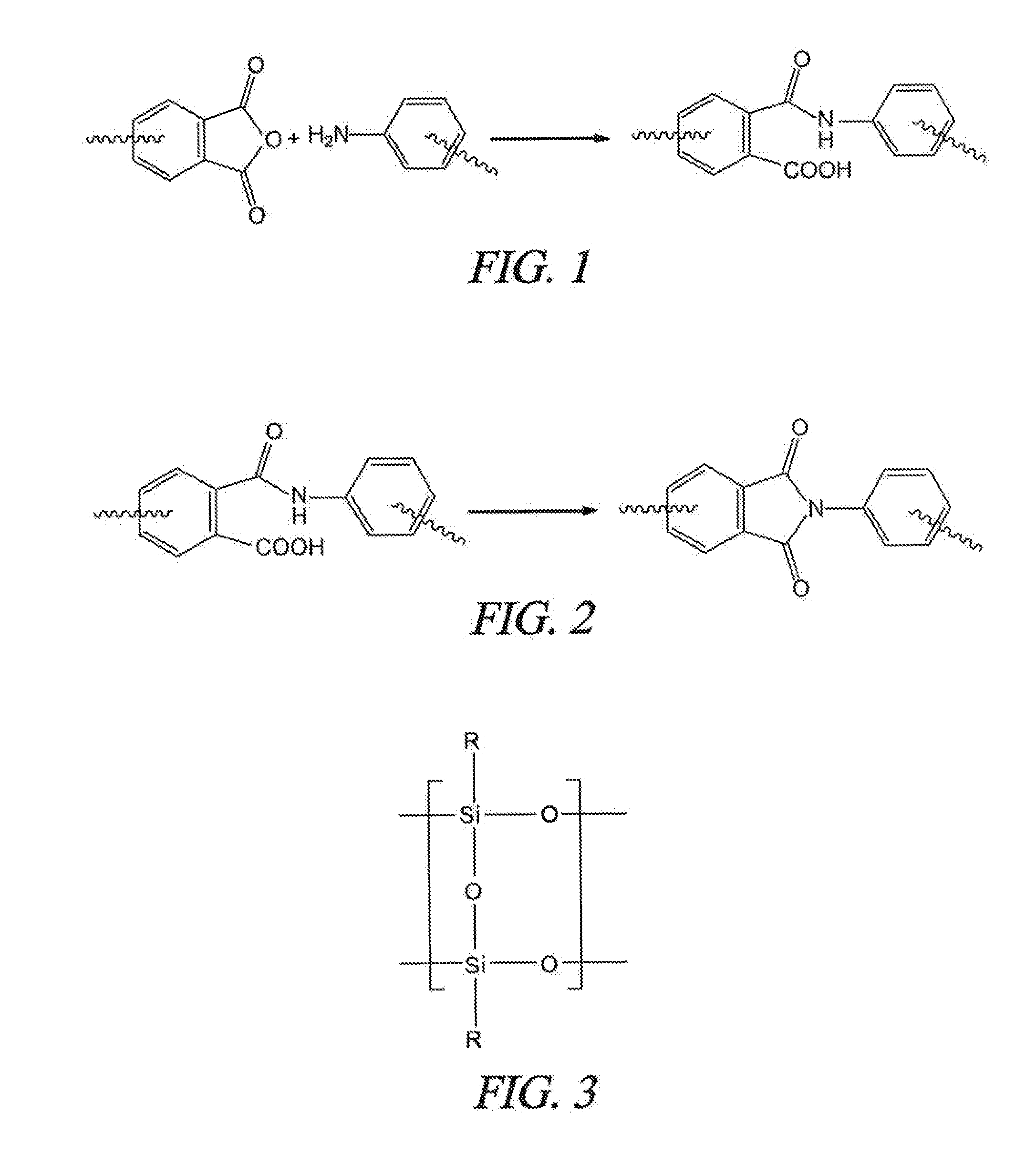
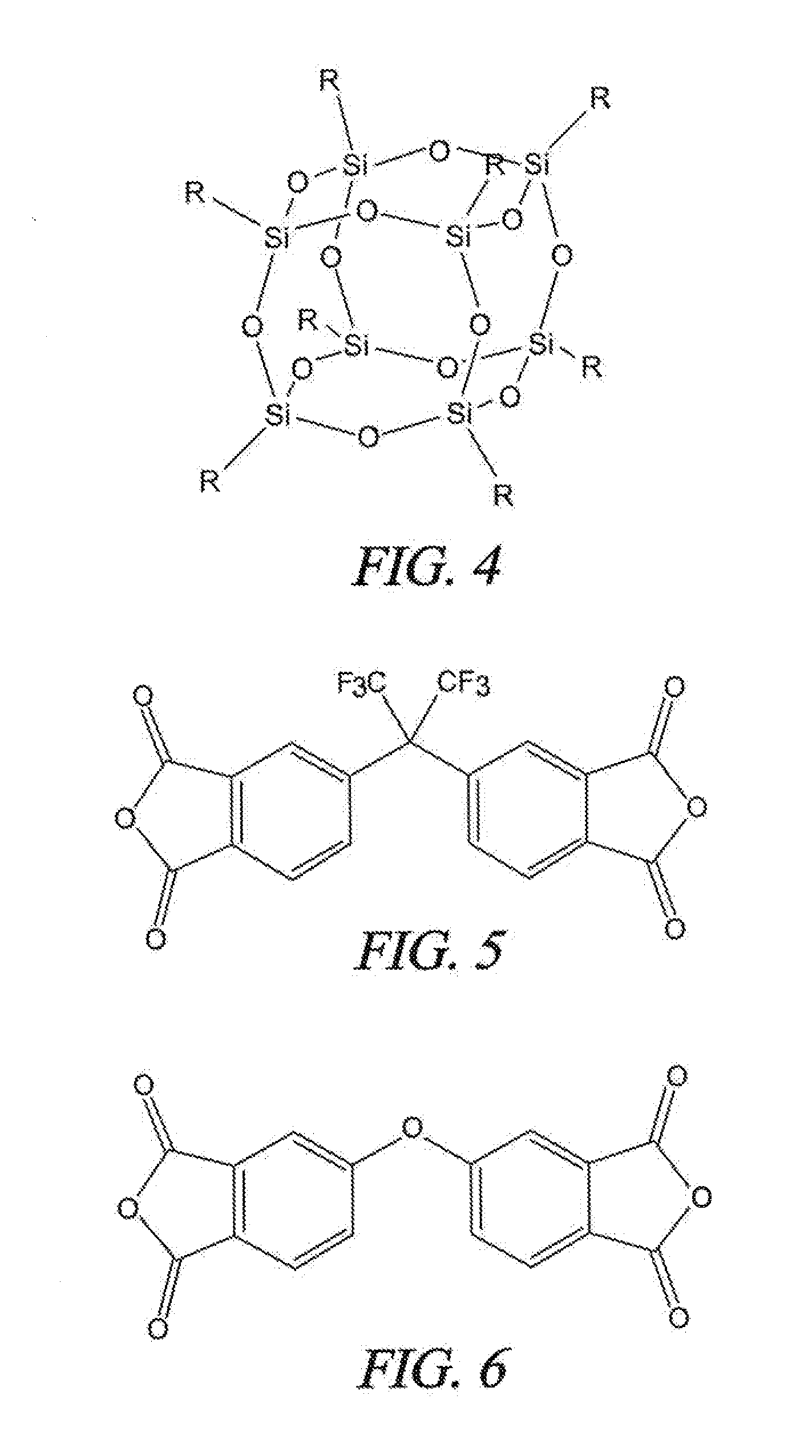
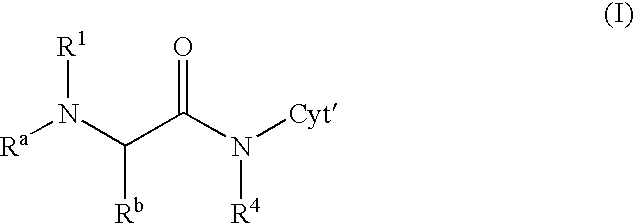
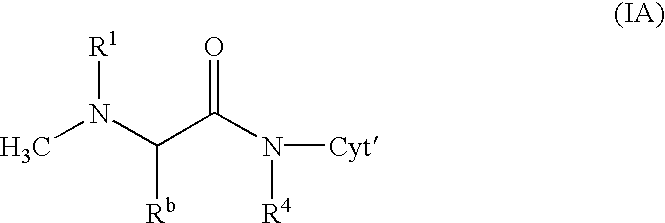
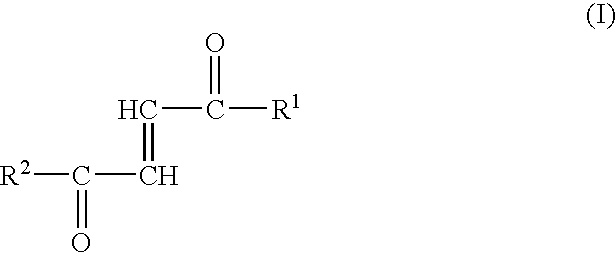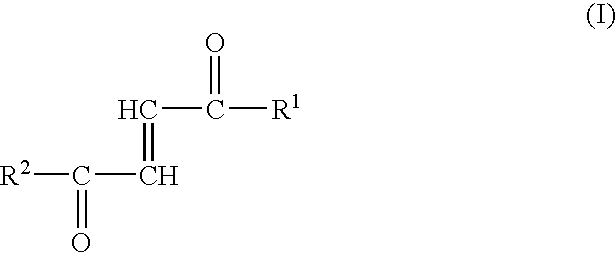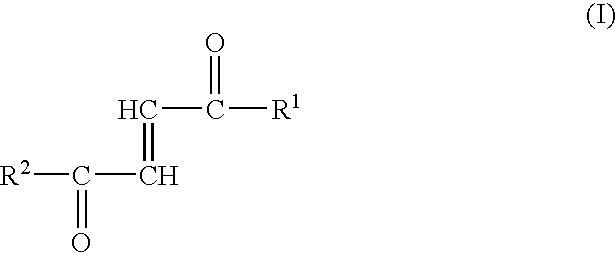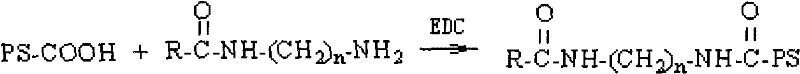Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1433 results about "Amide bonds" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
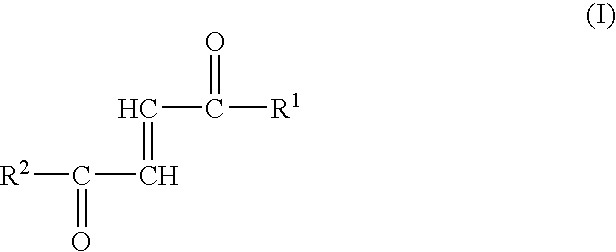
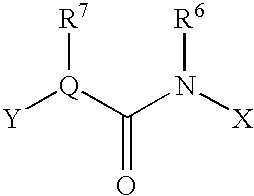
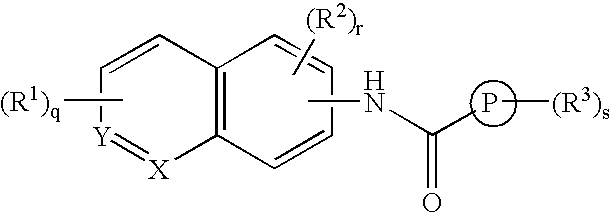
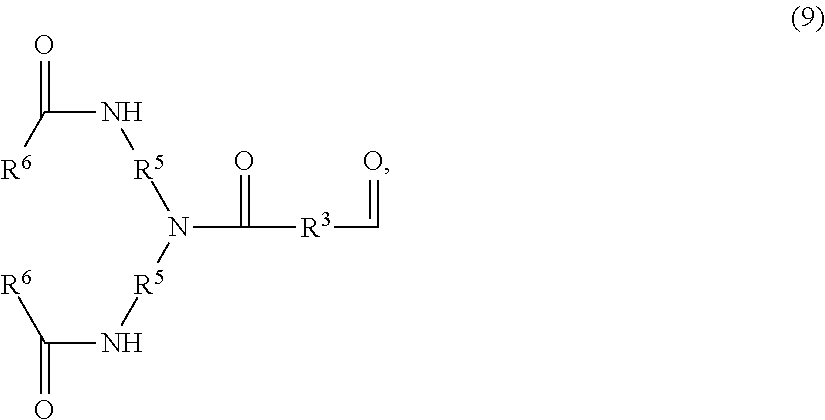
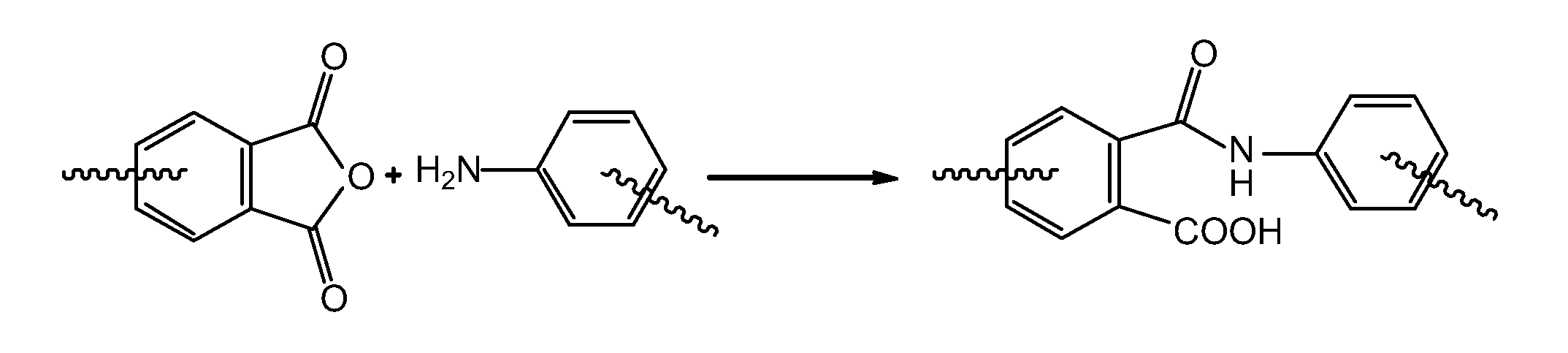
