Medicine composition containing Enalapril maleate, folic acid and acid stabilizer
A technology of enalapril maleate and acid stabilizer, which is applied in the directions of medical preparations containing active ingredients, medical preparations without active ingredients, and pill delivery, etc., can solve problems such as type II instability, and achieve improved performance. The effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] A suitable stabilizer was screened by selecting different acids, citric acid, fumaric acid, malic acid, arginine and maleic acid, and no acid was added in the control, and the results are shown in Table 1.
[0044] Table 1 Acid Stabilizer Selection
[0045]
[0046] Preparation Process:
[0047] (1) Weigh folic acid and starch through a 60-mesh sieve and mix in equal increments, add acid stabilizer citric acid, fumaric acid, malic acid, arginine or maleic acid, and mix with enalapril maleate Pass 60 mesh sieve;
[0048] (2) Weigh the prescription amount of disintegrant croscarmellose sodium and binder povidone K30, pass through a 60-mesh sieve and mix uniformly by equal increment method, and mix with microcrystalline cellulose 102 to pass through 60 mesh screen;
[0049] (3) Add lubricant magnesium stearate, mix and press into tablets to make 1000 tablets.
[0050] Related substance detection method: Waters high performance liquid chromatography e2695-2489 instru...
Embodiment 2
[0053] Table 2 maleic acid dosage screening
[0054]
[0055] The preparation method is the same as in Example 1.
[0056] Be 0.1%~5% by weight percentage of maleic acid dosage to the stability influence of preparation, the preparation that prescription Rx6~Rx12 obtains is placed 10 days under 60 ℃ high temperature, humidity RH 92.5% and 4500lx strong light irradiation conditions Influencing factor experiment. As a result, it was found that the impurities of enalapril diketone, enalaprilat, and capric acid in the preparation did not change significantly under the conditions of humidity RH 92.5% and strong light irradiation of 4500lx, but under the high temperature condition of 60°C, enalapril Diketones still increase. With the increase of the amount of maleic acid in the prescription, the impurity of enalapril diketone gradually decreased, but when the amount of maleic acid increased to 5%, the impurity no longer decreased. Calculated by weight percentage, when the malei...
Embodiment 3
[0060] Table 3 Filler Screening
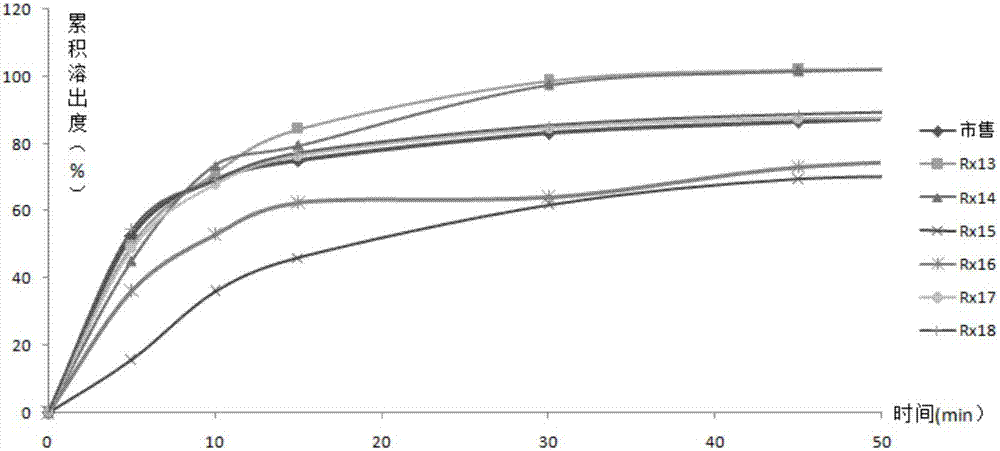
[0061] Element Rx13 Rx14 Rx15 Rx16 Rx17 Rx18 enalapril maleate 10.0g 10.0g 10.0g 10.0g 10.0g 10.0g folic acid 0.8g 0.8g 0.8g 0.8g 0.8g 0.8g starch 0 0 30.0g 30.0g 0 0 anhydrous lactose 80.0g 0 50.0g 0 0 0 lactose monohydrate 0 80.0g 0 50.0g 50.0g 50.0g Microcrystalline Cellulose 102 0 0 0 0 30.0g 0 Microcrystalline Cellulose 101 0 0 0 0 0 30.0g Croscarmellose Sodium 5.0g 5.0g 5.0g 5.0g 5.0g 5.0g Povidone K30 1.0g 1.0g 1.0g 1.0g 1.0g 1.0g maleic acid 2.0g 2.0g 2.0g 2.0g 2.0g 2.0g Magnesium stearate 1.0g 1.0g 1.0g 1.0g 1.0g 1.0g
[0062] Tablet preparation method is with embodiment 1.
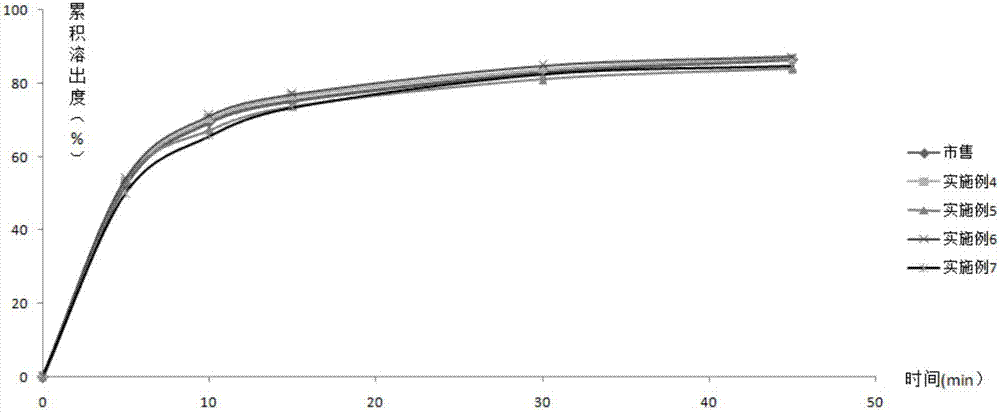
[0063] According to the second method of appendix XC of the Chinese Pharmacopoeia in 2010, 500mL of 0.1M hydrochloric acid solution is the dissolution medium, and the rotating speed is 50rpm. Rx11~Rx16 and commercially ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com