Irbesartan and hydrochlorothiazide pharmaceutical composition and preparation method thereof
A technology of hydrochlorothiazide and composition, which is applied in the direction of drug combination, pharmaceutical formula, medical preparations of non-active ingredients, etc., can solve the problems of unavailable tablet products, unreasonable prescription dosage, and low density of irbesartan, etc., and achieve Excellent dissolution performance, good quality stability, not easy to decompose and deteriorate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
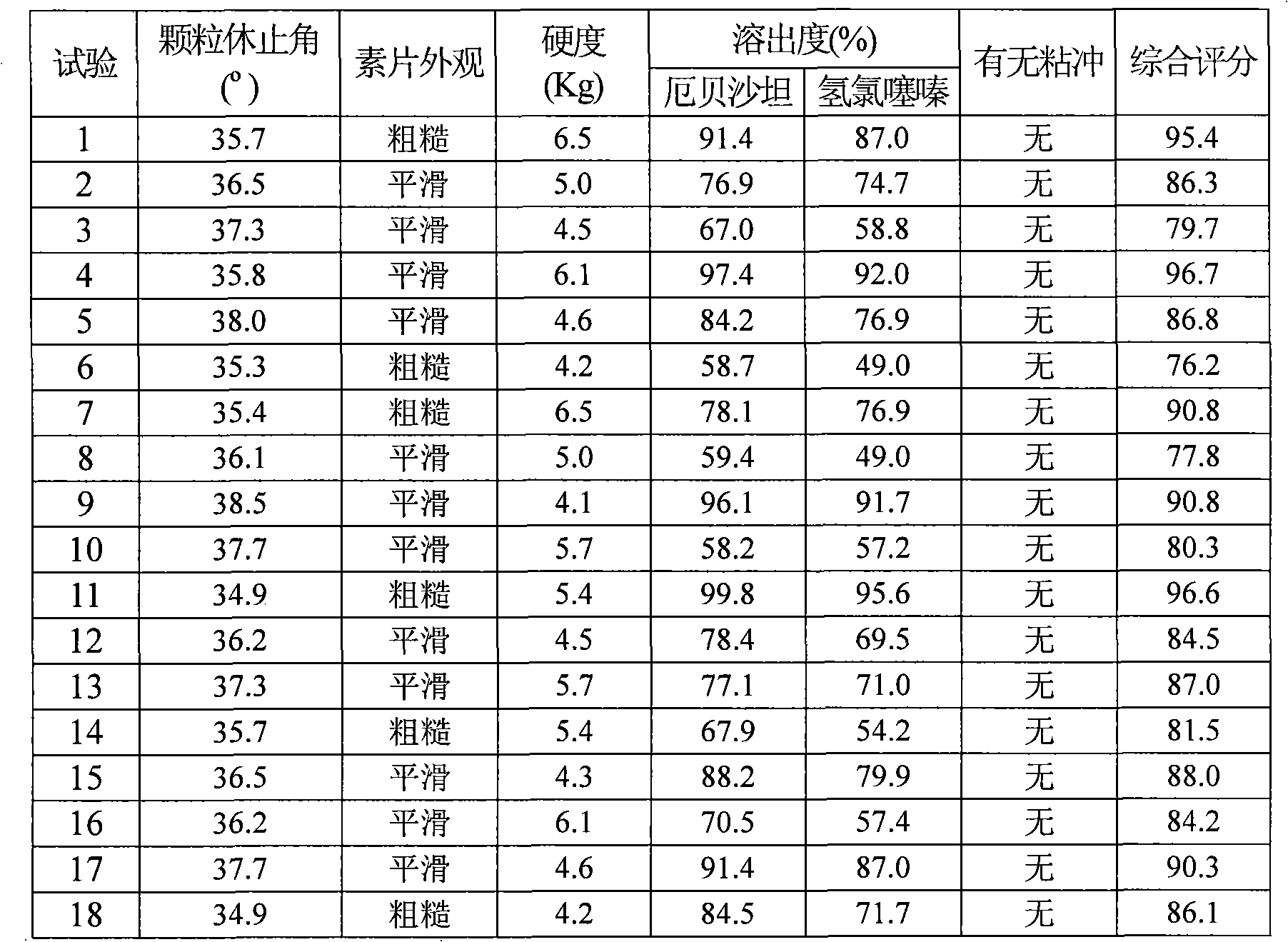
Examples
Embodiment 1
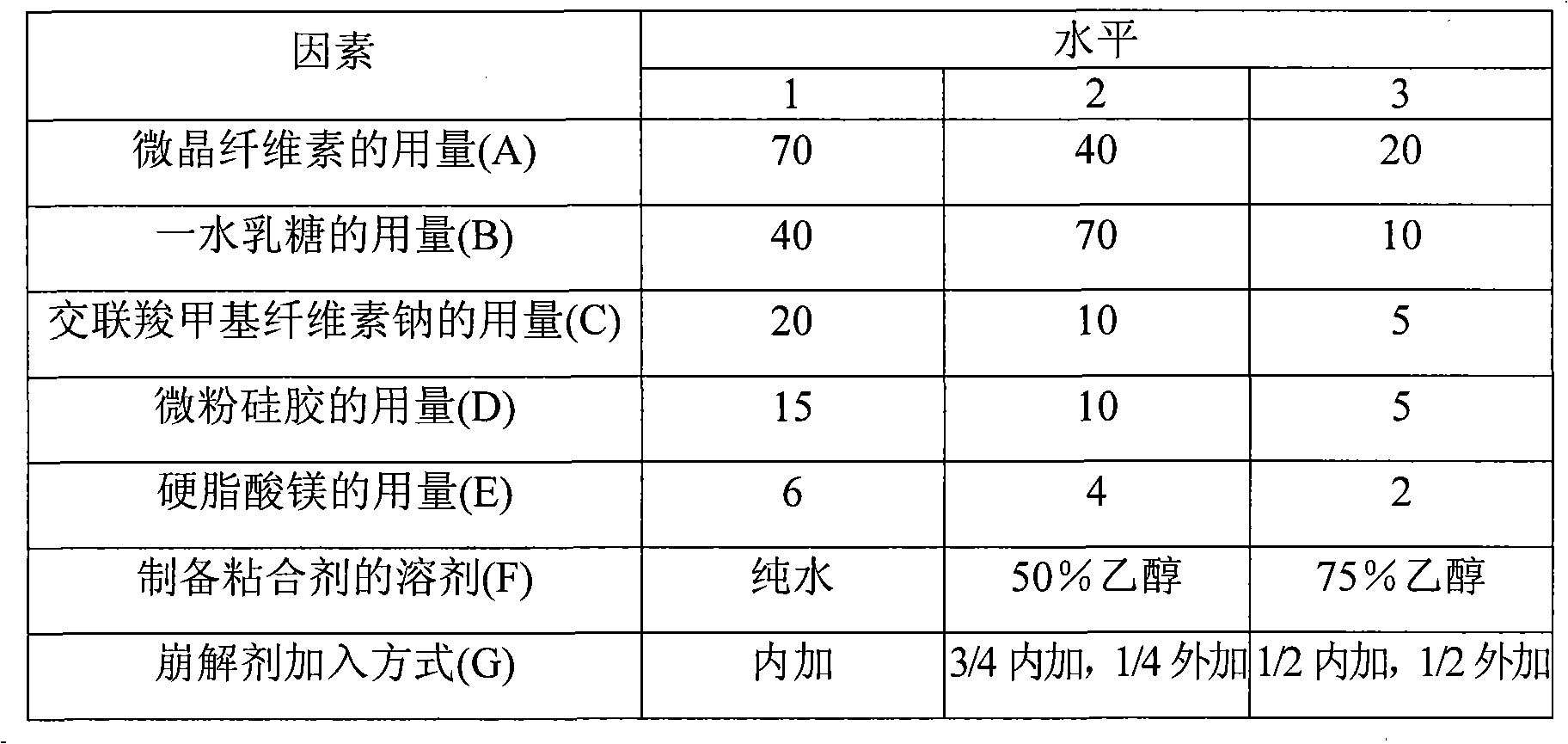
[0049] Prescription: Irbesartan 150g
[0050] Hydrochlorothiazide 12.5g
[0051] Microcrystalline Cellulose 40g
[0052] Lactose monohydrate 40g
[0053] Croscarmellose Sodium 20g
[0054] Micronized silica gel 5g
[0056] Hypromellose 3g
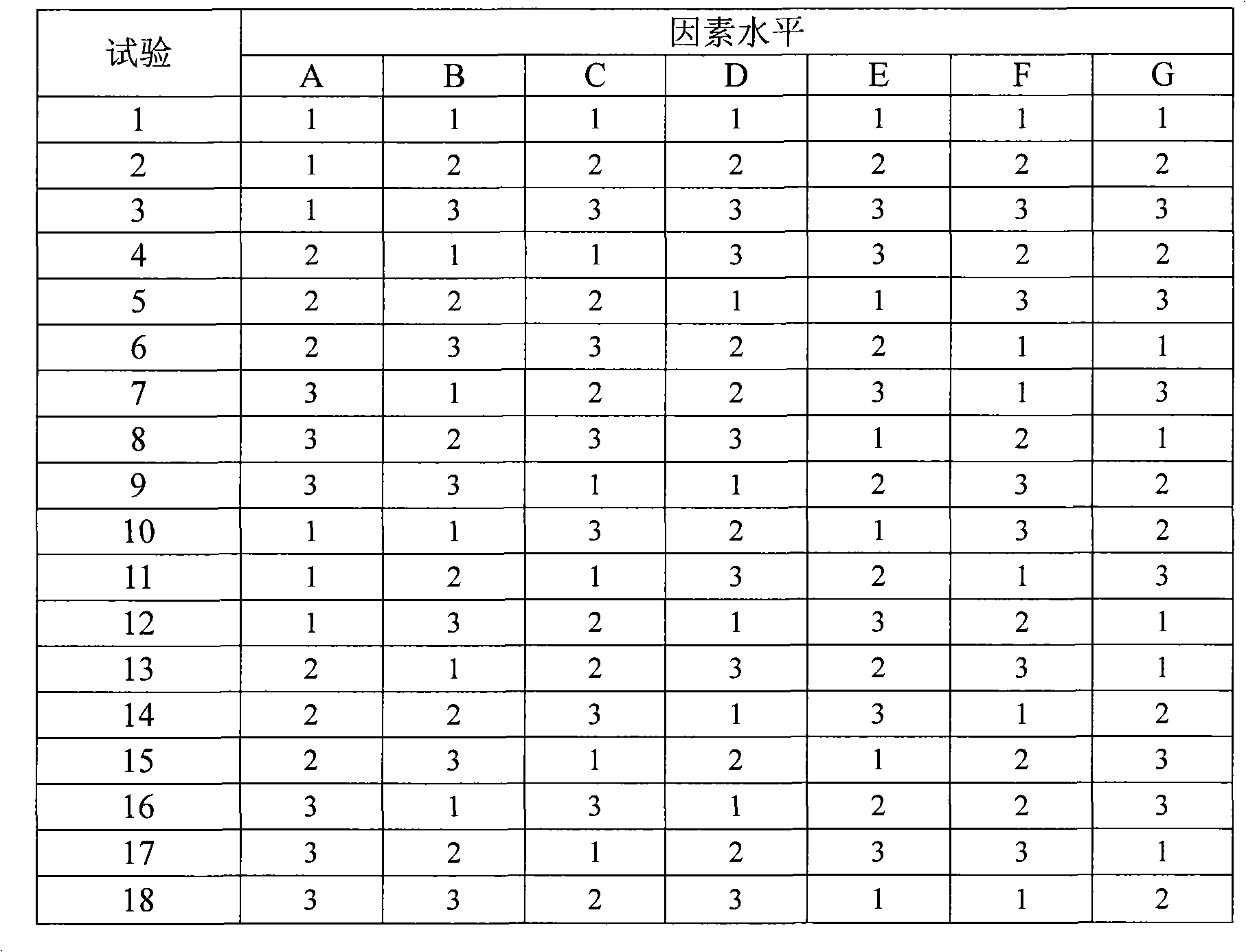
[0057] Mix irbesartan with 3 / 8 croscarmellose sodium and grind it into 500 mesh powder, then mix hydrochlorothiazide, 3 / 8 croscarmellose sodium and microcrystalline cellulose Grind to a 500-mesh powder, then mix the two powders