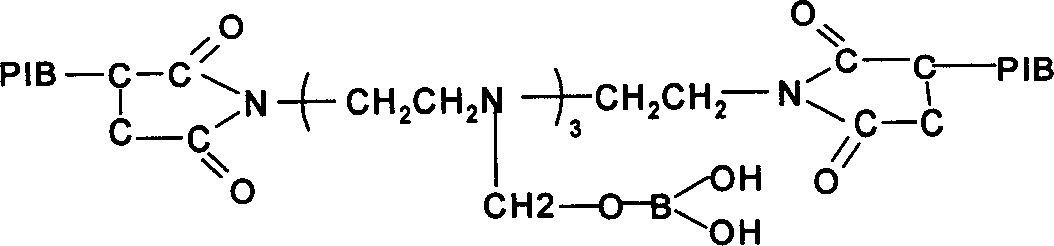
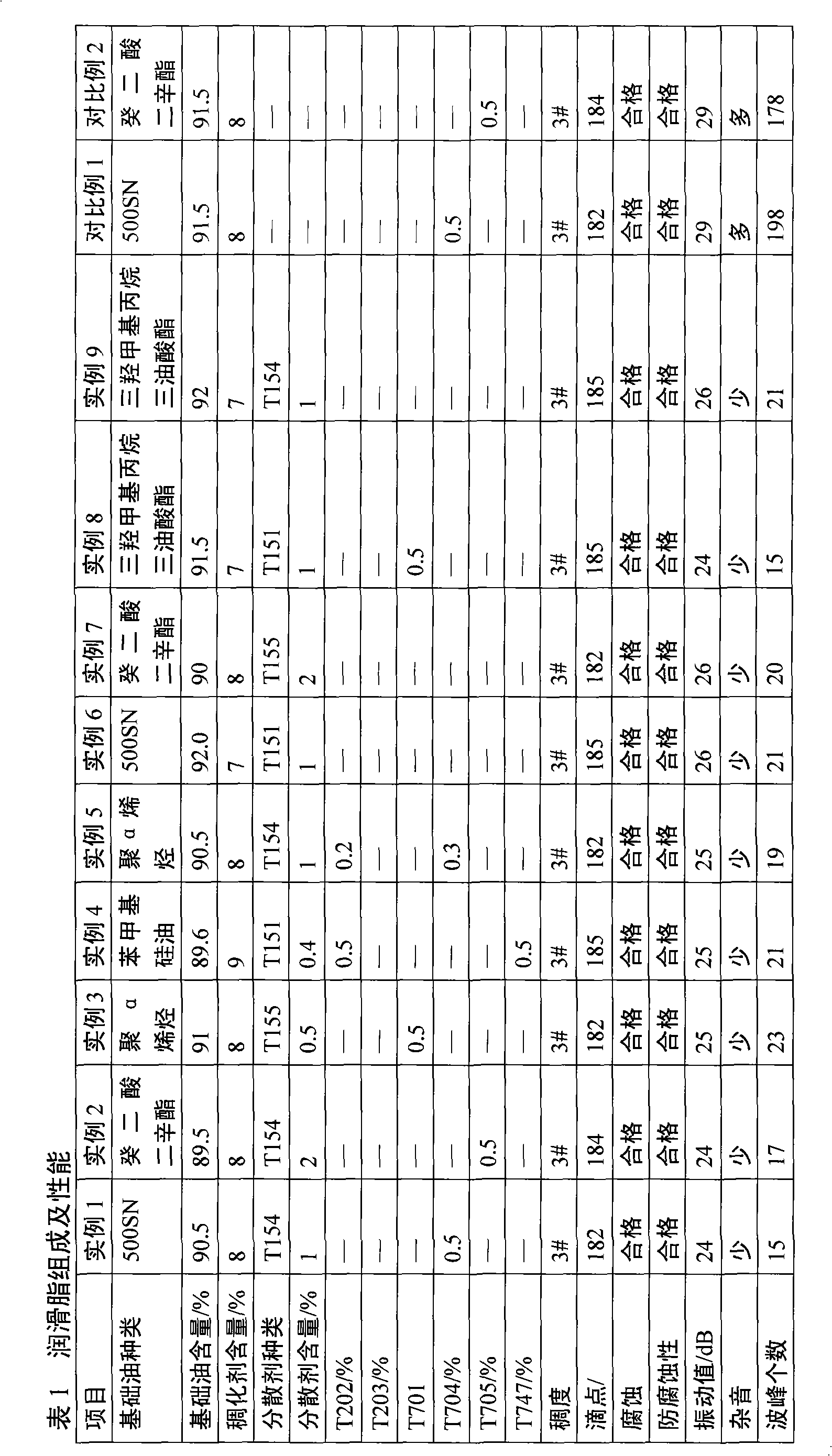
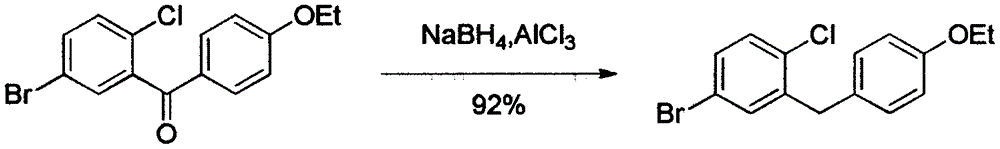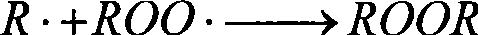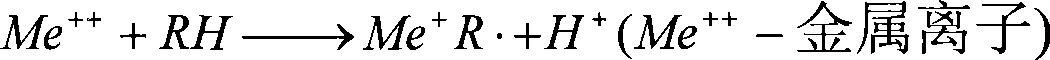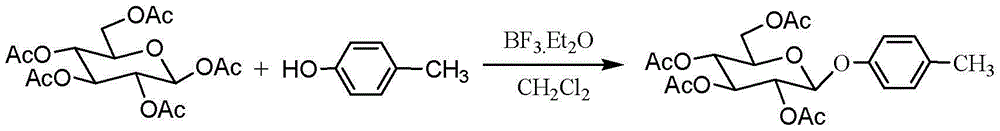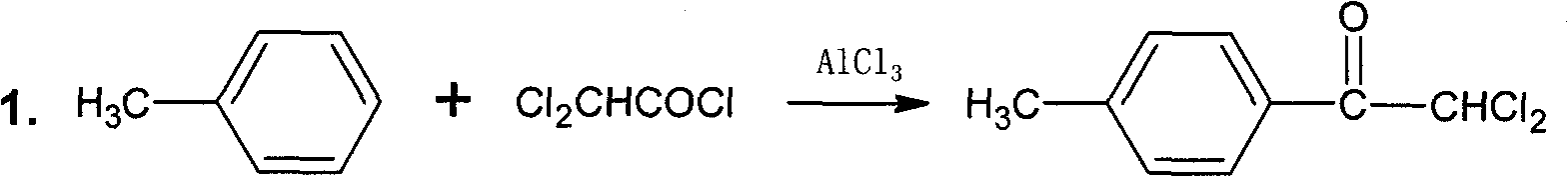Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1278 results about "Succinchlorimide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Succinchlorimide definition is - a crystalline compound C2H4(CO)2NCl that has an odor like that of chlorine and is used as a disinfectant and chlorinating agent; N-chloro-succinimide —not used systematically.
