Stable perindopril indapamide tablet and preparation technology
A technology for indapamide tablets and perindapamide, which is applied in the field of perindopril indapamide tablets and the preparation technology thereof, can solve the problem of insufficiency to ensure that perindopril tert-butylamine and indapamide are stable and clinically stable. It can solve the problems of curative effect, inter-batch variation, etc., to achieve the effect of solving the problem of content uniformity, excellent inter-batch variation, and excellent intra-batch variation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] A kind of perindopril indapamide tablet, described perindopril indapamide tablet is made up of following components according to parts by weight:
[0065] Alpha crystal form perindopril tert-butylamine: 4 parts;
[0066] Indapamide: 1.25 parts;
[0067] Lactose monohydrate: 79.65 parts;
[0068] 3.6 parts of hydroxypropyl cellulose;
[0069] 1.5 parts of sodium carboxymethyl starch;
[0070] 0.5 parts of colloidal silicon dioxide;
[0071] Magnesium stearate: 1 part; indapamide crystal form and literature Phamazie 2006, 61(12):99-1004
[0072] The reported crystal forms are the same;
[0073] The tablet weighing about 90mg of last system, its preparation process comprises the following steps:
[0074] (1) Take the lactose and dry it until the moisture content is lower than 5.5%, pass through a 50-mesh sieve together with colloidal silicon dioxide, and mix;
[0075] (2) adding sieved hydroxypropyl cellulose (passing 50 mesh), sodium carboxymethyl starch (passing 5...
Embodiment 2
[0092] A kind of perindopril indapamide tablet, described perindopril indapamide tablet is made up of following components according to parts by weight:
[0093] Alpha crystal form perindopril tert-butylamine: 2 parts;
[0094] Indapamide: 0.625 parts;
