Preparation method of melbinum osmotic pump controlled release tablets
An osmotic pump controlled-release, metformin technology, applied in the directions of non-active ingredients medical preparations, active ingredients-containing medical preparations, pharmaceutical formulas, etc., can solve the problems of large intestinal irritation, inconvenience in taking, poor compliance, etc. Achieve the effect of less administration times, stable drug release, and improved compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
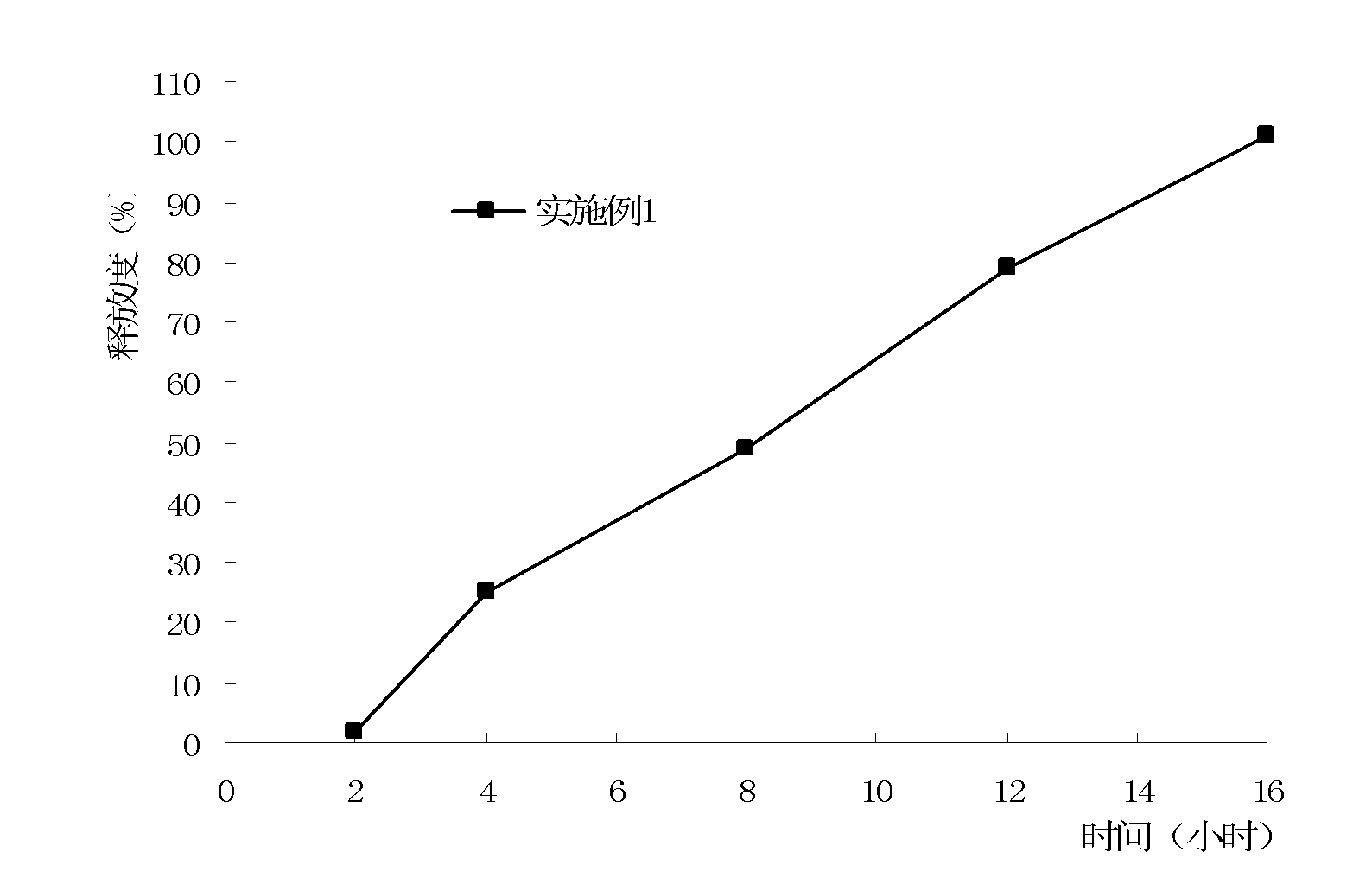
Embodiment 1
[0052] A method for preparing metformin osmotic pump controlled-release tablets, wherein steps (1) to (5) are the preparation process of the tablet core:
[0053] Tablet prescription:
[0054]
[0055] (1) Granulation: mix Poloxamer 188 (German BASF Company, the same below), hypromellose K4M (U.S. Dow Company) and metformin hydrochloride (Shandong Keyuan Pharmaceutical Co., Ltd., the same below), and place In the wet granulator, add 40% ethanol (V / V) as a wetting solution, granulate, and pass the wet granules through a 16-mesh sieve;
[0056] (2) Drying of wet granules: place the prepared granules in a fluidized bed, set the inlet air temperature to 65°C for drying, until the moisture content is within 5%;
[0057] (3) whole grain: whole grain, through 16 mesh sieve;
[0058] (4) Total mixing: add magnesium stearate (Liaocheng Ahua Pharmaceutical Co., Ltd., the same below), and mix well;
[0059] (5) Tablet compression: Take the above-mentioned granules and place them in...
