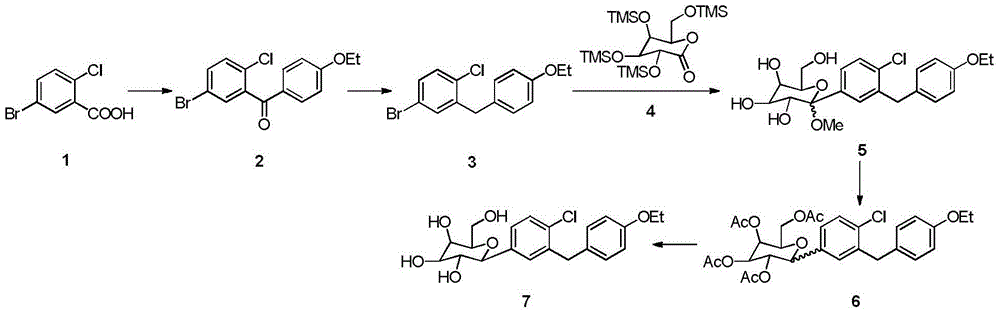
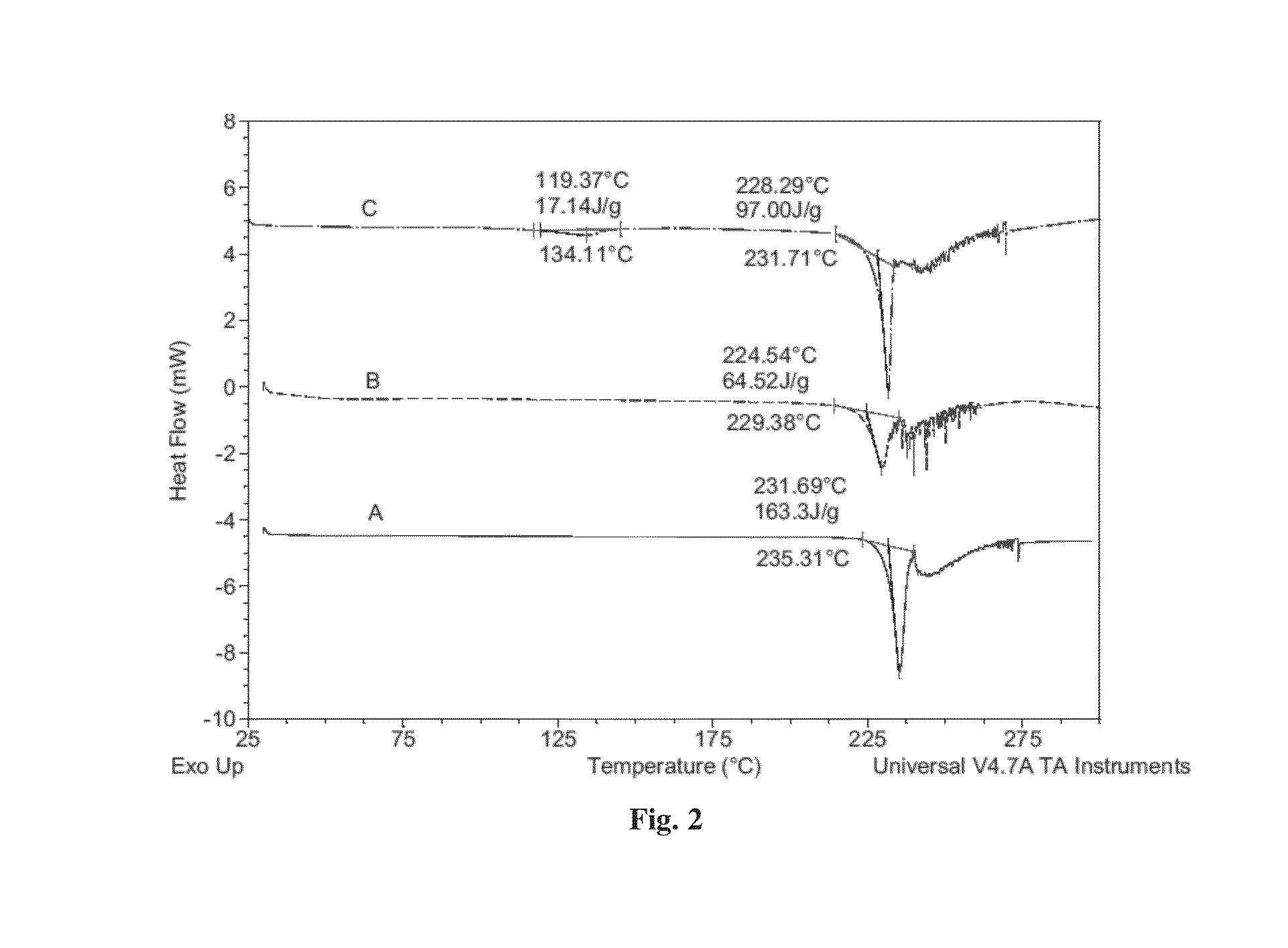
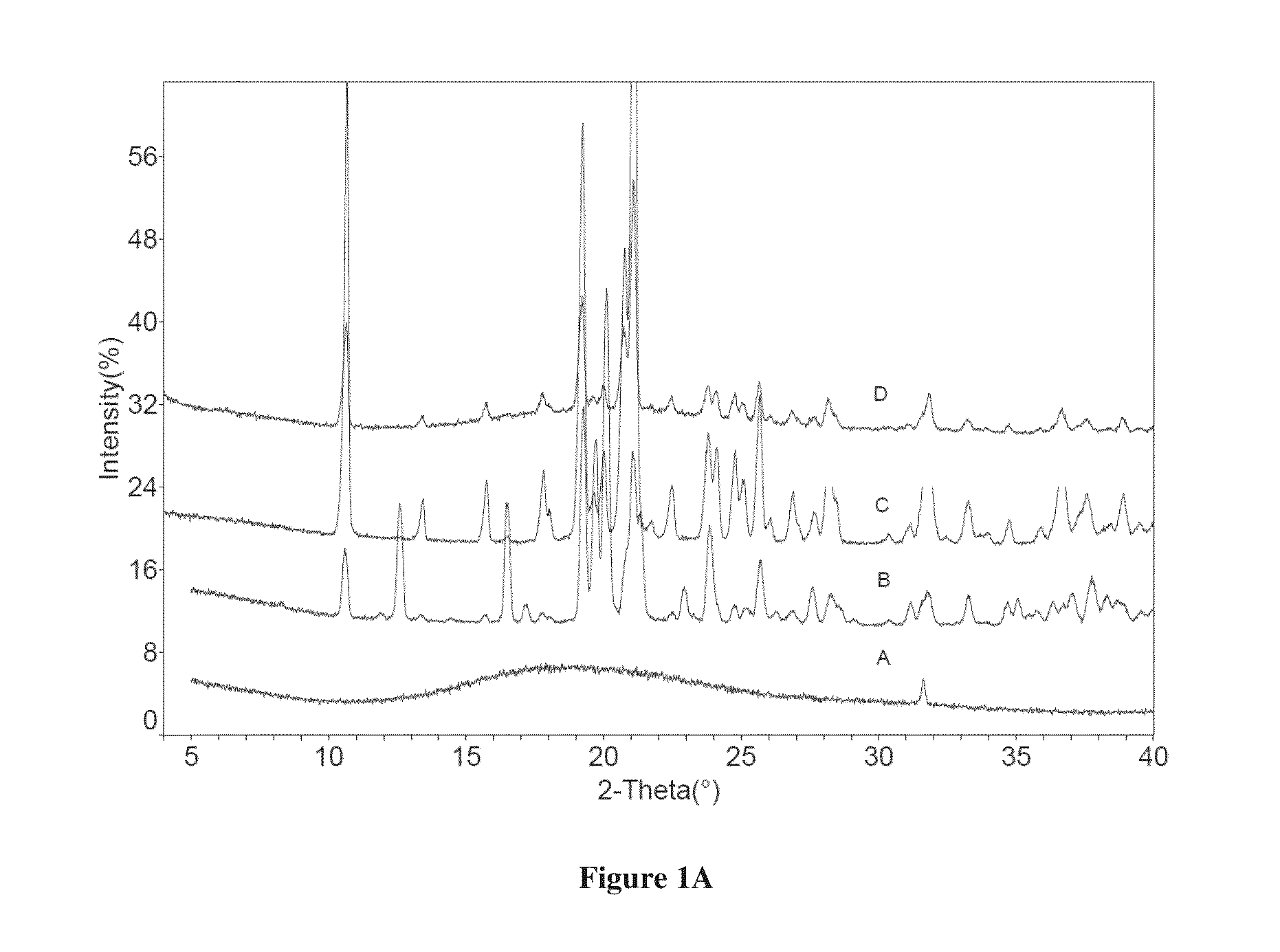
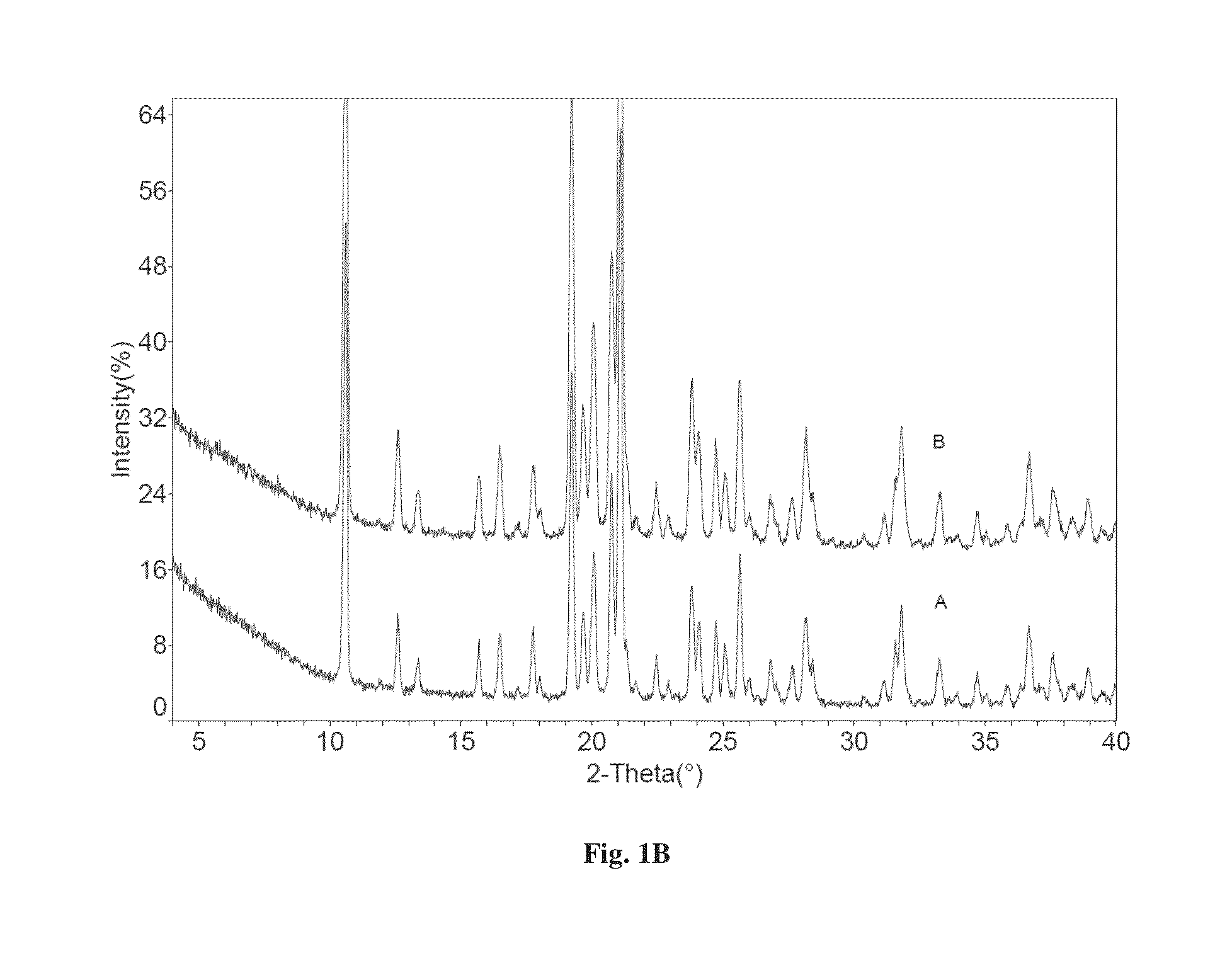
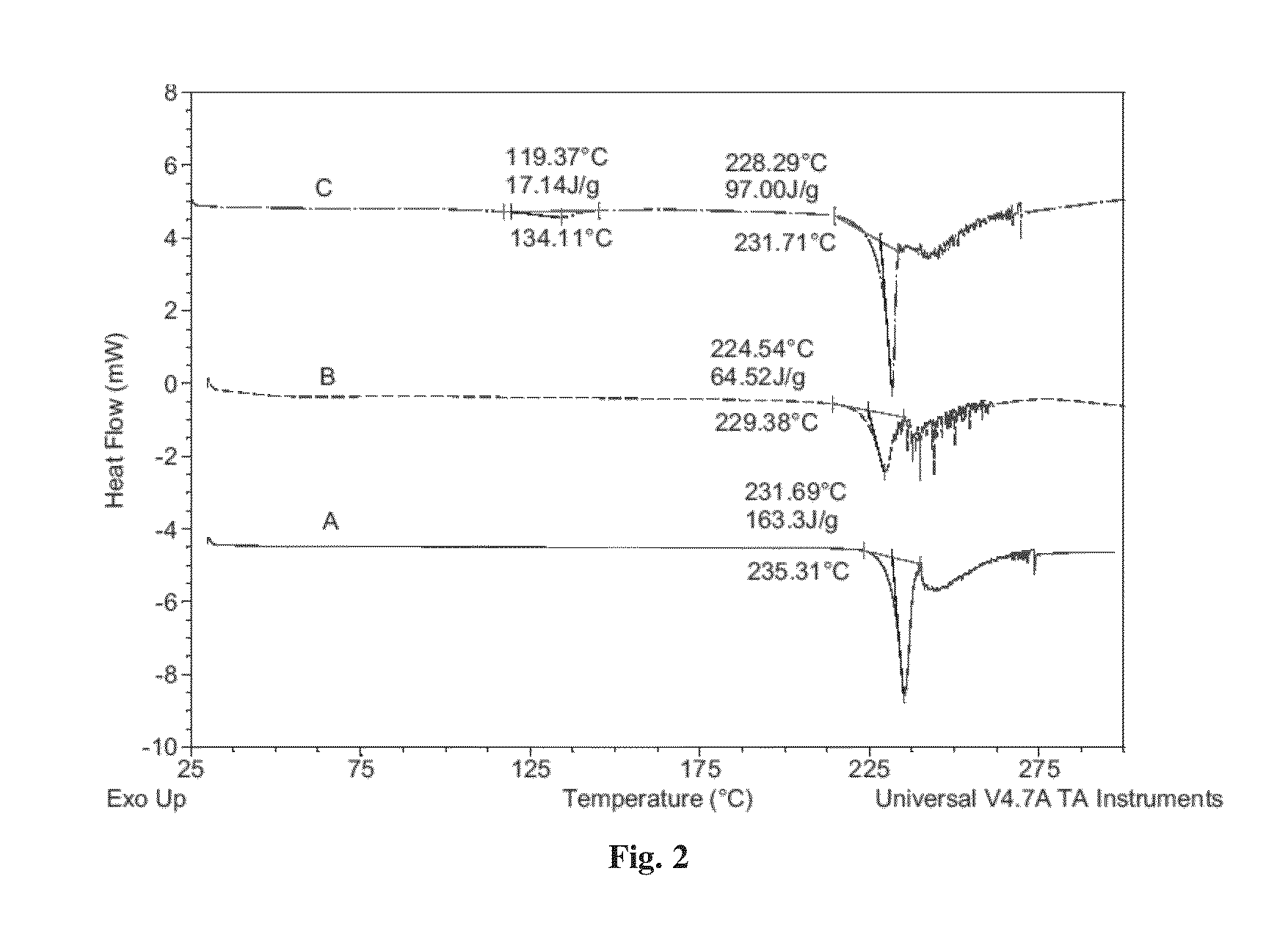
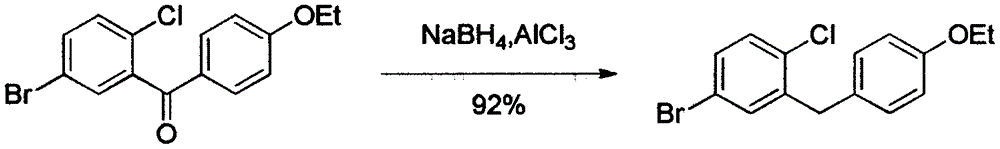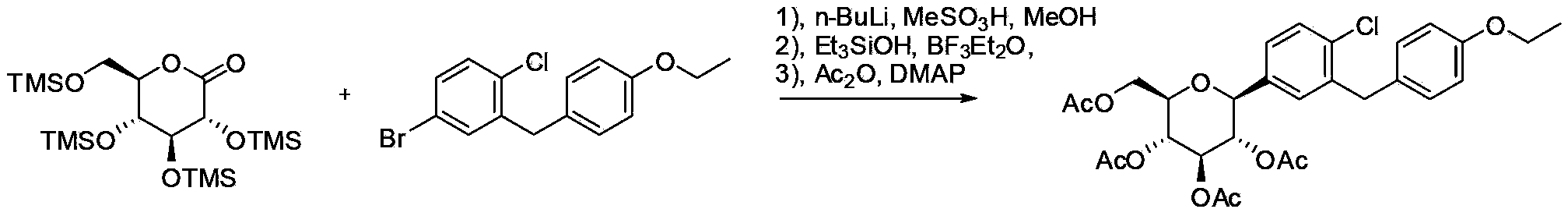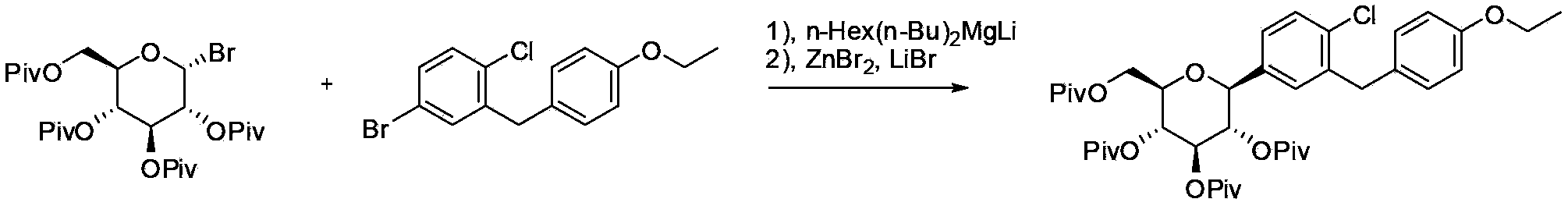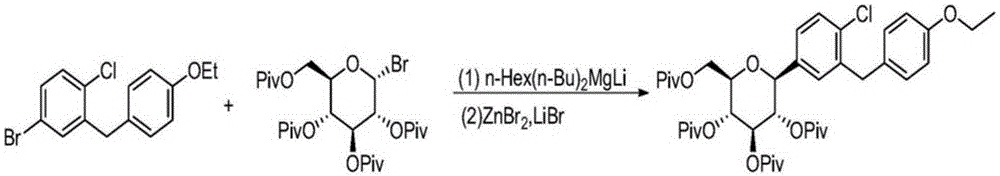Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
118 results about "Dapagliflozin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
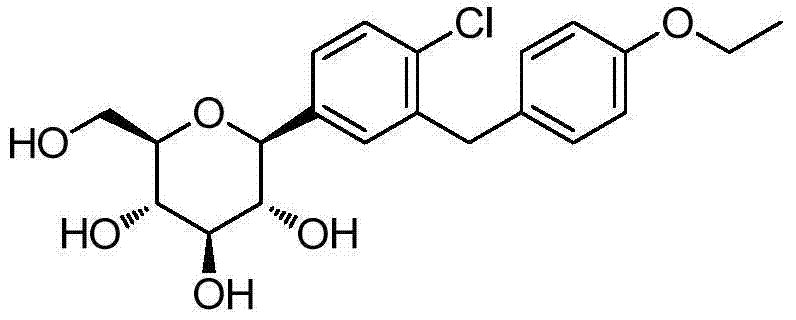
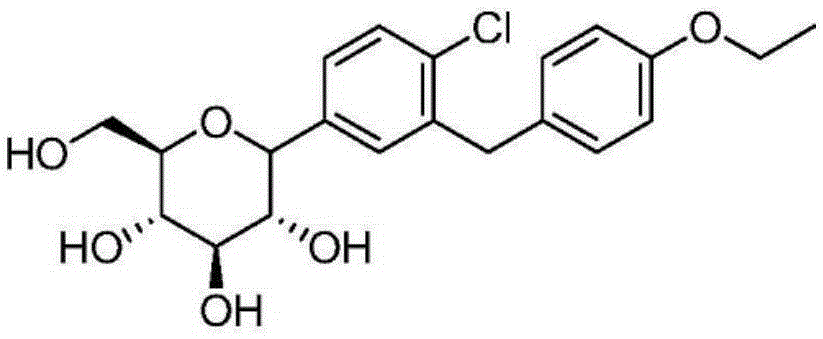
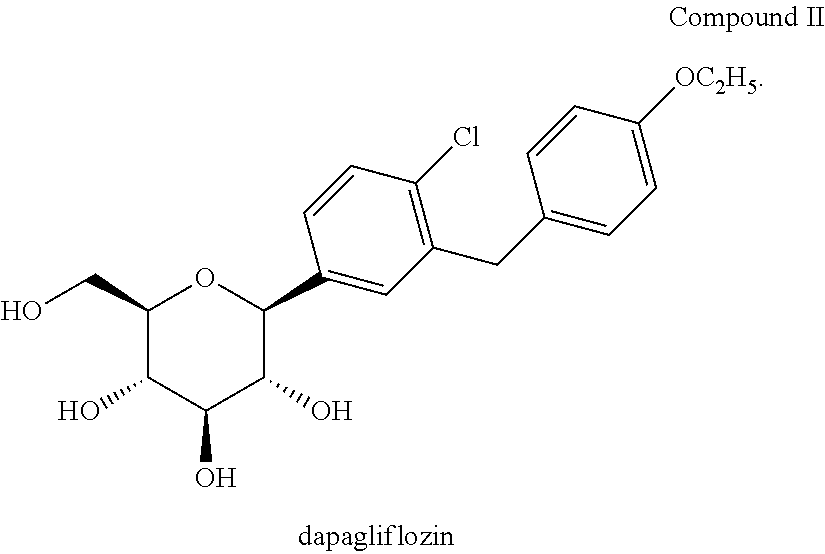
Dapagliflozin is used with a proper diet and exercise program to control high blood sugar in people with type 2 diabetes.
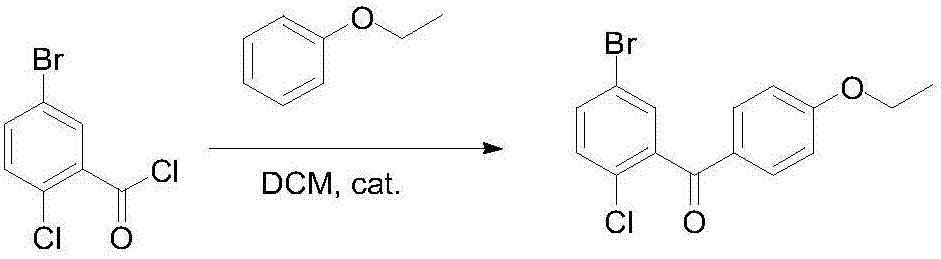
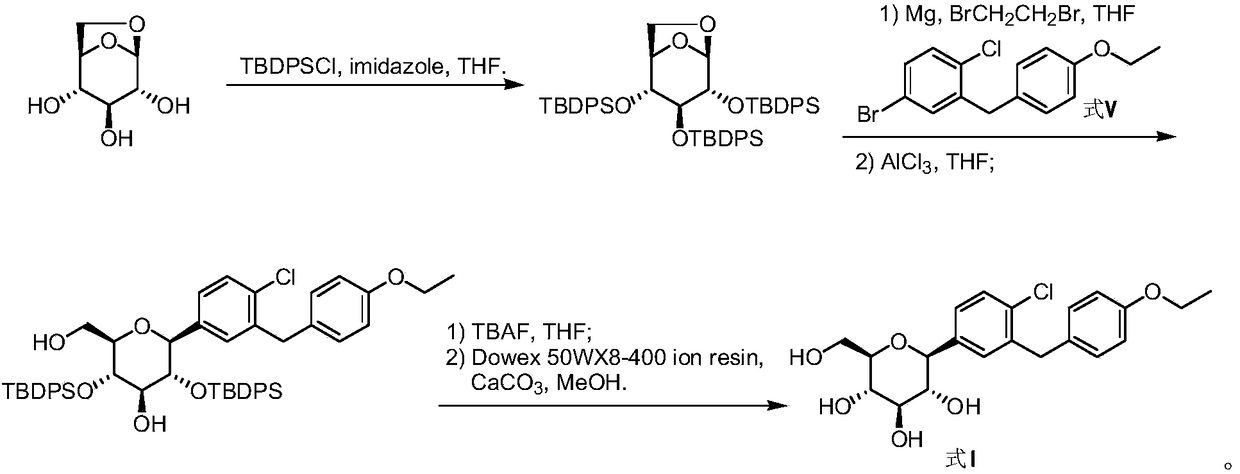
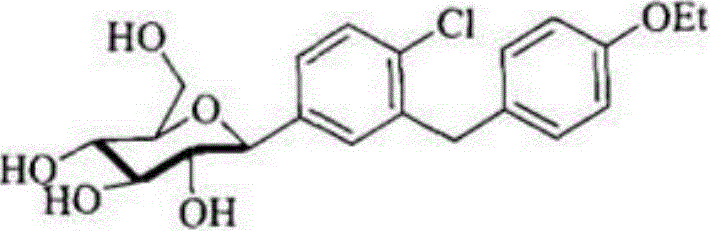
Preparation method of 5-bromine-2-chlorine-4'-ethyoxyl diphenylmethane
InactiveCN104478670ALow water requirementMild reaction conditionsOrganic chemistryOrganic compound preparationDiphenylmethaneAlkyl transfer
The invention relates to the chemical field and particularly relates to a novel synthesis method for preparing a key intermediate 5-bromine-2-chlorine-4'-ethyoxyl diphenylmethane of a drug dapagliflozin for treating diabetes mellitus II. The preparation method comprises the following steps: enabling a starting raw material ortho-toluidine to firstly perform bromization and then perform chlorination after diazotization on a benzene ring with N-bromo-succinimide; then, in the presence of a halogenating agent, performing halogenating reaction of beta-position; and finally, performing Friedel-Crafts alkylation synthesis with phenetole, thereby obtaining the key intermediate. The preparation method is simple and convenient, economical and relatively high in reaction yield in each step, and suitable for industrial production.
Owner:CHINA PHARM UNIV
Eutectic preparation method of sodium-glucose cotransporter 2 bulk pharmaceutical chemicals
InactiveCN102167715AFew reaction stepsHigh selectivitySaccharide with carbocyclic radicalsSugar derivativesGadolinium ethoxybenzyl DTPATriol
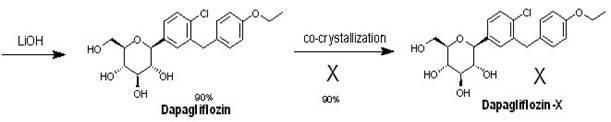
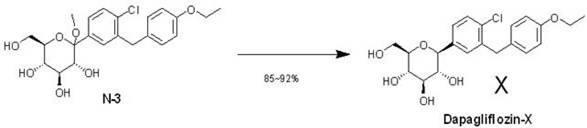