and lactose monohydrate evenly, the speed of the mixer is 15 rpm, and the mixing time is 30 minutes. Then the hydroxypropyl methylcellulose was dissolved in 50% ethanol to prepare a 2% hydroxypropylmethylcellulose solution. Get the mixed powder obtained above and add it to the hydroxypropyl methylcellulose solution, mix evenly, the speed of the mixer is 30 rpm, and the mixing time is 25 minutes. Then use a 24-mesh sieve to granulate, dry...
Embodiment 2
[0059] Prescription: Irbesartan 150g
[0060] Hydrochlorothiazide 12.5g
[0061] Microcrystalline Cellulose 20g
[0062] Lactose monohydrate 60g
[0063] Croscarmellose Sodium 15g
[0064] Micronized silica gel 2g
[0066] Hypromellose 3g
[0067] Mix irbesartan with 3 / 8 croscarmellose sodium and grind it into 400 mesh powder, then mix hydrochlorothiazide, 3 / 8 croscarmellose sodium and microcrystalline cellulose Grind to 400 mesh powder, then mix the two powders and lactose monohydrate evenly, the speed of the mixer is 20 rpm, and the mixing time is 25 minutes. Then the hydroxypropyl methylcellulose was dissolved in 50% ethanol to prepare a 2% hydroxypropylmethylcellulose solution. Get the mixed powder obtained