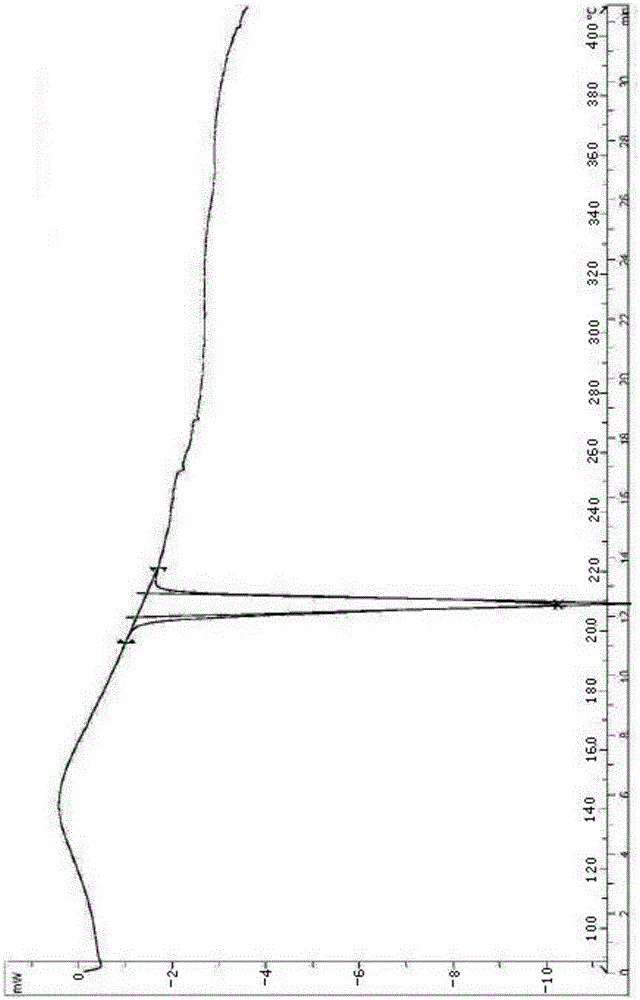
[0033] The rivaroxaban differential scanning calorimetry method determines that rivaroxaban has an endothermic peak at 204.3°C to 212.6°C, and the peak...
Embodiment 2
[0035] Embodiment 2 Preparation of rivaroxaban according to the present invention
[0036] Weigh 10g of the crude product of rivaroxaban, add it into 30ml of dioxane: acetonitrile: water = 1:2.5:1 mixed solvent, stir, heat up to reflux, completely dissolve, reflux for 20 minutes, at 10°C per hour Reduce the temperature of the reaction solution to 10°C at the cooling rate, stir at this temperature for 1 hour at a stirring speed of 50 rpm, filter, and dry the obtained solid at 35°C overnight to obtain 9.61 g of rivaroxaban. The rate is 96.1%.
[0037] The X-ray powder diffraction pattern, differential scanning thermogram, and thermogravimetric pattern of the rivaroxaban prepared in this example are the same as in Example 1.
Embodiment 3
[0038] Embodiment 3 Preparation of rivaroxaban according to the present invention
[0039] Weigh 10 g of the crude product of rivaroxaban, add it into 70 ml of dioxane: acetonitrile: water = 1:2.5:1 mixed solvent, stir, heat up to reflux, completely dissolve, reflux for 20 minutes, at 20 ° C per hour Reduce the temperature of the reaction solution to 10°C at a cooling rate of 10°C, stir at this temperature for 1 hour at a stirring speed of 50 rpm, filter, and dry the obtained solid at 35°C overnight to obtain 9.55 g of rivaroxaban. The rate is 95.5%.
[0040] The X-ray powder diffraction pattern, differential scanning thermogram, and thermogravimetric pattern of the rivaroxaban prepared in this example are the same as in Example 1.
[0041] Test Example 1 High Temperature Stability Test
[0042] Take the rivaroxaban prepared in the above-mentioned Examples 1-3, put it in a flat weighing bottle, spread it into a thin layer with a thickness of ≤5mm, and put it in a thermostat ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com