The invention relates to a preparation method of bulk pharmaceutical chemicals used for curing the type 2 diabete, in particular to a eutectic preparation method of sodium-glucose cotransporter 2 bulk pharmaceutical chemicals. In the invention, the technical problems of the existing preparation method that reaction steps are more, the methoxy-removing condition is strict and the yield of the obtained main product with beta configuration is low are solved. The technical scheme in the invention is as follows: the eutectic preparation comprises the following steps: adding chiral component (X) inN-3((3R,4S,5S,6R)-2-(4-chloro-3-(4-ethoxylbenzyl)phenyl)-6-(hydroxymethyl)-2-methoxytetrahydro-2H-pyran-3,4,5-triol, performing selective complexation with reductant to remove methoxy, then reducing the temperature to perform eutectic preparation, and adopting the one-pot method to obtain dapagliflozin-X. The eutectic preparation method is mainly used to prepare the sodium-glucose cotransporter 2bulk pharmaceutical chemicals.
Owner:SHANGHAI HUISI BIOTECH
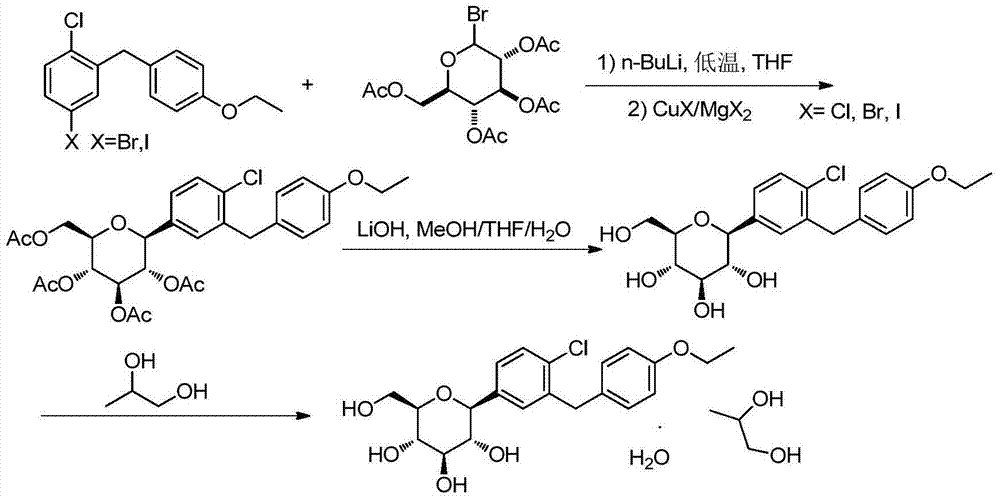
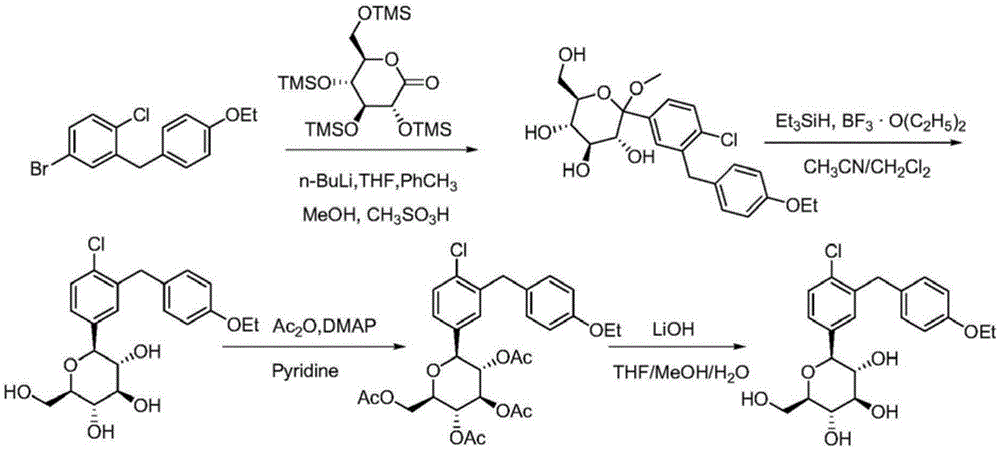
Synthesis method of dapagliflozin
InactiveCN104496952ASimple and fast operationEasy to purifyOrganic chemistrySynthesis methodsGrignard reagent
The invention relates to a synthesis method of dapagliflozin. The halogeno-benzene derivative and 2,3,4,6-tetraacetyloxy-alpha-D-glucopyranose bromide are used as the raw materials, thereby saving the reduction reaction and acetylation reaction in the original method, shortening the reaction processing steps and enhancing the total yield. The phenyl lithium reagent is prepared into the copper lithium reagent or Grignard reagent intermediate with mild reaction conditions to reduce the generation of the byproduct, so that the reaction temperature is controlled at -10 DEG C below.
Owner:HYBIO PHARMA
Preparation methods of SGLT-2 diabetes inhibitors and intermediates thereof
ActiveCN107163092AAvoid it happening againLower reaction yieldSugar derivativesSugar derivatives preparationSynthesis methodsCombinatorial chemistry
The invention provides a preparation method of an intermediate compound 7 of an SGLT-2 diabetes inhibitor dapagliflozin and an intermediate compound 8 of a SGLT-2 diabetes inhibitor empagliflozin, and new synthesis method of two final products. The preparation method comprises the following steps: carrying out carbonyl group reduction and hydroxyl group protection on a (5-halo-2-chlorophenyl)(4-ethoxyphenyl)ketone compound 1 used as an initial raw material to obtain a Grignard addition reaction key compound 4, and carrying out Grignard addition and acetylation to obtain the compound 7 and the compound 8. The dapagliflozin and the empagliflozin are respectively prepared from the compound 8. The methods have the advantages of simplicity in operation, high yield, high purity of the obtained products, and suitableness for amplified production.