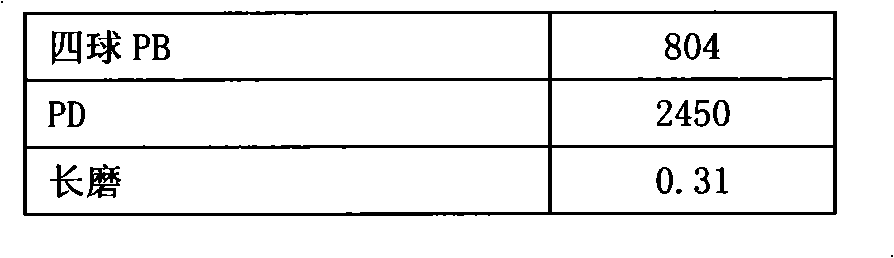
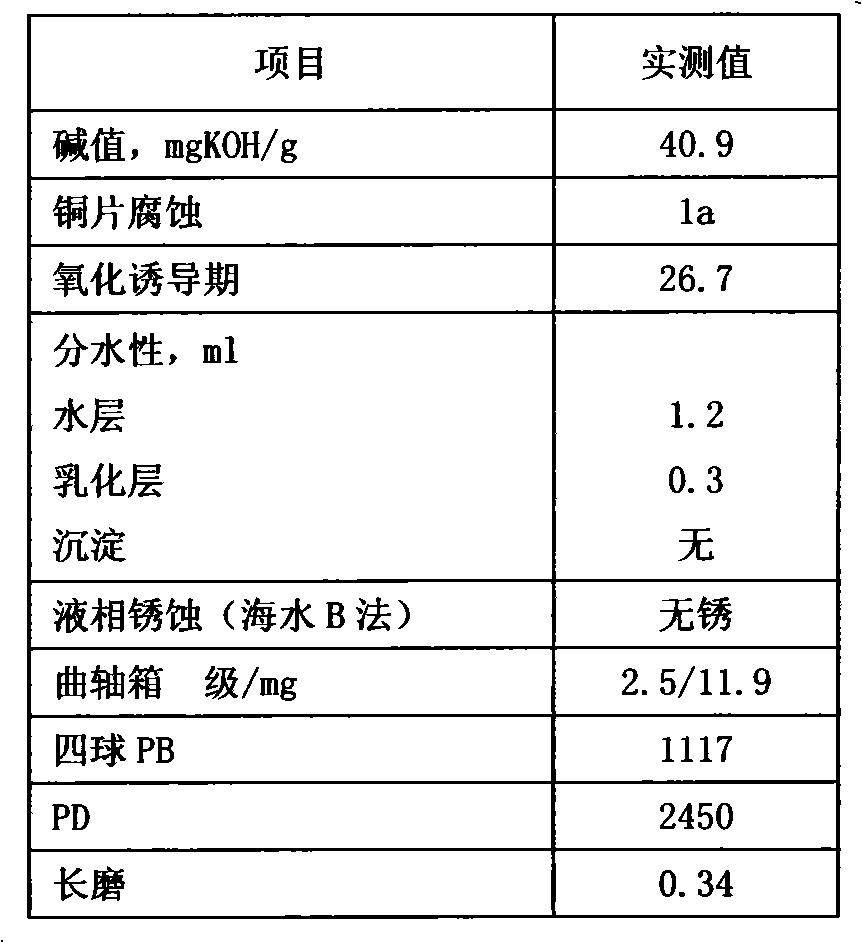
Prepn of antioxidant for lubricant oil
InactiveCN1191340CEffective protectionImprove solubilityAdditivesAntioxidantInternal combustion engine
The preparation of antioxidant for lubricant oil includes the condensation reaction between the mixture liquid containing shielding phenol, aldehyde and CS2 and added dialkyl amine at 40-120 deg.c and product separation and collection. The prepared antioxidant has the integrated structure of both free radical terminator and peroxide decomposing agent and has the capacity of trapping free radical and decomposing hydrogen peroxide to protect the oxidation safety of oil product. Containing S element in its structure makes it possess certain antiwear effect. The product of the present invention is liquid, easy to dissolve and compatible with other functional additives, and may be used in industrial lubricant oil and internal combustion engine oil.
Owner:CHINA PETROLEUM & CHEM CORP +1
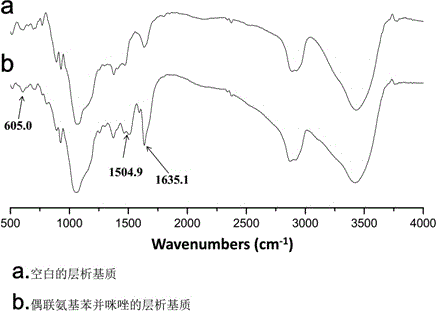
Chromatographic medium using amino benzimidazole as function ligand and preparation method thereof
ActiveCN104096544ALarge adsorption capacityReduce adverse effectsOther chemical processesPeptide preparation methodsChromatographic separationDesorption
The invention discloses a chromatographic medium using amino benzimidazole as a function ligand and a preparation method thereof. Hydrophilic porous microspheres are used as a chromatographic medium, activated by allyl bromide, and coupled with the amino benzimidazole to obtain a medium using the amino benzimidazole as the function ligand; dimethyl sulfoxide and the allyl bromide are sequentially added into a chromatographic matrix for activation; the activated chromatographic matrix is reacted with N-bromo-succinimide for bromo-alcoholization; the bromo-alcoholized chromatographic matrix is mixed with an amino benzimidazole solution for coupling the amino benzimidazole ligand; finally an aqueous ethanol amine solution is used for sealing unreacted bromo-alcoholized ends to obtain a hydrophobic charge induced chromatographic medium using the amino benzimidazole as the function group. The new chromatographic medium is simple in preparation process and high in antibody adsorption capacity, and has the characteristics of non salt dependent adsorption, can realize desorption and recovery by changing the solution pH to weak acid, and can be used for hydrophobic charge induction chromatographic separation of antibodies.
Owner:ZHEJIANG UNIV
Diesel oil multi-effect additive composition
InactiveCN1552829AImprove wear resistanceAntioxidantLiquid carbonaceous fuelsPhenolStabilizing Agents
A diesel multi-effect additive composition includes: components A and B at weight ratio of component A : B = 1:0.1-10, wherein, component A consisting of reacted product or mixture obtained by phenol acids and alkenyl succimide reacted under 60-200degC for 0.5-20 hours at 0.5-5:1 mole ratio in existence of organic solvent, and component B consisting of reacted product or mixture of, at least one selected from C6-C40 organic acid, alcohols, amine, alcohol amine or epoxides. The multi-effect additive achieves additive amount 50-2000ppm, good lubricity and resistance to oxidation as diesel anti-scuff agent, stabilizing agent and dispersing agent.
