Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
198results about "Saccharide with carbocyclic radicals" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
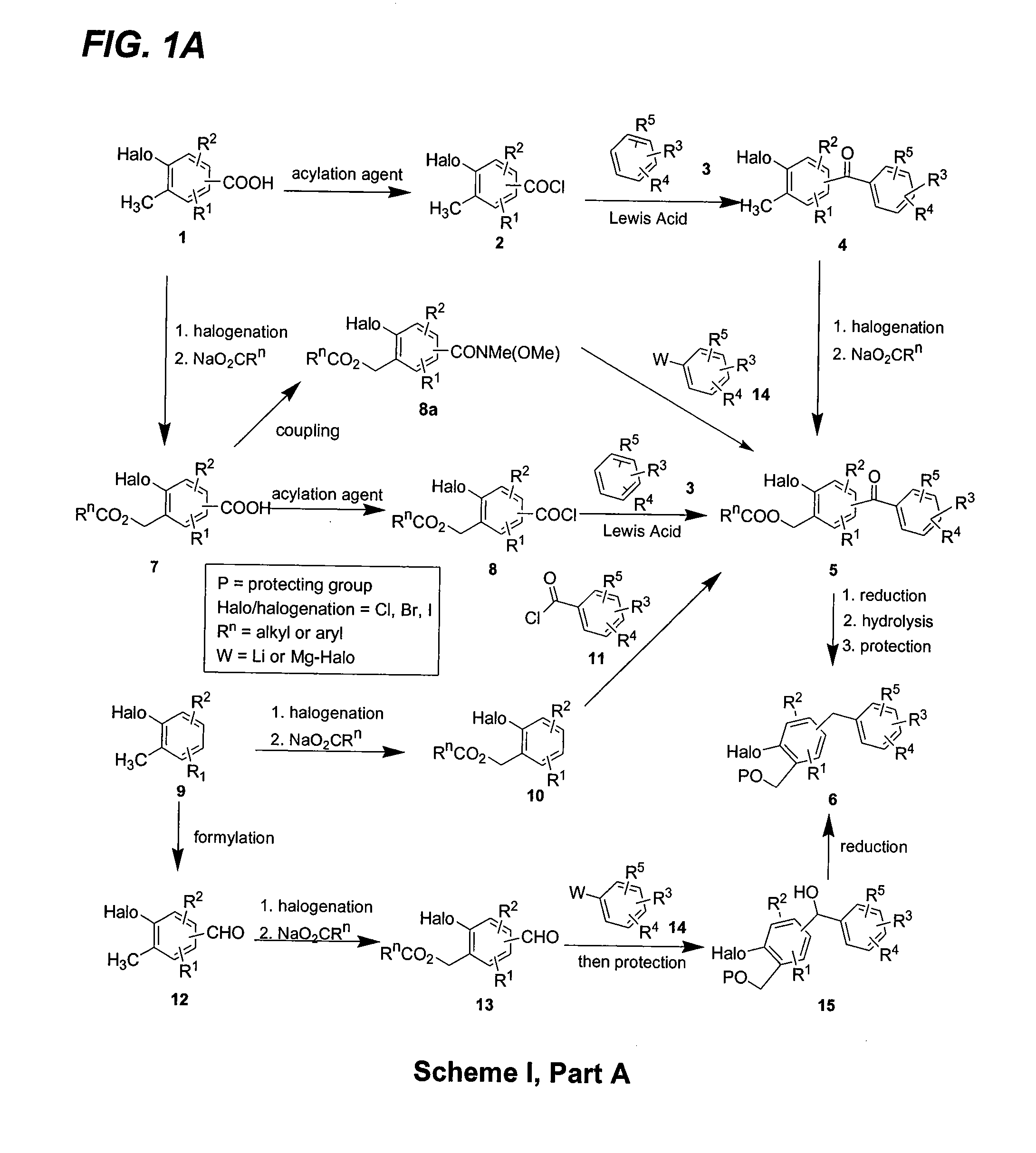
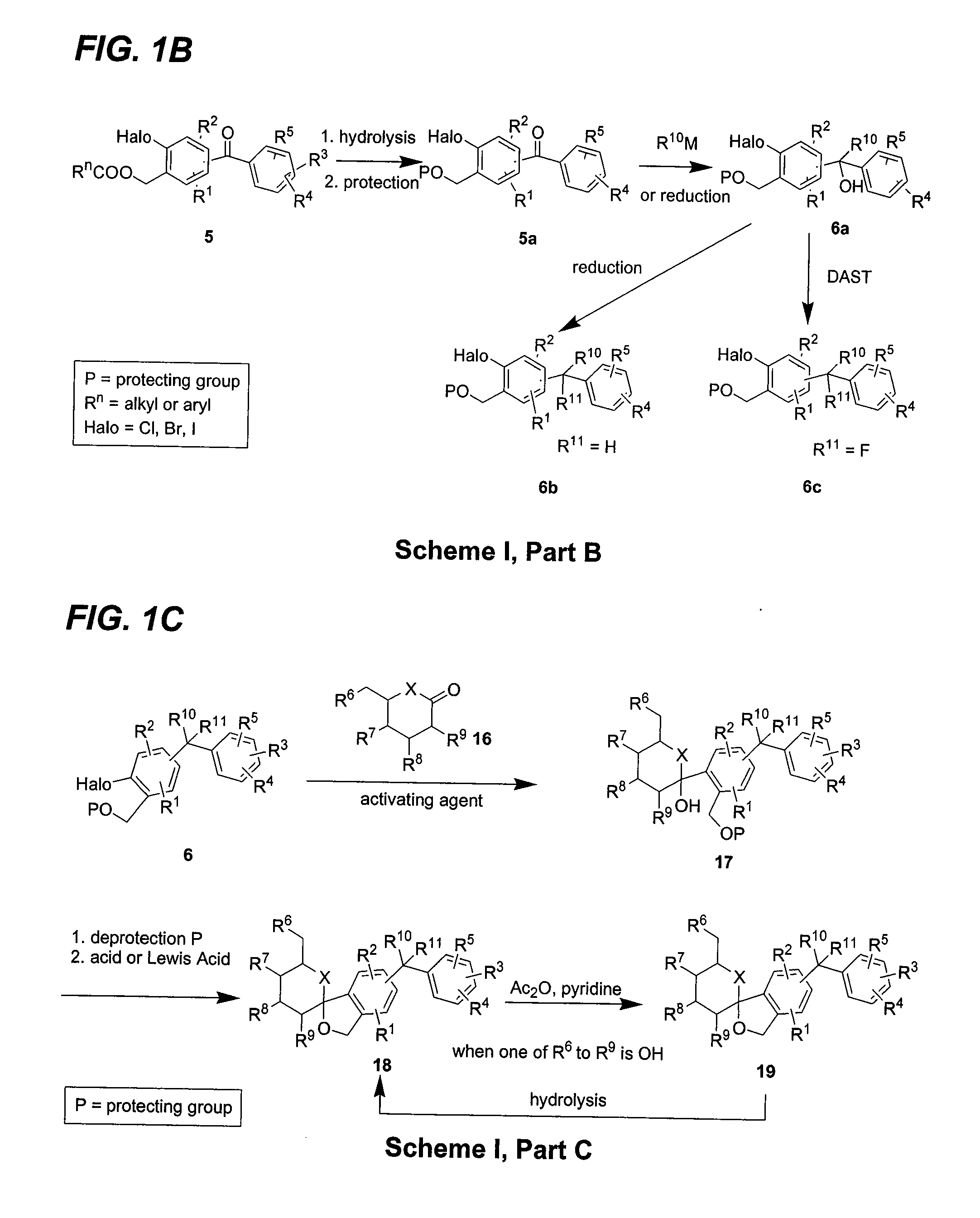
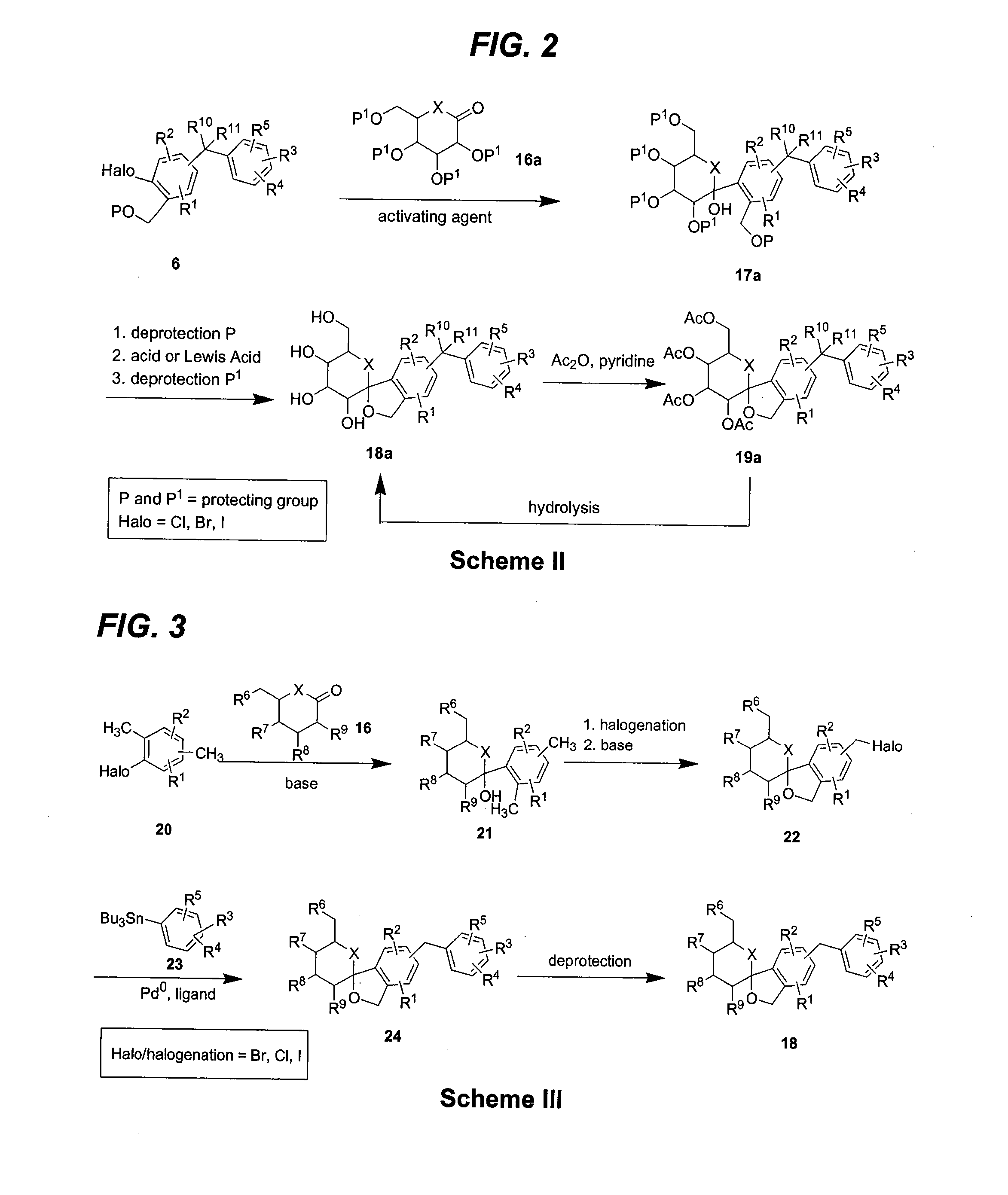
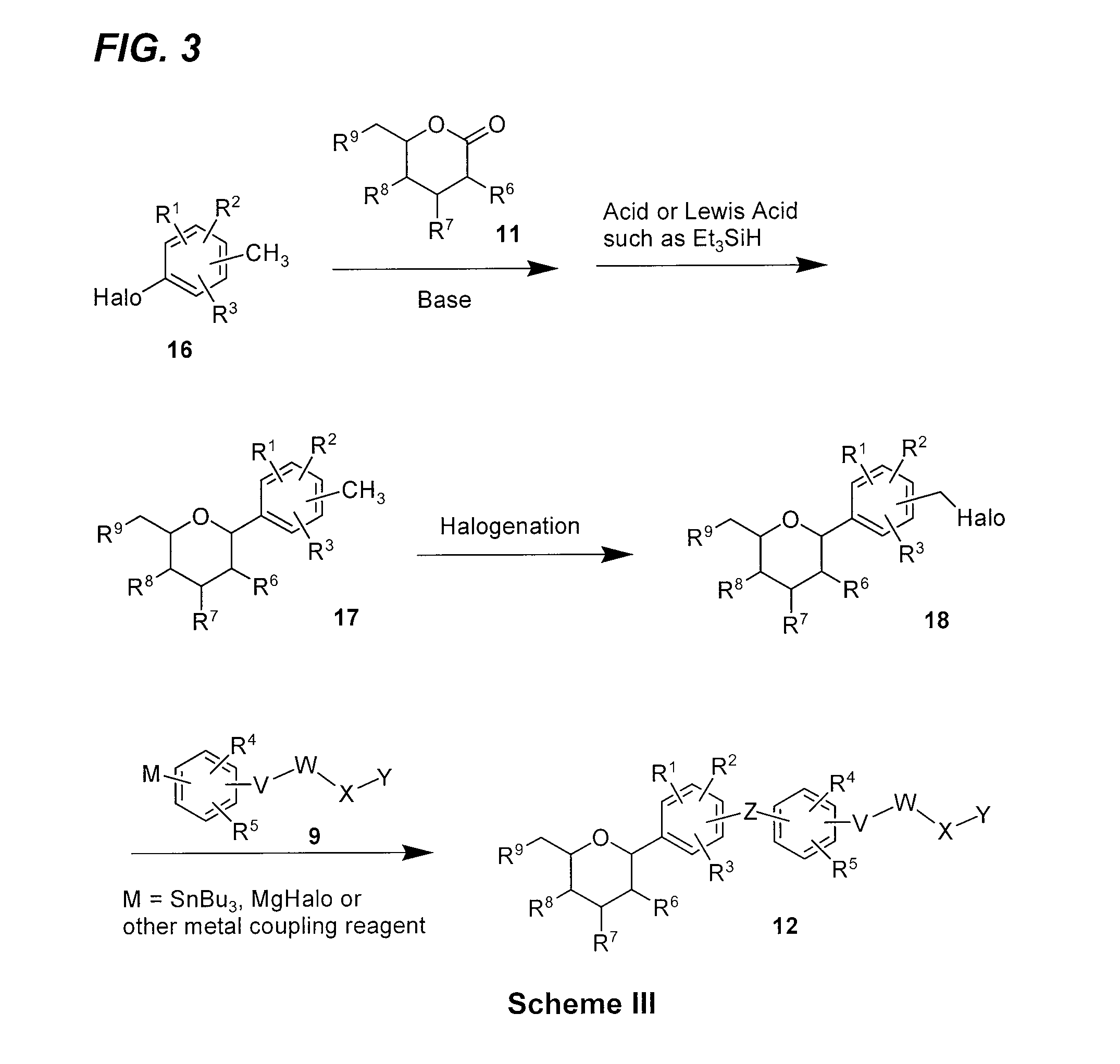
C-aryl glucoside SGLT2 inhibitors and method
Owner:BRISTOL MYERS SQUIBB CO
Glucose transport inhibitors and methods of use
Provided are compounds having an inhibitory effect on sodium-dependent glucose cotransporter SGLT. The invention also provides pharmaceutical compositions, methods of preparing the compounds, synthetic intermediates, and methods of using the compounds, independently or in combination with other therapeutic agents, for treating diseases and conditions which are affected by SGLT inhibition.
