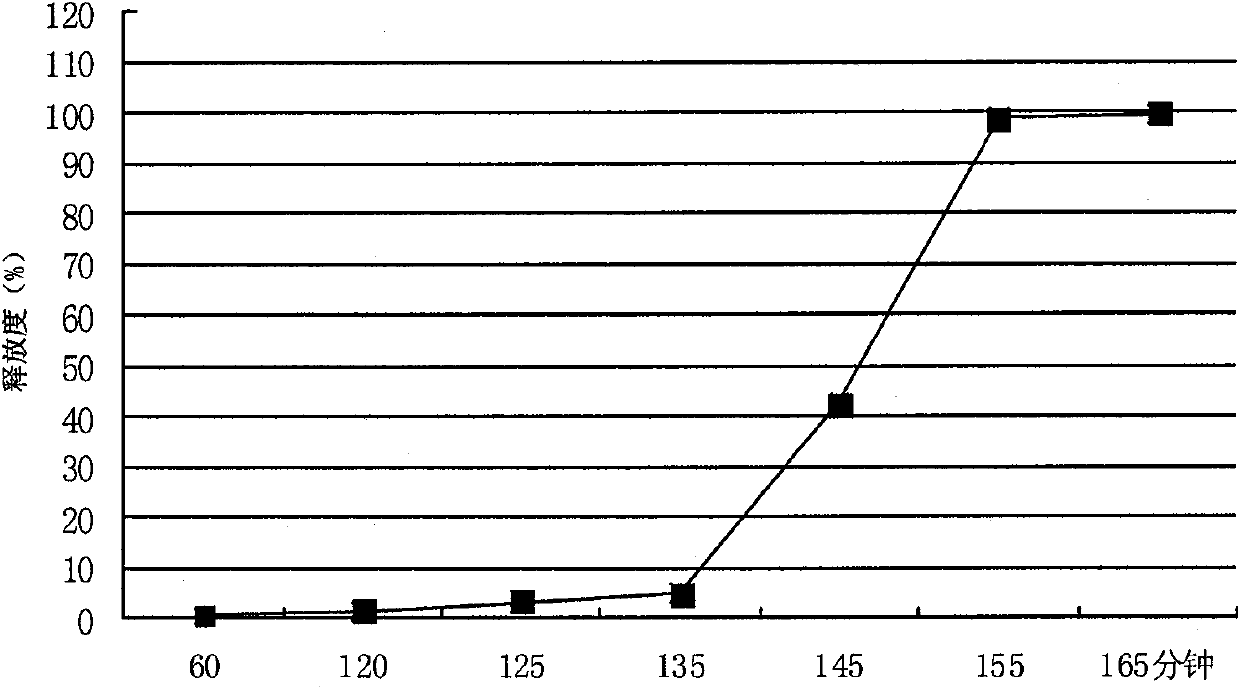
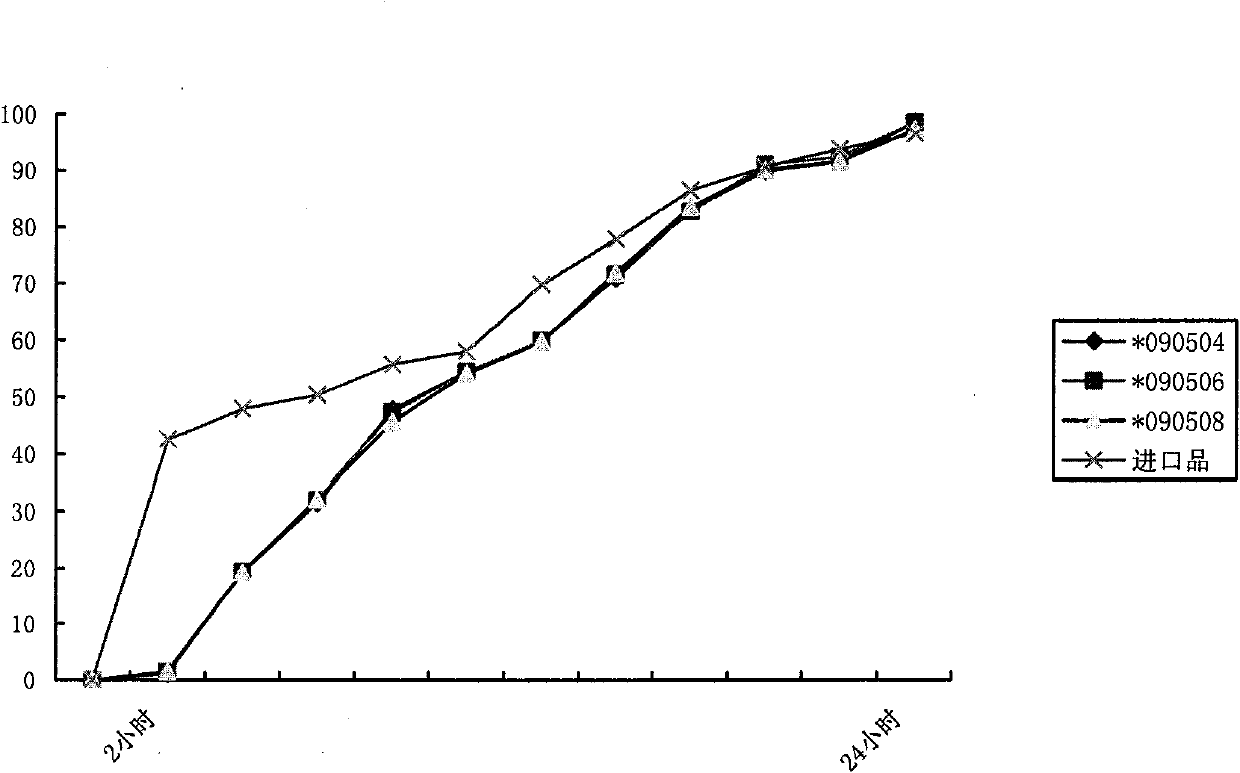
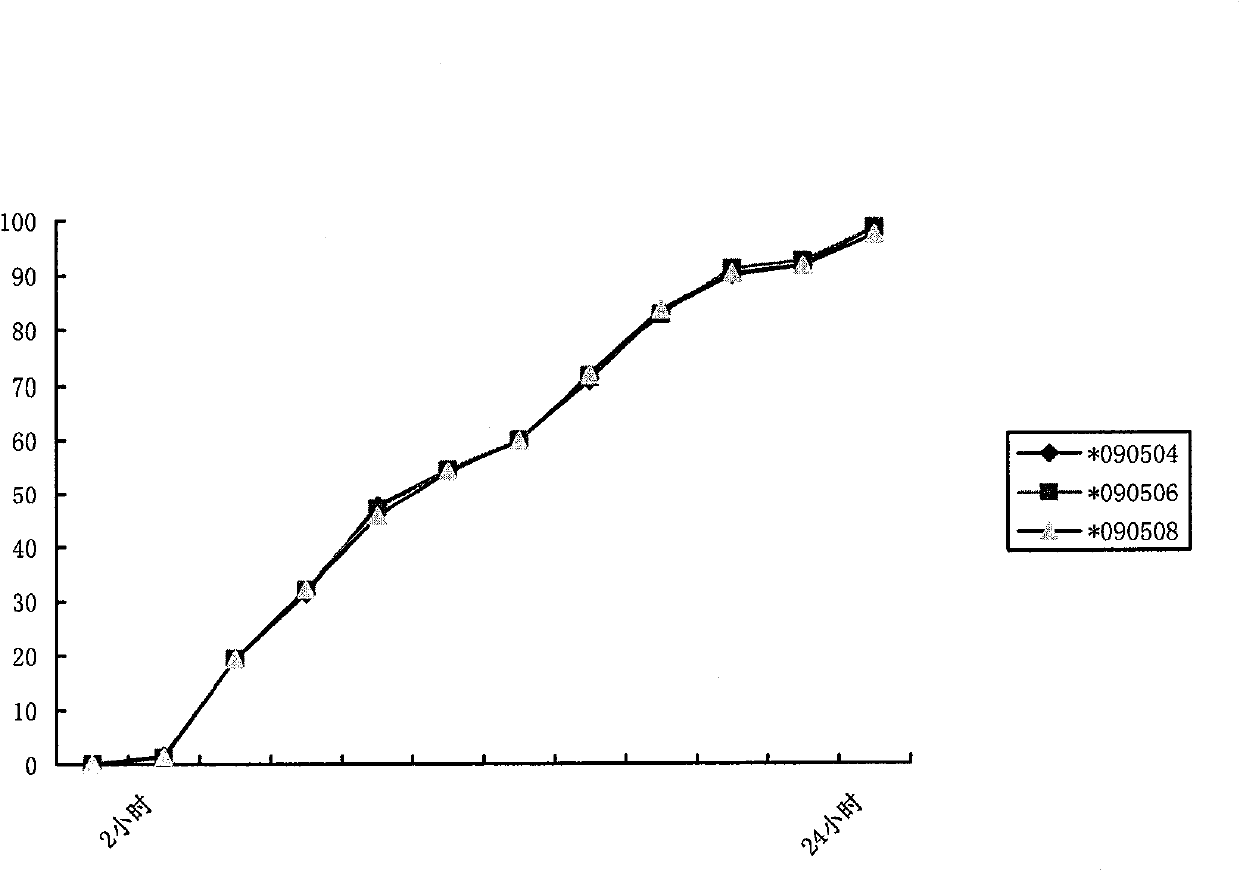
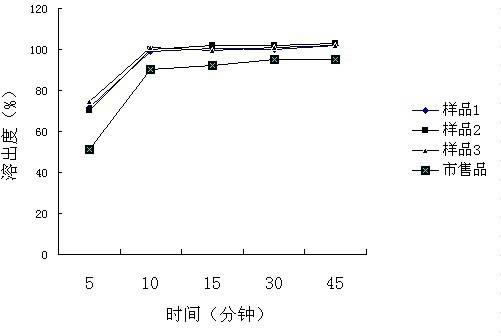
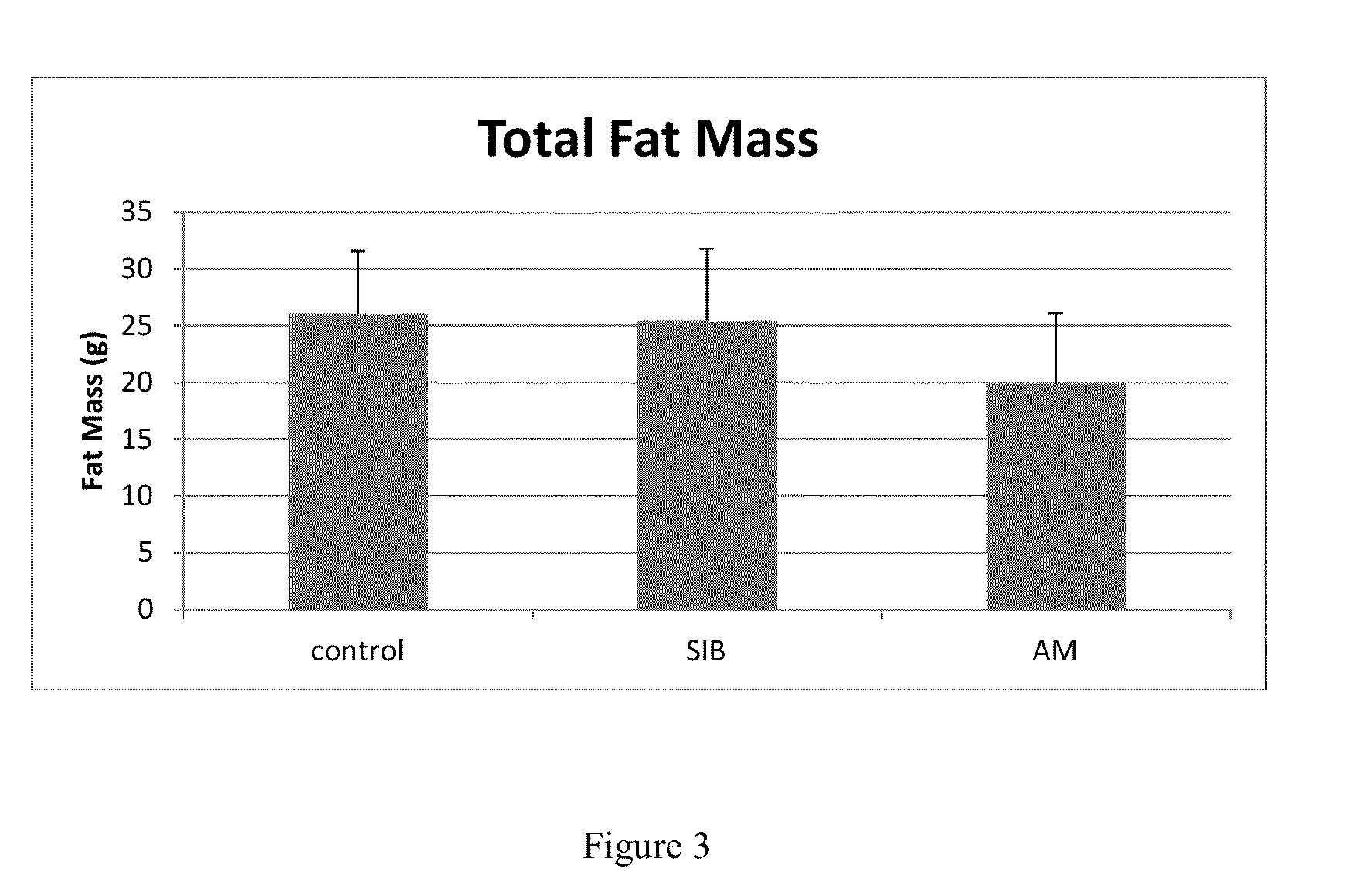
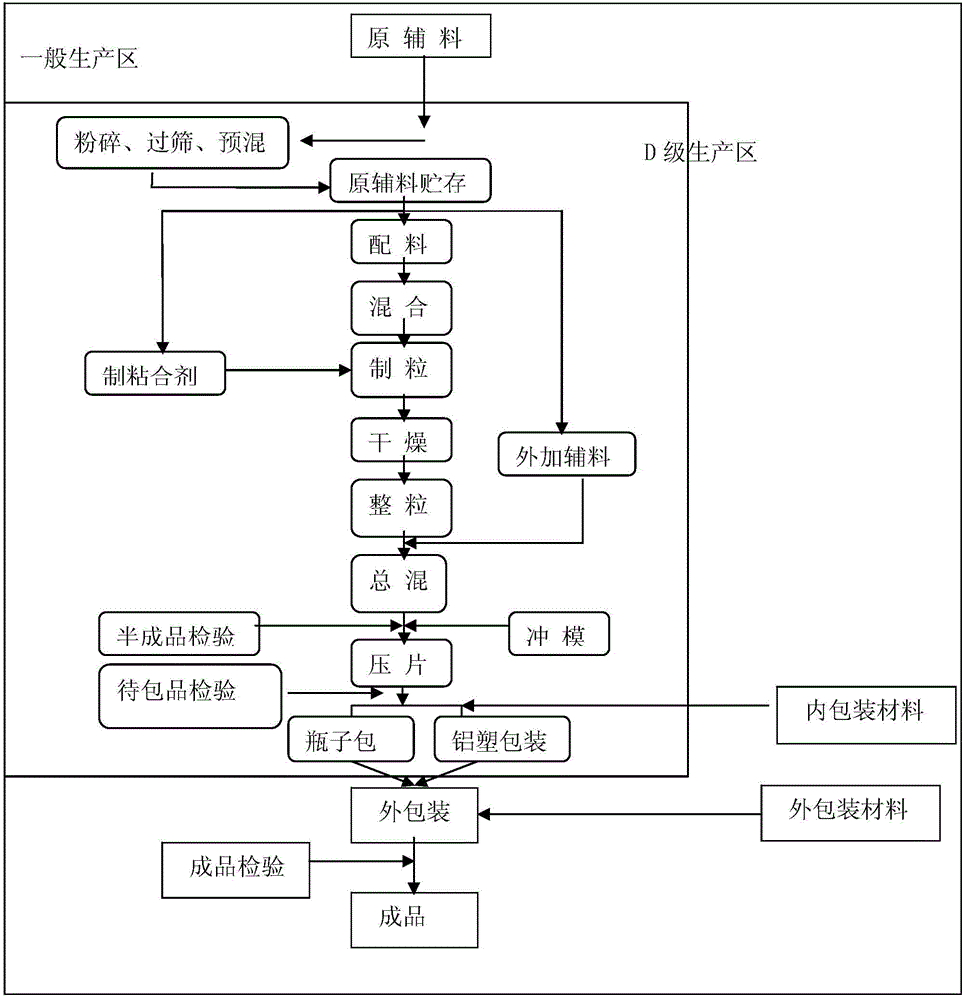
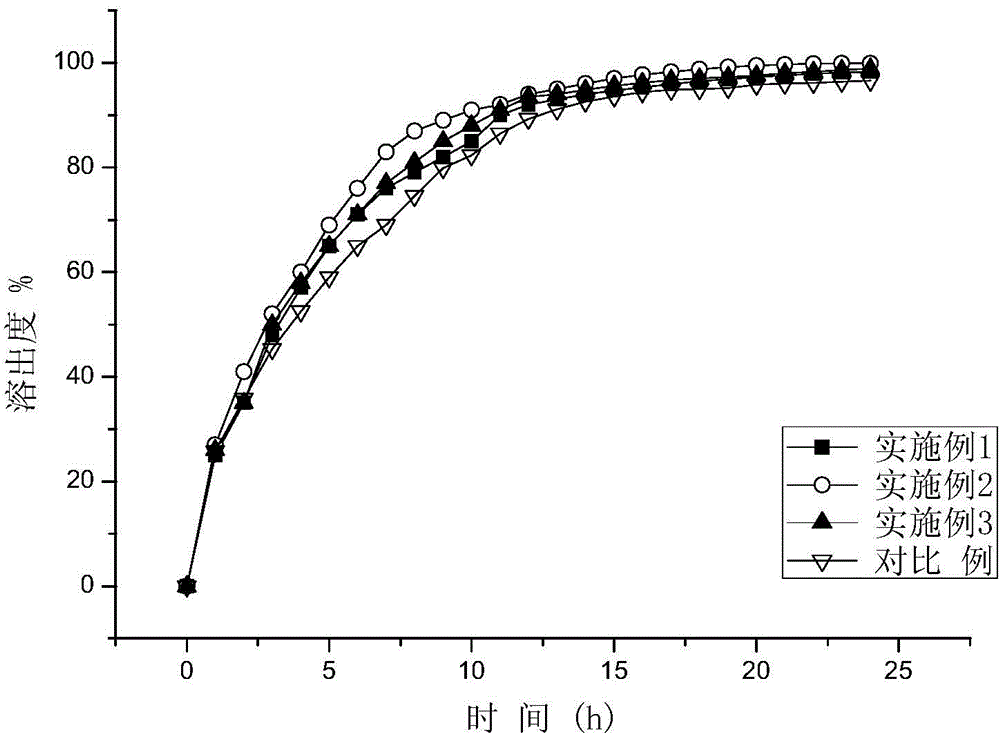
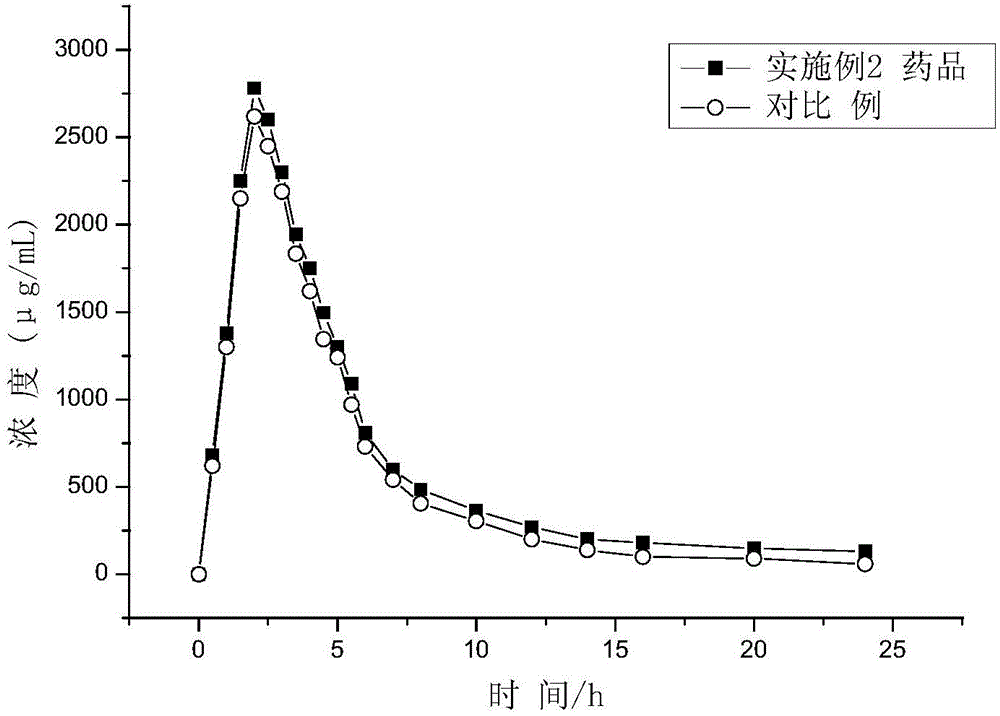
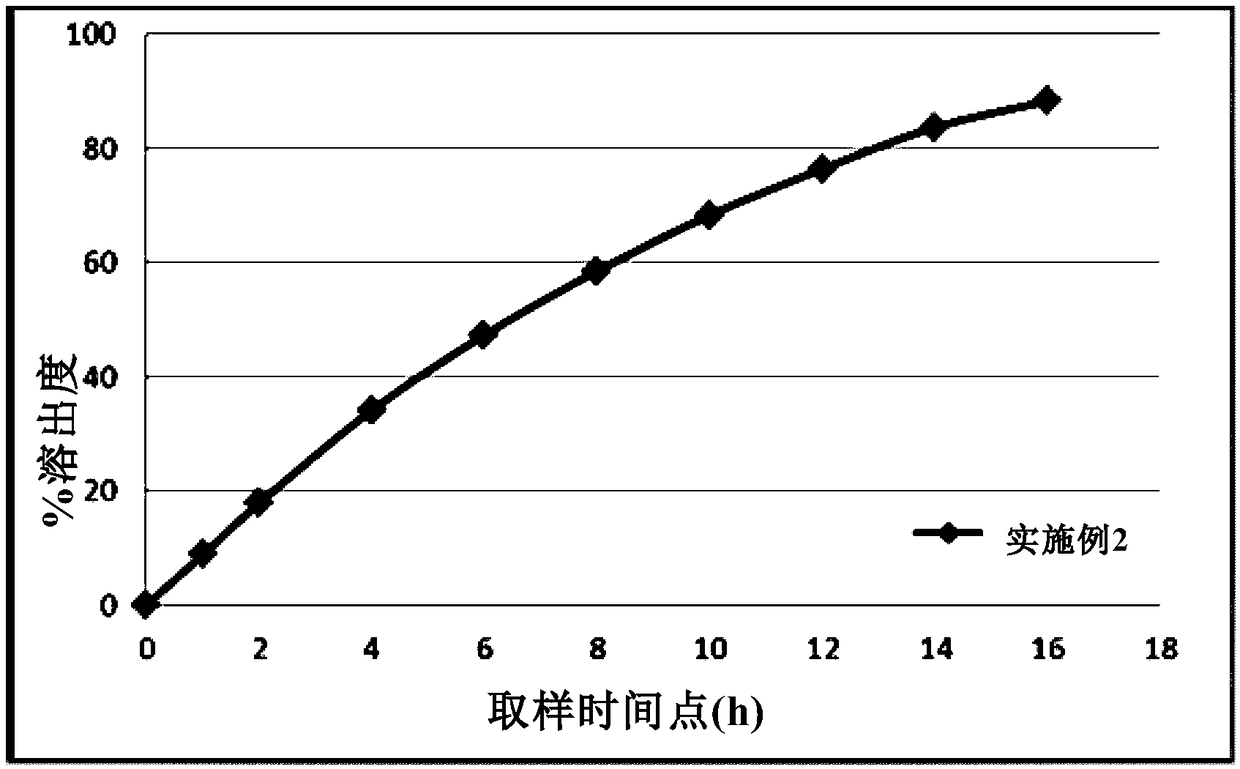
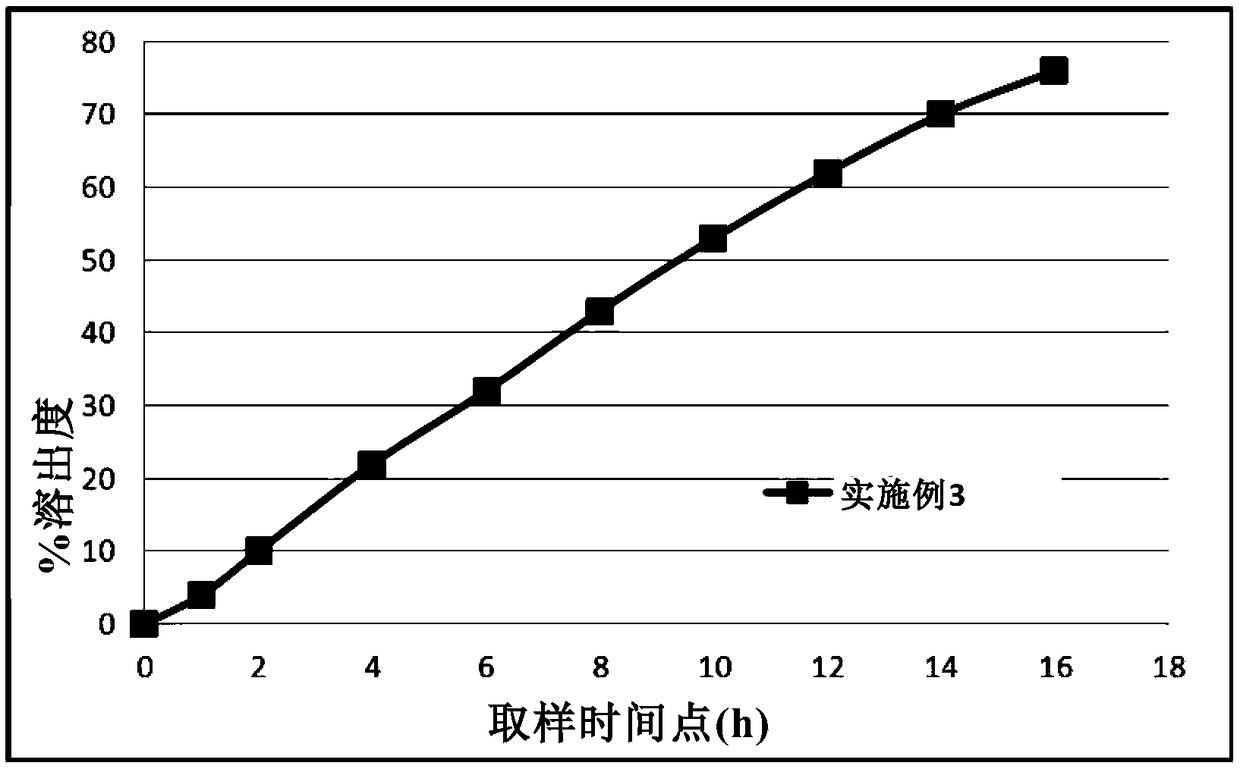
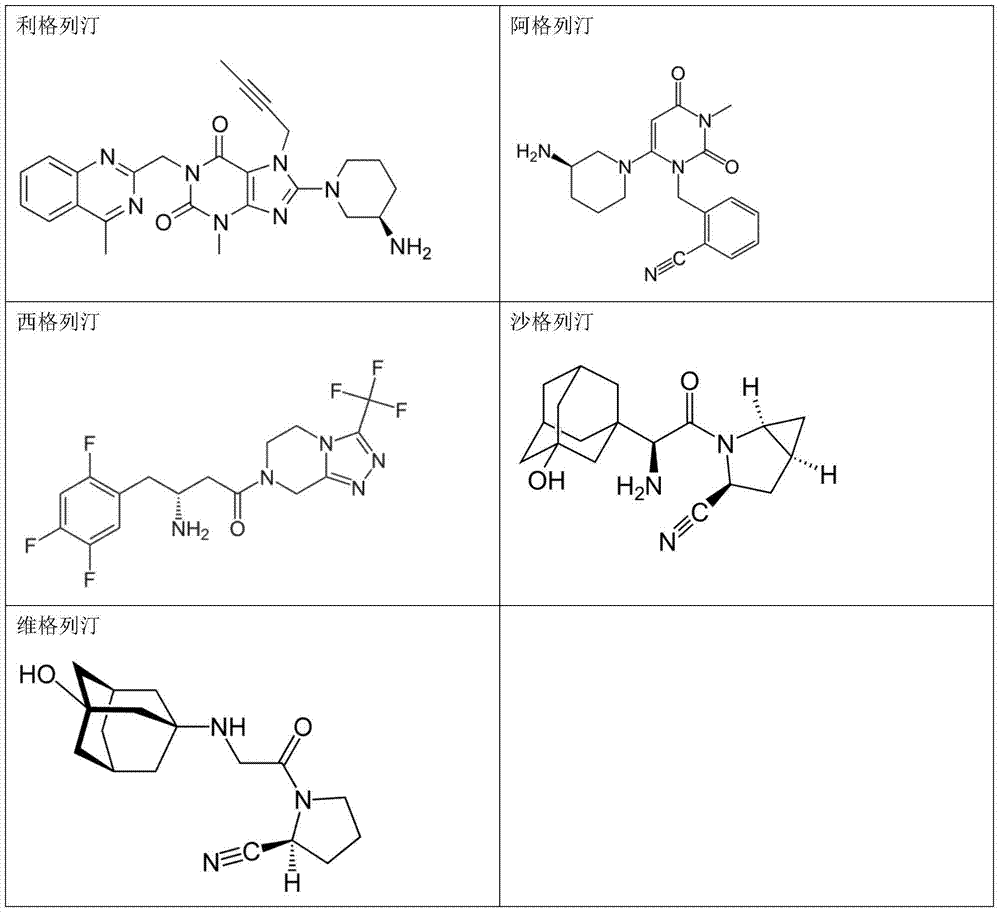
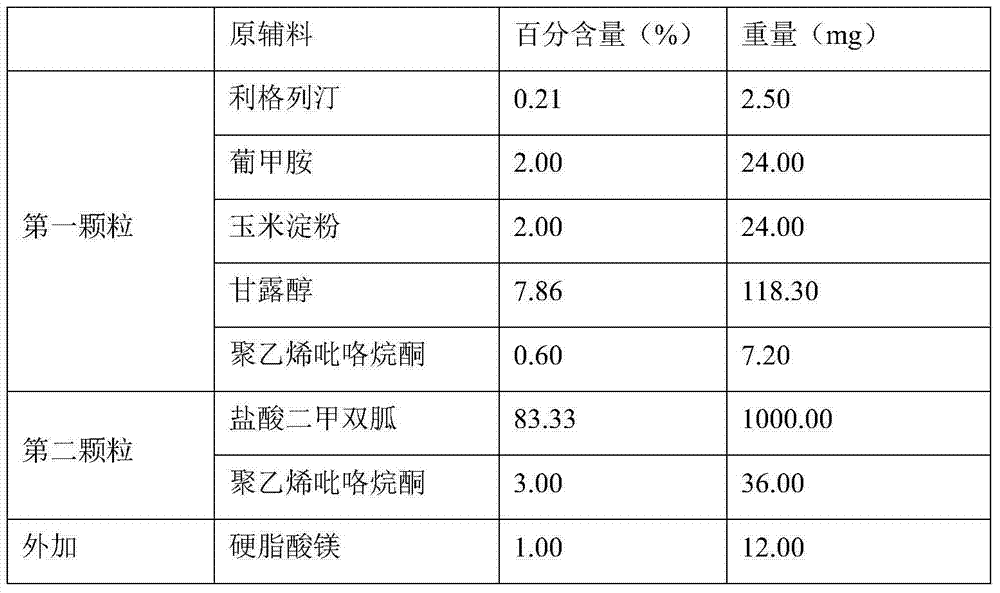
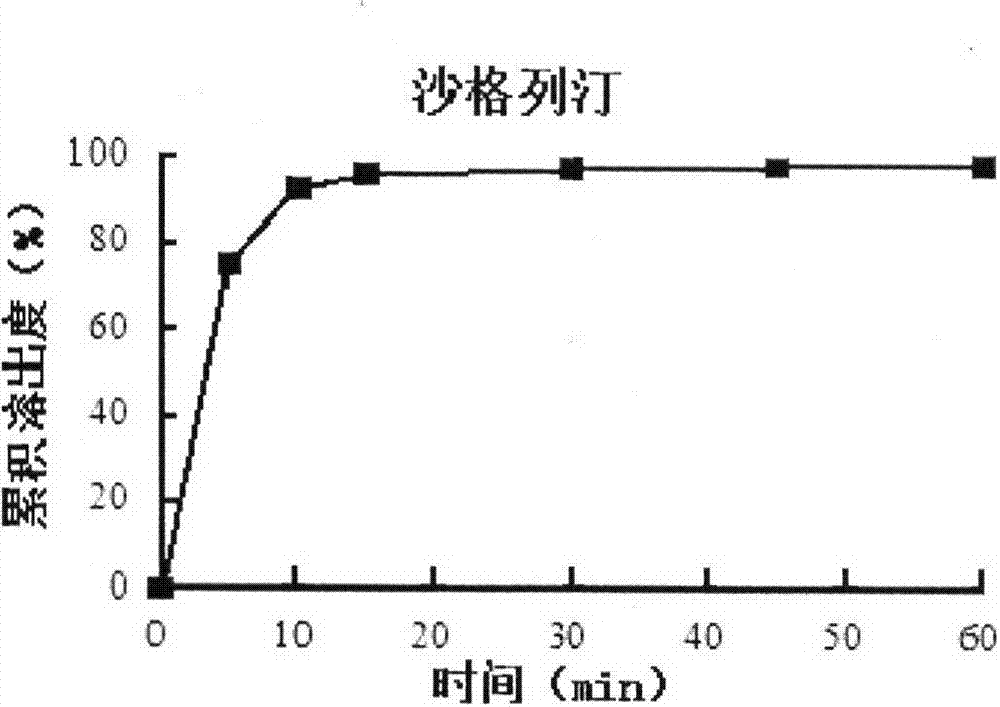
