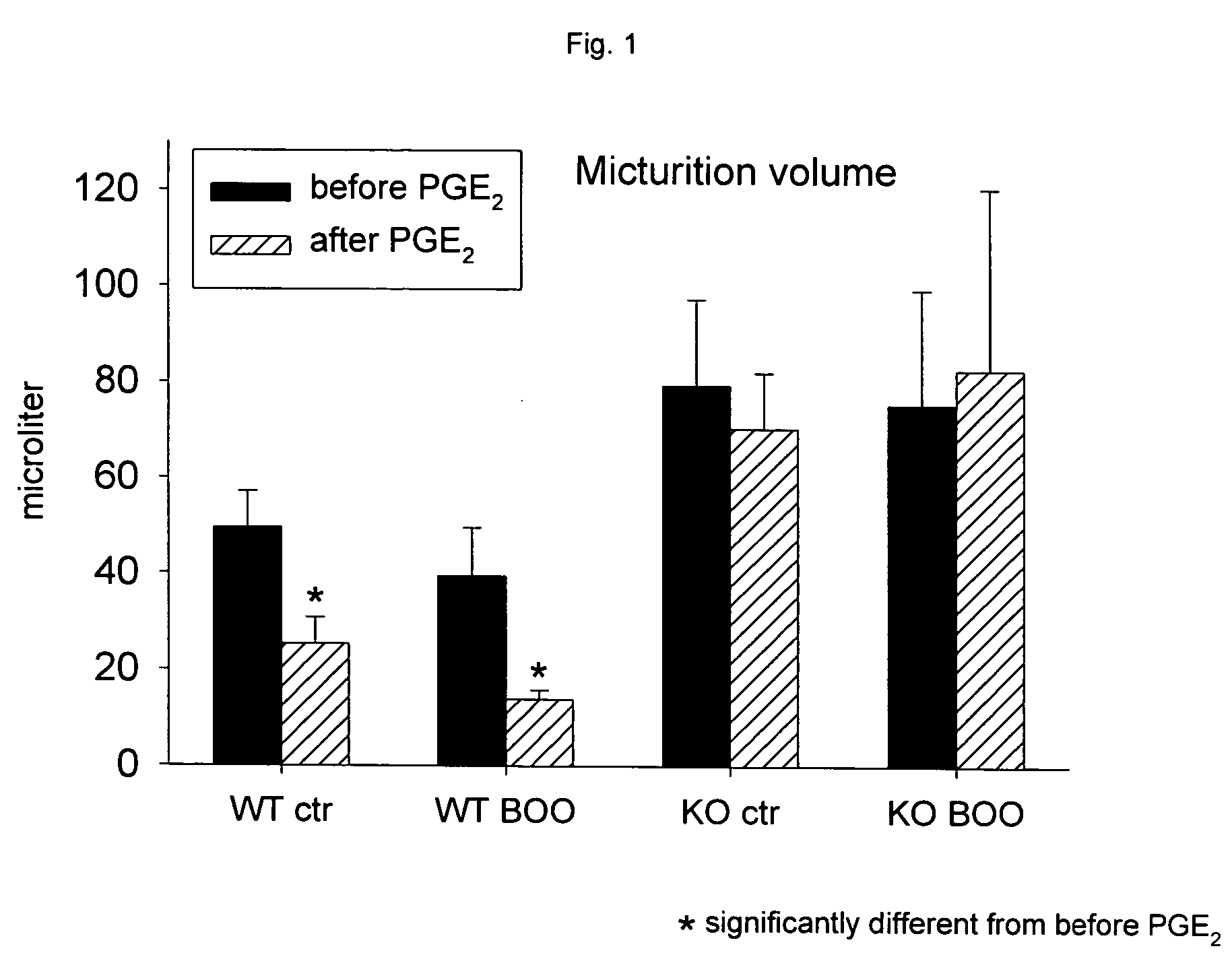
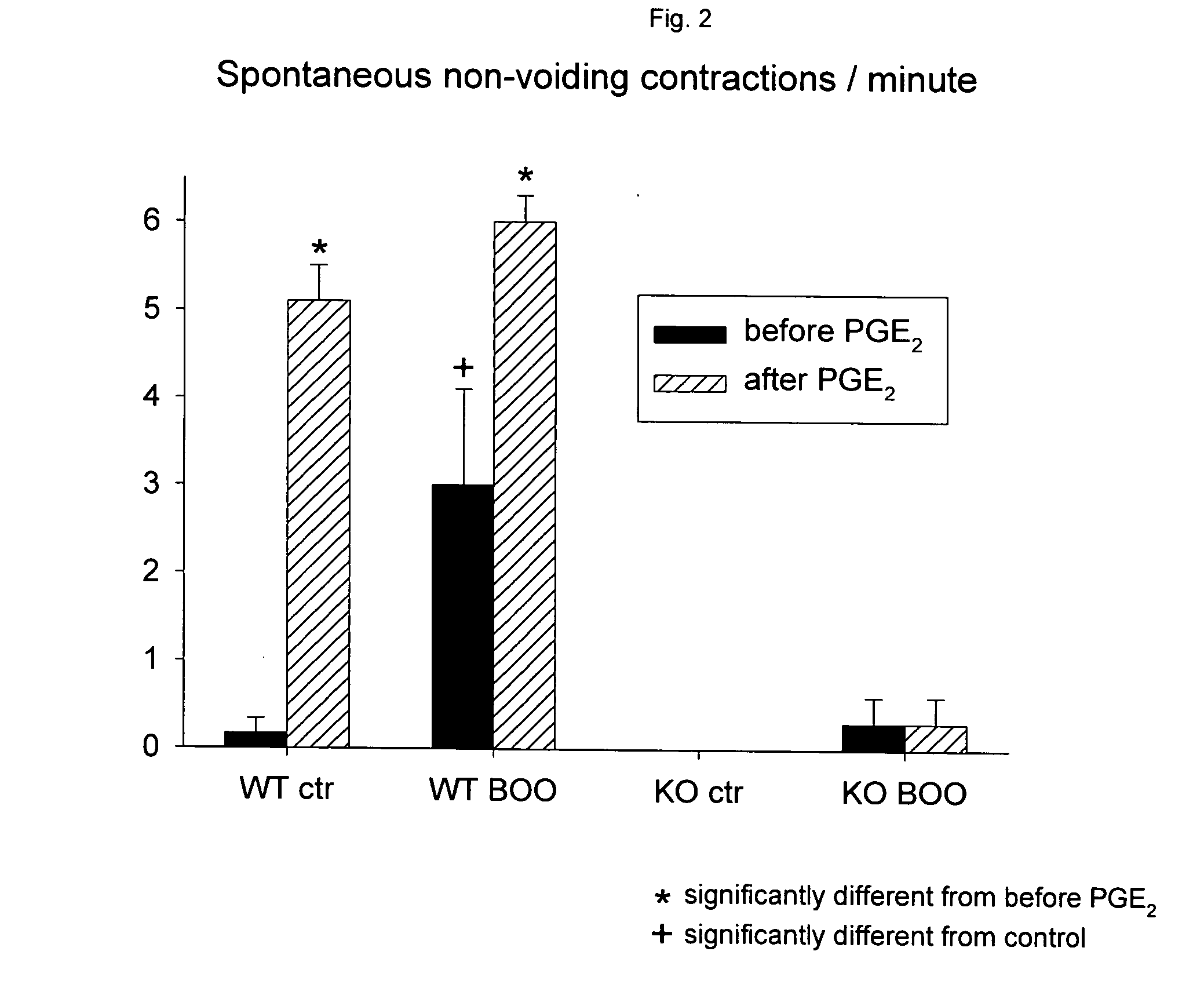
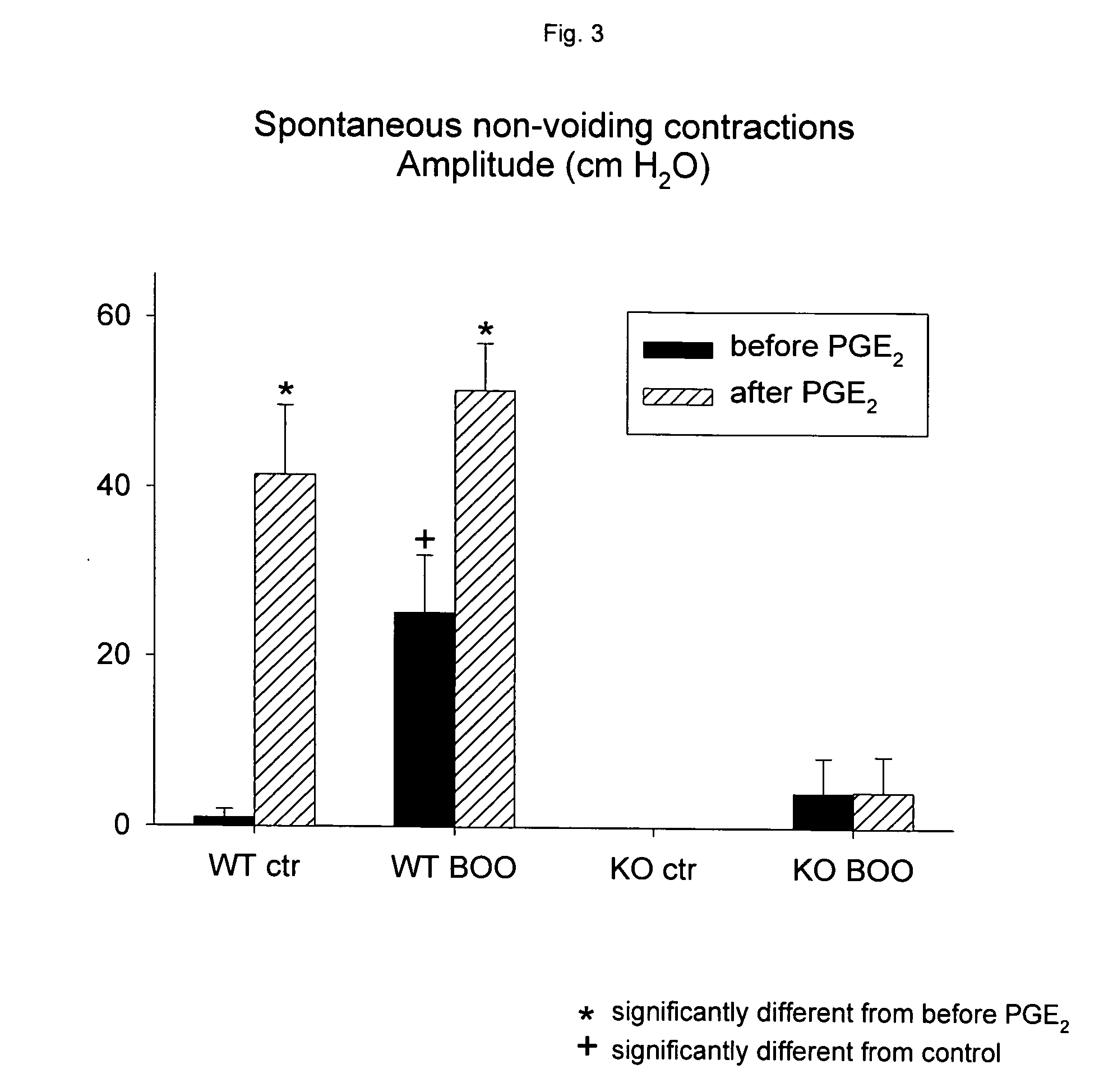
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
103 results about "Lower urinary tract symptoms" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lower urinary tract symptoms (LUTS) refer to a group of clinical symptoms involving the bladder, urinary sphincter, urethra and, in men, the prostate. Although LUTS is a preferred term for prostatism, and is more commonly applied to men, lower urinary tract symptoms also affect women.