[0095] Lactose monohydrate: 82.275 parts;
[0096] SSL hydroxypropyl cellulose 3.6 parts;
[0097] 1.5 parts of sodium carboxymethyl starch;
[0098] 0.5 parts of colloidal silicon dioxide;
[0099] Magnesium stearate: 1 part;
[0100] The crystal form of indapamide is the same as that reported in the literature Phamazie 2006, 61(12):99-1004.
[0101] The tablet weighing about 90mg of last system, its preparation process comprises the following steps:
[0102] (1) After drying the lactose until the water content is lower than 5.5%, pass through a 50-mesh sieve with colloidal silicon dioxide and mix;
[0103] (2) Do not add sieved hydroxypropyl cellulose (over 50 mesh) and sodium carboxymethyl starch (over 50 mesh) to mi...
Embodiment 3
[0127] A kind of perindopril indapamide tablet, described perindopril indapamide tablet is made up of following components according to parts by weight:
[0128] Alpha crystal form perindopril tert-butylamine: 4 parts;
[0129] Indapamide: 1.25 parts;
[0130] Lactose monohydrate: 76.25 parts;
[0131] SSL hydroxypropyl cellulose 1.5 parts;
[0132] 4.5 parts of sodium carboxymethyl starch;
[0133] 1 part of colloidal silicon dioxide;
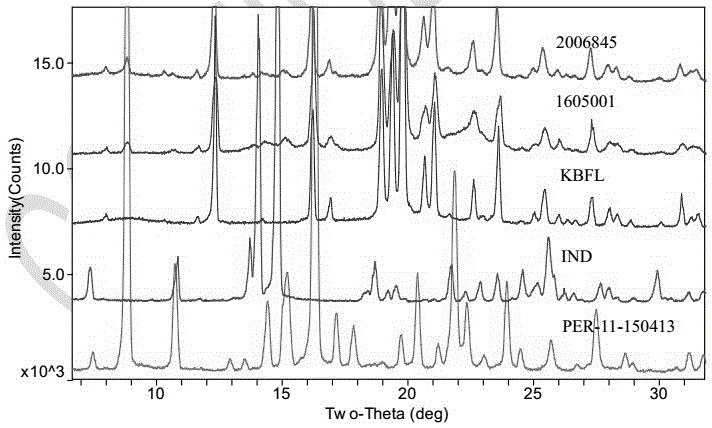
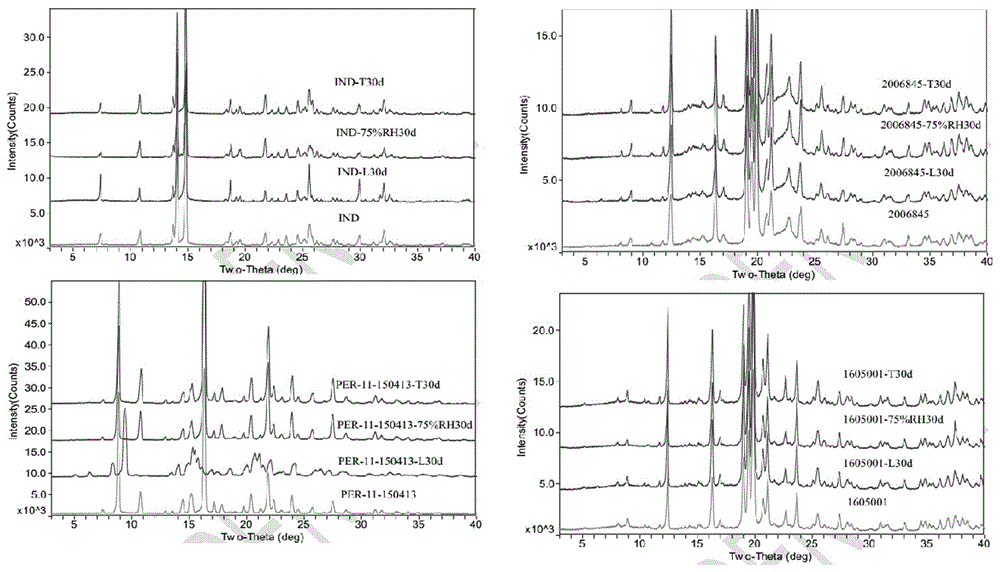
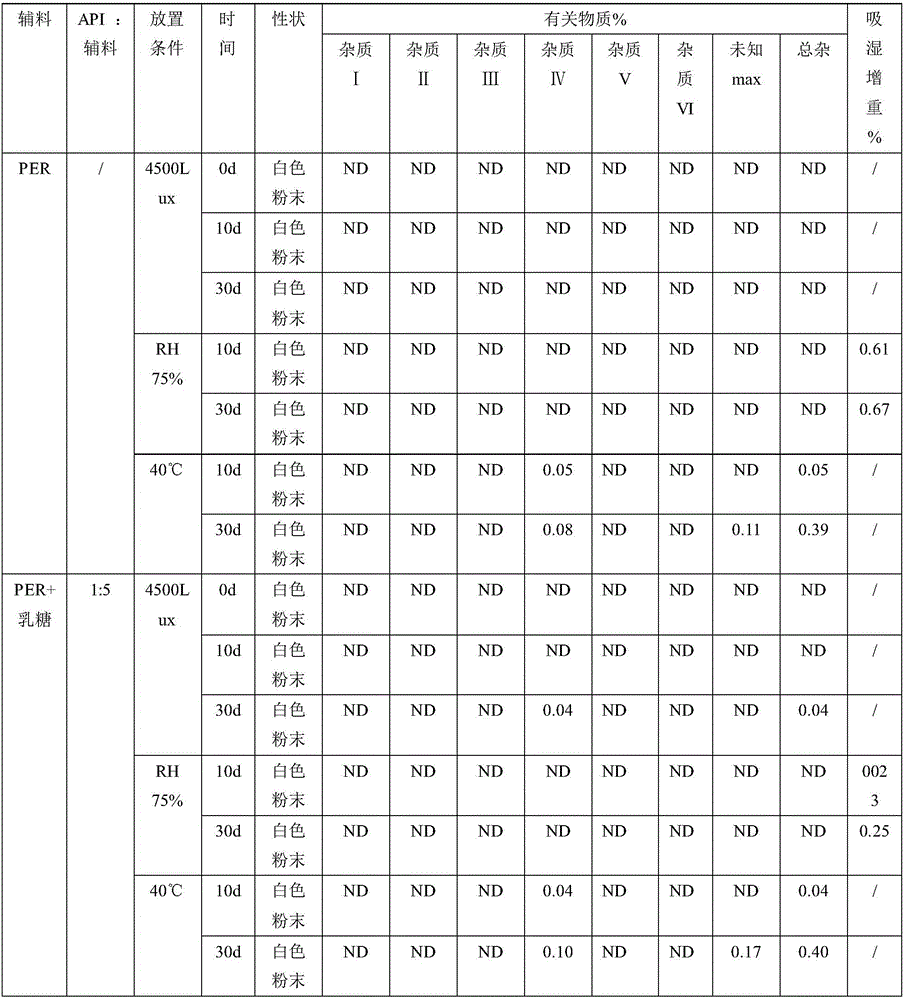
[0134] Magnesium stearate: 1.5 parts; the crystal form of indapamide is the same as the crystal form reported in the document Phamazie 2006, 61 (12): 99-1004. Finally, a tablet weighing about 90 mg is made, and its preparation process is as in Example 1 As mentioned above, the crystal form of the product obtained in the embodiment is consistent with that of the marketed product, and the key quality attributes such as properties, related substances, dissolution rate, content uniformity, moisture content, and content are all consistent.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com