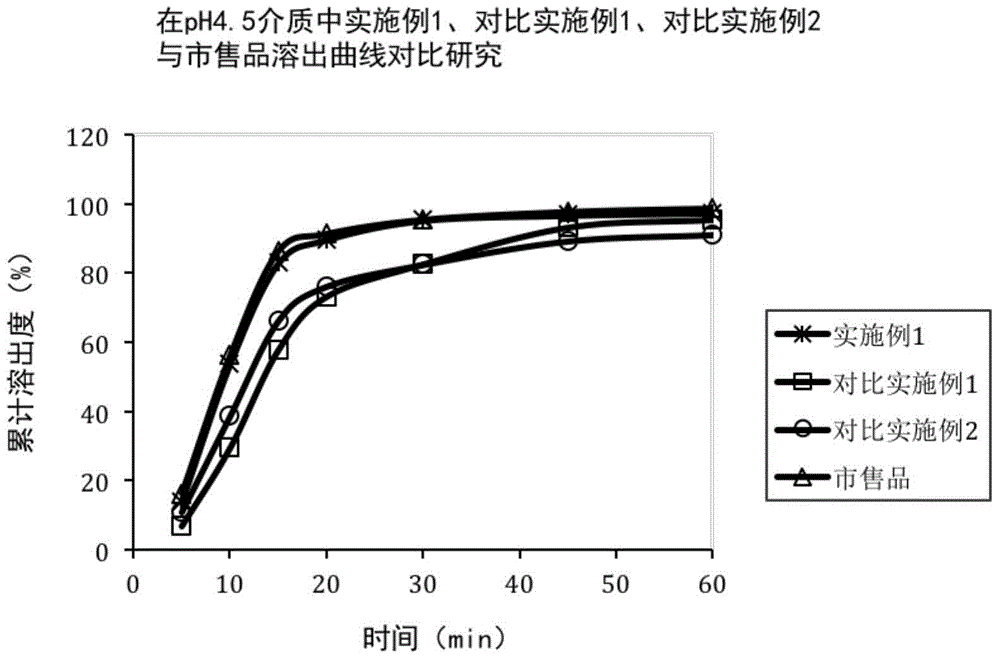
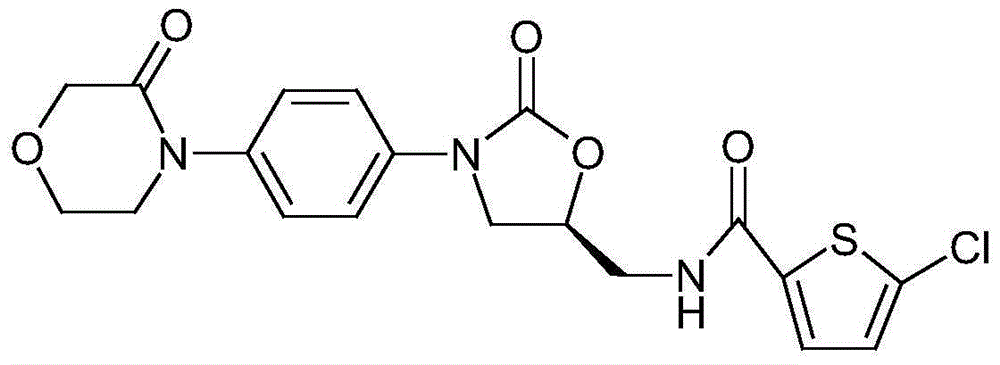
Rivaroxaban tablets and preparation method for same
A technology of rivaroxaban and prescription, which is applied in the direction of medical formula, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of slow disintegration and dissolution of azilsartan tablets, and achieve compression Good formability, uniform particle size, and the effect of improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] 10mg tablet specifications:
[0053] Element
Content (mg)
Rivaroxaban
10
lactose monohydrate
30
Microcrystalline Cellulose (ph101)
40
2
Hydroxypropyl Methyl Cellulose (5cps)
2.2
Croscarmellose sodium (additional)
1.8
Croscarmellose sodium (additional)
1.8
0.5
[0054] The preparation method is as follows:
[0055] 1) Micronizing the rivaroxaban raw material to a particle size D90≤15 μm;
[0056] 2) Lactose, microcrystalline cellulose, sodium lauryl sulfate, and croscarmellose sodium are passed through a 60-mesh sieve;
[0057] 3) Preparation of binder: prepare an 8% solution according to the ratio of hydroxypropyl methylcellulose (5cps):purified water=8:100 (W:V);
[0058] 4) Weigh and mix the processed rivaroxaban, lactose, microcrystalline cellulose, sodium lauryl sulfate, and partly added croscarmellose sodium acco...
Embodiment 2
[0067] 5mg tablet specifications:
[0068] Element
Content (mg)
Rivaroxaban
5
20
Microcrystalline Cellulose (ph101)
30
1
Hydroxypropyl Methyl Cellulose (5cps)
2
Croscarmellose sodium (additional)
1.5
Croscarmellose sodium (additional)
1.5
0.3
[0069] The preparation method is as follows:
[0070] 1) Micronizing the rivaroxaban raw material to a particle size D90≤15 μm;
[0071] 2) Lactose, microcrystalline cellulose, sodium lauryl sulfate, and croscarmellose sodium are passed through a 60-mesh sieve;
[0072] 3) Preparation of binder: prepare an 8% solution according to the ratio of hydroxypropyl methylcellulose (5cps):purified water=8:100 (W:V);
[0073] 4) Weigh and mix the processed rivaroxaban, lactose, microcrystalline cellulose, sodium lauryl sulfate, and partly added croscarmellose sodium accordin...
Embodiment 3
[0083] 20mg tablet specifications:
[0084] Element
Content (mg)
Rivaroxaban
20
40
Microcrystalline Cellulose (ph101)
50
Sodium dodecyl sulfate
3
Hydroxypropyl Methyl Cellulose (5cps)
2.5
Croscarmellose sodium (additional)
2
Croscarmellose sodium (additional)
2
Magnesium stearate
0.8
[0085] The preparation method is as follows:
[0086] 1) Micronizing the rivaroxaban raw material to a particle size D90≤15 μm;
[0087] 2) Lactose, microcrystalline cellulose, sodium lauryl sulfate, and croscarmellose sodium are passed through a 60-mesh sieve;
[0088] 3) Preparation of binder: prepare an 8% solution according to the ratio of hydroxypropyl methylcellulose (5cps):purified water=8:100 (W:V);
[0089] 4) Weigh and mix the processed rivaroxaban, lactose, microcrystalline cellulose, sodium lauryl sulfate, and partly added croscarmellose sodium accordin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com