Owner:山东科巢生物制药有限公司
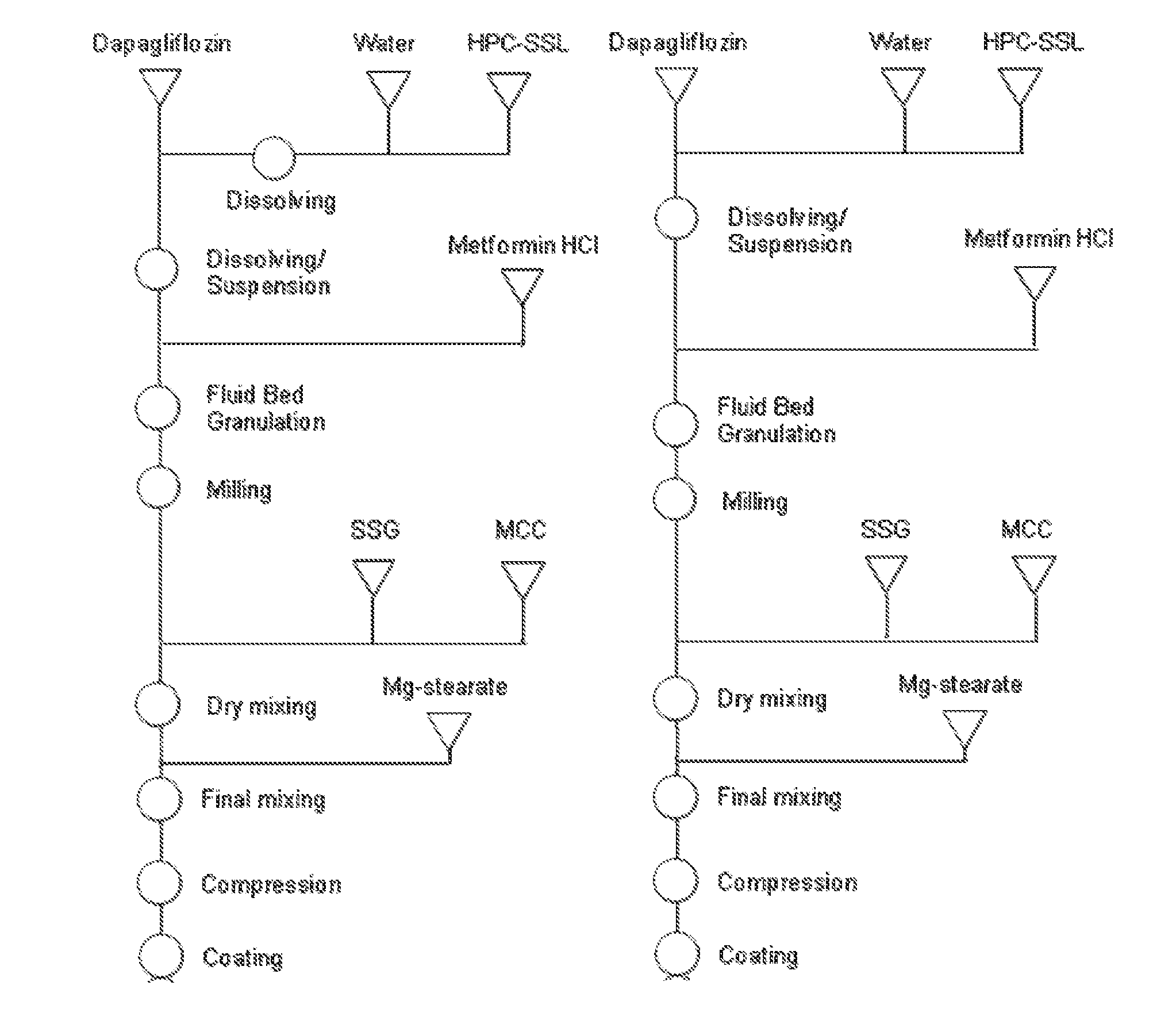
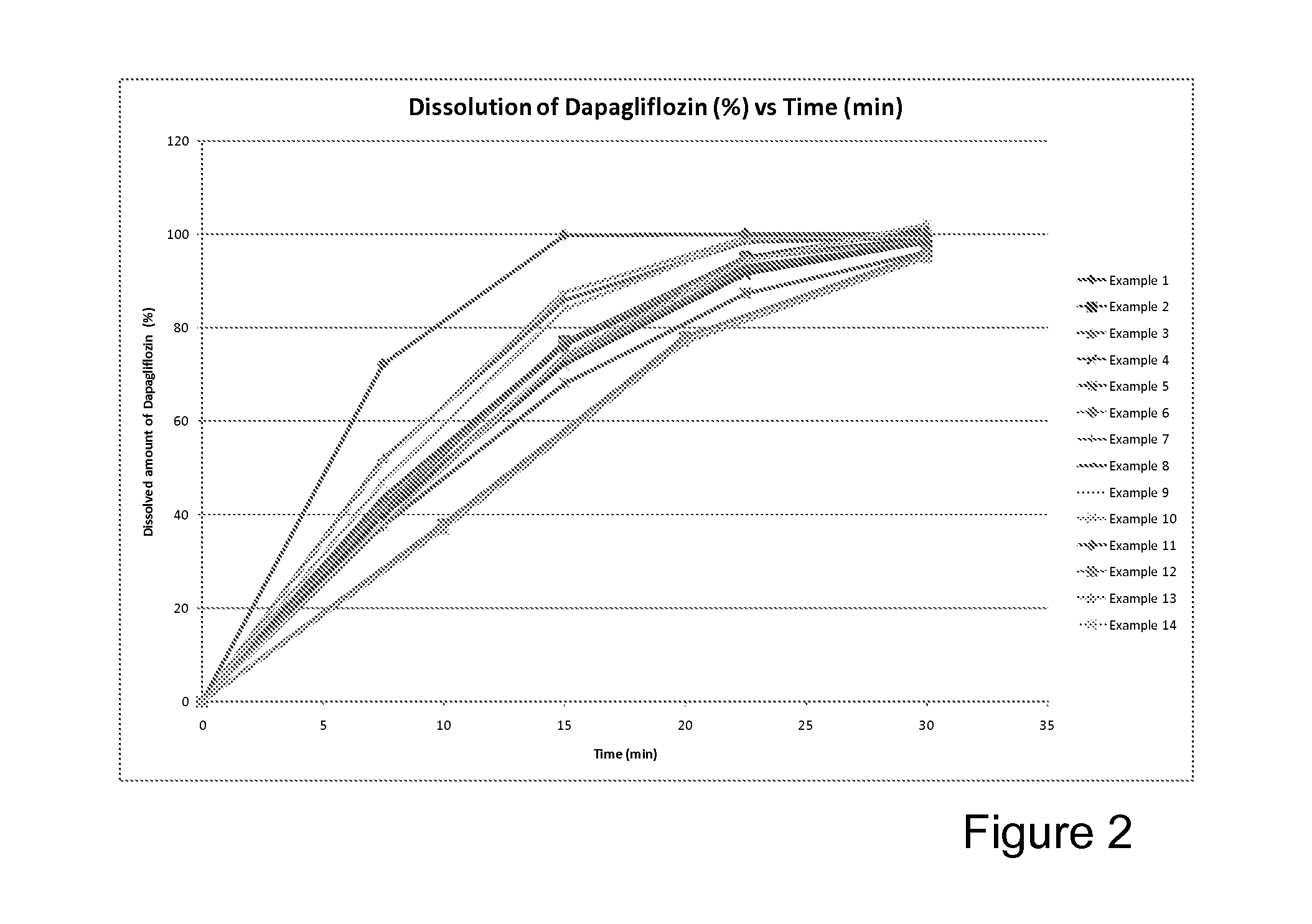
Immediate release tablet formulations
InactiveUS20130034606A1Increased blood levelsImprove the level ofBiocideSenses disorderDiseaseSodium dependent
The present invention provides an immediate release pharmaceutical formulation which includes a tablet or capsule formulation comprising metformin and the sodium dependent glucose transporter (SGLT2) inhibitor dapagliflozin or its propylene glycol hydrate. The present invention also provides methods of preparing the formulations and methods of treating diseases or disorders associated with SGLT2 activity employing these formulations.
Owner:ASTRAZENECA AB
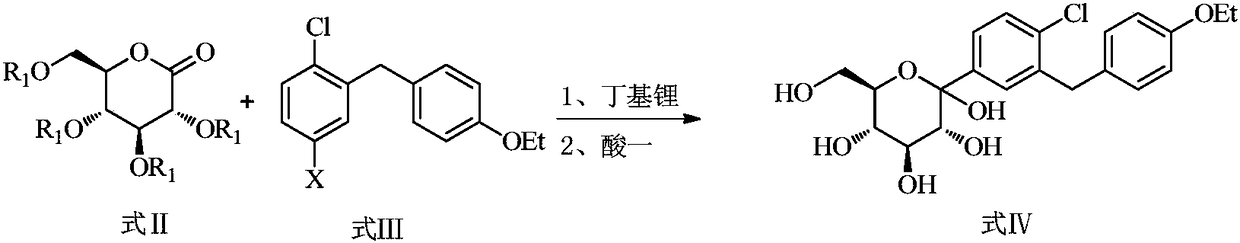
Preparation method of antidiabetic dapagliflozin intermediate
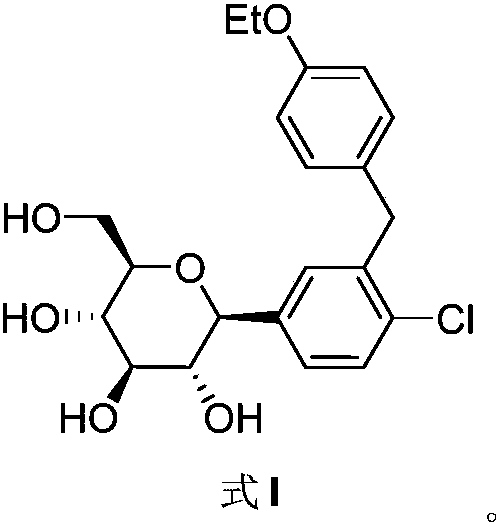
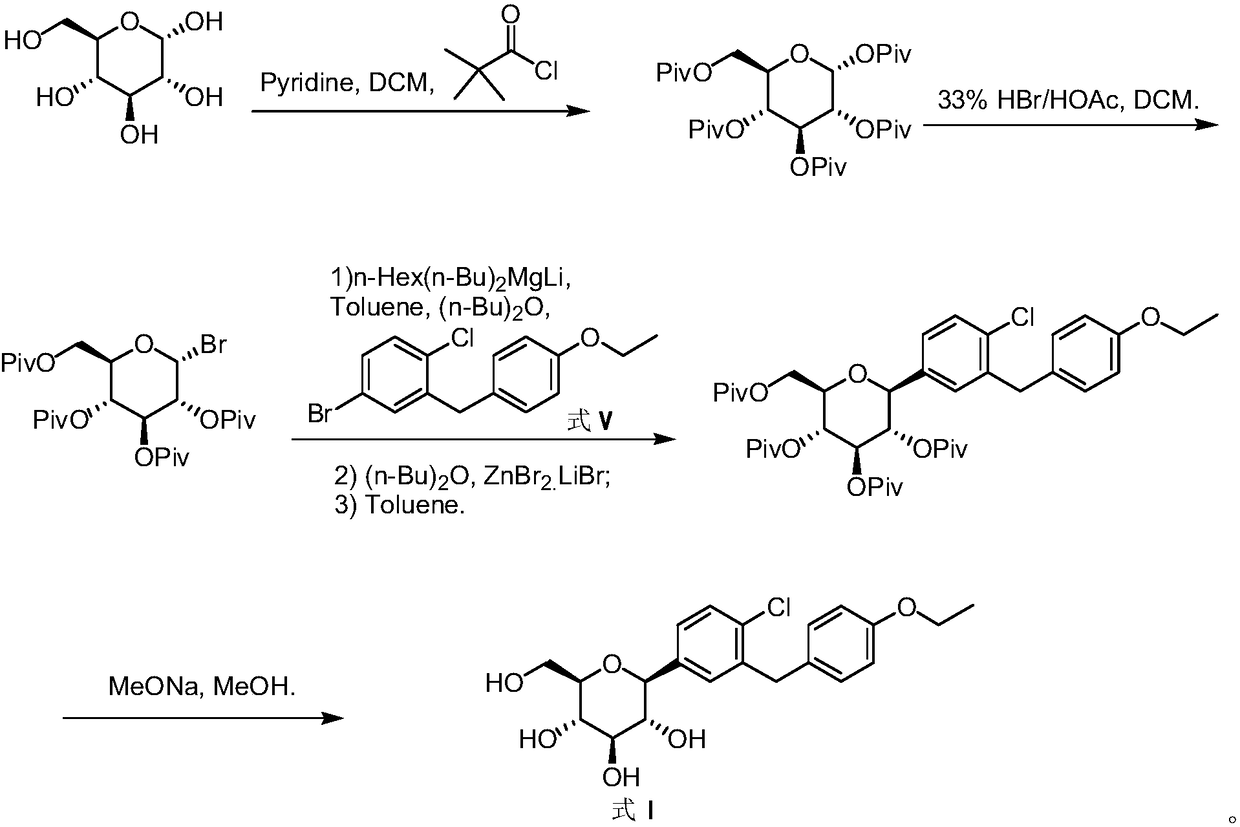
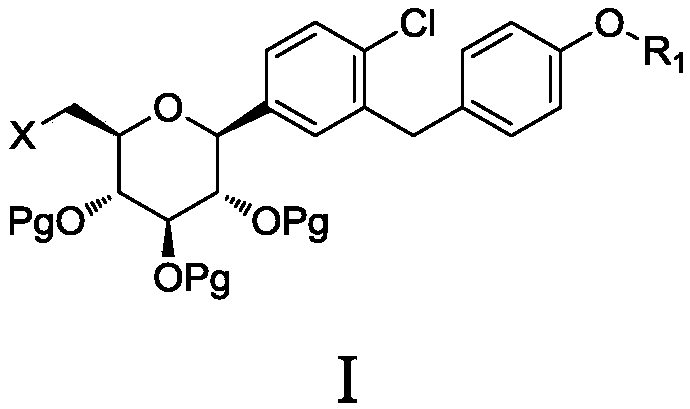
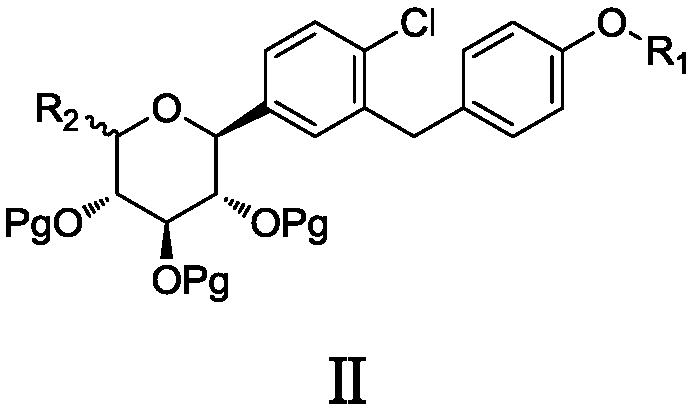
The invention discloses a preparation method of antidiabetic dapagliflozin intermediate, hydroxyl protected ALPHA-D-bromo-glucopyranose as shown in formula II is used as a starting material for reacting with a Grignard reagent as shown in formula III, and the reaction is carried out in the presence of a cobalt catalyst and a ligand to prepare a dapagliflozin senior intermediate as shown in formula I. The raw material and reagents required in the preparation method are relatively cheap in price, and the preparation method has the advantages of simple operation, low cost, safe and convenient operation, good yield, less environmental pollution, and very good economic effect and is suitable for industrial production.
Owner:SHANGHAI FANGNAN PHARMA
Synthesis method of dapagliflozin
InactiveCN104478839AEasy to operateMild reaction conditionsOrganic chemistrySynthesis methodsDapagliflozin
The invention provides a synthesis method of dapagliflozin. A synthesis route of the synthesis method is shown as the following formulae which are as shown in the specification. The synthesis method of dapagliflozin comprises the following steps: (1) carrying out friedel-crafts acylation on a raw material 1 to generate a compound 2; (2) carrying out reduction reaction on the compound 2 to generate a compound 3; (3) carrying out condensation reaction on the compound 3 and a compound 4 to generate a compound 5; (4) removing methoxy of the compound 5 to generate a compound 6; and (5) carrying out deprotection on the compound (6) to generate a compound 7. The synthesis method of dapagliflozin at least has the following beneficial effects of being simple in operation steps and mild in reaction condition; the dapagliflozin is easy to extract and separate and high in yield; the production cost is greatly reduced; the industrial production is facilitated.
