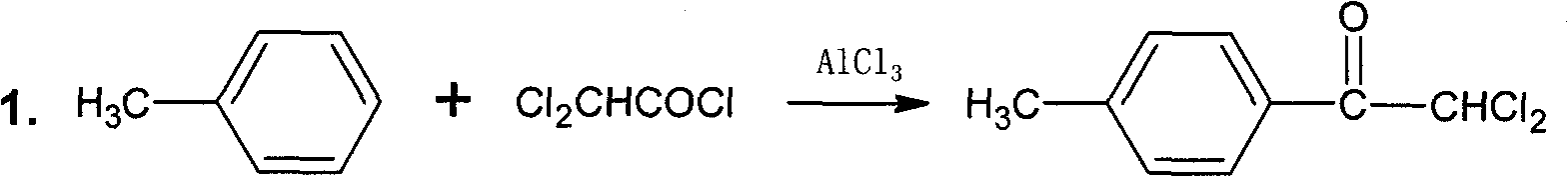Synthesis method of 4-chloro-2-cyano-1-dimethylamino-sulfonyl-5-(4-methylphenyl)imidazo
A technology of dimethylaminosulfonyl and oximemethyne imidazole, which is applied in the field of synthesis of 4-chloro-2-cyano-1-dimethylaminosulfonyl-5-imidazole, can solve solvent waste, Long response time, increased waste water discharge and other problems, to achieve the effect of saving costs, reducing waste water discharge, and reducing environmental pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] a. Synthesis of 2,2-Dichloro-4′-methylacetophenone
[0018] In a 1000ml four-necked flask equipped with a thermometer, agitator and reflux condenser, add 450ml of toluene, 80.1g of anhydrous aluminum trichloride, add 88.5g of dichloroacetyl chloride dropwise, and control the temperature at 25±5°C. After the addition, the temperature was raised to 80°C and reacted for 2 hours. The reaction solution was poured into 500g of crushed ice, stirred until the ice was completely dissolved, separated, the organic phase was evaporated to remove the toluene, and recrystallized with 50ml of absolute ethanol to obtain 115.7g of white solid.
[0019] b. Synthesis of 1-hydroxy-4(5)-(4-methylphenyl)-2-oximinomethine imidazole-3-oxo
[0020] Add 64.1g of 2,2-dichloro-4'-methylacetophenone, 104.3g of hydroxylamine hydrochloride, 200ml of methanol, and 100ml of water into a 1000ml four-necked flask equipped with thermometer, stirrer and reflux condenser, and heat to reflux , React for 2 hours, ...
Embodiment 2
[0027] a. Synthesis of 2,2-Dichloro-4′-methylacetophenone
[0028] In a 1000ml four-necked flask equipped with a thermometer, agitator and reflux condenser, add 450ml of toluene, 80.1g of anhydrous aluminum trichloride, add 88.5g of dichloroacetyl chloride dropwise, and control the temperature at 25±5°C. After the addition, the temperature was raised to 80°C and reacted for 2 hours. The reaction solution was poured into 500g of crushed ice, stirred until the ice was completely dissolved, separated, the organic phase was evaporated to remove toluene, and recrystallized with 50ml of absolute ethanol to obtain 115.7g of white solid.
[0029] b. Synthesis of 1-hydroxy-4(5)-(4-methylphenyl)-2-oximinomethine imidazole-3-oxo
[0030] Add 64.1g of 2,2-dichloro-4'-methylacetophenone, 104.3g of hydroxylamine hydrochloride, 200ml of methanol, and 100ml of water into a 1000ml four-necked flask equipped with thermometer, stirrer and reflux condenser, and heat to reflux , React for 2 hours, add ...
Embodiment 3
[0035] The method is basically the same as that of Example 1, except that in step b, "hydroxylamine hydrochloride" is replaced with hydroxylamine sulfate. The total yield of cyanoxaazole is 59.5% and the content is 98.4%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com