Owner:CHINA PETROLEUM & CHEM CORP +1
Preparation method of 5-bromine-2-chlorine-4'-ethyoxyl diphenylmethane
InactiveCN104478670ALow water requirementMild reaction conditionsOrganic chemistryOrganic compound preparationDiphenylmethaneAlkyl transfer
The invention relates to the chemical field and particularly relates to a novel synthesis method for preparing a key intermediate 5-bromine-2-chlorine-4'-ethyoxyl diphenylmethane of a drug dapagliflozin for treating diabetes mellitus II. The preparation method comprises the following steps: enabling a starting raw material ortho-toluidine to firstly perform bromization and then perform chlorination after diazotization on a benzene ring with N-bromo-succinimide; then, in the presence of a halogenating agent, performing halogenating reaction of beta-position; and finally, performing Friedel-Crafts alkylation synthesis with phenetole, thereby obtaining the key intermediate. The preparation method is simple and convenient, economical and relatively high in reaction yield in each step, and suitable for industrial production.
Owner:CHINA PHARM UNIV
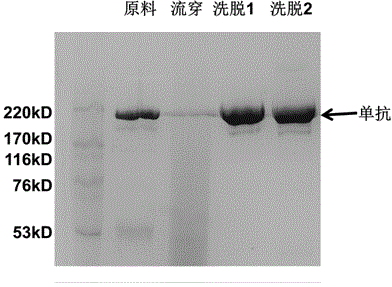
Affinity chromatography medium employing tetrapeptide as functional ligand and preparation method of affinity chromatography medium
ActiveCN104645949AHigh affinityGood choiceOther chemical processesSolid sorbent liquid separationSodium acetateAcetic anhydride
The invention discloses an affinity chromatography medium employing tetrapeptide as a functional ligand and a preparation method of the affinity chromatography medium. The method comprises the following steps: adding dry matrix and allyl bromide to a dimethyl sulfoxide solution, activating, and reacting activating matrix with N-bromo succinimide; enabling bromo alcoholized matrix to react with hexamethylendiamine to obtain amino activating matrix; sequentially washing with deionized water, absolute ethyl alcohol and anhydrous N,N-dimethyl formamide, adding an N,N-dimethyl formamide solution containing tetrapeptide, 2-(7-azobenzotriazole)-N,N,N',N'-te-tramethyluronium hexafluorophosphate and N,N-diisopropylethylamine, and coupling a tetrapeptide ligand; and putting a medium coupled to tetrapeptide in a mixed liquid of sodium acetate and acetic anhydride to obtain the affinity chromatography medium employing tetrapeptide as the functional ligand. According to the novel chromatography medium developed by the method, a functional group is tetrapeptide composed of tyrosine, phenylalanine, arginine and histidine, and is designed on the basis of a protein A binding site of an antibody Fc segment; the antibody binding selectivity is greatly improved; and the affinity chromatography medium can be applied to efficient separation of an antibody.
Owner:ZHEJIANG UNIV
Anti-tartar detersive for quenching oil column of ethylene unit
ActiveCN101445749AImprove bindingInhibition of polymerizationHydrocarbon purification/separationHydrocarbonsPhenolic antioxidantRoom temperature
The invention relates to an anti-tartar detersive for a quenching oil column of an ethylene unit. In order to solve the problems of the anti-tartar and cleaning of the quenching oil column, the invention provides the anti-tartar detersive for the quenching oil column. The anti-tartar detersive can inhibit the generation of dirt from the source; meanwhile, the detersive can also eliminate the generated dirt in the quenching oil column on line, thus providing necessary conditions for the long period operation of the ethylene unit. The anti-tartar detersive for the quenching oil column of the ethylene unit of the invention is characterized in that according to weight percentage, phenolic antioxidant is 1-18%, phosphorus metal ion deactivator is 8-20%, amine inhibitive substance is 5-35%, thiazolinyl succinimide dispersant is 2-18%, solvent is 30-70%; the substances are put into a container for heating, stirred and mixed evenly and cooled to room temperature, and then filtered to obtain the product.
Owner:浙江杭化科技股份有限公司
Lubricating oil composition of diesel engine
The invention relates to a lubricating oil composition of an engine, in particular to a lubricating oil composition for a heavy-load diesel engine, characterized by containing a great amount of hydrotreating base oil. The composition of the invention also contains suitable amounts of alkyl salicylates, sulfurized alkyl sulfide salts, sulphonate, macromolecular ashless dispersant, polyisobutylene-bis-succinimide ashless dispersant, zinc dialkyl dithiophosphate, phenolic antioxidant, amine-type antioxidant and sulfur-bearing antioxidant antiwear agent. The lubricating oil composition can satisfy the specification requirement of diesel engine oil CF-4, which is specified by GB11122-2006. The composition has simple prescription, low cost and convenient modulation.
Owner:PETROCHINA CO LTD
Lubricating oil
The invention discloses a lubricating oil, which contains base oil, a detergent and a dispersing agent and an additive, wherein the additive is unnecessary; the dispersing agent contains butanimide and ester succinate. With the adoption of the lubricating oil in an engine, the oil sludge generation can be greatly reduced in the using process of the lubricating oil, and the lubricating oil can perform stable functions for a long time, so as to have good maintenance function for the engine.
Owner:BYD CO LTD
Soluble branch substituted anthracene molecule blue material as well as preparation method and uses thereof
InactiveCN101200634AEasy to synthesizeEasy to purifySolid-state devicesSemiconductor/solid-state device manufacturingSolubilityN-Bromosuccinimide
The present invention discloses soluble branch substituted anthracene molecular blue light-emitting material and a preparation method and an application thereof. The anthracene molecular blue light-emitting material considers anthracene as a center, and soluble branch group Dendron and rigid group Ar1 are respectively accessed at the two ends of the anthracene to ensure that the prepared emitting material with an asymmetrical structure has certain solubility and can be purified by solution method. When the anthracene molecular blue light-emitting material is prepared, 9-bromoanthracene or 9-anthracene boric acid ester is used as reaction raw material, the soluble branch group Dendron is introduced by the palladium catalysis Suzuki coupling reaction, then N-bromosuccinimide is used for bromizing at 10-position of the anthracene to obtain an anthracene molecular bromide; the Ar1 is introduced into the obtained bromide or boric acid prepared from the bromide by the palladium catalysis Suzuki coupling reaction to obtain a target product. The material has the advantages of synthesis and purification and has important application prospect at electroluminescence display, illumination and laser.
