Lipid prodrug of drug containing guanidino and pharmacosome thereof
A technology of prodrugs and drugs, which is applied in the field of amphiphilic lipid prodrugs and their drug plastids, can solve problems such as difficult to achieve and low bioavailability, optimize the operation process, slow down blood drug concentration fluctuations, reduce Effects of gastrointestinal irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 2
[0023] Example 1. Preparation of glyceryl dipalmitate
[0024]
[0025] Heat the palmitic acid to melt (60°C), and add excess SOCl to it 2 , After stirring and reacting for several hours, distill, first distill out SOCl at low temperature 2 , Vacuum distillation to obtain colorless palmitoyl chloride, sealed and waterproof.
[0026] The dilute sulfuric acid was added dropwise to the hot water dissolved in calcium glycerate, and a large amount of white precipitate was immediately formed. After the dripping is completed, the white precipitate is filtered off hot while maintaining the temperature to obtain an aqueous solution of glyceric acid. The water was evaporated under reduced pressure, and the heating was stopped when the mother liquor became a viscous white liquid. Add anhydrous acetone, shake to dissolve, add anhydrous magnesium sulfate and dry overnight. The next day, filter to remove the desiccant and steam to remove acetone to obtain glyceric acid.
[0027] Dissolve palmi...
Embodiment 2 2
[0028] Example 2. Synthesis of dipalmitoyl glycerate-metformin
[0029]
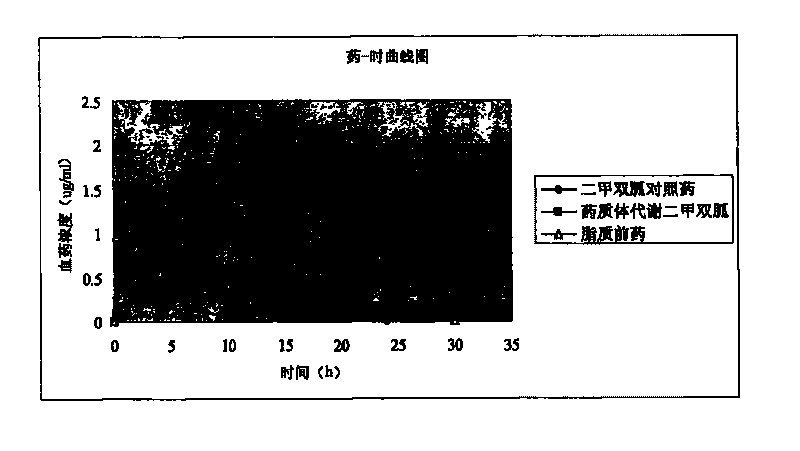
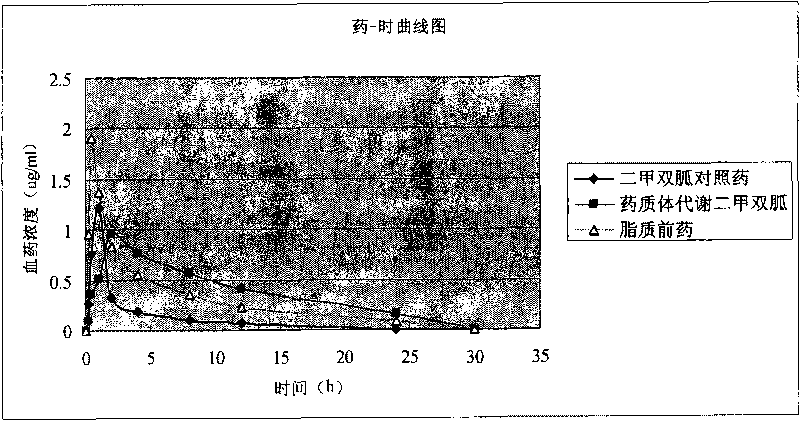
[0030] Put 0.58 g (1 mmol) of dipalmitoyl glycerate in a 50 ml flask, and add 5 ml SOCl to it 2 . Reflux for 8 hours, pump down the generated HCl and SOCl 2 . 5 ml of anhydrous methylene chloride was added, and 0.1 ml of anhydrous pyridine and 0.13 g of metformin were dissolved in 3 ml of methanol. The methanol solution was added dropwise to the reaction flask, stirred at room temperature for 24 hours, refluxed for 4 hours, and cooled. The resulting solution was spin-dried directly, 30 ml of dichloromethane was added, and the insoluble matter was filtered off. Wash twice with 10 ml of water, dry the organic layer and spin dry. Ethyl acetate was added to precipitate 0.45 g of white solid. 1 H-NMR(CDCl 3 ): 0.85 (t, 6H), 1.23 (s, 48H), 1.52 (m, 4H), 2.27 (t, 2H), 2.32 (t, 2H), 2.50 (s, 6H) 4.34 (m, 1H), 4.43 (m, 1H), 5.2 (m, 1H).
Embodiment 3
[0031] Example 3. Synthesis of palmitoyl-metformin
[0032]
[0033] Metformin hydrochloride (0.33 g, 2 mmol) was added to 2 ml of water and stirred to dissolve, then sodium hydroxide (0.08 g, 2 mmol) was added, and the mixture was stirred at room temperature for 1 hour. An acetone solution of palmitoyl chloride (0.7 g, 2.5 mmol) was added dropwise and stirred at room temperature for 2 hours. Add acetone until the precipitate no longer precipitates, filter, and spin-dry the filtrate. Add 5 ml of dichloromethane to dissolve, filter to remove insolubles. Wash twice with 5 ml of water, dry the organic layer and spin dry. Ethyl acetate was added to precipitate 0.48 g of white solid. 1 H-NMR(CDCl 3 ): 0.96 (t, 3H), 1.31 (s, 24H), 1.57 (m, 2H), 2.18 (t, 2H), 2.47 (s, 6H).
PUM
| Property | Measurement | Unit |
|---|---|---|
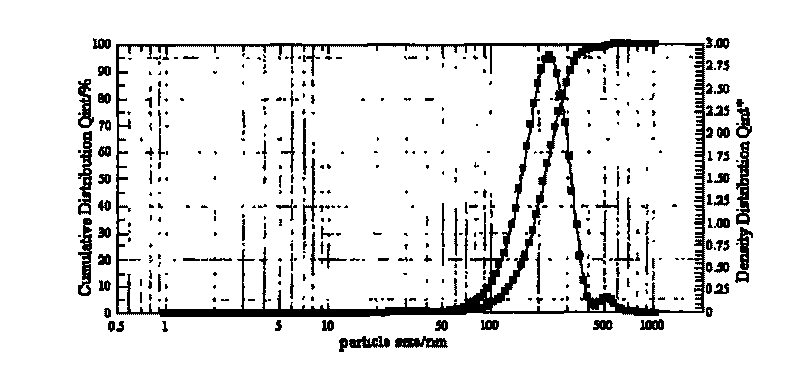
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com