Indole derivative having piperidine ring
InactiveUS20050256103A1High affinityEnhanced inhibitory effectBiocideNervous disorderHydrogen atomSerotonin 1A Receptor
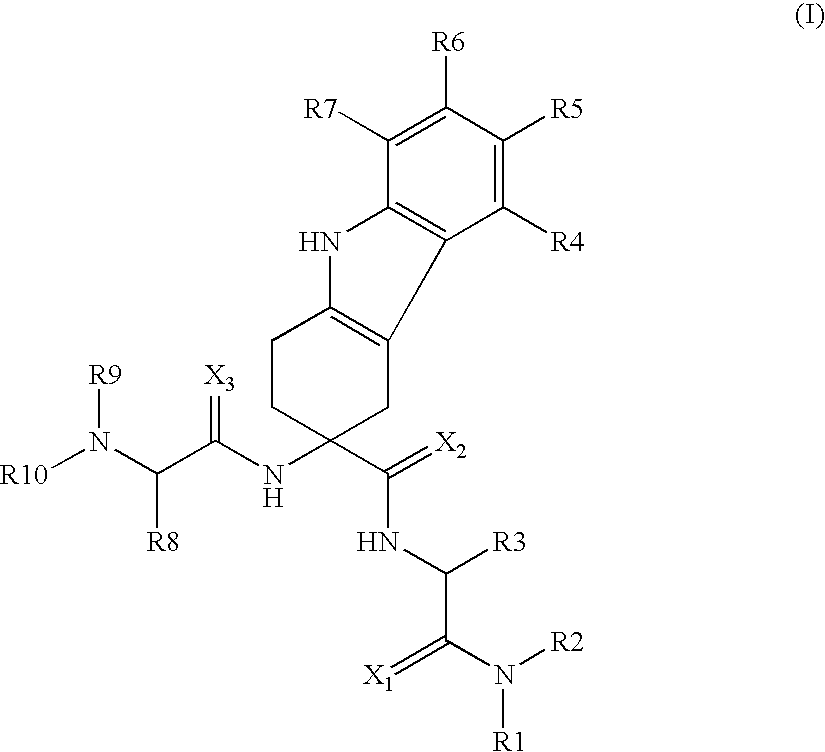
The present invention relates to a compound represented by the following formula, a pharmacologically acceptable salt thereof, or a use thereof as a pharmaceutical: wherein R1 and R2 are substituents adjacent to each other, and together with two carbon atoms to each of which they attach, form a 5- to 7-membered non-aromatic carbocyclic group or the like, which may be substituted by 1 to 4 substituents selected from (1) an oxo group, (2) a hydroxyl group, and the like; R3 represents a hydrogen atom or the like; and R6 represents a hydrogen atom or the like. It is an object of the present invention to discover an agent for treating or preventing lower urinary tract symptoms, and particularly symptoms regarding urinary storage, which has a superior strength of binding to a 5-HT1A receptor and an antagonism to the receptor.
Owner:EISIA R&D MANAGEMENT CO LTD
Treatment Of Interstitial Cystitis
ActiveUS20100260775A1Immunoglobulins against growth factorsAntibody ingredientsAnti ngfLower urinary tract symptoms
The present invention relates to the use of an anti-NGF antibody in the treatment or prevention of pain and / or a lower urinary tract symptom (LUTS) associated with interstitial cystitis and / or painful bladder syndrome and / or bladder pain syndrome.
Owner:PFIZER LTD
Treatment of BPH
InactiveUS20050020646A1Bladder capacity decreasedReduce volume capacityBiocideCompound screeningScreening methodProstate hyperplasia
The present invention relates to the use of EP1 receptor antagonists for the treatment of lower urinary tract symptoms (LUTS) associated with benign prostatic hyperplasia (BPH). The invention also includes screening methods to identify compounds useful for the treatment of LUTS associated with BPH.
Owner:PFIZER INC
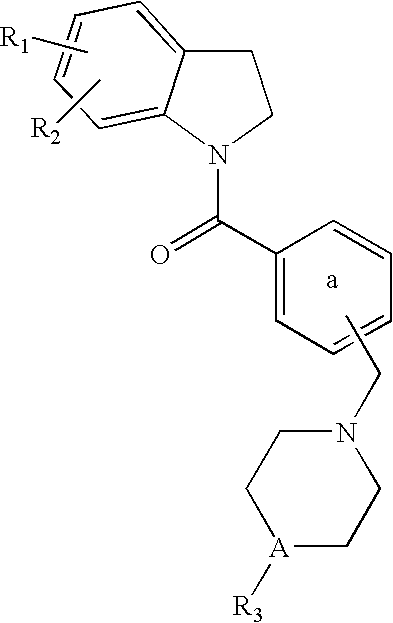
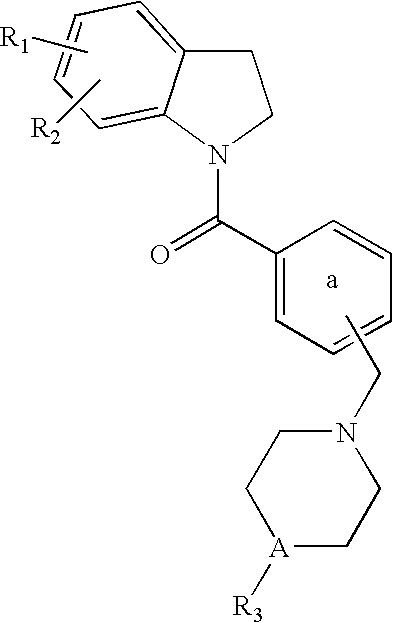
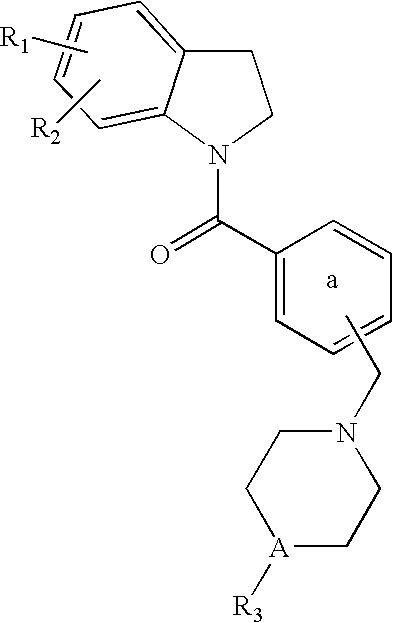
Dihydroindolyl methanones as alpha 1a/1d adrenoreceptor modulators for the treatment of benign prostatic hypertrophy and lower urinary tract symptoms
InactiveUS20060183902A1Affects blood pressureEffective amountBiocideOrganic chemistryEccentric hypertrophyDisease