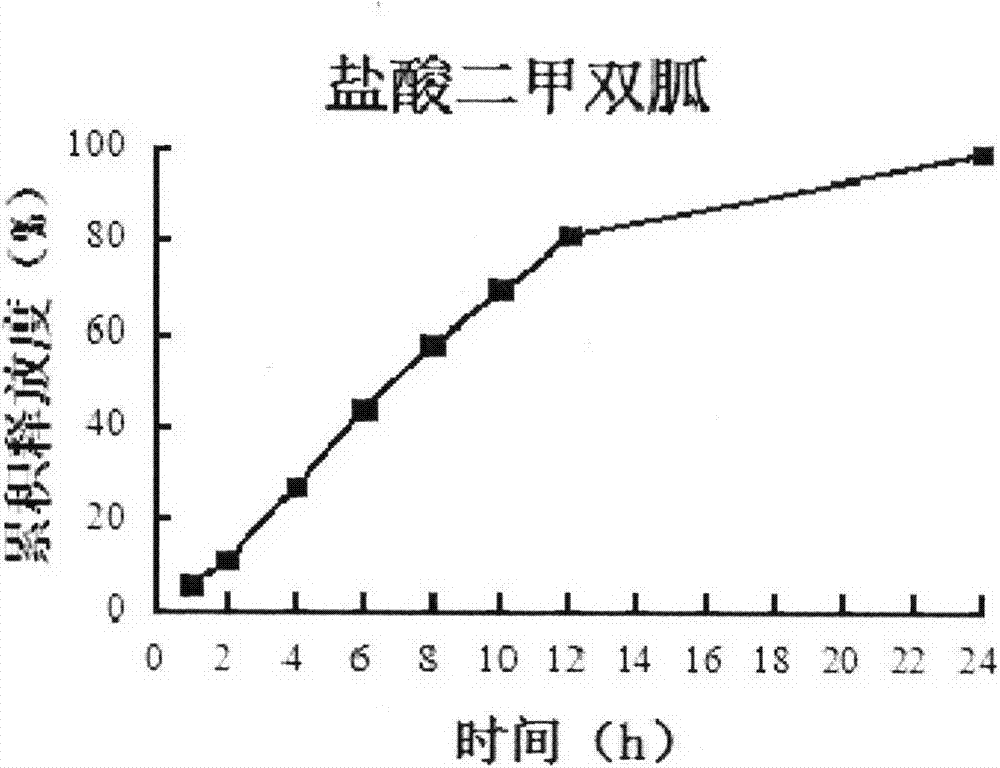
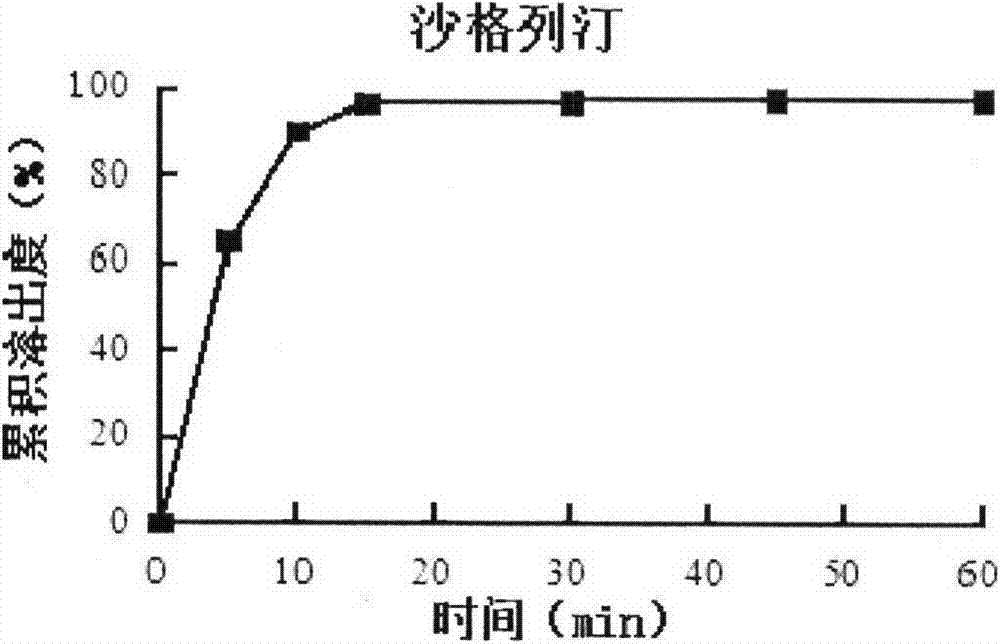
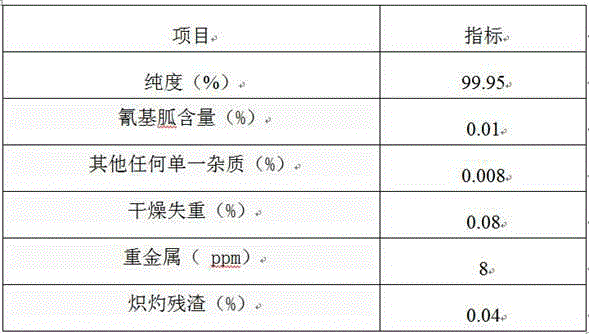
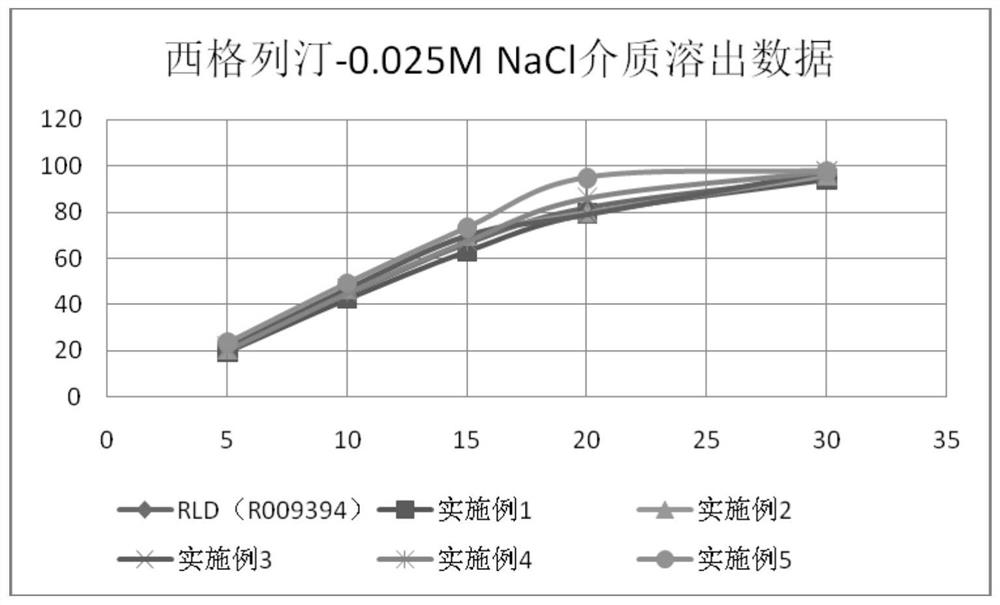
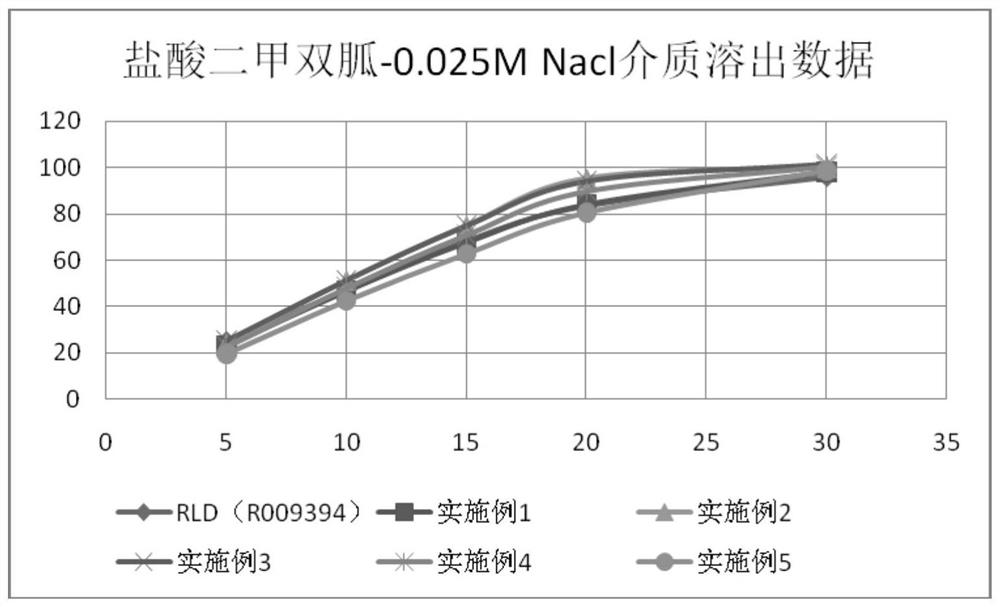
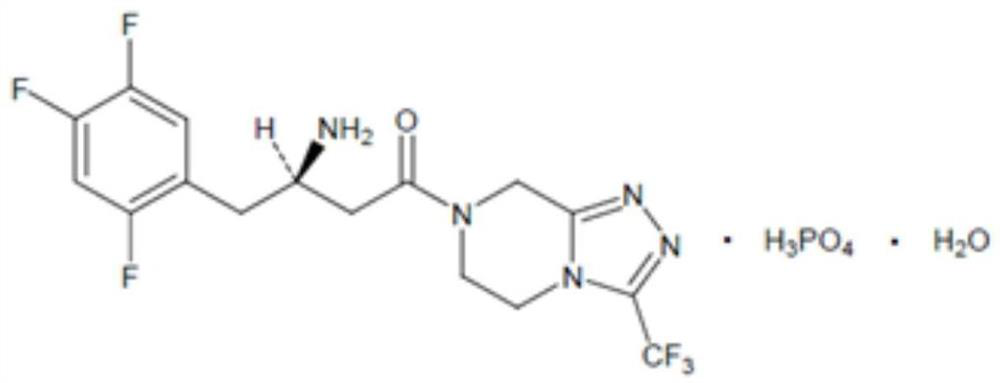
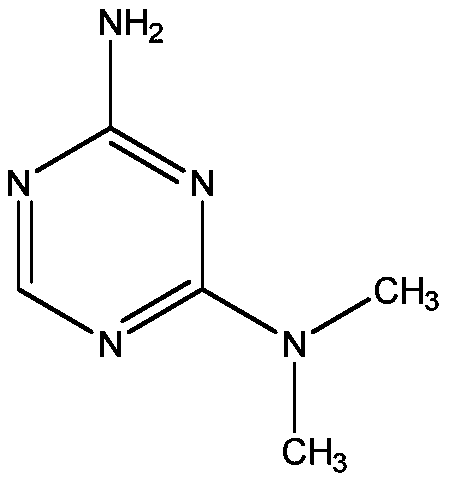
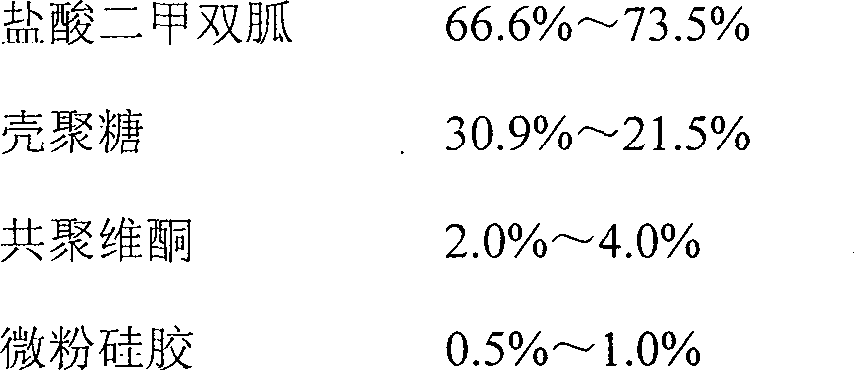Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
130 results about "Metformin hcl" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
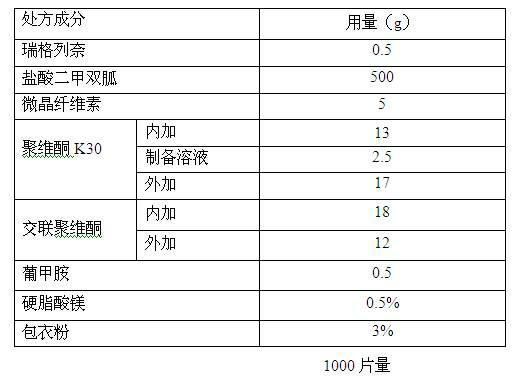