Owner:GUANG ZHOU NEW VISION OPTO ELECTRONICS TECH
Lubricating oil for natural gas and gasoline dual purpose fuel engine
Owner:CHANGAN UNIV
Synthesis method of 5-bromo-2-chloro benzoic acid
ActiveCN105622382AEasy to operateHigh purityOrganic compound preparationCarboxylic compound preparationBenzoic acidChlorobenzilate
The invention provides a synthesis method of 5-bromo-2-chloro benzoic acid.The method includes the following steps of A, making 2-chlorine benzotrichloride and bromide reagents react under the effect of a catalyst to obtain 2-chloro-5-bromine benzotrichloride, wherein bromide reagents include one or more of bromine, N-bromosuccinimide, dibromohydantoin and hydrobromic acid; B, conducting hydrolysis reaction on 2-chloro-5-bromine benzotrichloride in the step A under the acid condition to obtain 5-bromo-2-chloro benzoic acid.According to the method, 2-chlorine benzotrichloride which is low in price and easy to obtain is adopted as the raw material, operation is easy, intermediates do not need to be purified, 5-bromo-2-chloro benzoic acid is synthesized through a one-pot method, purity is high, yield is high, three-waste emission is little, and production cost is low.It is shown through experiment results that 2-chloro-5-benzoic acid obtained according to the synthesis method has yield larger than 95% and purity of 80-92%.
Owner:苏州正济药业有限公司
Low-concentration weakly alkaline cyanide-free copper plating and bath solution preparing method
The invention relates to a bath solution preparing method and a low-concentration weakly alkaline cyanide-free copper plating which can ensure that the bonding force exists between a plating layer and a matrix, the plating solution has best stability, better dispersing capacity and deep plating capacity and simple process maintenance. The low-concentration weakly alkaline cyanide-free copper plating comprises a main salt, a conductive salt, a main complexing agent and a secondary complexing agent, wherein the main salt is copper chloride, the conductive salt comprises a mixture of sodium chloride, potassium chloride and ammonium chloride, the main complexing agent is a mixture of tartrate, citrate, nitrilotris(methylene)]tris-phosphonic acid salt, (1-Hydroxyethylidene)bis-phosphonic acid tetrasodium salt and ethylenediamine tetra(methylenephosphonic acid) salt; and the secondary complexing agent is a mixture of ortho-hydroxybenzoic acid, succinimide and derivatives thereof and dimethyl hydantoin. The invention has the advantages of capability of ensuring the bonding force of the plating and the matrix, best stability and better dispersing capacity and deep plating capacity and simple process maintenance.
Owner:HANGZHOU HAISHANG TECH
Organic fuel cell anti-freeze cooling liquid with low conductivity and ultra-long acting and preparation method of anti-freeze cooling liquid
ActiveCN108102616AImprove conductivityReduce conductivityHeat-exchange elementsFuel cellsOrganic fuel8-Hydroxyquinoline
The invention discloses an organic fuel cell anti-freeze cooling liquid with low conductivity and ultra-long acting and a preparation method of the anti-freeze cooling liquid in the field of anti-freeze cooling fluids. The anti-freeze liquid is prepared from components in percentage by weight as follows: 10wt%-70wt% of ethylene glycol, 0.001wt%-0.01wt% of 8-hydroxyquinoline, 0.005wt%-0.02wt% of uracil, 0.01wt%-0.03wt% of 4-acetaminophen, 0.01wt%-0.05wt% of benzotriazole octadecylamine, 0.005wt%-0.05wt% of N-bromosuccinimide, 0.001wt%-0.01wt% of inosine and the balance of deionized water. The preparation method of the anti-freeze liquid comprises the steps as follows: all the components of the anti-freeze cooling fluid are put in a reaction kettle in percentage by mass; the components are stirred and mixed for 30-90 min, so that all components are fully dissolved and mixed uniformly, the mixed solution passes through anion and cation mixed exchange resin by the aid of a pressure pump, and the fuel cell anti-freeze cooling fluid is obtained. The organic fuel cell anti-freeze cooling liquid with low conductivity and ultra-long acting is weakly alkaline, is anti-freezing and anti-boiling and has performance of low conductivity, ultra-long acting and high corrosion inhibition.
Owner:扬州中德汽车零部件有限公司
Prepn of antioxidant for lubricant oil
InactiveCN1453347AEffective protectionImprove solubilityAdditivesAntioxidantInternal combustion engine
The preparation of antioxidant for lubricant oil includes the condensation reaction between the mixture liquid containing shielding phenol, aldehyde and CS2 and added dialkyl amine at 40-120 deg.c and product separation and collection. The prepared antioxidant has the integrated structure of both free radical terminator and peroxide decomposing agent and has the capacity of trapping free radicaland decomposing hydrogen peroxide to protect the oxidation safety of oil product. Containing S element in its structure makes it possess certain antiwear effect. The product of the present invention is liquid, easy to dissolve and compatible with other functional additives, and may be used in industrial lubricant oil and internal combustion engine oil.
Owner:CHINA PETROLEUM & CHEM CORP +1
Preparation method for nucleoside compounds
InactiveCN101148450ARaw materials are easy to getHigh yieldOrganic chemistryBulk chemical productionPurineCyclic compound
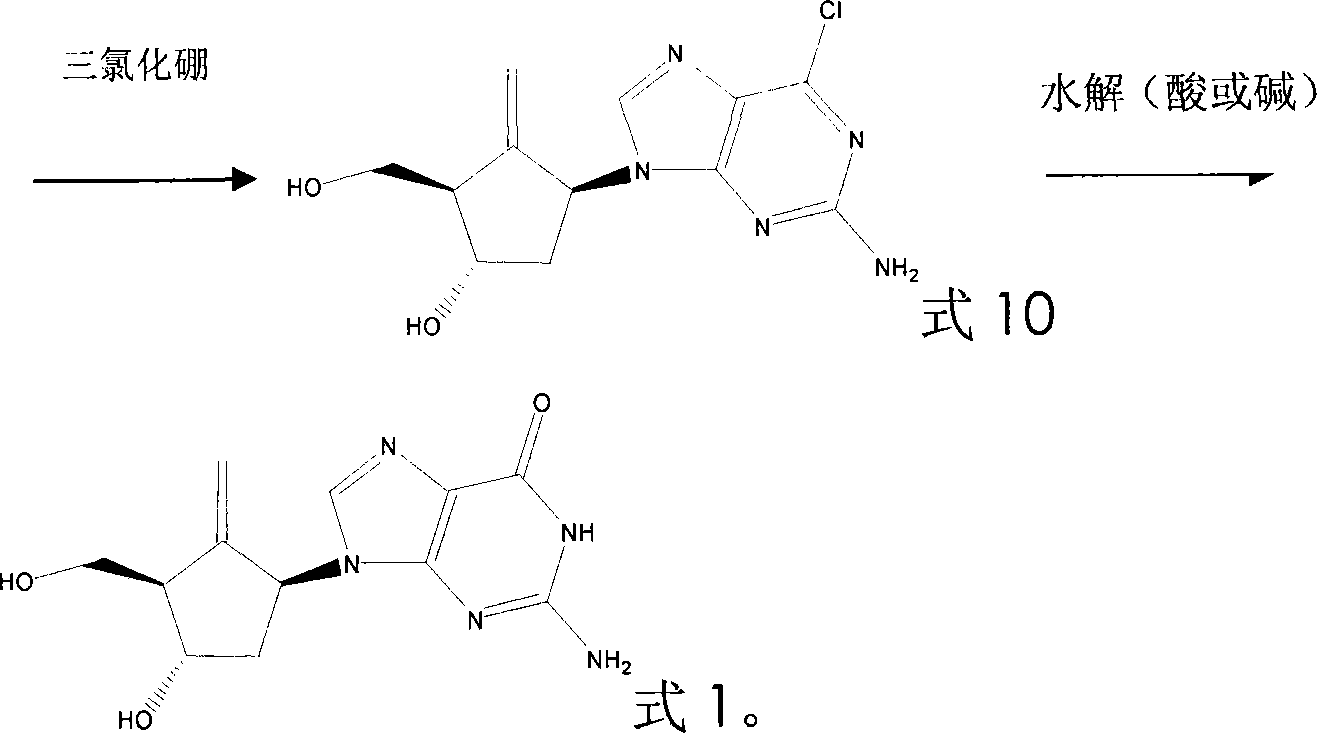
The present invention relates to nucleoside compound preparing process, and is especially preparation process of 2-amino-9-[(1S, 3S, 4S)-4-hydroxy-3-hydroxymethyl-2-methyleneamyl]-1, 9-dihydro-6-H-purine-6-one. The initial materials [1s-(1alpha, 2beta, 3alpha, 5alpha)]-3-(benzylozy)-2-[( benzylozy) methyl]-6-oxa cyclic compound [3.1.0] and hexane are aminated, dess-mardin oxidized and nysted methylenated to obtain one intermediate compound; the intermediate compound is condensated with 2-amino-4-chloro-5-nitropyrimidinyl one as one other antiviral intermediate; and the condensation product is finally reacted through cyclization, hydrolysis, etc to obtain the target compound. The present invention has facile material and high product yield.