The present invention relates to new compounds of Formula (I): and pharmaceutically acceptable forms thereof, use of the compounds as α1a and / or α1d adrenoreceptor modulators, including use of a pharmaceutical composition, medicine or medicament comprising said compounds, a process to prepare said compounds and a method for treating an α1a and / or α1d adrenoreceptor mediated disorder.
Owner:JANSSEN PHARMA NV
Kits and improved compositions for treating lower urinary tract disorders
PendingUS20120196830A1Remarkable effectSufficient quantityAntibacterial agentsBiocideDiseaseSevere pain
Superior buffered formulations and their kits for treating lower urinary tract symptoms and disorders are provided in the invention. In particular superior buffered formulations have demonstrated improvement for treating lower urinary tract symptoms of patients experiencing severe pain and / or urgency of the bladder and associated areas of the lower urinary tract.
Owner:PLATINUM MONTAUR LIFE SCI LLC & AS AGENT
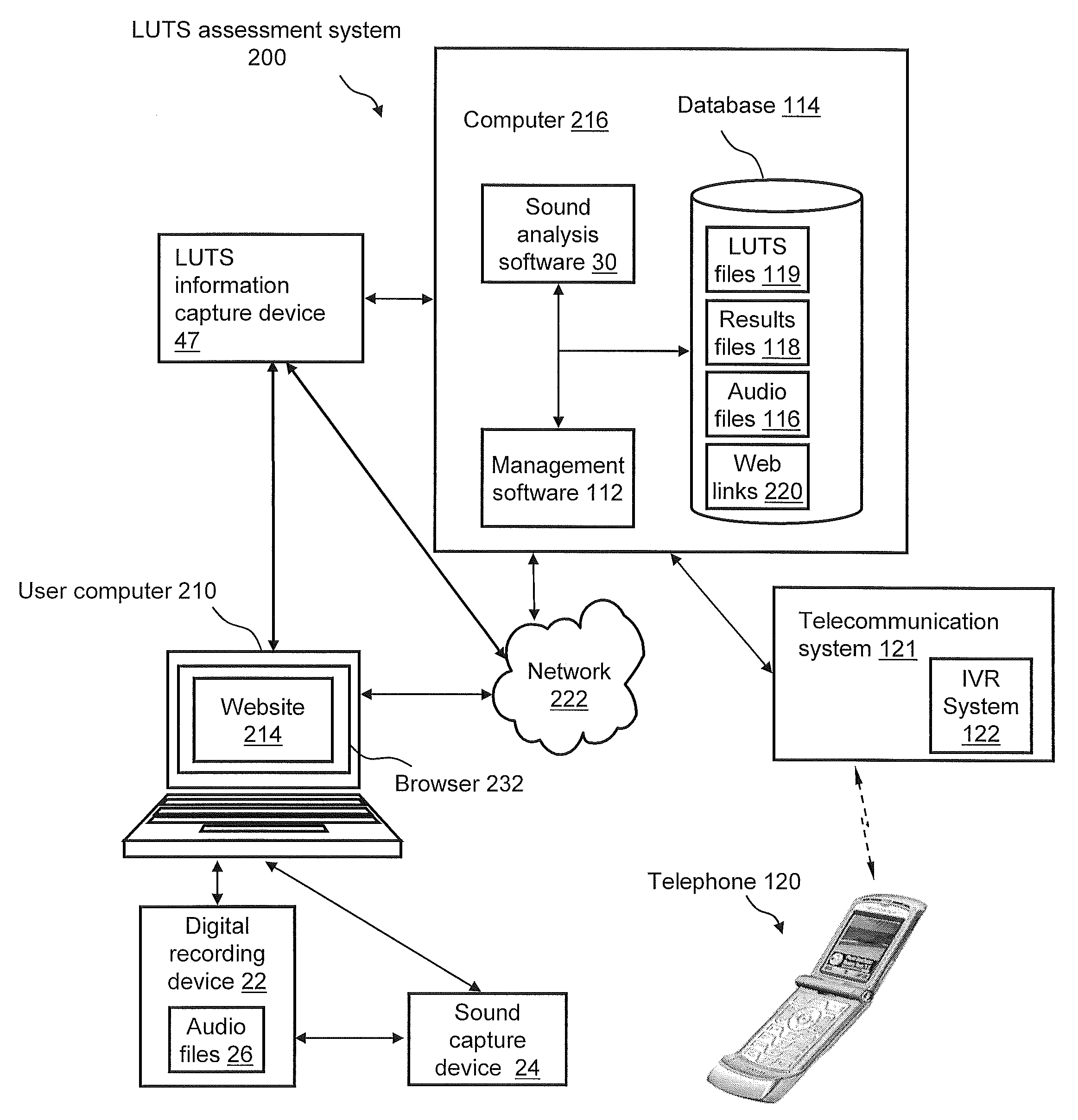
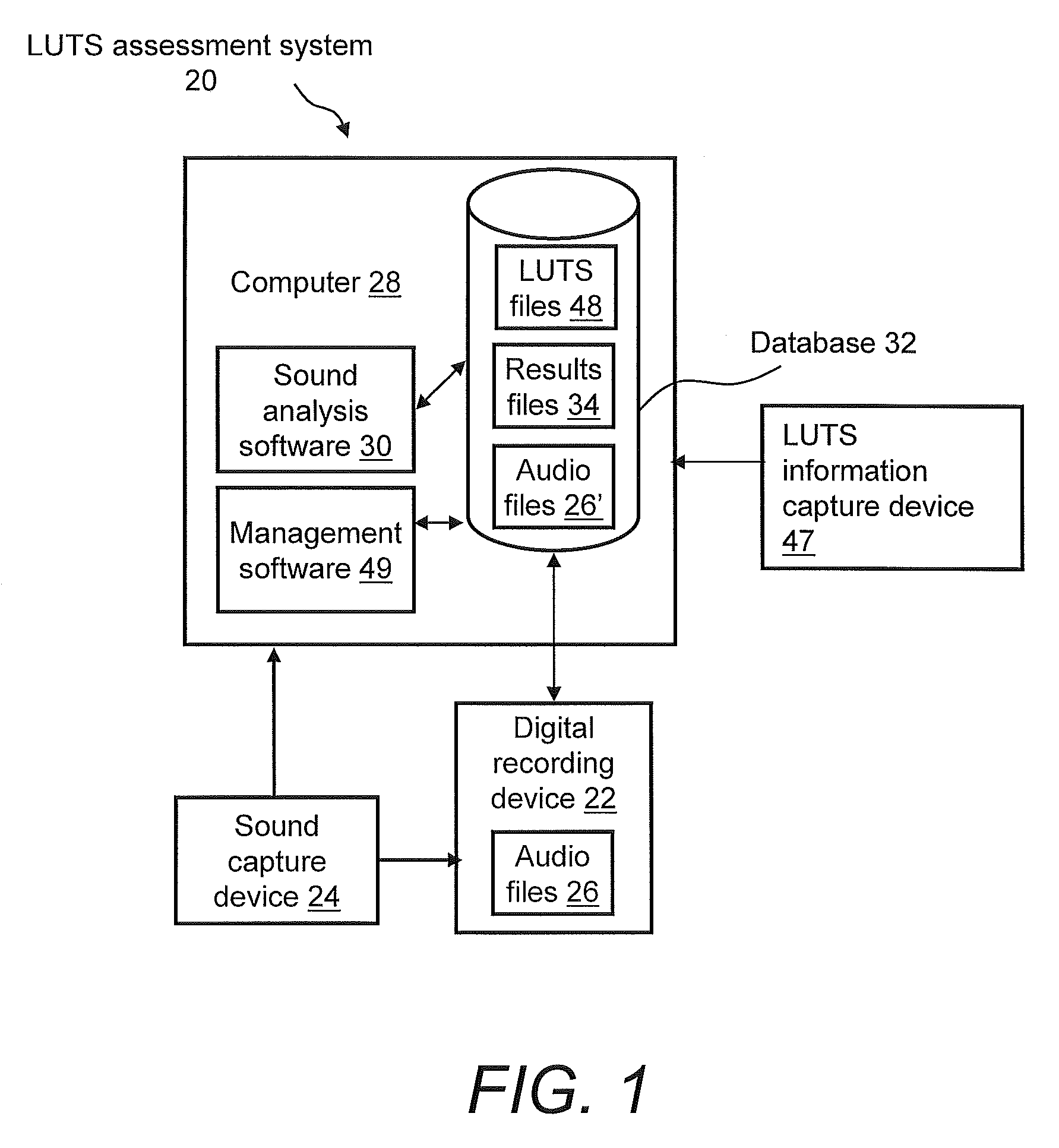
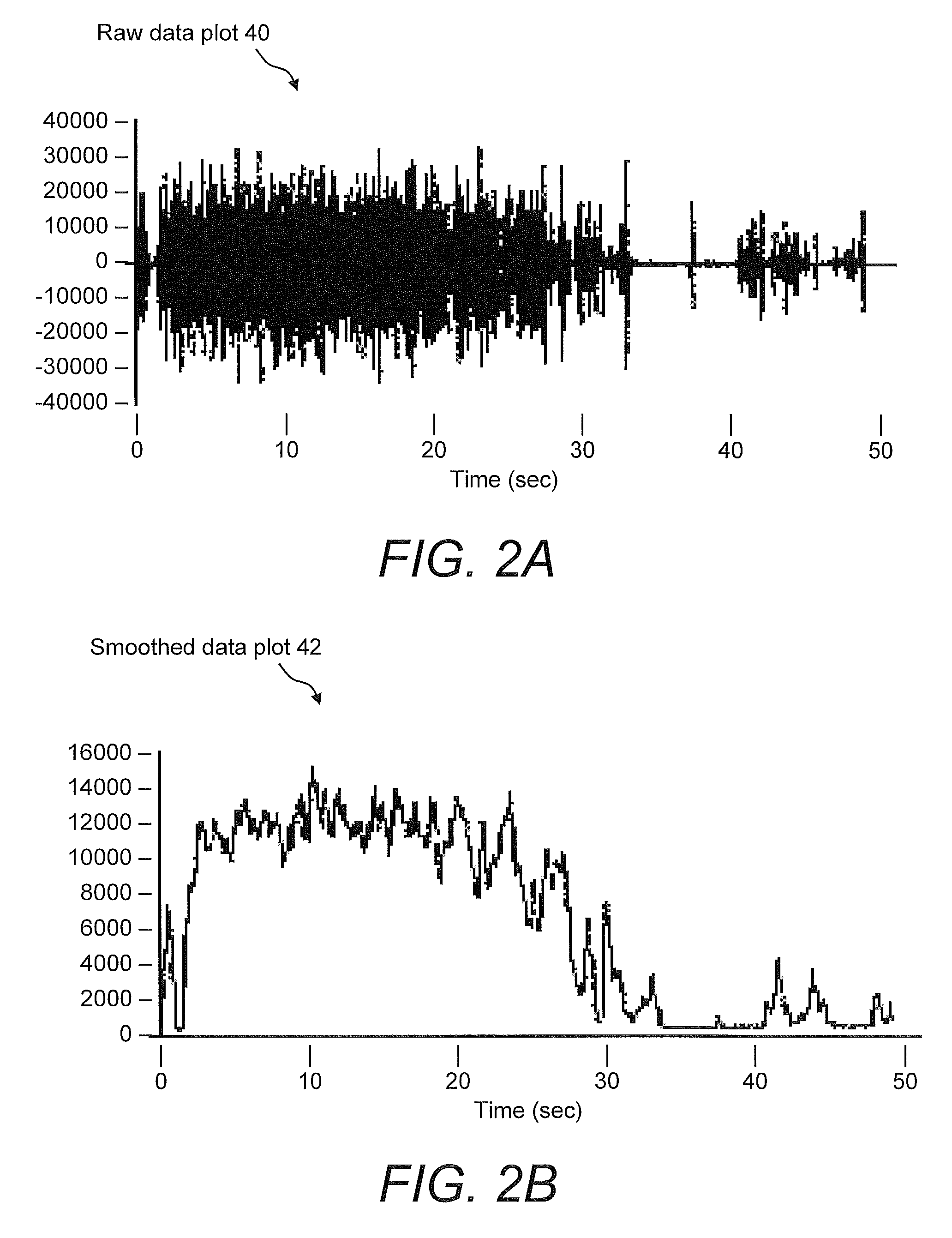
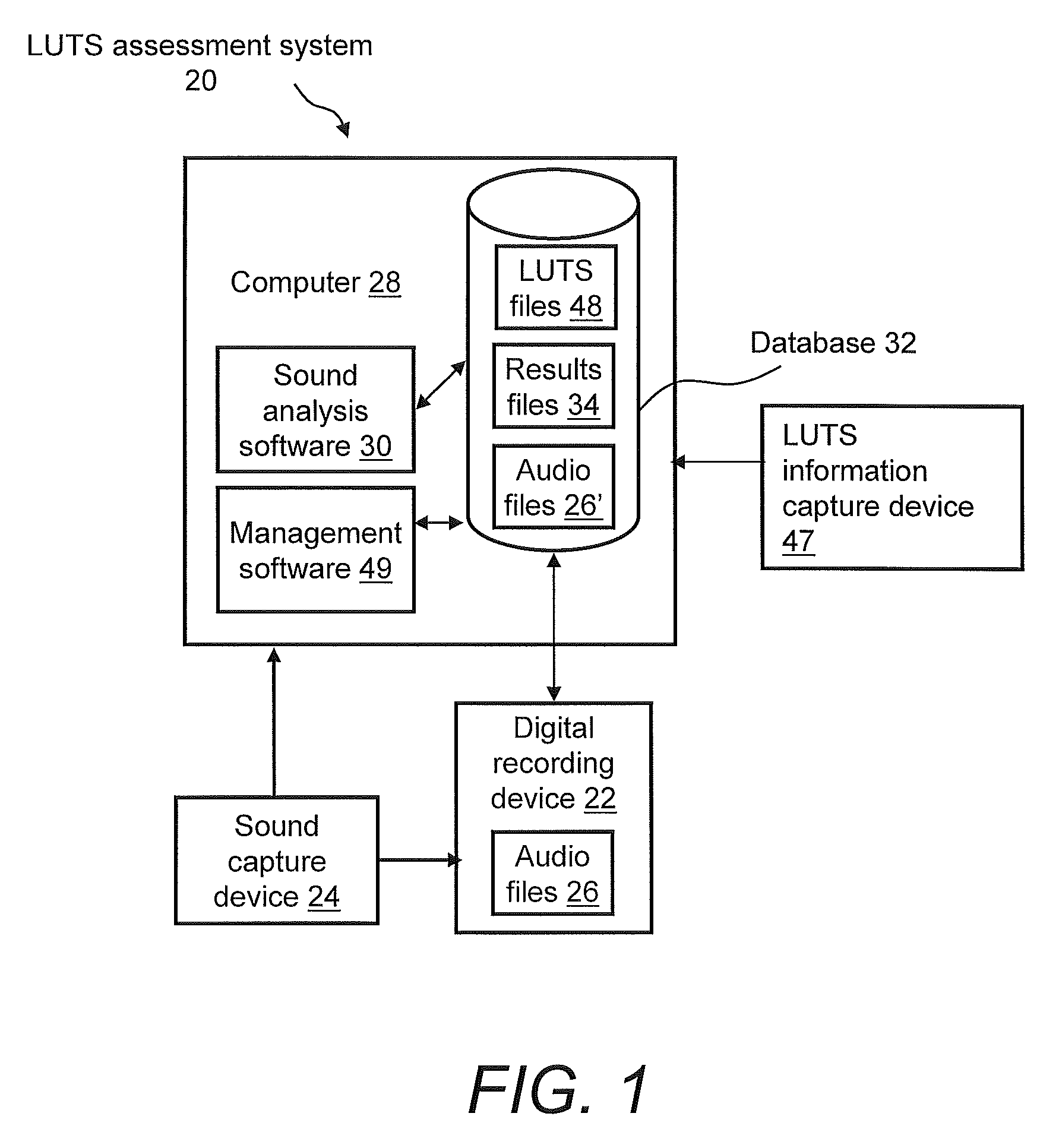
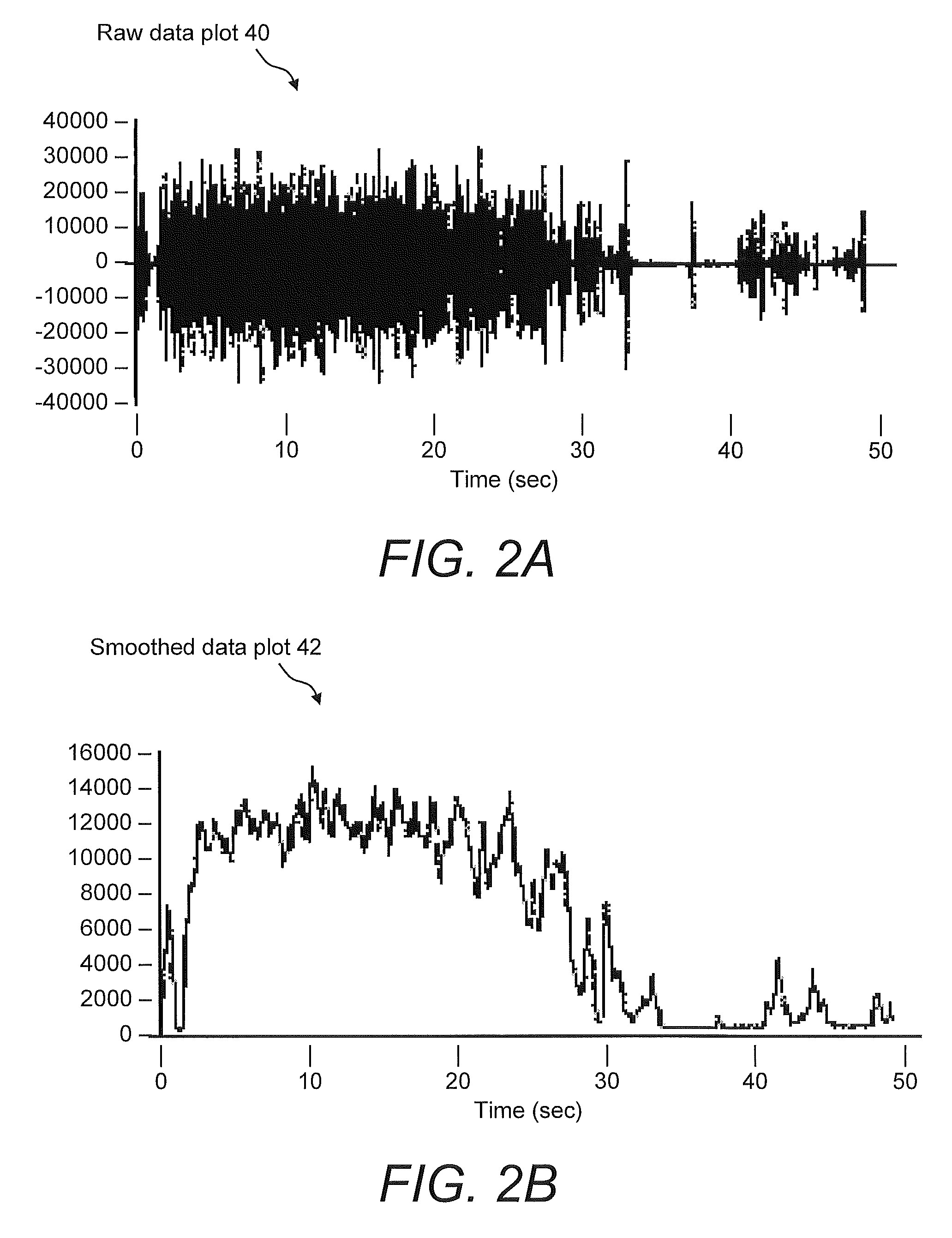
Systems For And Methods Of Assessing Lower Urinary Tract Function Via Sound Analysis
Systems for and methods of assessing lower urinary tract function from urinary flow data via sound analysis and user-provided information regarding the lower urinary tract symptoms (LUTS) of frequency, urgency and urge incontinence. Embodiments of the LUTS assessment systems include a computer and a telephone or a digital recording mechanism to capture the sound of one or more urination events, which are stored as audio files in a database. The LUTS assessment systems may include sound analysis software for analyzing the strength and duration of each urination event and may include a web-based software application for viewing the results via the Internet or other network. The database stores information from multiple urination events, and combined with information regarding the lower urinary tract symptoms, serves as an objective tool to assess bladder function and monitor progression of disease or therapy effectiveness.