Owner:THERAKOS INC
C-aryl glucoside SGLT2 inhibitors and method
Owner:BRISTOL MYERS SQUIBB CO
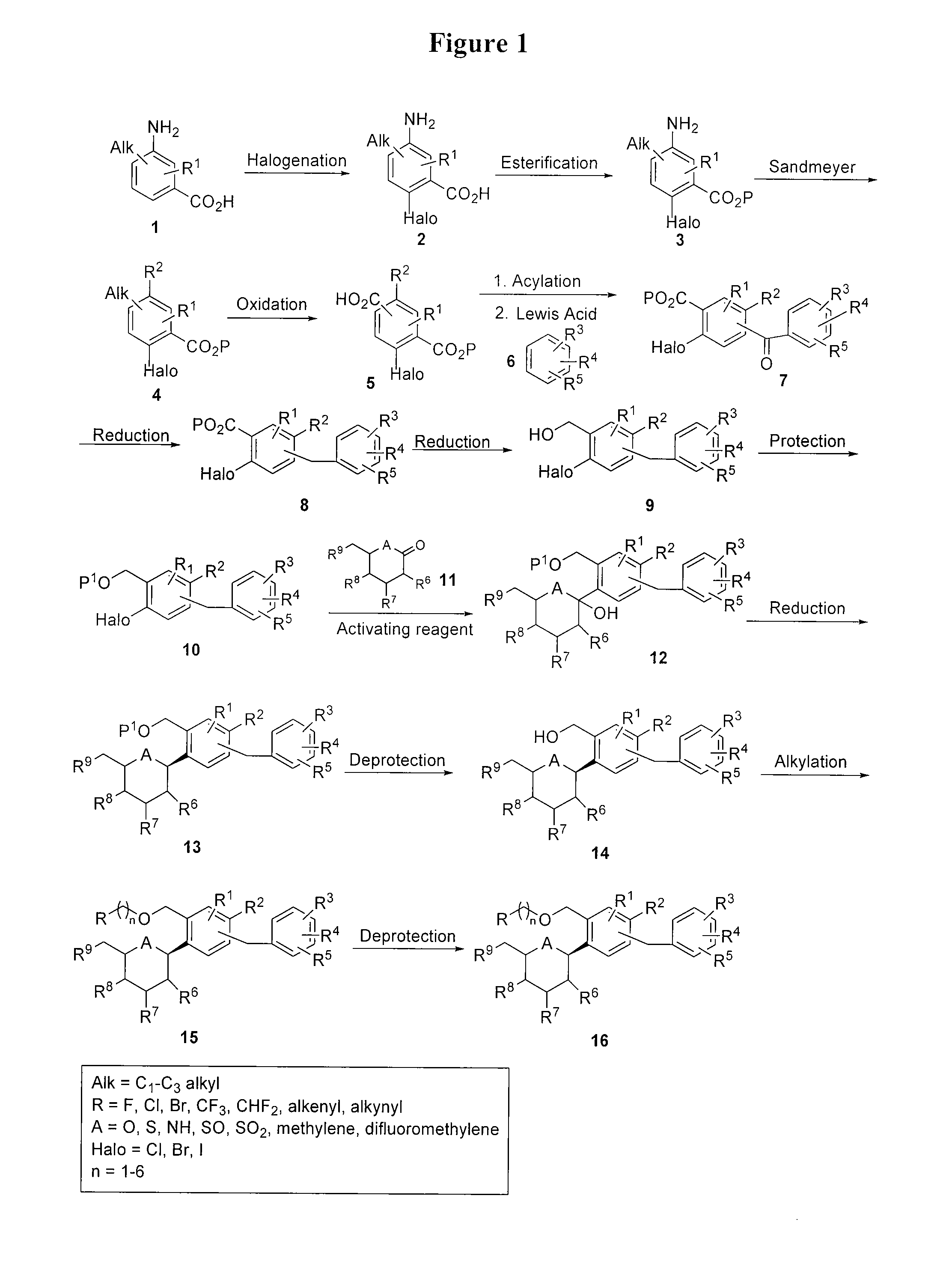
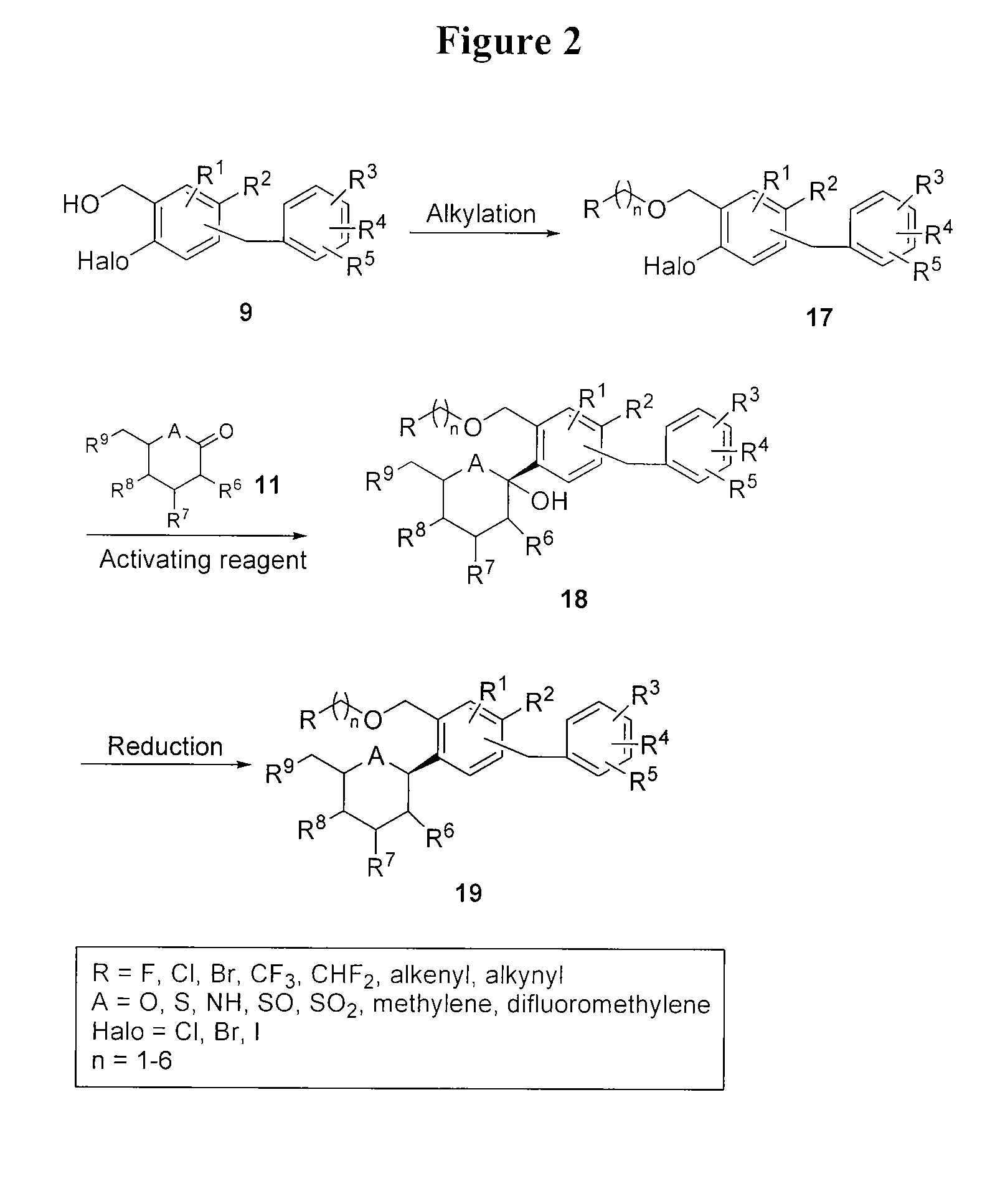
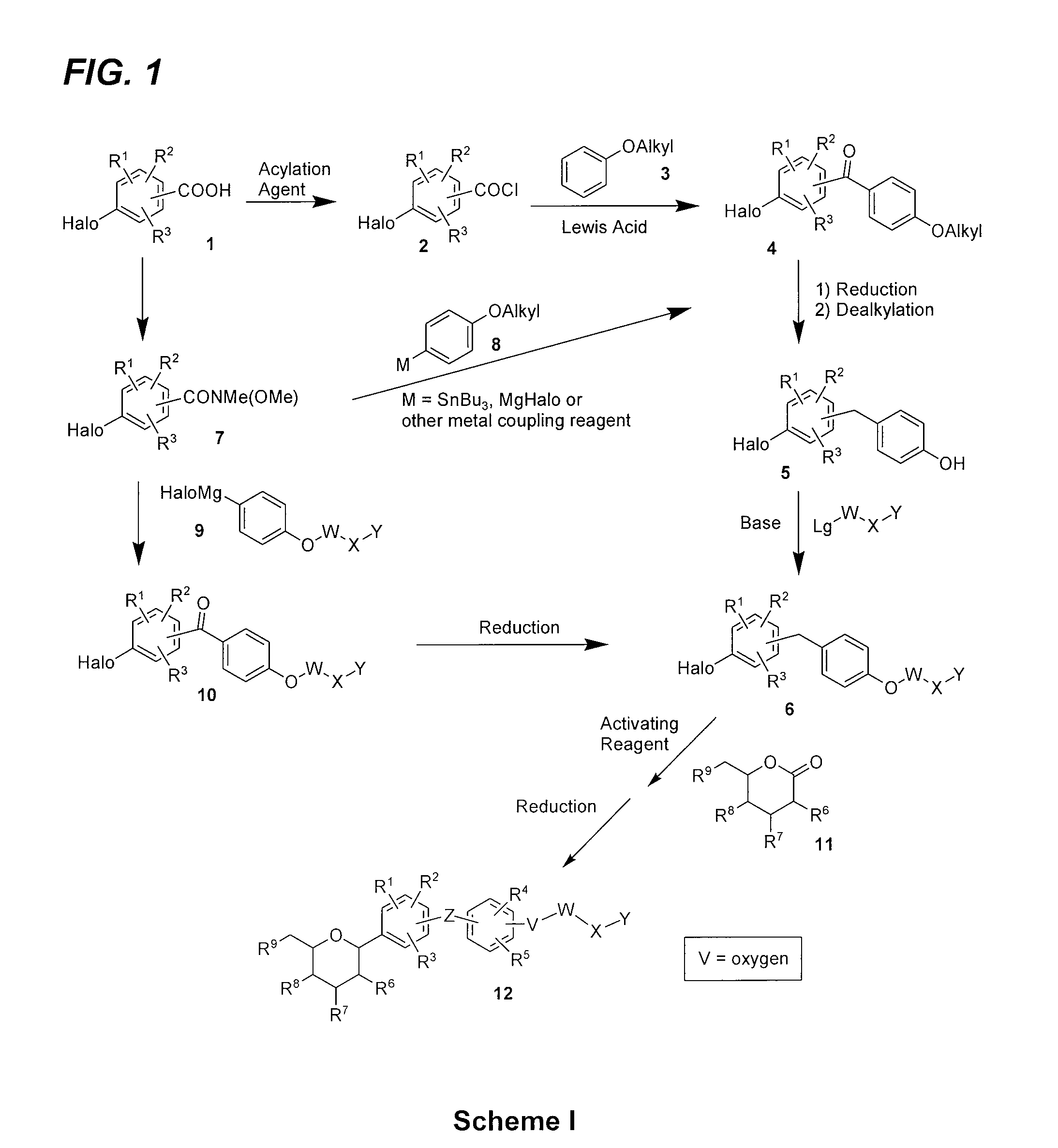
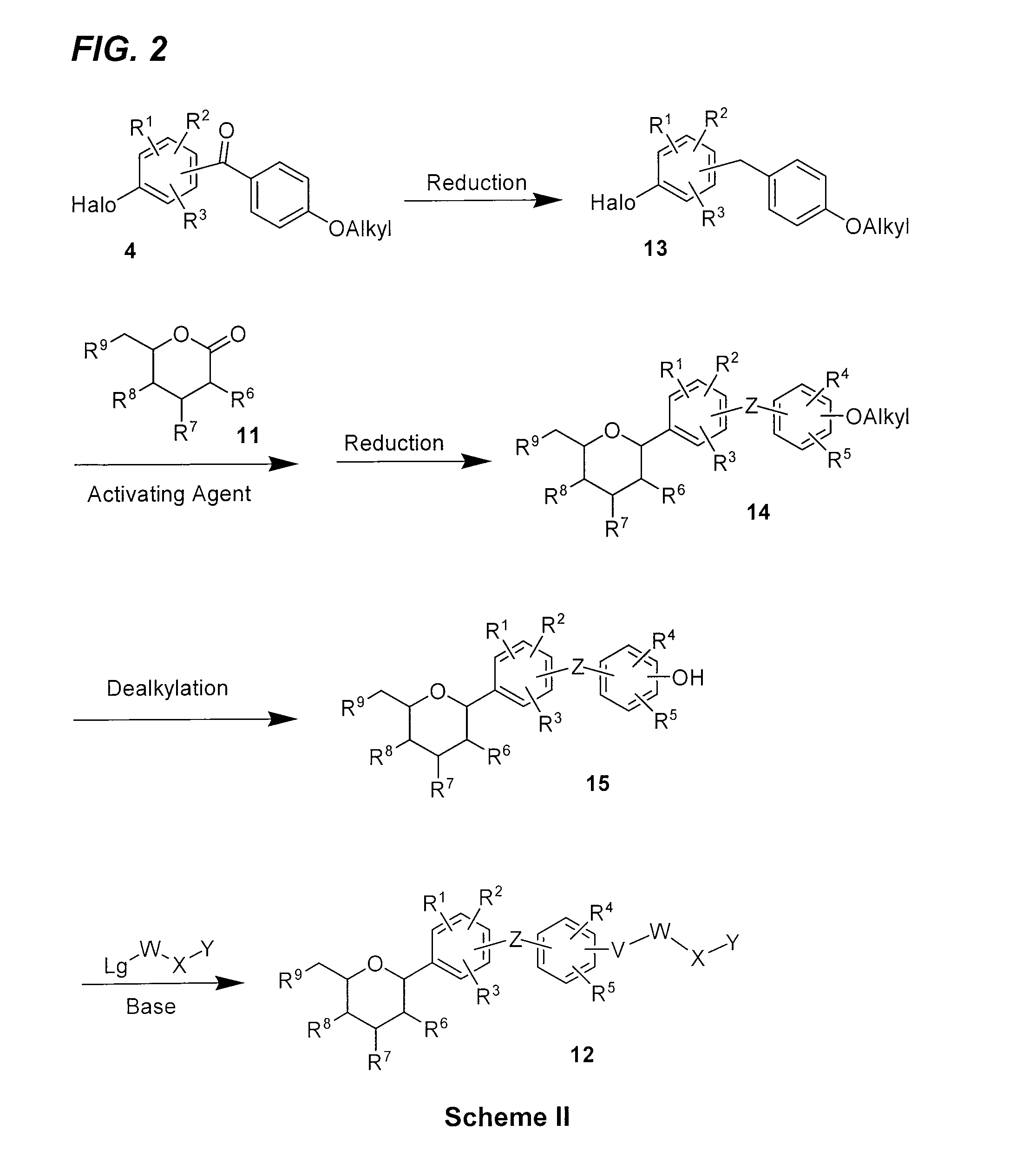
Benzylic glycoside derivatives and methods of use
Provided are compounds having an inhibitory effect on sodium-dependent glucose cotransporter SGLT. The invention also provides pharmaceutical compositions, methods of preparing the compounds, synthetic intermediates, and methods of using the compounds, independently or in combination with other therapeutic agents, for treating diseases and conditions which are affected by SGLT inhibition.
Owner:THERAKOS INC
Benzylbenzene derivatives and methods of use
Provided are compounds having an inhibitory effect on sodium-dependent glucose cotransporter SGLT. The invention also provides pharmaceutical compositions, methods of preparing the compounds, synthetic intermediates, and methods