Owner:严红芳
Silver-graphitic electrical contact composite plating layer and method of preparing the same
InactiveCN101256903AEvenly distributedFlat surfaceContact materialsConductive materialAdditive ingredientMetallic materials
Silver -black lead electrical contact composite plating and manufacture method thereof, which belongs to metallic material technology field. The steps are: confecting compound plating solution, black lead granule dispersing, and substrate preprocess, compound plating solution ingredient: methane sulfonic acid silver 0.05M / L, succinimide 0.15M / L-1.5M / L, composite plating. boracic acid 0.5M / L-0.5M / L, black lead content in solution 2g / L-50g / L. The composite plating is made up of silver and black lead, wherein the black lead volume percent is: 1-15%, silver volume fraction 85%-99%, contact resistance 1.2-2m Omega, hardness 70-100Hv, density more than 99.9%. The present invention plating solution is protection environment and non-toxic; the ultrasonic energy promoting adequate distribution of black lead granule, the produced composite plating has smooth surface and high density.
Owner:SHANGHAI JIAO TONG UNIV
Method for perparing anticoagulant composed of chitosan-arginine
InactiveCN1519035AImprove anticoagulant performanceLow cytotoxicityPharmaceutical containersMedical packagingHemocompatible MaterialsCross-link
A Chitosan-arginine conjugate as anticoagulant material is prepared through dissolving chitosan in the solution of N, N, N'-tetramethyl ethanediamine, adding 1-ethyl-3-(3-dimethylamino propyl) carbodiimine, N-hydroxy-butanediimide and arginine, reacting while magnetic stirring, dialyzing, drying to obtain said conjugate, adding glucosyl aldehyde oxide, and cross-linking reaction to become film. Its advantages are high effect and low poison to cell.
Owner:TIANJIN UNIV
Lubricating oil composition for marine medium-speed cylindrical diesel engine
The invention relates to a lubricating oil composition for a marine medium-speed cylindrical diesel engine, which comprises the following components in percentage by weight: 12-22% of high temperature detergent, 1-3% of ashless dispersant, 0.5-1.5% of antioxidant, 0.002-0.005% of antifoam agent and the balance of base oil, wherein the high temperature detergent is compounded from the following components in percentage by weight: 30-70% of high base number alkyl calcium salicylate and 30-70% of high base number sulfurized calcium alkyl phenate; the ashless dispersant is bis-succinimide; the antioxidant is zinc dialkyl dithiophosphate; and the antifoam agent is methylsilicone oil. The invention can satisfy the operational requirements of advanced marine medium-speed cylindrical piston diesel engine oil; and the lubricating oil composition has the function of inhibiting the generation of black oil sludge, reduces the faults of the medium-speed cylindrical piston diesel engine due to bad lubrication and the like, prolongs the service life of the engine, and has the advantages of accessible raw materials, simple production technique and low cost.