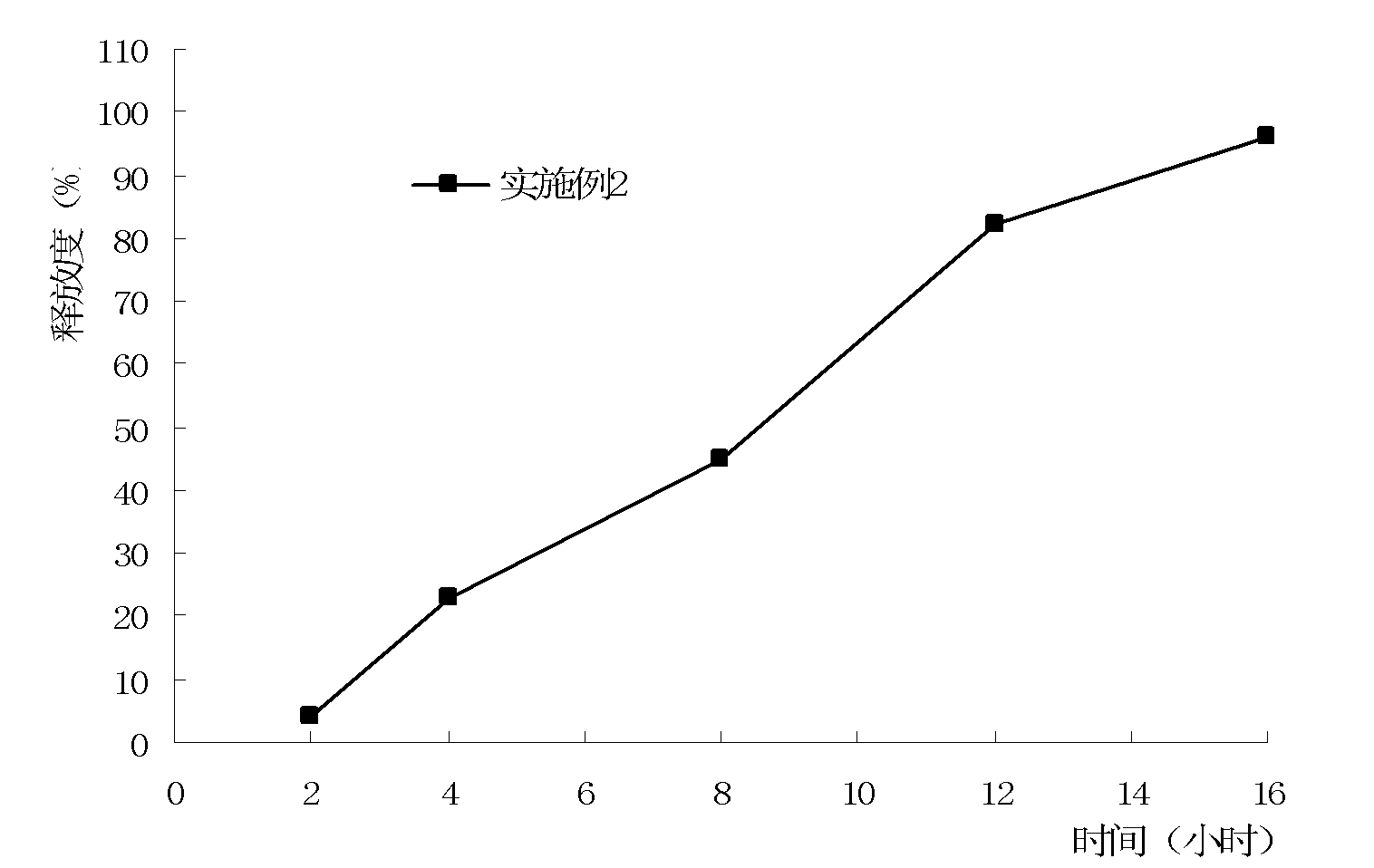
Embodiment 2
[0071] A method for preparing metformin osmotic pump controlled-release tablets, wherein steps (1) to (5) are the preparation process of the tablet core:
[0072] Tablet prescription:
[0073] Metformin Hydrochloride 1000g
[0074] Poloxamer 407 52g
[0075] water 400g
[0077] (1) Granulation: poloxamer 407 (Germany BASF company, the same below) and metformin hydrochloride were placed in a wet granulator, mixed, added water, granulated, and the wet granules were passed through a 16-mesh sieve;
[0078] (2) Drying of wet granules: place the prepared granules in a fluidized bed, set the inlet air temperature to 70°C for drying, until the moisture content is within 5%;
[0079] (3) whole grain: whole grain, through 16 mesh sieve;
[0080] (4) Total blending: add magnesium stearate and mix well;
[0081] (5) Tablet compression: Take the above granules, place them in a rotary tablet press, use oval punched tablets with a diameter of 9×18 mm, a...
