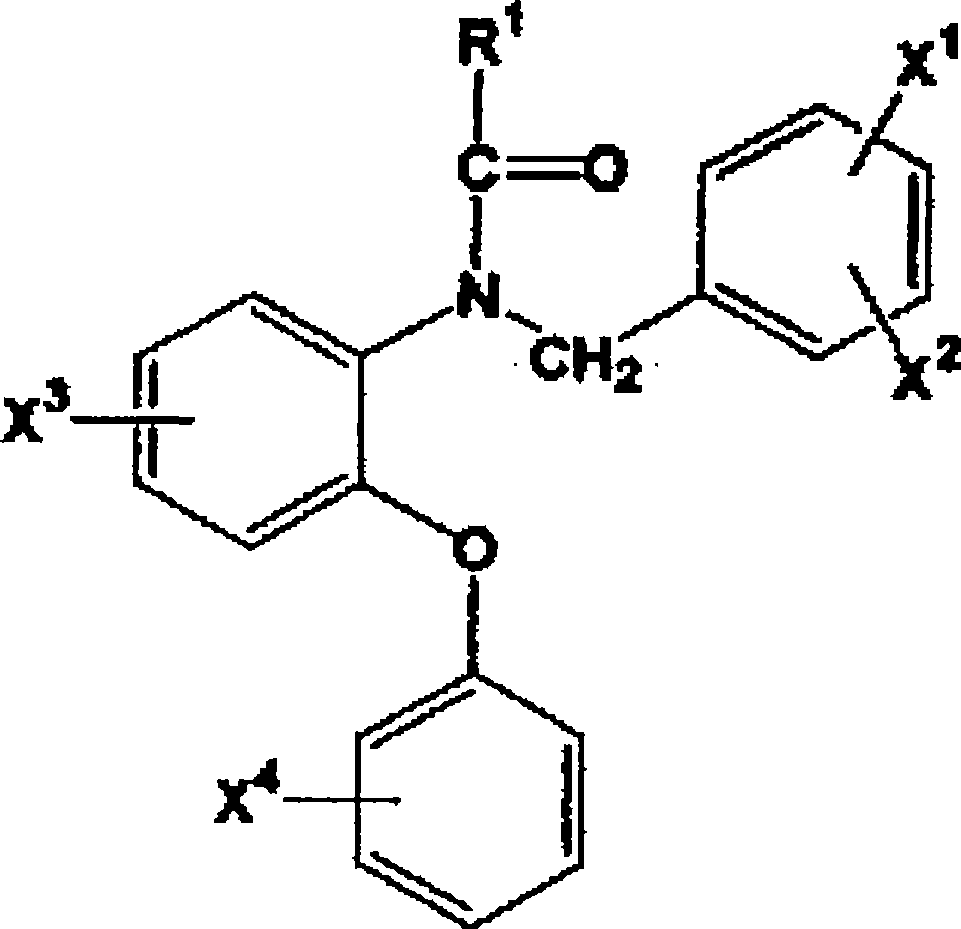
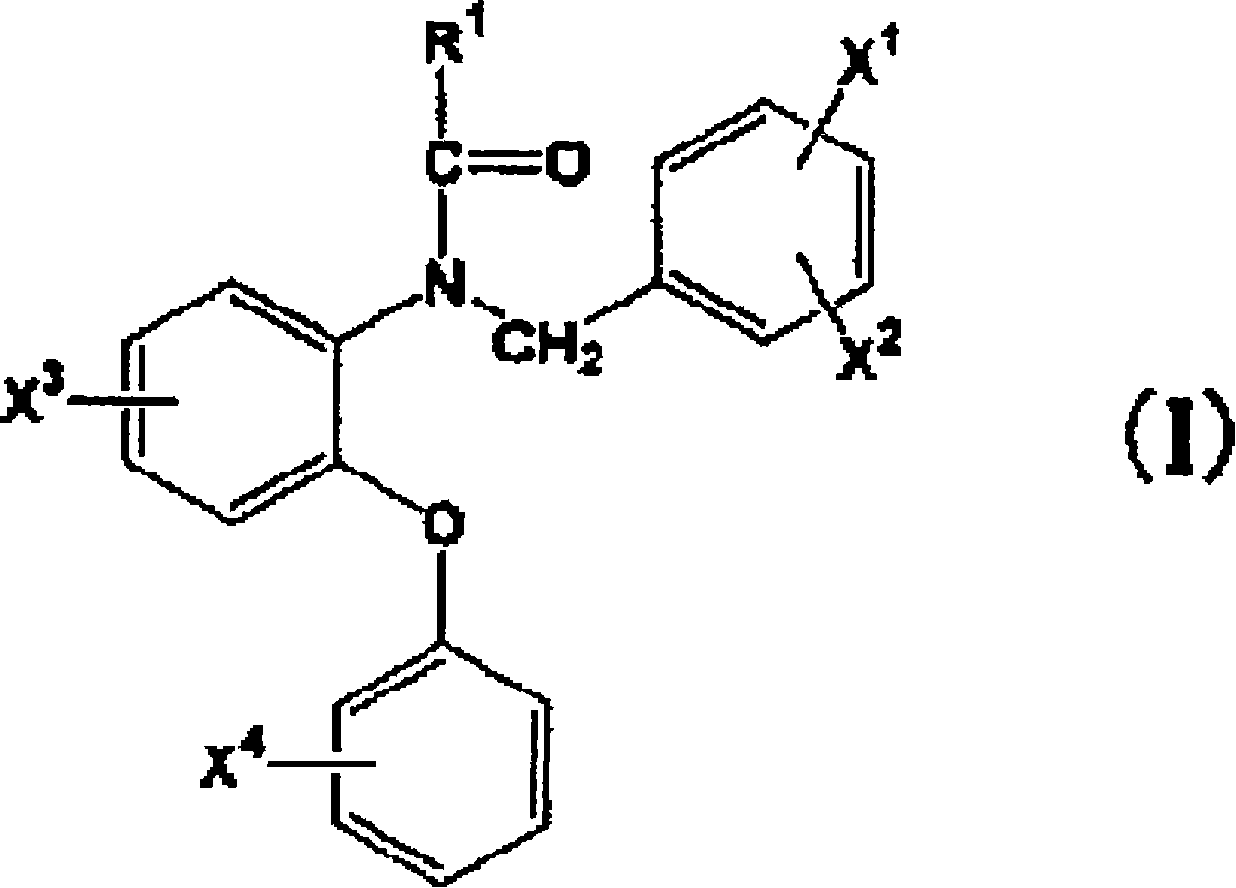
Radioactive halogen-labeled phenyloxyaniline derivatives
A phenoxyaniline and radioactive technology, applied in the field of phenoxyaniline derivatives, can solve problems such as quantitative analysis problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] preparation[ 131 1]-N-(2,5-dimethoxybenzyl)-N-(5-iodo-2-phenoxyphenyl)acetamide (hereinafter referred to as [ 131 I]-1IDAA1106)
[0034] 1-1) Dissolve N-(2,5-dimethoxybenzyl)-N-(5-bromo-2-phenoxyphenyl)acetamide (510 mg, 1.12 mmol) in toluene, and wash with hexa Butylditin(IV) and dichlorobis(triphenylphosphine)palladium(0) were refluxed for 4 days. After removing toluene, the reaction product was purified by silica gel column chromatography (eluted by hexane:ethyl acetate=1:4) to obtain 320 mg (43%) of N-(2,5-dimethoxybenzyl)-N -(5-tributylstannyl-2-phenoxyphenyl)acetamide.
[0035] FABMS C 36 h 48 FNO 3 Sn(m / z)680.5(m + +1)
[0036] To a solution of N-(2,5-dimethoxybenzyl)-N-(5-tributylstannyl-2-phenoxyphenyl)acetamide (55 mg, 0.083 mmol) in chloroform was added 100 mg of solid iodine, the resulting reaction solution was stirred at room temperature for 1 hour, and then a saturated aqueous solution of sodium thiosulfate was added to the reaction solution until...
Embodiment 2
[0039] Preparation of N-(2-[ 131 I] iodo-methoxybenzyl)-N-(5-fluoro-2-phenoxyphenyl)acetamide (hereinafter referred to as [ 131 I]-2IDAA1106)