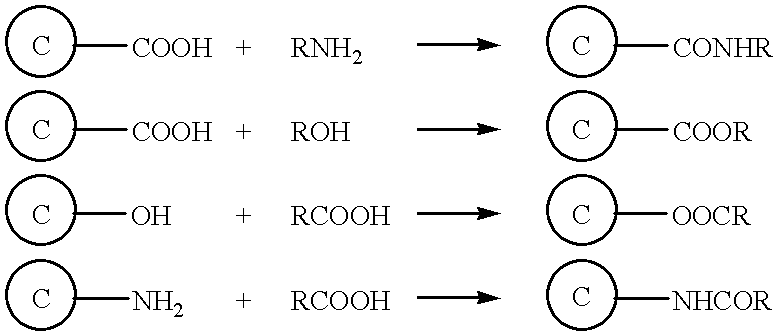
An amide bond is a chemical bond that occurs between a hydroxyl group of a carboxylic group (-COOH) of one molecule and a hydrogen of an amino group (-NH2) of another molecule. Whereas, the peptide bond is a type of amide bond which occurs between two amino acids during the synthesis...
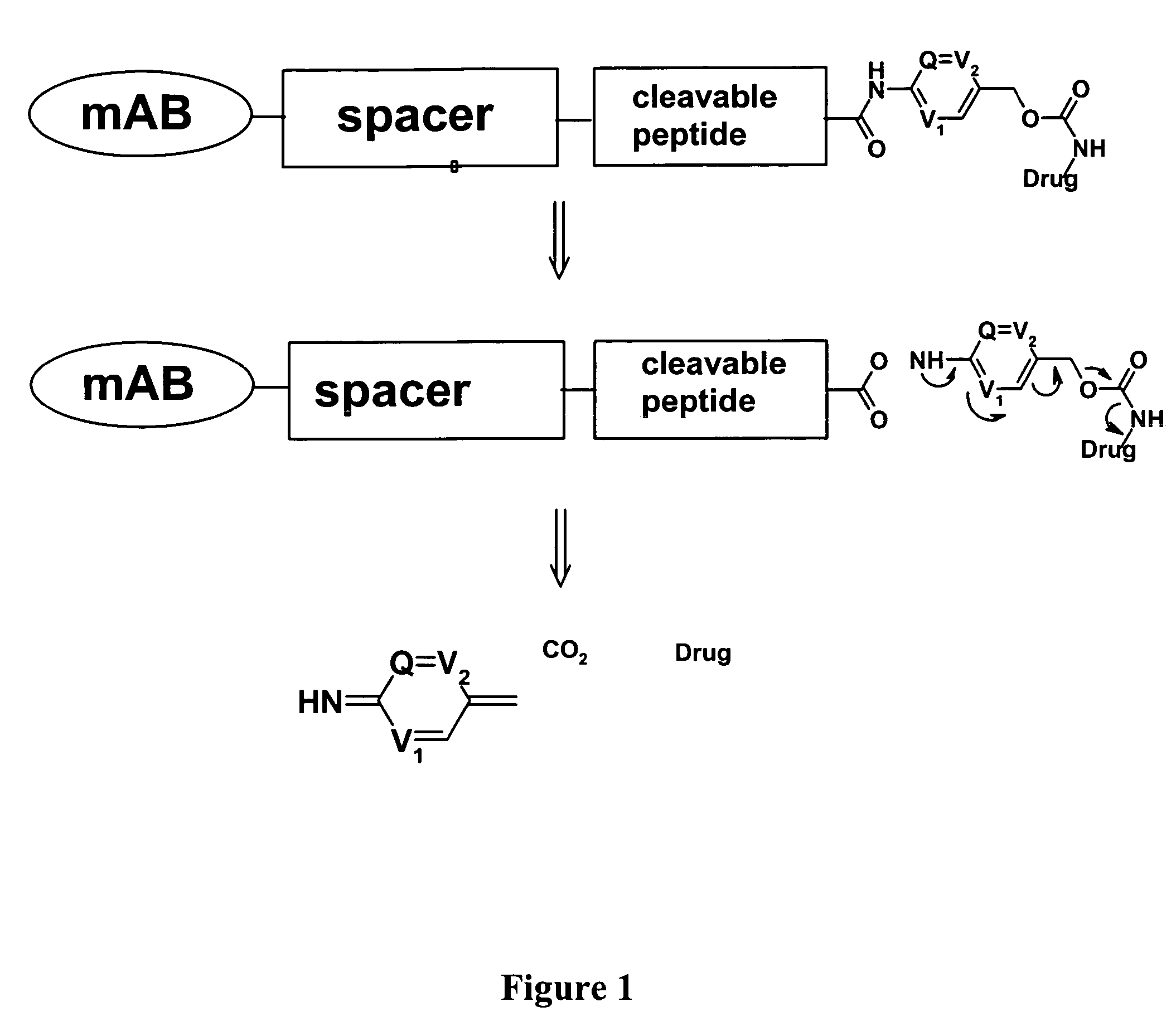
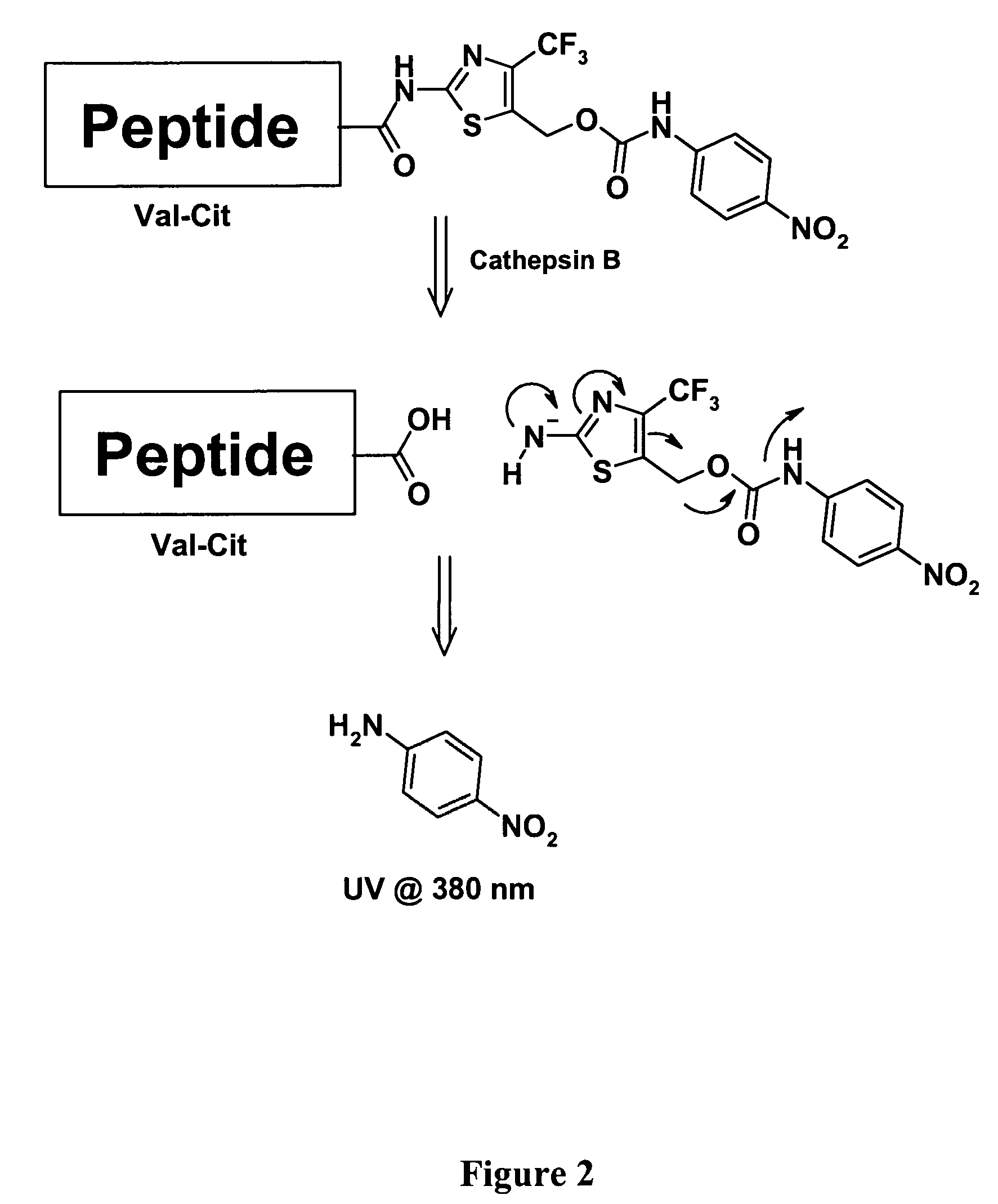
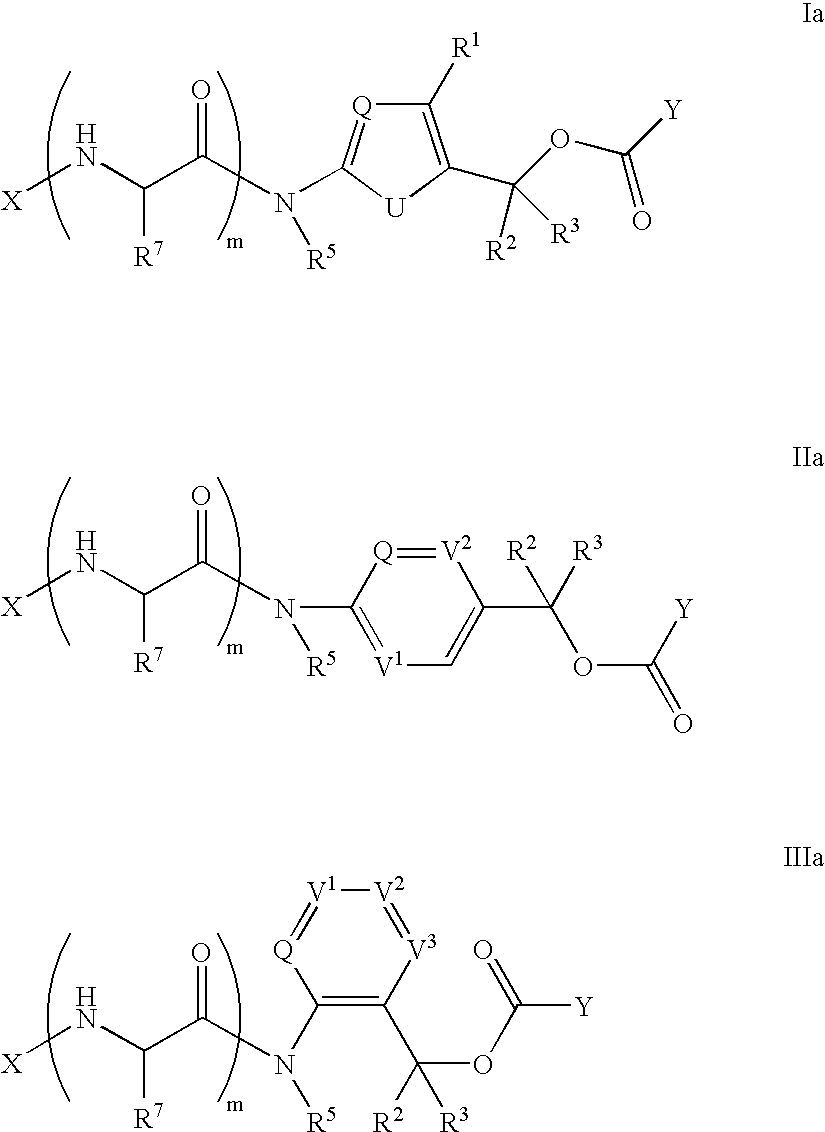
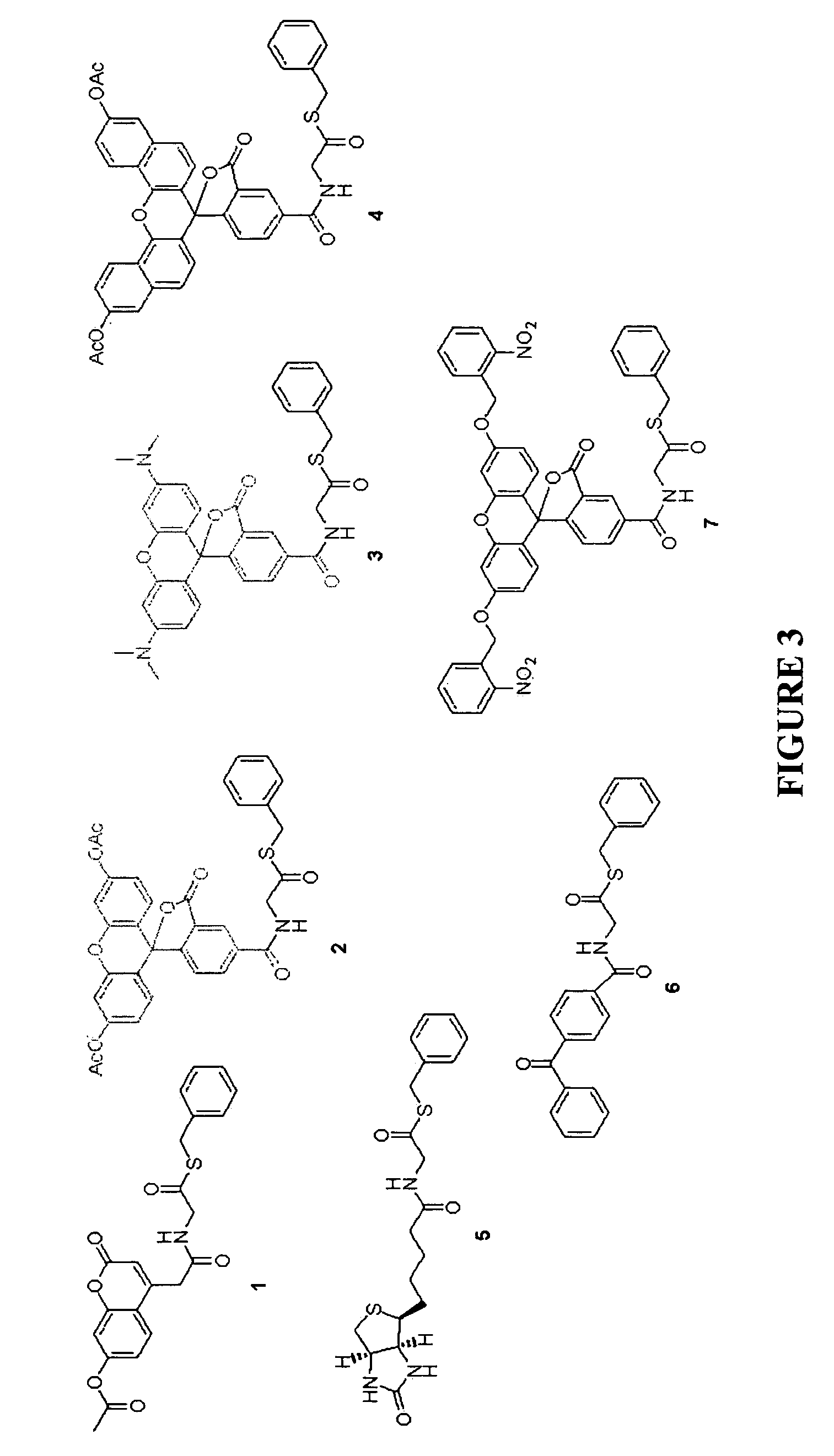
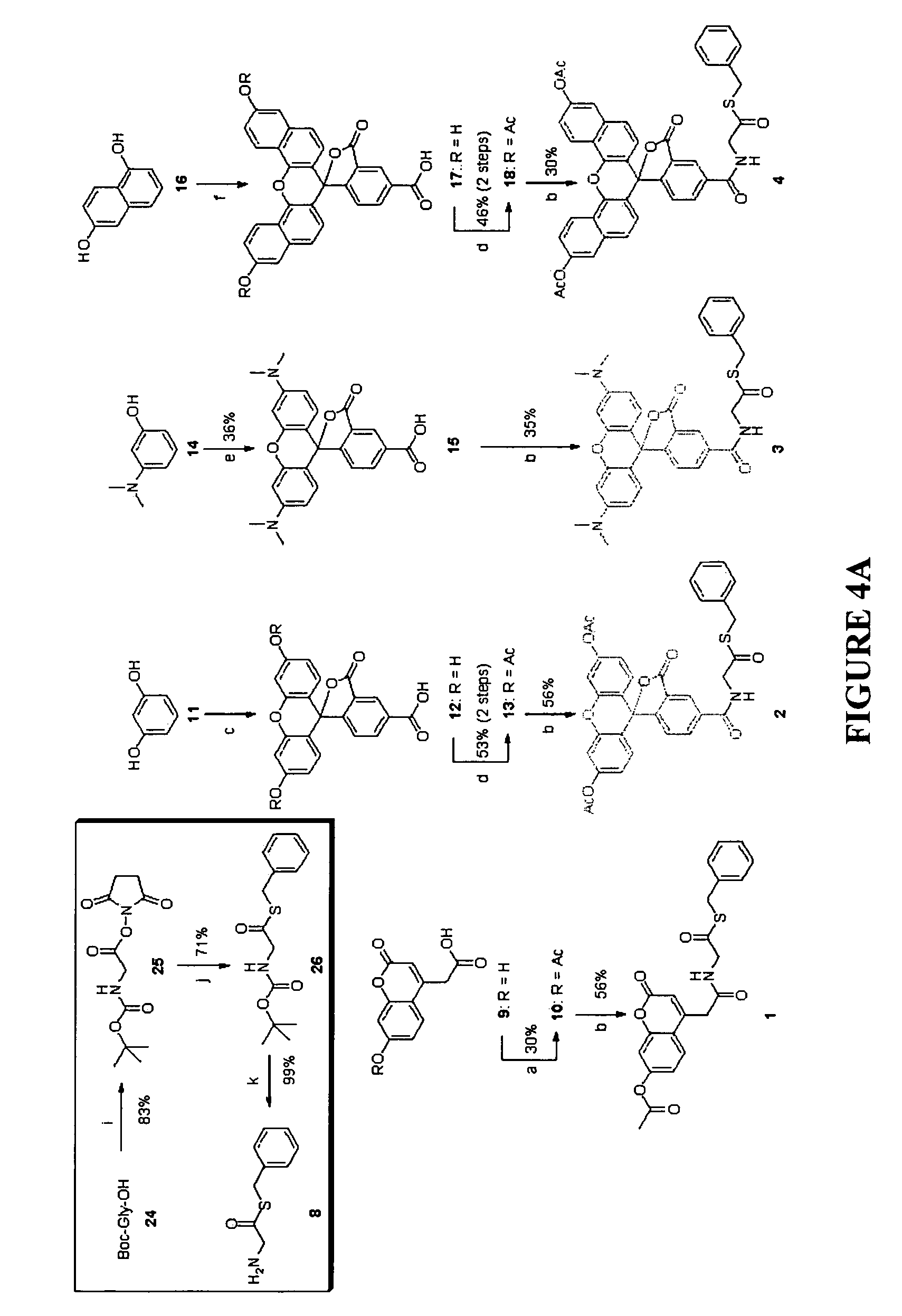
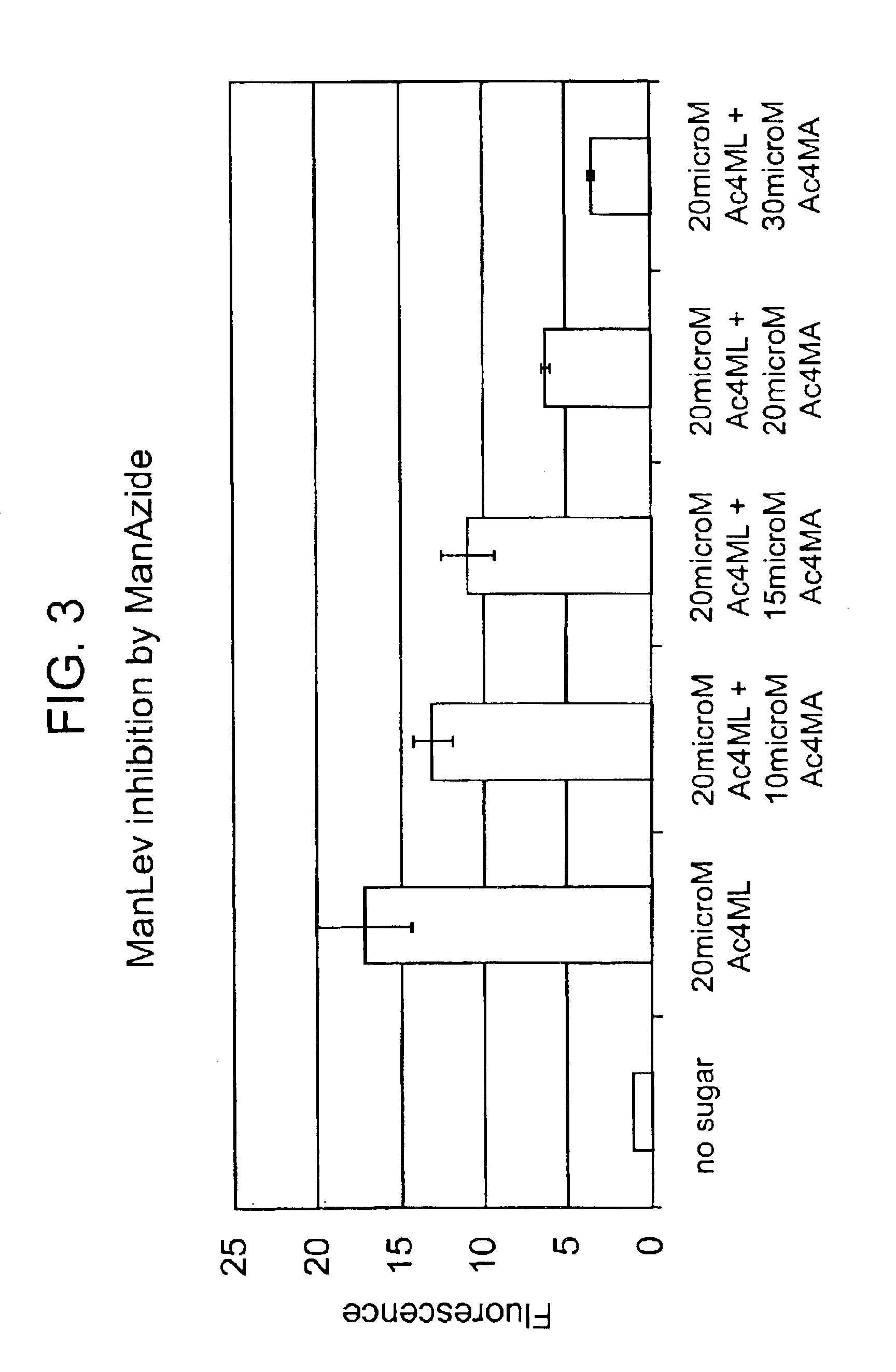
Heterocyclic self-immolative linkers and conjugates
The present invention provides heterocyclic linker compounds useful for linking drug moieties to ligands. The compounds also include drug-ligand conjugates comprising a ligand capable of targeting a selected cell population, and a drug connected to the ligand by a heterocyclic linker moiety. The linker moiety comprises a peptide sequence that is a substrate for an intracellular enzyme, for example a cathepsin, that cleaves the peptide at an amide bond. The peptide further contains a self-immolating moiety which connects the drug and the protein peptide sequence. Upon cleavage of the peptide sequence by an intracellular enzyme the self-immolating moiety cleaves itself from the drug moiety such that the drug moiety is in an underivatized and active form.