Owner:SUZHOU JONATHAN NEW MATERIALS TECH
New C-aryl glycosidase SGLT2 (sodium glucose transporter type-2) inhibitor
ActiveCN104327027AEasy dischargeSGLT2 inhibitory activity is goodOrganic active ingredientsOrganic chemistryArylSGLT2 Inhibitor
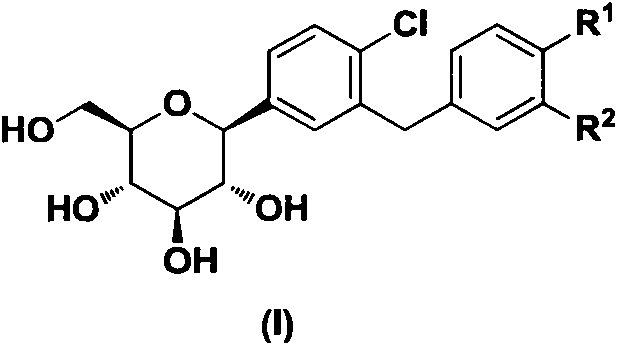
The invention discloses a new C-aryl glycosidase SGLT2 (sodium glucose transporter type-2) inhibitor, relates to the field of medicines associated with diabetes, and in particular relates to a new C-aryl glycosidase derivative shown as the general formula (I), a preparation method thereof, compositions thereof, and use thereof in preparing hypoglycemic drugs. The C-aryl glucoside derivative has excellent effect of promoting urine discharge and in-vivo hypoglycemic activity, and compounds with equal or better in-vivo hypoglycemic activity than that of dapagliflozin are screened out, and can be used for the prevention or treatment of diabetes.
Owner:CHINA PHARM UNIV
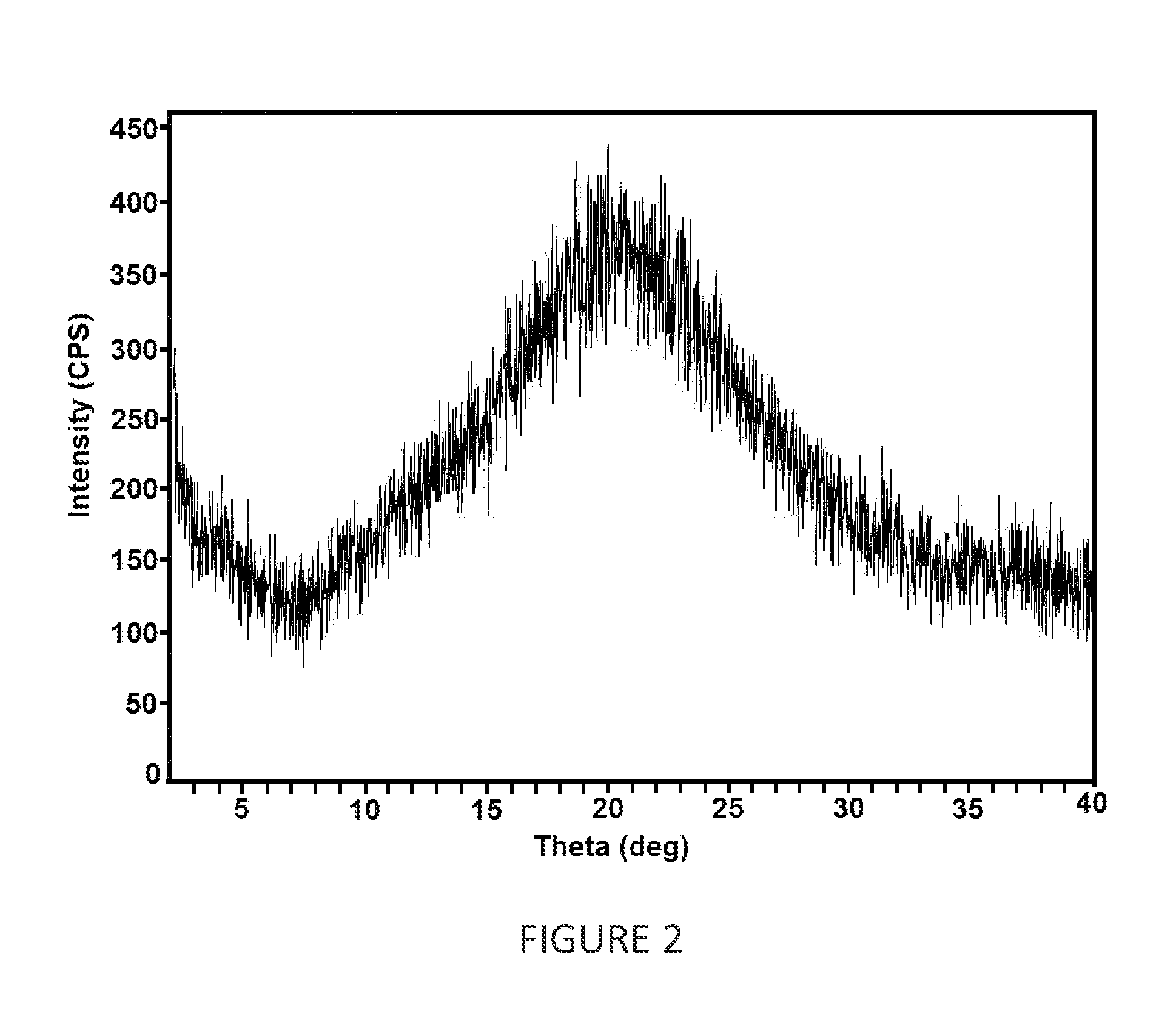
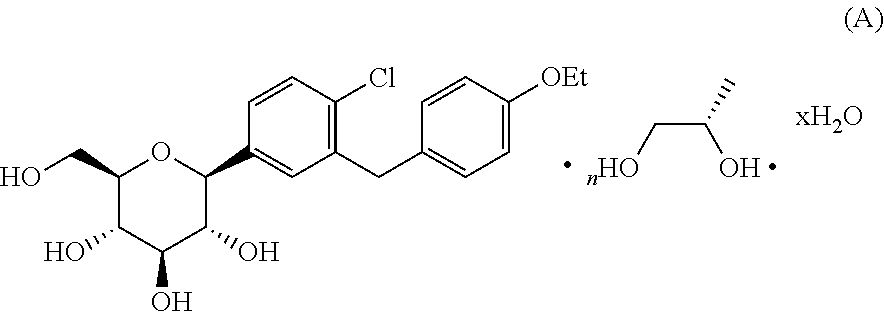
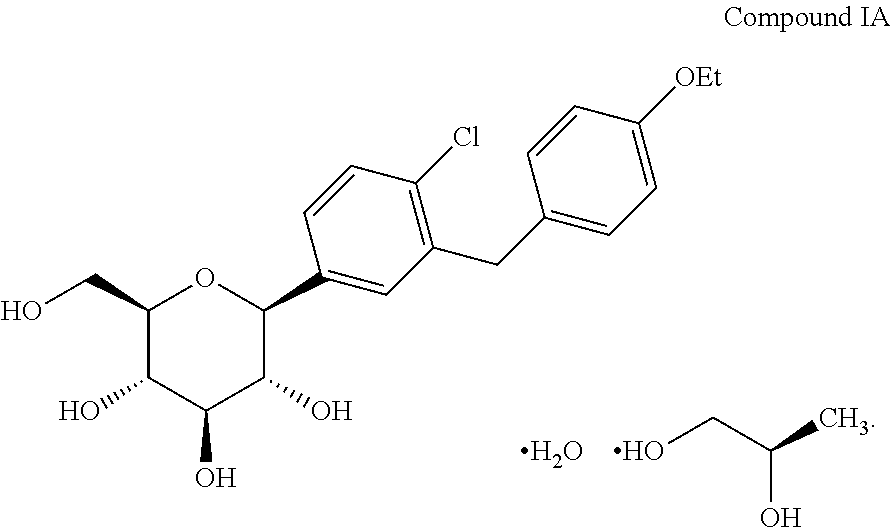
Amorphous form of dapagliflozin 1,2-propanediol
The invention provides an amorphous form of dapagliflozin 1,2-propanediol of Formula (A) or hydrates thereof and their process for preparation. The present invention also provides a pharmaceutical composition comprising art amorphous solid dispersion containing dapagliflozin 1,2-propanediol or hydrates thereof.
Owner:CADILA HEALTHCARE LTD
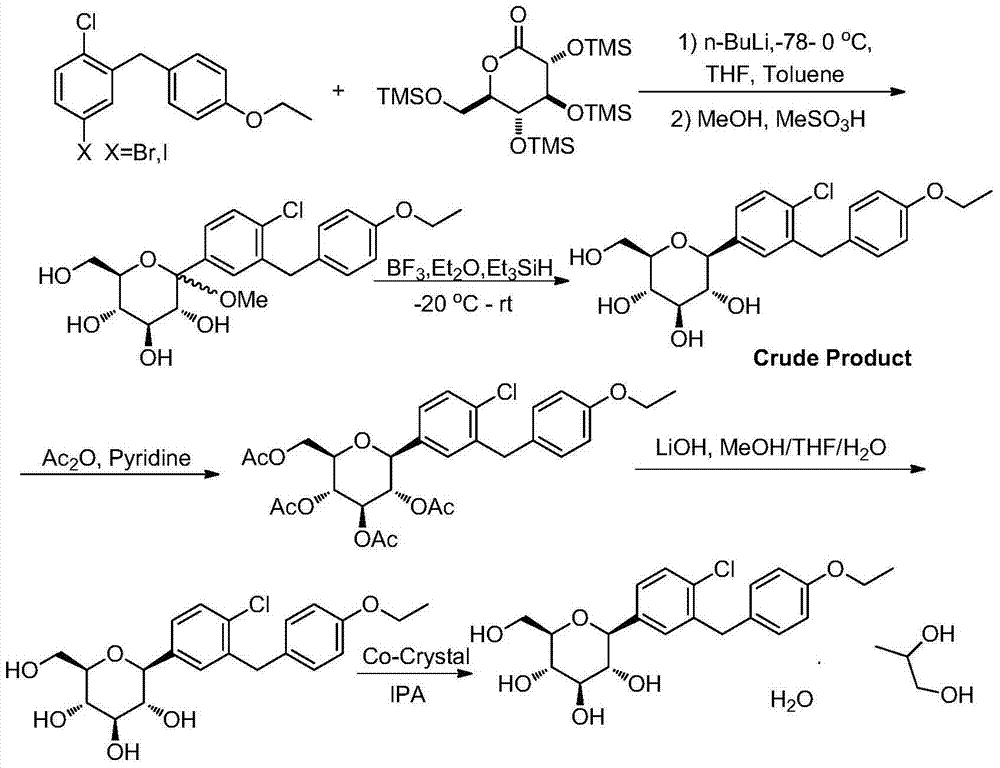
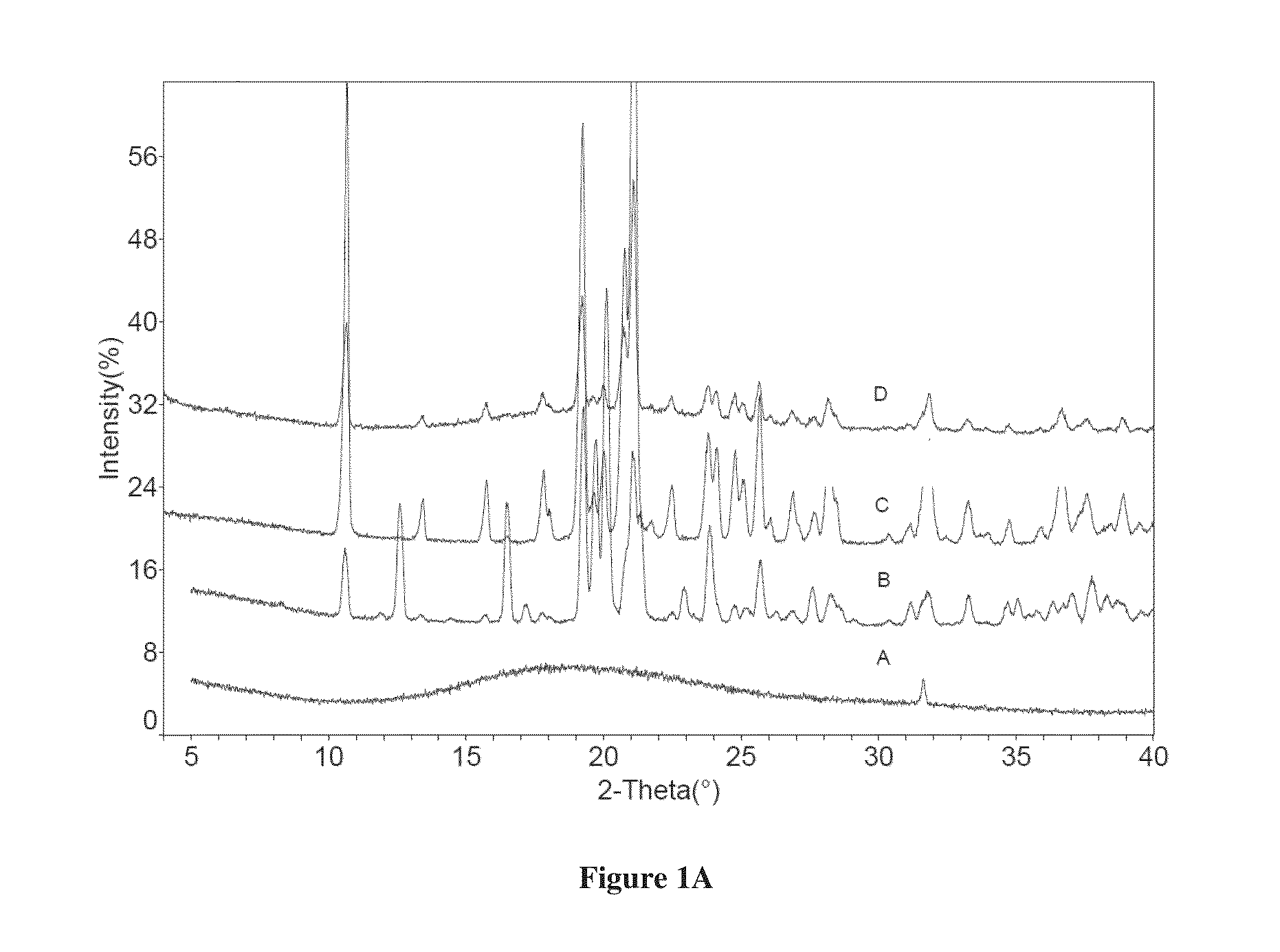
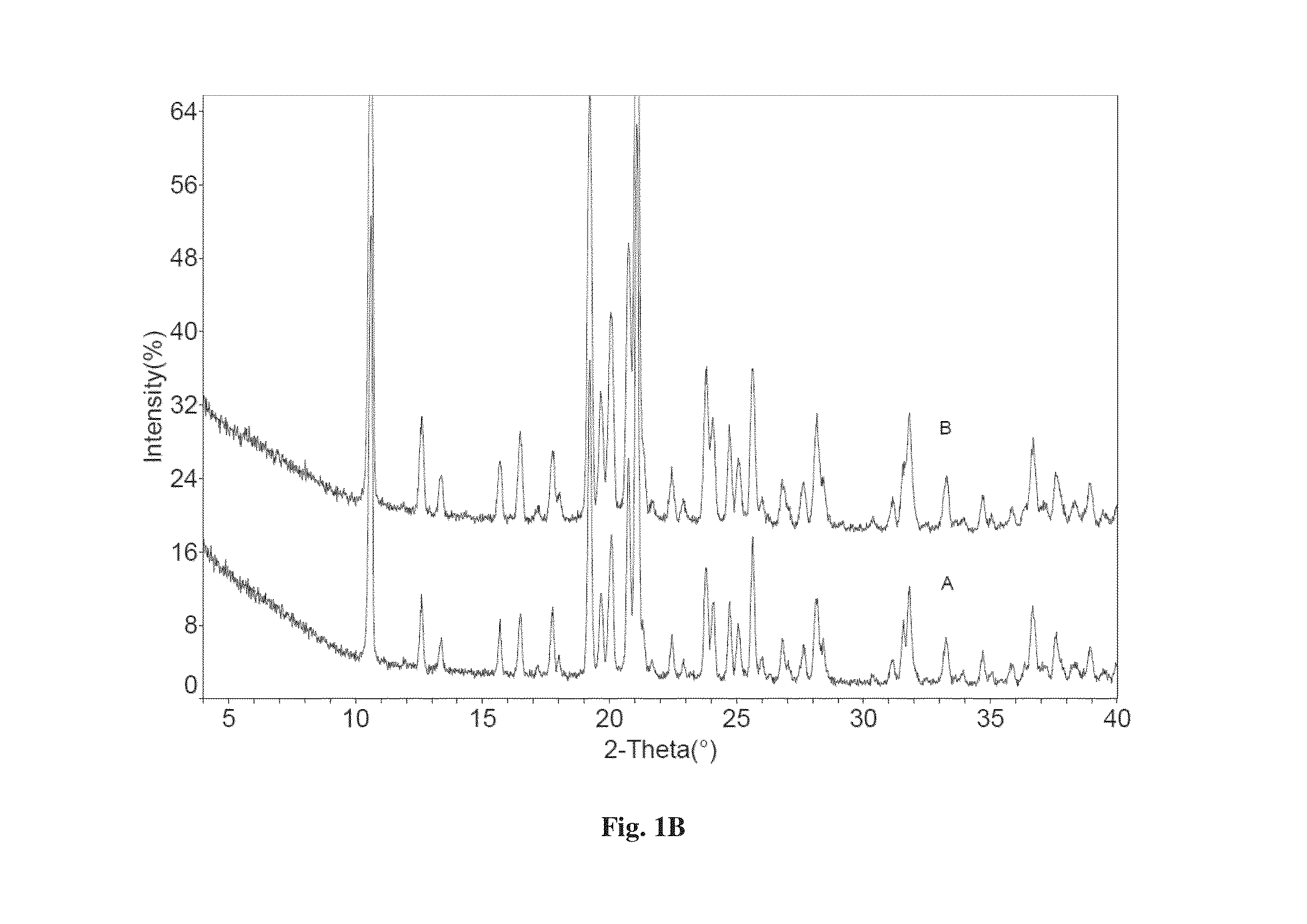
Co-crystals of dapagliflozin
ActiveUS20140343010A1Adequate stability characteristicBetter physicochemical propertyBiocideOrganic active ingredientsLactosePharmacology
The present invention provides novel co-crystal forms of dapagliflozin, namely a dapagliflozin lactose co-crystal and a dapagliflozin asparagine co-crystal, to pharmaceutical compositions comprising same, methods for their preparation and uses thereof for treating type 2 diabetes.
