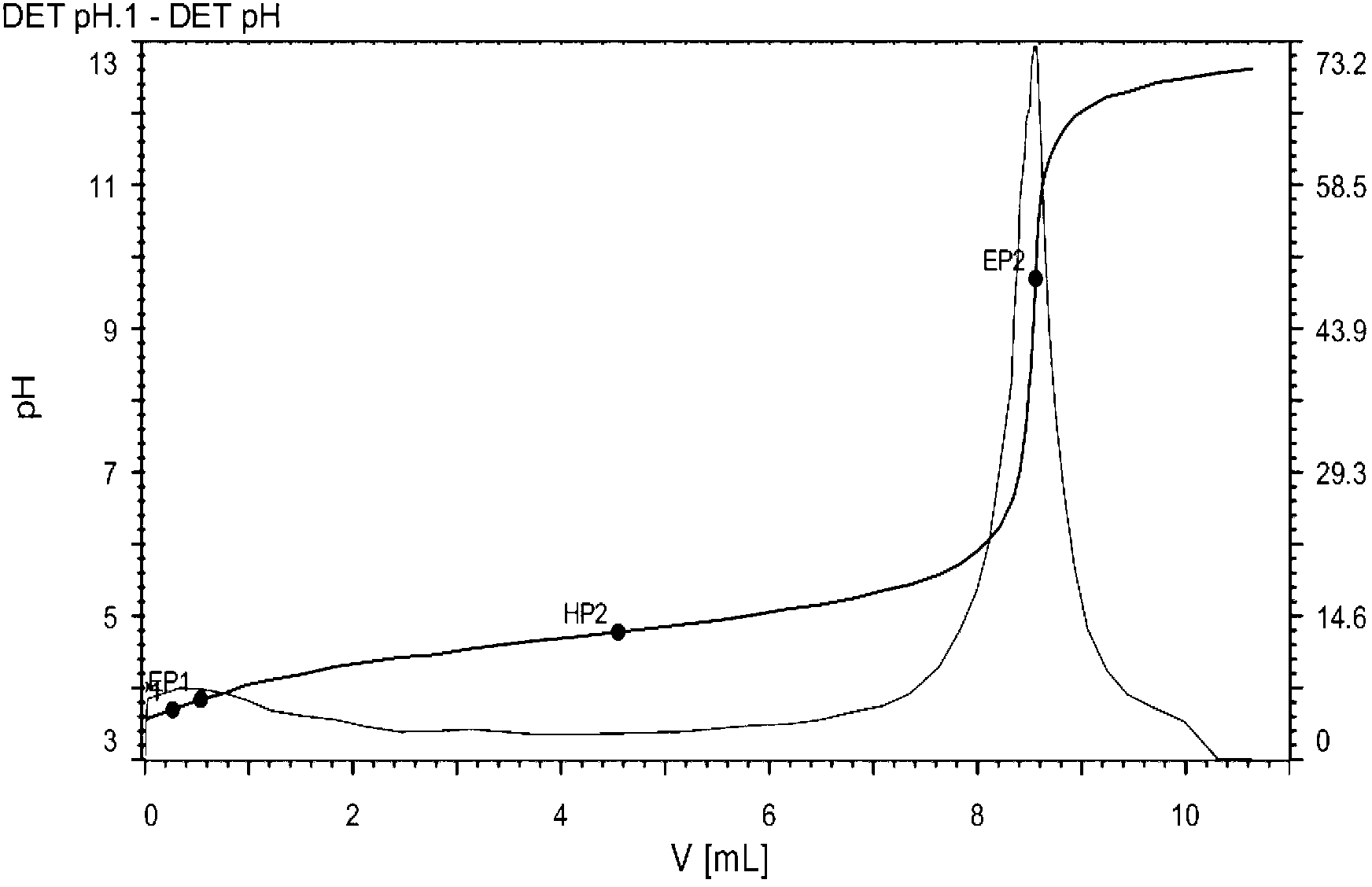
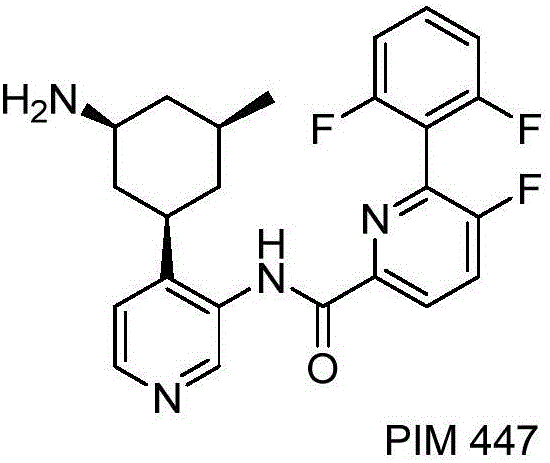
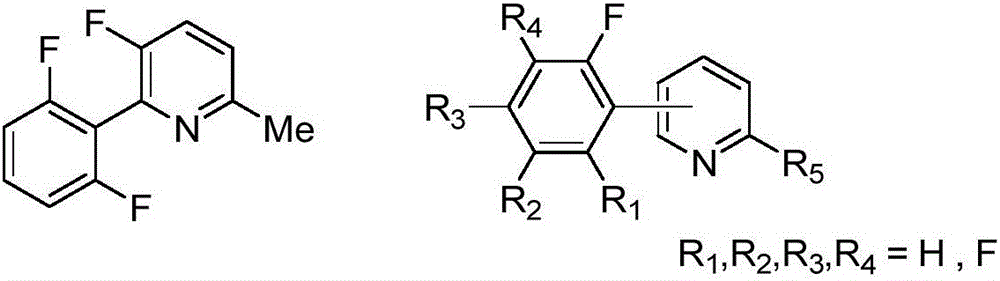
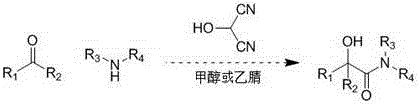
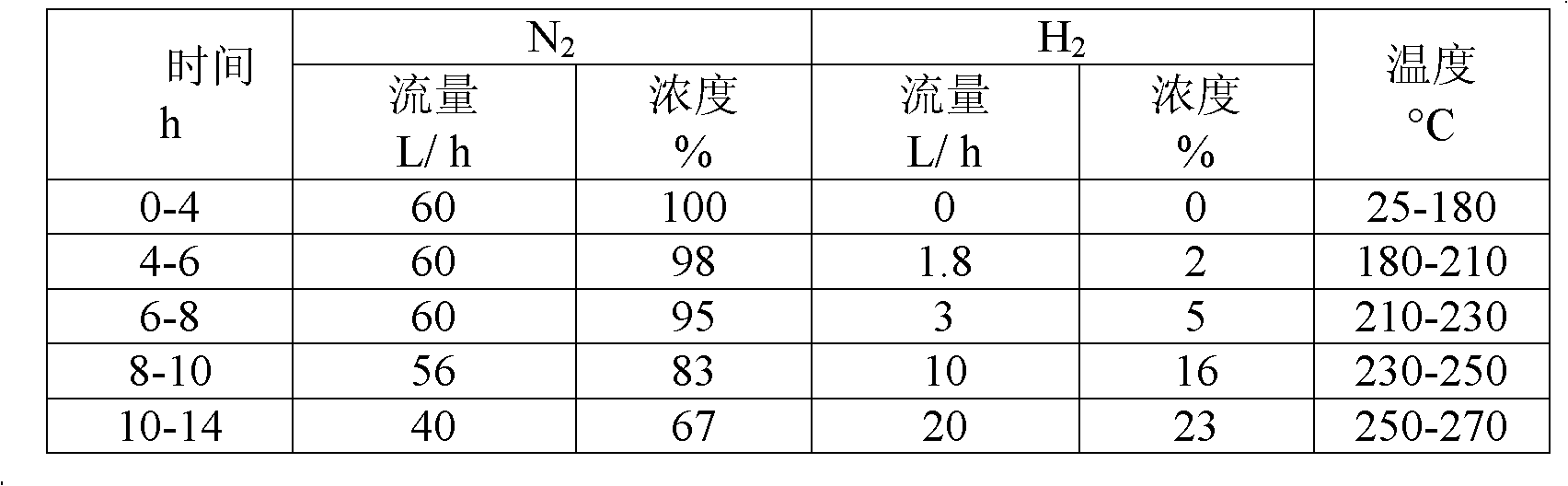
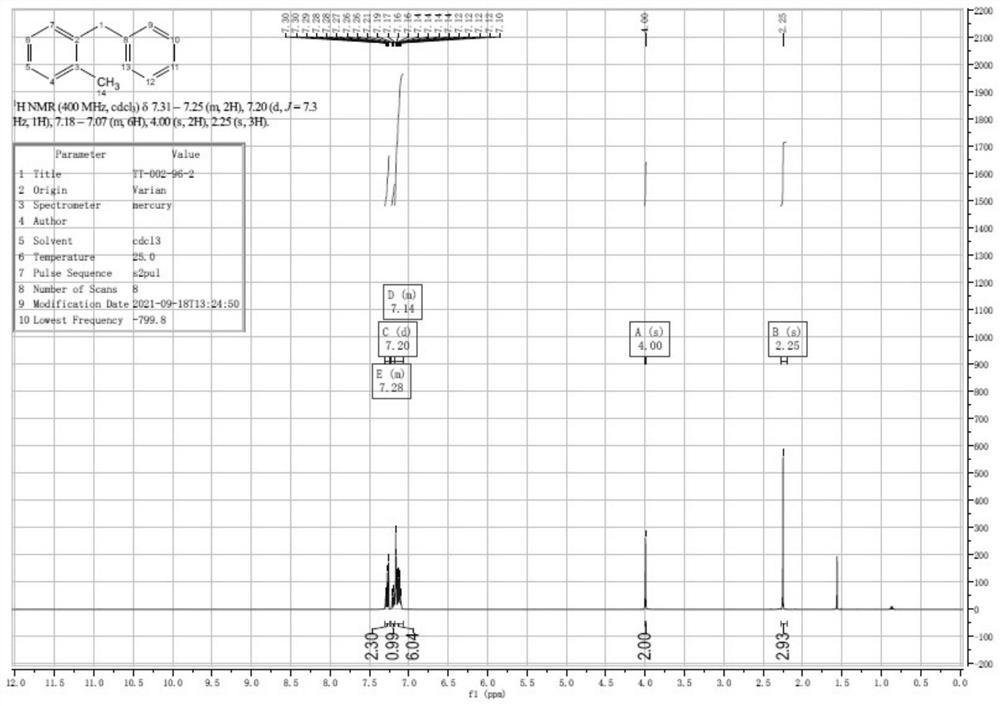
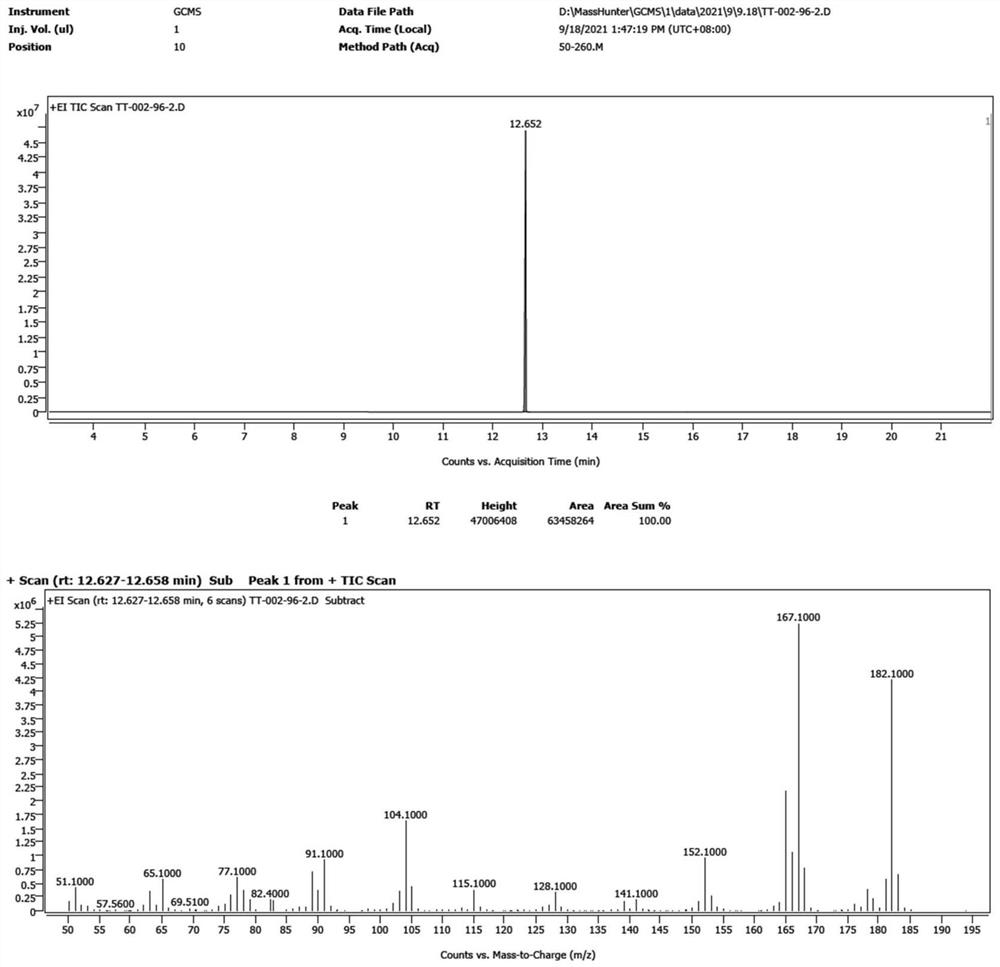
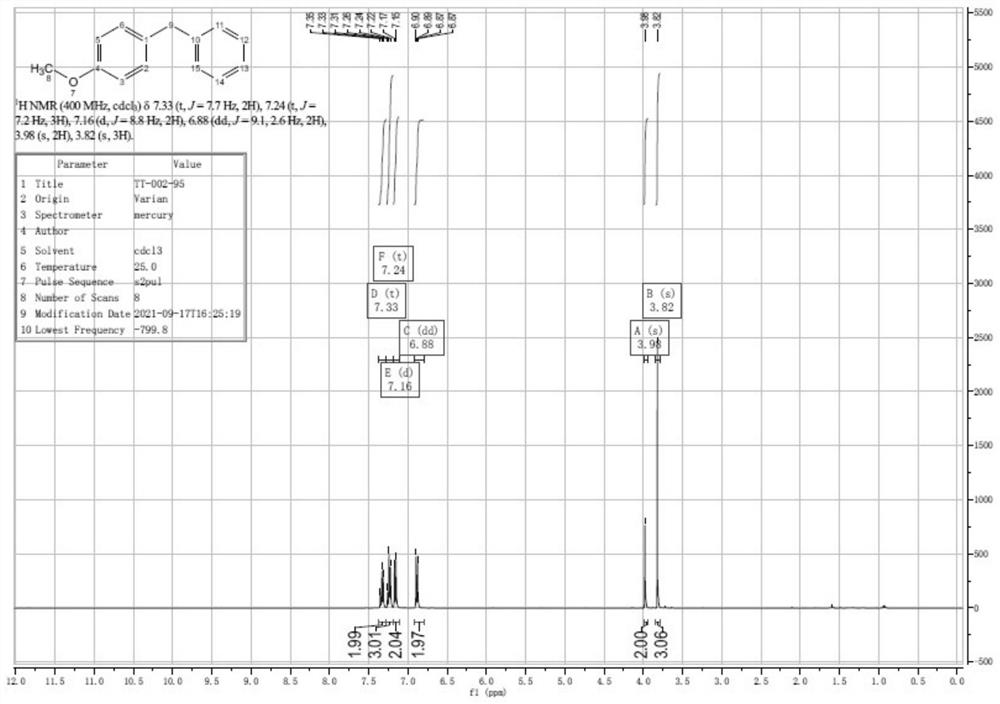
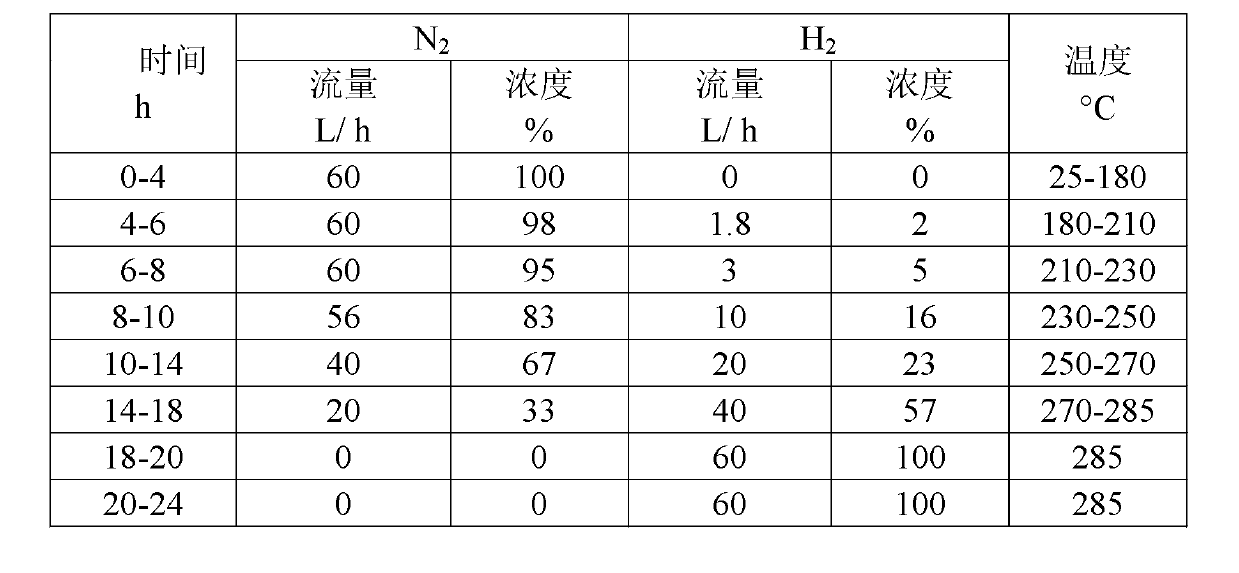
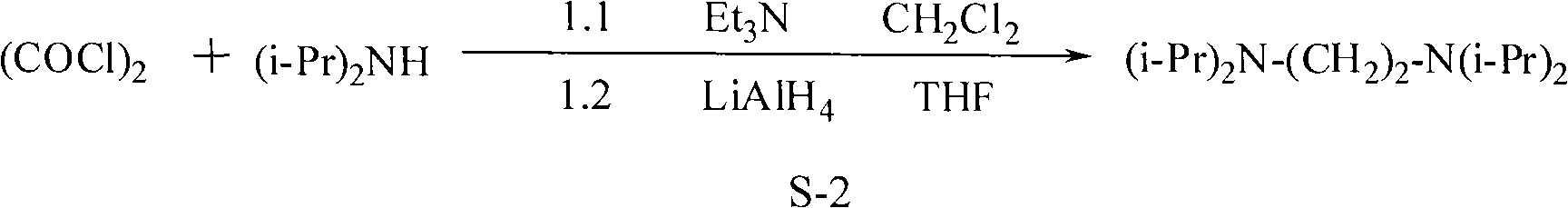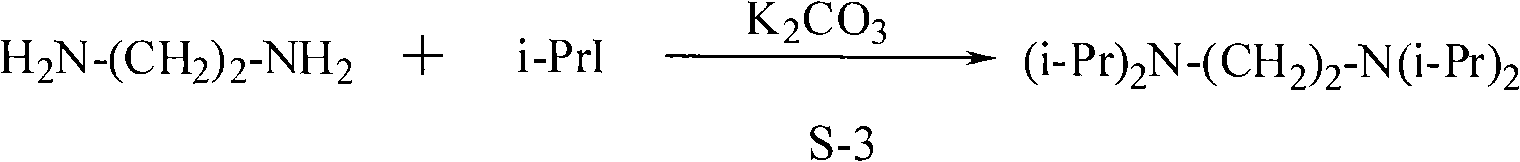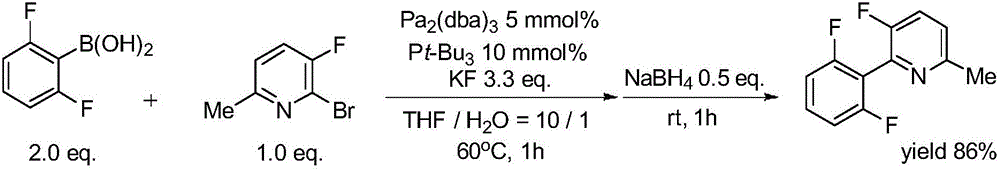Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
49results about How to "React clean" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
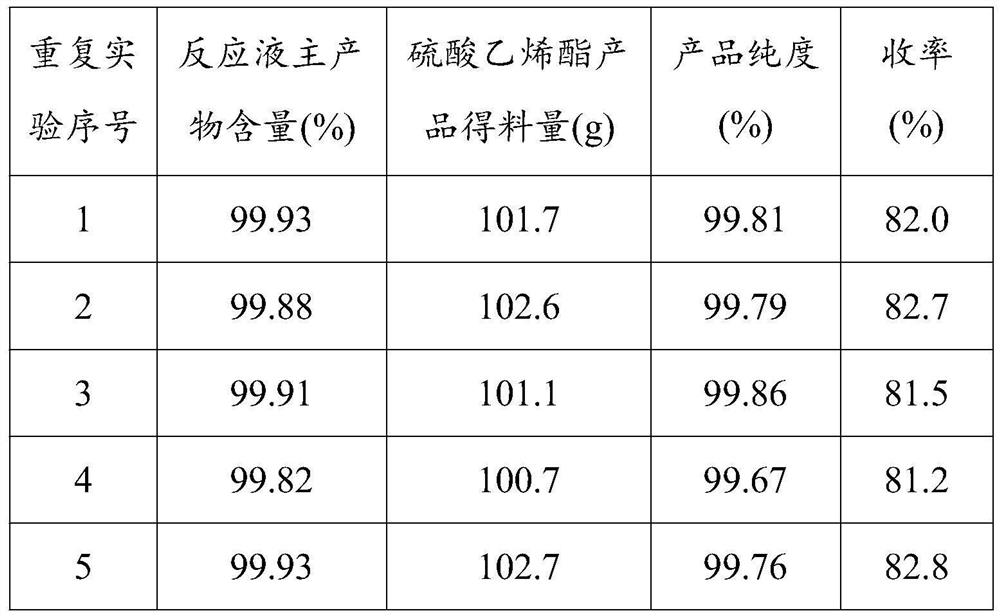
Method for preparing cyclic sulfate by directly oxidizing hydrogen peroxide
PendingCN111909129ALess impuritiesHigh purityOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsPtru catalystCatalytic oxidation
The method comprises the following steps: dropwise adding hydrogen peroxide into a mixture of cyclic sulfite, an organic solvent and a solid catalyst to carry out catalytic oxidation reaction, filtering out the solid catalyst after the reaction is finished, standing filtrate for layering, taking an organic layer, and performing distilling and concentrating to obtain a cyclic sulfate product. Cheaphydrogen peroxide is used for directly catalyzing and oxidizing cyclic sulfite to prepare cyclic sulfate, so that on one hand, the reaction is mild and easy to control, and the reaction conversion rate is high; on the other hand, no waste salt is generated, the evaporation capacity of water is small, energy consumption is low, generated waste water is little, and the production process is more environmentally friendly; the used solid catalyst contains an active component, an active auxiliary agent and an oxide carrier, and can be recycled, so that the consumption of noble metals is reduced, and the production cost is greatly reduced; the cyclic sulfate prepared by the method is few in impurities, high in purity and wide in market prospect.