Owner:UNIVERSITY OF VERMONT
Treatment of interstitial cystitis
ActiveUS8591898B2Immunoglobulins against growth factorsAntibody ingredientsBladder Pain SyndromeAnti ngf
The present invention relates to the use of an anti-NGF antibody in the treatment or prevention of pain and / or a lower urinary tract symptom (LUTS) associated with interstitial cystitis and / or painful bladder syndrome and / or bladder pain syndrome.
Owner:PFIZER LTD
Treatment of chronic prostatitis
ActiveUS8481036B2Immunoglobulins against growth factorsAntibody ingredientsSurgeryPelvic pain syndrome
The present invention relates to the use of an anti-NGF antibody in the treatment or prevention of pain and / or a lower urinary tract symptom (LUTS) associated with chronic prostatitis and / or chronic pelvic pain syndrome.
Owner:PFIZER INC
Treatment of Chronic Prostatitis
ActiveUS20110097341A1Significant economic impactEconomic impactImmunoglobulins against growth factorsAntibody ingredientsSurgeryAnti ngf
The present invention relates to the use of an anti-NGF antibody in the treatment or prevention of pain and / or a lower urinary tract symptom (LUTS) associated with chronic prostatitis and / or chronic pelvic pain syndrome.
Owner:PFIZER INC
Indole Derivative Having Piperidine Ring
InactiveUS20070219179A1Enhanced inhibitory effectIncrease frequencyBiocideNervous disorderHydrogen atomSerotonin 1A Receptor
The present invention relates to a compound represented by the following formula, a pharmacologically acceptable salt thereof, or a use thereof as a pharmaceutical: wherein R1 and R2 are substituents adjacent to each other, and together with two carbon atoms to each of which they attach, form a 5- to 7-membered non-aromatic carbocyclic group or the like, which may be substituted by 1 to 4 substituents selected from (1) an oxo group, (2) a hydroxyl group, and the like; R3 represents a hydrogen atom or the like; and R6 represents a hydrogen atom or the like. This compound has a superior strength of binding to a 5-HT1A receptor and an antagonism to the receptor, and is useful as an agent for treating or preventing lower urinary tract symptoms, and particularly symptoms regarding urinary storage.
Owner:EISIA R&D MANAGEMENT CO LTD
Combined Use of an Alpha-Adrenergic Receptor Antagonist and an Anti-Muscarinic Agent
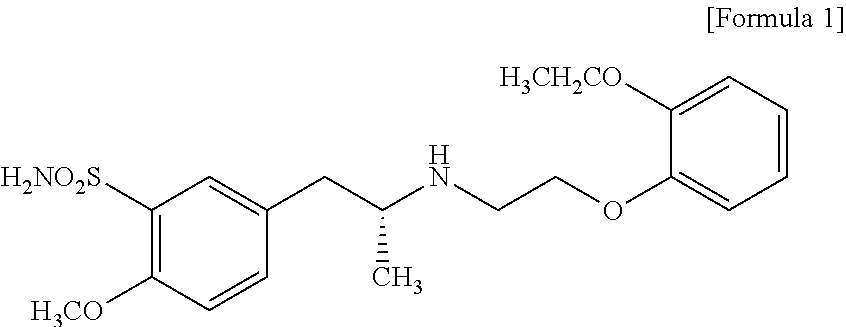
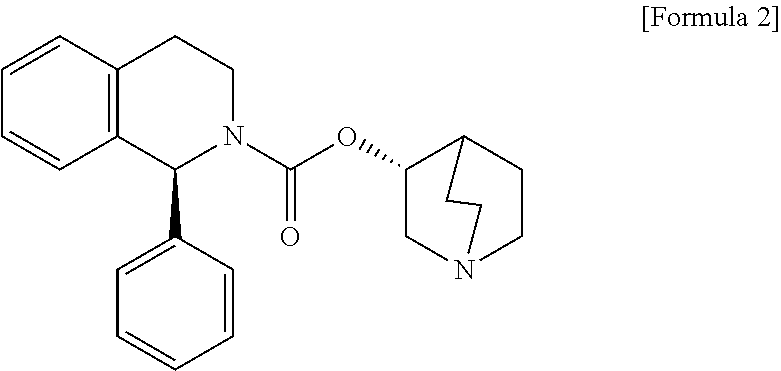
The combined use of (R)-5-(2-{[2-(2-ethoxyphenoxy)ethyl]amino}propyl)-2-methoxybenzene-1-sulfonamide (tamsulosin), or its pharmaceutically acceptable salt, and (1S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline-2-carboxylic acid (3R)-quinuclidin-3-yl ester (solifenacin), or its pharmaceutically acceptable salt, for the preparation Of a medicament for the improvement of lower urinary tract symptoms associated with benign prostatic hyperplasia (LUTS / BPH) with a substantial storage component is provided.
Owner:ASTELLAS IRELAND
Implantable devices and methods to treat benign prostate hyperplasia (BPH) and associated lower urinary tract symptoms (LUTS)
ActiveUS20180318114A1Avoids inadvertentAvoids misplaced deploymentStentsIncision instrumentsUrethraMinimally invasive procedures
The invention is devices and methods to treat benign prostatic hyperplasia (BPH) and associated lower urinary tract symptoms infections (LUTS). The devices are intra-urethral implants placed in a patient in need thereof by minimally invasive procedures, preferably under local anesthesia in an office environment. The devices are sized and designed for atraumatic insertion and expansion within the urethra to engage and retract enlarged prostatic tissue proximate to the urethra that is leading to adverse symptoms associated with BPH. The methods include steps to deploy the implant devices of the invention using a delivery system of the invention and at target prostatic tissue that is visualized during the procedure and yields a reduction in the symptoms of BPH.
Owner:PRODEON MEDICAL CORP
Methods of treating benign prostatic hyperplasia or lower urinary tract symptoms by using PDE 5 inhibitors
The use of PDE 5 inhibitors in methods for the treatment of benign prostatic hyperplasia or lower urinary tract symptoms and other physiological disorders, as a monotherapy and in combination with other active agents is disclosed. For example, a representative compound useful in the methods of the invention is:
Owner:SCHERING CORP
Systems for and methods of assessing lower urinary tract function via sound analysis
Systems for and methods of assessing lower urinary tract function from urinary flow data via sound analysis and user-provided information regarding the lower urinary tract symptoms (LUTS) of frequency, urgency and urge incontinence. Embodiments of the LUTS assessment systems include a computer and a telephone or a digital recording mechanism to capture the sound of one or more urination events, which are stored as audio files in a database. The LUTS assessment systems may include sound analysis software for analyzing the strength and duration of each urination event and may include a web-based software application for viewing the results via the Internet or other network. The database stores information from multiple urination events, and combined with information regarding the lower urinary tract symptoms, serves as an objective tool to assess bladder function and monitor progression of disease or therapy effectiveness.
Owner:UNIVERSITY OF VERMONT
Medicament for preventive and/or therapeutic treatment of lower urinary tract symptom
InactiveUS20080207768A1Good prevention effectGood treatment effectBiocidePeptide/protein ingredientsBenzoic acidDisease
A medicament for preventive and / or therapeutic treatment of a lower urinary tract symptom caused by a lower urinary tract disorder, which comprises as an active ingredient a retinoid such as, for example, 4-[(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)carbamoyl]benzoic acid
Owner:KEMPHYS
Methods of Treating Interstitial Cystitis
InactiveUS20110287018A1Antibody ingredientsImmunoglobulinsBladder Pain SyndromeLower urinary tract symptoms
The present invention relates to the use of IL-12 and / or IL-23 inhibitors for the treatment of a pain and / or a lower urinary tract symptom(s) (LUTS) associated with interstitial cystitis and / or painful bladder syndrome and / or bladder pain syndrome.