Owner:MAPI PHARMA
Co-crystals of dapagliflozin
ActiveUS9006188B2Adequate stability characteristicMaintain good propertiesPowder deliveryBiocideLactosePharmacology
The present invention provides novel co-crystal forms of dapagliflozin, namely a dapagliflozin lactose co-crystal and a dapagliflozin asparagine co-crystal, to pharmaceutical compositions comprising same, methods for their preparation and uses thereof for treating type 2 diabetes.
Owner:MAPI PHARMA
Preparation method for dapagliflozin
ActiveCN105294624AHigh puritySimple operation processOrganic chemistryChemical industryD-Glucopyranose
The invention belongs to the field of medicine chemical industry, and concretely relates to a preparation method for dapagliflozin. 2,3,4,6-tetra-O-acetyl-alpha-D-glucopyranosyl hydroxide is taken as a raw material and is subjected to a sulfonylation reaction, a nucleophilic substitution reaction and a acetyl removal reaction, so that dapagliflozin is obtained. The preparation method possesses the advantages of mild reaction conditions, simple and reasonable technological process, short reaction time, simple post-processing, high product quality, high yield and the like.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
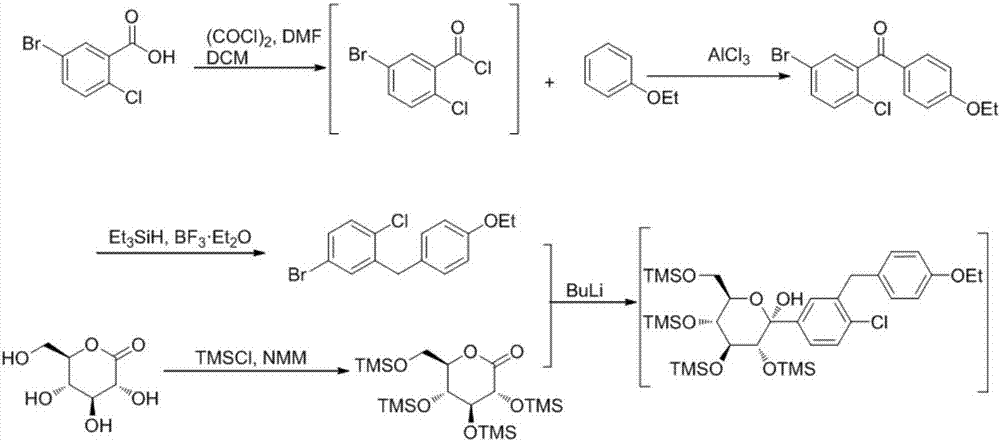
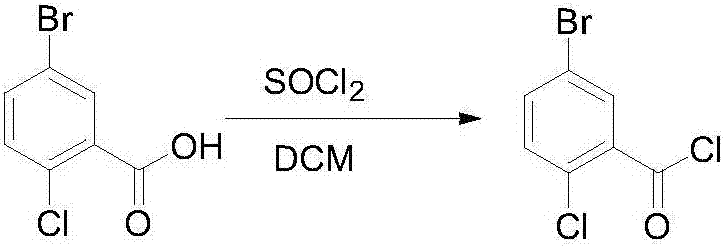
Novel method for synthesizing dapagliflozin intermediate compound
InactiveCN107417515AEasy to operateReact cleanOrganic compound preparationCarbonyl compound preparation by condensation2-Chlorobenzoic acidSolid acid
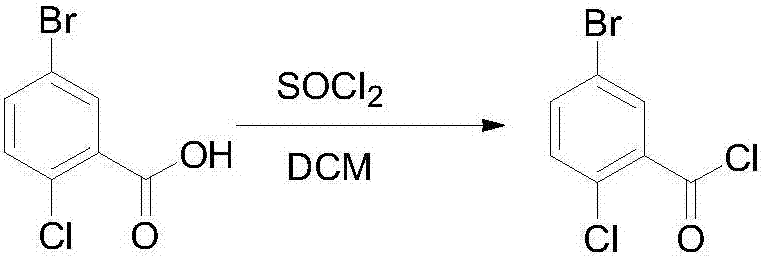
The invention discloses a novel method for synthesizing a dapagliflozin intermediate compound. The method comprises the following steps: (1) by taking dichloromethane as a solvent and pyridine as a catalyst, reacting 5-bromo-2-chlorobenzoic acid and thionyl chloride to obtain 5-bromo-2-chlorobenzoyl chloride; (2) by taking dichloromethane as a solvent and solid acid as a catalyst, reacting phenetole and the 5-bromo-2-chlorobenzoyl chloride to obtain 5-bromo-2-chloro-4-ethoxydiphenylketone; and (3) by taking THF as a solvent, adding the 5-bromo-2-chloro-4-ethoxydiphenylketone concentrated solution obtained in the step (2); by taking acetic acid and aluminum trichloride as catalysts, adding sodium borohydride, and performing a reduction reaction; and after the reaction is completed, adding a saturated saline solution, and performing quenching at 25 DEG C or below to obtain 5-bromo-2-chloro-4-ethoxydiphenylmethane. The novel method disclosed by the invention has the advantages of cheap and available raw materials, simple and easy operation, no discharge of three wastes, high reaction yield and the like.
Owner:上海常丰生物医药科技有限公司
Method for preparing dapagliflozin
InactiveCN108530408AEase of commercializing bulk purchasesEasy to operateOrganic chemistryTrimethylsilylStrong acids
The invention aims at providing a method for more effectively synthesizing dapagliflozin with shorter steps. The method comprises the steps: catalyzing 5-bromo-2-chloro-4'-ethoxybenzophenone serving as a starting material by using a strong acid to finish ketal protection, treating the obtained diaryl ketal compound by using magnesium, then, reacting the treated diaryl ketal compound with trimethylsilyl-protected glucolactone, and reducing a hemiketal compound obtained after dilute acid quenching to finish the preparation of dapagliflozin.
Owner:SOUTHEAST UNIV +1
Methods for treating extreme insulin resistance in patients resistant to previous treatment with other anti-diabetic drugs employing an SGLT2 inhibitor and compositions thereof
Owner:ASTRAZENECA AB
Preparation method for Dapagliflozin
The invention relates to a preparation method for Dapagliflozin. The preparation method comprises the following steps that a compound 2 and phenetole are mixed to obtain a mixture, and then the mixture is dropped into a suspended aluminum trichloride solution, so that the content of generated ortho isomer impurities is smaller than 1 percent; a compound 4 in the preparation method is firstly reacted with butyl lithium to generate a compound 5 in the following formula; then the compound 5 is reacted with a compound 1, so that generation of sulphonate type genetic toxic impurities is avoided; therefore the utilization rate of raw materials is increased to a certain extent, the problem of complicated post-treatment is avoided, and the purity and the yield of products are improved. According to the preparation method, a compound 6 is reduced by using hydroboron and sulfuric acid, so that the reduction reaction and deprotection can be simultaneously carried out, and the pollution is greatly alleviated. The raw materials and the reagents which are adopted in the preparation method are relatively cheap and low in cost; meanwhile, the reaction conditions of the whole preparation process are mild, and the operation is simple and safe; industrial production can be conveniently realized.
Owner:ZHEJIANG MENOVO PHARMA
6-halogenated glucose C-glycoside as well as preparation method and application thereof
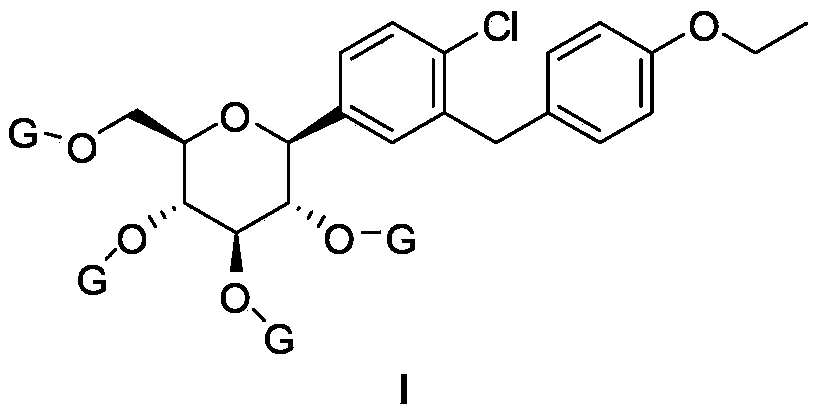
ActiveCN108675976AHigh purityHigh yieldOrganic chemistryBulk chemical productionC-glycosideD-Glucose
The invention discloses a 6-halogenated glucose C-glycoside as well as a preparation method and application thereof. A structure of 6-halogenated glucose C-glycoside is shown in formula I; an intermediate can be synthesized efficiently with cheap and easily available raw materials; meanwhile, when the raw material is used for synthesizing Jardiance, dapagliflozin and the like, a reaction yield ishigh, and an obtained product has high purity and relatively high industrial application prospect.
Owner:ZHEJIANG HONGYUAN PHARMA
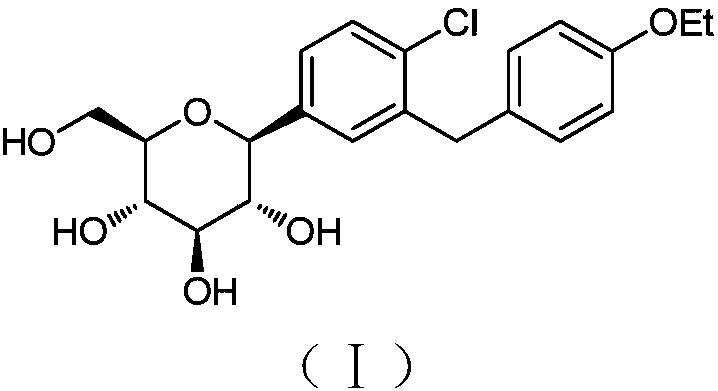
Process for the preparation of dapagliflozin
Dapagliflozin compounds and process for dapagliflozin preparation are described. Pharmaceutical compositions comprising dapagliflozin or solvates of dapagliflozin, for the treatment of diabetes are also described.
Owner:ZYDUS LIFESCIENCES LTD
Preparation method of Dapagliflozin intermediate used for treating II-type diabetes
InactiveCN107200683AHigh yieldGood reserve supportOrganic compound preparationCarbonyl compound preparation by condensationTert-butyldimethylsilyl chloride2-Chlorobenzoic acid
The invention discloses a preparation method of a Dapagliflozin intermediate used for treating II-type diabetes. The preparation method comprises the following steps: 1) performing a reaction on 5-bromine-2-chlorobenzoic acid and oxalyl chloride in anhydrous dichloromethane under the catalysis of DMF (dimethyl formamide), so as to obtain 5-bromine-2-chloro-benzoyl chloride; 2) under the condition that tert-Butyldimethylsilyl chloride exists, performing a reaction on 5-bromine-2-chloro-benzoyl chloride obtained in step 1) and phenetole under the catalysis of ferric trichloride, so as to obtain 5-bromine-2-chloro-4'-ethyoxyl benzophenone. According to the preparation method provided by the invention, no ortho-by-product is generated, and the yield of a target product is high, so that the good support of storage of raw materials is provided for Dapagliflozin. Additionally, the preparation method is mild in conditions and short in reaction time, therefore, the preparation method is suitable for industrial production and promotion.
Owner:QINGDAO CHENDA BIOLOGICAL SCI & TECH
Dapagliflozin medicinal composition and preparation method thereof
InactiveCN106727368ANon-fibrousLiquidityOrganic active ingredientsMetabolism disorderMedicineAdhesive
The invention provides a dapagliflozin medicinal composition. The dapagliflozin medicinal composition is characterized by consisting of the following components in percentage by mass: 1 to 95 percent of a dapagliflozin and microcrystalline cellulose composition, 0 to 95 percent of a filling agent / an adhesive, 0 to 20 percent of a disintegrant, 0.1 to 5 percent of a lubricating agent and 0 to 10 percent of a flow aid. According to the dapagliflozin medicinal composition, microcrystalline cellulose serves as a carrier material; according to a method provided by the invention, the dapagliflozin and microcrystalline cellulose are mixed and heated so that the dapagliflozin is molten; the dapagliflozin covers the surface of the microcrystalline cellulose in a liquid form; after the dapagliflozin is cooled, the dapagliflozin is solidified on the surface of the microcrystalline cellulose to form a dapagliflozin and microcrystalline cellulose compound; the specific surface area of the dapagliflozin is increased, so that the medicine dissolving speed can be improved and the quality is stable; the preparation method provided by the invention is simple and feasible, and is suitable for industrial production.