of using the compounds, independently or in combination with other therapeutic agents, for treating diseases and conditions which are affected by SGLT inhibition.
Owner:THERACOSBIO LLC
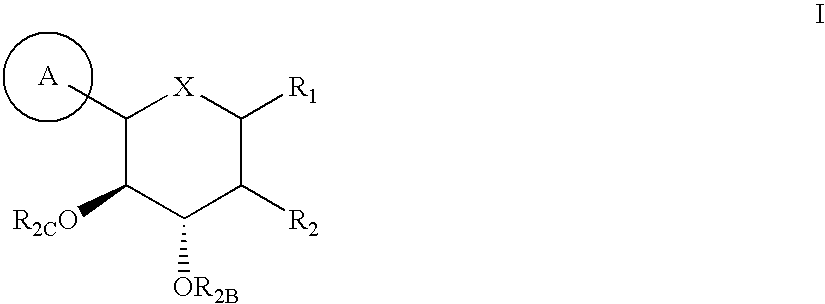
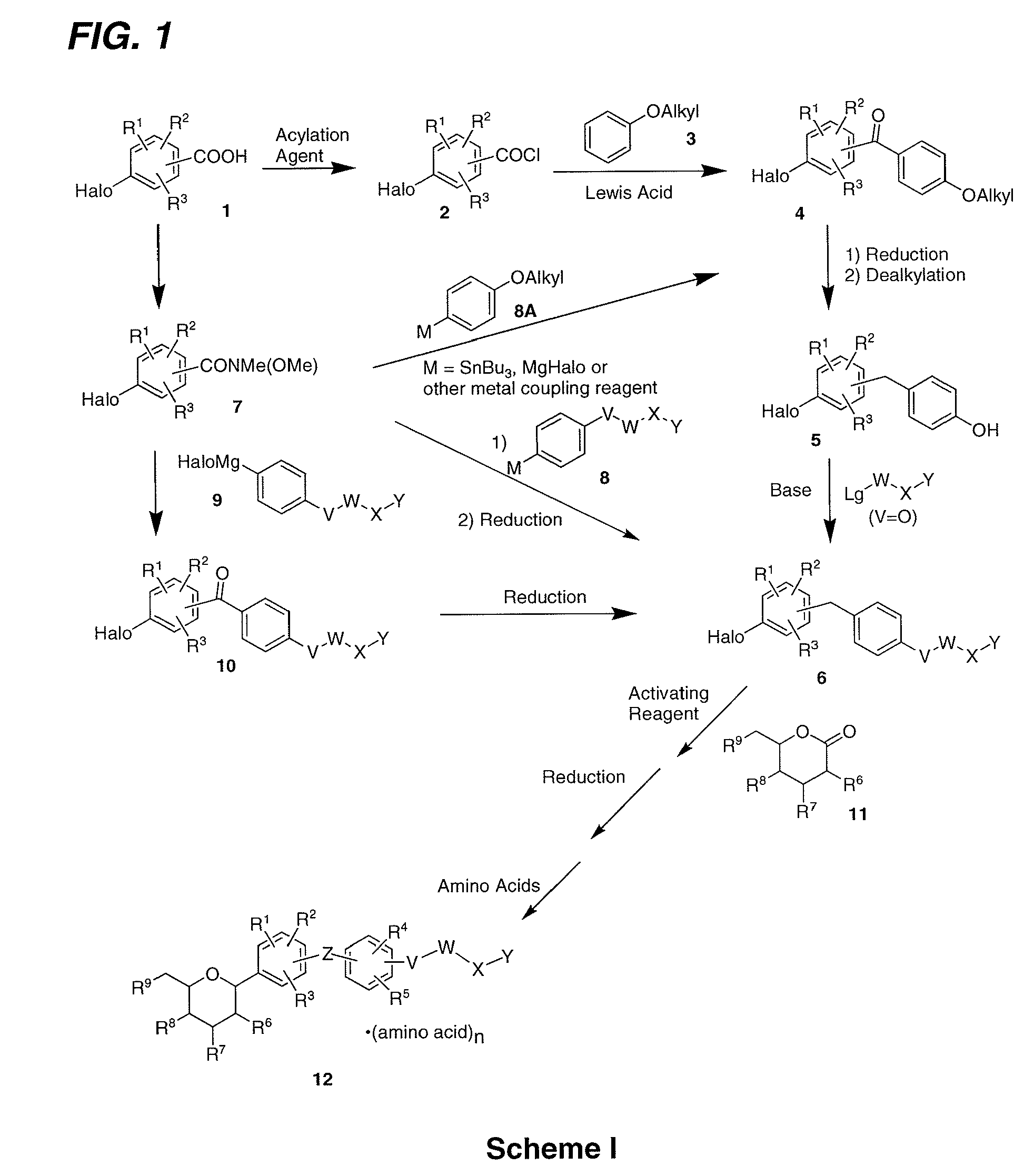
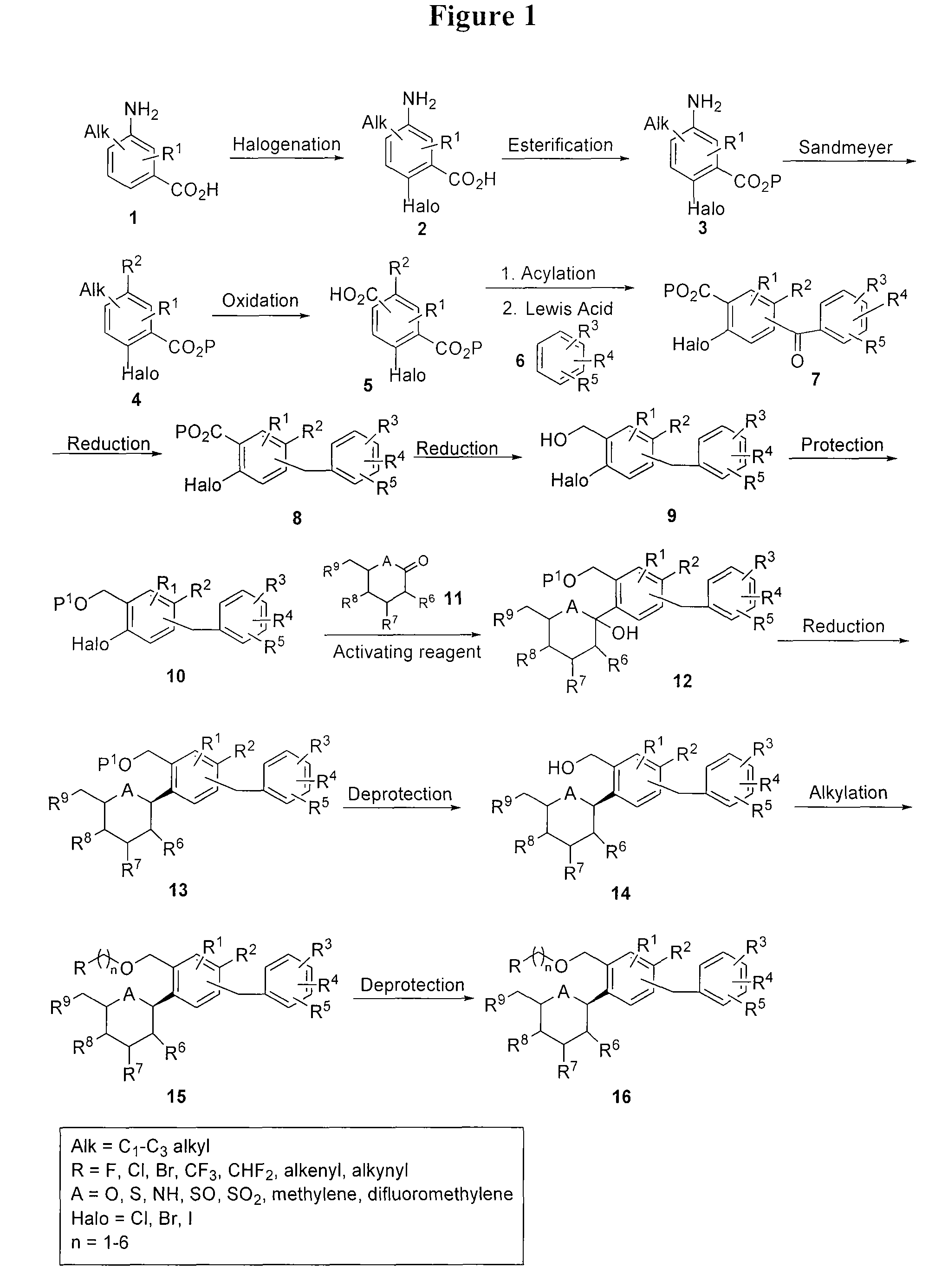
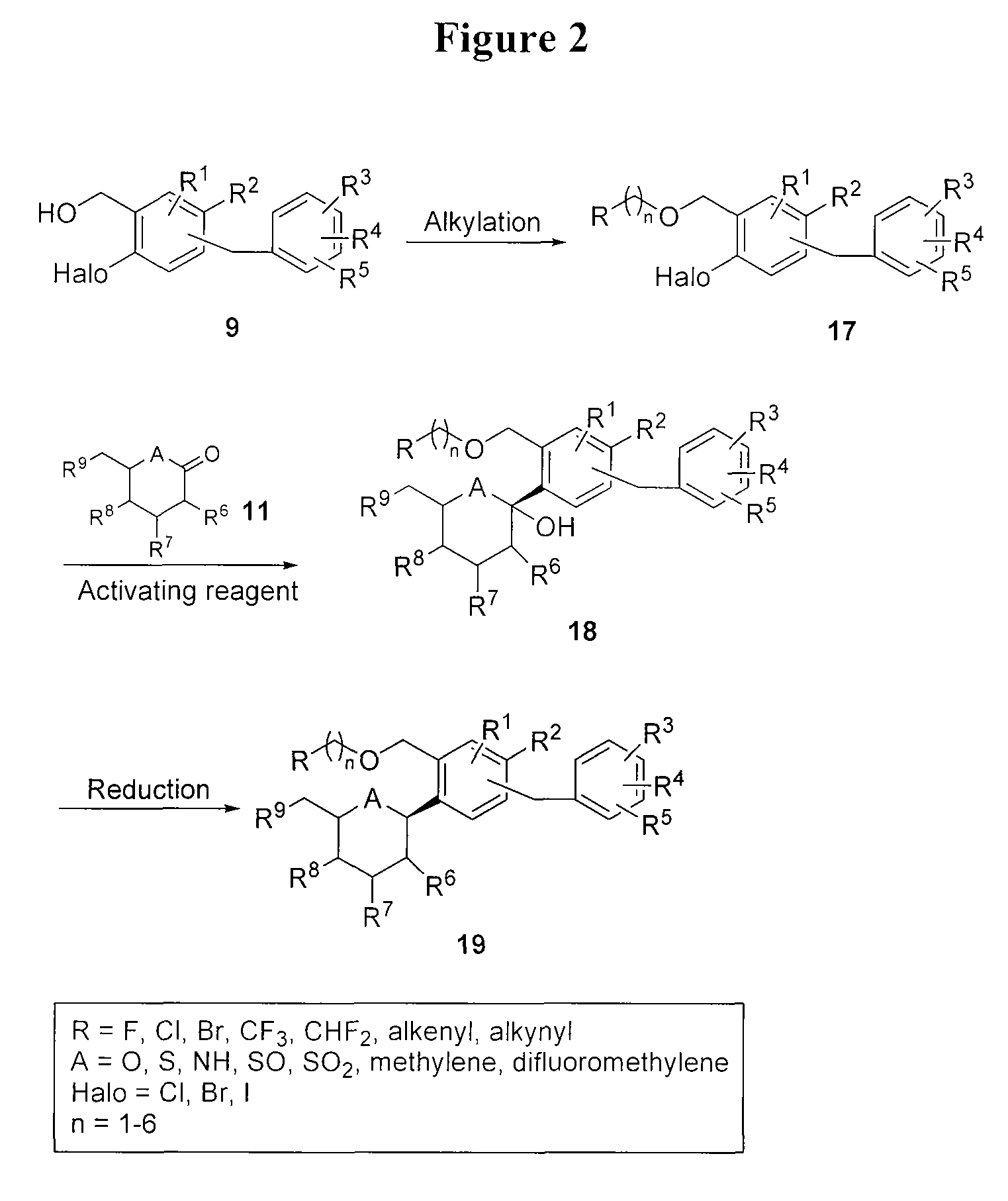
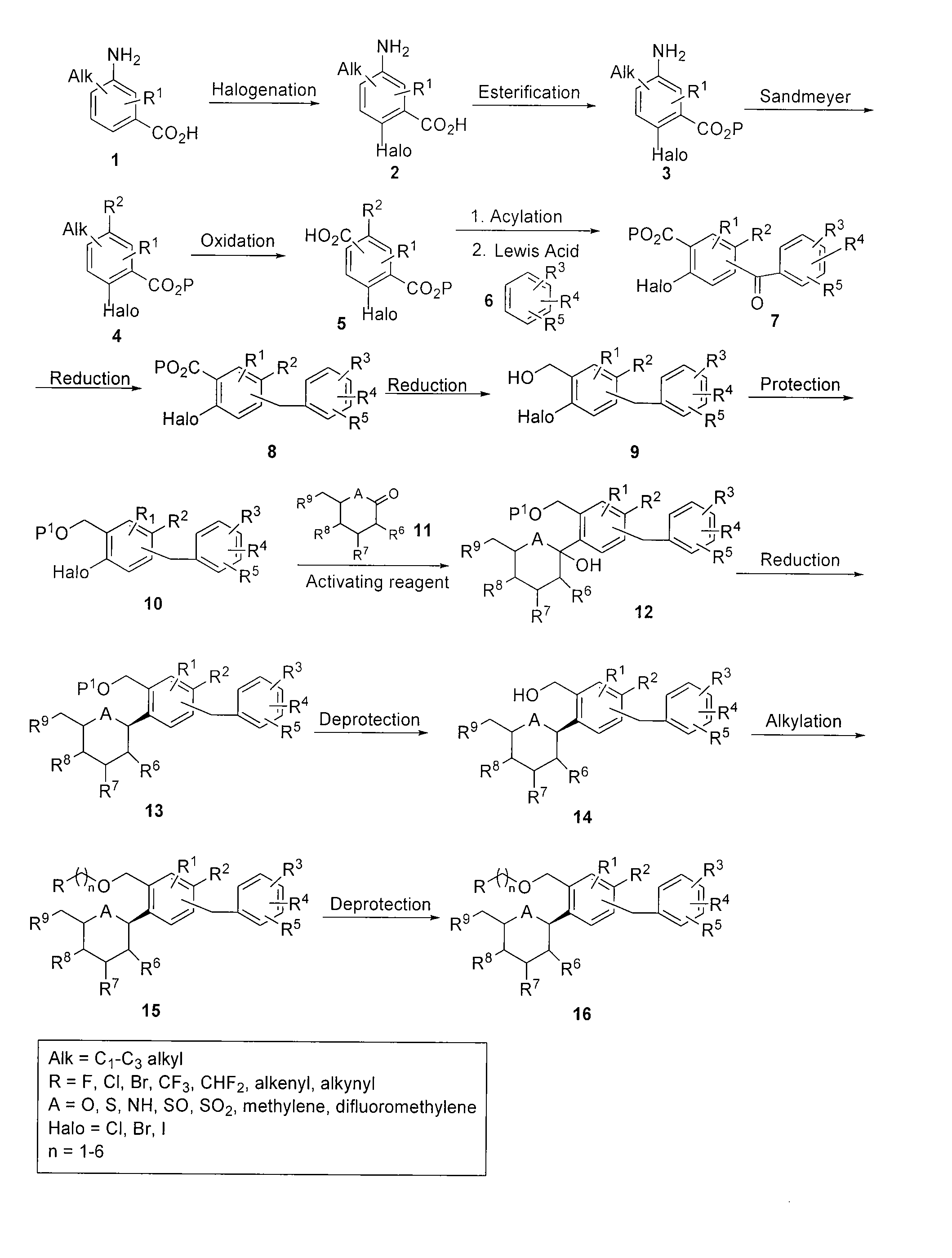
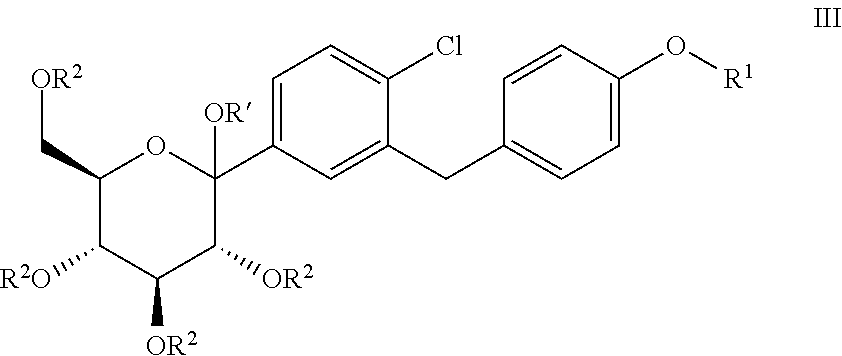
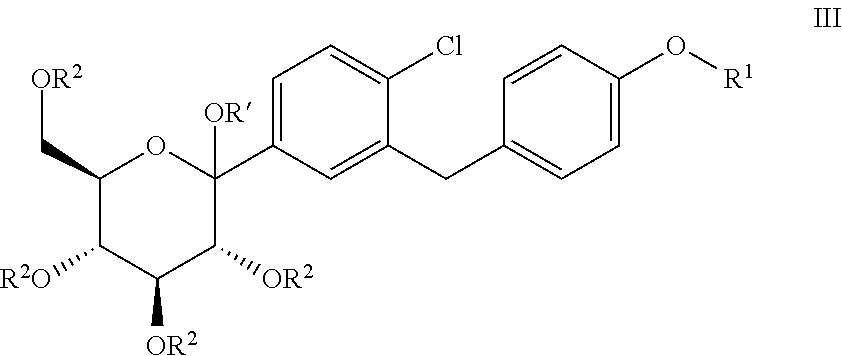
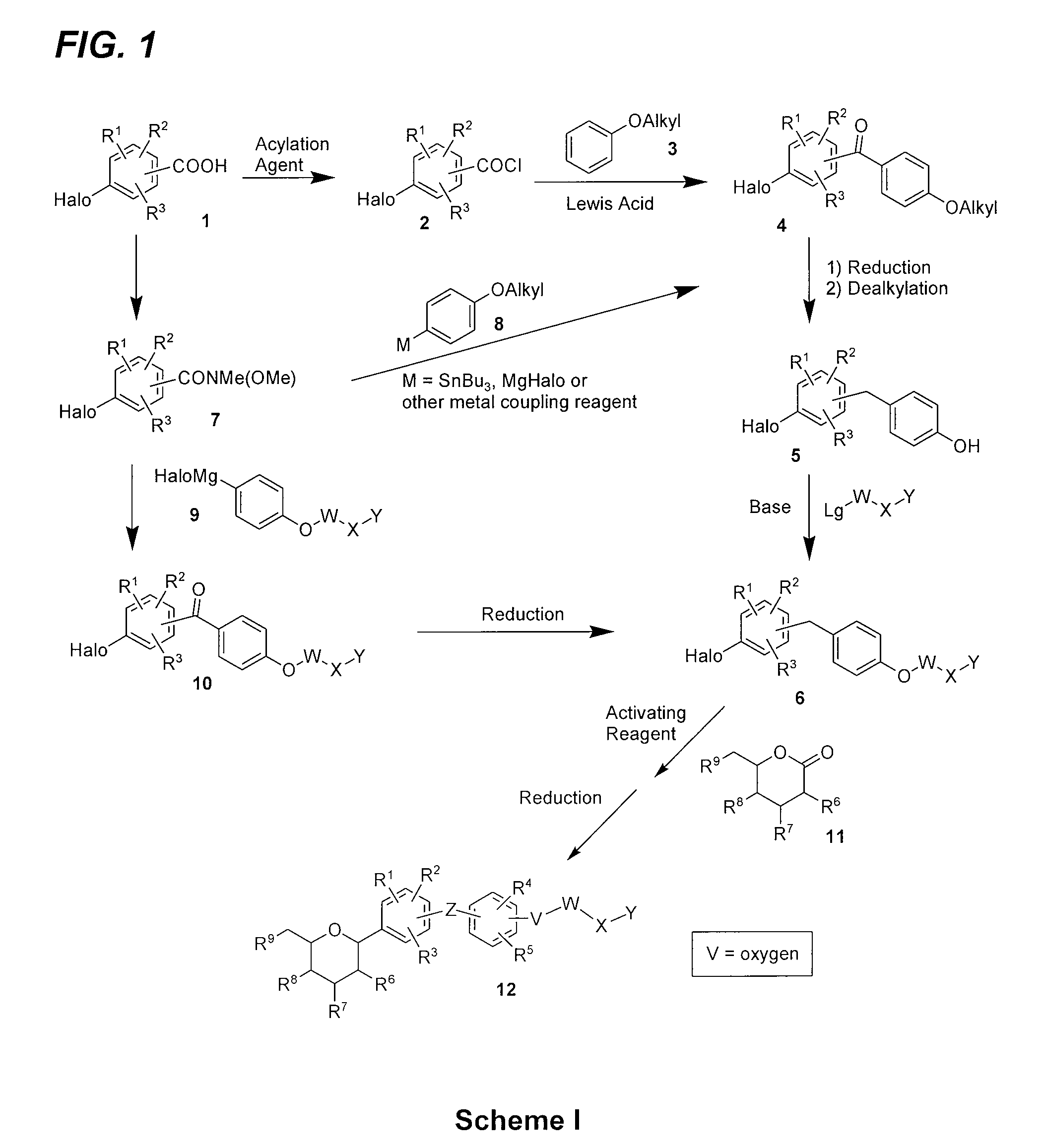
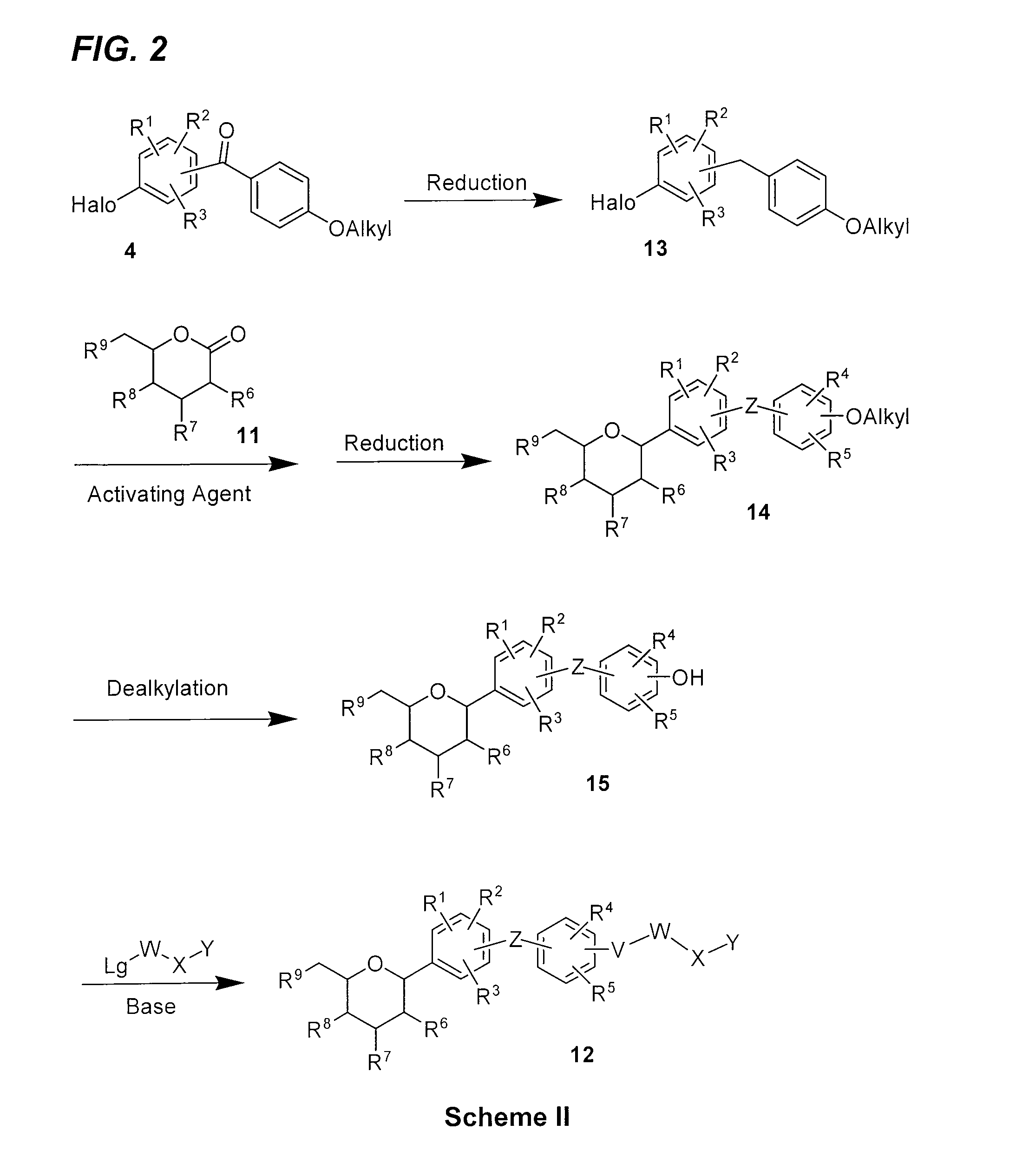
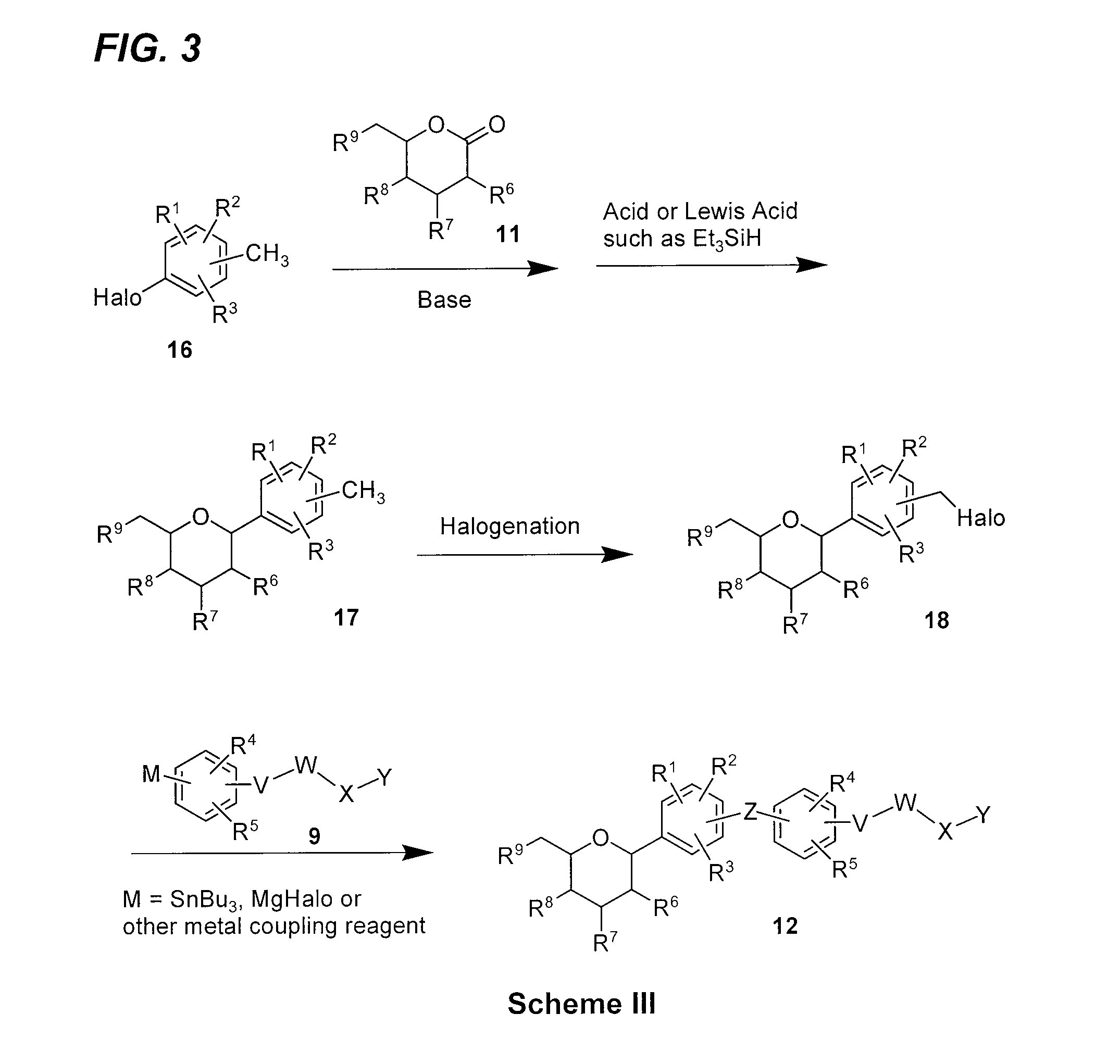
Processes for preparing of glucopyranosyl-substituted benzyl-benzene derivatives
ActiveUS20110237789A1High yieldHigh puritySaccharide with carbocyclic radicalsSugar derivativesCombinatorial chemistryPerylene derivatives
The present invention relates to processes for preparing a glucopyranosyl-substituted benzyl-benzene derivative of general formula III,wherein R1 is defined according to claim 1.