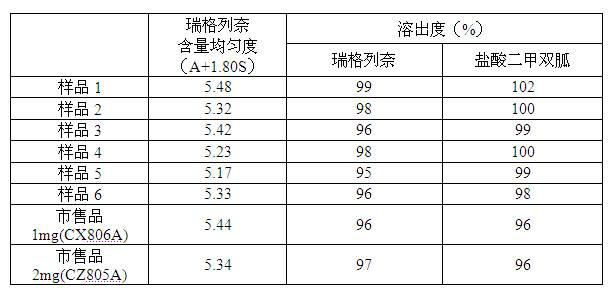
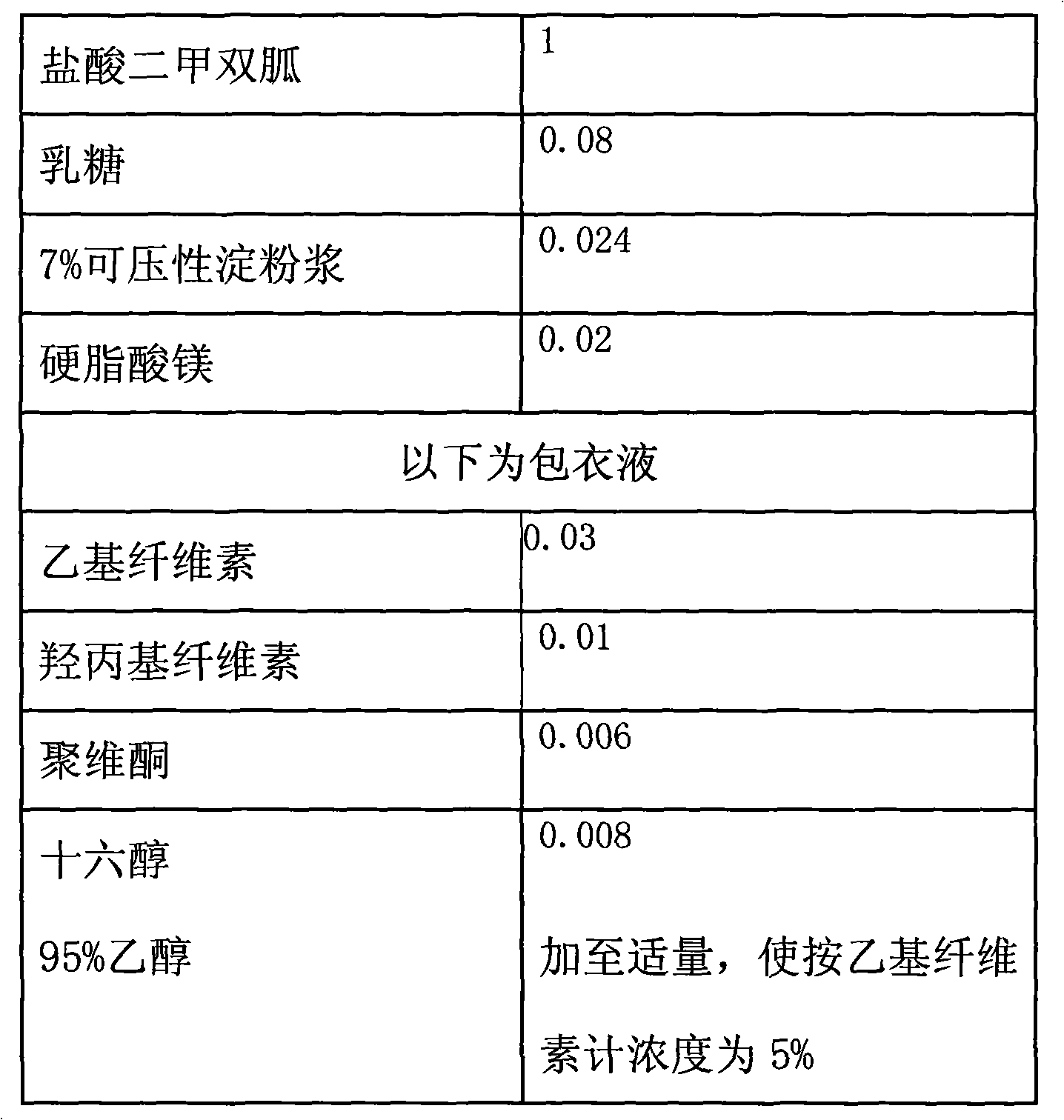
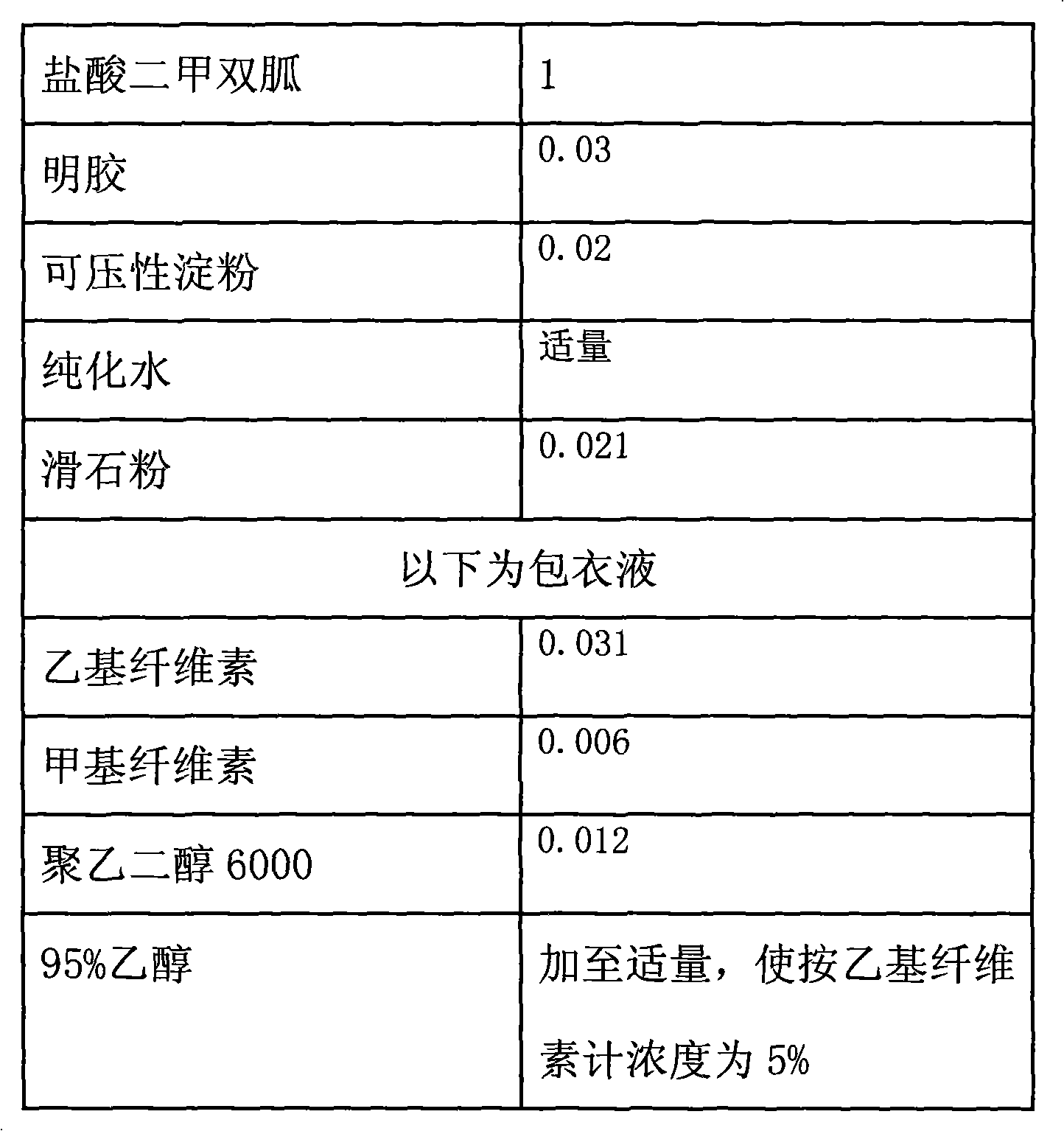
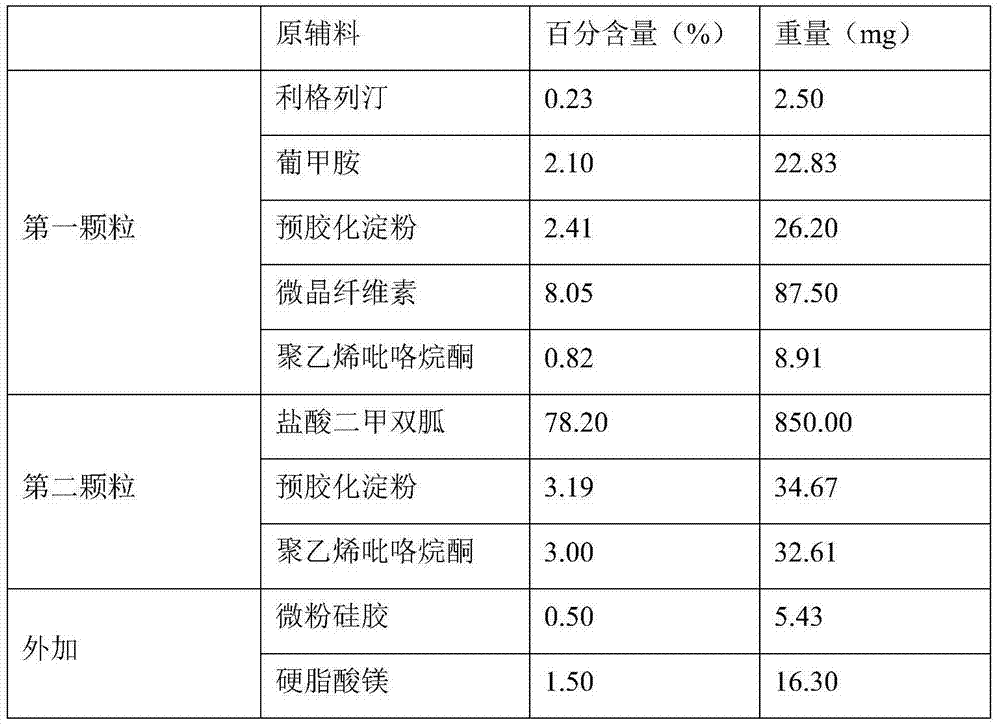
Directly compressible extended-release matrix formulation for metformin hydrochloride
InactiveUS6524618B1Acceptable degreeAcceptable of friabilityPowder deliveryBiocideMetformin HydrochlorideDissolution
An extended-release matrx formulation capable of being directly compressed into tablets comprising metformin hydrochloride blended with specific excipients. The excipients used in the formulation enhance the flow and compaction properties of the drug and insure that the formulation is directly compressible into a tablet containing about 100 mg to about 800 mg, preferably about 250 mg to about 750 mg, of metformin hydrochloride in unit dosage form. Each tablet produced by direct compression of the formulaton has the desired hardness and dissolution characteristics such that the drug is released in the body of the subject over an extended period of time.