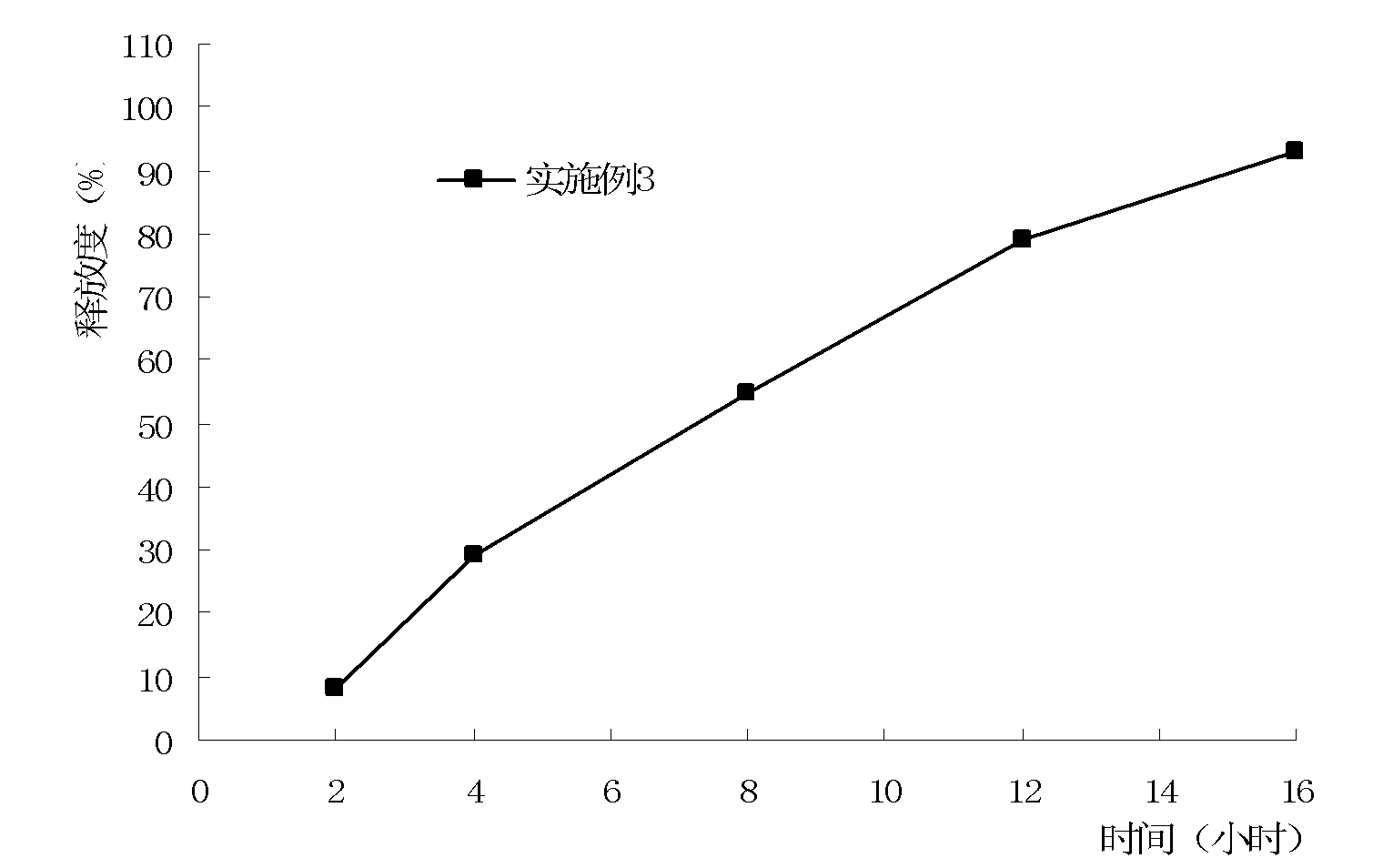
Embodiment 3
[0103] A kind of preparation method of metformin osmotic pump controlled-release tablet, preparation method is the same as embodiment 1, tablet core prescription is as follows:
[0104] Tablet prescription:
[0105] Metformin Hydrochloride 750g
[0106] Poloxamer 188 60g
[0107] 60% ethanol (V / V) 160mL
[0109] The above can be made into each tablet containing 750mg of metformin hydrochloride.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com