Owner:SEAGEN INC
Natural rubber master batch, production method thereof, and natural rubber composition
InactiveUS6841606B2Easy to processImprove propertiesPigmenting treatmentSpecial tyresPolymer scienceSlurry
The natural rubber master batch of the present invention is prepared by mixing a natural rubber latex having its amide linkages cleaved and an aqueous slurry having a filler dispersed in water. Alternatively, the natural rubber master batch is prepared by a method including a step for mixing a natural rubber latex and an aqueous slurry containing dispersed fillers having a specific particle size distribution and a limited range of 24M4DBP absorption. The natural rubber composition of the present invention is prepared by compounding natural rubber, which contains non-rubber components prepared by cleaving amide linkages of natural rubber latex, with silica and / or a particular inorganic filler.
Owner:BRIDGESTONE CORP
Heptapeptide oxytocin analogues
InactiveUS6143722AImprove stabilityAdequate potencyNervous disorderPeptide/protein ingredientsOxytocinAmide bonds
PCT No. PCT / SE97 / 01968 Sec. 371 Date Aug. 2, 1999 Sec. 102(e) Date Aug. 2, 1999 PCT Filed Nov. 21, 1997 PCT Pub. No. WO98 / 23636 PCT Pub. Date Jun. 4, 1998Heptapeptide analogues or pharmaceutically acceptable salts thereof consist of a hexapeptide moiety S and a C-terminal beta -aminoalcohol residue Z bound to the moiety S by an amide bond, wherein the beta -aminoalcohol Z is -NR-CH(Q)-CH2OH, Q is (CH2)n-NH-A is H or -C(=NH)NH2, and R is CH3 or C2H5, and the moiety S wherein H is a D-aromatic alpha -aminoacid and Y is an aliphatic alpha -aminoacid and have oxytocin antagonist activity. Also disclosed is: a method of their synthesis; pharmaceutical compositions containing these analogues; the synthesis of such compositions; a method of control of uterine contractions.
Owner:FERRING BV
Fumaric acid amides
InactiveUS7157423B2More resistant to hydrolysisEasy to handleAntibacterial agentsSenses disorderDiseaseSide chain
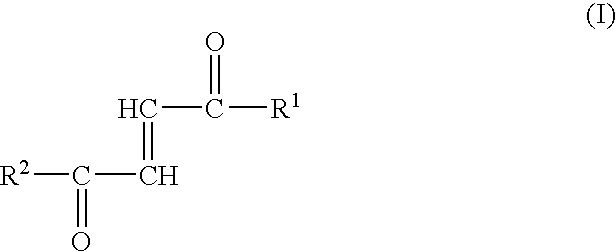
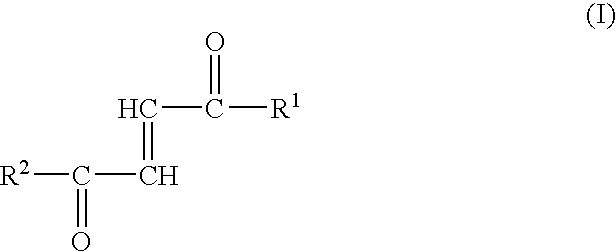
Fumaric acid amides of the general formula (I)wherein R1 represents OR3 or a D- or L-amino acid radical —NH—CHR4—COOH bonded via an amide bond, wherein R3 is hydrogen, a straight-chained or branched, optionally substituted C1-24 alkyl radical, a phenyl radical or C6-10 aralkyl radical and R4 is a side chain of a natural or synthetic amino acid and R2 represents a D- or L-amino acid radical —NH—CHR5—COOH bonded via an amide bond or a peptide radical comprising 2 to 100 amino acids bonded via an amide bond, wherein R5 is a side chain of a natural or synthetic amino acid, are used for preparing a drug (1) for the therapy of an autoimmune disease; (2) for use in transplantation medicine; (3) for the therapy of mitochondrial diseases; or (4) for the therapy of NF-kappaB mediated diseases.
Owner:BIOGEN INT
Fumaric Acid Amides
InactiveUS20060205659A1Easy to handleMore resistant to hydrolysisAntibacterial agentsOrganic active ingredientsAutoimmune conditionHydrogen
Fumaric acid amides of the general formula (I) wherein R1 represents OR3 or a D- or L-amino acid radical —NH—CHR4—COOH bonded via an amide bond, wherein R3 is hydrogen, a straight-chained or branched, optionally substituted C1-24 alkyl radical, a phenyl radical or C6-10 aralkyl radical and R4 is a side chain of a natural or synthetic amino acid and R2 represents a D- or L-amino acid radical —NH—CHR5—COOH bonded via an amide bond or a peptide radical comprising 2 to 100 amino acids bonded via an amide bond, wherein R5 is a side chain of a natural or synthetic amino acid, are used for preparing a drug (1) for the therapy of an autoimmune disease; (2) for use in transplantation medicine; (3) for the therapy of mitochondrial diseases; or (4) for the therapy of NF-kappaB mediated diseases.
Owner:BIOGEN INT
Dual phase drug release system
The present invention relates to conjugate comprising a carrier substituted with one or more occurrences of a moiety having the structure (I): wherein each occurrence of M is independently a modifier having a molecular weight ≦10 kDa; denotes direct of indirect attachment of M to linker LM; and each occurrence of LM is independently an optionally substituted succinamide-containing linker, whereby the modifier M is directly or indirectly attached to the succinamide linker through an amide bond, and the carrier is linked directly or indirectly to each occurrence of the succinamide linker through an ester bond. In another aspect, the invention provides compositions comprising the conjugates, methods for their preparation, and methods of use thereof in the treatment of various disorder, including, but not limited to cancer.
Owner:THE GENERAL HOSPITAL CORP
Modification of peptide and protein
PCT No. PCT / JP95 / 01994 Sec. 371 Date Sep. 8, 1997 Sec. 102(e) Date Sep. 8, 1997 PCT Filed Sep. 29, 1995 PCT Pub. No. WO96 / 10089 PCT Pub. Date Apr. 4, 1996A process for modifying a physiologically active peptide or a physiologically active protein which comprises reacting a physiologically active peptide or a physiologically active protein having at least a glutamine residue with a substance having an amino donor in the presence of a transglutaminase originating in a microorganism to thereby form an acid amide bond at the gamma -position acid amide group of the glutamine residue with the amino group of the amino donor; and the product of modification obtained thereby.
Owner:AJINOMOTO CO INC
Benzamide derivative or salt thereof
InactiveUS20070167444A1Strong inhibitory activityBiocideNervous disorderNeurogenic painOveractive bladder
There is provided a compound having a capsaicin receptor VR1 inhibitory activity and useful as a therapeutic agent for various pains including inflammatory pain and neurogenic pain, migraine, cluster headache, bladder diseases including overactive bladder, and the like. A benzamide derivative or a salt thereof wherein a benzene ring is attached to a D ring (a monocyclic or bicyclic hydrocarbon ring or a monocyclic or bicyclic heteroaromatic ring) through an amide bond, the benzene ring is directly bonded to an E ring (a monocyclic or bicyclic hydrocarbon ring or a monocyclic or bicyclic heteroaromatic ring), and the benzene ring is further bonded to A (an amino moiety, a monocyclic or bicyclic heterocycle) through L (a lower alkylene).
Owner:ASTELLAS PHARMA INC
Cationic lipids
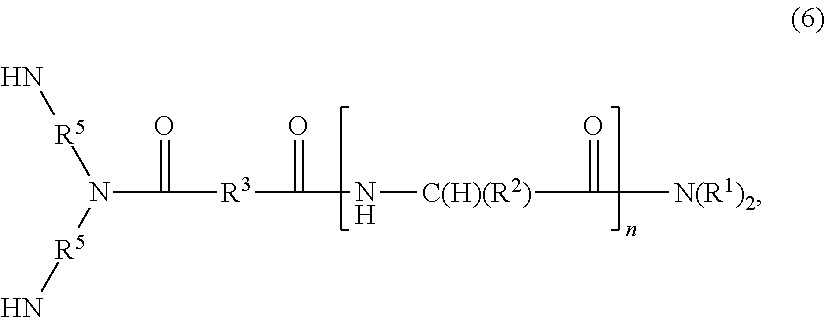
The invention provides a cationic lipid comprising:(i) one head group, comprising one or more amino acids, in which at least one amino acid has a side chain that comprises a cationic moiety or a cationic precursor;(ii) a linking moiety of formula (5):—(HNR5)2NC(O)R3C(O)— (5),wherein:each R5 is independently an optionally substituted C1-4 alkylene moiety; andR3 is an optionally substituted alkylene or alkenylene moiety; and(iii) two lipophilic moieties,wherein the head group and each of the lipophilic moieties are connected to the linking moiety through amide linkages.