Owner:PHARMALOGIX
Metformin hydrochloride enteric-coated sustained release tablet and preparation method thereof
ActiveCN101785763AOrganic active ingredientsMetabolism disorderPatient complianceSustained-Release Preparations
The invention discloses a metformin hydrochloride enteric-coated sustained release tablet which is prepared by enteric coating the metformin hydrochloride sustained release tablet. Compared with the prior art, the sustained release tablet integrates with the enteric coating technology to prepare a new form of the metformin hydrochloride enteric-coated sustained release tablet. By using the enteric coating technology, the metformin hydrochloride does not disintegrate in the stomach or stimulate the gastric mucosa, and the adverse reaction of nausea, stomachache and diarrhea caused by medicine taking can be avoided; meanwhile, the metformin hydrochloride is prevented from being damaged by gastric juice, and the bioavailability is improved. The product is a sustained release preparation, the medicine can stably release in vivo, the effective blood concentration can be maintained for a long time, the toxic and side effects caused by over-high blood concentration in a short time are avoided, the medicine taking frequency is decreased, and the patient compliance is improved as well.
Owner:贵州天安药业股份有限公司
Preparation method of metformin hydrochloride
ActiveCN103435518AHigh recovery rateImprove securityOrganic chemistryOrganic compound preparationMetformin hclPharmacology
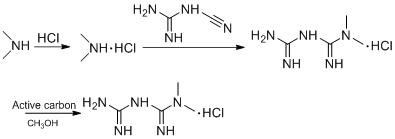
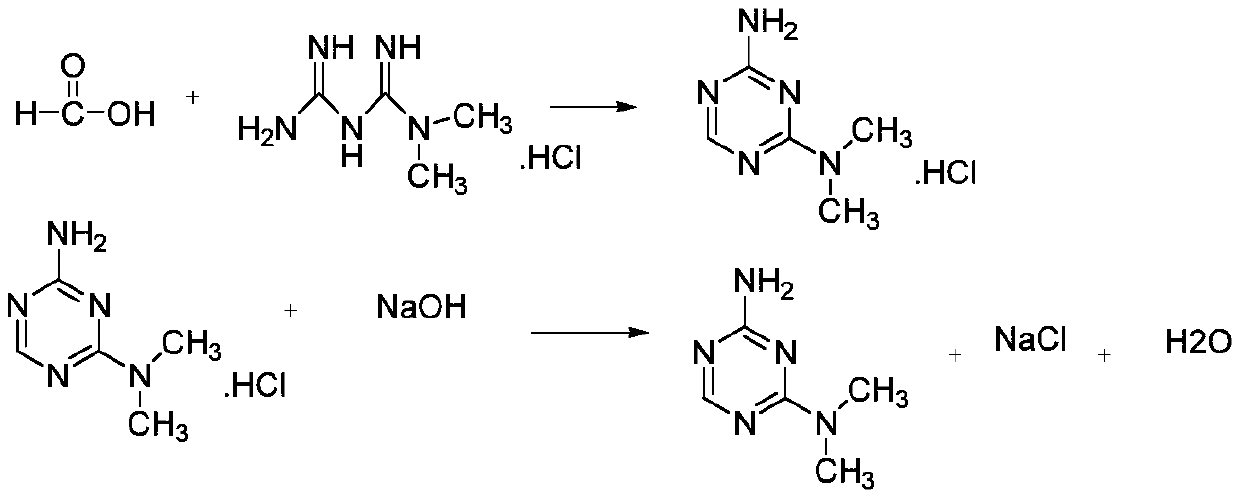
The invention discloses a preparation method of metformin hydrochloride. The preparation method is characterized by using dicyandiamide and dimethylamine hydrochloride as raw materials, feeding the materials in a mole ratio of (1:1)-(1:1.2), using N,N-dimethylacetamide or dimethyl sulfoxide 2-4 times dicyandiamide by weight as a solvent, and reacting for 4-8 hours at 140+ / -5 DEG C to prepare a crude metformin hydrochloride product; recrystallizing the crude product with ethanol, regulating the pH value to be 5-6, decoloring the crystal, cooling the crystal to minus 10-0 DEG C while stirring, precipitating the crystal, obtaining a refined metformin hydrochloride product through filtering and drying, and recovering the solvent from filtrate. The qualified product has yield of 80-85% and high purity. The preparation method has the advantages that as the selected reaction solvent has a relatively high boiling point, the recovery rate of the solvent is high; the phenomenon of material surging can be effectively avoided, so that the preparation method has the advantages of mild reaction conditions, simplicity in operation and high safety.
Owner:QINGDAO HUANGHAI PHARM CO LTD
Composition containing repaglinide and metformin hydrochloride and preparation thereof
ActiveCN102319245AReduce dosageFacilitated releaseOrganic active ingredientsMetabolism disorderMetformin hclPharmaceutical Aids
The invention relates to a composition containing repaglinide and metformin hydrochloride and a preparation method thereof, belonging to the technical field of medicines. The composition containing repaglinide and metformin hydrochloride consists of repaglinide, metformin hydrochloride, a filling agent, a disintegrating agent, a bonding agent, a latent solvent and a lubricant, wherein the repaglinide is added into the metformin hydrochloride and auxiliary materials in the form of aqueous solution for wet granulation. The preparation method has the advantages of simple and efficient process, small using quantity of auxiliary materials, capability of solving the problem of poor content uniformity caused by small using amount of the repaglinide and increase in the dissolution rate; and moreover, a preparation has a smaller unit quantity and can be applied to industrial mass production.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST
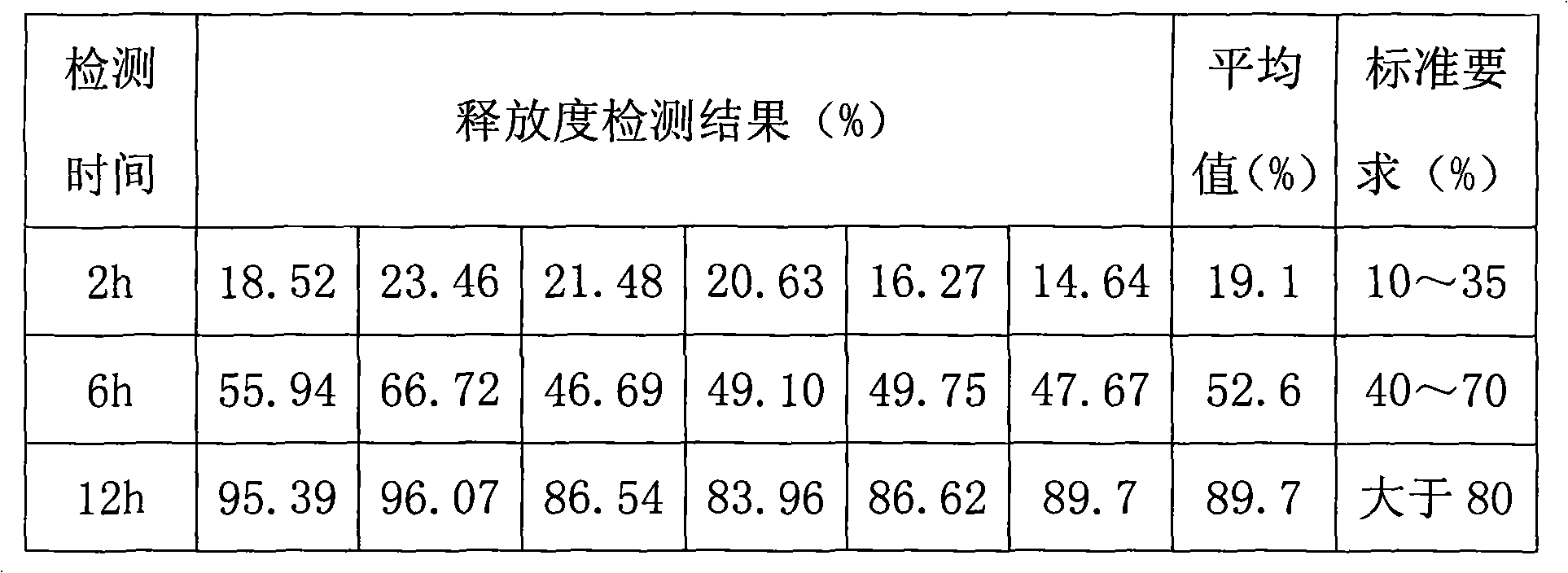
Metformin hydrochloride controlled-release tablet and preparation method thereof
ActiveCN101579325ALess weight gainIncrease production capacityOrganic active ingredientsMetabolism disorderPharmaceutical industryMetformin Hydrochloride
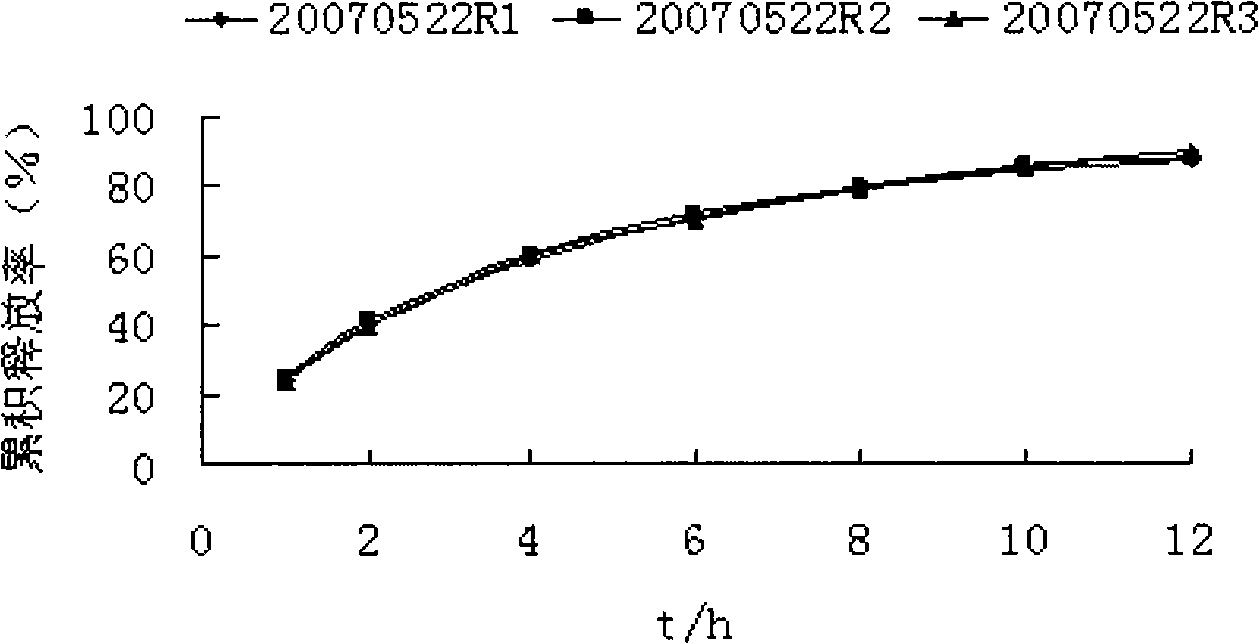
The invention provides a metformin hydrochloride controlled-release tablet, which comprises a metformin hydrochloride controlled-release tablet with effective dose and pharmaceutical accessories and is characterized in that the metformin hydrochloride controlled-release tablet uses the common tabletting, and the controlled-release effect is controlled by totally depending on the film coating technique. The coating adopted by the invention is a releasing system comprising the prescription which can cause the medicine to reach release degree standard in vitro. The preparation method in the invention is simple and convenient; the process conditions are easy to control and suitable for batch production, can use conventional production equipment in pharmaceutical industries for economically and conveniently producing the metformin hydrochloride controlled-release tablet on a large scale, can effectively and stably cause the release degree of the metformin hydrochloride controlled-release tablet in the second hour to be 10% to 35%, the release degree to be 40% to 70% in the sixth hour and the release degree to be more than 80% in the twelfth hour.