Owner:BOEHRINGER INGELHEIM INT GMBH
Process for preparing compounds useful as sglt inhibitors
InactiveCN102264714AEsterified saccharide compoundsOrganic active ingredientsBiochemistryGlucose transporter
The present invention relates to a novel process for the preparation of compounds having inhibitory activity on sodium-dependent glucose transporter (SGLT) present in the intestine or kidney.
Owner:JANSSEN PHARMA NV +1
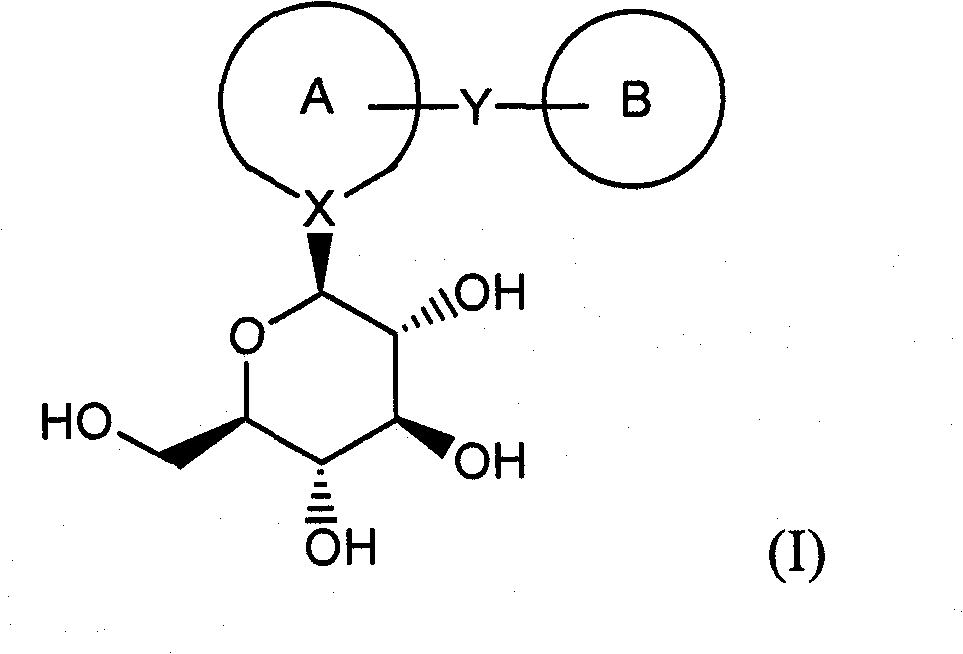
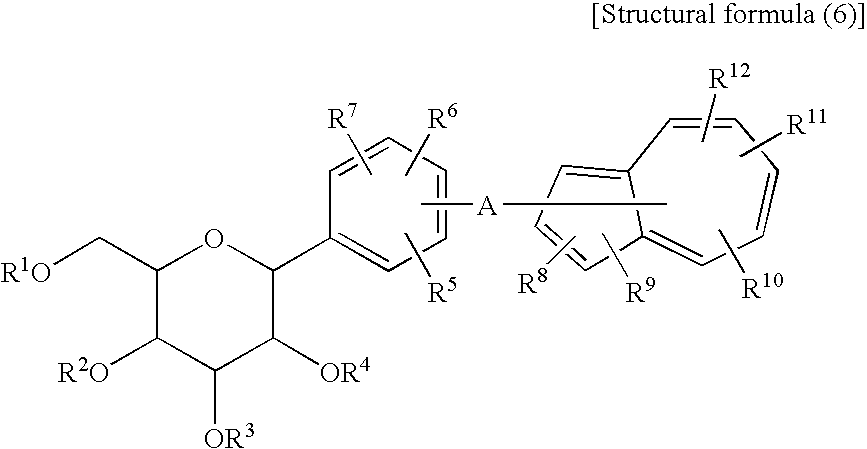
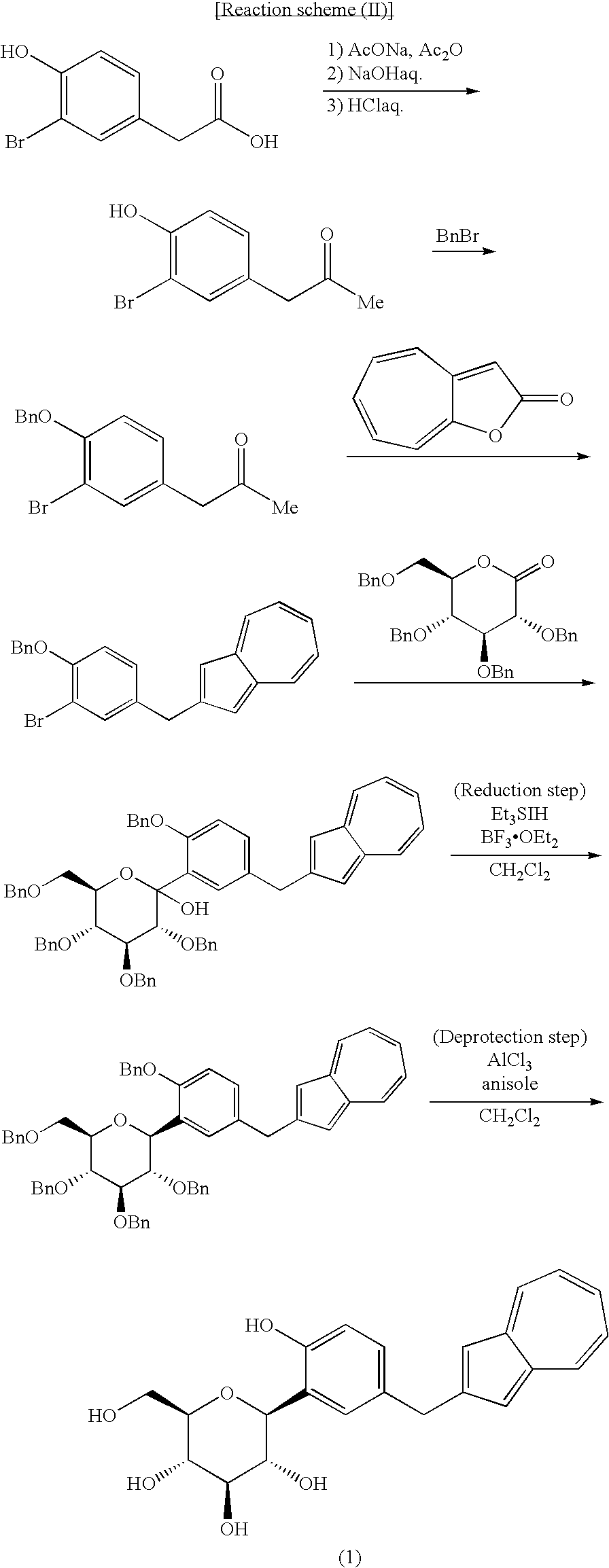
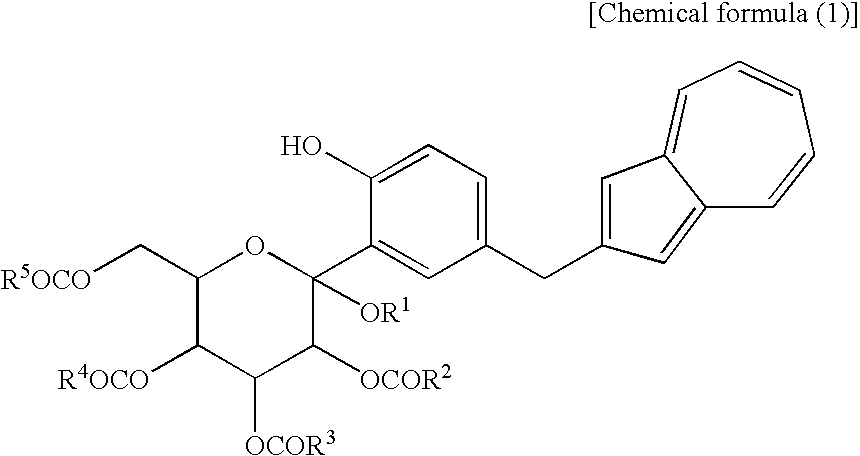
Process for Production of Azulene Derivatives and Intermediates for the Synthesis of the Same
InactiveUS20070293690A1High yieldEasy to operateSaccharide with carbocyclic radicalsSugar derivativesC-glycosideAcyl group
A process for producing an azulene derivative useful as a Na+-glucose cotransporter inhibitor, which is high in yield, is simple in operation, is low in cost, is suited for environmental protection, and is advantageous industrially, the process being characterized by reducing and deprotecting at least one compound selected from penta-acyl compounds and tetra-acyl compounds or salts thereof to obtain a C-glycoside compound; and a useful intermediate for synthesis of such an azulene derivative, obtained in the course of the above process.
Owner:ASTELLAS PHARMA INC +1
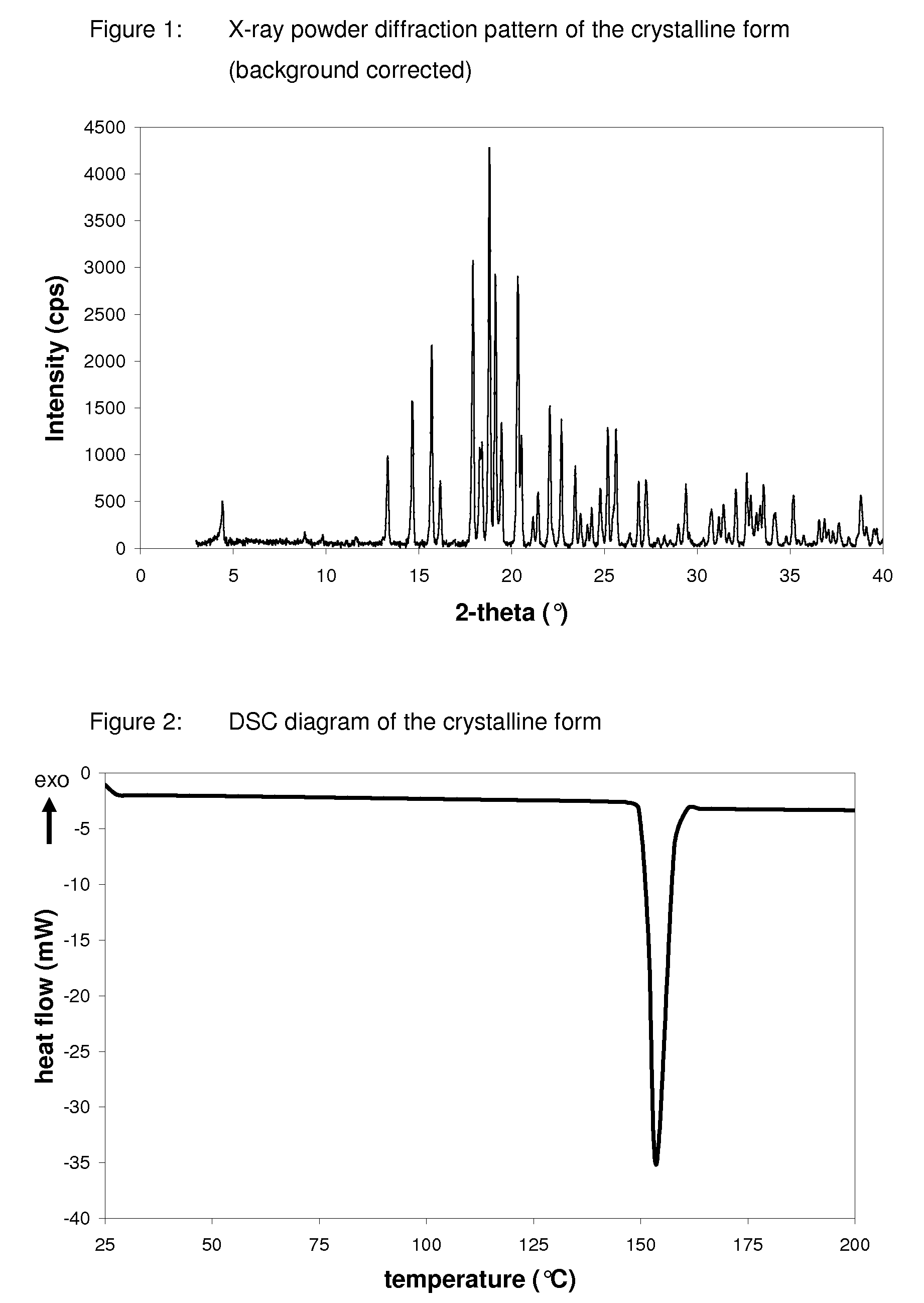
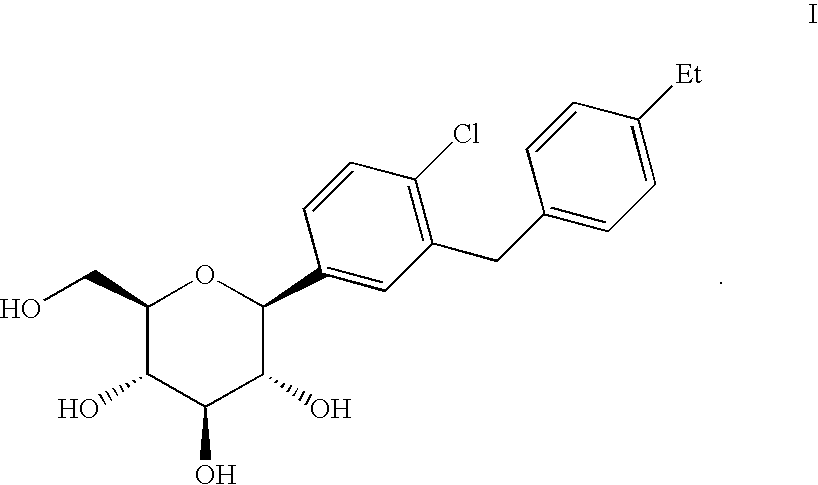
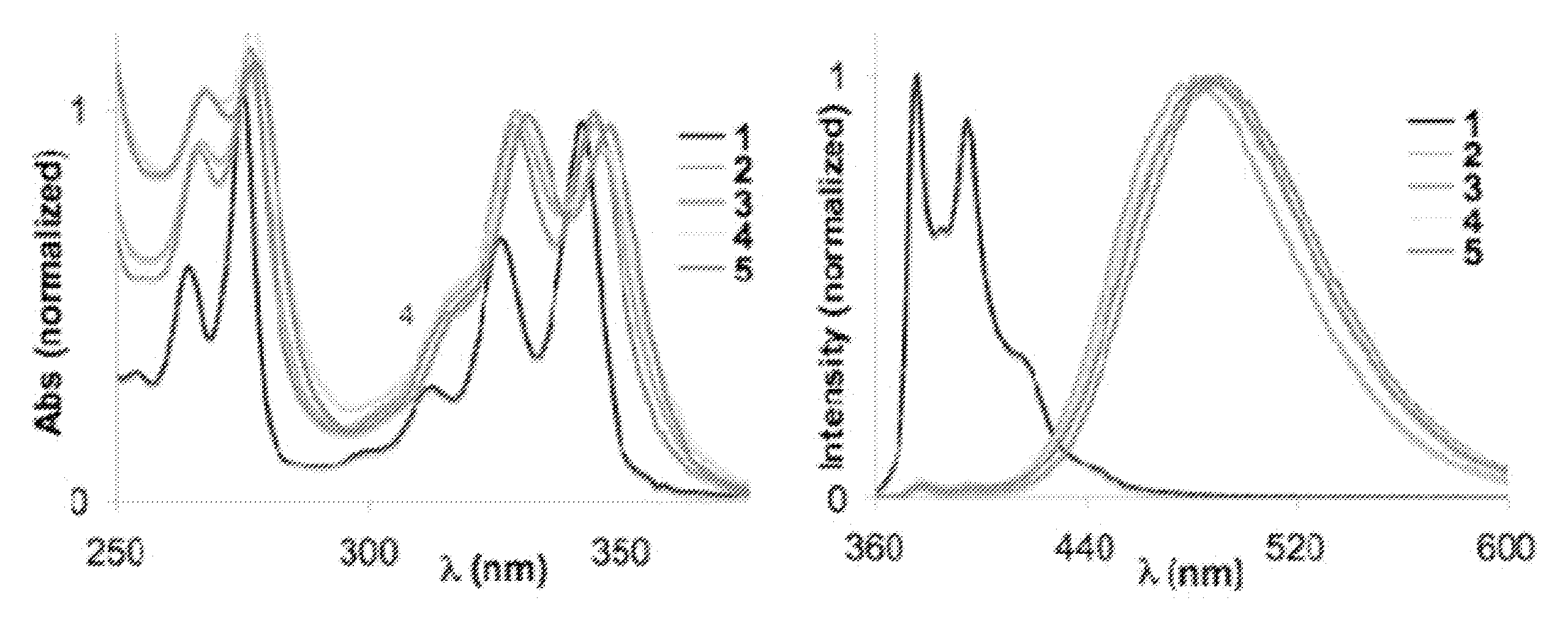
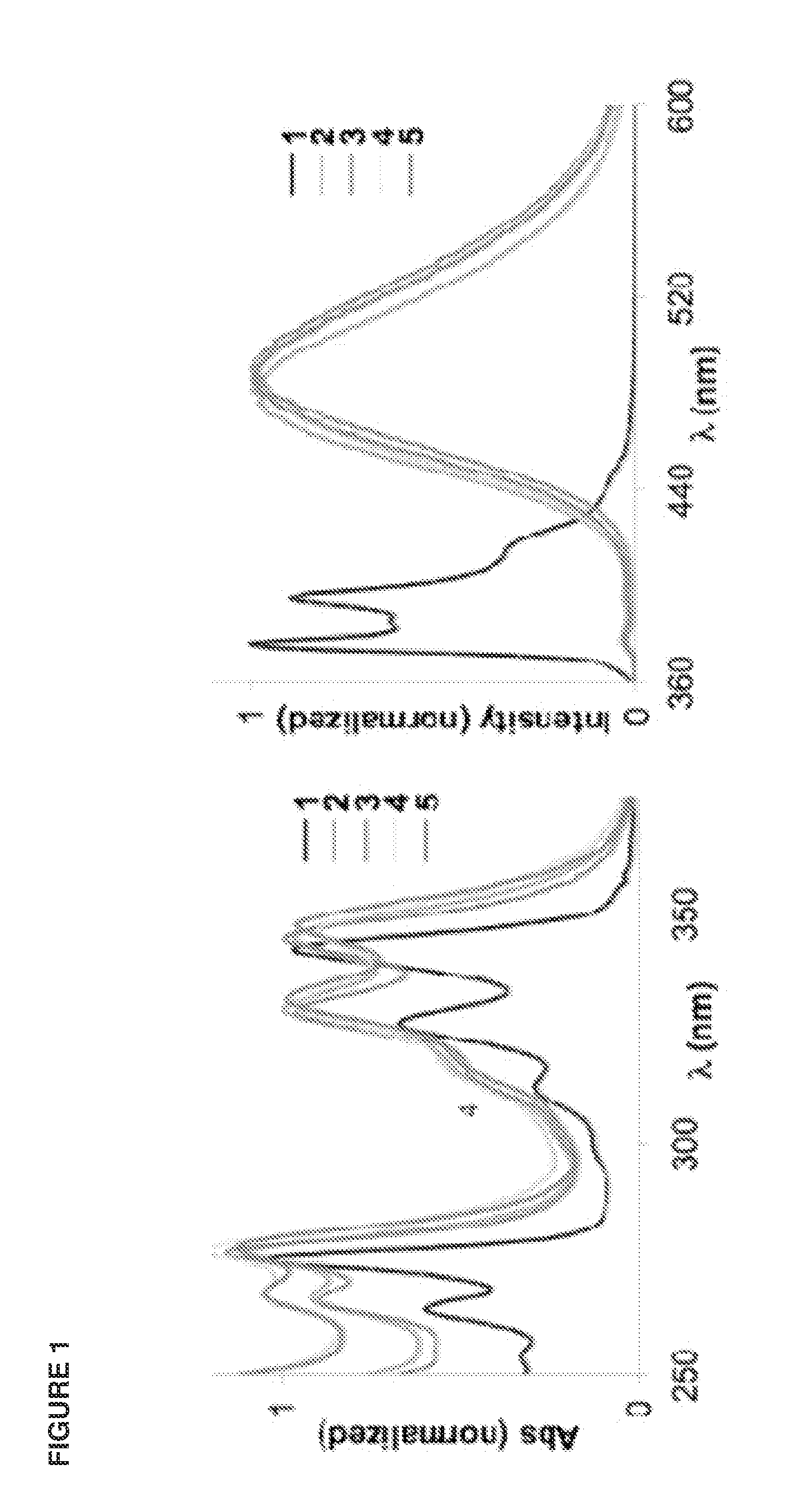
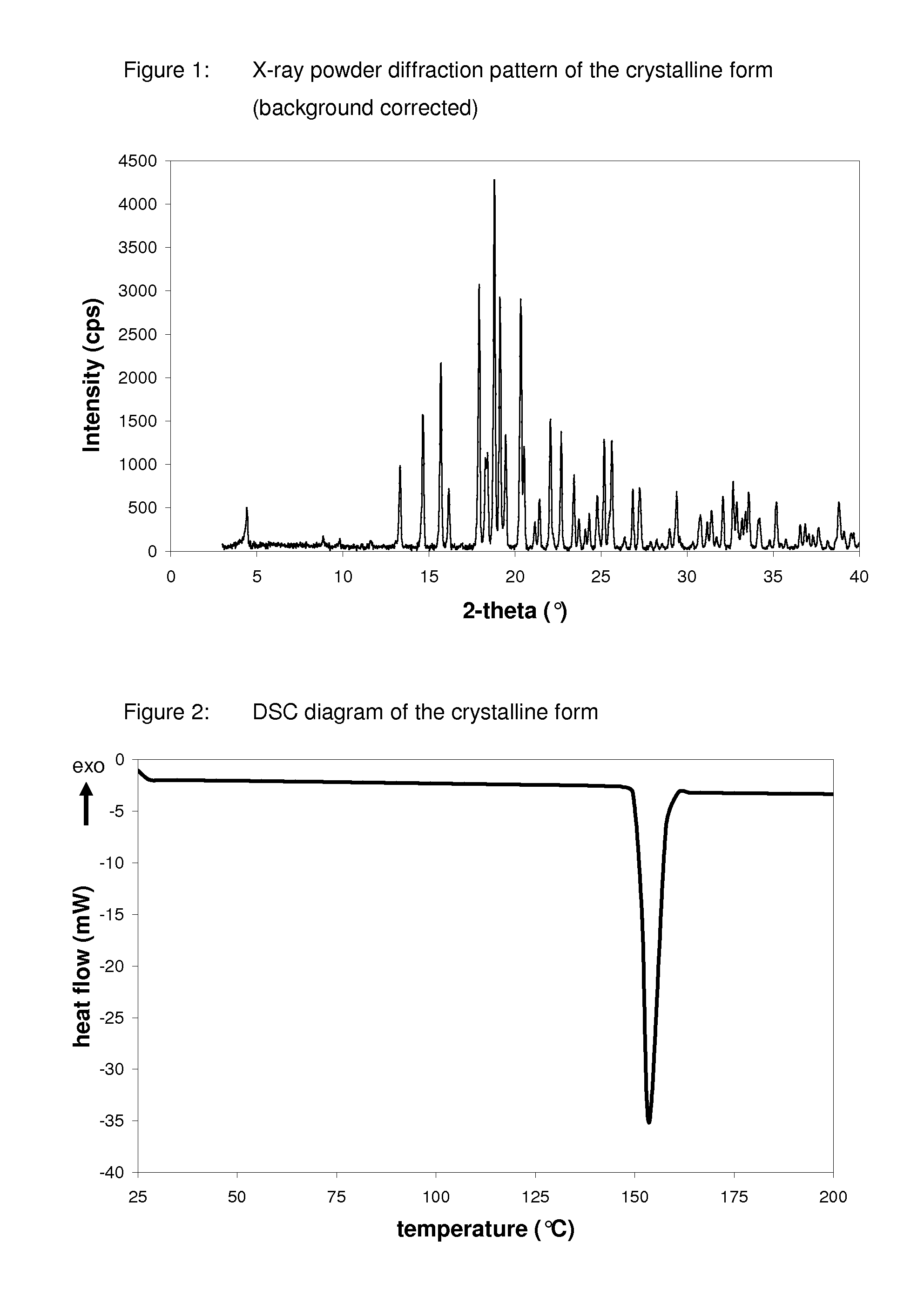
Crystalline form of 1-(beta-D-glucopyransoyl)-4-methyl-3-[5-(4-fluorophenyl)-2- thienylmethyl]benzene hemihydrate
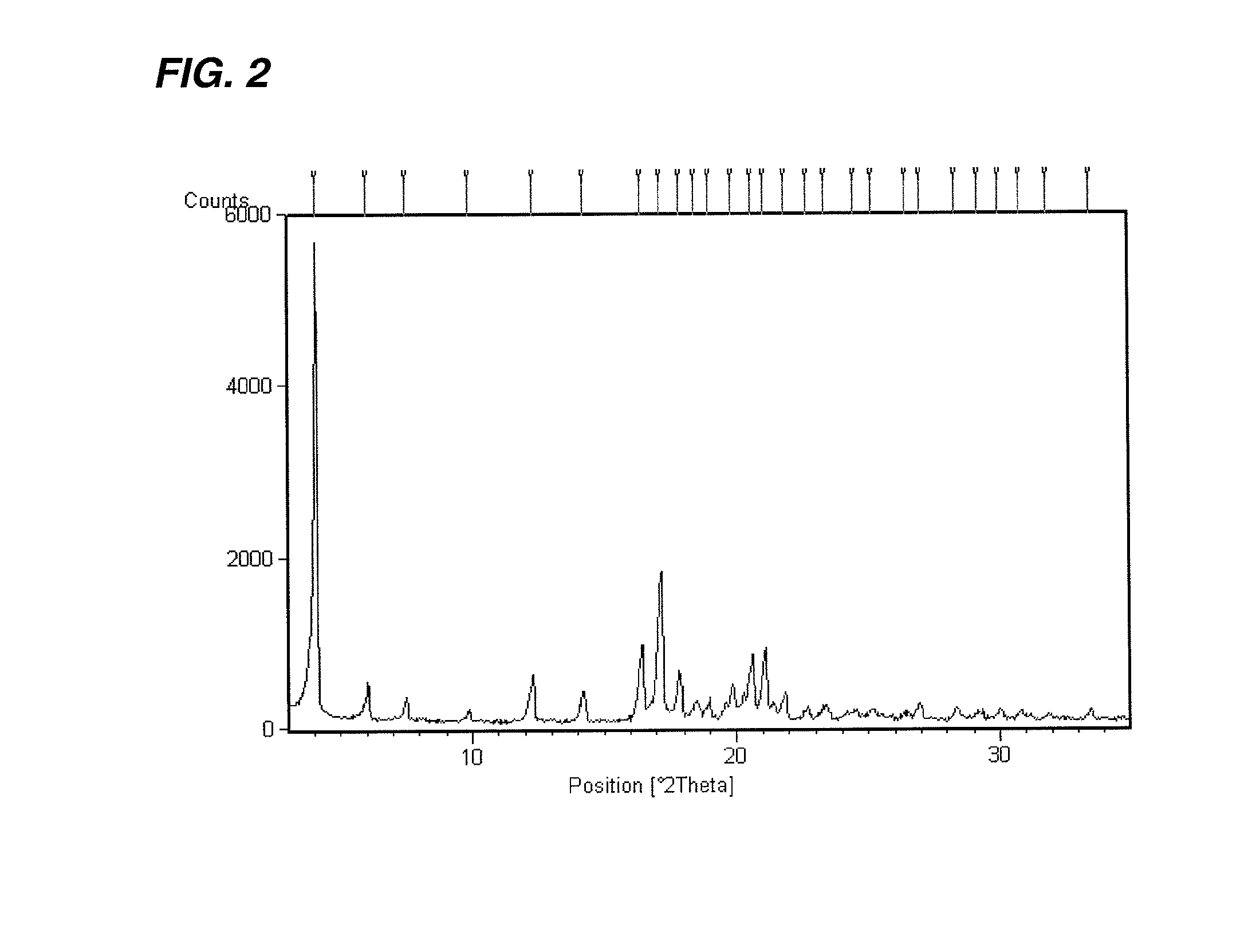
A novel crystal form of 1-(β-D-glucopyranosyl)-4-methyl-3-[5-(4-fluorophenyl)-2-thienylmethyl]benzene hemihydrate, and having favorable characteristics, is characterized by its x-ray powder diffraction pattern and / or by its infra-red spectrum.
Owner:MITSUBISHI TANABE PHARMA CORP