above and add it to the hydroxypropyl methylcellulose solution, mix evenly, the speed of the mixer is 25 rpm, and the mixing time is 30 minutes. Then use a 24-mesh sieve to granulate, dry a...
Embodiment 3
[0069] Prescription: Irbesartan 150g
[0070] Hydrochlorothiazide 12.5g
[0071] Microcrystalline Cellulose 60g
[0072] Lactose monohydrate 20g
[0073] Croscarmellose Sodium 22g
[0074] Micronized silica gel 3g
[0076] Hypromellose 5g
[0077] Mix irbesartan with 3 / 8 croscarmellose sodium and grind it into 500 mesh powder, then mix hydrochlorothiazide, 3 / 8 croscarmellose sodium and microcrystalline cellulose Grind to a 500-mesh powder, then mix the two powders and lactose monohydrate evenly, the speed of the mixer is 10 rpm, and the mixing time is 30 minutes. Then the hydroxypropyl methylcellulose was dissolved in 50% ethanol to prepare a 2% hydroxypropylmethylcellulose solution. Get the mixed powder obtained above and add it to the hydroxypropyl methylcellulose solution, mix evenly, the speed of the mixer is 35 rpm, and the mixing time is 20 minutes. Then use a 24-mesh sieve to granulate, dry...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com