Owner:PETROCHINA CO LTD
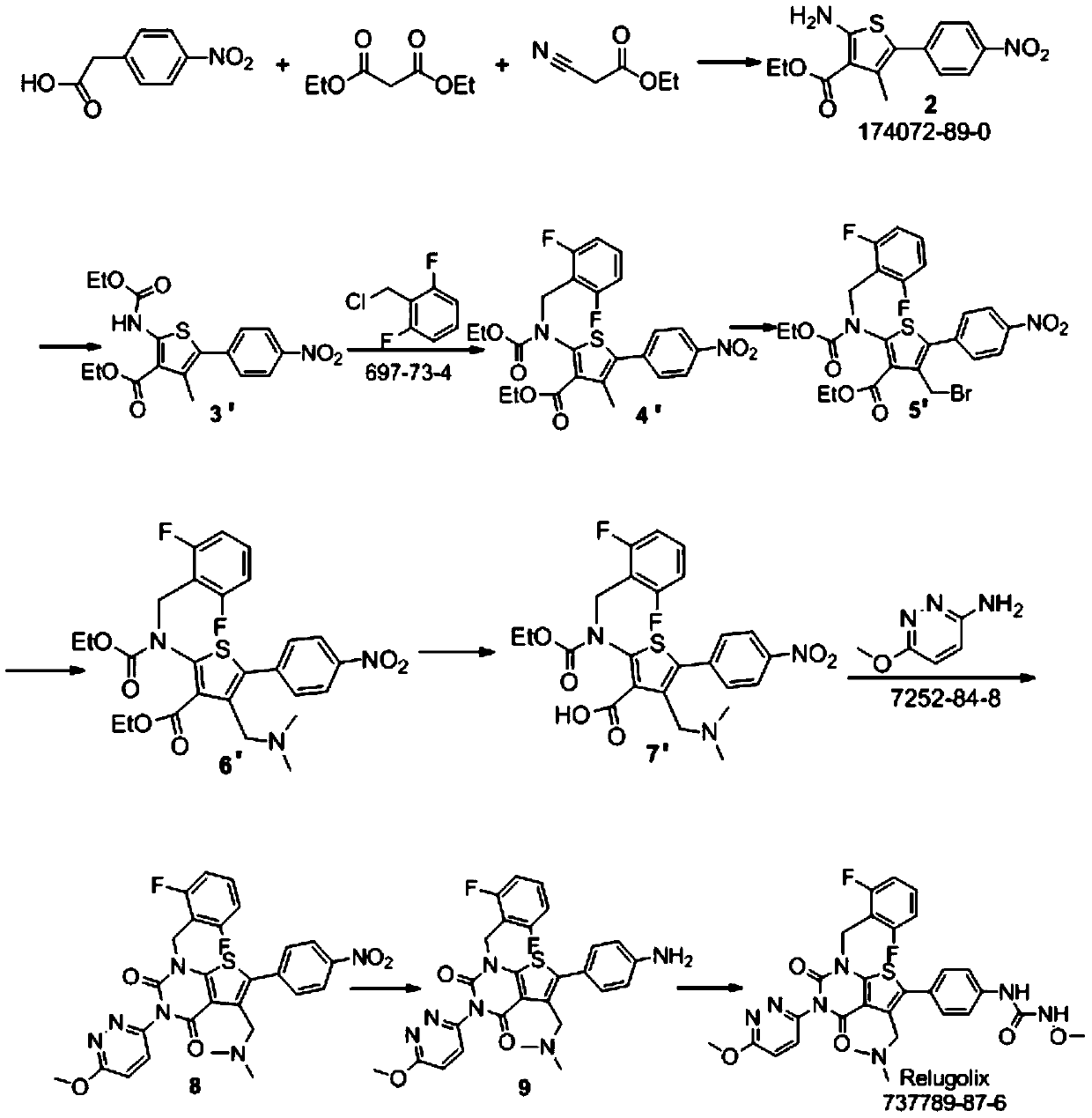
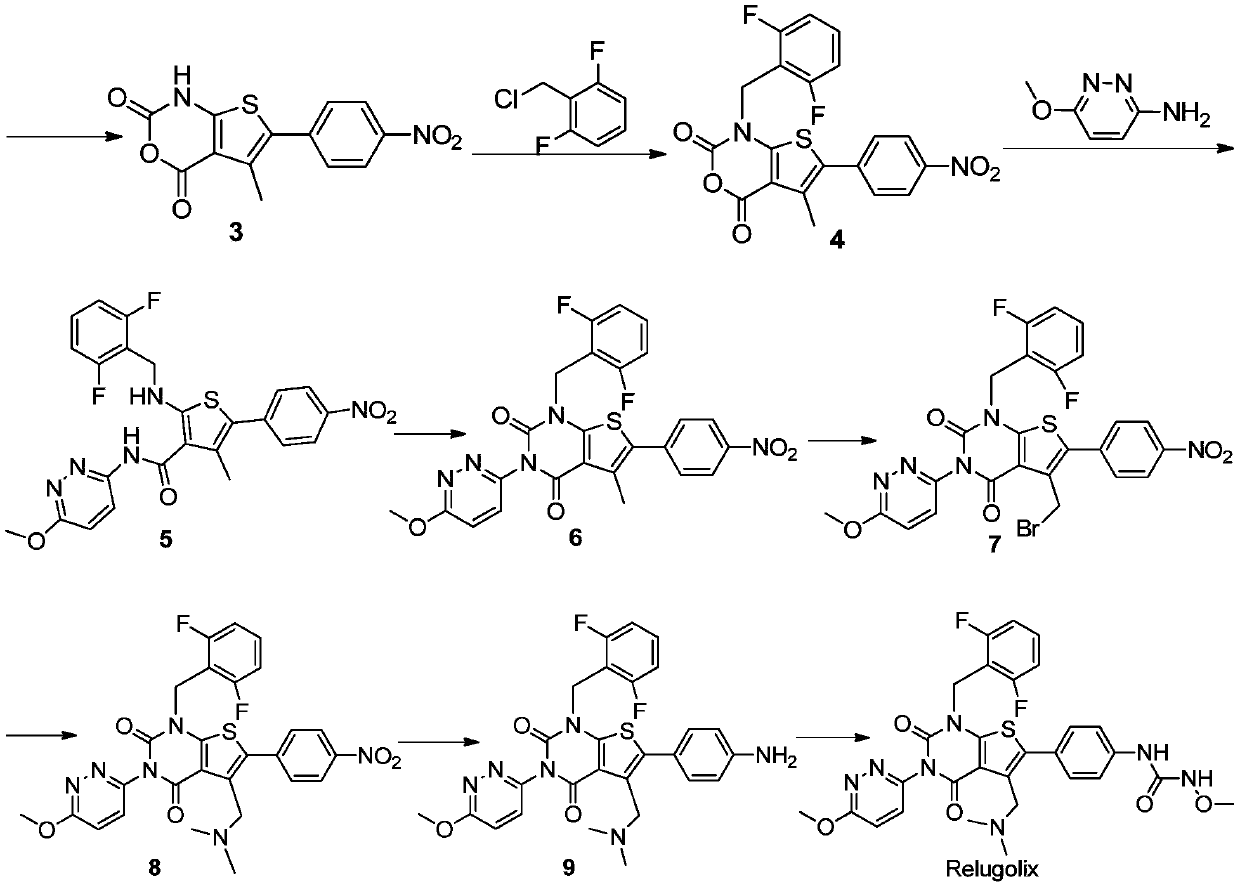
Relugolix synthesis method
The present invention provides a method for preparing a relugolix intermediate compound 8. The method comprises: (a) carrying out a reaction on a compound 2 and N,N'-carbonyldiimidazole to obtain a compound 3; (b) carrying out a reaction on the compound 3 and 2,6-difluorobenzyl chloride to obtain a compound 4; (c) carrying out a reaction on the compound 4 and 3-amino-6-methoxypyridazine to obtaina compound 5; (d) carrying out a reaction on the compound 5 and N,N'-carbonyldiimidazole to obtain a compound 6; (e) carrying out a reaction on the compound 6, N-bromosuccinimide and azobisisobutyronitrile to obtain a compound 7; and (f) carrying out a reaction on the compound 7 and dimethylamine hydrochloride to obtain a compound 8. The invention further provides a relugolix preparation method, which comprises: (g) carrying out a reaction on the compound 8 obtained by the method and hydrogen under a catalyst to obtain a compound 9; and (h) carrying out a reaction on the compound 9, N,N'-carbonyldiimidazole and methoxy amine hydrochloride to obtain relugolix. According to the present invention, the method adopts the route sequentially comprising loop closing and coupling, such that the method has characteristics of simple operation, less side-reaction, mild reaction condition, high yield, high product purity and easy product purification, and is suitable for commercial scale production.
Owner:四川伊诺达博医药科技有限公司
Preparation method of hydrophilic polymer-modified polyurethane foaming plastic carrier
ActiveCN103627015ASimple processEasy to operateBiological water/sewage treatmentCross-linkPolymer modified
The invention discloses a preparation method of a hydrophilic polymer-modified polyurethane foaming plastic carrier. The preparation method comprises steps of: 1) cutting polyurethane flexible foam into pieces; 2) preparing an aqueous starch solution having a proper concentration, an aqueous sodium carboxymethylcellulose solution having a proper concentration, a N,N-dimethyl formamide solution of polybutanimide having a proper concentration; 3) dipping polyurethane foaming plastic into the aqueous starch solution, the aqueous sodium carboxymethylcellulose solution and the N,N-dimethyl formamide solution of the polybutanimide; and then transferring the polyurethane foaming plastic into an aqueous cross-linking agent solution and heating; and 4) taking the foaming plastic out, washing and drying. The preparation method is simple in technology and convenient in operation. A microbial carrier which has an advantage of cost performance and is used for sewage treatment can be obtained after modification.