Owner:SHANGHAI SUNTECH PHARMA
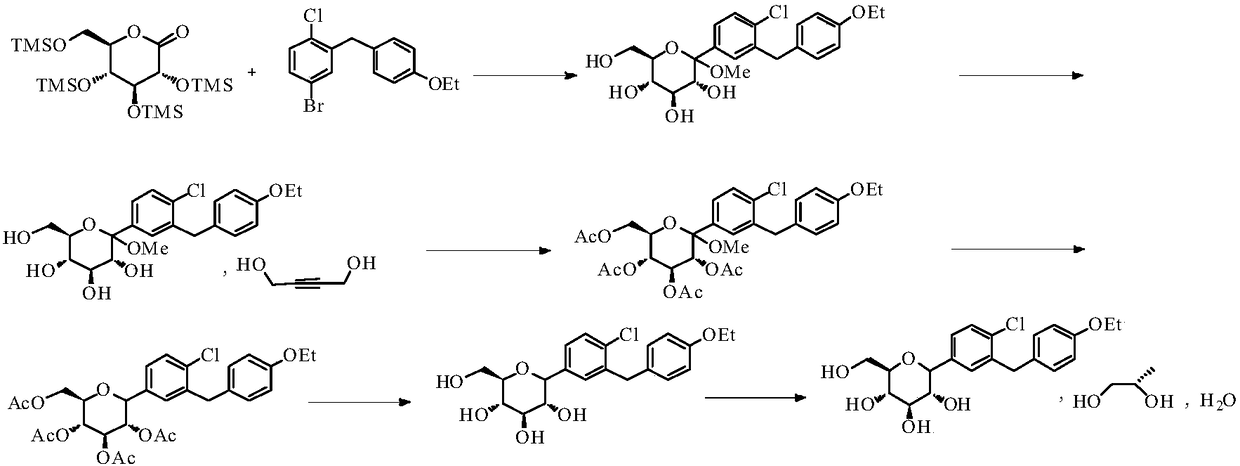
Synthetic method of dapagliflozin
ActiveCN109400561AEfficient synthesisMild reaction conditionsOrganic chemistry methodsChemical/physical/physico-chemical microreactorsSynthesis methodsReaction temperature
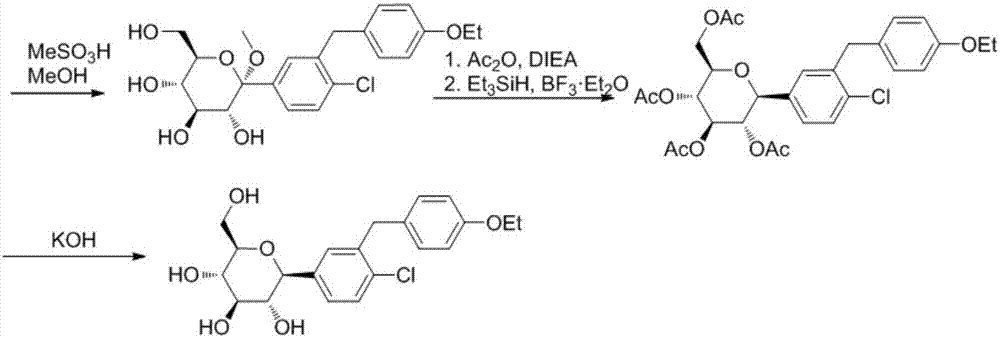
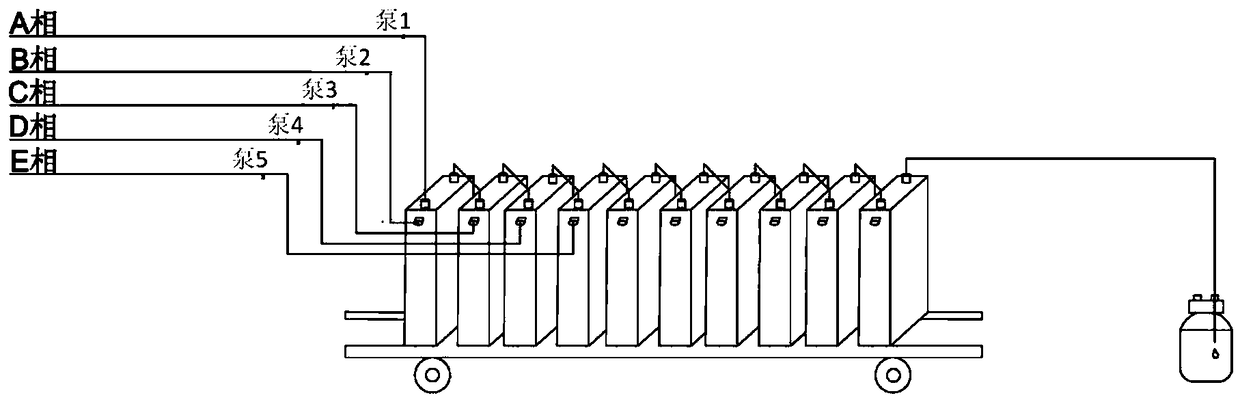
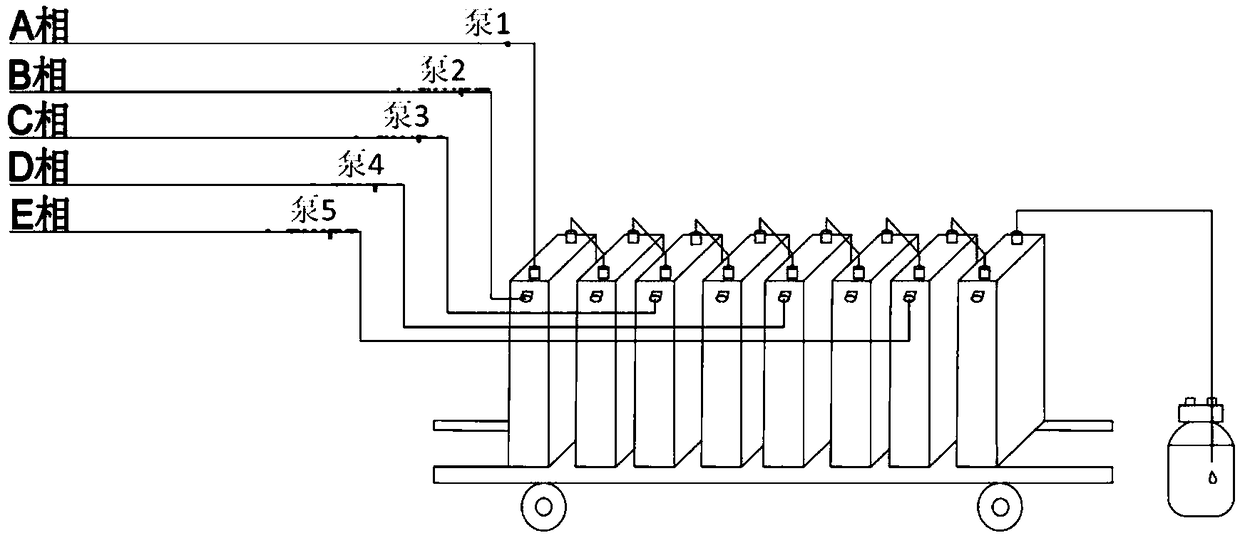
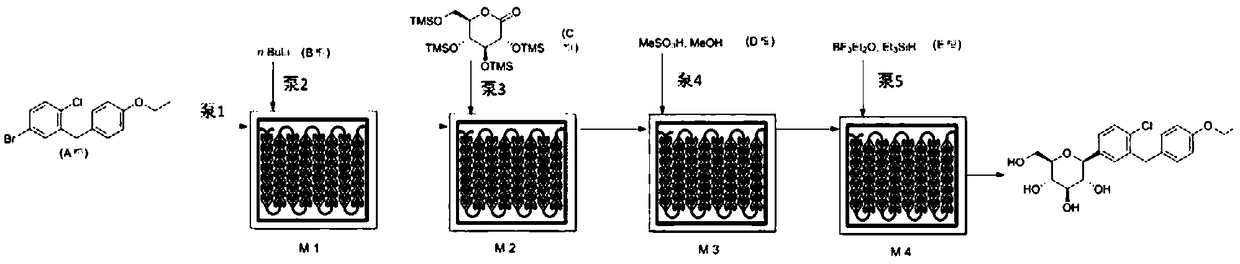
Disclosed is a synthesis method of dapagliflozin. The method includes providing a microreactor having at least 4 reaction units in series, delivering a 5-bromo-2-chloro-4'-ethoxydiphenylmethane solution and an alkyllithium solution into a first reaction unit and controlling the reaction temperature in the first reaction unit to be -5 to -40 DEG C; after the reaction is completed, making a reactionsolution flow into a second reaction unit, and delivering trimethylsilyl protected gluconolactone or a solution thereof to the second reaction unit; after the reaction is completed, making a reactionsolution flow into a third reaction unit and delivering a mixed solution of methanol and methane sulfonic acid to the third reaction unit; making a reaction solution flow into a fourth reaction unitafter the reaction is completed and delivering a mixed solution of boron trifluoride diethyl etherate and Triethylsilane into the fourth reaction unit.
Owner:SHANDONG HIMILE CHEM TECH
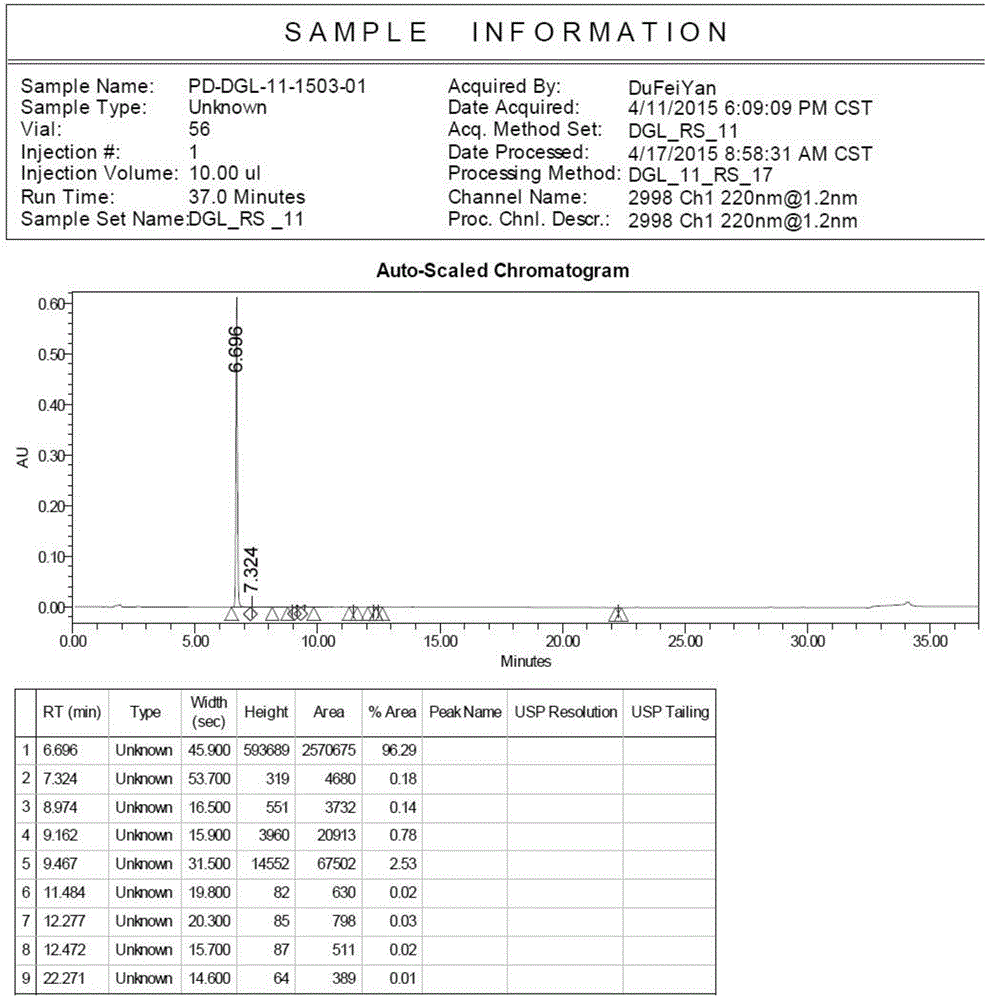
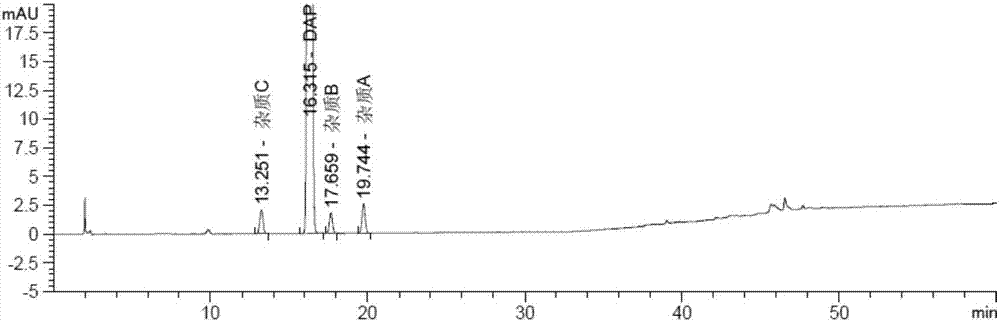
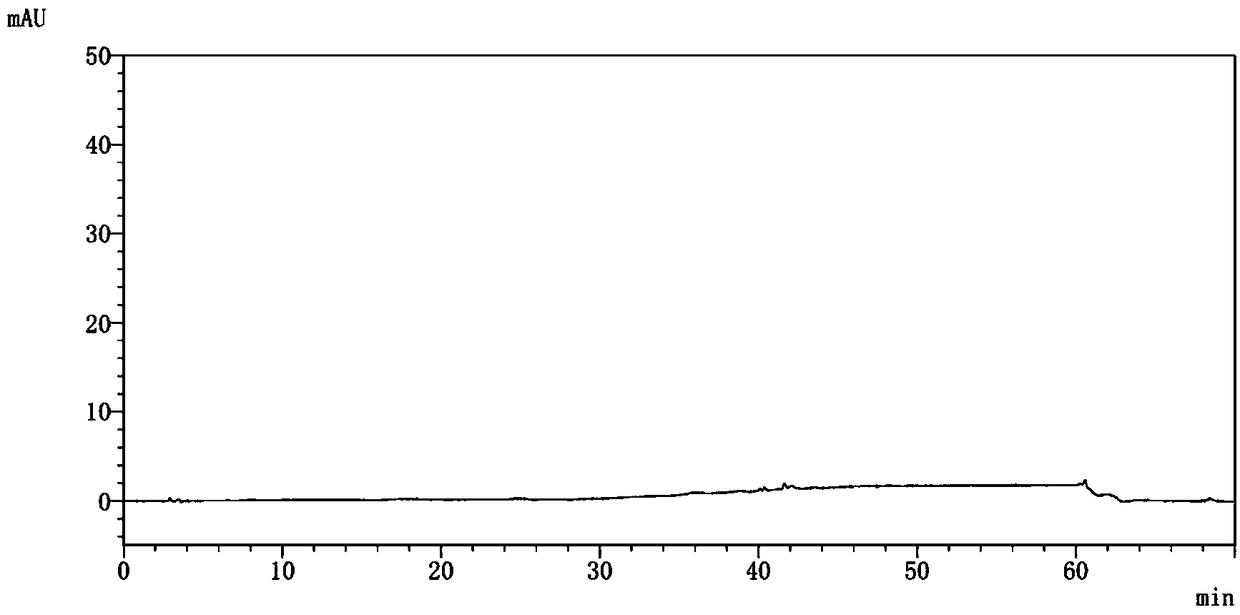
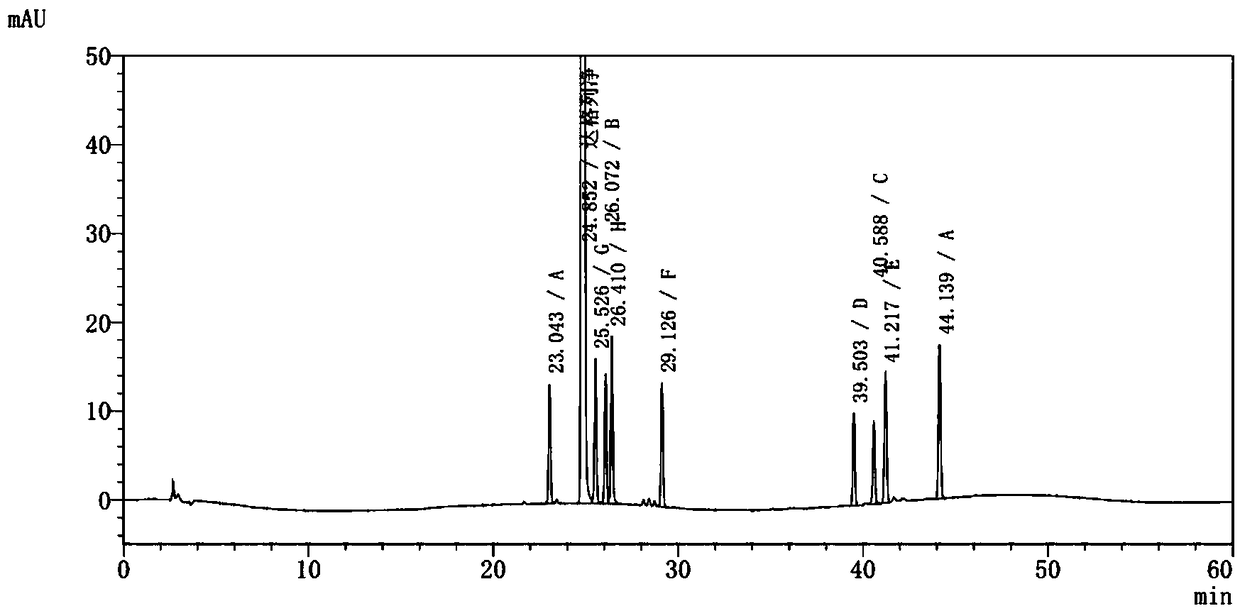
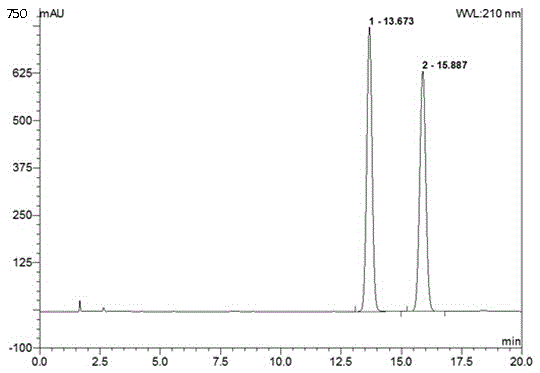
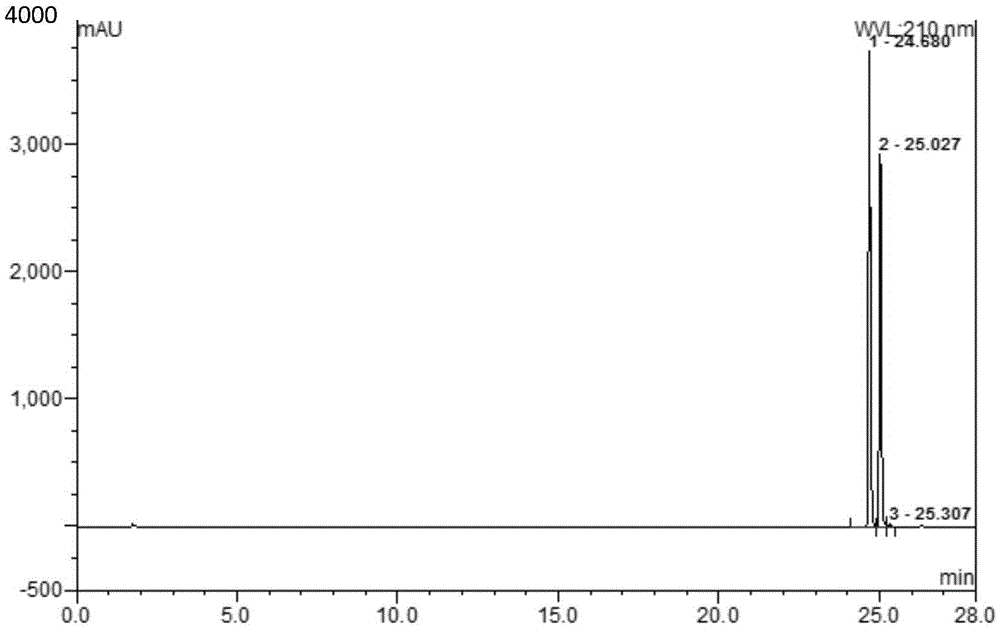
Method for determining dapagliflozin and its related substance by high performance liquid chromatograph
ActiveCN107515255AEffective quality controlQuality improvementComponent separationOrganic solventGradient elution
The invention discloses a method for determining dapagliflozin and its related substance by a high performance liquid chromatograph. The high performance liquid chromatography utilizes an octadecylsilane bonded silica gel column as a chromatographic column, an acidic aqueous solution as a mobile phase A and a first organic solvent as a mobile phase B for gradient elution. The method has high sensitivity, high specificity, high precision, high accuracy, simple and quick operation and wide applicability range, realizes separation and determination of the dapagliflozin bulk drug and related substances of a dapagliflozin preparation under the same chromatographic conditions and effectively controls the quality of drugs.