Owner:CHONGQING CONQUER PHARML
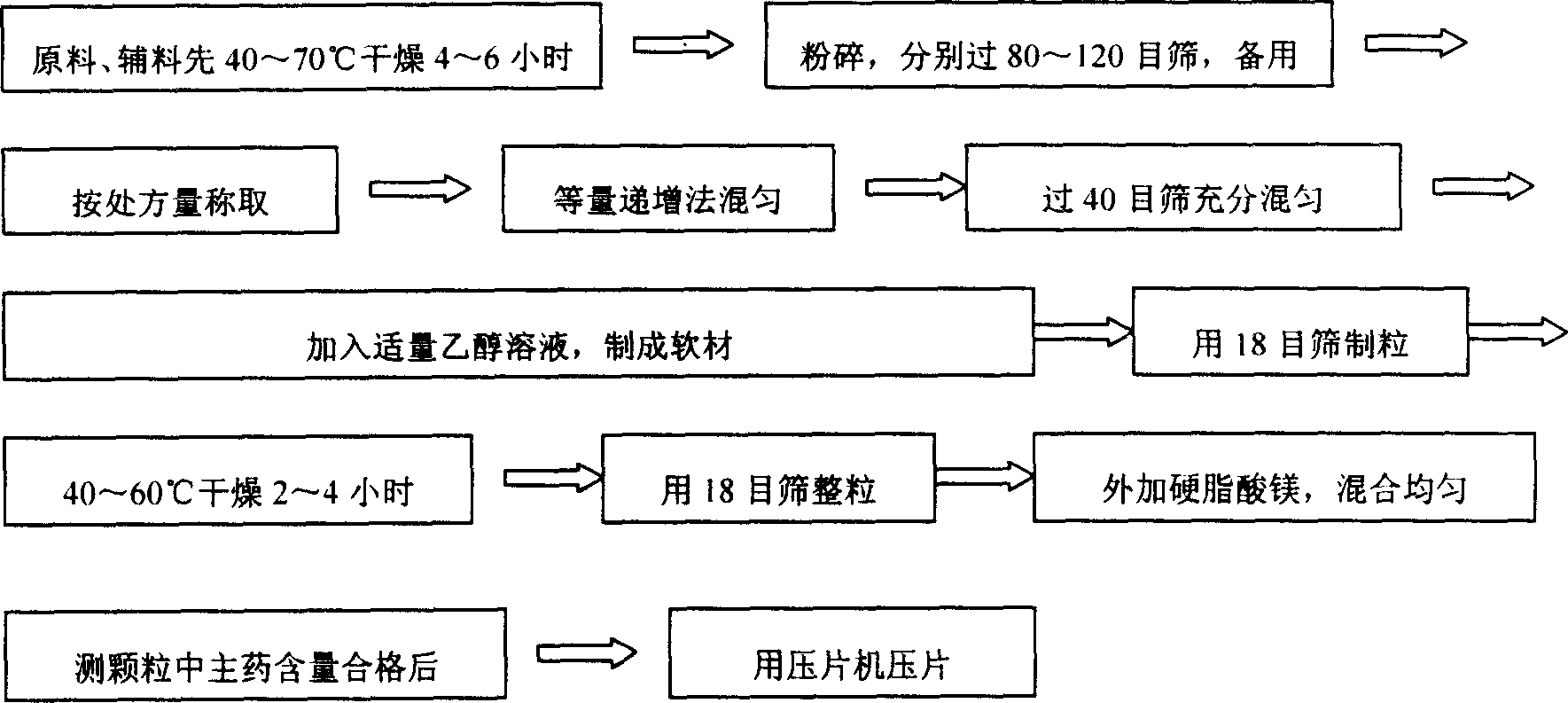
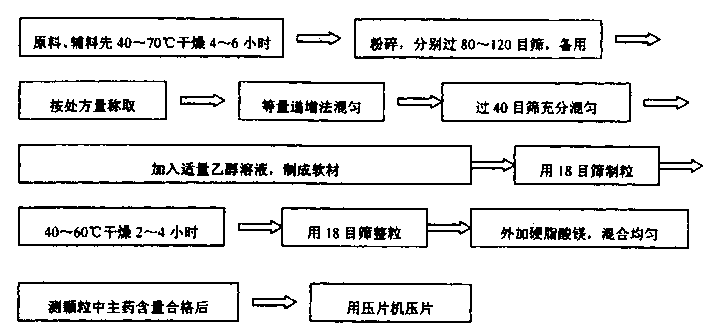
Metformin hydrochloride slowly released tablet and its preparation method
InactiveCN1415288APromote aerobic metabolismIncrease intakeOrganic active ingredientsMetabolism disorderExtended release tabletsAlcohol
A slowly-releasing mellitin tablet is prepared from fluamine, excipient, adhesive and lubricant through drying at 40-70 deg.C for 4-6 hrs, pulverizing, sieving by 80-120 meshes, proportionally mixing, mixing with alcohol solution, granularating by 18-mesh sieving, drying at 40-60 deg.C for 2-4 hrs, mixing with lubricant, and tabletting. Its advantages are long half-life (0.9-2.6 hr), low dosage and low by-effect.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD
Pharmaceutical composition comprising an ampk activator and a serotonergic agent and methods of use thereof
InactiveUS20140350064A1Easy to transportImprove toleranceBiocideOrganic active ingredientsDiseaseMetformin Hydrochloride
The present invention is based on the unexpected discovery that a combination of certain known drugs exhibits synergistic effects in treating metabolic syndrome and various other diseases. In particular, the invention comprises a pharmaceutical composition comprising: (1) a therapeutically effective quantity of a first agent that is an AMPK activator; and (2) a therapeutically effective quantity of a second agent that possesses or maintains serotonin activity. A preferred composition comprises metformin hydrochloride and melatonin. The invention further comprises methods for the use of these compositions for the treatment of metabolic syndrome, hyperproliferative diseases including cancer, and other diseases and conditions.
Owner:ALS MOUNTAIN
Metformin hydrochloride sustained-release tablet
ActiveCN103816130ALow costGood slow releaseOrganic active ingredientsMetabolism disorderSustained Release TabletMetformin Hydrochloride
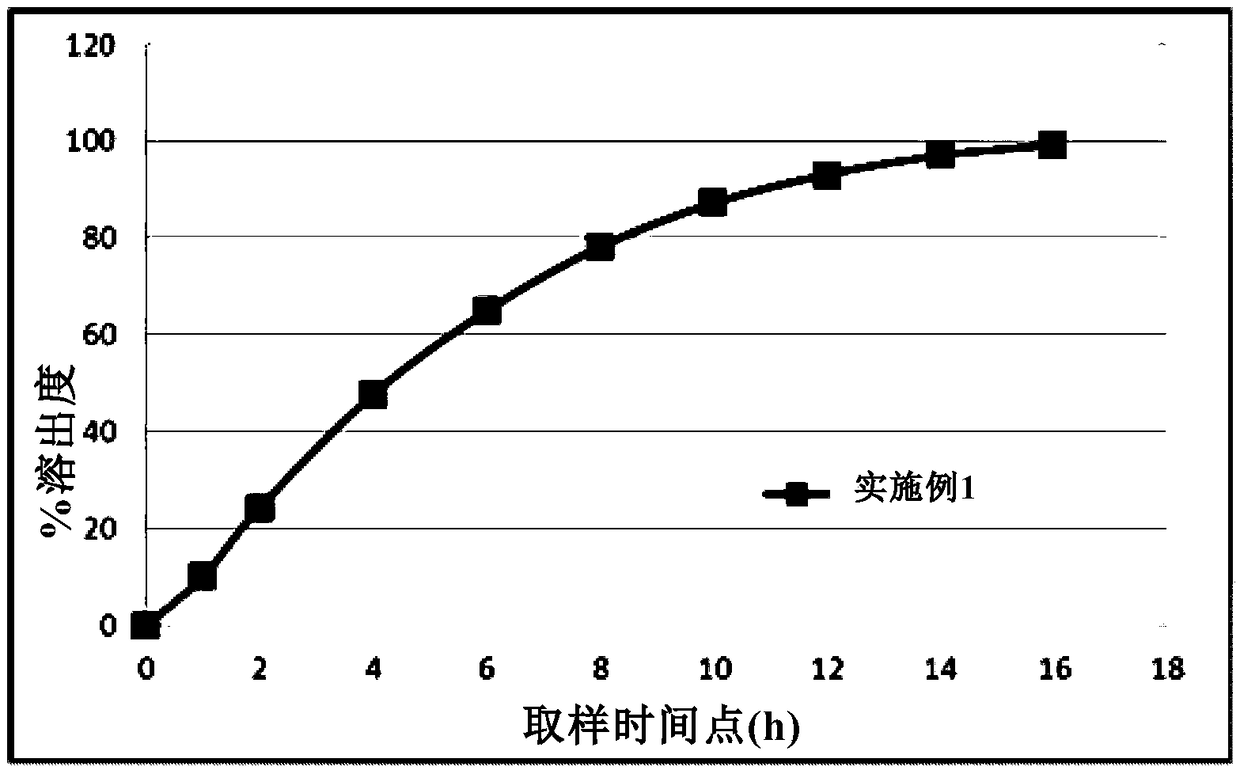
The invention provides a metformin hydrochloride sustained-release tablet. The metformin hydrochloride sustained-release tablet is prepared from the components by weight: 400-600 parts of metformin hydrochloride, 30-60 parts of sodium carboxymethylcellulose, 200-250 parts of hydroxypropyl methylcellulose, 180-220 parts of ethyl acrylate-methyl methacrylate copolymer aqueous dispersion and 5-10 parts of magnesium stearate. Suitable auxiliary materials and a suitable preparation method are adopted to prepare the metformin hydrochloride sustained-release tablet with lower raw material cost and simpler process, and the sustained-release performance of the obtained product is good, the release amounts at 1 hour, 3 hours and 10 hours are respectively 20-45 percent, 45-75 percent and above 80 percent, and the drug has good stability and can be preserved for 24 months at the room temperature.
Owner:YOUCARE PHARMA GROUP
Pharmaceutical composition, methods for treating and uses thereof
InactiveUS20180344647A1Reduce riskImprove blood sugar controlOrganic active ingredientsMetabolism disorderImmediate releaseMetformin Hydrochloride
The invention relates to solid pharmaceutical dosage forms comprising an extended release core comprising metformin hydrochloride and one or two immediate release coatings comprising linagliptin and / or empagliflozin.
Owner:BOEHRINGER INGELHEIM INT GMBH
High-purity metformin hydrochloride preparation method
InactiveCN104788345AAvoid affecting product qualityLower the temperature of the addition reactionOrganic chemistryOrganic compound preparationMetformin hclPyrrolidinones
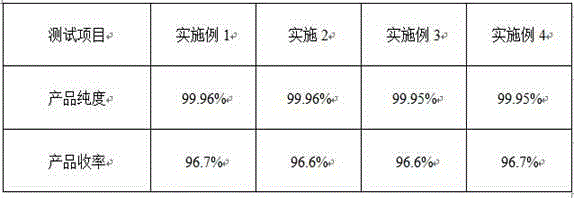
The invention discloses a high-purity metformin hydrochloride preparation method. N-methyl pyrrolidone is used as a solvent, dicyandiamide and dimethylamine hydrochloride are taken as a solute, the solvent and the solute are added into a synthesis kettle for reaction, and metformin hydrochloride is prepared through steps of stirring, cooling, spin-drying, washing, crystallizing and drying. The metformin hydrochloride prepared through the preparation method provided by the invention has the advantages that the yield is higher than 96.4 percent, and the product purity is higher than 99.93 percent.
Owner:TAISHAN MEDICAL UNIV
Metformin hydrochloride and Glipizide sustained-release pellet and method of preparing the same
ActiveCN101278919AAvoid Difficulty ScreeningAvoid screening timeOrganic active ingredientsMetabolism disorderSustained release pelletsBlood concentration
The invention discloses a metformin hydrochloride and glipizide sustained-release pellet and a preparation method thereof. In the preparation method, a sustained release coated pellet of the metformin hydrochloride and a sustained release pellet of the glipizide are prepared respectively; and the two pellets are filled in capsules in a proportion of 250g-500g of the metformin hydrochloride and 2.5g-10g of the glipizide in every 1000 capsules; wherein, the coated pellet of the metformin hydrochloride is prepared by pill pericardium sustained release coating membrane; the sustained release pellet of the glipizide is prepared directly by extrusion-spheronization method. In the invention, the two pellets are filled into one capsule, thereby being convenient for quality control; octodecyl alcohol is taken as sustained release material for the sustained-release pellet of the glipizide, which is convenient for the forming of the pellet so as to reduce bursting release effectively. The metformin hydrochloride and the glipizide slowly release a drug within 12 hours, which reduces the frequency of taking medicine, stabilizes blood concentration better and reduces untoward effect, thereby having good marketing prospect.
Owner:国药控股星鲨制药(厦门)有限公司
Metformin hydrochloride floating sustained-release tablet and preparation method thereof
InactiveCN108969501AGood floating performanceGood sustained release effectOrganic active ingredientsMetabolism disorderDrugMetformin Hydrochloride
The invention provides a metformin hydrochloride floating sustained-release tablet and a preparation method thereof. The metformin hydrochloride floating sustained-release tablet comprises a tablet core and a non-gastric-soluble coating film wrapped outside the tablet core, wherein the tablet core comprises metformin hydrochloride, a floating material, an adhesive and other excipients, and non-gastric-soluble coating film comprises a film forming material and other excipients. The tablet core is prepared by matching the above materials, and is matched with the non-gastric-soluble coating filmoutside the tablet core, and the density of the tablet core is less than 1g / cm<3>, so that the metformin hydrochloride floating sustained-release tablet can immediately float in water or gastric juice, thereby reducing the possibility of the tablet being discharged from the stomach into the duodenum, and prolonging the time in which the metformin hydrochloride sustained-release tablet floats in the stomach; the metformin hydrochloride floating sustained release tablet can float until the contents of the stomach are emptied, thereby ensuring that the medicine is released in the stomach; the metformin hydrochloride floating sustained-release tablet provided by the invention does not swell substantially in water or gastric juice, thereby reducing the possibility of abdominal distension feeling of a patient.