Owner:山东青素环保材料有限责任公司
Lubricating oil composition for diesel engine
InactiveCN1523090AImprove detergencyGood soot dispersion performanceAdditivesBase-materialsChemical compositionAntioxidant
The present invention relates to a luboil composition for heavy-load diesel oil engine. It is characterized by that it contains lots of hydrogenated base oil. Said invented composition also contains proper quantity of high base number salicylate, low base number salicylate, sulfurized alkylphenol salt, boronized disuccinyl imine ashless dispersant, high-molecular ashless dispersant, zinc dialkyl dithiophosphate and phenolic and amine auxiliary antioxidants. Said luboil composition can meet the specification requirements of SAE J300 and API CF-4 diesel engine oil.
Owner:PETROCHINA CO LTD
Low-noise lithium-based lubricating grease composition and preparation method thereof
The invention relates to a lithium-based lubricating grease composition. Based on the total weigh of lubricating grease, the lithium-based lubricating grease composition comprises the following components: (1) 75 to 94 percent of base oil; (2) 5 to 15 percent of lithium soap thickening agent; and (3) 0.1 to 4 percent of alkenyl diimide dispersing agent. The lubricating grease composition has excellent bearing vibrating performance, can be applied to medium and small-sized sealing bearings and is applicable to lubrication of motor bearings of official automated equipment and household appliances.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for chemically synthesising gastrodin
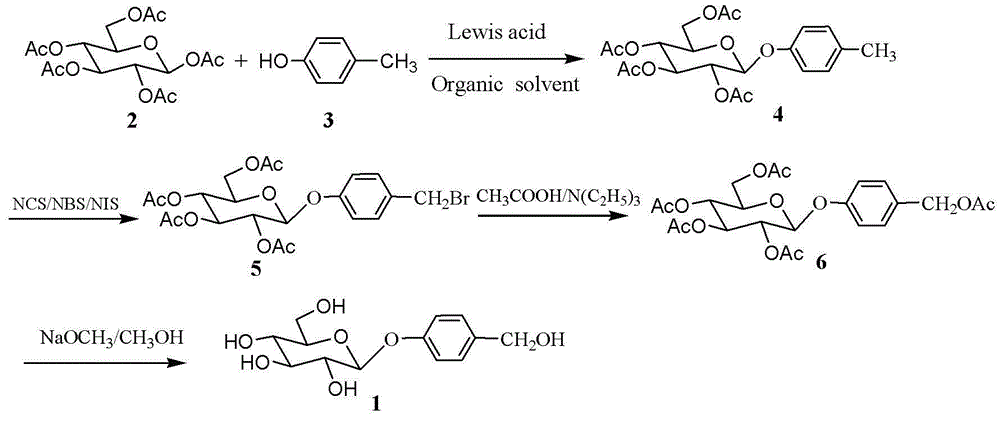
InactiveCN102977161AImprove stabilityAvoid harmSugar derivativesSugar derivatives preparationChemical synthesisLewis acid catalysis
The invention discloses a method for chemically synthesising gastrodin, comprising the following steps of: in the presence of a molecular sieve, under the catalysis of Lewis acid, and performing glycosylation reaction on pent-acetyl-b-D-glucose and p-cresol in an organic solvent with to generate 4-methylphenyl-2,3,4,6-O-tetra-acetyl-b-D-glucopyranoside; then preparing 4-halomethylphenyl-2,3,4,6-O-tetra-acetyl-b-D-glucopyranoside from 4-methylphenyl-2,3,4,6-O-tetra-acetyl-b-D-glucopyranoside and N-halosuccinimide in the presence of an initiator, and then reacting 4-halomethylphenyl-2,3,4,6-O-tetra-acetyl-b-D-glucopyranoside with the mixed solution of glacial acetic acid and tertiary amine to obtain 4-acetoxylmethylphenyl-2,3,4,6-O-tetra-acetyl-b-D-glucopyranoside; and finally removing the acetyl protecting group from the 4-acetoxylmethylphenyl-2,3,4,6-O-tetra-acetyl-b-D-glucopyranoside in an alkaline condition to obtain gastrodin. Compared with the traditional method, the method disclosed by the invention has easily available raw materials, and is short in reaction time, capable of preparing the reaction product in each step via recrystallization, simple, and more suitable for industrialized production for gastrodin.
Owner:QINGDAO AGRI UNIV
Synthetic method of deuterium-marked sulfanilamide
ActiveCN102603655ARaw materials are easy to getMild reaction conditionsOrganic chemistrySulfonyl chlorideN-Chlorosuccinimide
The invention relates to a synthetic method of deuterium-marked sulfanilamide. The method comprises the following steps of: reacting deuterated sulfonic acid with N-chlorosuccinimide (NCS) to obtain deuterated sulfonyl chloride which is not required to be separated; and adding amine for undergoing an amination reaction under the action of a metal catalyst to obtain deuterium-marked sulfanilamide. Compared with the prior art, the method has the advantages that: acyl chloride synthesis and the amination reaction are performed with a 'one pot process', operation is simple and convenient, the yield is high, and a synthesized product comprises sulfapyridine-benzene ring-D4, sulfadimidine-benzene ring-D4, sulfameter-benzene ring-D4, sulfaquinoxaline-benzene ring-D4, sulfisoxazole-benzene ring-D4 and sulfamethoxazole-benzene ring-D4.
Owner:SHANGHAI RES INST OF CHEM IND
Method for preparing deuterated diphenyl urea
ActiveCN102675018AOrganic compound preparationIsotope introduction to organic compoundsMedicinal chemistrySuccinchlorimide
The invention relates to a method for preparing deuterated diphenyl urea. Specifically, the invention provides an intermediate N-(1, 1, 1-3 deuterated methyl) benzo succinimide which can be used for preparing a deuterated diphenyl urea compound and an application of the intermediate in the preparation of the deuterated diphenyl urea compound. The method can be used for conveniently preparing the intermediate with the high purity and various deuterated diphenyl urea compounds in high efficiency.
Owner:SUZHOU ZELGEN BIOPHARML
Synthesis method of 4-chloro-2-cyano-1-dimethylamino-sulfonyl-5-(4-methylphenyl)imidazo
ActiveCN102424671AEmission reductionShort reaction timeOrganic chemistrySodium dithioniteN-Chlorosuccinimide
The invention discloses a synthesis method of 4-chloro-2-cyano-1-dimethylamino-sulfonyl-5-(4-methylphenyl)imidazo, which aims to solve the technical problems of long reaction time, low yield and environment pollution of the prior art. The method is used for preparing 2,2-dichloro-4'-methyl acetophenone as an intermediate through an acylation reaction of methylbenzene and dchloroethanoyl chloride and preparing 4(5)-chloro-2-cyano-5(4)-(4-methylphenyl)imidazo as an intermediate by taking ethyl acetate and the like as solvent, N-succinchlorimide as chlorinating agent and sodium dithionite and the like as reductant. The synthesis method has short reaction time, high yield and total yield up to 61.7% and is mainly used for preparing the 4-chloro-2-cyano-1-dimethylamino-sulfonyl-5-(4-methylphenyl)imidazo.