Owner:湖北石河医药科技有限公司
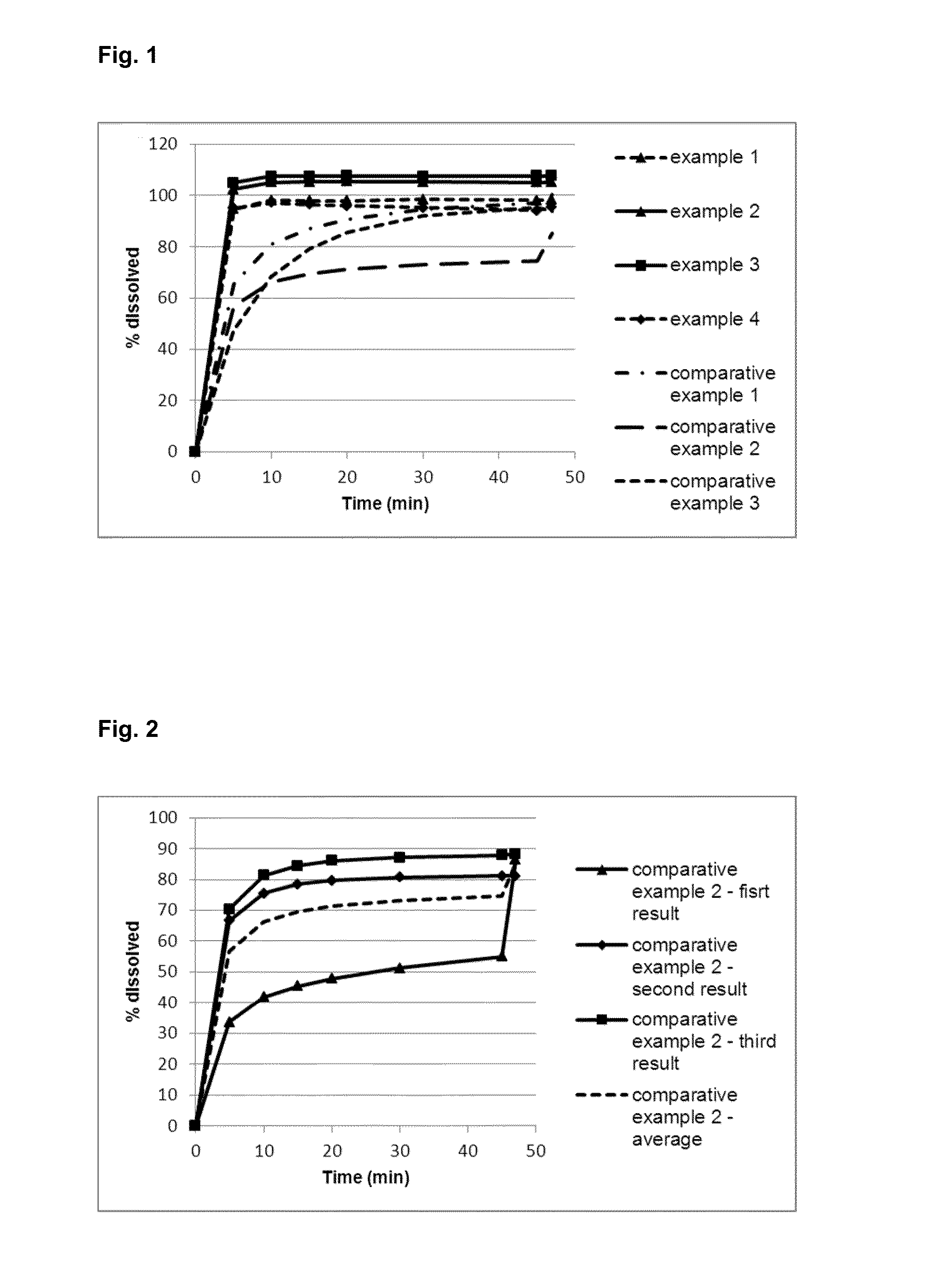
Formulations Containing Amorphous Dapagliflozin
InactiveUS20160256433A1Improve performanceGood chemical stabilityMetabolism disorderCarbohydrate active ingredientsMedicinePharmaceutical industry
The present invention belongs to the field of pharmaceutical industry and relates to an amorphous solid dispersion comprising at least one polymer and dapagliflozin, to a pharmaceutical composition comprising said solid dispersion, to a process for the preparation thereof, and to the solid dispersion and pharmaceutical composition respectively obtainable by said process. Further, the present invention refers to an adsorbate comprising dapagliflozin and to a pharmaceutical composition comprising said adsorbate, as well as to a process for the preparation thereof. Finally, the present invention relates to the solid dispersion, the adsorbate or the pharmaceutical composition for use in the treatment of diseases related to hypoglycemia.
Owner:SANDOZ AG
Dapagliflozin preparation method
InactiveCN107540648ACheap and easy to getEasy to industrializeOrganic chemistryAlkyl transferTrimethylsilyl
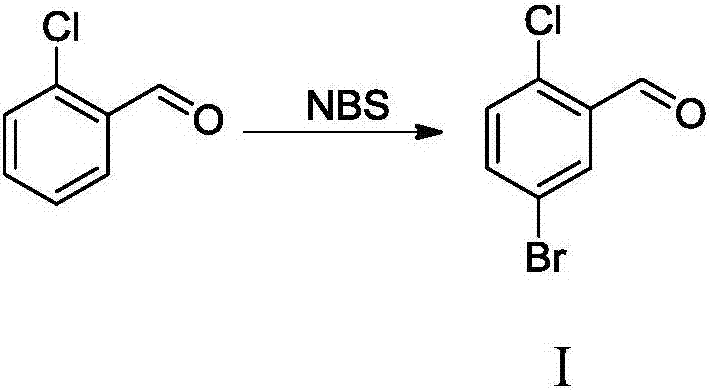
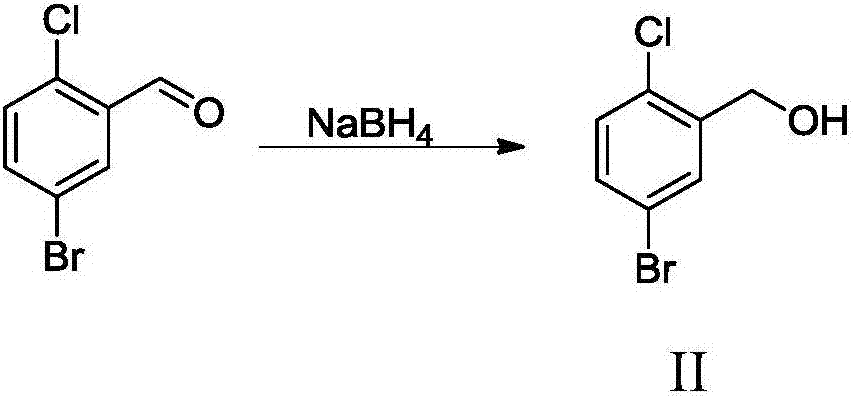
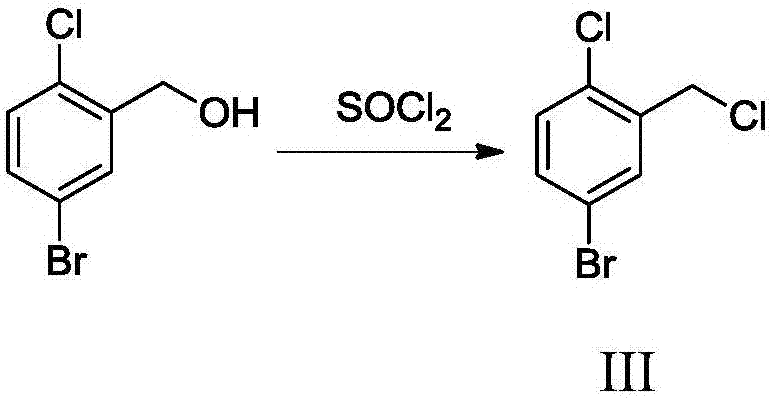
The invention relates to a Dapagliflozin preparation method, which comprises the following steps: using 2-chlorobenzaldehyde as a starting material, carrying out bromination, reducing, chlorinating tosynthesize 5-bromo-2-chlorobenzyl chloride, carrying out Friedel-Crafts alkylation reaction between 5-bromo-2-chlorobenzyl chloride and phenetole to synthesize 5-bromo-2-chloro-4'-ethyoxyldiphenylmethane, conducting condensation between 5-bromo-2-chloro-4'-ethyoxyldiphenylmethane and 2,3,4,6-tetra-O-trimethylsilyl-D-glucolactone, carrying out trimethylsilyl deprotection, conducting etherification, and reducing for demethylation to obtain a hypoglycemic drug Dapagliflozin. The invention has the following advantages: according to the Dapagliflozin preparation method, 2-chlorobenzaldehyde, whichis used as a starting material, is cheaper and easily available in comparison with 5-bromo-2-chlorobenzoic acid, and the technology is easy for industrialization; during the synthetic process, no rawmaterials which cause severe toxicity will be used and furthermore there is no dangerous process; the synthetic route is short and novel and the operation is simple; and through the synthetic route,purity of the final product can be raised, and the purity can reach 99% and above.
Owner:IANGSU COLLEGE OF ENG & TECH
Immediate release tablet formulations
The present invention provides an immediate release pharmaceutical formulation which includes a tablet or capsule formulation comprising metformin and the sodium dependent glucose transporter (SGLT2) inhibitor dapagliflozin or its propylene glycol hydrate. The present invention also provides methods of preparing the formulations and methods of treating diseases or disorders associated with SGLT2 activity employing these formulations.
Owner:ASTRAZENECA AB
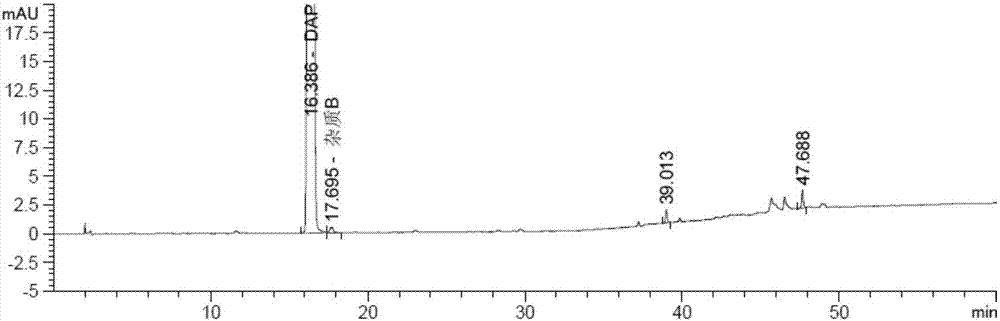
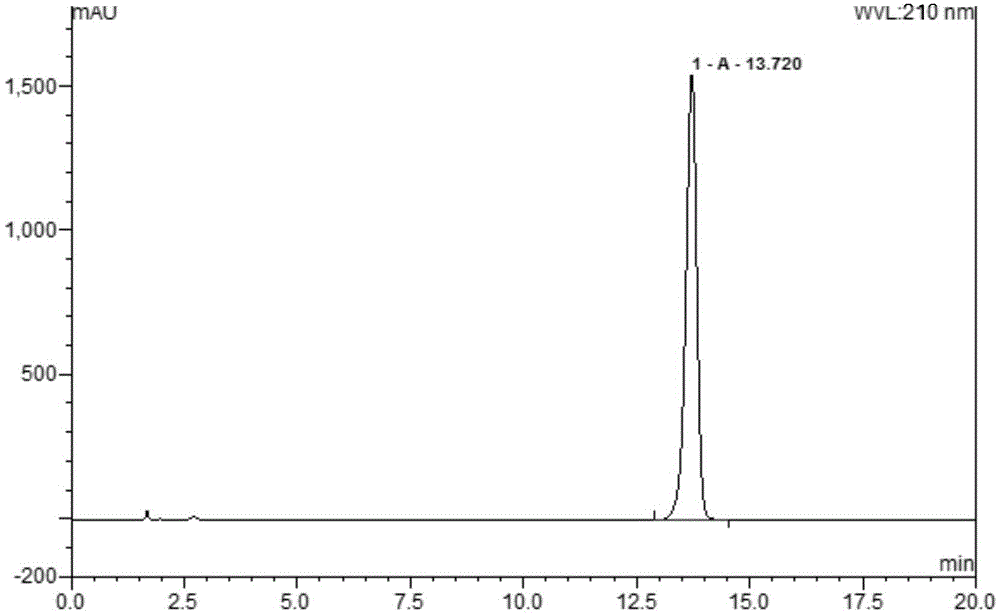
Method for separating and measuring dapagliflozin crude drug related substance through HPLC
ActiveCN109374784AQuality improvementEfficient separationComponent separationUltraviolet detectorsColumn temperature
The invention provides a method for separating and measuring dapagliflozin crude drug related substance through a HPLC. The method comprises the following steps: performing gradient elution by takingoctadecylsilane chemically bonded silica as a filler, water as a flowing phase A and acetonitrile as a flowing phase B, ensuring that the flow rate is 0.8 to 1.2ml / min and the column temperature is 25to 40 DEG C, adopting an ultraviolet detector to detect the dapagliflozin crude drug related substance, and ensuring that the detection wavelength of the ultraviolet detector is 205 to 230nm. The method ensures that detection results are optimized through overall consideration of comprehensive influence on separation and detection by an analytical column, flowing phases, a gradient elution program, the flow rate and the column temperature, thereby effectively controlling the quality of crude drugs. In addition, the method has the advantages of high rapidness, simpleness, sensitivity, specificity, precision, accuracy, reliability and convenience in operation, and is applied to separation and measurement of the dapagliflozin crude drug related substance.