Owner:BOSCH PHILIP
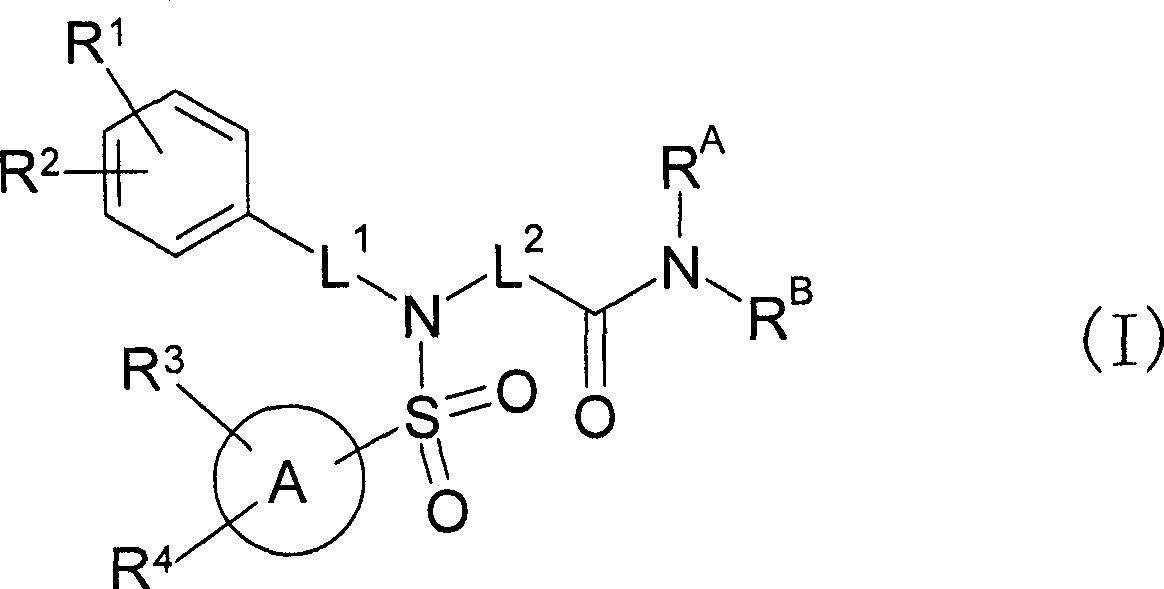
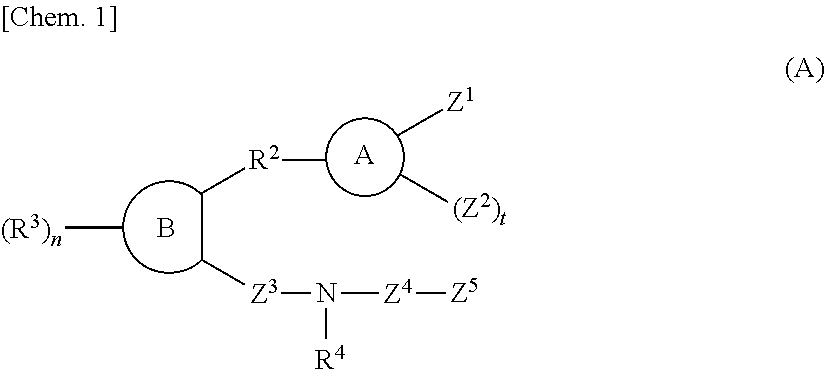
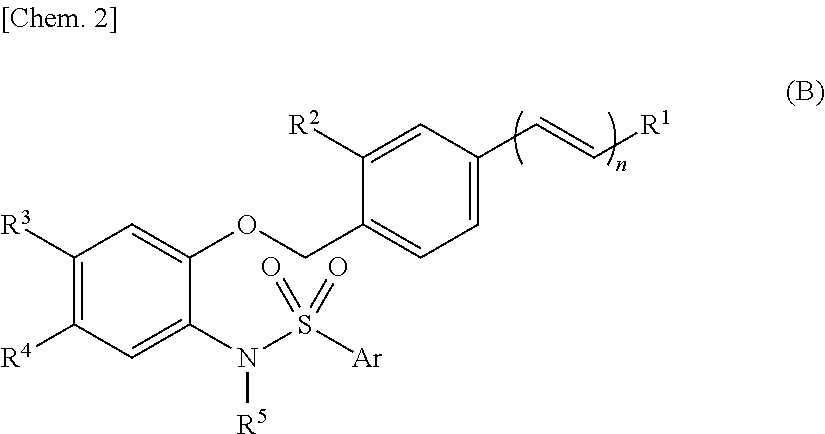
Sulfonamide compound or salt thereof
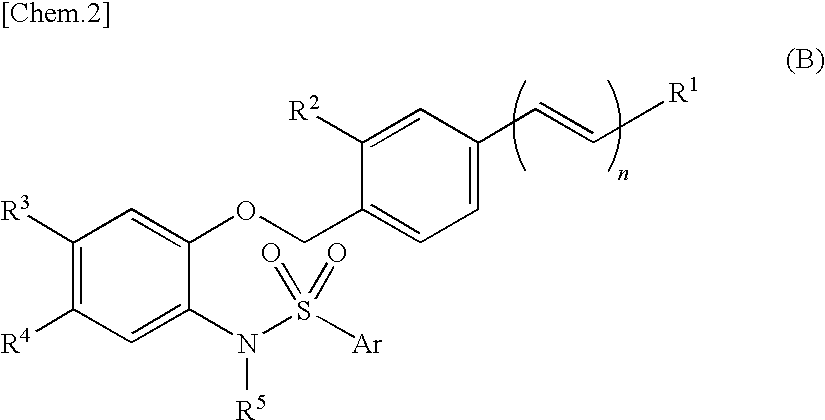
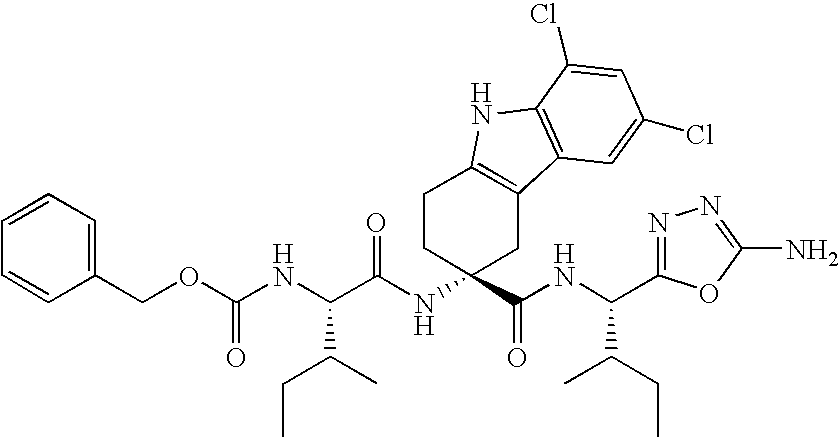
Disclosed is a sulfonamide compound having a chemical structure characterized in that the compound has an amide structure wherein a carbon atom in the amide binds to a nitrogen atom in a sulfonamide through a lower alkylene, or a salt of the compound. The compound or the salt has a potent antagonistic activity against an EP1 receptor and is therefore useful as a therapeutic agent for a disease associated with an EP1 receptor, particularly lower urinary tract symptom.
Owner:ASTELLAS PHARMA INC
Sulfonamide compound or salt thereof
InactiveUS20090312328A1Potent EP receptor antagonistic activityBiocideNervous disorderDiseaseReceptor
[Object] A compound that can be used as an agent for treating a disease associated with an EP1 receptor, in particular, a lower urinary tract symptom.[Means for Solution] It was confirmed that a sulfonamide compound having an amide structure and characterized by a chemical structure in which a carbon atom in the amide bonds to the N atom in sulfonamide through lower alkylene, or a salt thereof, has a potent EP1 receptor antagonistic activity, accomplishing the present invention.Since the sulfonamide compound of the present invention or a pharmaceutically acceptable salt thereof has a potent EP1 receptor antagonistic activity, it is useful as an agent for treating a disease associated with an EP1 receptor, in particular, a lower urinary tract symptom.
Owner:ASTELLAS PHARMA INC
Treatment of benign prostatic hypertrophy and lower urinary tract symptoms
InactiveUS20070093493A1Reduce frequencyReduce severityBiocideUrinary disorderPhosphodiesteraseEccentric hypertrophy
A method of treating symptoms of benign prostatic hypertrophy and a method of treating lower urinary tract symptoms, are disclosed. The method includes administering to a mammal about 1 to about 20 milligrams of an agent that inhibits cyclic guanosine 3,5-monophosphate specific phosphodiesterase type 5.
Owner:LILLY ICOS LLC
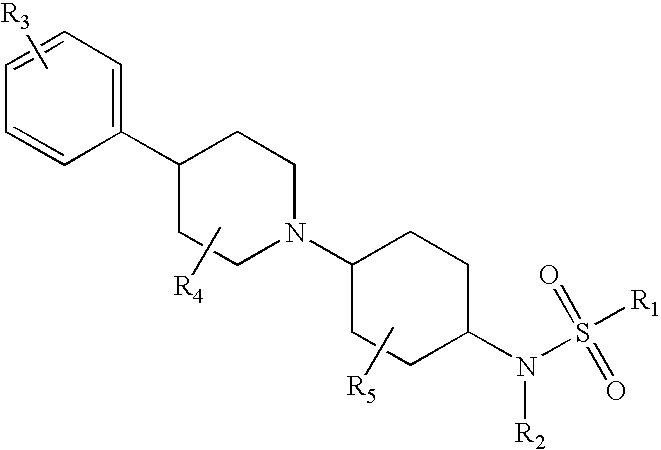
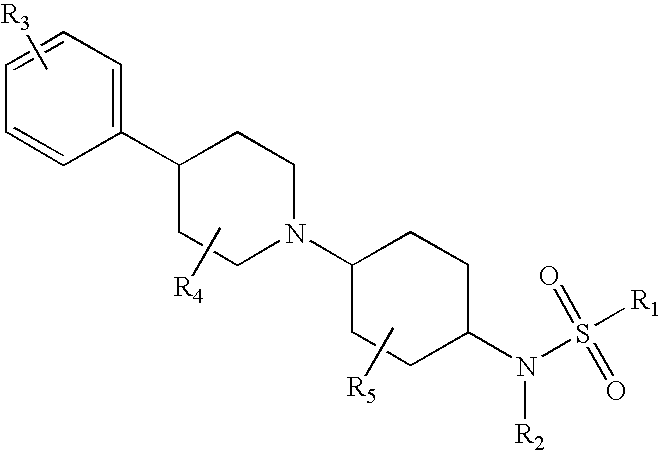
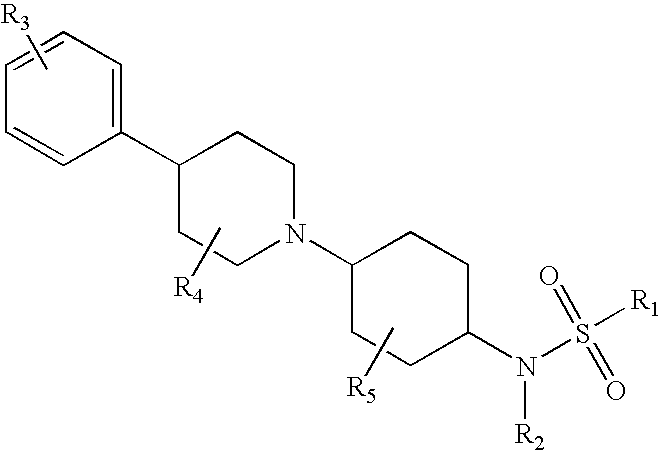
Piperidinyl substituted cyclohexane-1,4-diamines
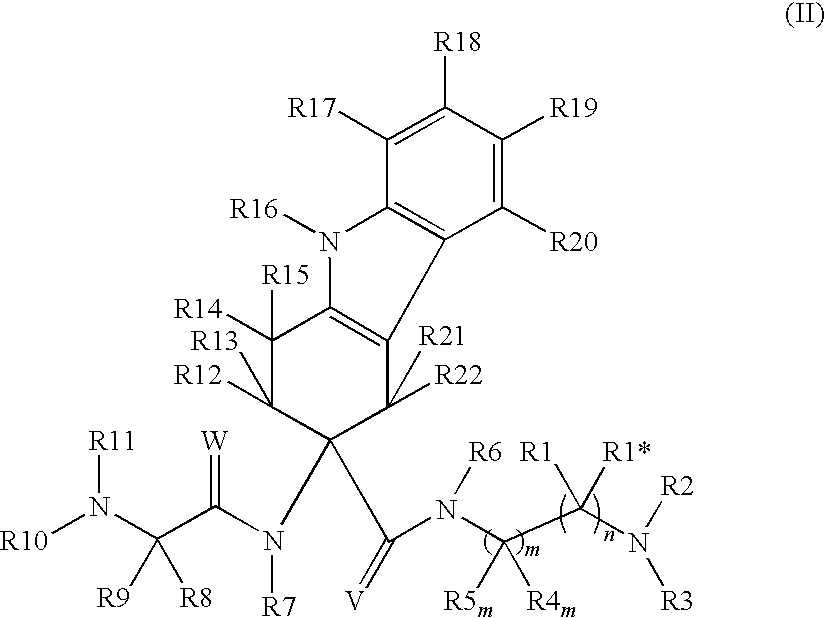
InactiveUS20060217419A1Inhibition of contractionBiocideOrganic chemistryEccentric hypertrophyAdrenergic receptor sites
The present invention relates to piperidine substituted cyclohexane-1,4-diamine compounds of Formula (I) and pharmaceutically acceptable forms thereof, as α1a / α1d adrenoreceptor modulators for the treatment of benign prostatic hypertrophy and lower urinary tract symptoms. The present invention also relates to pharmaceutical compositions comprising said new compounds, new processes to prepare these new compounds and new uses as a medicine as well as method of treatments.
Owner:JANSSEN PHARMA NV
Phosphodiesterase-5 inhibitor
The invention belongs to the technical field of medicines and specifically relates to a phosphodiesterase-5 inhibitor as shown in a general formula (I) and a pharmaceutically acceptable salt or a stereoisomer thereof, wherein R1, R2, R3, R4, R5, L and W are as defined in the description. The invention further relates to preparation methods of compounds, pharmaceutical compositions containing the compounds, and applications of the compounds and the pharmaceutical compositions in preparation of medicaments for treating and / or preventing diseases related to sexual dysfunction and lower urinary tract symptoms.
Owner:XUANZHU BIOPHARMACEUTICAL CO LTD
Lhrh antagonists for the treatment of lower urinary tract symptoms
InactiveUS20090075937A1Low urinary tract symptomNegative hormone withdrawal symptoms are minimized and/or preventedBiocideOrganic chemistryMammalLhrh antagonist
The present invention provides at least one LHRH antagonist for the treatment or prophylaxis of at least one lower urinary tract symptom in mammals which is to be administered in an intermediate dose, which does not cause chemical (hormonal) castration.
Owner:AETERNA ZENTARIS GMBH
Method of treatment for bladder dysfunction
InactiveUS20100104631A1Improve urinary tract symptomDecreasing bladder irritationPeptide/protein ingredientsUrinary disorderLower riskLiposome
Liposomes are used for intravesical drug delivery, especially delivery of botulinum toxin (BoNT) to help improve lower urinary tract symptoms by decreasing bladder irritation and frequency. The system uses a lower and safer dose of BoNT with lower risk of urinary retention. A simple instillation of liposome-BoNT as a liquid formulation into the bladder, in a typical volume of 30-60 ml, will achieve efficacy similar to that currently achieved with cystscopic needle injection of BoNT. The dose may be lower than that done by injection, thereby causing significantly less risk of urinary retention. Liposome-BoNT can protect the BoNT from bladder and urine breakdown. Liposome-BoNT instillation is more comfortable for the patients and allows many more doctors' offices to offer this form of treatment that would otherwise be restricted to doctors skilled and certified in cystoscopic BoNT injection.
