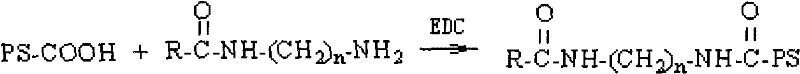Polysaccharide conjugate of carboxylic acid drug, preparation method thereof and application thereof
A technology of carboxylic acid drugs and conjugates, which can be used in pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. Low, difficult to achieve the dosage and other issues, to achieve the effects of good stability, excellent biodegradability, and excellent biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: the synthesis of all-trans retinoic acid-heparin
[0050] Take 10mmol all-trans retinoic acid, 12mmol dicyclohexylcarbodiimide (DCC), 15mmol hydroxysuccinimide (NHS), dissolve in 30ml N,N-dimethylformamide, protect from light and nitrogen , reacted in ice bath for 30min, and then rose to room temperature for 24h. After the reaction, the precipitate was filtered off, and a large amount of ethyl acetate was added to wash the precipitate. The filtrate was extracted, the ethyl acetate layers were combined, and the solvent was removed by rotary evaporation to obtain the activated intermediate ester of all-trans retinoic acid. Dissolve 1mmol of all-trans retinoic acid activated intermediate ester in 10ml of dichloromethane, slowly drop into 3mmol / ml of ethylenediamine in dichloromethane solution under ice-bath conditions, and monitor the reaction by thin layer chromatography (TLC method) After completion, the reaction liquid is extracted, the organic layers ar...
Embodiment 2
[0051] Embodiment 2: the synthesis of baicalin-chondroitin
[0052] Take 10mmol baicalin, 16mmol dicyclohexylcarbodiimide (DCC), 16mmol hydroxysuccinimide (NHS), dissolve in 25ml tetrahydrofuran, keep away from light, under the protection of nitrogen, react in ice bath for 45min, then rise to room temperature for reaction After 24 hours, the precipitate was filtered off, and a large amount of ethyl acetate was added to wash the precipitate. The filtrate was extracted, the ethyl acetate layers were combined, and the solvent was removed by rotary evaporation to obtain the activated intermediate ester of baicalin. Dissolve 2mmol of baicalin activated intermediate ester in 20ml of dichloromethane, and slowly drop into 6mmol / ml of ethylenediamine in dichloromethane solution under ice-bath conditions. After TLC monitors the reaction until complete, extract the reaction solution and combine organic layer, and separate and purify the resulting product to obtain the active intermediat...
Embodiment 3
[0053] Embodiment 3: the synthesis of baicalin-carboxymethyl chitosan
[0054] Take 10mmol baicalin, 16mmol dicyclohexylcarbodiimide (DCC), 16mmol hydroxysuccinimide (NHS), dissolve in 25ml tetrahydrofuran, keep away from light, under the protection of nitrogen, react in ice bath for 45min, then rise to room temperature for reaction After 24 hours, the precipitate was filtered off, and a large amount of ethyl acetate was added to wash the precipitate. The filtrate was extracted, the ethyl acetate layers were combined, and the solvent was removed by rotary evaporation to obtain the activated intermediate ester of baicalin. Dissolve 2mmol of baicalin activated intermediate ester in 20ml of dichloromethane, and slowly drop into 6mmol / ml of ethylenediamine in dichloromethane solution under ice-bath conditions. After TLC monitors the reaction until complete, extract the reaction solution and combine organic layer, and separate and purify the resulting product to obtain the active ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com