Owner:CHANGSHU CHANGJI CHEM +1
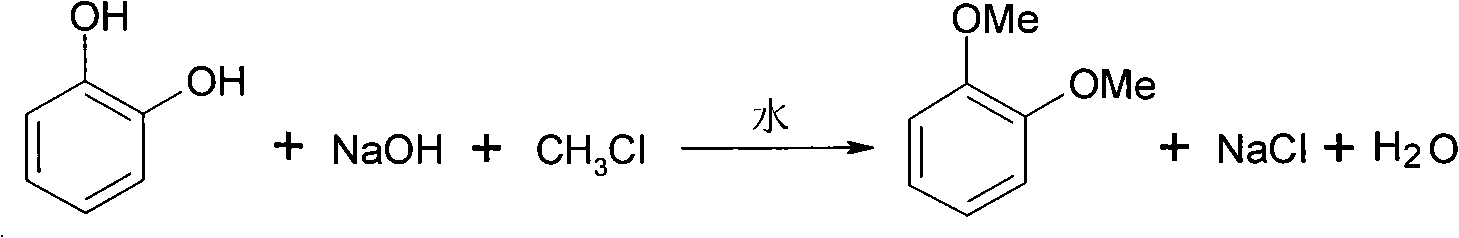
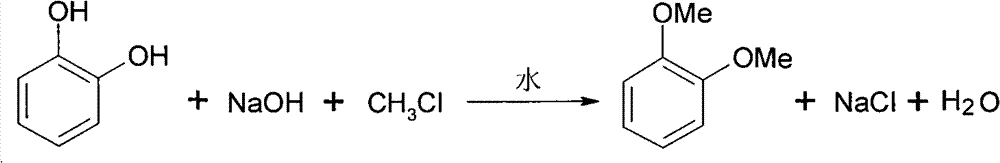
Synthesizing method for 1,2-dimethoxy benzene
InactiveCN101811942AReact cleanReduce pollutionEther preparation by ester reactionsBenzeneReaction temperature
The invention discloses a synthesizing method for 1,2-dimethoxy benzene, which utilizes pyrocatechol, strong base and chloromethane as raw materials and water as solvent to react in a high pressure autoclave; wherein the mol ratio of strong base to pyrocatechol is 2-3:1, the mole ratio of chloromethane to pyrocatechol is 2-3:1, the reaction temperature is 50-100 DEG C, the reaction pressure is 0.3-0.5 Mpa, the reaction time is 4-10h, and the 1,2-dimethoxy benzene is obtained through carrying out liquid separating and rectification on reaction liquid. The method of the invention is adopted to synthesize 1,2-dimethoxy benzene, thus having the characteristics of environment friendless, simple process and high yield.
Owner:ZHEJIANG UNIV
Compound I and (R)-3-aminopiperidine hydrochloride II, preparation method and application in Linagliptin synthesis
ActiveCN104387315AHigh optical purityHigh process reproducibilityOptically-active compound separationOrganic racemisationDipeptidyl peptidase3-Aminopyridine
The invention discloses a preparation method of 3-aminopiperidine and its derivative with optical activity and an application of the compound and its derivative in synthesis of a dipeptidyl peptidase-IV inhibitor Linagliptin. According to the preparation method, 3-aminopyridine is used as a raw material to prepare a compound I by 3- amino protection, catalytic hydrogenation reduction of pyridine ring and chiral reagent resolution, and deprotection is then carried out to obtain (R)-3-aminopiperidine hydrochloride II. In the application, a compound III prepared from the compound I is an important intermediate for synthesis of Linagliptin, and (R)-3-aminopiperidine hydrochloride II also can directly be used for synthesis of high-purity Linagliptin. The raw material used in the invention is low-cost and is easily-available in the market; each step is simple to operate; requirements on equipment are low; safety is high; and the preparation method is easy for industrial production.
Owner:2Y CHEM
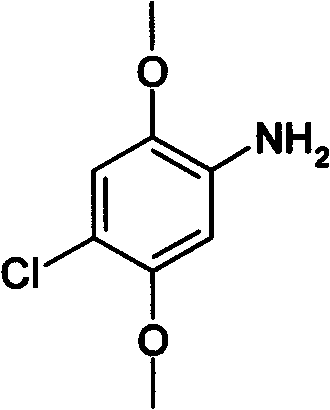
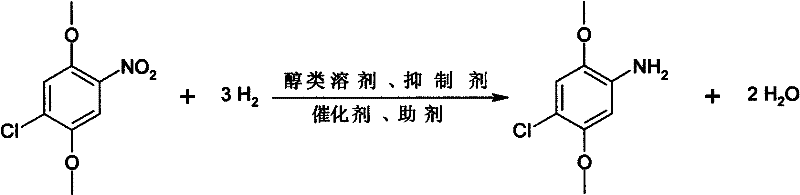
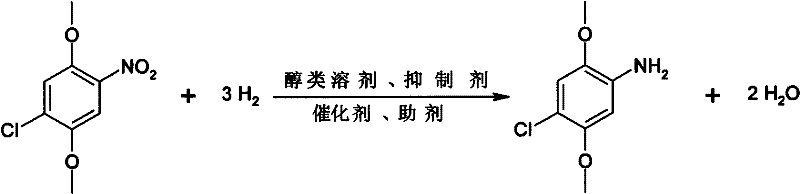
Method for preparing 2,5-dimethoxy-4-chloroaniline by using liquid-phase catalytic hydrogenation method
InactiveCN102344380AReact cleanReduce pollutionOrganic compound preparationAmino-hyroxy compound preparationSolventHigh pressure
The invention relates to a method for preparing 2,5-dimethoxy-4-chloroaniline by using a liquid-phase catalytic hydrogenation method, which comprises the following steps: (a) under the solvent condition, performing a catalytic hydrogenation reaction by taking raneys nickel as a catalyst, dimethyl sulfoxide as an auxiliary agent, organic amine as a dehalogenation inhibitor and 2,5-dimethoxy-4-chloroaniline as a raw material in a high pressure reaction vessel; (b) carrying out decolorizing by using active carbon and filtering to a reaction solution obtained in a step (a), and then adding a protective agent into the reaction solution, cooling, crystallizing and filtering; (c) adding the protective agent into mother liquor filtered in a step (b), concentrating, cooling, crystallizing and filtering, then merging the obtained products filtered in the step (b) and step (c) to obtain the white 2,5-dimethoxy-4-chloroaniline. The invention has the advantages of clean reaction, less pollution, simple process, easy operation, less reaction energy consumption and low cost.
Owner:江苏力达宁化工有限公司
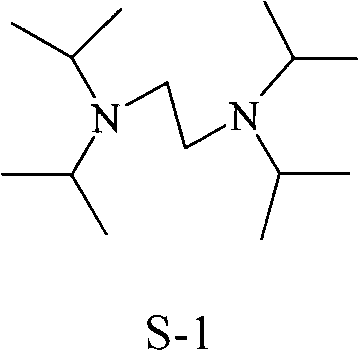
Synthesis method of N, N, N', N'-tetraisopropyl ethylene diamine
ActiveCN101928222AReact cleanReduce pollutionAmino preparation by functional substitutionDichloroethaneAqueous solution
The invention discloses a synthesis method of N, N, N', N'-teraisopropyl ethylene diamine. Diisopropylamine and dichloroethane are taken as raw materials, reaction is carried out in a high pressure autoclave under the conditions of no solvent and no catalyst, the mol ratio of diisopropylamine and [to] dichloroethane is 1: 1-4, reaction temperature is 100-200 DEG C, reaction pressure is 0.2-1.1Mpa, reaction time is 4-10 hours, so as to obtain solid liquid mixture; alkaline aqueous solution is added into the solid liquid mixture until pH value is 11.5-13.5; and finally separation and reduced pressure rectification are carried out, thus obtaining N, N, N', N'-teraisopropyl ethylene diamine. The method for synthesizing N, N, N', N'-teraisopropyl ethylene diamine of the invention has the characteristics of simple process and high yield and is environmentally friendly.