Strongly quenching oligomeric excimer/quencher pairs for detection schemes
InactiveUS20100129820A1High efficiency quenchingEfficiently quenchedSaccharide with carbocyclic radicalsSugar derivativesOligomerPerylene
Compositions and systems are provided for the high efficiency quenching small water-soluble oligomers, or oligofluors, of from about 1-10 kd in size, where the oligofluors comprise multiple excimeric or exciplex forming fluorophores arranged on a scaffold, which are efficiently quenched by a quencher entity linked to the oligomer through a cleavable moiety. Fluorophores of interest include, without limitation, aromatic fluorophores such as pyrenes, e.g. benzopyrene, perylene, pyrene, etc. In some embodiments the oligofluor / quencher combination provides for a Stern-Vollmer constant (KSV) of greater than about 106 M−1, and may be greater than about 107 M−1, greater than about 108 M−1, or more. In some embodiments of the invention, the scaffold is a phosphodiester / glycoside backbone, e.g. an analog of a polynucleotide. The system of oligofluors and quenchers can be used in qualitative and quantitative screening and detection methods to detect any enzymatic, chemical or catalytic activity that can cleave the moiety between the quencher and scaffold.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
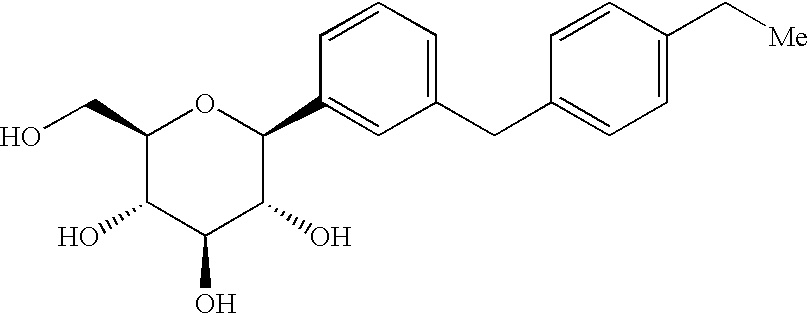
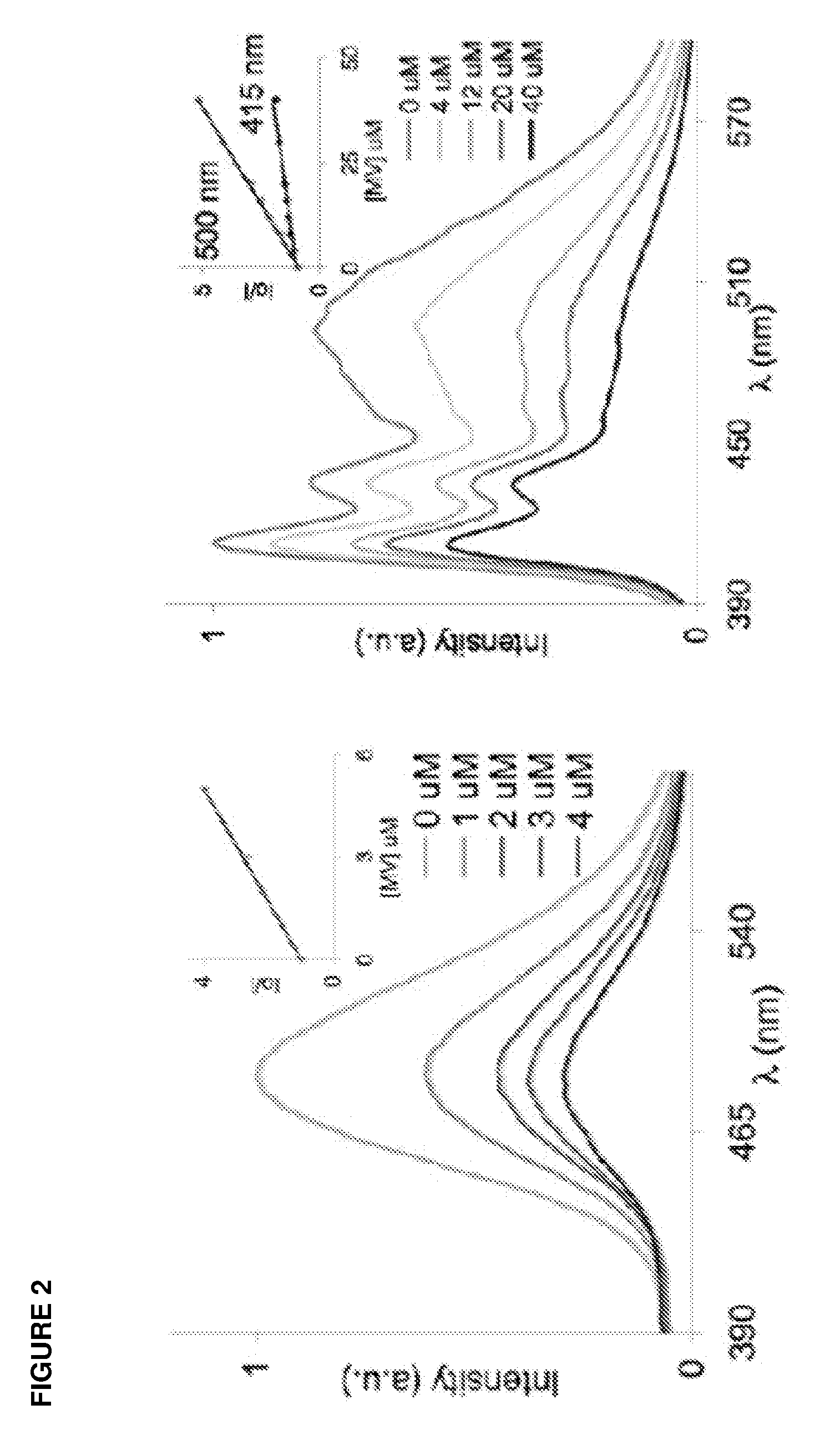
Crystalline form of 4-(beta-d-glucopyranos-1-yl)-1-methyl-2-[4-((s)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments
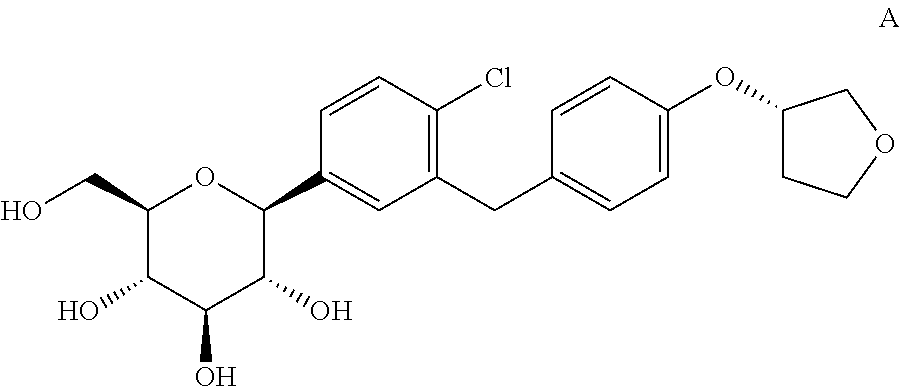
ActiveUS20100317847A1Prevent degradationImproving and restoring functionalitySaccharide with heterocyclic radicalsBiocideBenzeneCrystallography
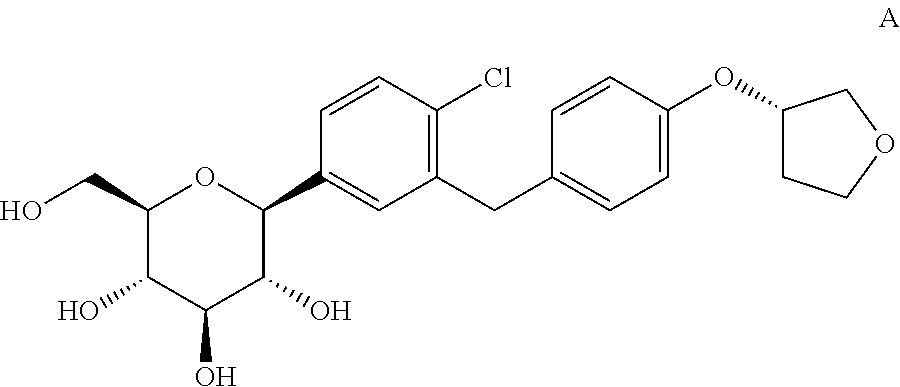
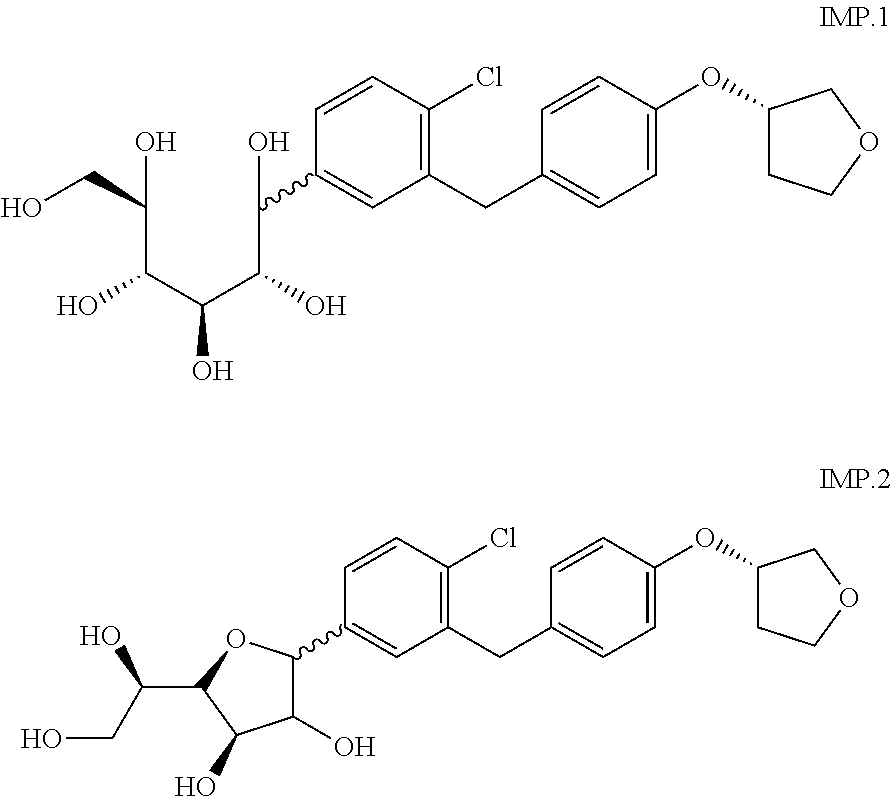
The invention relates to a crystalline form of 4-(β-D-glucopyranos-1-yl)-1-methyl-2-[4-((S)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, to a method for the preparation thereof, as well as to the use thereof for preparing medicaments.
