Multi-component crystalline particles for inhalation therapy
A multi-component crystallization and particle technology, applied in inhalers, drug combinations, drug devices, etc., to reduce the impact of the system, eliminate deposition and accumulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1: Glycopyrronium bromide (GB) and formoterol fumarate (FF)
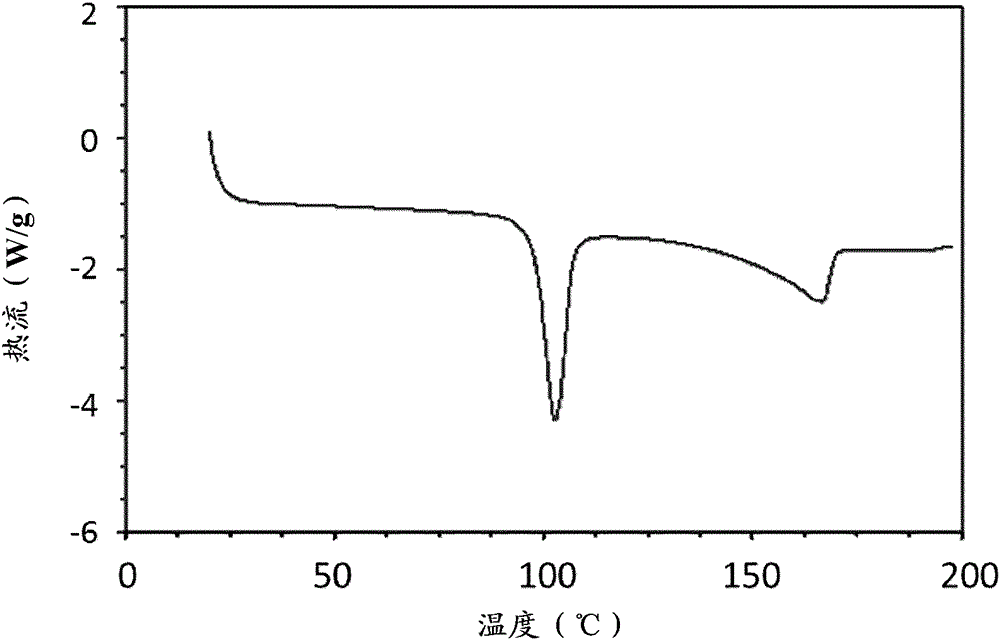
[0064] A methanolic solution of GB / FF was prepared and added to recirculating TBME at room temperature using a crude probe based system at 0.5ml / min addition rate, solution / non-solvent 1 / 20, using 40w ultrasonic power. Immediate recrystallization and formation of a homogeneous slurry was observed in all cases. The material isolated by filtration was crystalline as shown by differential scanning calorimetry (DSC).
[0065] For GB:FF (7.5:1) in MeOH / TBME, the experimental parameters are as follows.
[0066] Solution concentration: 25% (6.8g, in 27ml methanol)
[0067] Volume TBME : 648ml
[0068] Solution-non-solvent ratio: 1 / 24 V / V
[0069] Reaction vessel temperature : 7.4 + / - 0.2 ℃
[0070] Solution adding rate: 0.5ml / min
[0071] Solution adding speed: 0.042m / s
[0072] Solution feeding tube diameter: 0.5mm
[0073] Adding time: 60min
[0074] Recirculation rate: 0.9L / min
[0075] Rec...
Embodiment 2
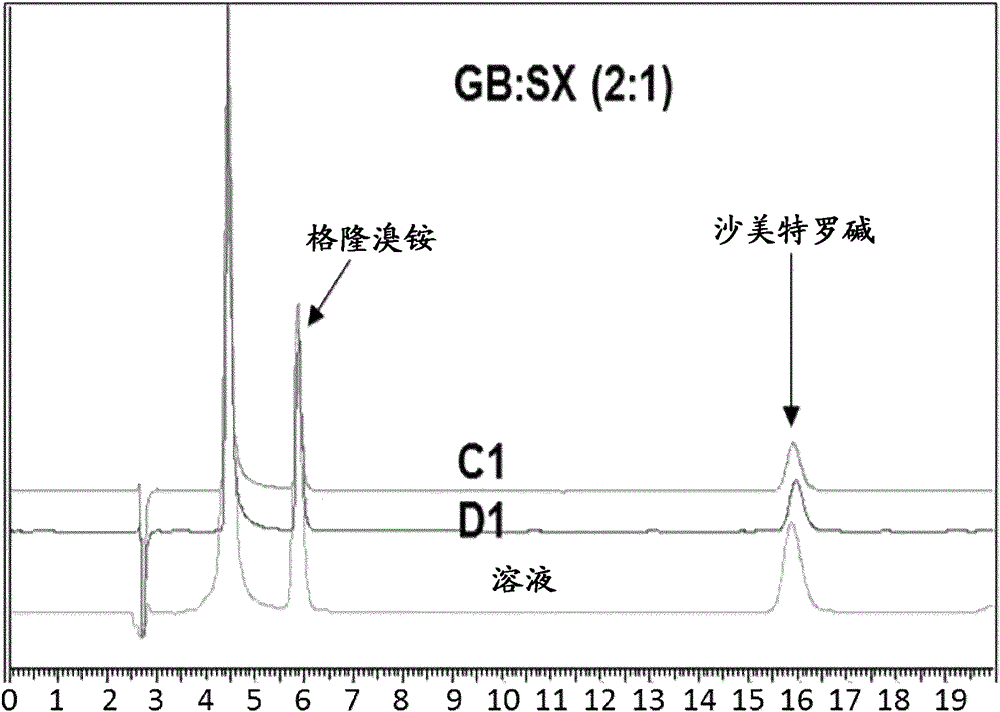
[0093] Embodiment 2: Glycopyrronium bromide (GB) and salmeterol xinafoate (SX)
[0094] Methanol solutions of GB / SX were prepared at different ratios (4:1, 2:1 and 1:1) and using a crude probe based system using 40W US power at 0.5ml / min addition rate, solution / non-solvent 1 / 20, added to recycle DIPE at room temperature. Immediate recrystallization and formation of a homogeneous slurry was observed in all cases. Material isolated by filtration was crystalline as shown by DSC.
[0095] For GB:SX (2:1) in MeOH / DIPE, the experimental parameters are as follows.
[0096] Solution concentration: 25% (6.8g, in 27ml methanol)
[0097] Volume DIPE : 648 ml
[0098] Solution-non-solvent ratio: 1 / 24 V / V
[0099] Reaction vessel temperature : 7.4 + / - 0.2 ℃
[0100] Solution adding rate: 0.5ml / min
[0101] Solution adding speed: 0.042 m / s
[0102] Solution feeding tube diameter: 0.5mm
[0103] Adding time: 60mins
[0104] Recirculation rate: 2.63L / min
[0105] Recirculatio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com