Owner:THE UNIV COURT OF THE UNIV OF EDINBURGH
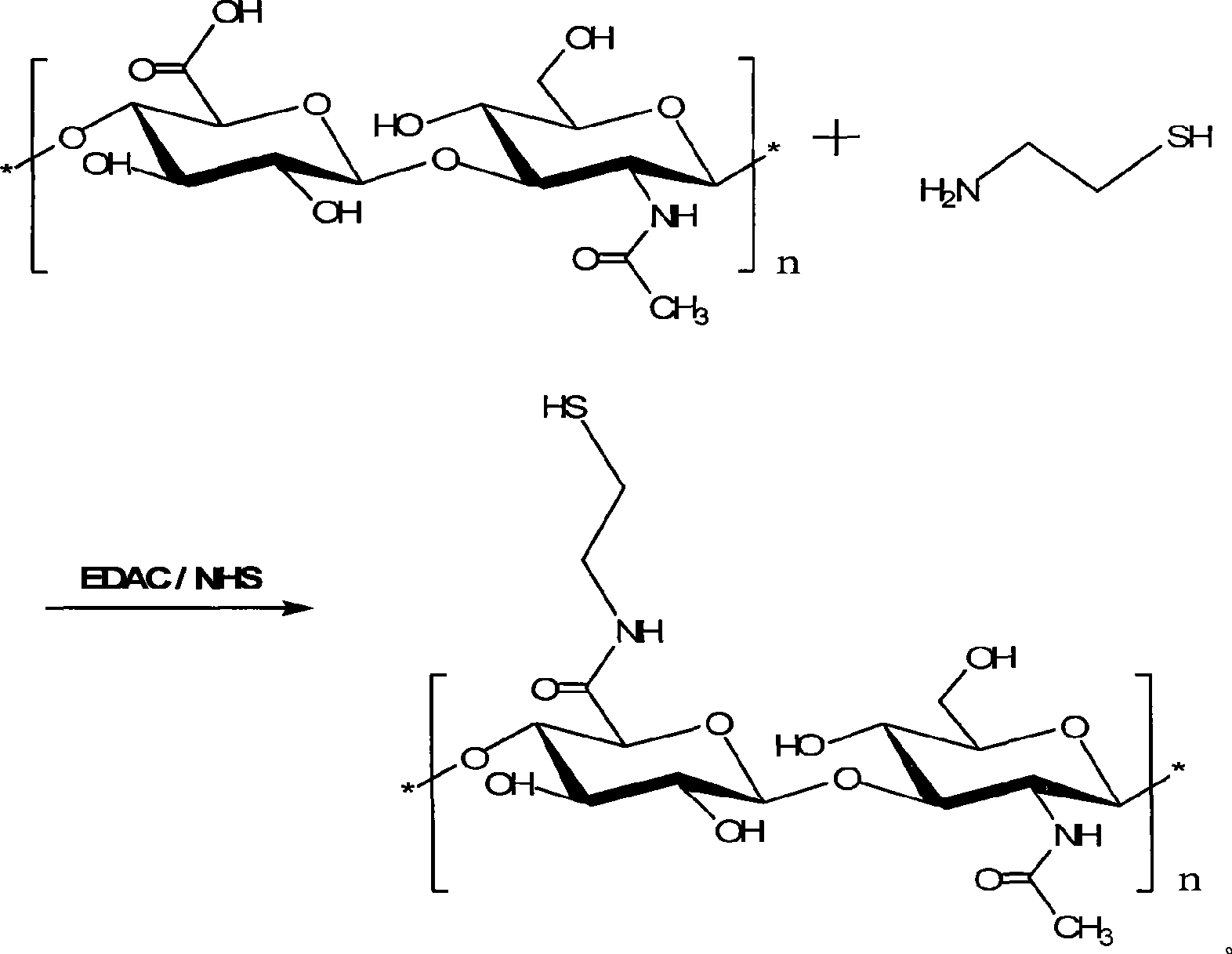
Cysteamine modified sulfhydryl hyaluronic acid couplet, preparation and application thereof
InactiveCN101367884ANon-antigenic and immunogenicGood biocompatibilityAnimal cellsPharmaceutical non-active ingredientsCYSTEAMINE HYDROCHLORIDEChemistry
The invention belongs to the technical field of high polymer materials, in particular to a thiolated hyaluronic acid conjugate modified by cysteamine, a preparation method and an application thereof. The method comprises the steps of adding 1-ethyl-3-(3-dimethylamino propyl)-carbodiimide (EDAC) and N-hydroxysuccinimide (NHS) in the hyaluronic acid solution to activate the carboxyl of the hyaluronic acid solution, adding cysteamine hydrochloride to make the amino of cysteamine hydrochloride and the carboxyl of hyaluronic acid to form amido link, regulating the proportions of each material and reaction conditions to obtain the thiolated hyaluronic acid conjugates with different sulfhdryl contents. The invention widens the application range of the thiolated reaction. The adhesive properties of the mucosa and the biocompatibility of the conjugate are good. The invention has strong in-situ gel property and supplies good bio-adhesive materials for non-injected preparations, tissue engineering and the like.
Owner:FUDAN UNIV
Temperature-responsive polymer compound and process for producing the same
InactiveUS6956077B1Improve hydrophobicityComponent separationOther chemical processesChemical compoundSide chain
A temperature-responsive polymer and polymer material which has ester bond(s) and / or acid amide bond(s) respectively at one or more sites in the side chain and can be arbitrarily controlled by varying the side chain is provided.
Owner:GE HEALTHCARE BIO SCI CORP
Chemoselective ligation
InactiveUS20050148032A1Esterified saccharide compoundsSugar derivativesIn vivoChemoselective ligation
The present invention features a chemoselective ligation reaction that can be carried out under physiological conditions. In general, the invention involves condensation of a specifically engineered phosphine, which can provide for formation of an amide bond between the two reactive partners resulting in a final product comprising a phosphine moiety, or which can be engineered to comprise a cleavable linker so that a substituent of the phosphine is transferred to the azide, releasing an oxidized phosphine byproduct and producing a native amide bond in the final product. The selectivity of the reaction and its compatibility with aqueous environments provides for its application in vivo (e.g., on the cell surface or intracellularly) and in vitro (e.g., synthesis of peptides and other polymers, production of modified (e.g., labeled) amino acids).
Owner:RGT UNIV OF CALIFORNIA
Site-specific labelling of proteins
InactiveUS7449558B2Hydrolysed protein ingredientsPeptide preparation methodsThioester synthesisCombinatorial chemistry
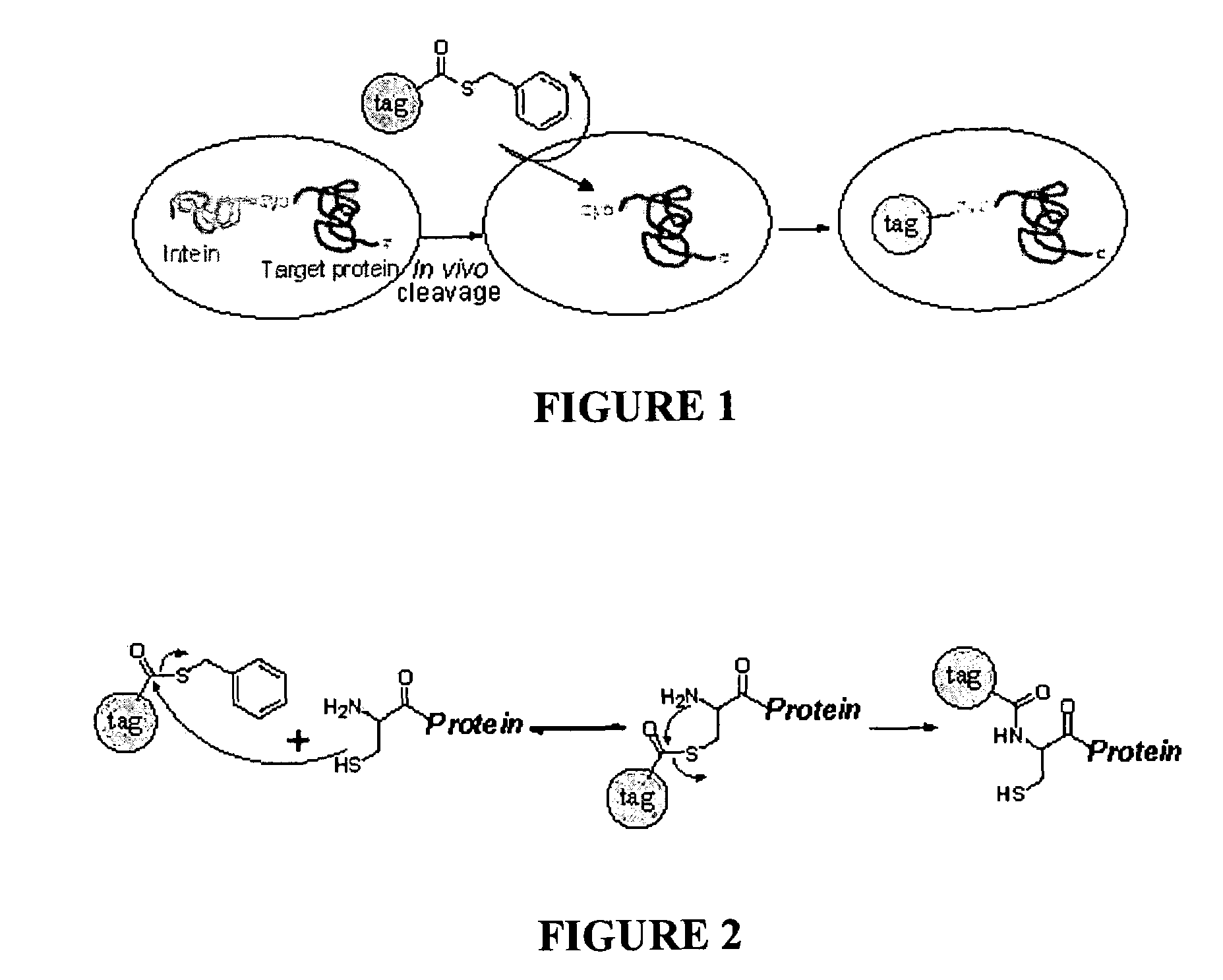
An in vivo method for labelling a protein having an N-terminal cysteine is disclosed. A probe comprising a thioester group and a detectable label is introduced into a cell expressing the N-terminal cysteine protein, allowing for the N-terminal cysteine to cleave the thioester bond and resulting in the label being covalently attached to the N-terminus of the protein by an amide bond.
Owner:NAT UNIV OF SINGAPORE
Chemoselective ligation
The present invention features a chemoselective ligation reaction that can be carried out under physiological conditions. In general, the invention involves condensation of a specifically engineered phosphine, which can provide for formation of an amide bond between the two reactive partners resulting in a final product comprising a phosphine moiety, or which can be engineered to comprise a cleavable linker so that a substituent of the phosphine is transferred to the azide, releasing an oxidized phosphine byproduct and producing a native amide bond in the final product. The selectivity of the reaction and its compatibility with aqueous environments provides for its application in vivo (e.g., on the cell surface or intracellularly) and in vitro (e.g., synthesis of peptides and other polymers, production of modified (e.g., labeled) amino acids).