Owner:BOEHRINGER INGELHEIM INT GMBH
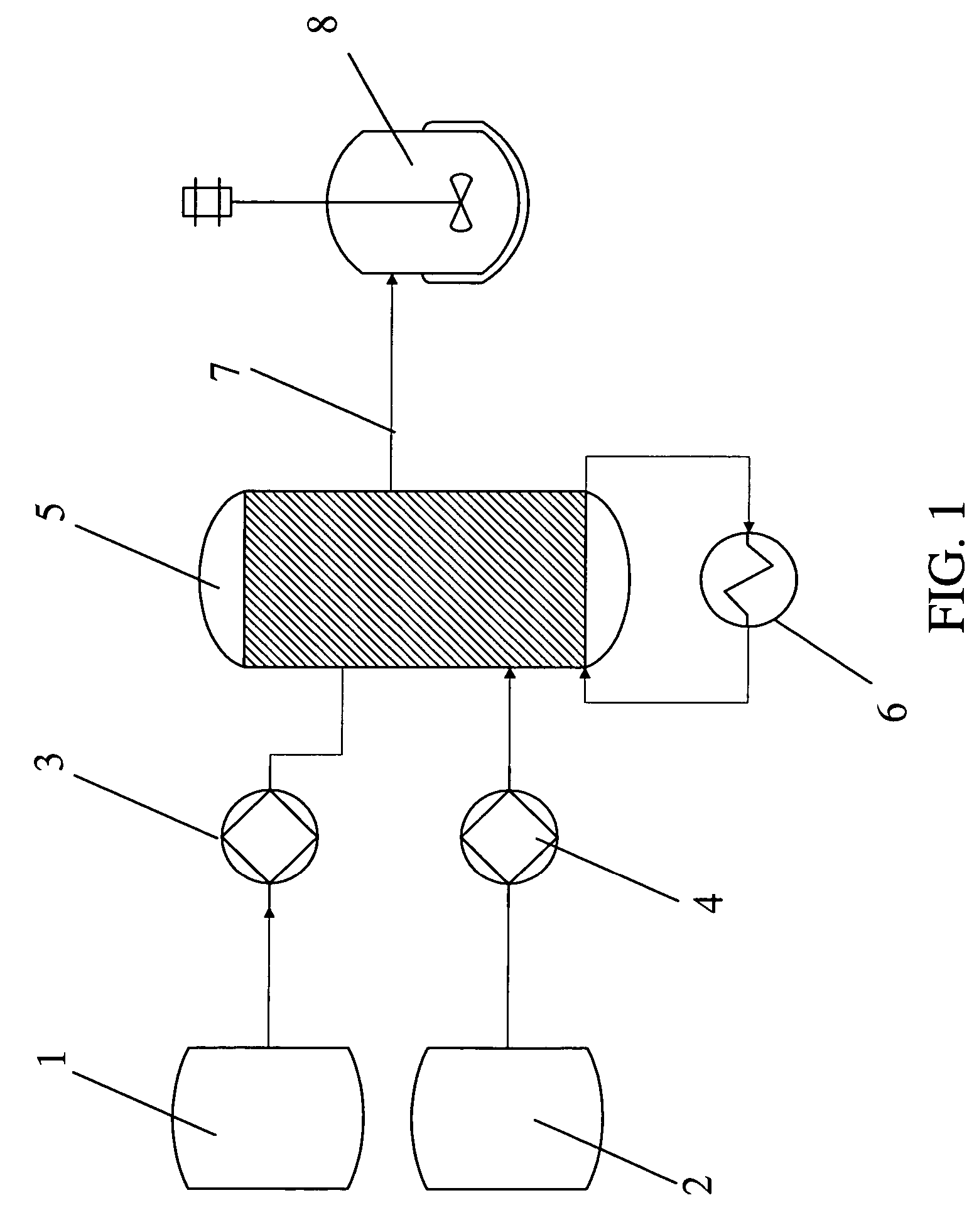
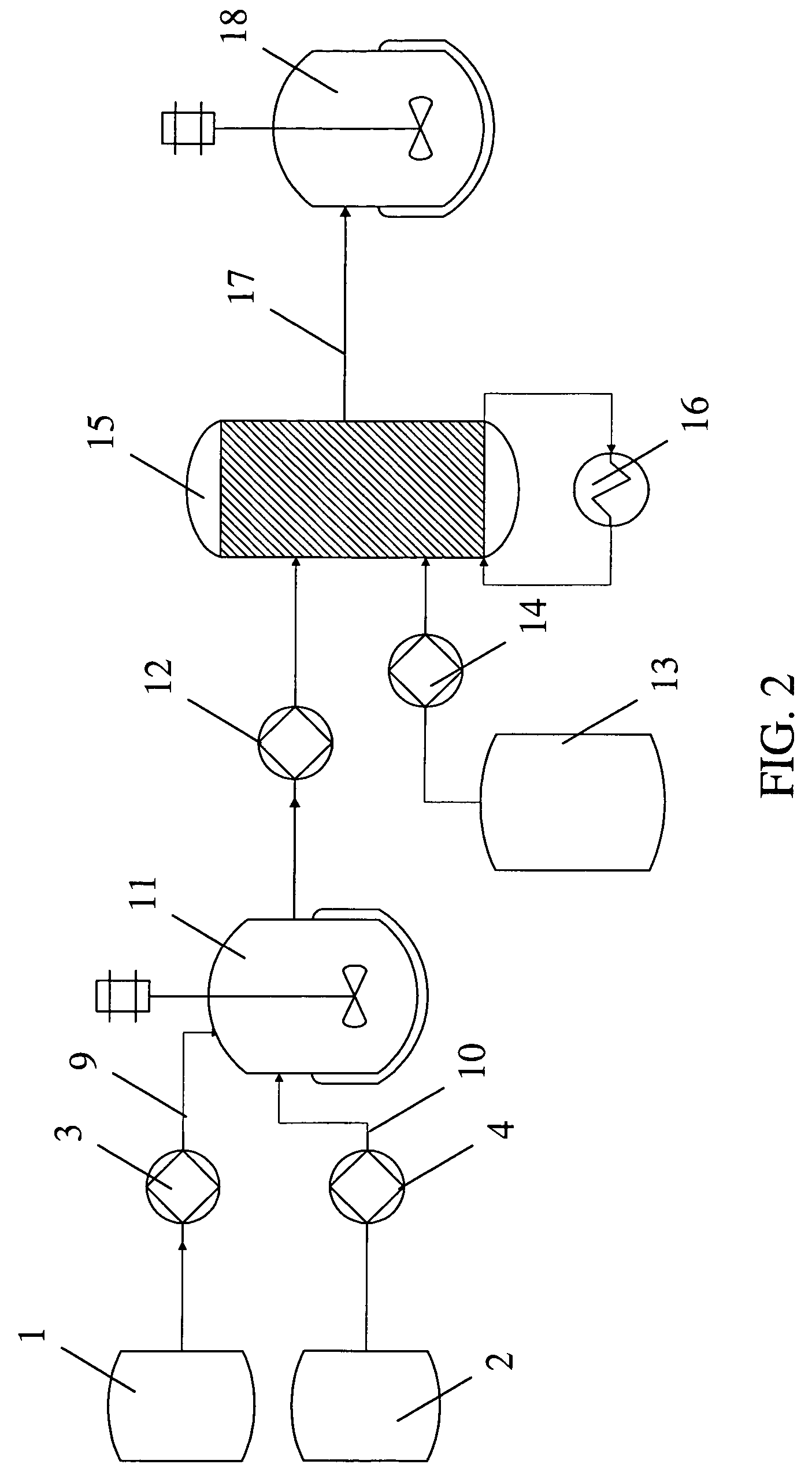
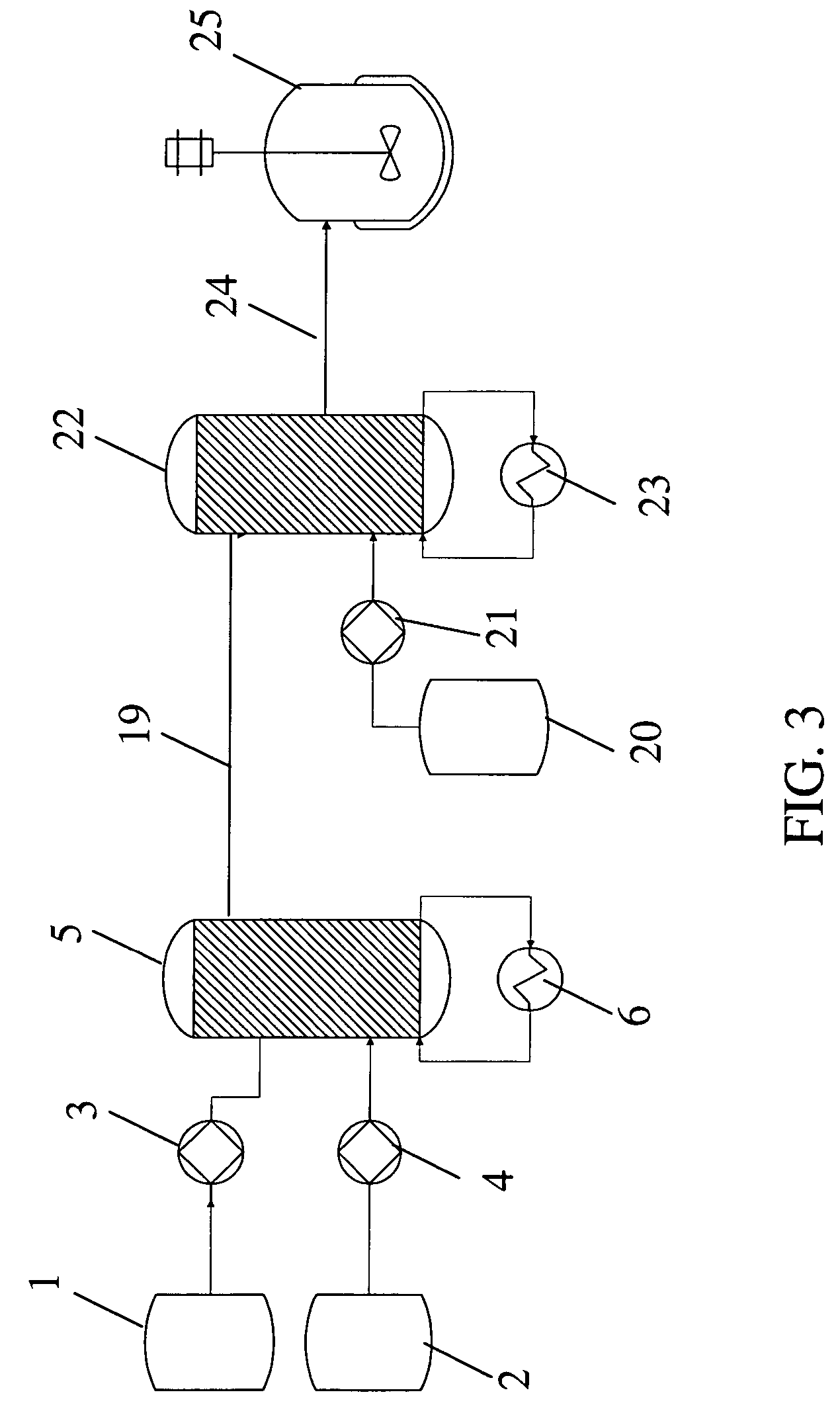
Method for the preparation of a crystalline form
ActiveUS20110237526A1High purityHigh yieldBiocideSaccharide with heterocyclic radicalsBenzeneCrystallography
The invention relates to a method for the preparation for a crystalline form of 1-chloro-4-(β-D-glucopyranos-1-yl)-2-[4-((S)-tetrahydrofuran-3-yloxy)-benzyl]-benzene. In addition the invention relates to a crystalline form obtainable by this method, to a pharmaceutical composition and to the use thereof for preparing medicaments.
Owner:BOEHRINGER INGELHEIM INT GMBH
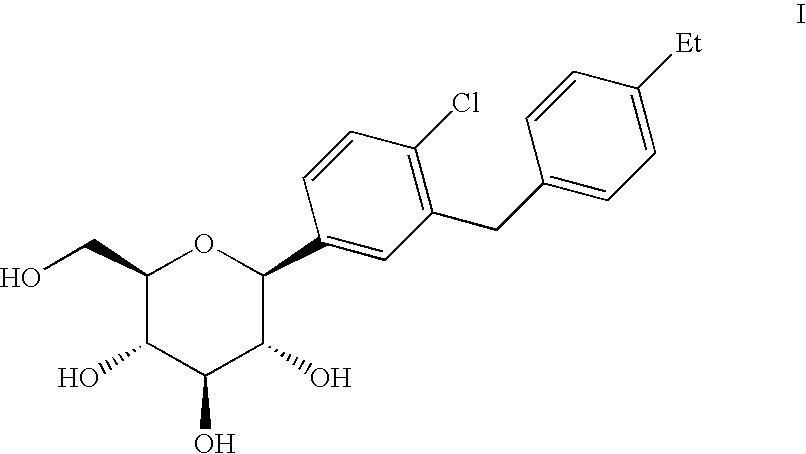
Crystalline form of 1-(β-D-glucopyransoyl)-4-methyl-3-[5-(4-fluorophenyl)-2- thienylmethyl]benzene hemihydrate
A novel crystal form of 1-(β-D-glucopyranosyl)-4-methyl-3-[5-(4-fluorophenyl)-2-thienylmethyl]benzene hemihydrate, and having favorable characteristics, is characterized by its x-ray powder diffraction pattern and / or by its infra-red spectrum.
Owner:MITSUBISHI TANABE PHARMA CORP
Benzylbenzene derivatives and methods of use
Provided are compounds having an inhibitory effect on sodium-dependent glucose cotransporter SGLT. The invention also provides pharmaceutical compositions, methods of preparing the compounds, synthetic intermediates, and methods of using the compounds, independently or in combination with other therapeutic agents, for treating diseases and conditions which are affected by SGLT inhibition.
Owner:THERACOSBIO LLC
C-aryl glucoside SGLT2 inhibitors and method
Owner:ASTRAZENECA AB
Crystalline form of 4-(beta-D-glucopyranos-1-yl)-1-methyl-2-[4-((S)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments
The invention relates to a crystalline form of 4-(β-D-glucopyranos-1-yl)-1-methyl-2-[4-((S)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, to a method for the preparation thereof, as well as to the use thereof for preparing medicaments.
Owner:BOEHRINGER INGELHEIM INT GMBH
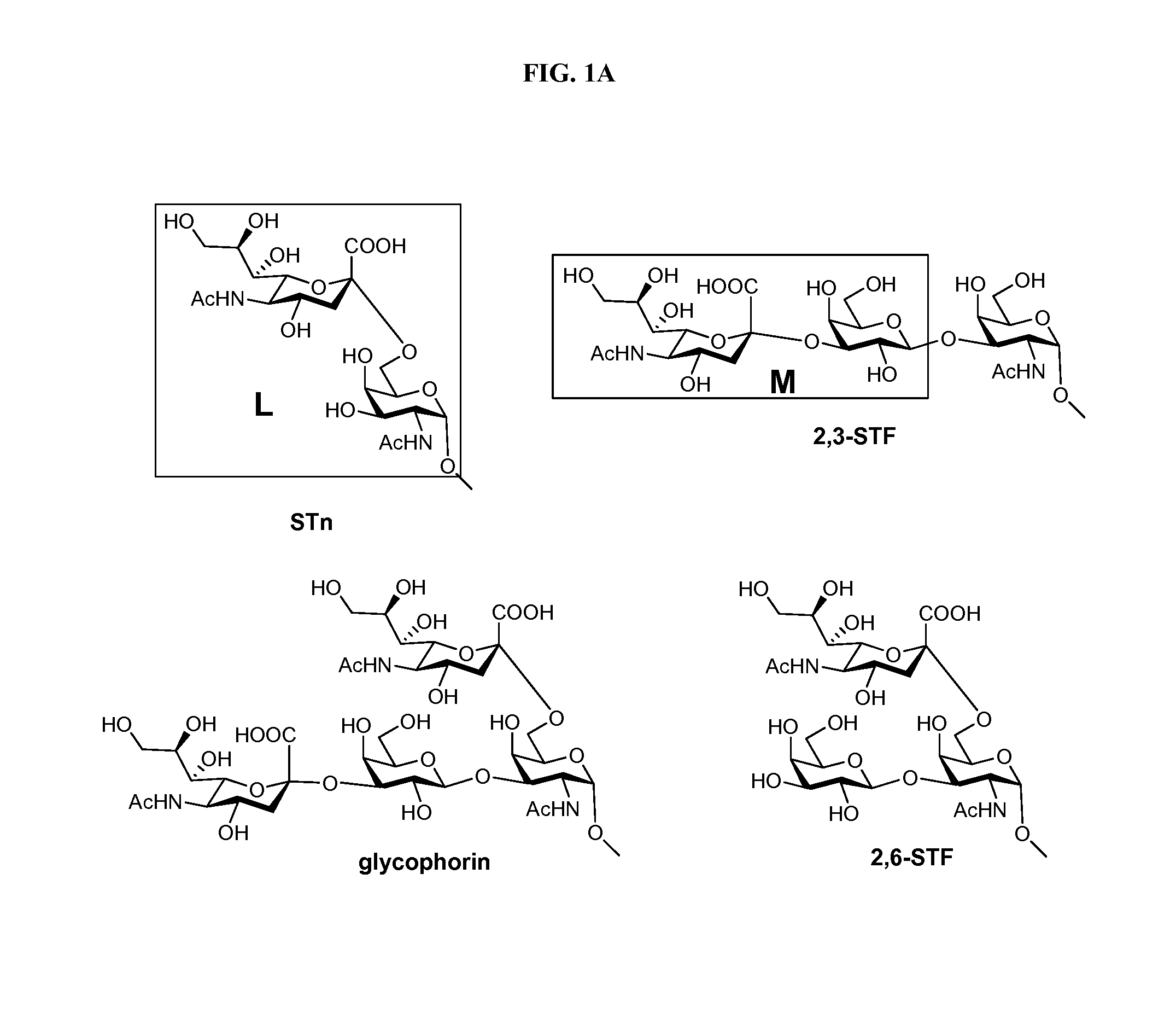
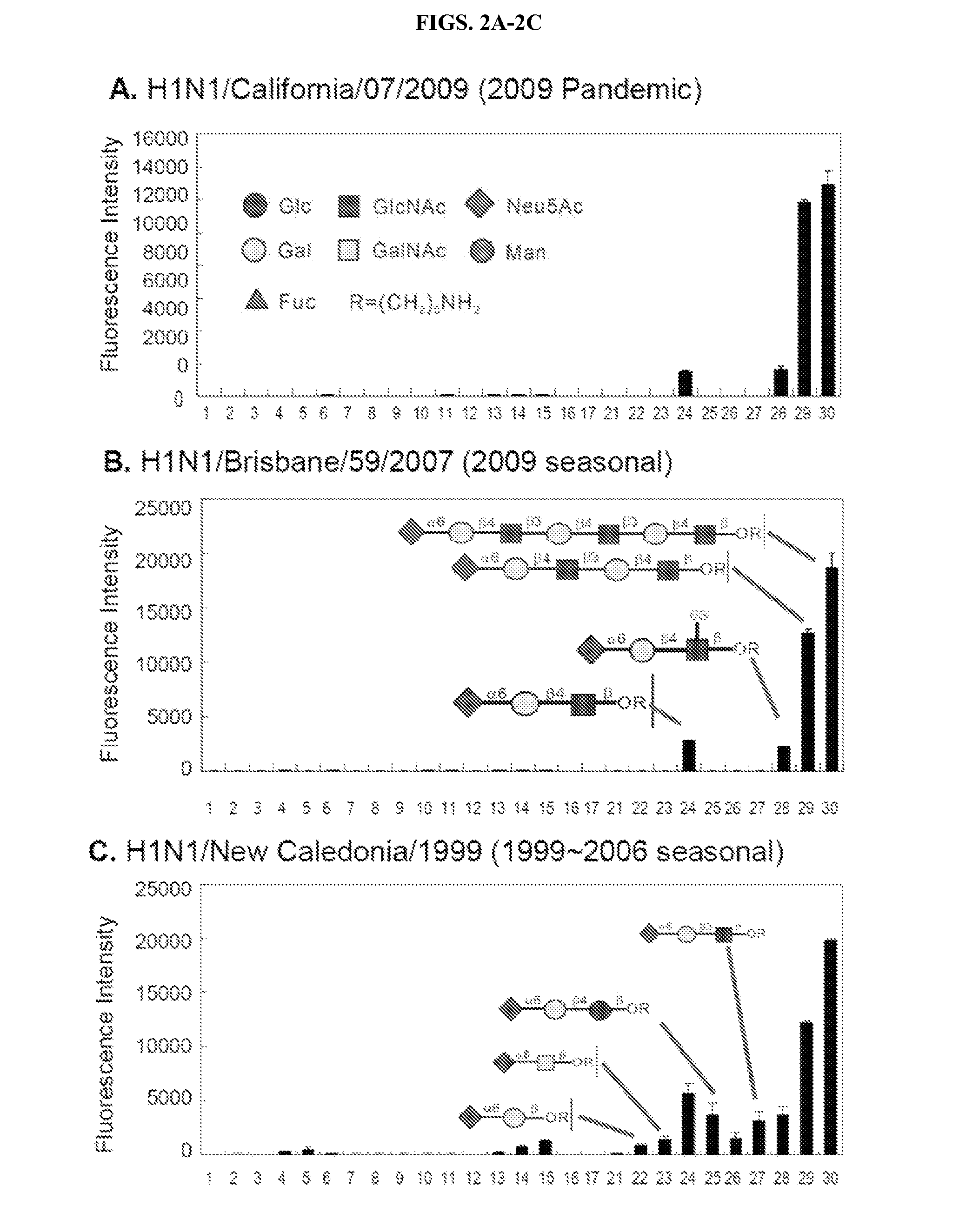
Alpha-selective sialyl phosphate donors for preparation of sialosides and sialoside arrays for influenza virus detection
ActiveUS8507660B2Esterified saccharide compoundsSaccharide with carbocyclic radicalsHemagglutininCancer cell
A novel N-acetyl-5-N,4-O-carbonyl-protected dibutyl sialyl phosphate donor for sialylation of both primary and sterically hindered secondary acceptors to prepare sialosides with high yield and α-selectivity is disclosed. Methods for making disaccharide building blocks comprising α(2→3), α(2→6), α(2→8), α(2→8) / α(2→9) alternate, and α(2→9) sialosides are provided. methods for one-pot synthesis of complex sialosides are disclosed. Libraries of sialosides and methods for using the libraries for detection and receptor binding analysis of surface glycoproteins or pathogens and cancer cells are disclosed. Methods for distinguishing between hemagglutinin (HA) from various strains of influenza are provided.