Owner:CHINA CONSTR IND & ENERGY ENG GRP CO LTD +1
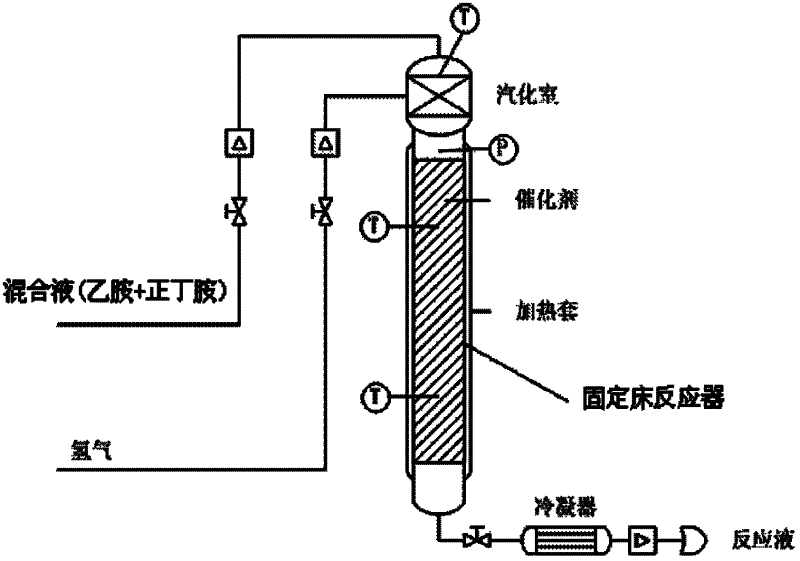
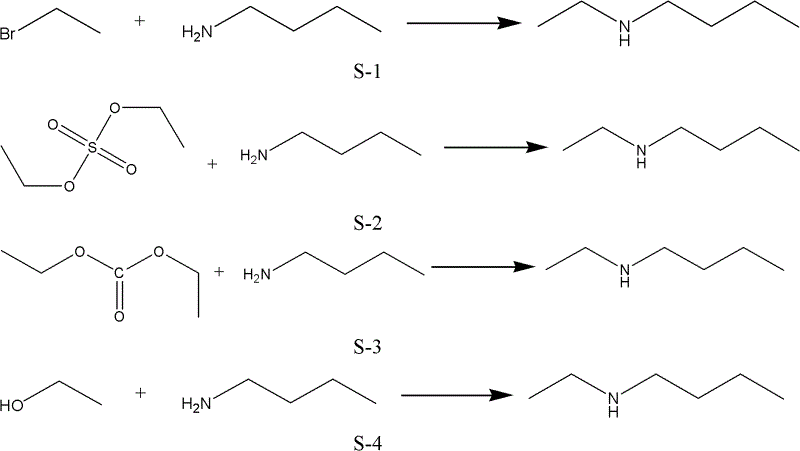
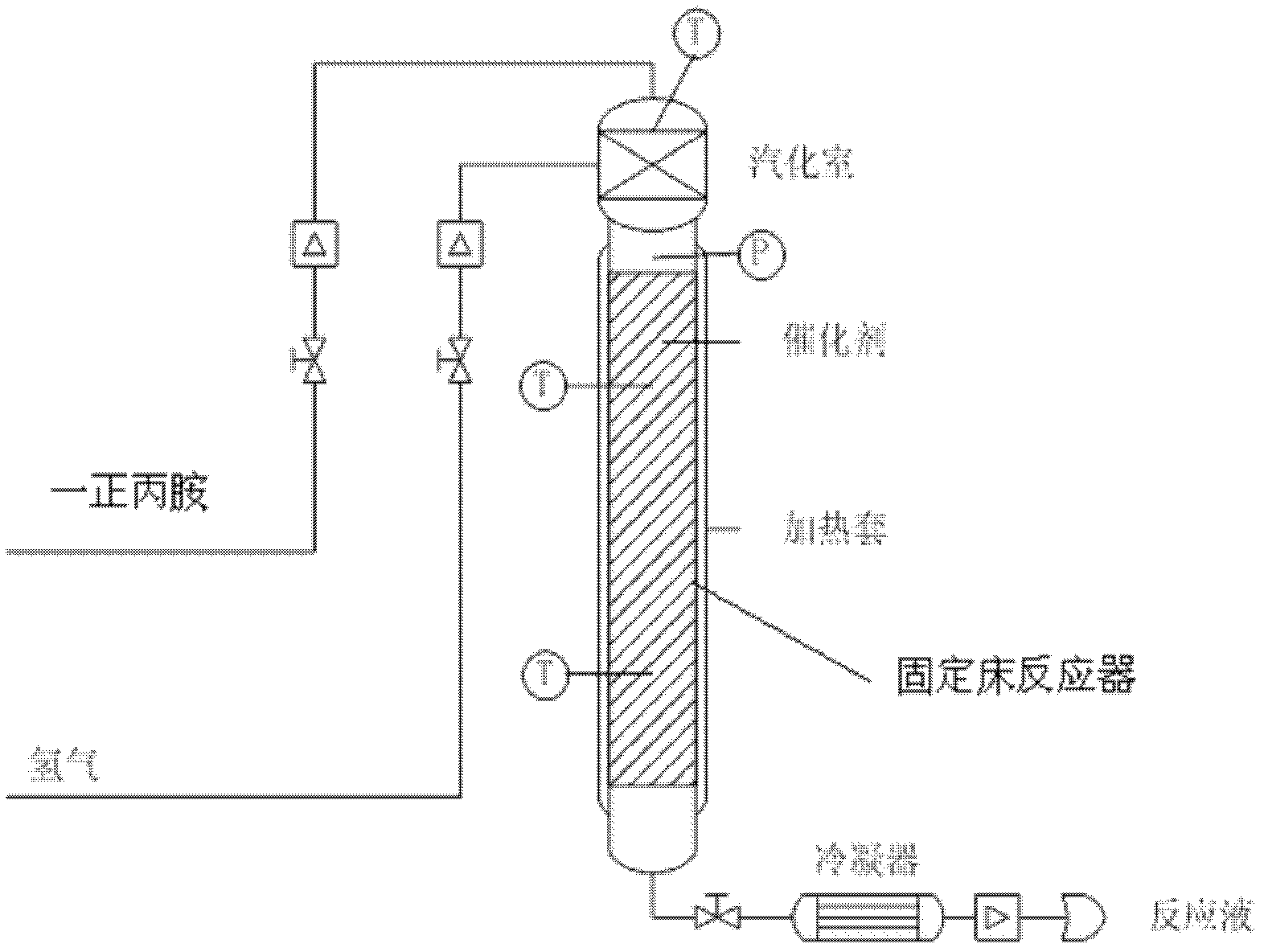
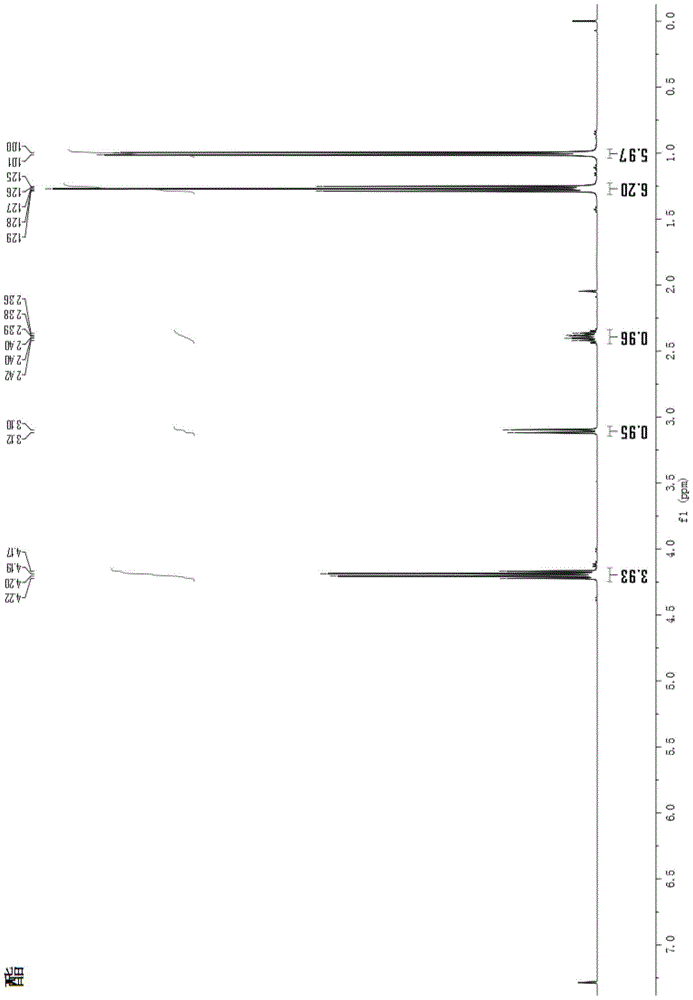
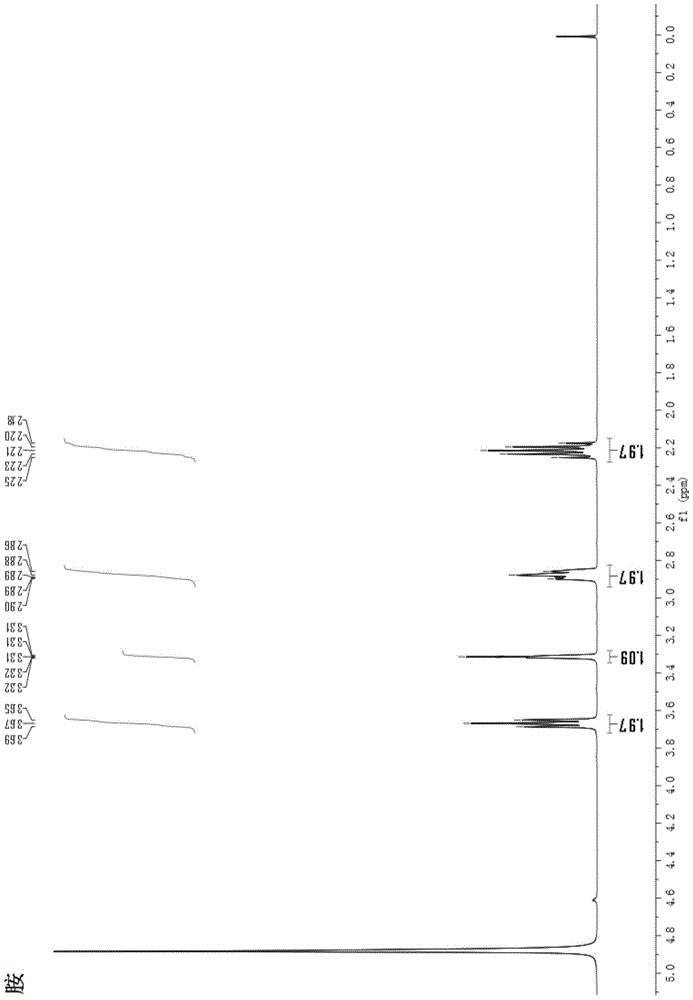
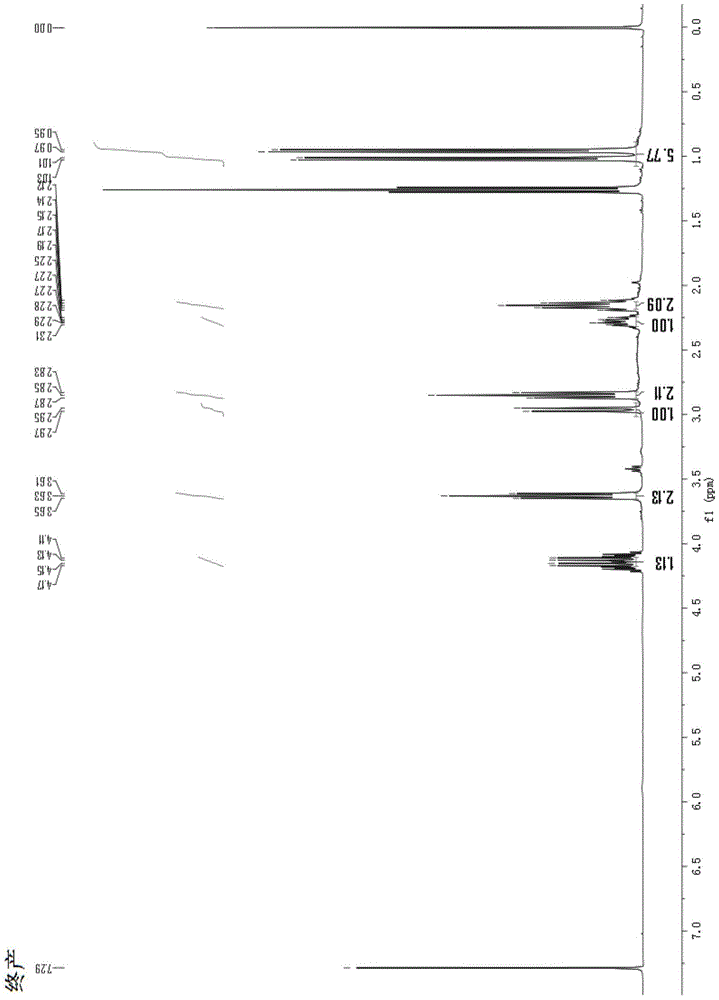
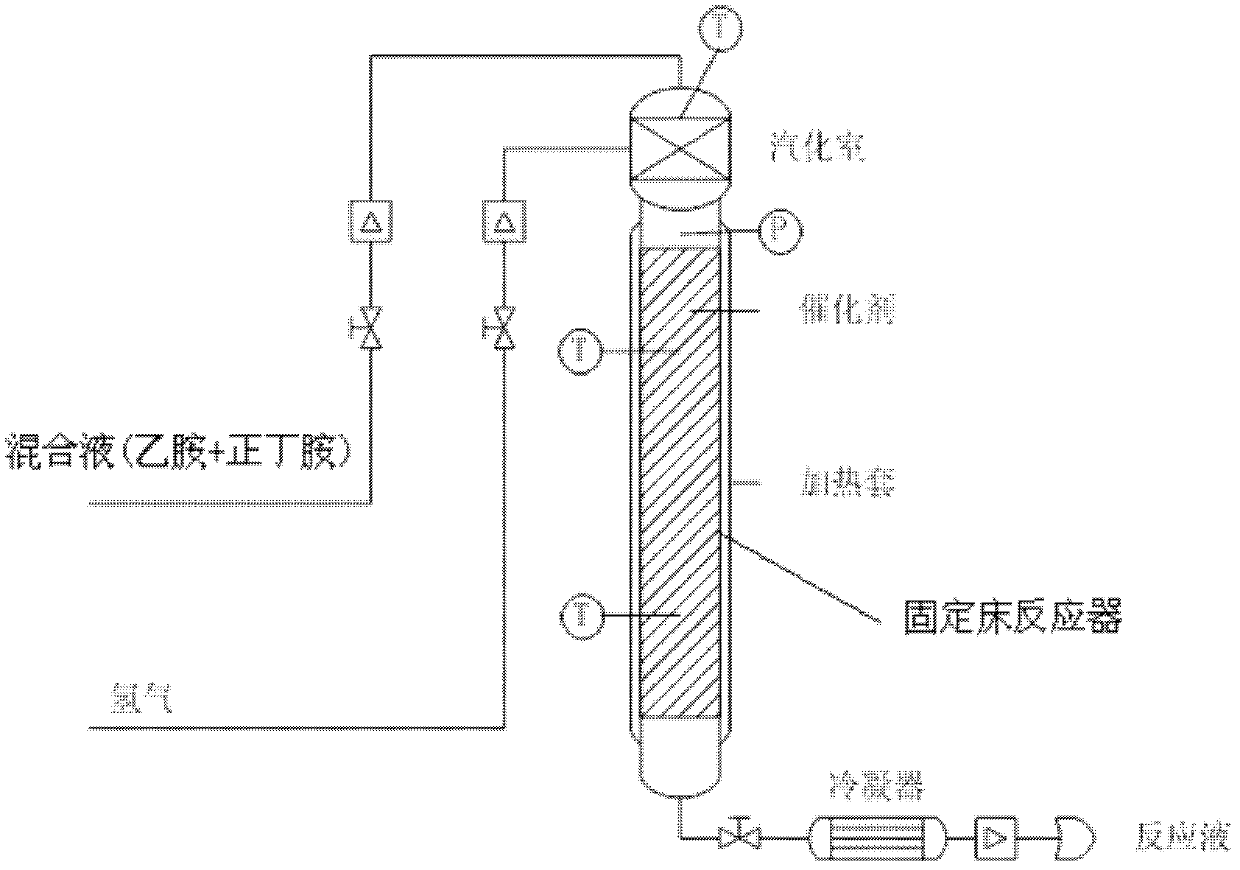
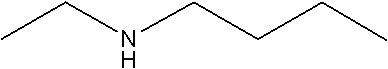
Method and used catalytic agent for synthesizing N-butylethylamine
ActiveCN102614881AAvoid generatingAvoid compositionMetal/metal-oxides/metal-hydroxide catalystsAmino compound preparation by disproportionationActive componentFixed bed
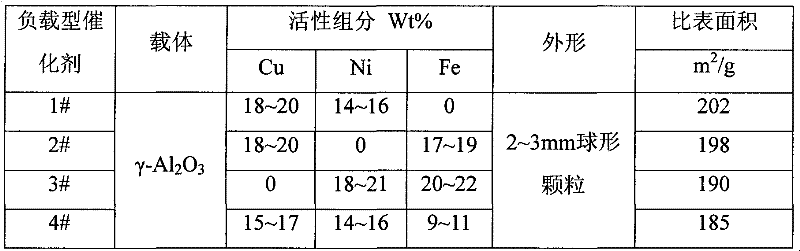
The invention discloses a loading-type catalytic agent for synthesizing N-butylethylamine. Gamma-alumina after roasting serves as a carrier, active components composed of Cu and Ni are loaded on the carrier, sum of weight of the Cu, the Ni and the gamma-AI2O3 after roasting is total weight, sum of weight of the Cu and the Ni occupies 25%-38% of the total weight, and ratio of amount of substance of the Cu and the Ni is 0.9-1.5 / 1. The invention further provides a preparation method of the loading-type catalytic agent. A method for synthesizing the N-butylethylamine by using the loading-type catalytic agent is further provided, ethylamine and the n-butylamine are mixed in a mixing tank to obtain mixed liquor which passes through a fixed bed reactor containing activated loading-type catalyticagent after being vaporized, an amine disproportionated reaction is carried out in a hydrogenation condition, products are collected after condensation, and rectification is carried out to obtain theN-butylethylamine.