Owner:RGT UNIV OF CALIFORNIA
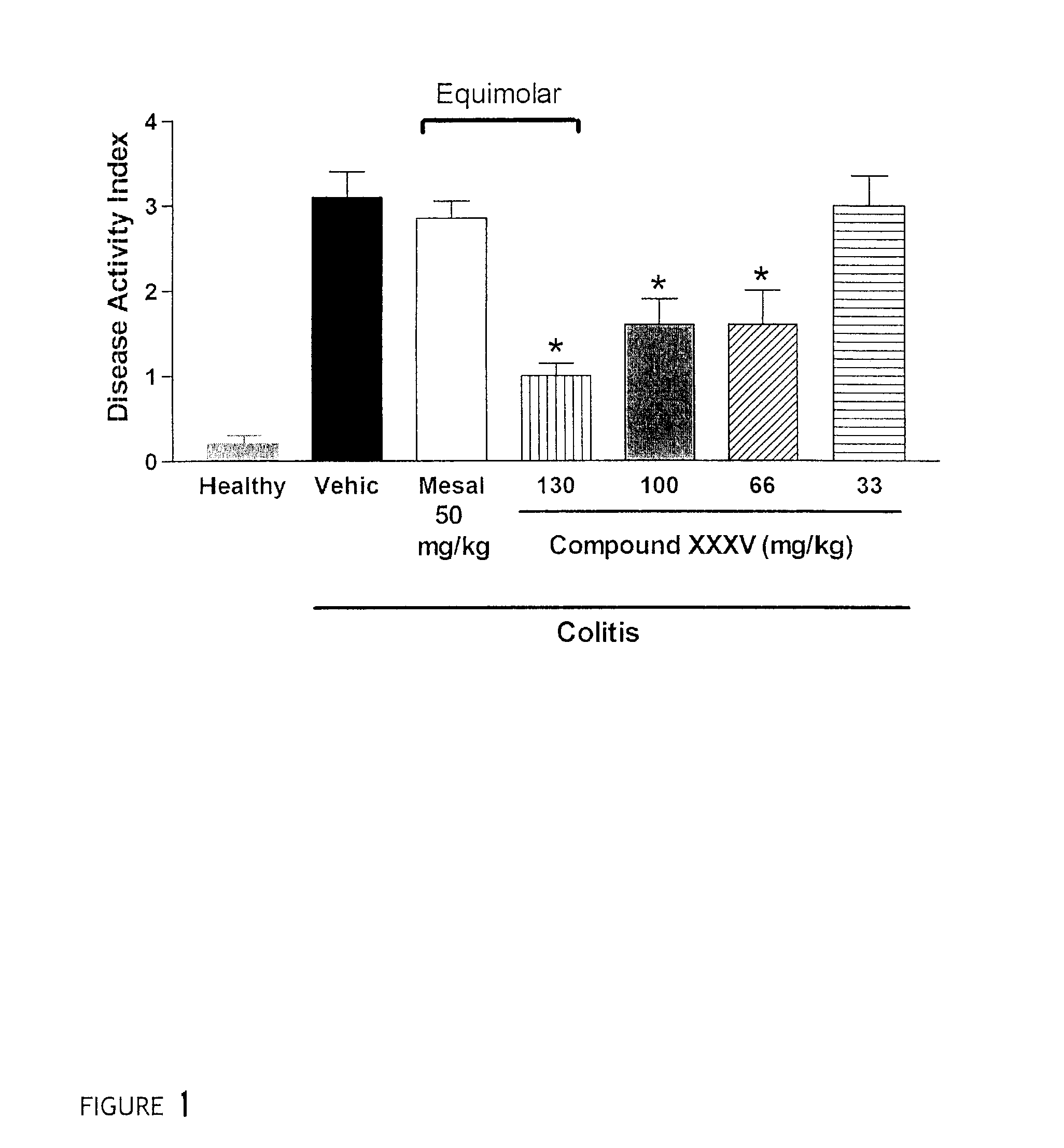
Derivatives of 4- or 5-aminosalicylic acid
InactiveUS7910568B2Easily reach colonPromote absorptionBiocideAntipyreticSalicylic acidBULK ACTIVE INGREDIENT
Owner:ANTIBE THERAPEUTICS INC
Lanthanide Chelates and Use Thereof in Bioanalysis
InactiveUS20110136242A1Improve stabilityGood spectral characteristicsChemiluminescene/bioluminescenceGroup 3/13 element organic compoundsFluorescenceQuantitative determination
Novel chemical compounds, with application in fluorometric analytical methods, for qualitative and quantitative determination of biomolecules. The aim of the invention is to identify and prove the suitability of such compounds. Said aim is achieved with compounds of formula (1) where R′ is an antenna function, R2 is a chelate forming agent, containing a coordinated lanthanide(III)ion, X is —OH or a group with affinity for the biomolecule, bonded to a carboxylate group of the chelate forming agent by means of an amide bond and Y is —H or a group with affinity for the biomolecule, coupled to the antenna function.
Owner:SENSIENT IMAGING TECH GMBH
Polymeric coating for protecting objects
ActiveUS20100063244A1Provide protectionCosmonautic power supply systemsCosmonautic thermal protectionPhenyl groupPolyimide
A protective polymeric coating is applied to the surface of various objects which are to be exposed to a harsh environment. The protective polymeric coating covers the exposed surface, where the polymeric coating includes a polyimide polymer. The polyimide polymer in the polymeric coating has a backbone with at least one non-terminal phenyl group. A linkage is connected to the non-terminal phenyl group, where the linkage can be an amide linkage or an ester linkage. An oligomeric silsesquioxane compound is connected to the linkage through an organic substituent, where the oligomeric silsesquioxane is not incorporated into the polymer backbone. The polymeric coating provides protection to the underlying object.
Owner:NEXOLVE HLDG CO LLC
New effectors of dipeptidyl peptidase iv for topical use
InactiveUS20030092630A2Reduced activityOrganic active ingredientsPeptide/protein ingredientsDiseaseSide chain
Abstract of Disclosure The invention relates to compounds for topically influencing the activity of dipeptidyl peptidase of the general formula wherein A is an amino acid having at least one functional group in the side chain;B is a chemical compound covalently bound to a functional group of the side chain of A, chosen from the group consisting of (a) oligopeptides having a chain length of up to 20 amino acids, (b) homopolymers of glycine consisting of up to 6 glycine monomers, and (c) polyethylene glycols having molar masses of up to 20 000 g / mol; and C is a group amide-bonded to A chosen from the group consisting of thiazolidine, pyrrolidine, cyanopyrrolidine, hydroxyproline, dehydroproline or piperidine. The invention further relates to the use of said compounds for targeted intervention in local immunological processes (chemotaxis, inflammatory processes, autoimmune diseases), as well as effective and targeted treatment of pathophysiological and physiological processes related thereto (psoriasis, periodontitis, arthritis, allergies, inflammation), inter alia.
Owner:VIVORYON THERAPEUTICS NV
Derivaitves of 4-Or 5-Aminosalicylic Acid
InactiveUS20080207564A1Less readily absorbedEasily reach colonBiocideAntipyreticThioester synthesisSalicylic acid
The present invention provides new derivatives of 4- or 5-aminosalicylic acid, and a pharmaceutical composition containing these derivatives of 4- or 5-aminosalicylic acid as active ingredients, useful for the treatment of intestinal diseases such as inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS) and for the prevention / treatment of colon cancer. More particularly, these derivatives comprise a hydrogen sulfide releasing moiety linked via an azo, an ester, an anhydride, a thioester or an amide linkage to a molecule of 4- or 5-aminosalicylic acid. Furthermore, the present invention provides a process for preparing these compounds and their use for treating IBD and IBS and the prevention / treatment of colon cancer.
Owner:ANTIBE THERAPEUTICS INC
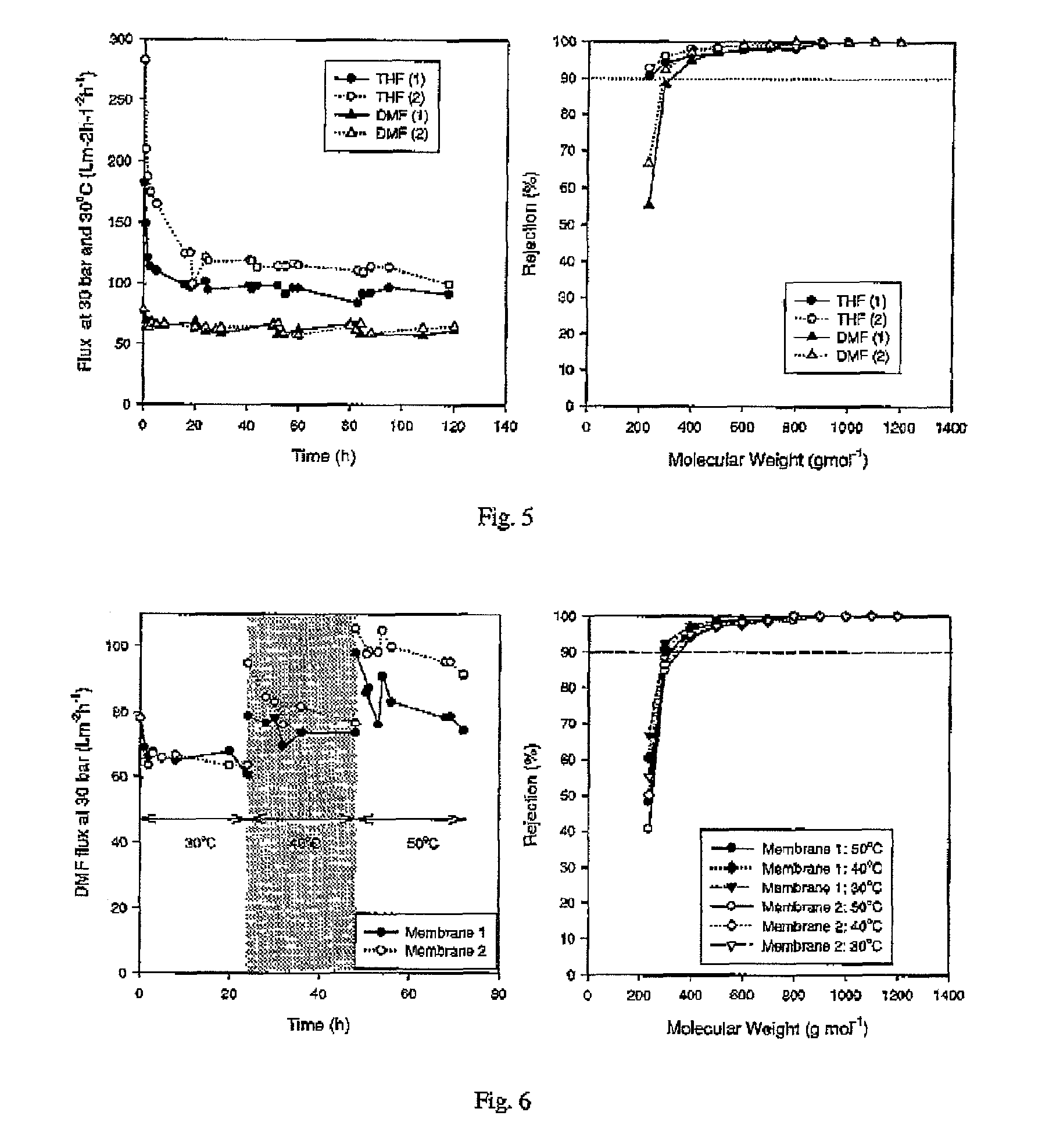
Asymmetric membranes for use in nanofiltration
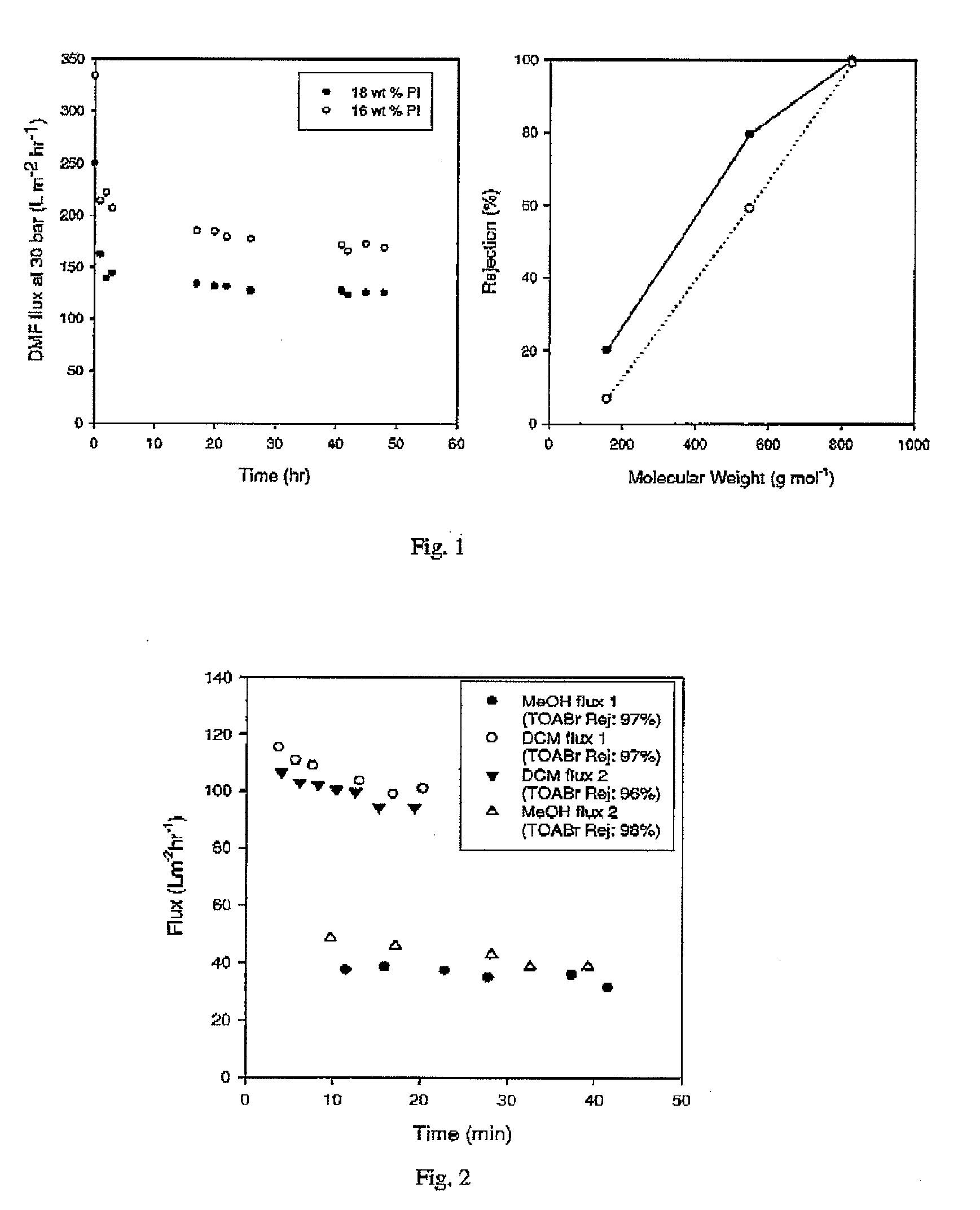
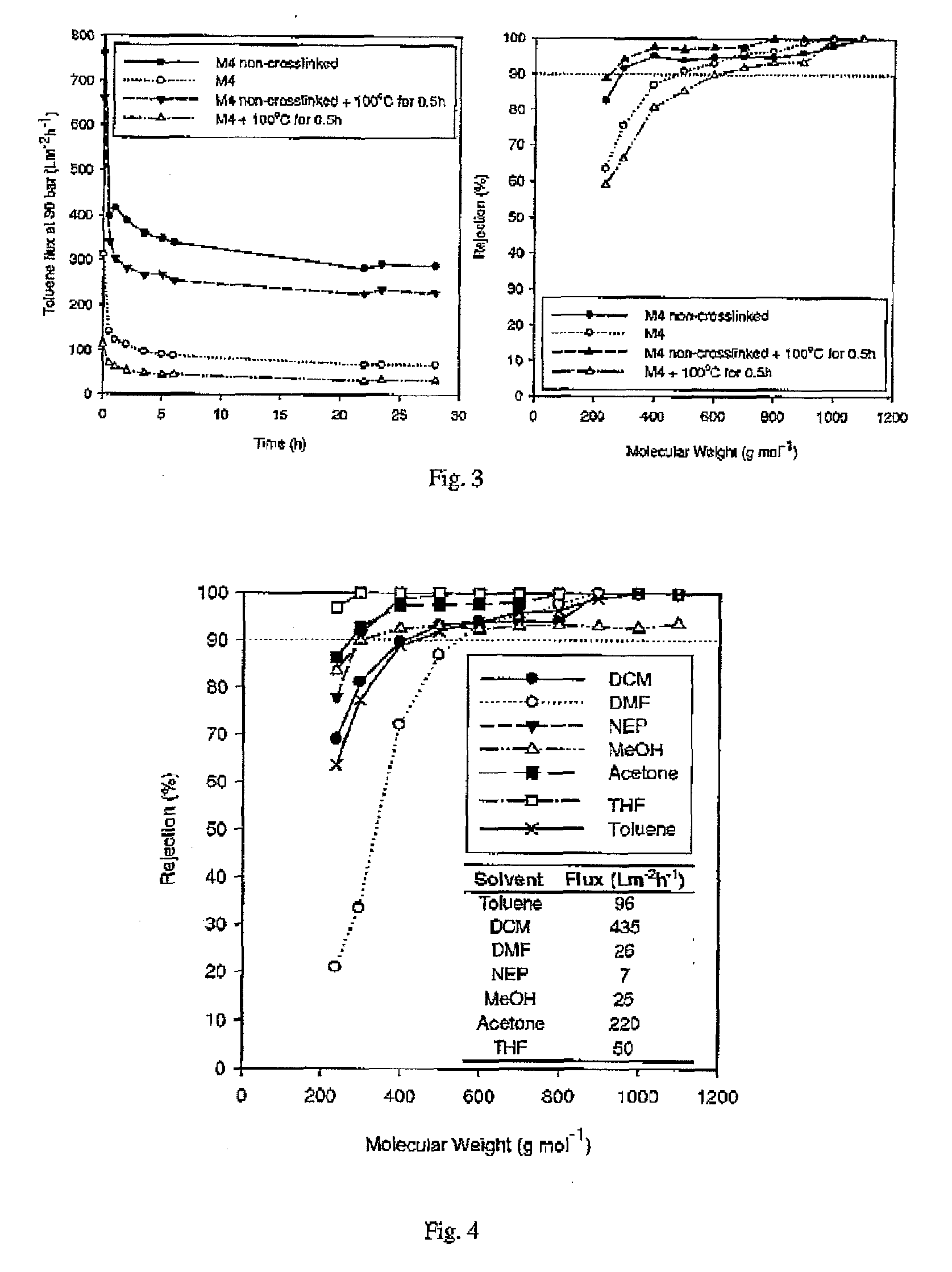
ActiveUS20100038306A1Low and no fluxImprove throughputSemi-permeable membranesMembranesImideOrganic solvent
Improved integrally skinned asymmetric membranes for organic solvent nanofiltration, and their methods of preparation and use are disclosed. Membranes are formed from polyimides by phase inversion and are then crosslinked by addition of amine crosslinking agents that react with the imide groups of the polyimide, creating amide bonds. These stabilise the membranes and allow solvent nanofiltration to be maintained even in the solvents from which the membranes were formed by phase phase inversion.
Owner:IP2IPO INNOVATIONS LTD
Insulin Derivatives
The present invention relates to insulin derivatives having a side chain attached either to the -amino group of the N-terminal amino acid residue of the B chain or to the amino group of a Lys residue present in the B chain of the parent insulin via an amide bond which side chain comprises at least one aromatic group; at least one free carboxylic acid group or a group which is negatively charged at neutral pH, a fatty acid moiety with 4 to 22 carbon atoms in the carbon chain; and possible linkers which link the individual components in the side chain together via amide bonds.