[0040] Add 100 μL of acetic acid / 30% hydrogen peroxide (3 / 1) to N-(2-tributylstannyl-5-methoxybenzyl)-N-(5-fluoro-2-phenoxyphenyl ) acetamide (0.1 mg) in ethyl acetate (100 μL) and mix. Add to it [ 131 I] NaI (0.1 mCi), let stand for 1 minute. After the reaction was completed, the reaction mixture was injected into a reverse-phase semi-preparative HPLC (YMC J'sphere ODS-H80 column, 10mmID x 250mm). Using CH at a flow rate of 4 mL / min 3 CN / H 2 O(9 / 1) was used as the mobile phase to obtain [ 131 I] Fraction of 1IDAA1106. The solvent was removed from this fraction by distillation under reduced pressure, and the residue was dissolved in physiological saline (1 mL), which was then passed through a 0.22 μm Millipore filter to successfully obtain [ 131 I] 2IDAA1106 (0.095 mCi).
[0041] (experimental example)
[0042] ex vivo autor...
Embodiment 3
[0060] N-(4-Chloro-2-phenoxyphenyl)-N-(2-[2- 11 C] isopropoxybenzyl) acetamide ([ 11 C]3)
[0061] Ultra-high-purity nitrogen (1.5 MPa) containing 0.01% oxygen is irradiated with 18.5 MeV protons from a cyclotron by 14 N(p,α) 11 The C nuclear reaction produces carrier-free [ 11 C]CO 2 .
[0062] After radiation, through N 2 (50mL / min) [ 11 C]CO 2 , and concentrated until liquid N 2 The radioactivity throughout the coil reaches a steady state in a stainless steel coil cooled to -150°C. The concentrated [ 11 C]CO 2 , so that dry N 2 (2mL / min) inflow containing CH at -5°C 3 In the loop of MgBr. When the transfer of radioactivity is complete, stop the N 2 flow, and then hold the loop at 25°C for 5 minutes to perform the Grignard reaction. Subsequently, LiAlH 4 A solution of THF (0.2M, 500 μL) was passed through the loop and the reaction mixture was moved to a heated reactor at 180° C. for 1 min. After the reactor was cooled at 50-60°C, an aqueous solution of HI (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com