Owner:LIPELLA PHARMA
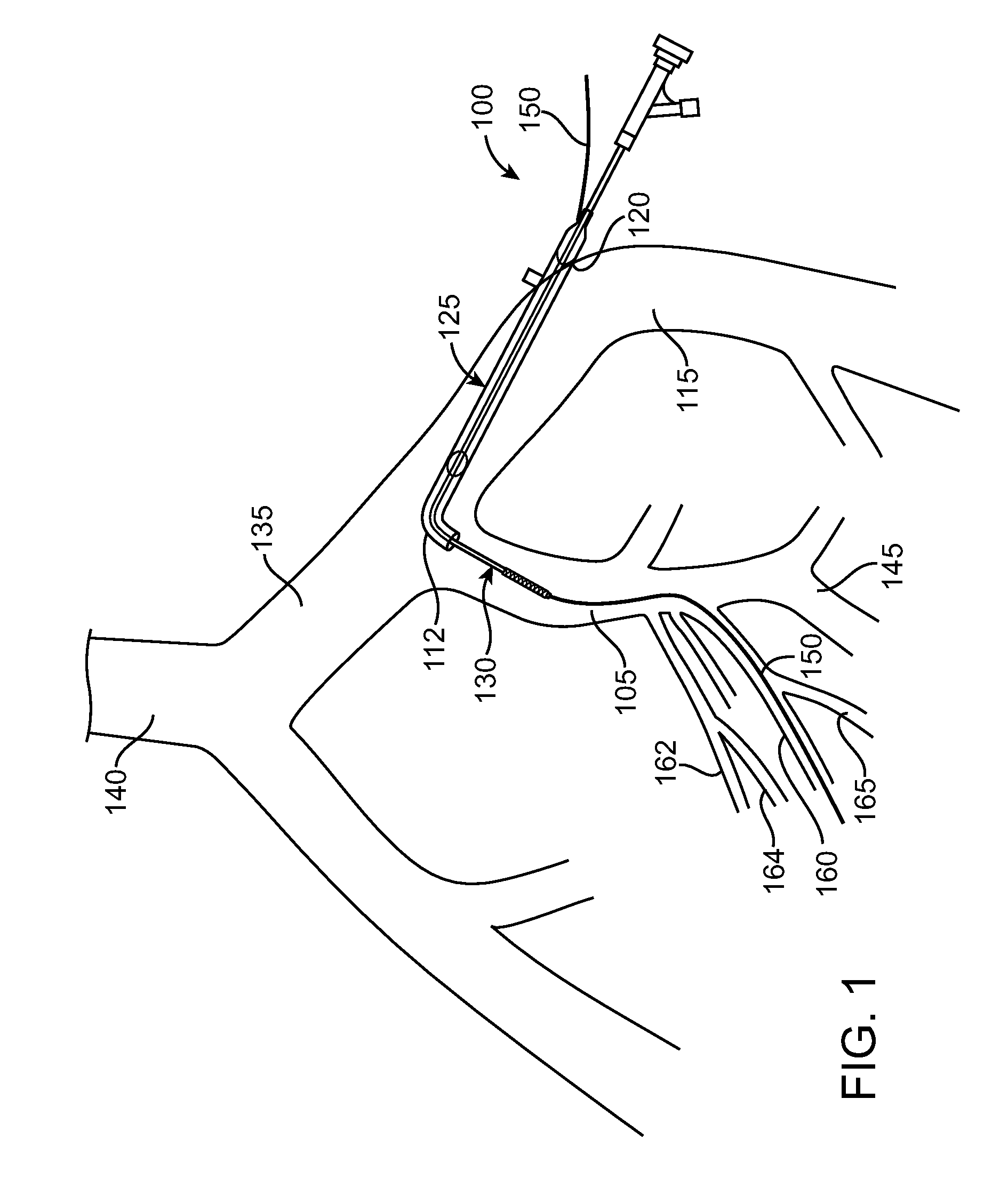
Method of Diagnosing and Treating Lower Urinary Tract Symptoms
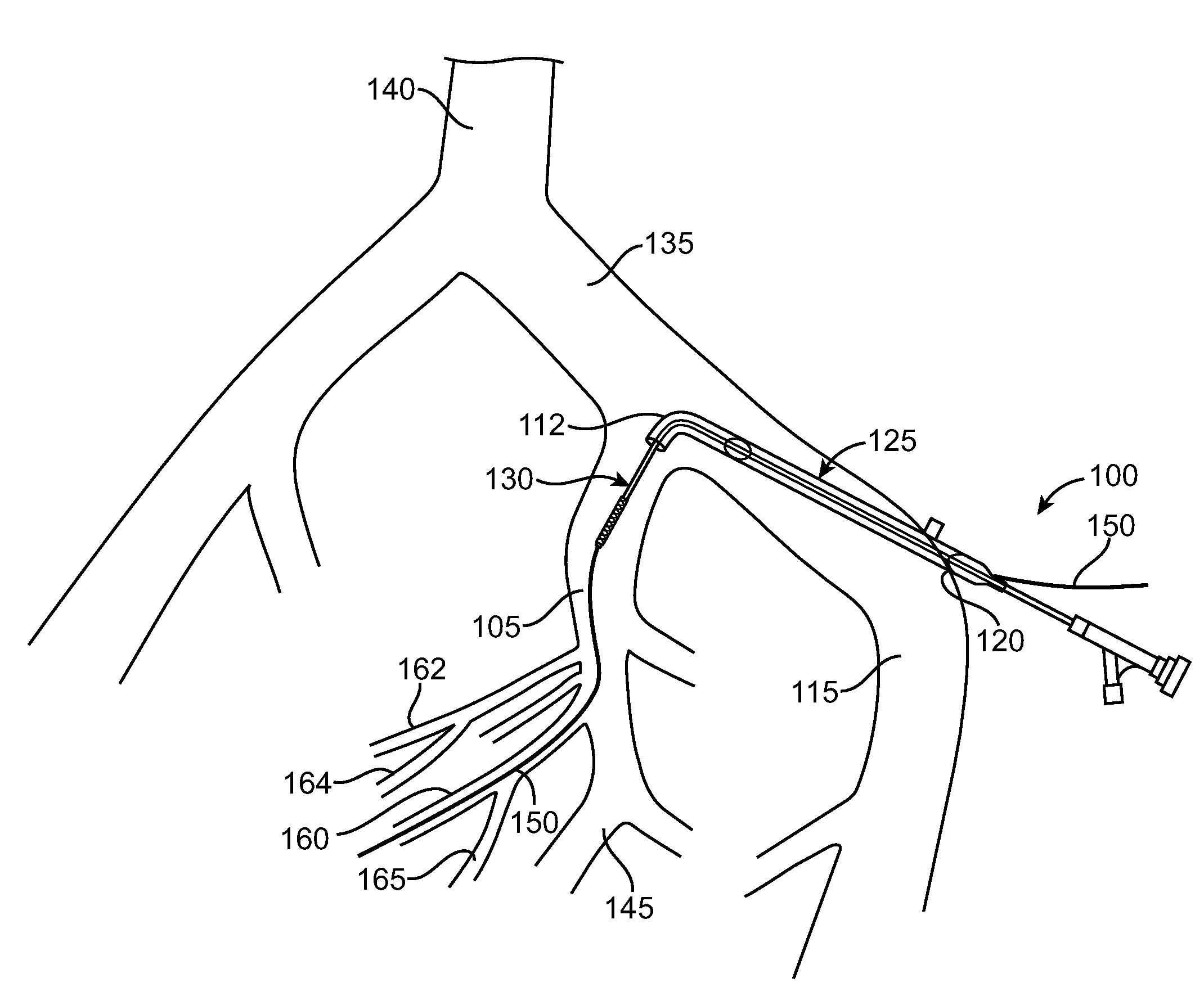
A method of diagnosing and treating a patient having lower urinary tract symptoms caused by insufficient blood flow to the urinary bladder, the urethra, a nerve innervating the urinary bladder, or a nerve innervating the urethra due to atherosclerosis of a pelvic artery is disclosed. A method of diagnosing the patient's condition includes determining if a stenosis exists within a pelvic vessel. A method of treating the patient's condition may include placing a stent within the stenosed pelvic artery.
Owner:MEDTRONIC VASCULAR INC
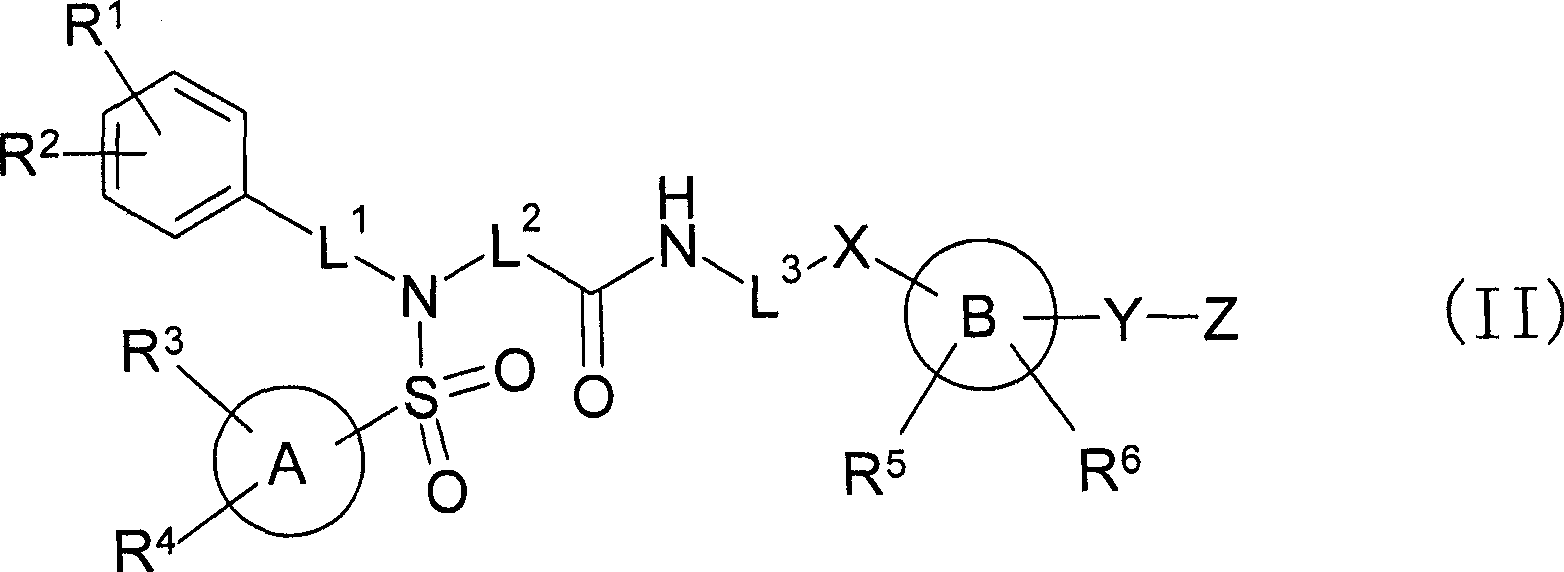
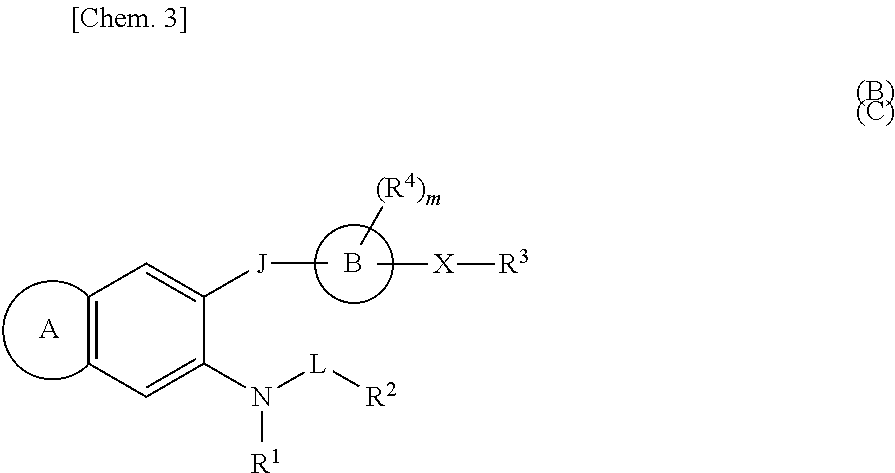
Sulfonamide compounds or salts thereof
ActiveUS20110201616A1Potent EP receptor antagonistic actionLow urinary tract symptomBiocideOrganic chemistryMedicinal chemistryLower urinary tract symptoms
[Object] A compound which is useful as an EP1 receptor antagonist is provided.[Means for Solution] The present inventors investigated EP1 receptor antagonists, and confirmed that a compound having a sulfonamide structure, in which the nitrogen atom of the sulfonamide structure is substituted with 2-fluoropropyl group, 3-fluoro-2-methylpropyl group or the like, has a potent EP1 receptor antagonistic action, thereby completing the present invention. The sulfonamide compound of the present invention has a potent EP1 receptor antagonistic action and can be used as an agent for preventing and / or treating a lower urinary tract symptom or the like.