Owner:安徽联创生物医药股份有限公司
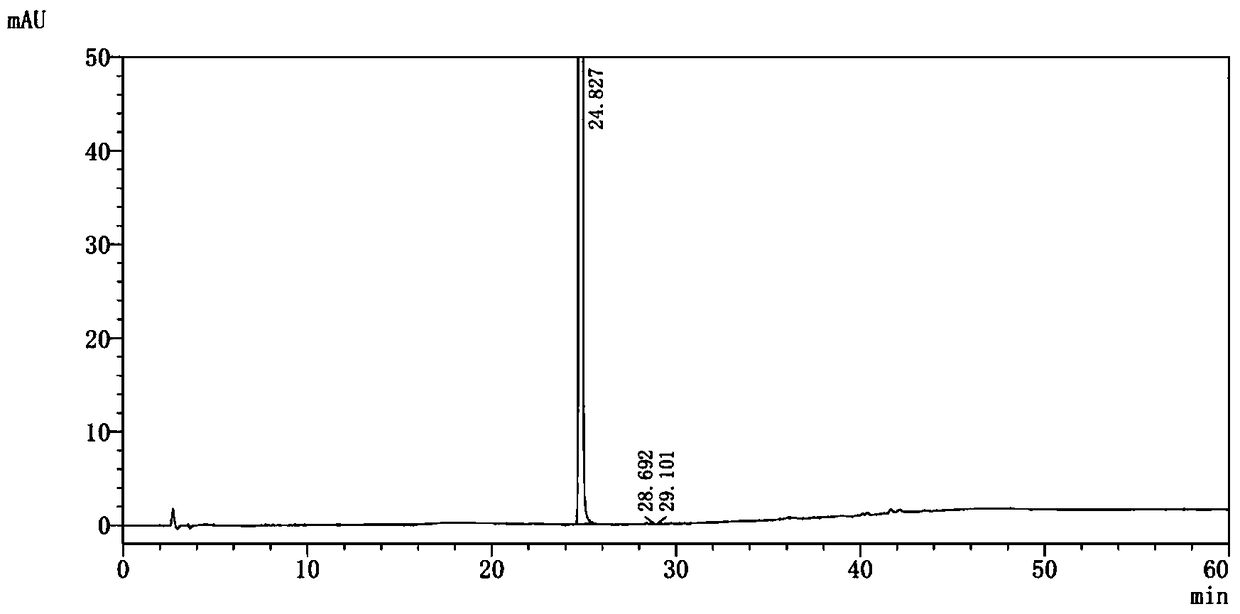
Method for separating dapagliflozin and alpha-isomer thereof
ActiveCN105486767AEfficient separationHigh feasibilityComponent separationUltraviolet detectorsColumn temperature
The invention belongs to the technical field of pharmaceutical analysis, and provides a method for separating dapagliflozin and an alpha-isomer thereof. A high performance liquid chromatographic analyzer is adopted, a chromatographic column with octadecyl bonded silica as a filler is adopted, and the specification is 4.6mm*250mm, 5[mu]m; the mobile phase is an acetonitrile and water mixed liquid or a methanol and water mixed liquid, a volume ratio of acetonitrile to water is 32-42:58-68, and a volume ratio of methanol to water is 60-80:20-40; the flow velocity is 0.6-1.2mL / min; the column temperature is 20-40DEG C; the wavelength of an ultraviolet detector is 205-260nm; and the sample introduction volume is 10[mu]m, a compound having the after coming peak is the alpha-isomer, and a compound having the first-out peak is dapagliflozin. The method has the advantages of just use of a common liquid chromatograph, low device requirements, common and easily available media selected as the mobile phase, high feasibility, and simple and convenient operation process.
Owner:上海柏狮生物科技有限公司
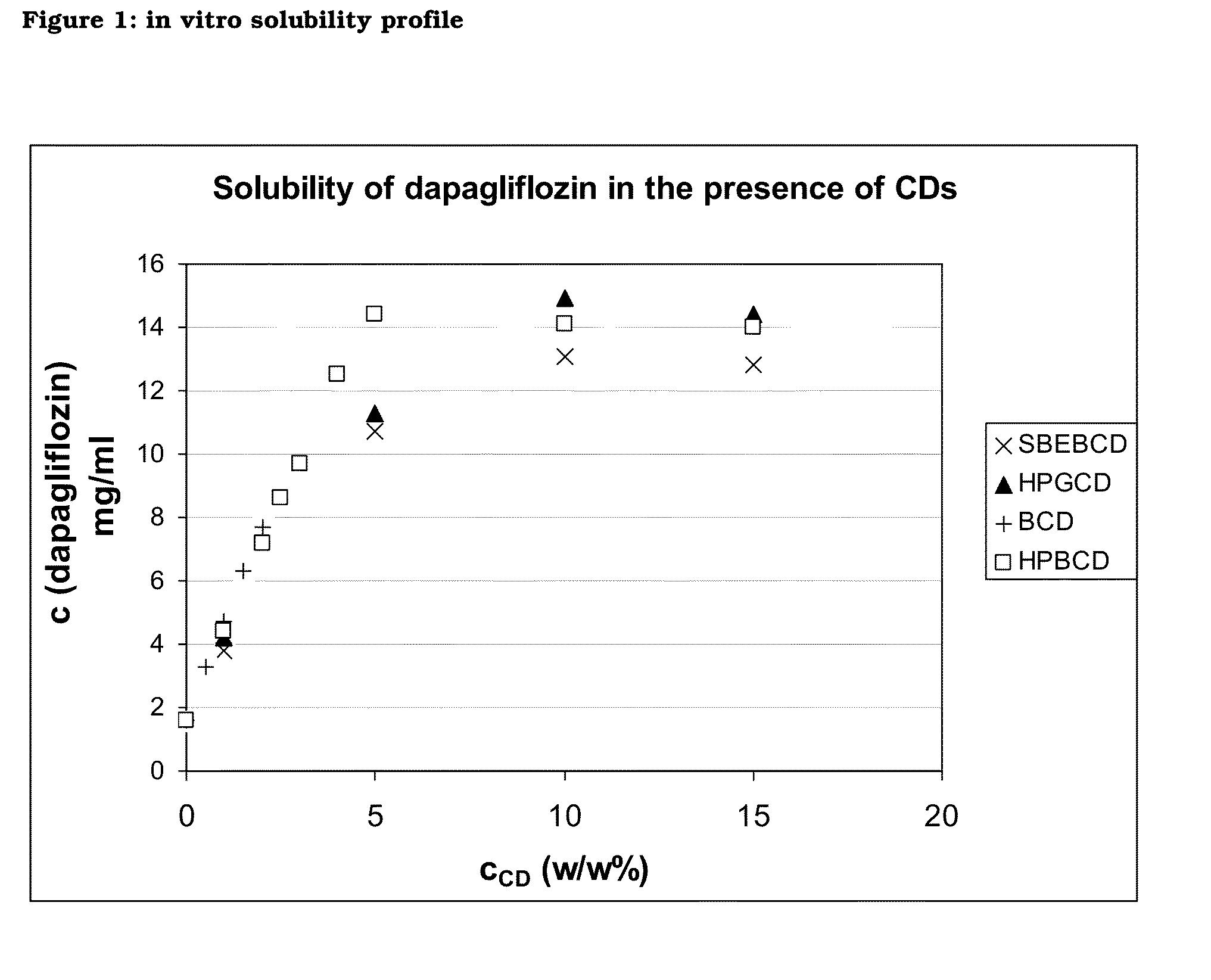
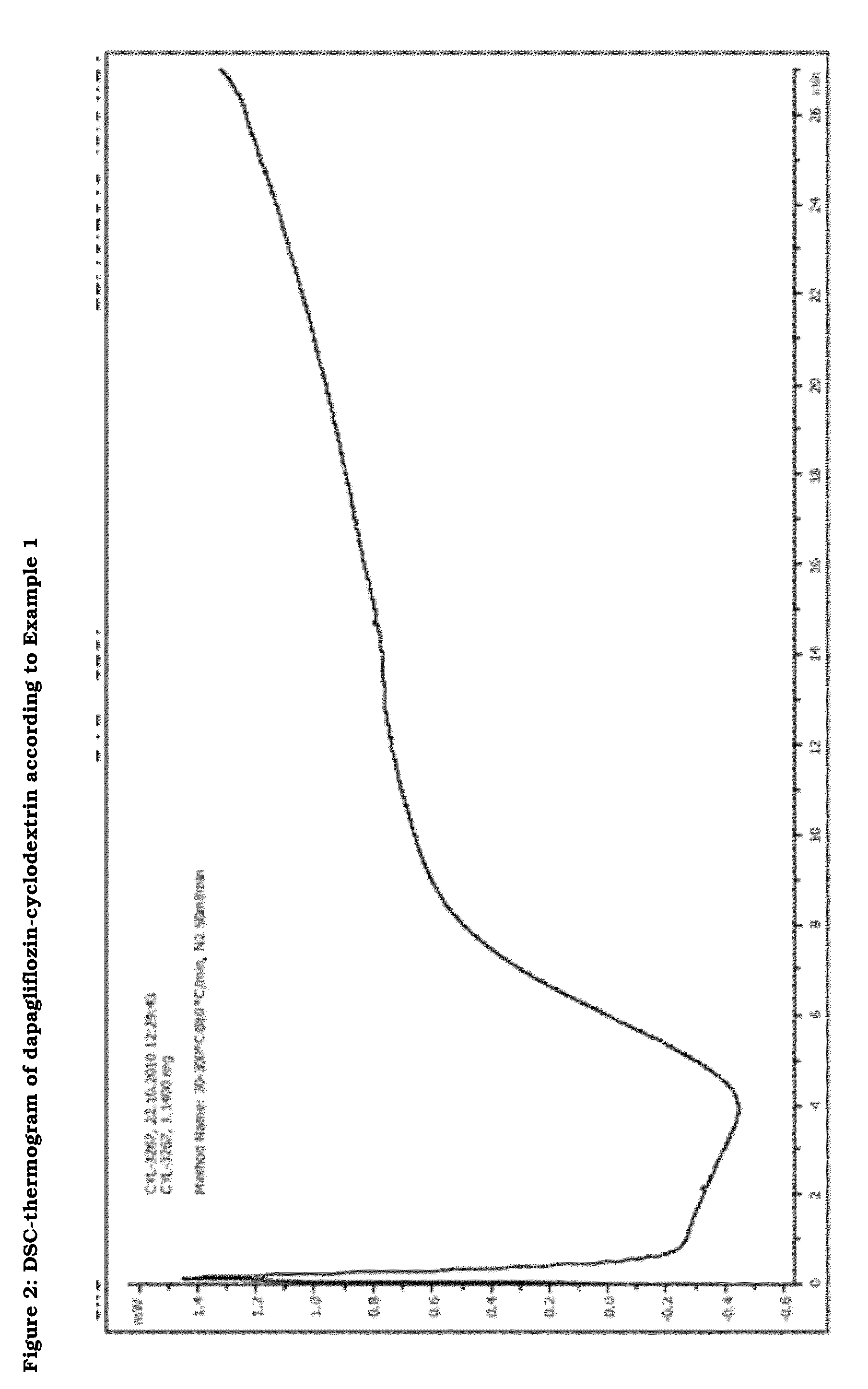
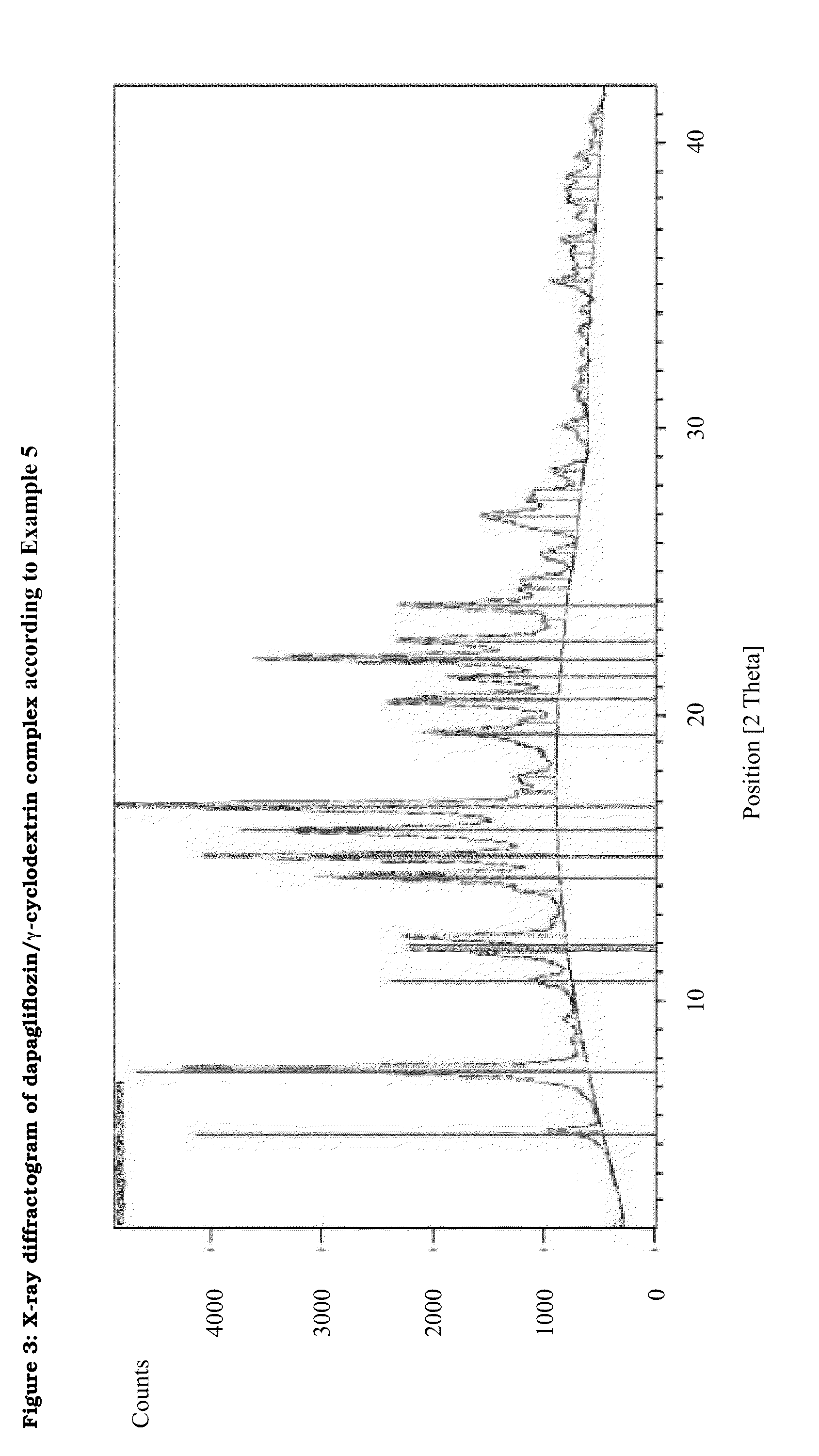
Pharmaceutical Composition Comprising Dapagliflozin and Cyclodextrin
The present invention relates to pharmaceutical compositions comprising dapagliflozin and cyclodextrin, preferably (2-hydroxy)propyl-b-cyclodextrin or γ-cyclodextrin, preferably as inclusion complex. The invention further relates to a process for producing said pharmaceutical compositions. Finally, the invention relates to the use of cyclodextrin for producing dapagliflozin-containing dosage forms and to methods of purification of dapagliflozin.
Owner:RATIOPHARM GMBH
Dapagliflozin agent composition
InactiveCN107714667ASolve the stability problem of content uniformityHigh dissolution rateOrganic active ingredientsMetabolism disorderPolyethylene glycolLactose
The invention relates to a dapagliflozin agent composition, belonging to the technical field of pharmaceutical preparations. The technical scheme of the composition is that: the dapagliflozin agent composition is prepared from 5-10mg of dapagliflozin with D50 of 32-46 microns, 26-35 mg of lactose, 48-60mg of microcrystalline cellulose, 5-8mg of polyethylene glycol 6000, 9-12mg of sodium citrate, 3-8mg of povidone K30, 3-8mg of polyvinylpolypyrrolidone, 0.9-1.2mg of lauryl sodium sulfate and 1-1.5mg of magnesium stearate. The preparation method provided by the invention is used for solving thestable problem of content uniformity of the dapagliflozin.
Owner:WEIHAI GUANBIAO INFORMATION TECH
Method for the preparation of dapagliflozin
The invention relates to a preparation method of dapagliflozin. The preparation method takes a compound of the formula II and a compound of the formula III as a starting material, and the dapagliflozin is obtained by the steps of condensation, ethyl ether, reduction and the like, and can be used for treating type II diabetes. The preparation method of dapagliflozin has the advantages of reasonableprocess design, high reaction yield and high purity of the prepared dapagliflozin, and is very suitable for industrial production of the dapagliflozin.
Owner:JIANGSU HANSOH PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com