Owner:ZHEJIANG JIANYE CHEM
Method of surface grafting modification for nylon fabric
InactiveCN103074769AImprove anti-dripping performanceFacilitate the grafting reactionFibre treatmentNylon materialMicrowave method
The invention relates to a method of surface grafting modification for nylon fabric with a microwave irradiation method. The grafting reaction conducted with the microwave method has the advantages of short response time, uniform reaction and small influence on a property of the fabric. As hydroxyethyl methacrylate serves as a grafting monomer, the hygroscopicity of the grafted nylon fabric is improved obviously, and the dripping resistance is good; in addition, the reaction is combined with chemical bonds; an effect is stable and lasting; and the washing resistance is high.
Owner:BEIJING UNIV OF CHEM TECH
Preparation method of 1,3-diene derivatives having aggregation-induced emission property
InactiveCN104326861AGreen synthesisEfficient synthesisPreparation from carboxylic acid saltsPreparation from carboxylic acid halidesAggregation-induced emissionOxygen
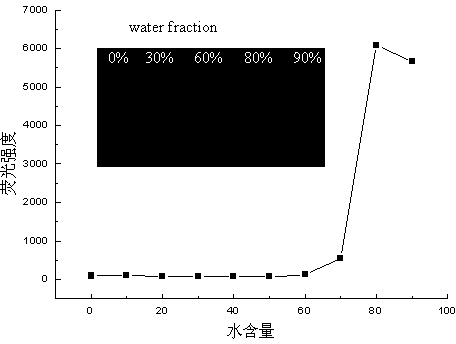
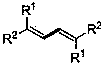
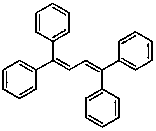
The invention discloses a preparation method of a 1,3-diene derivative having aggregation-induced emission property and belongs to the technical field of synthesis of compounds. The derivatives have strong aggregation-induced emission property and the preparation of the derivatives is achieved by carrying out oxydehydrogenation coupling reaction on a substrate olefin under the catalysis of palladium in the presence of oxygen serving as an oxidant. The method has the characteristics of mild reaction condition, simple operation, use of easily available raw materials, high selectivity and environmental friendliness and is used for solving the deficiencies in the prior art that the synthetic efficiency of the compounds having the aggregation-induced emission property is low and the cost is high.
Owner:GUANGDONG OCEAN UNIVERSITY
Microwave synthesis method for diarylamine compound
InactiveCN103214385ARealize sustainable development and utilizationShort reaction timeOrganic compound preparationAmino-carboxyl compound preparationMicrowave methodIsomerization
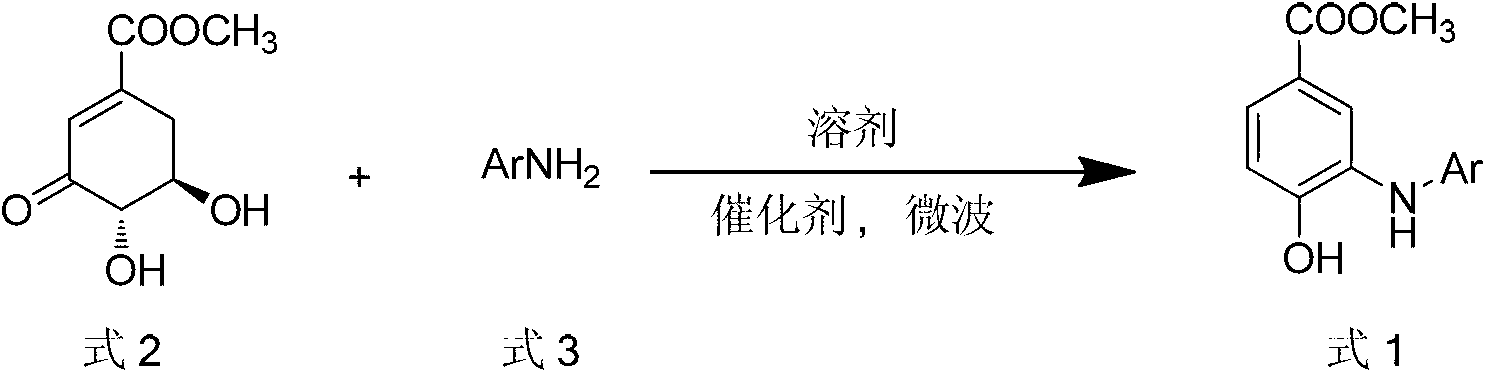
The invention discloses a microwave synthesis method for a diarylamine compound. The method comprises the following steps of: subjecting 3-methyl dehydroshikimic acid and a primary arylamine compound to a condensation reaction, an isomerization reaction and a dehydration reaction under the action of an organic solvent, a catalyst and microwaves to enable a hexatomic ring framework to be aromatized; and then, cooling a reaction liquid, pouring the reaction liquid into saturated brine, rapidly stirring the solution, separating out a solid, and carrying out suction filtration, drying and recrystallization to obtain a 3-amido-4-hydroxybenzoic acid methyl ester compound. The raw material 3-methyl dehydroshikimic acid adopted in the method is a non-aromatic compound and can be prepared from shikimic acid by using a simple and convenient method without depending on fossil resources; sustainable exploitation and utilization can be realized; in addition, the microwave synthesis method adopts a microwave method which is short in reaction time, simple and convenient in operation, convenient in after-treatment and high in yield; and the microwave synthesis method is clean in reaction, friendly to the environment and low in energy consumption.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
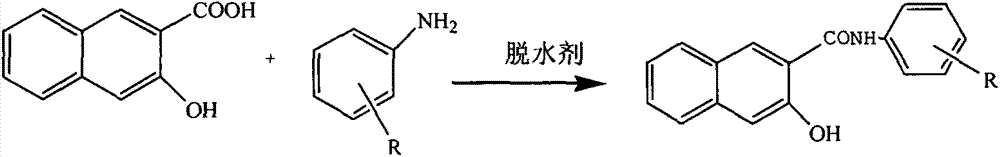
Synthesis process of novel green naphthol chromophore type products
InactiveCN104844470AHigh speedShort reaction timeOrganic compound preparationCarboxylic acid amides preparationMicrowaveSolvent
The present invention discloses a synthesis process of novel green naphthol chromophore type products. The synthesis process is characterized by comprising the following steps: 1) putting and dissolving materials of 2, 3-acid and aniline compounds into a suitable solvent; 2) pumping a resulting solution into a microwave reactor for reaction; 3) transferring water in time because 1 equivalent of the water can be drained in the process of the reaction; 4) upon completion of the reaction, distilling off the solvent through rectification; and 5) discharging bottom products. The microwave reaction and the reaction rectification are combined, so that the product synthesis efficiency is improved, and zero discharge of pollutants is realized.
Owner:JIANGSU HUAYI TECH
Method for removing nitrogen oxides in industrial waste gas
InactiveCN104056539AEfficient removalAdjust the solubility at willDispersed particle separationReaction temperatureNitrogen oxide
The invention discloses a method for removing nitrogen oxides in industrial waste gas. The method comprises the following steps: solution preparation, wherein solid ammonium bicarbonate is added into a mixing tank with industrial water through a hopper and mixed with the industrial water under the stirring effect of a stirrer of the mixing tank to generate a 20% ammonium bicarbonate solution; delivery, wherein the ammonium bicarbonate solution is delivered to an addition site through a delivery pump and then added into a waste gas generator at a set flow through regulation of a flow meter and a control valve; reaction, wherein the ammonium bicarbonate solution is atomized to be dispersed into tiny liquid drops, the reaction temperature is controlled to be within 800-850 DEG C, the pressure in the waste gas generator is controlled to be normal pressure or micro negative pressure, it is guaranteed that the reaction time is no shorter than 1 min, and ammonia gas, water and carbon dioxide are generated after the reaction. By means of the method, the nitrogen oxides in the industrial waste gas are effectively removed, and compounds which can influence the environment or pollute a system will not be generated.