Owner:ASTELLAS PHARMA INC
Sulfonamide compounds or salts thereof
ActiveUS8314240B2Potent EP receptor antagonistic actionLow urinary tract symptomBiocideOrganic chemistryMedicinal chemistryReceptor antagonist
[Object] A compound which is useful as an EP1 receptor antagonist is provided.[Means for Solution] The present inventors investigated EP1 receptor antagonists, and confirmed that a compound having a sulfonamide structure, in which the nitrogen atom of the sulfonamide structure is substituted with 2-fluoropropyl group, 3-fluoro-2-methylpropyl group or the like, has a potent EP1 receptor antagonistic action, thereby completing the present invention. The sulfonamide compound of the present invention has a potent EP1 receptor antagonistic action and can be used as an agent for preventing and / or treating a lower urinary tract symptom or the like.
Owner:ASTELLAS PHARMA INC
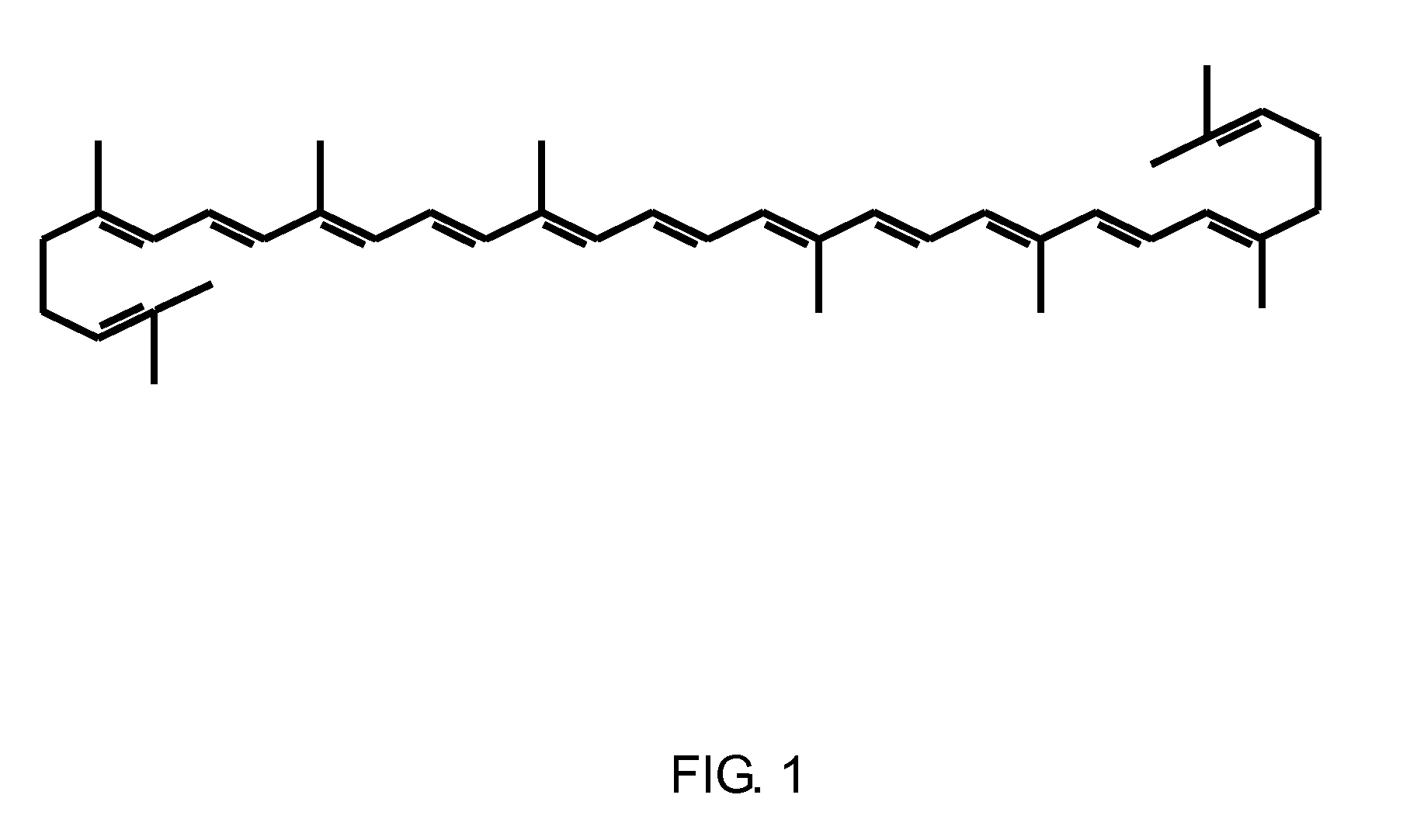
Multi-carotenoids compositions and uses therefor
Methods for ameliorating the effects of benign prostate hyperplasia (BPH)-related lower urinary tract symptoms (LUTS) in men, comprising orally administering an effective amount of multi-carotenoids compositions. Multi-carotenoids composition for oral administration comprising about 71% by weight, of a tomato extract containing therein about 2% to 10% by weight of lycopene, about 0.25% to 2% by weight of phytoene, and about 0.2% to 2% by weight of phytofluene, and about 29% by weight, of a suitable encapsulating matrix. A suitable encapsulating matrix is an edible oil exemplified by soya oil, pumpkin seed oil, grape-seed oil and the like. The tomato extract may additionally comprise one or more of at least one carotene selected from the group comprising β-carotene, γ-carotene, and δ-carotene, a phytosterol, a tocopheral and a phospholipid. Use of multi-carotenoids compositions for the treatment of urinary tract malfunctions including benign prostate hyperplasia and lower urinary tract symptoms.
Owner:HEALTH EVER BIOTECH CO LTD
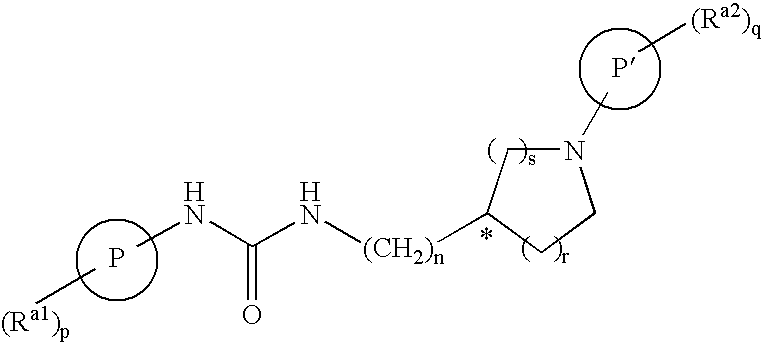
Bicyclic Amide, Carbamate or Urea Derivatives as Vanilloid Receptor Modulators