Owner:奕利制药有限公司
Repaglinide-metformin hydrochloride tablet and preparing method thereof
InactiveCN104337811AUse less excipientsSolve the problem of insoluble in waterOrganic active ingredientsMetabolism disorderMetformin HydrochlorideSolvent
The invention discloses a prescription of a repaglinide-metformin hydrochloride tablet and a preparation technology thereof. In particular, a solid dispersion water solution technology is used; the problem that repaglinide is insoluble in water can be solved only through common wet granulations; dissolved objects and related matter are both superior to those of foreign objects. Microcrystalline celluloses and sorbitol serve as fillers; polyvinylpolypyrrolidone serves as disintegrating agents; PVPk30 serves as bonding agents; silicon dioxide serves as lubricating agents; meglumine serves as saltiness agents; and poloxamer 188 serves as solubilizers. The repaglinide-metformin hydrochloride tablet prepared with the technology is fewer in use auxiliary material; the high dissolving performance and the high stability can be kept; the preparation technology is simple; the technology cost is lower; and the repaglinide-metformin hydrochloride tablet is suitable for industrial production.
Owner:JIANGSU CAREFREE PHARM CO LTD
Antidiabetic pharmaceutical composition and preparation method thereof
InactiveCN104840960AOrganic active ingredientsMetabolism disorderActive componentMetformin Hydrochloride
The invention relates to an antidiabetic pharmaceutical composition, more specifically to a pharmaceutical composition containing a DPP-4 inhibitor and metformin hydrochloride and a preparation method thereof. The composition contains DPP-4 inhibitor and metformin hydrochloride two active components, and meglumine as the stabilizer. The pharmaceutical composition has excellent storage stability and excellent dissolution characteristics. The invention also provides a preparation method of the composition, the auxiliary materials of the preparation technology are easily available and low in price, thus being suitable for industrial production.
Owner:SUNSHINE LAKE PHARM CO LTD
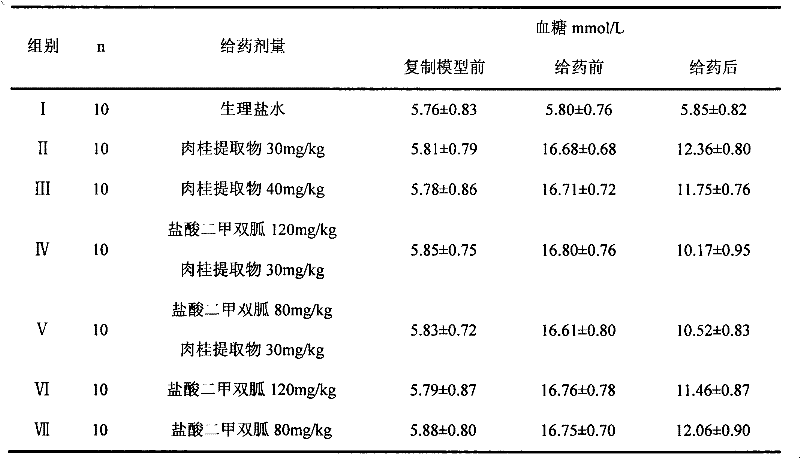
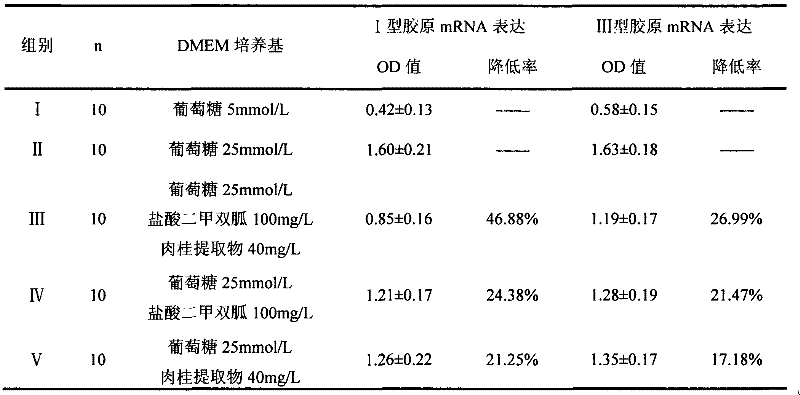
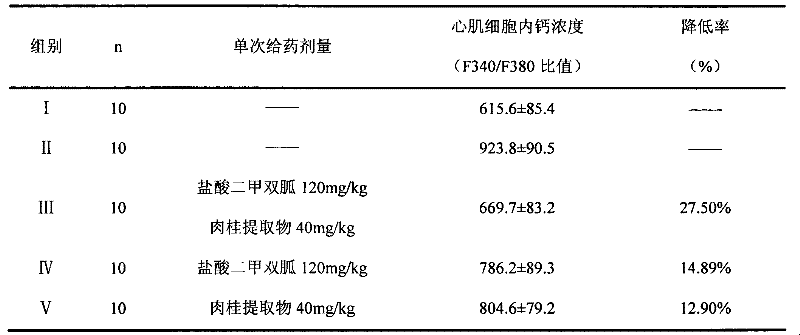
A pharmaceutical composition for the prevention and treatment of type 2 diabetes mellitus and its complications
InactiveCN102266388AOrganic active ingredientsSenses disorderMetformin HydrochlorideDiabetic complication
The invention discloses a pharmaceutical composition for treating diabetes and controlling diabetic complications and application thereof in pharmaceutics, belonging to the technical field of medicines. The pharmaceutical composition provided in the invention comprises a chemical medicine and a Chinese herbal medicine extract, and specifically comprises metformin hydrochloride and cinnamon extract. The pharmaceutical composition in the invention has a good effect on treating diabetes, can effectively control occurrence of diabetic complications, produces low adverse reaction; the utilization of the pharmaceutical composition is more safe and reliable compared to single utilization of chemical medicines and enables usage amount of metformin hydrochloride to be reduced.
Owner:天津市聚星康华医药科技有限公司
Compound preparation including DPP-4 inhibitor and metformin hydrochloride and preparation method thereof
ActiveCN103933031AOrganic active ingredientsMetabolism disorderControlled Release TabletMetformin Hydrochloride
The invention relates to a compound preparation including a DPP-4 (Dipepitidyl Peptidase-4) inhibitor and metformin hydrochloride and a preparation method of the compound preparation. According to the compound preparation, the metformin hydrochloride is a controlled release part, and made into a controlled release tablet by using an osmotic pump technology; the DDP-4 inhibitor is a quick release part, and made into a quick release coating layer by adopting a medicine coating method. The compound preparation structurally and sequentially comprises a metformin hydrochloride core, a controlled release film with small medicine release holes and a DPP-4 inhibitor quick release coating layer from inside to outside.
Owner:广西中恒创新医药研究有限公司
Composition containing pioglitazone hydrochloride and metformin hydrochloride and preparation thereof
ActiveCN101721414AImprove initial dissolutionGood initial dissolutionOrganic active ingredientsMetabolism disorderBiguanideChemistry
The invention provides a composition containing pioglitazone hydrochloride and metformin hydrochloride and preparation thereof. By controlling the particle diameter of about 80% of powder at 50-75mu m and the particle diameter of the rest powder below 50mu m by weight after the raw material of the pioglitazone hydrochloride is pulverized and selecting the recipe, the invention overcomes the low original dissolution rate of the pioglitazone hydrochloride in the original compound preparation and provides a compound pioglitazone hydrochloride and dimethyl biguanide preparation with higher original dissolution rate.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST +1
Method for preparation of high purity and high yield metformin hydrochloride by two-component solvent
ActiveCN104829495AHigh purityGood crystal shapeOrganic compound preparationAmino compound preparationMetformin hclMetformin hydrochloride product
The invention discloses a method for preparation of high purity and high yield metformin hydrochloride by a two-component solvent. The method includes: preparation of dimethylamine hydrochloride and preparation of metformin hydrochloride. During preparation of dimethylamine hydrochloride, dimethylamine gas and 31% hydrochloric acid are adopted as the raw materials to carry out reaction, through co-cooling of a cooler and a reaction kettle jacket, pumping circulating, spraying absorption of dimethylamine tail gas and absorption of dimethylamine tail gas by hydrochloric acid, the utilization rate of the raw materials is maximumly ensured, the loss of raw materials is reduced, and the product yield is improved. During preparation of metformin hydrochloride, dimethylformamide and ethylene glycol monopropyl ether two components are used as the solvent, the synergistic effect of the two solvents with different natures are utilized, and the prepared metformin hydrochloride product has yield of over 95% and purity of more than 99.90%.
Owner:山西津华晖星制药有限公司
Method for preparing fluorescent carbon quantum dots based on metformin as precursor
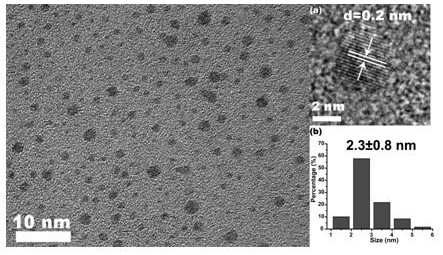
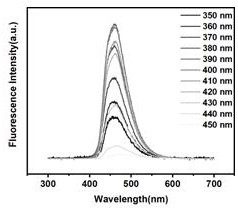
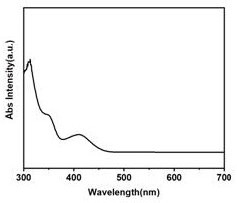
InactiveCN111607393ALarge scale preparationEnhanced fluorescence emission intensityNanoopticsNano-carbonBenzoic acidNatural organic matter
The invention relates to a method for preparing fluorescent carbon quantum dots by taking metformin as a precursor. The method comprises the following steps: step 1, carrying out hydrothermal reactionon metformin hydrochloride and dithiodibenzoic acid to generate a carbon quantum dot stock solution; step 2, filtering out impurities from the carbon quantum dot stock solution to form filtrate; step3, after the filtrate is subjected to heating and magnetic stirring treatment, attaching carbon quantum dots in the filtrate to a first filter membrane through the first filter membrane; and step 4,drying the first filter membrane to obtain carbon quantum dot powder. The purity of the final product is higher than that of fluorescent carbon dots with natural organic matter as a carbon source, thereaction process and structure can be analyzed, no toxic reagent is added, the operation method is simple and convenient, and finally the fluorescent carbon quantum dots with high fluorescence emission intensity can be prepared on a large scale.