Owner:ACAD SINIC
Inhibitors of sodium glucose co-transporter 2 and methods of their use
Compounds and pharmaceutical compositions comprising them are disclosed that may be useful for the treatment of diseases and disorders such as diabetes and obesity.
Owner:LEXICON PHARM INC
Synthesis method of 2, 5-disubstituted thiophene compound
ActiveCN102115468AWide variety of sourcesSimple and fast operationOrganic active ingredientsSaccharide with carbocyclic radicalsState of artReaction conditions
Owner:TOPHARMAN SHANDONG
Non-cryogenic process for forming glycosides
InactiveUS7164015B2Increase productionReduce manufacturing costSaccharide with carbocyclic radicalsSugar derivativesChemistryCarbonyl group
The present invention provides a method for making glycoside compounds including the steps of: (a) lithiating an aromatic reactant having a leaving group using a lithium reagent in a first microreactor under non-cryogenic conditions to form a lithiated anion species, and (b) coupling the lithiated anion species with a carbonyl substituted reactant to form a glycoside.
Owner:ASTRAZENECA AB
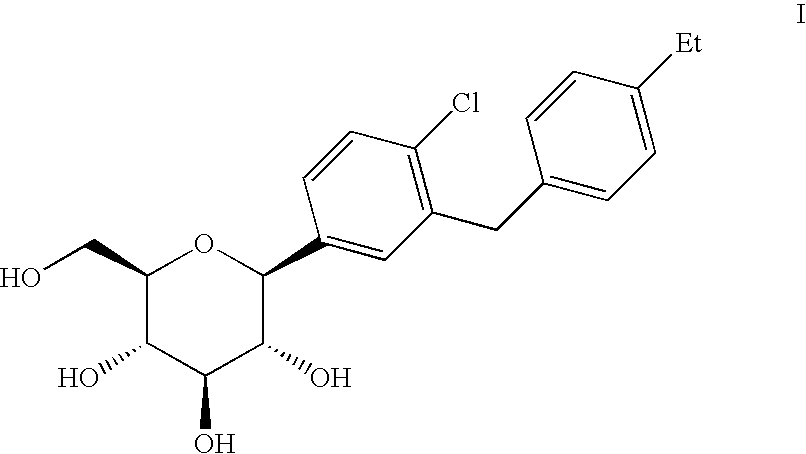
Inhibitor of sodium-dependent glucose transport protein and preparation method therefor and use thereof
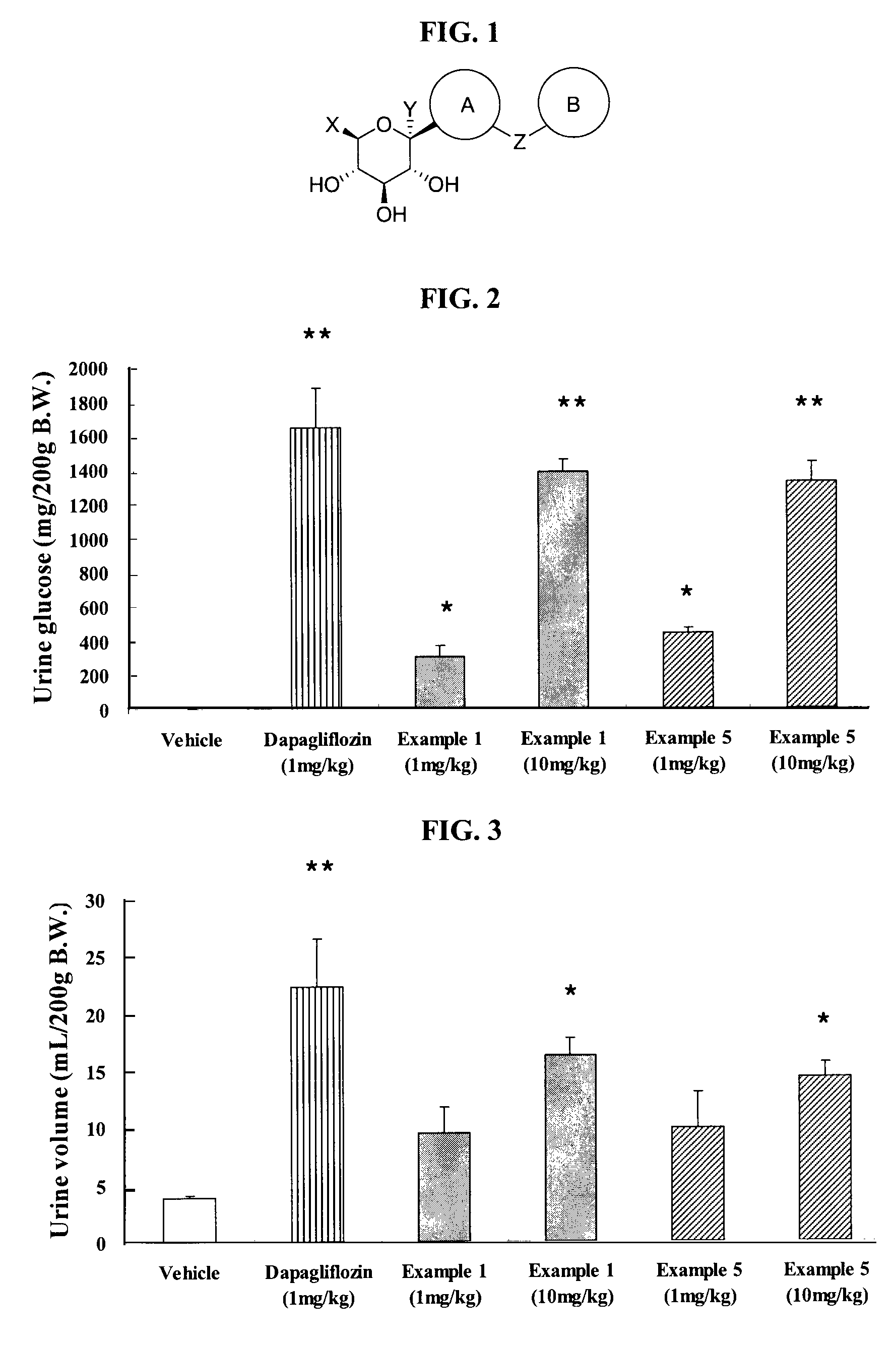
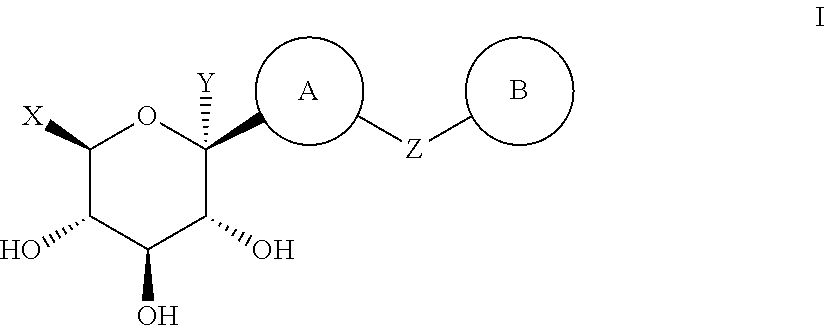
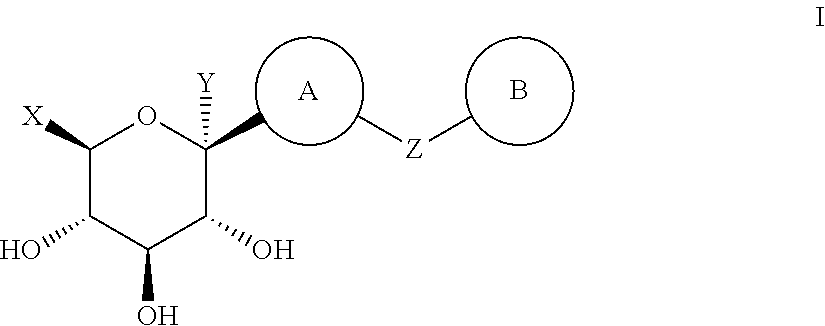
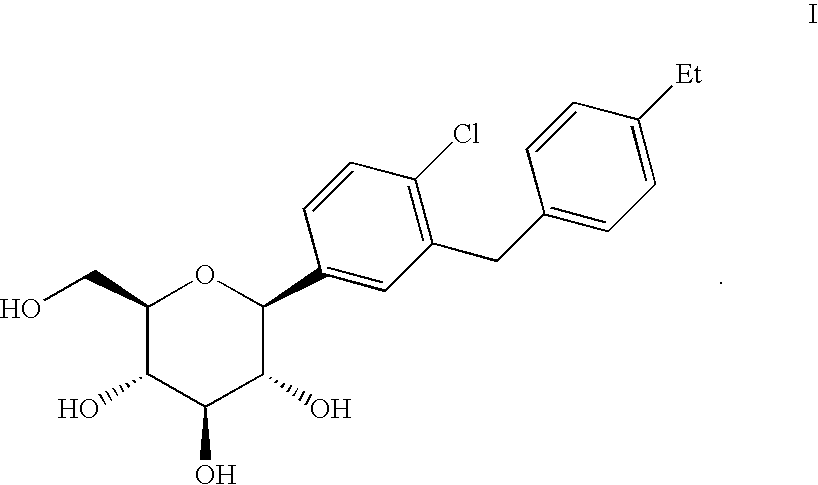
ActiveCN102757415AExtended half-lifePromote absorptionSenses disorderSaccharide with carbocyclic radicalsDiseaseSodium dependent
A compound represented by formula I, or a pharmaceutically acceptable salt, solvate, polymorph, enantiomer or racemic mixture thereof, wherein, R1 and R2 are independently hydrogen, -OH, alkyl, -CF3, -OCHF2, -OCF3 or halogen,R3 is cycloalkyl, -OCH2CF3, -OCH2CHF2, -OCH2CH2F or -OCH2CH3,R4 is hydrogen, -OH, -O aryl, -OCH2 aryl, alkyl, cycloalkyl, -CF3, -OCHF2, -OCF3, -OCH2CF3, -OCH2CHF2, -OCH2CH2F or halogen,A is -CX1X2, wherein X1 and X2 are independently H, F and Cl, and when both X1 and X2 are H, R3 is other than -OCH2CH3. The compound has an activity of inhibitors of sodium-dependent glucose transport protein. A method for preparing the compound. A pharmaceutical composition comprising the compound. A use of the compound and pharmaceutical composition thereof in the preparation of medicaments of SGLT2 inhibitors for treating a disease of interest.
Owner:BEIJING PRELUDE PHARM SCI & TECH
Methods of treatment, pharmaceutical compositions and uses thereof
InactiveUS20130137646A1Increase body weightReduce weightBiocideNervous disorderSGLT2 InhibitorNeuroleptic agents
Owner:BOEHRINGER INGELHEIM INT GMBH
Thiazole derivatives as SGLT2 inhibitors and pharmaceutical composition comprising same
ActiveUS8586550B2Prevention and/or treatment of metabolic disordersReduce usageBiocideSaccharide with heterocyclic radicalsSodium dependentSGLT2 Inhibitor
The present invention relates to a novel compound with thiazole ring having an inhibitory activity against sodium-dependent glucose cotransporter 2 (SGLT2) being present in the intestine and kidney, and a pharmaceutical composition comprising the same as an active ingredient, which is useful for preventing or treating metabolic disorders, particularly diabetes.
Owner:THE GREEN CROSS CORP
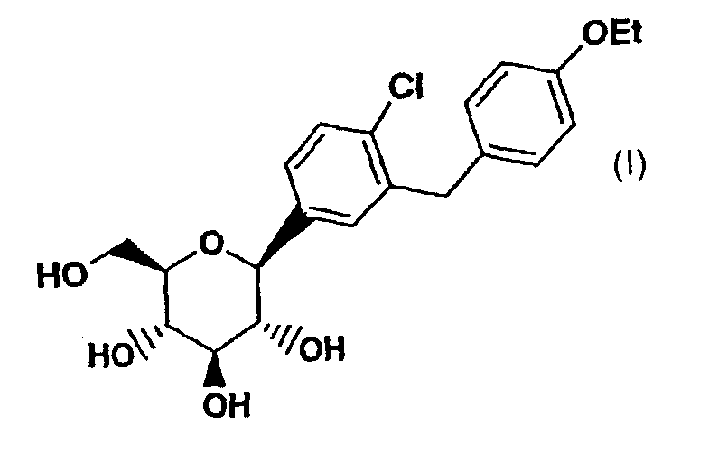
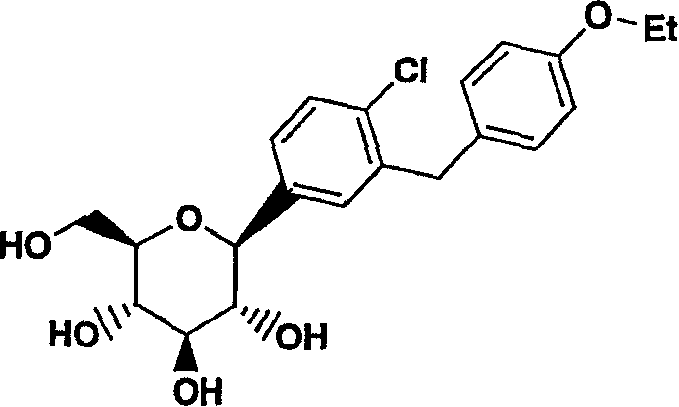
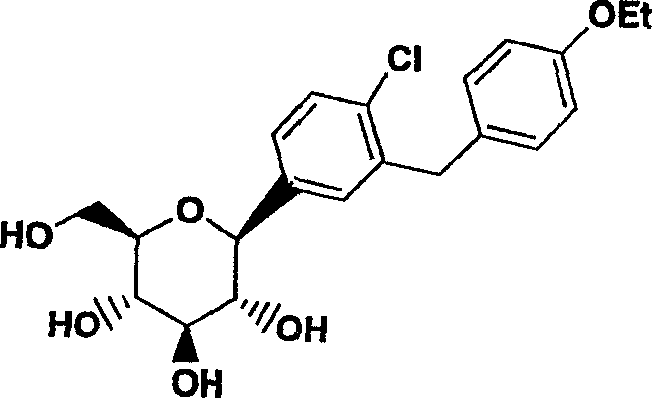
Method for the preparation of a crystalline form
ActiveUS8802842B2High purityHigh yieldBiocideSaccharide with carbocyclic radicalsCrystallographyBenzene
Owner:BOEHRINGER INGELHEIM INT GMBH
Processes for the preparation of SGLT2 inhibitors
ActiveUS8283454B2Organic active ingredientsSaccharide with carbocyclic radicalsSodium dependentSGLT2 Inhibitor
Provided are processes for the preparation of complexes that are useful in purifying compounds having an inhibitory effect on sodium-dependent glucose cotransporter SGLT. The processes can reduce the number of steps needed to obtain the target compounds and the complexes formed in the processes are typically provided in a crystalline form.