This invention relates to bicyclic amide, carbamate or urea derivatives and salts thereof which are useful as active ingredients of pharmaceutical preparations. The bicyclic amide, carbamate or urea derivative of the present invention has vanilloid receptor (VR1) antagonistic activity, and can be used for the prophylaxis and treatment of diseases associated with VR1 activity, in particular for the treatment of urological diseases or disorders, such as detrusor overactivity (overactive bladder), urinary incontinence, neurogenic detrusor oeractivity (detrusor hyperflexia), idiopathic detrusor overactivity (detrusor instability), benign prostatic hyperplasia, and lower urinary tract symptoms; chronic pain, neuropathic pain, postoperative pain, rheumatoid arthritic pain, neuralgia, neuropathies, algesia, nerve injury, ischaemia, neurodegeneration, stroke, and inflammatory disorders such as asthma and chronic obstructive pulmonary (or airways) disease (COPD).
Owner:BAYER SCHERING PHARMA AG
PDE Inhibitors and Combinations Thereof for the Treatment of Urological Disorders
The invention provides pharmacological compositions comprising PDE-5 and PDE-4 inhibitors, alone or in combination, for the treatment of urological disorders comprising Benign Prostate Hyperplasia (BPH), Lower Urinary Tract Symptoms (LUTS) and in particular irritative symptoms caused by BPH-induced bladder outlet obstruction (BOO). The invention also provides methods of screening for such PDE-5 and PDE-4 inhibitors for use, alone and in combination, in the preparation of medicaments for the treatment of said urological disorders.
Owner:BAYER SCHERING PHARMA AG
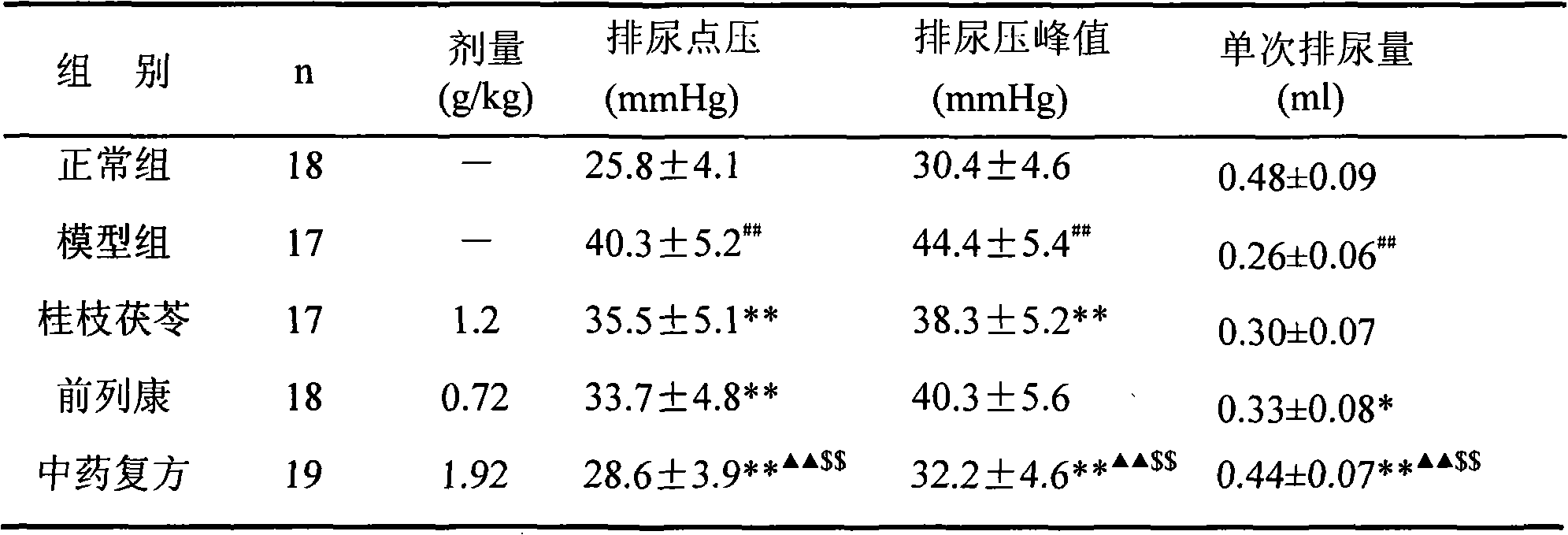
TCM composite for treating benign prostatic hyperplasia
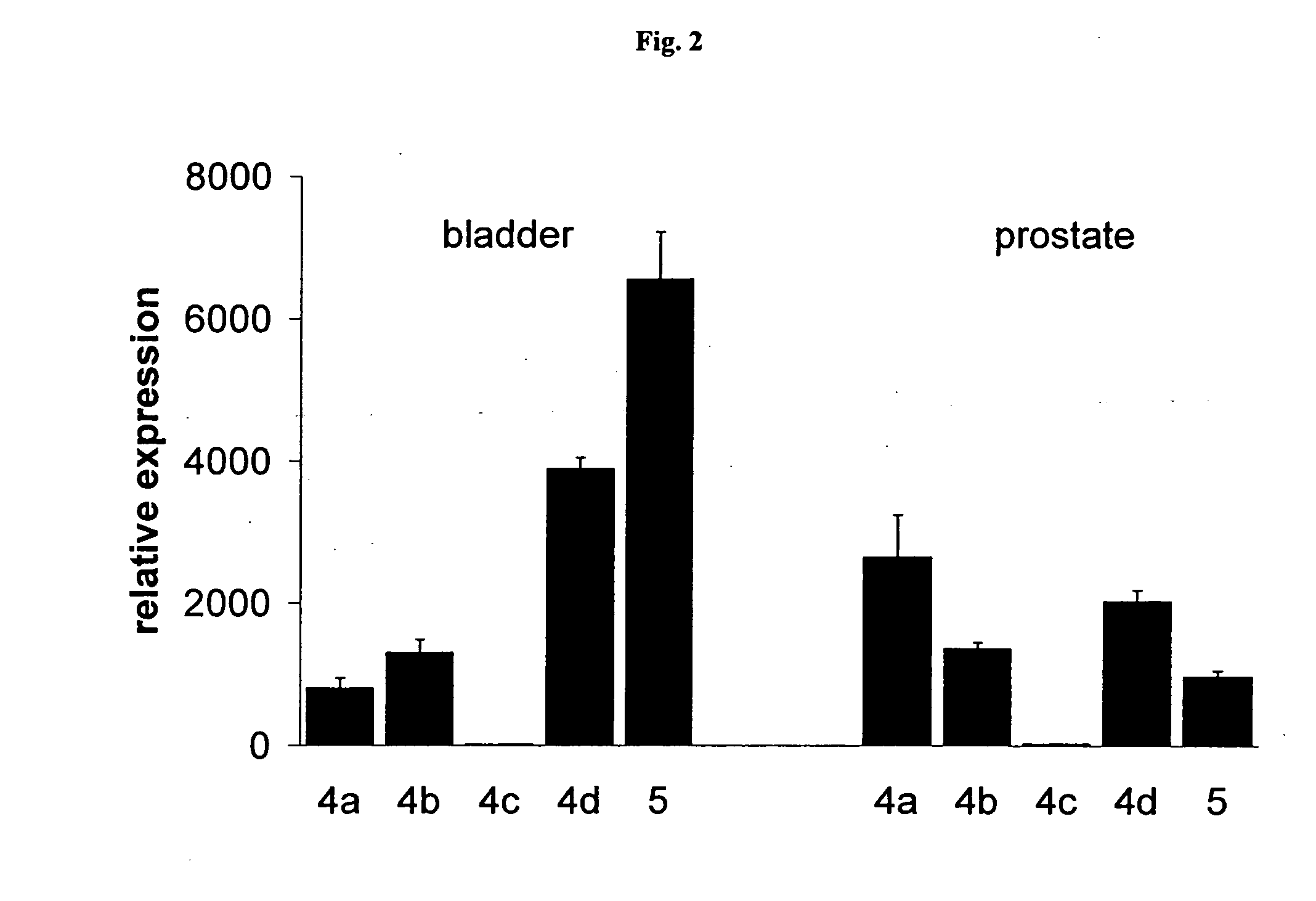
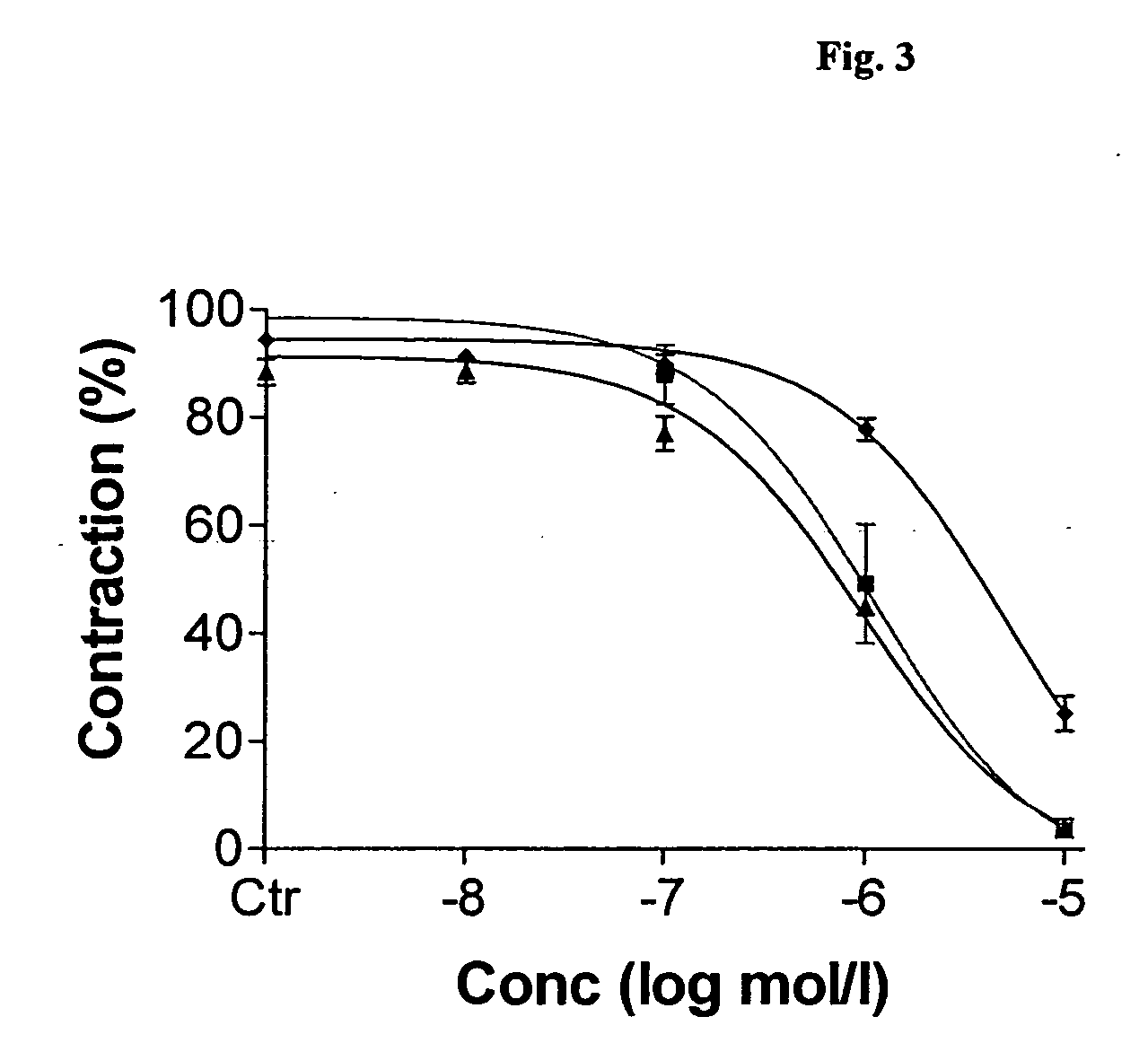
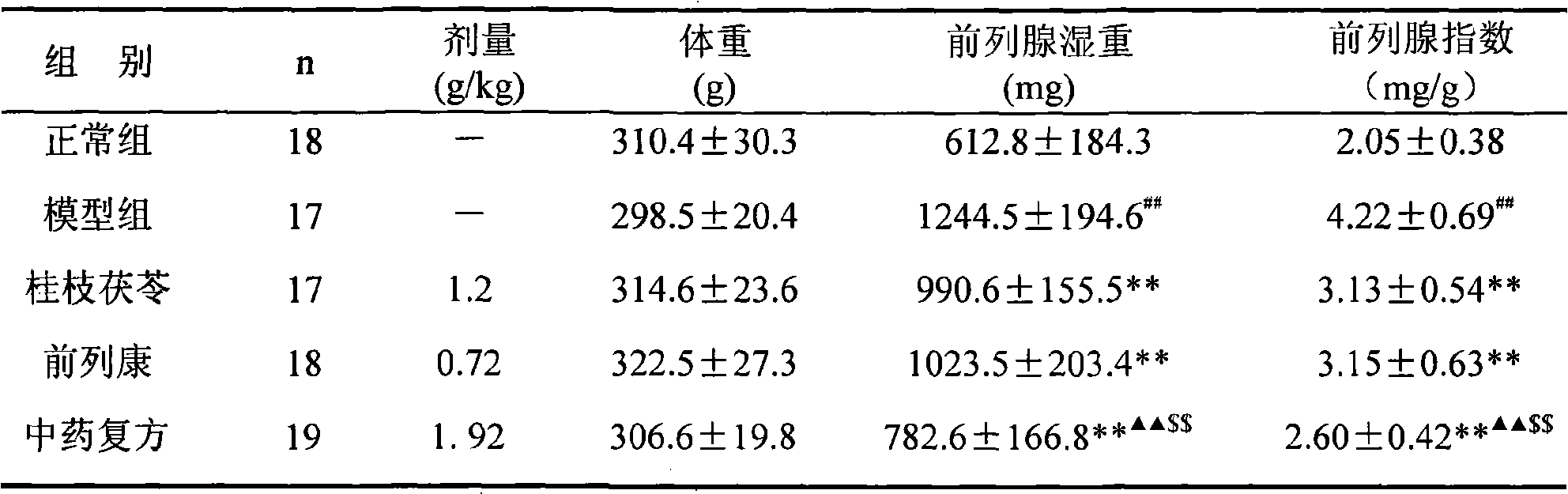
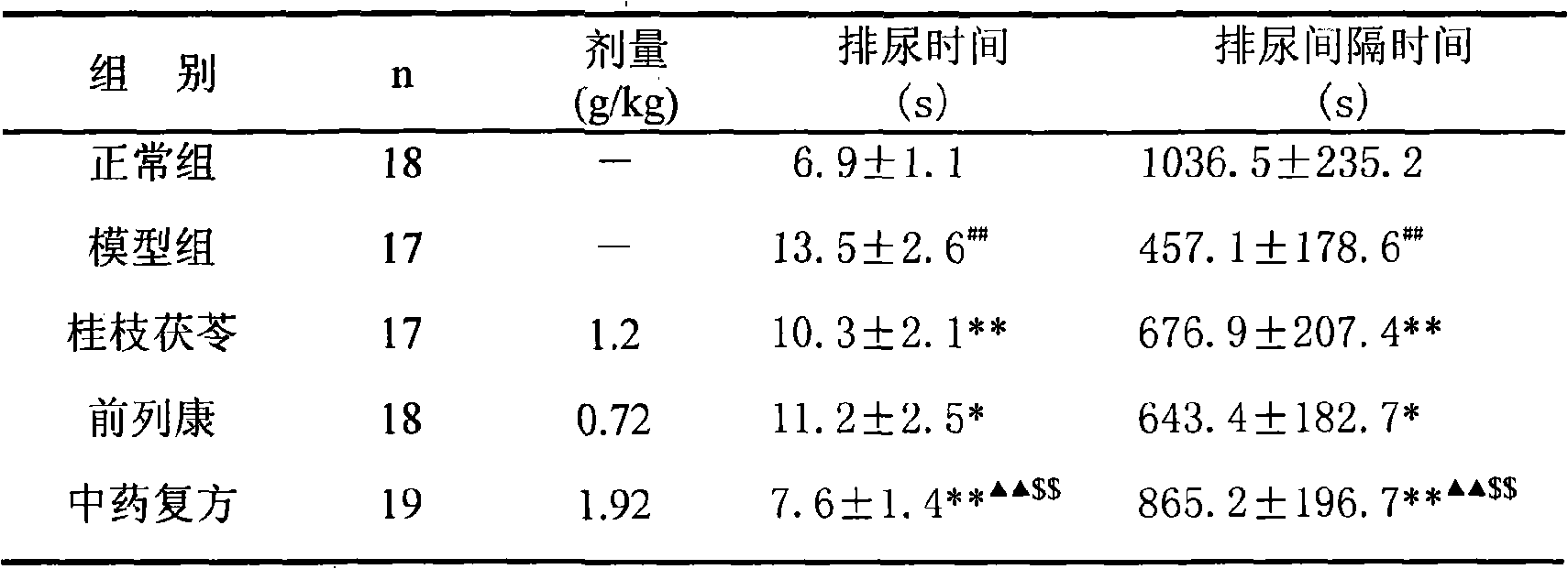
ActiveCN101590126AImprove complianceEasy to take medicineAnthropod material medical ingredientsPharmaceutical delivery mechanismDiseaseAdemetionine
The invention discloses a new TCM composite composed of six medicinal materials: cassia twig, Tuckahoe, peach seed, salivia chinensis, radix paeoniae alba, rape bee pollen and provides the application of the composite in preparing medicine for treating benign prostatic hyperplasia and medicine for treating related diseases.The invention can effectively reduce the volume of prostate and improve lower urinary tract symptoms.In addition, the invention provides an effective preparation method for preparing the TCM composite.
Owner:深圳泰乐德医学检验实验室
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com