Owner:NORTHEAST NORMAL UNIVERSITY
Solid compound preparation containing metformin hydrochloride and glimepiride, preparation method and application thereof
ActiveCN103505466AHigh dissolution rateImprove stabilityMetabolism disorderSulfonylurea active ingredientsWater insolubleFiller Excipient
The invention provides a solid compound preparation containing metformin hydrochloride and glimepiride. The solid compound preparation is prepared from metformin hydrochloride granules and glimepiride granules. The metformin hydrochloride granules contain metformin hydrochloride and an adhesive, and the glimepiride granules contain glimepiride, a water soluble filler, a disintegrating agent and an adhesive. The solid compound preparation does not contain water insoluble filler. The invention also provides a preparation method and application of the solid compound preparation. Compared with common preparations and preparations adopting a water insoluble filler, the solid compound preparation provided by the invention can significantly improve the dissolution rate of glimepiride, and can effectively reduce interaction of the two main drugs, thereby reducing the increase of related impurities in a placement process. The product has stable quality and good uniformity, so that the effectiveness and safety of drug use by patients can be improved.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
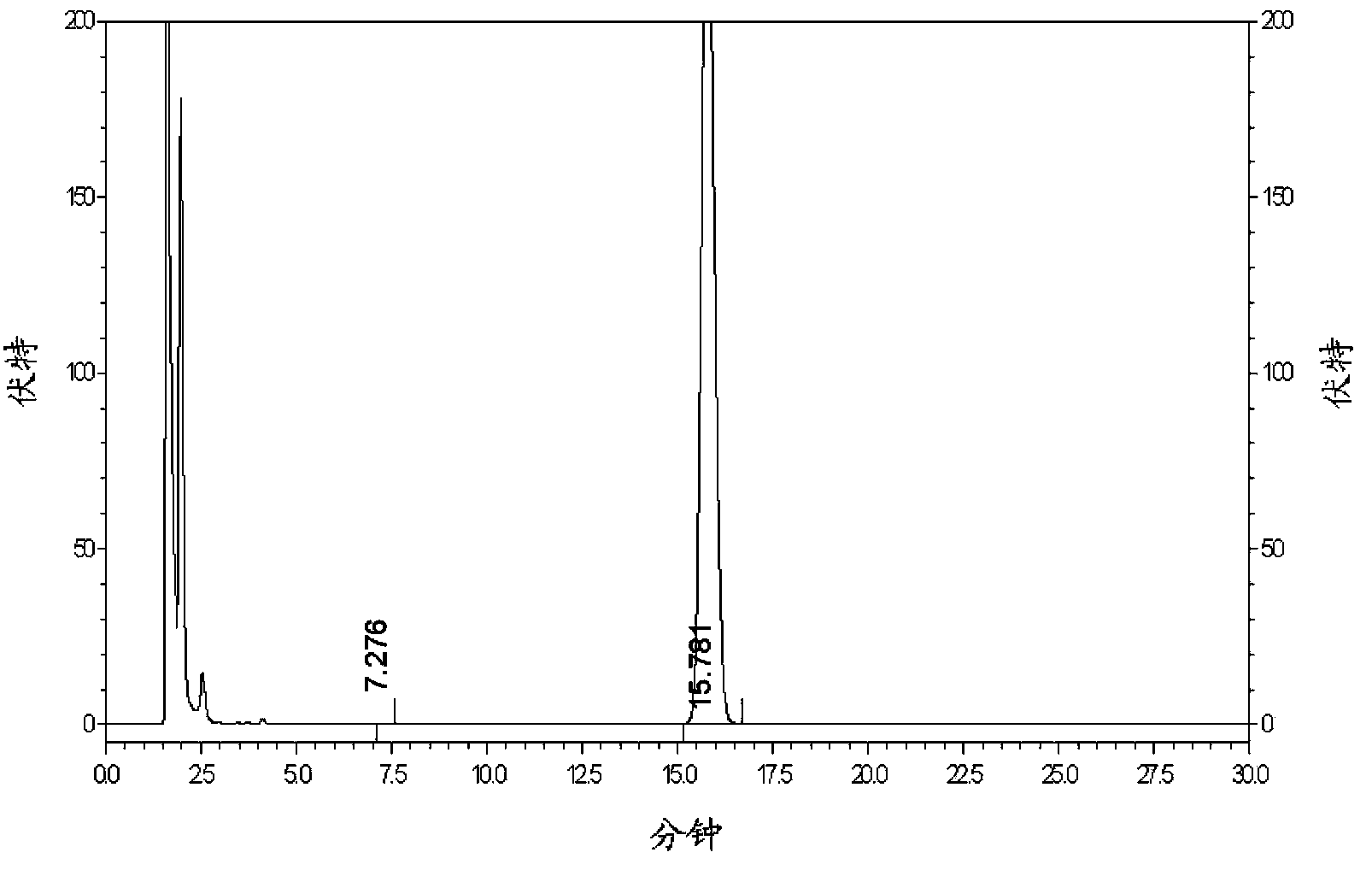
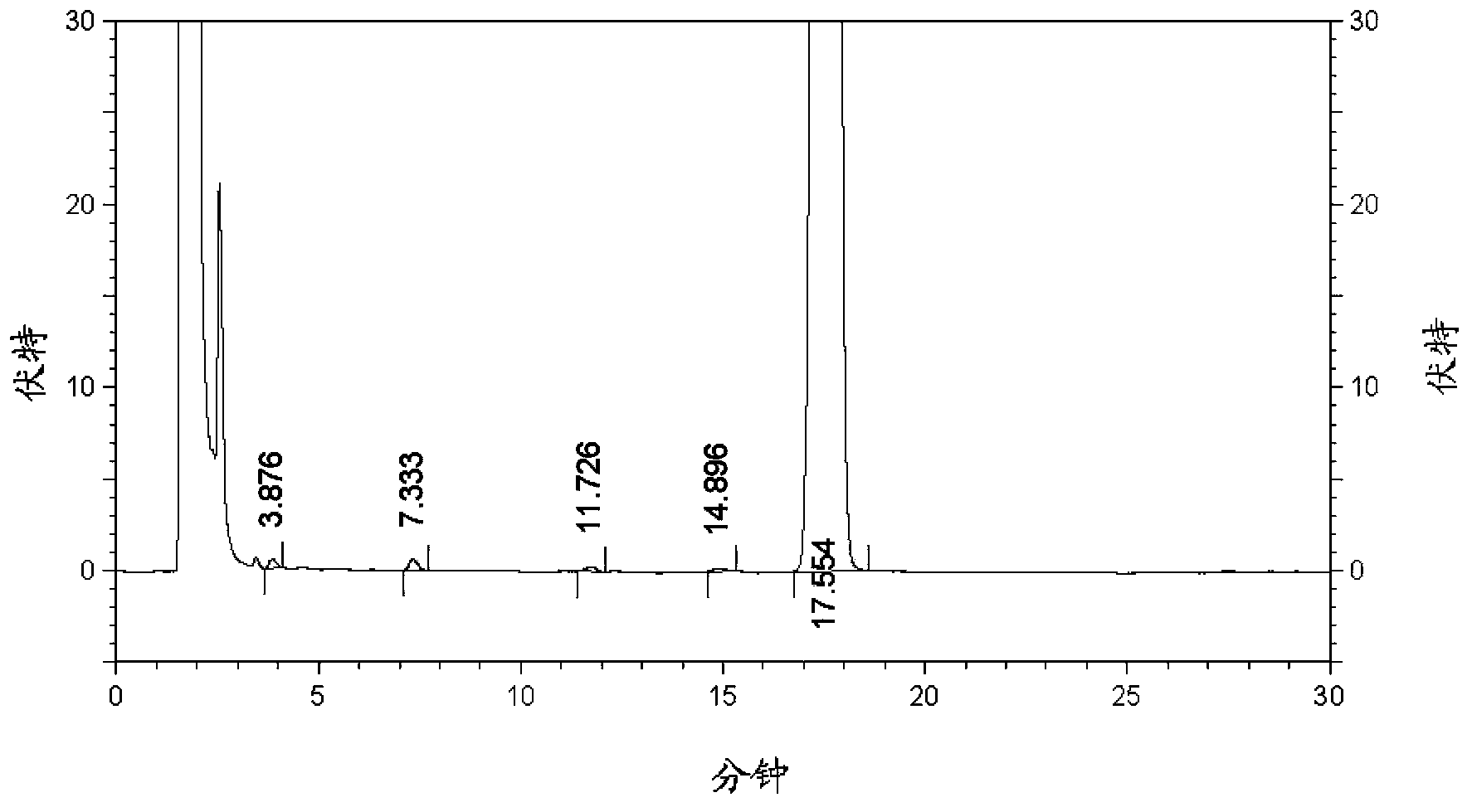
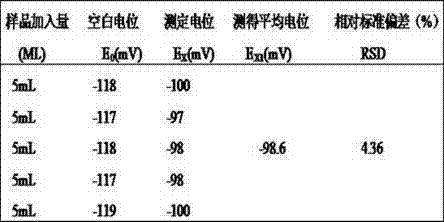
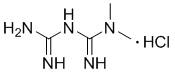
New metformin hydrochloride detection method
The present invention provides a new method for determining metformin hydrochloride content by using an ion selective electrode method. According to the present invention, a pCL-1Q9 type chloride ion selective electrode is adopted as a work electrode, a 217 type saturated calomel electrode is adopted as a reference electrode, a stirring rate is 120 rpm / min, a stirring time is 3 min, the pH value is 4-6, a temperature is 18 DEG C, a buffer solvent is a total ionic strength adjustment buffer solution comprising potassium nitrate and sodium citrate, and a standard curve method is adopted as the method for quantitative analysis of the drug metformin hydrochloride effective component content; and compared with the traditional method, the method of the present invention has characteristics of simple experiment equipment, low test cost, simpleness, rapidness, accurate determination result, wide linear range and high sensitivity, and is the easiest and feasible analysis method for determining the metformin hydrochloride content.
Owner:大连杰信生物科技有限公司
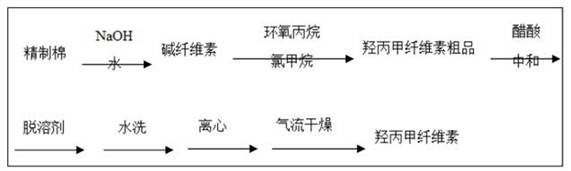
Method for controlling genotoxic impurities in metformin hydrochloride sustained release tablet preparation process
InactiveCN113081990ASimple control methodPrecise control methodOrganic active ingredientsMetabolism disorderNitrosoMetformin hcl
The invention relates to the technical field of preparation processes, in particular to a method for controlling genotoxic impurities in a metformin hydrochloride sustained release tablet preparation process, which comprises the following steps of: controlling the content of impurity dimethylamine in a raw material medicine metformin hydrochloride and the content of impurity nitrite in an auxiliary material hydroxypropyl methylcellulose; effective control of the genotoxic impurity N-nitrosodimethylamine is achieved, side reactions of medication of patients are reduced, and medication safety of the patients is guaranteed to a certain extent; the content of the genetic toxic impurity N-nitrosodimethylamine of the metformin hydrochloride sustained-release tablet is far lower than that of the State Food and Drug Administration and an acceptable limit specified by FDA (Food and Drug Administration).
Owner:SHANDONG INST FOR FOOD & DRUG CONTROL +1
Medicine composition for treatment of diabetes, and preparation method and application thereof
InactiveCN109528706ABirth controlEasy to take medicineOrganic active ingredientsMetabolism disorderSGLT2 InhibitorDrug
The invention relates to a medicine composition for treatment of diabetes, which includes dapagliflozin and metformin hydrochloride according to mass ratio of 1:50 to 1:200. The invention also provides a preparation method and an application of the medicine composition of dapagliflozin and metformin hydrochloride. The medicine composition is composed of, in specific ratio, the SGLT2 inhibitor dapagliflozin and the metformin hydrochloride, so that the medicine composition can control the generation rate of blood glucose more effectively in order to synergistically treat the diabetes. The medicine composition brings convenience to patients and improves compliance. In the method, by control the particle sizes of both the metformin hydrochloride particles and the dapagliflozin, mixing uniformity of the two active components is guaranteed, and flowability and tabletability of the particles are ensured. The method is suitable for industrial large-scale production. The medicine composition can be used for treatment on the diabetes type II.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Novel metformin hydrochloride slow-releasing tablet and preparation method thereof
ActiveCN102119931BReduce dosageControl smooth releaseOrganic active ingredientsMetabolism disorderExtended release tabletsMetformin hcl
The invention provides a novel metformin hydrochloride slow-releasing tablet and a preparation method thereof. The slow-releasing tablet comprises the following components by weight percent: 66.6%-73.5% of metformin hydrochloride, 30.9%-21.5% chitosan, 2.0%-4.0% of copolymerization and 0.5%-1.0% of superfine silica powder. The dry granulating technique is adopted in the preparation method and the preparation method comprises the following steps: uniformly mixing the metformin hydrochloride, chitosan, copolymerization and superfine silica powder in proportion according to the prescription, granulating by using a dry granulating machine, adding superfine silica powder and uniformly mixing, and then pressing the mixture into tablet, thereby acquiring the novel metformin hydrochloride slow-releasing tablet.
Owner:SHOUGUANG FUKANG PHARMA
Preparation method of metformin hydrochloride
ActiveCN103435518BHigh recovery rateImprove securityOrganic chemistryOrganic compound preparationMetformin hclMetforminum
Owner:QINGDAO HUANGHAI PHARM CO LTD
Anti-breast cancer powder containing metformin hydrochloride and gdc 0941
InactiveCN105147695ASignificant antitumor activitySmall side effectsOrganic active ingredientsPowder deliveryMetformin hclPharmaceutical medicine
The invention discloses anti-breast cancer powder containing metformin hydrochloride and gdc0941. Active components of the anti-breast cancer powder are the metformin hydrochloride and gdc 0941. The anti-breast cancer powder further comprises pharmaceutically acceptable auxiliary materials. The anti-breast cancer powder is simple to prepare, easy to operate, lower in cost and economical and practical; the anti-breast cancer powder disperses easily and takes effect fast, and dosage of the powder is easily controllable; in-vitro antitumor tests show that the combination of the metformin hydrochloride and gdc 0941 provides higher anti-cancer activity and the powder is worthy of clinical popularization.
Owner:李荣勤
Sitagliptin metformin tablet preparation and preparation method thereof
PendingCN114306267AGood content uniformityReduce usageOrganic active ingredientsMetabolism disorderMetformin hclHot melt
The invention relates to the technical field of pharmaceutical preparations, and discloses a sitagliptin metformin tablet preparation and a preparation method thereof. The preparation is prepared from metformin hydrochloride, sitagliptin phosphate monohydrate, an adhesive and a lubricant through a hot melt extrusion method. According to the invention, a hot melt extrusion technology is adopted, metformin hydrochloride and an adhesive are mixed, the mixture is melted, extruded and conveyed in a hot melt extruder to obtain solid dispersion strips, the solid dispersion strips are cut off, crushed and sieved, the sieved solid dispersion strips are mixed with sitagliptin phosphate monohydrate, and then a lubricant is added for mixing, so that prepared particles are uniform and have better fluidity and good compressibility; in the production process, few auxiliary materials are needed, the production cost is reduced, the production procedures are reduced, the content and content uniformity of the obtained product far exceed the product quality requirements, the reproducibility is good, continuous operation can be achieved, continuous production is achieved, and the difference between batches is reduced.
Owner:HYBIO PHARMA
Peroral solid preparation of metformin hydrochloride and preparation method
InactiveCN1795846AUniform appearanceHigh mechanical strengthOrganic active ingredientsMetabolism disorderDiabetes mellitusAdditive ingredient
Owner:RADIOLOGY INST ACAD OF MILITARY MEDICINE SCI PLA
A kind of method that the production solid waste of metformin hydrochloride is recycled
The invention relates to a method for recycling solid waste generated in metformin hydrochloride production. The method includes the following steps that firstly, a reaction solvent is added into metformin hydrochloride solid waste, heated, dissolved and reacted with fatty acid; secondly, solid-liquid separation is conducted through cooling, neutralization, sedimentation and suction filtration; thirdly, the solid serves as a melamine derivative and has a good flame-retardant property, filtrate contains a small quantity of pollutants, and pollution-free discharge is achieved basically. Compared with the prior art, recycling of the metformin hydrochloride solid waste can be achieved, emission of odor, waste liquid and liquid residues is reduced, the environment pollution risk is reduced, and clean production is achieved.
Owner:SHOUGUANG FUKANG PHARMA +5
Anti-breast cancer granules combining metformin hydrochloride and gdc 0941 and a preparation method thereof
InactiveCN105147696ASignificant antitumor activitySmall side effectsOrganic active ingredientsGranular deliveryMetformin hclPharmaceutical medicine
The invention discloses anti-breast cancer granules combining metformin hydrochloride and gdc 0941. Active components of the anti-breast cancer granules are metformin hydrochloride and gdc 0941. The anti-breast cancer granules further comprise pharmaceutically acceptable auxiliary materials. The anti-breast cancer granules are simple to prepare, easy to operate, lower in cost and economical and practical; the anti-breast cancer granules are portable, dissolve fast, are well absorbable and take effect fast. In-vitro antitumor tests show that anti-cancer activities of the metformin hydrochloride and gdc 0941 are synergistic, the combination of the metformin hydrochloride and gdc 0941 provides high anti-cancer activity, high cost of a single dose can be decreased, and the anti-breast cancer granules are worthy of clinical popularization.
Owner:李荣勤
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com