Owner:THERACOSBIO LLC
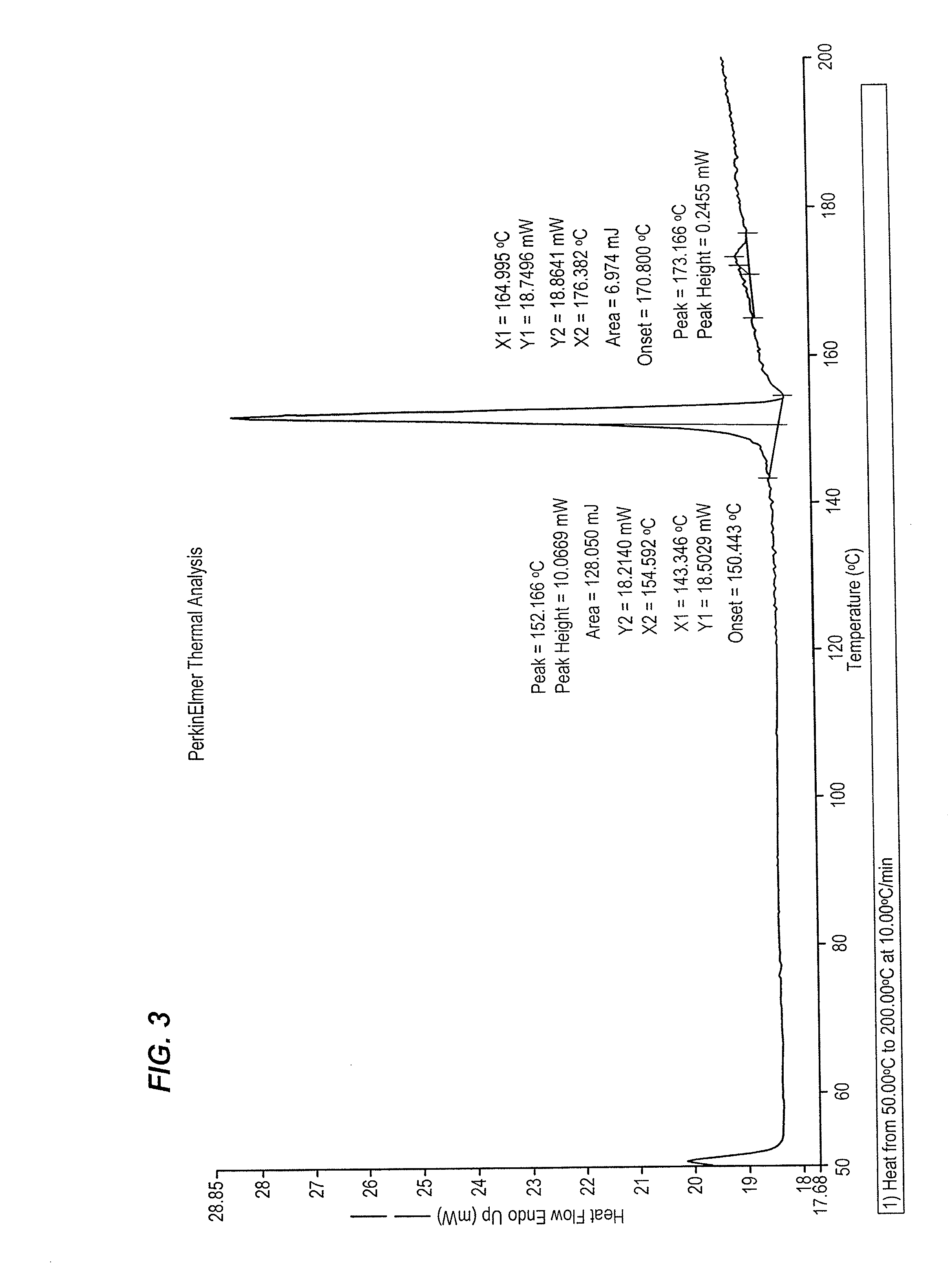
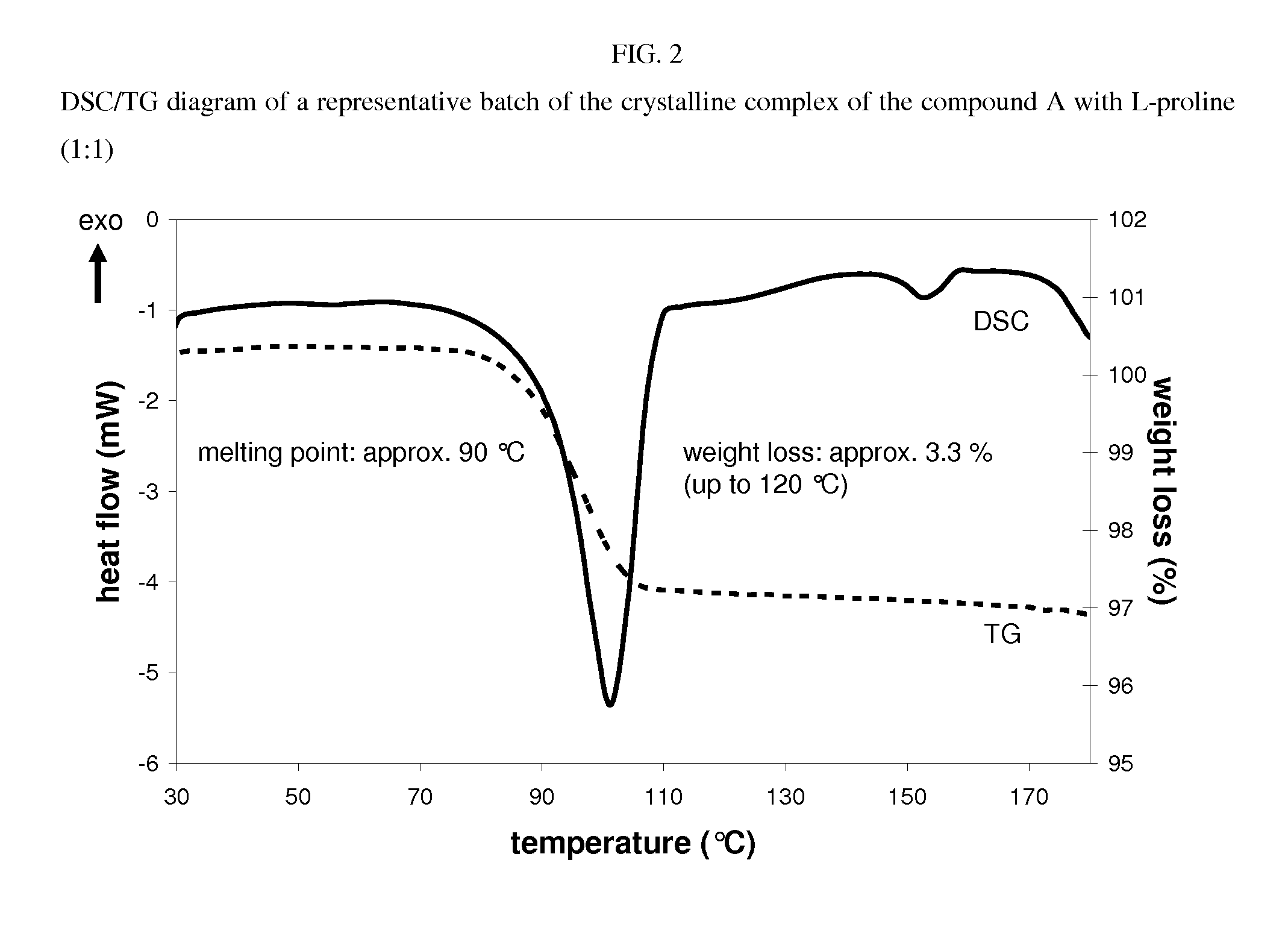
Crystalline complex of 1-cyano-2-(4-cyclopropyl-benzyl)-4-(ss- d-glucopyranos-1-yl)-benzene, methods for its preparation and the use thereof for preparing medicaments
The invention relates to a crystalline complex of 1-cyano-2-(4-cyclopropyl-benzyl)-4-(β-D-glucopyranos-1-yl)-benzene and a natural amino acid, to methods for the preparation thereof, as well as to uses thereof for preparing medicaments.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Benzylic glycoside derivatives and methods of use
Owner:THERAKOS INC
Method for extracting and refining hydroxysafflor yellow A from safflower
ActiveCN102675379AThe purification process is stable and controllableHigh puritySaccharide with carbocyclic radicalsSugar derivativesFreeze-dryingCarthamus yellow
The invention discloses a method for extracting and refining hydroxysafflor yellow A from safflower. The hydroxysafflor yellow A is a compound with a chalcone monoside structure, and is abundant in traditional Chinese medicinal material safflower (CARTHAMI FLOS). The hydroxysafflor yellow A with content of over 80 percent is obtained through the following five steps of: extracting form the traditional Chinese medicine safflower; purifying through ion exchange resin; purifying through neutral polarity macro-porous adsorption resin; purifying through non-polar macro-porous adsorption resin; and freeze-drying, wherein the transfer rate is over 20 percent.
Owner:HEBEI YILING MEDICINE INST
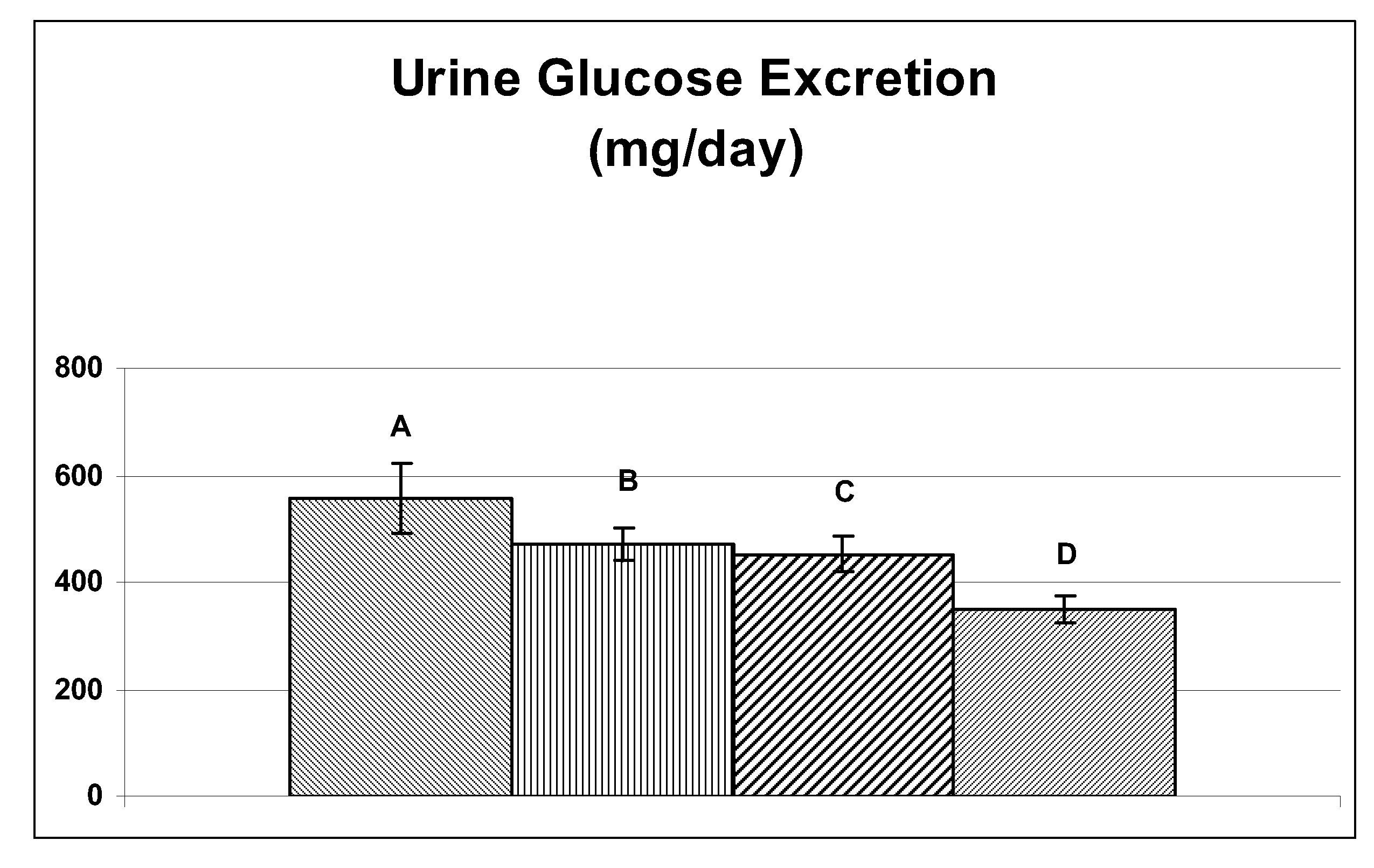
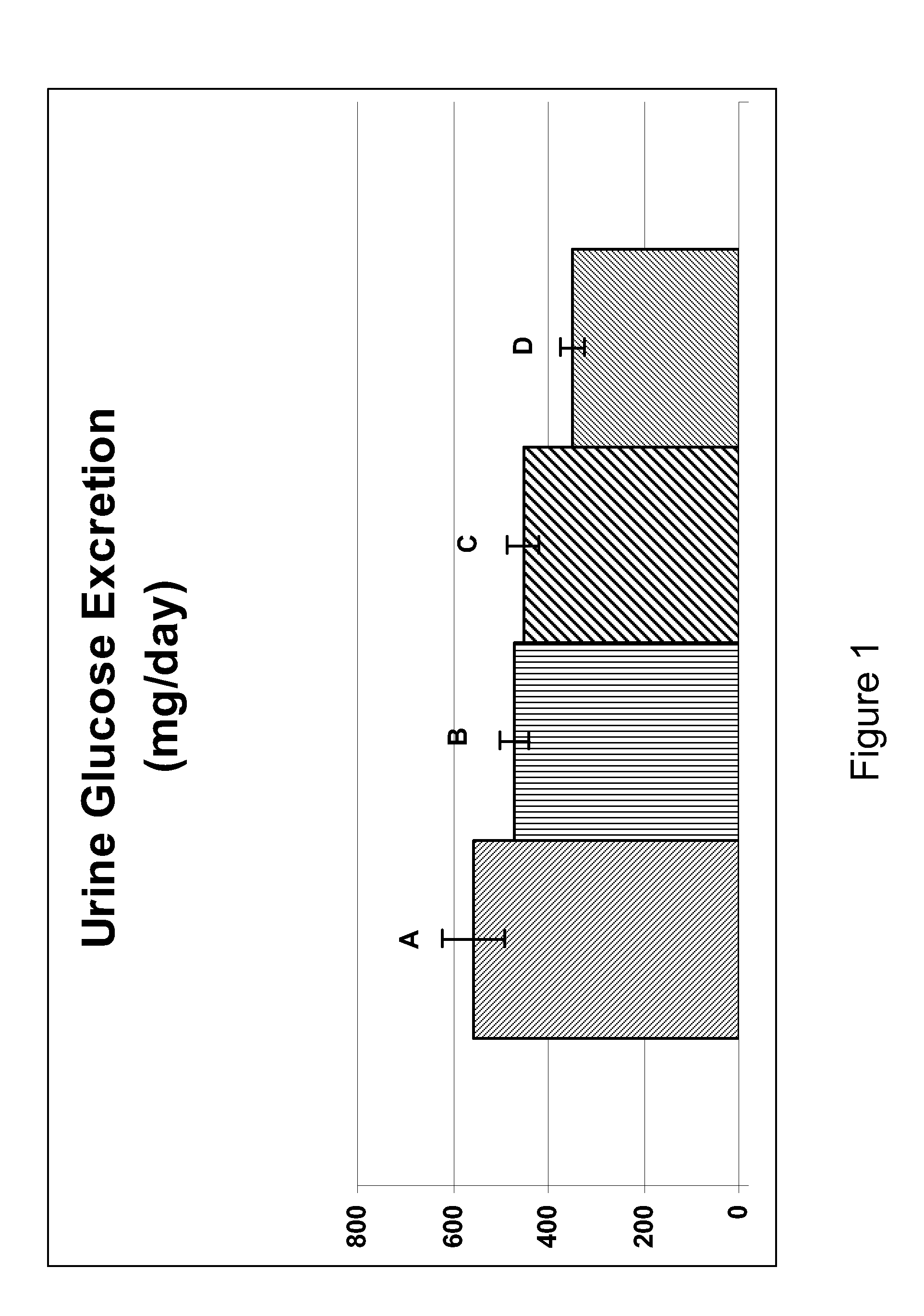
Treatment of metabolic disorders in feline animals
ActiveUS20150164856A1Reduce doseReduce frequencyBiocideNervous disorderAcute hyperglycaemiaDyslipidemia
The present invention relates to one or more SGLT2 inhibitors or pharmaceutically acceptable forms thereof for use in the treatment and / or prevention of a metabolic disorder in a feline animal, preferably wherein the metabolic disorder is one or more selected from the group consisting of: ketoacidosis, pre-diabetes, diabetes mellitus type 1 or type 2, insulin resistance, obesity, hyperglycemia, impaired glucose tolerance, hyperinsulinemia, dyslipidemia, dysadipokinemia, subclinical inflammation, systemic inflammation, low grade systemic inflammation, hepatic lipidosis, atherosclerosis, inflammation of the pancreas, neuropathy and / or Syndrome X (metabolic syndrome) and / or loss of pancreatic beta cell function and / or wherein the remission of the metabolic disorder, preferably diabetic remission, is achieved and / or maintained.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
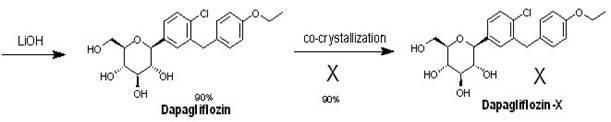
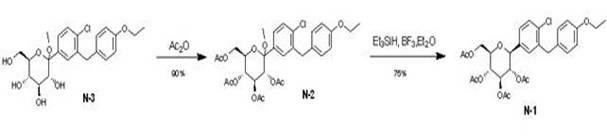
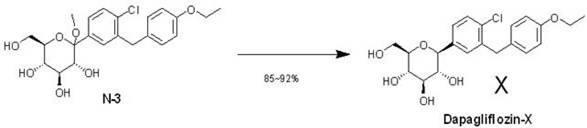
Eutectic preparation method of sodium-glucose cotransporter 2 bulk pharmaceutical chemicals
InactiveCN102167715AFew reaction stepsHigh selectivitySaccharide with carbocyclic radicalsSugar derivativesGadolinium ethoxybenzyl DTPATriol
The invention relates to a preparation method of bulk pharmaceutical chemicals used for curing the type 2 diabete, in particular to a eutectic preparation method of sodium-glucose cotransporter 2 bulk pharmaceutical chemicals. In the invention, the technical problems of the existing preparation method that reaction steps are more, the methoxy-removing condition is strict and the yield of the obtained main product with beta configuration is low are solved. The technical scheme in the invention is as follows: the eutectic preparation comprises the following steps: adding chiral component (X) inN-3((3R,4S,5S,6R)-2-(4-chloro-3-(4-ethoxylbenzyl)phenyl)-6-(hydroxymethyl)-2-methoxytetrahydro-2H-pyran-3,4,5-triol, performing selective complexation with reductant to remove methoxy, then reducing the temperature to perform eutectic preparation, and adopting the one-pot method to obtain dapagliflozin-X. The eutectic preparation method is mainly used to prepare the sodium-glucose cotransporter 2bulk pharmaceutical chemicals.
Owner:SHANGHAI HUISI BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com



![Crystalline form of 1-(beta-D-glucopyransoyl)-4-methyl-3-[5-(4-fluorophenyl)-2- thienylmethyl]benzene hemihydrate Crystalline form of 1-(beta-D-glucopyransoyl)-4-methyl-3-[5-(4-fluorophenyl)-2- thienylmethyl]benzene hemihydrate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6684e991-76ee-477e-8225-4b4dd6d2c62b/US20080146515A1-20080619-D00000.png)
![Crystalline form of 1-(beta-D-glucopyransoyl)-4-methyl-3-[5-(4-fluorophenyl)-2- thienylmethyl]benzene hemihydrate Crystalline form of 1-(beta-D-glucopyransoyl)-4-methyl-3-[5-(4-fluorophenyl)-2- thienylmethyl]benzene hemihydrate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6684e991-76ee-477e-8225-4b4dd6d2c62b/US20080146515A1-20080619-D00001.png)
![Crystalline form of 1-(beta-D-glucopyransoyl)-4-methyl-3-[5-(4-fluorophenyl)-2- thienylmethyl]benzene hemihydrate Crystalline form of 1-(beta-D-glucopyransoyl)-4-methyl-3-[5-(4-fluorophenyl)-2- thienylmethyl]benzene hemihydrate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6684e991-76ee-477e-8225-4b4dd6d2c62b/US20080146515A1-20080619-D00002.png)
![Crystalline form of 4-(beta-d-glucopyranos-1-yl)-1-methyl-2-[4-((s)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments Crystalline form of 4-(beta-d-glucopyranos-1-yl)-1-methyl-2-[4-((s)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a953bddb-f666-4d40-ac83-f0dd8c295572/US20100317847A1-20101216-D00001.png)
![Crystalline form of 4-(beta-d-glucopyranos-1-yl)-1-methyl-2-[4-((s)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments Crystalline form of 4-(beta-d-glucopyranos-1-yl)-1-methyl-2-[4-((s)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a953bddb-f666-4d40-ac83-f0dd8c295572/US20100317847A1-20101216-C00001.png)
![Crystalline form of 4-(beta-d-glucopyranos-1-yl)-1-methyl-2-[4-((s)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments Crystalline form of 4-(beta-d-glucopyranos-1-yl)-1-methyl-2-[4-((s)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a953bddb-f666-4d40-ac83-f0dd8c295572/US20100317847A1-20101216-C00002.png)

![Crystalline form of 1-(β-D-glucopyransoyl)-4-methyl-3-[5-(4-fluorophenyl)-2- thienylmethyl]benzene hemihydrate Crystalline form of 1-(β-D-glucopyransoyl)-4-methyl-3-[5-(4-fluorophenyl)-2- thienylmethyl]benzene hemihydrate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/27687691-4375-4e2e-8939-94523a6100cc/US07943582-20110517-D00000.png)
![Crystalline form of 1-(β-D-glucopyransoyl)-4-methyl-3-[5-(4-fluorophenyl)-2- thienylmethyl]benzene hemihydrate Crystalline form of 1-(β-D-glucopyransoyl)-4-methyl-3-[5-(4-fluorophenyl)-2- thienylmethyl]benzene hemihydrate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/27687691-4375-4e2e-8939-94523a6100cc/US07943582-20110517-D00001.png)
![Crystalline form of 1-(β-D-glucopyransoyl)-4-methyl-3-[5-(4-fluorophenyl)-2- thienylmethyl]benzene hemihydrate Crystalline form of 1-(β-D-glucopyransoyl)-4-methyl-3-[5-(4-fluorophenyl)-2- thienylmethyl]benzene hemihydrate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/27687691-4375-4e2e-8939-94523a6100cc/US07943582-20110517-D00002.png)
![Crystalline form of 4-(beta-D-glucopyranos-1-yl)-1-methyl-2-[4-((S)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments Crystalline form of 4-(beta-D-glucopyranos-1-yl)-1-methyl-2-[4-((S)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9af34e3e-cad4-4b67-b618-8a019eb52d2e/US08283326-20121009-D00001.png)
![Crystalline form of 4-(beta-D-glucopyranos-1-yl)-1-methyl-2-[4-((S)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments Crystalline form of 4-(beta-D-glucopyranos-1-yl)-1-methyl-2-[4-((S)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9af34e3e-cad4-4b67-b618-8a019eb52d2e/US08283326-20121009-C00001.png)
![Crystalline form of 4-(beta-D-glucopyranos-1-yl)-1-methyl-2-[4-((S)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments Crystalline form of 4-(beta-D-glucopyranos-1-yl)-1-methyl-2-[4-((S)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9af34e3e-cad4-4b67-b618-8a019eb52d2e/US08283326-20121009-C00002.png)