Owner:NOVO NORDISK AS
Surface modified carbonaceous materials
Grafting a polymer at the surface of a carbonated material containing carboxyl, amine and / or hydroxyl functions at its surface. This material is suspended in a solution comprising the polymer to be grafted, which includes a carboxyl, amine and / or hydroxyl function, the solution also comprising a solvent of the polymer. This is followed by a treatment causing dehydration into a carboxyl function, an amide and / or hydroxyl function and the polymer is thus grafted on the carbonated material by means of ester or amide bonds. Utilization in the cathode or anode of an electrochemical generator, in a polymer material with low polarity, in an ink, and as a conductive deposit on flexible plastic used as electrical contact, electromagnetic protection and antistatic protection.
Owner:HYDRO QUEBEC CORP +1
Cationic lipid having improved intracellular kinetics
ActiveUS9708628B2Stable structureFacilitated releaseOrganic chemistryMicroencapsulation basedLipid formationCarbamate
The present invention relates to a compound represented by the formula (1)wherein Xa and Xb are each independently X1 or X2;s is 1 or 2,R4 is an alkyl group having 1-6 carbon atoms,na and nb are each independently 0 or 1,R1a and R1b are each independently an alkylene group having 1-6 carbon atoms,R2a and R2b are each independently an alkylene group having 1-6 carbon atoms,Ya and Yb are each independently an ester bond, an amide bond, a carbamate bond, an ether bond or a urea bond, andR3a and R3b are each independently a sterol residue, a liposoluble vitamin residue or an aliphatic hydrocarbon group having 12-22 carbon atoms,and use thereof.
Owner:NOF CORP +1
Amino-modified mesoporous silica with dual drug-loading effects
InactiveCN104027814AIncrease load factorImprove bioavailabilityOrganic active ingredientsPharmaceutical non-active ingredientsMicrosphereUrsolic acid
The invention relates to the technical field of nano materials and specifically relates to amino-modified silica with dual drug-loading effects. According to the technical scheme, firstly, mesoporous silica nanoparticles are prepared by a template method; secondly, surface amino modification is carried out onto the mesoporous silica nanoparticles by 3-aminopropyltriethoxy silane; thirdly, ursolic acid is chemically coupled to the spherical mesoporous silica nanoparticles by amido bonds; and fourthly, the coupled nanoparticles are dispersed in DMF (dimethyl formamide) solution; a proper amount of ursolic acid is added, mixed and stirred; and finally vacuum drying is carried out to obtain the amino-modified silica with dual drug-loading effects. The mesoporous nano material with dual drug-loading effects prepared by the mesoporous silica disclosed by the invention is high in loading rate, can achieve controlled release effect for the loading drug ursolic acid, so that drug effect lasts for a long period time, and therefore, bioavailability of the ursolic acid is greatly improved.
Owner:FUZHOU UNIV
Erythropoietin conjugates
InactiveUS7128913B2Easy to synthesizePeptide/protein ingredientsMammal material medical ingredientsRed blood cellBone marrow cell
The present invention refers to conjugates of erythropoietin with poly(ethylene glycol) comprising an erythropoietin glycoprotein having an N-terminal α-amino group and having the in vivo biological activity of causing bone marrow cells to increase production of reticulocytes and red blood cells and selected from the group consisting of human erythropoietin and analogs thereof which have the sequence of human erythropoietin modified by the addition of from 1 to 6 glycosylation sites or a rearrangement of at least one glycosylation site; said glycoprotein being covalently linked to one poly(ethylene glycol) group of the formula—CO—(CH2)x—(OCH2CH2)m—ORwherein the —CO of the poly(ethylene glycol) group forms an amide bond with said N-terminal α-amino group; and wherein R is lower alkyl; x is 2 or 3; and m is from about 450 to about 1350.
Owner:F HOFFMANN LA ROCHE INC
Common carrier material for targeting anticancer drug and gene and preparation and application
InactiveCN102949727AGood biocompatibilityEnhanced Osmotic Retention EffectGenetic material ingredientsInorganic non-active ingredientsTumor targetingResponse control
The invention relates to a common carrier material based on graphene oxide for a targeting anticancer drug and a gene and application and application. Folic acid, lactobionic acid and other tumor cell targeting or liver targeting molecules and part of amino groups of soluble chitosan are connected by amide bonds to prepare a conjugate, the conjugate is then connected with graphene oxide, quaternization is performed by using an epoxy compound with a quaternary ammonium group, and gene molecules are loaded by the quaternizationquaternized part of the chitosan through electrostatic attraction; and then the anticancer drug is loaded by pi-pi conjugates, hydrogen bonds and hydrophobic effects in a non-covalent bond method. By adopting the targeting performance of targeting molecules and effects of graphene oxide of a particular size to enhance penetration and retention in tumor tissues and combining the performance of the graphene oxide for pH response control release of the loaded drug, the drug can be realized released in a tumor cell, an intelligent delivery system for the common carrier of the tumor targeting or liver targeting anticancer drug and the gene is synthesized from the perspective of synergetic medication, and a theoretical basis and a method basis are provided for combined therapy of tumor.