Owner:XIAN MODERN CHEM RES INST
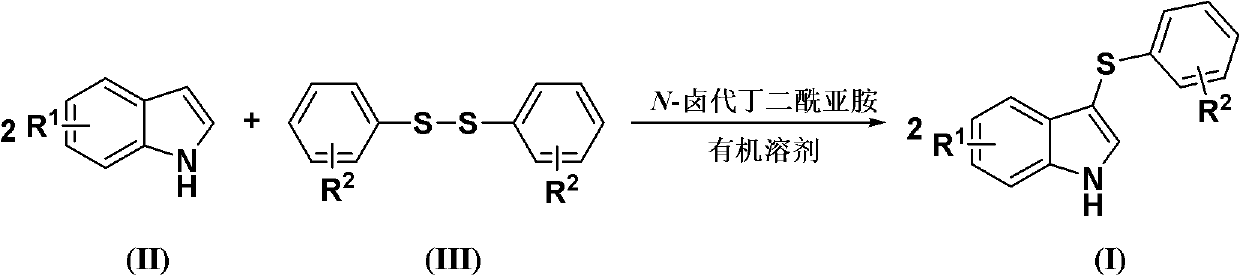
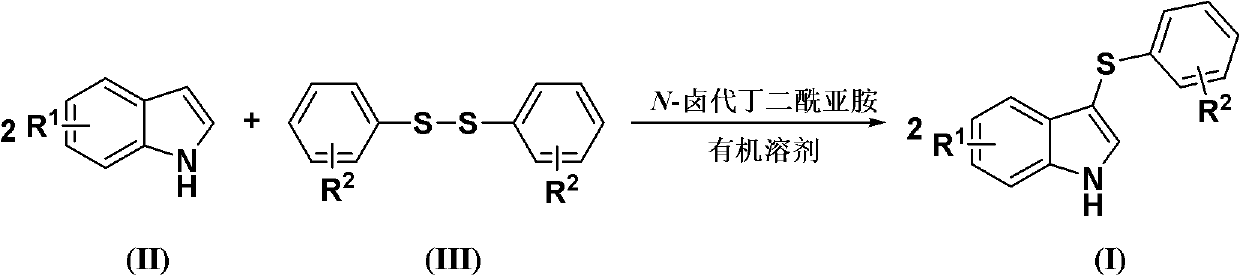
Method for synthesizing 3-aryl sulfydryl indole compound
The invention discloses a method for synthesizing a 3-aryl sulfydryl indole compound which is shown as a formula (I). According to the method for synthesizing the 3-aryl sulfydryl indole compound, an indole compound which is shown as a formula (II) and diaryl disulfides which are shown as a formula (III) are taken as raw materials, and the 3-aryl sulfydryl indole compound is obtained by fully reacting the indole compound and the diaryl disulfides in an organic solvent at the temperature of between -30 and 40 DEG C in the presence of N-halogenate succinimide; and the N-halogenate succinimide is one of N-chlorobutanimide, N-bromosuccinimide, and N-Iodosuccinimide. According to the method, a nonmetal low-toxicity reaction system is used, and cost is low; reaction selectivity and yield are high; a process route is advanced and reasonable, and reaction conditions are mild; and the method has great implementation value and social and economic benefit.
Owner:WENZHOU UNIVERSITY
Lubricating oil additive containing ultrafine rare-earth powder
InactiveCN103642569AAvoid direct contactReduce harmful ingredientsLubricant compositionPhosphateOil additive
The invention relates to a lubricating oil additive containing ultrafine rare-earth powder. The additive consists of the following components in percentage by mass: natural serpentine mineral powder, ultrafine rare-earth powder, surfactant, a dispersing agent, a friction modifier, an auxiliary and base oil, wherein the ultrafine rare-earth powder is one of cerium hydroxide, neodymium oxide and cerium fluoride; the surfactant is borate, span-60 or oleic acid; the dispersing agent is either polyisobutene dibutyl succinimide or high molecular weight polyisobutene dibutyl succinimide; the friction modifier is one of melamine cyanurate, boron nitride and calciuym nitride; the auxiliary comprises 72% of dialkyl disulfo oxygen molybdenum phosphate sulfide, 18% of polymethyl tetradecyl acrylate and 10% of a vinyl-propylene copolymer; and the base oil is 500SN base oil. The additive has obvious corrosion resistance; and after an engine is treated technically by using the self-repairing additive, the dry friction coefficient of the machine is reduced from original 0.05 to be less than 0.005 and therefore the surface abrasion is reduced.
Owner:ENERGY & ENVIRONMENT RES INST OF HEILONGJIANG PROVINCE +2
Succinimide silver plating solution and electroplating method
The invention discloses a succinimide silver plating solution and an electroplating method. The succinimide silver plating solution comprises the following components: 10-20g / L silver nitrate and 130-150g / L succinimide by referring silver content, 30-40g / L methylsulfonate by referring methanesulfonic acid radical, 20-30g / L carbonate and 1-2g / L polyethyleneimine by referring carbonate radical. According to the invention, succinimide is taken as a coordination agent, methylsulfonate is selected as an additive for increasing compactness and smoothness of a plating layer, polyethyleneimine is taken as a brightener, so that the plating liquid has good stability, and the plating layer has discolouration resistance and strong weldability.
Owner:WUXI XUEJIANG ENVIRONMENT ENG EQUIP
Automobile exhaust PM2.5 pollutant treatment agent
InactiveCN102559336AIncrease oxygen contentBurn fullyLiquid carbonaceous fuelsFuel additivesSuccinic acidInternal combustion engine
The invention relates to an automobile exhaust PM2.5 pollutant treatment agent added in fuel oil. The formula of the treatment agent comprises the following components by weight percent: 10%-30% of methanol, 0.03%-0.07% of ferrocene, 13%-16% of butanol, 0.5%-0.8% of nanometer lanthanum oxide, 0.6-0.9% of nanometer cerium oxide, 0.05%-0.08% of potassium permanganate, 13%-15% of methyl isopropyl ether, 0.1%-0.5% of aluminum acetylacetonate, 12%-18% of dodecyl alkenyl succinic acid, 1%-3% of urea, 5%-10% of isopropanol, 1%-5% of multi-alkenyl succinimide, 0.02%-0.05% of manganese dioxide and 10%-30% of ethanol. The treatment agent is prepared through simple processes such as mixing, stirring, precipitating and filtering. The automobile exhaust PM2.5 pollutant treatment agent is non-toxic, has no bad smell, does not corrode the equipment and can be used to increase the power of the engine and greatly reduce the emission of PM2.5 in the exhaust; and after 10-20g of the automobile exhaust PM2.5 pollutant treatment agent added in fuel oil is added in 1L of oil and the engine runs for 10min, the effect can be realized and the PM2.5 concentration of the exhaust discharged by the kerosene internal combustion engine can be reduced from 330mg / m<3> to 60mg / m<3>.
Owner:洛阳万山高新技术应用工程有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com