Owner:TIANJIN MEDICAL UNIV
Polymeric coating for the protection of objects
ActiveUS20120052293A1Provide protectionCosmonautic vehiclesCosmonautic radiation protectionPhenyl groupPolyimide
A protective polymeric coating is applied to the surface of various objects which are to be exposed to a harsh environment. The protective polymeric coating covers the exposed surface, where the polymeric coating includes a polyimide polymer. The polyimide polymer in the polymeric coating has a backbone with at least one non-terminal phenyl group. A linkage is connected to the non-terminal phenyl group, where the linkage can be an amide linkage or an ester linkage. An oligomeric silsesquioxane compound is connected to the linkage through an organic substituent, where the oligomeric silsesquioxane is not incorporated into the polymer backbone. The polymeric coating provides protection to the underlying object.
Owner:NEXOLVE HLDG CO LLC
FAP-activated anti-tumor compounds
InactiveUS20020155565A1Sugar derivativesPeptide/protein ingredientsAbnormal tissue growthCytotoxicity
The invention relates to a prodrug that is capable of being converted into a drug by the catalytic action of human fibroblast activation protein (FAPalpha), said prodrug having a cleavage site which is recognized by FAPalpha, and said drug being cytotoxic or cytostatic under physiological conditions, wherein said prodrug comprises an oligomeric part comprising at least two amino carboxylic residues, and a cytotoxic or cytostatic part, wherein the C-terminal amino carboxylic residue of the oligomeric part is an acyclic amino acid, the nitrogen atom of the amino function thereof is attached to a substituent being different from a hydrogen atom, and the C-terminal carboxy function thereof is linked to the cytotoxic or cytostatic part by an amide bond.
Owner:BOEHRINGER INGELHEIM PHARM KG
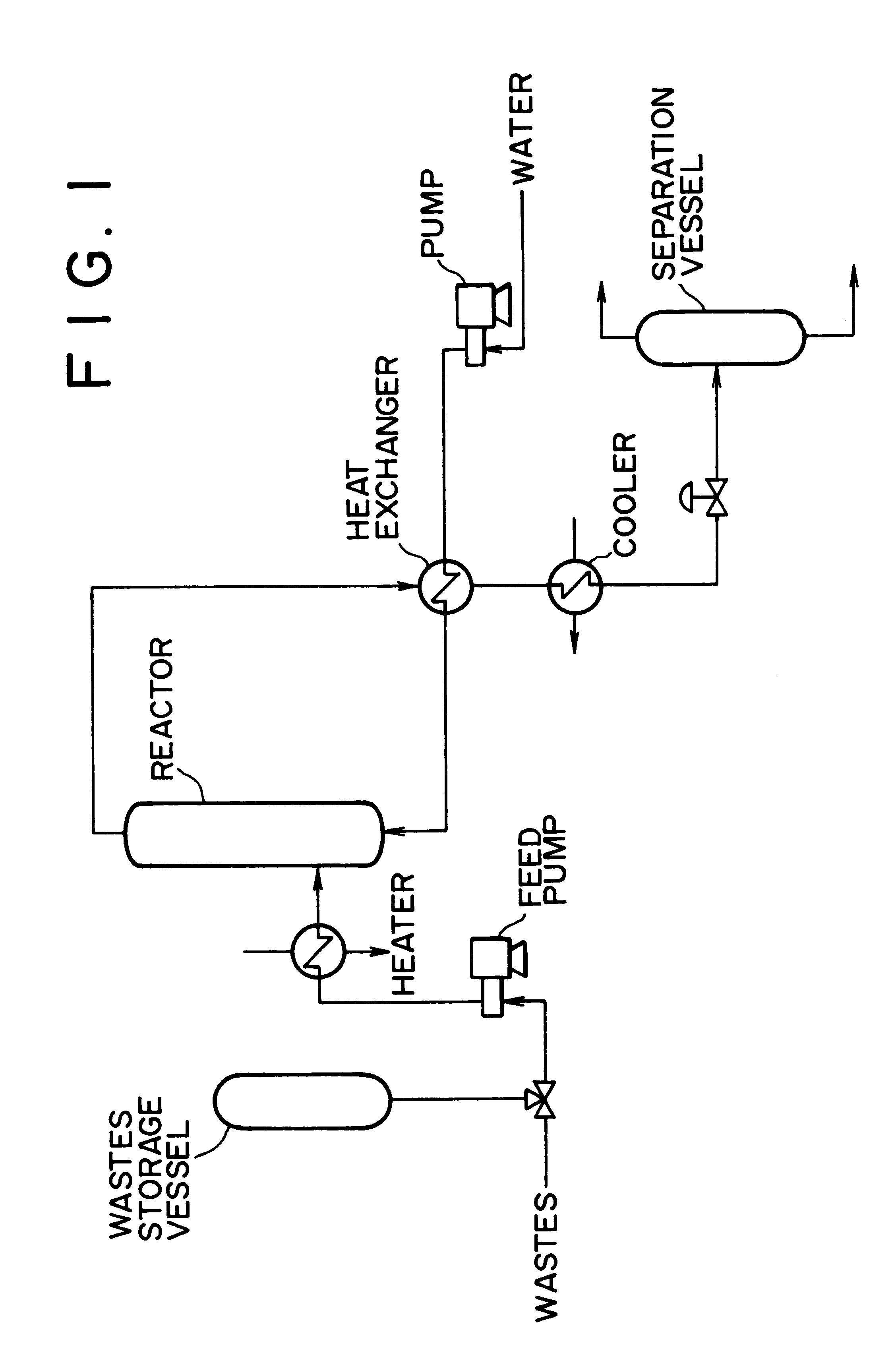
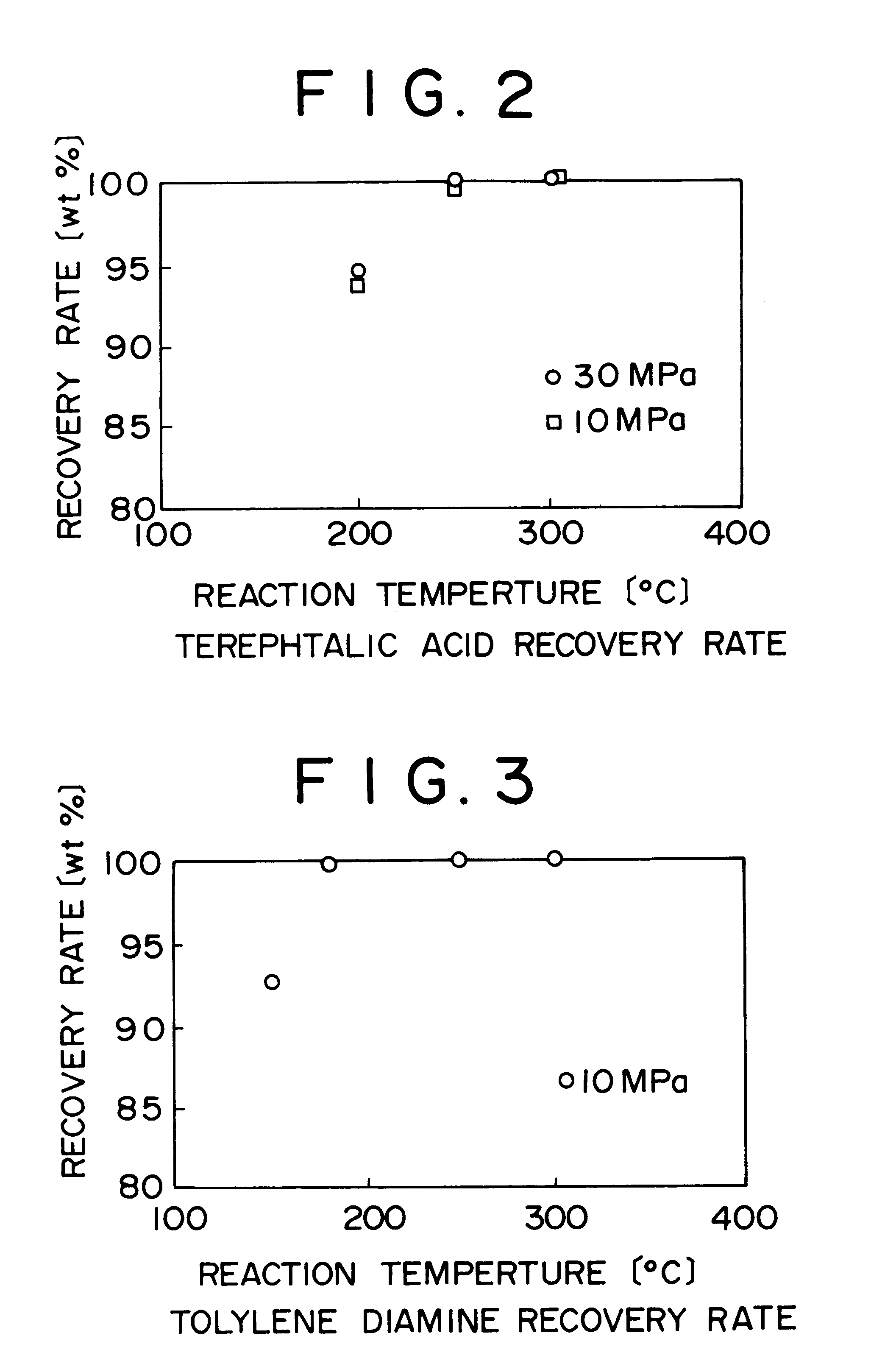
Method of and apparatus for decomposing wastes
InactiveUS6255529B1Useful operationResultant decomposition products can be utilized effectivelySolid waste disposalIndirect heat exchangersMolten stateLiquid state
A method of decomposing wastes containing target compounds having one or more of hydrolyzable bonds of ether bond, ester bond, amide bond and isocyanate bond wherein the method comprises continuously supplying the wastes in a molten state or liquid state to a reactor, continuously supplying super-critical water or high pressure / high temperature water to the reactor, bringing the water into contact with the wastes, thereby decomposing the target compounds and then recovering them as raw material compounds or derivatives thereof for the target compounds. Target compounds contained in wastes in chemical plants which could not be utilized but merely incinerated or discarded so far are continuously decomposed into raw material compounds or derivatives thereof for the aimed compound and can be reutilized effectively.
Owner:TAKEDA CHEM IND LTD 50
Polysaccharide conjugate of carboxylic acid drug, preparation method thereof and application thereof
InactiveCN101745119AMild conditionsFew reaction stepsPharmaceutical non-active ingredientsPharmaceutical active ingredientsCarboxylic acidPolysaccharide
The invention discloses a polysaccharide conjugate of carboxylic acid drug, a preparation thereof and application thereof. In the technical scheme, sub-alkyl diamine with 2-12 carbon atoms is used as a connecting arm, and a carboxylic acid drug and polysaccharide carboxyl are connected with each other through an amido link. Compared with the original carboxylic acid drug, the conjugate enhances the pharmacological effect, reduces the adverse effect and improve the safety. In addition, the conjugate can have the amphipathic performance through using the hydrophobic carboxylic acid drug, so as to be used as a carrier of a slightly soluble or sparingly soluble drug. The preparation method of the invention is simple, the process is mature, the yield is high, and the preparation method is applicable to industrial production.
Owner:CHINA PHARM UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com