Owner:SCIP SITA WASTE SERVICES
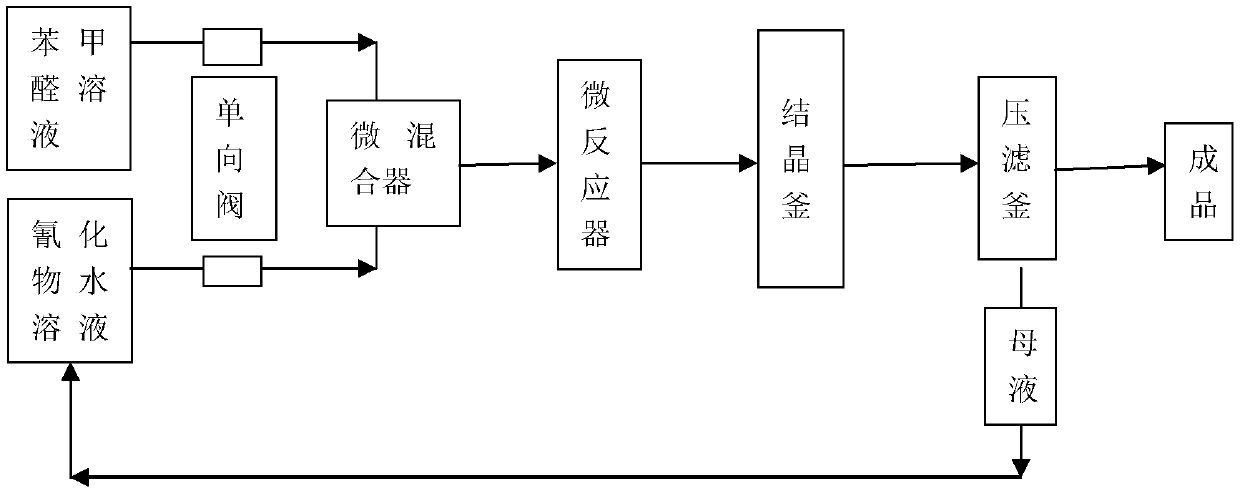
Method for preparing benzoin in micro-reaction device
ActiveCN111018681AReduce dosageReduce riskChemical/physical/physico-chemical microreactorsCarbonyl compound preparation by condensationMicroreactorBenzaldehyde
The invention relates to a method for preparing benzoin in a microreactor. According to the invention, benzaldehyde and sodium cyanide are used as raw materials, and benzoin is prepared through benzoin condensation. According to the preparation method for benzoin provided by the invention, the consumption of cyanide is greatly reduced, and the whole reaction process is totally closed and is thus relatively safe and reliable; the efficient heat and mass transfer capacity of the micro-channel reactor is used, so reaction temperature is reduced, side reactions such as oxidation, polymerization and self-disproportionation of benzaldehyde are reduced, reaction yield is high, a yield coefficient is high, and industrial production is easy to realize.
Owner:TIANJIN JIURI NEW MATERIALS CO LTD
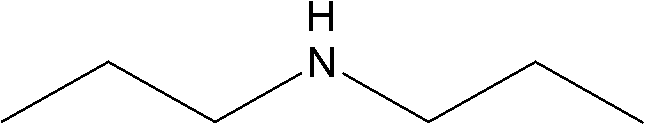
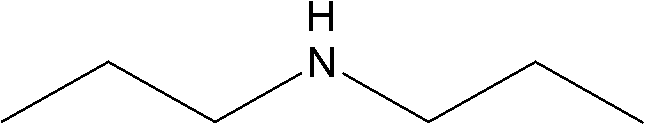
Method for combining di-n-propylamine through n-propylamine disproportionated reaction and used catalyst
ActiveCN102614895AReact cleanReduce pollutionMetal/metal-oxides/metal-hydroxide catalystsAmino compound preparation by disproportionationChemistryNickel
The invention discloses a load type catalyst used for combining di-n-propylamine, which utilizes roasted gama-alumina as a carrier. An active component is loaded on the carrier and is composed of nickel, copper, zinc and ruthenium. The weight sum of the nickel, the copper, the zinc, the ruthenium and the roasted gama-alumina is the total weight. The nickel occupies 15% to 25% of the total weight, the copper occupies 5% to 12% of the total weight, the zinc occupies 0.5% to 1% of the total weight, and the ruthenium occupies 0.5% to 1% of the total weight. A preparation method of the load type catalyst for combining the di-n-propylamine and a method for preparing the di-n-propylamine by using the load type catalyst are further disclosed. N-propylamine is added in a raw material cylinder, is gasified and then passes through a fixed bed reactor containing an activated load type catalyst, catalyst disproportionated reaction is conducted under the hydrogen condition, a product is collected after condensation, and rectification is conducted to obtain the di-n-propylamine.
Owner:ZHEJIANG JIANYE CHEM
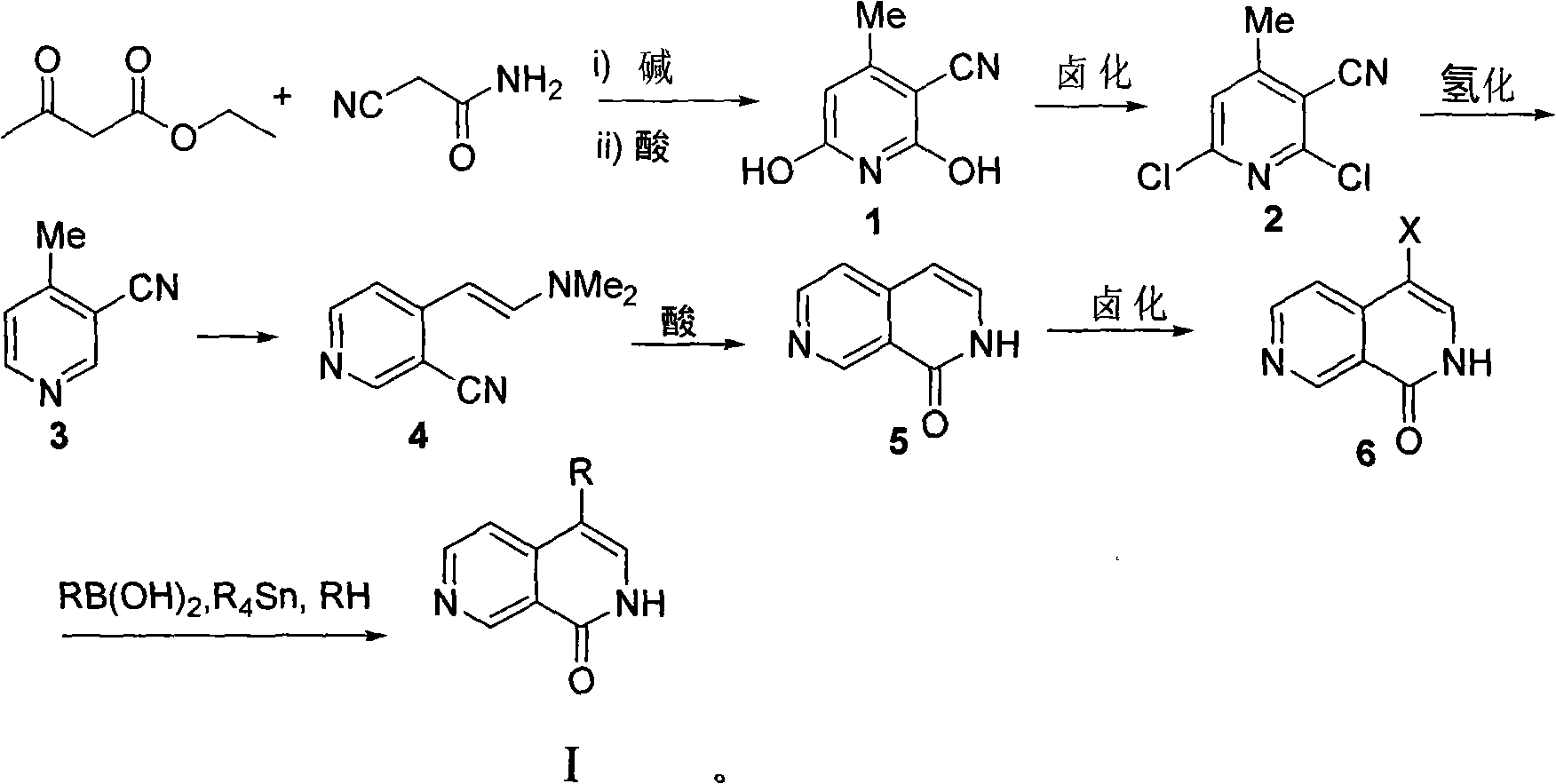
4-substituted 2,7-phthalazine compound, and preparation and usethereof
InactiveCN101328172AReact cleanHigh yieldOrganic active ingredientsNervous disorderHalogenSide chain
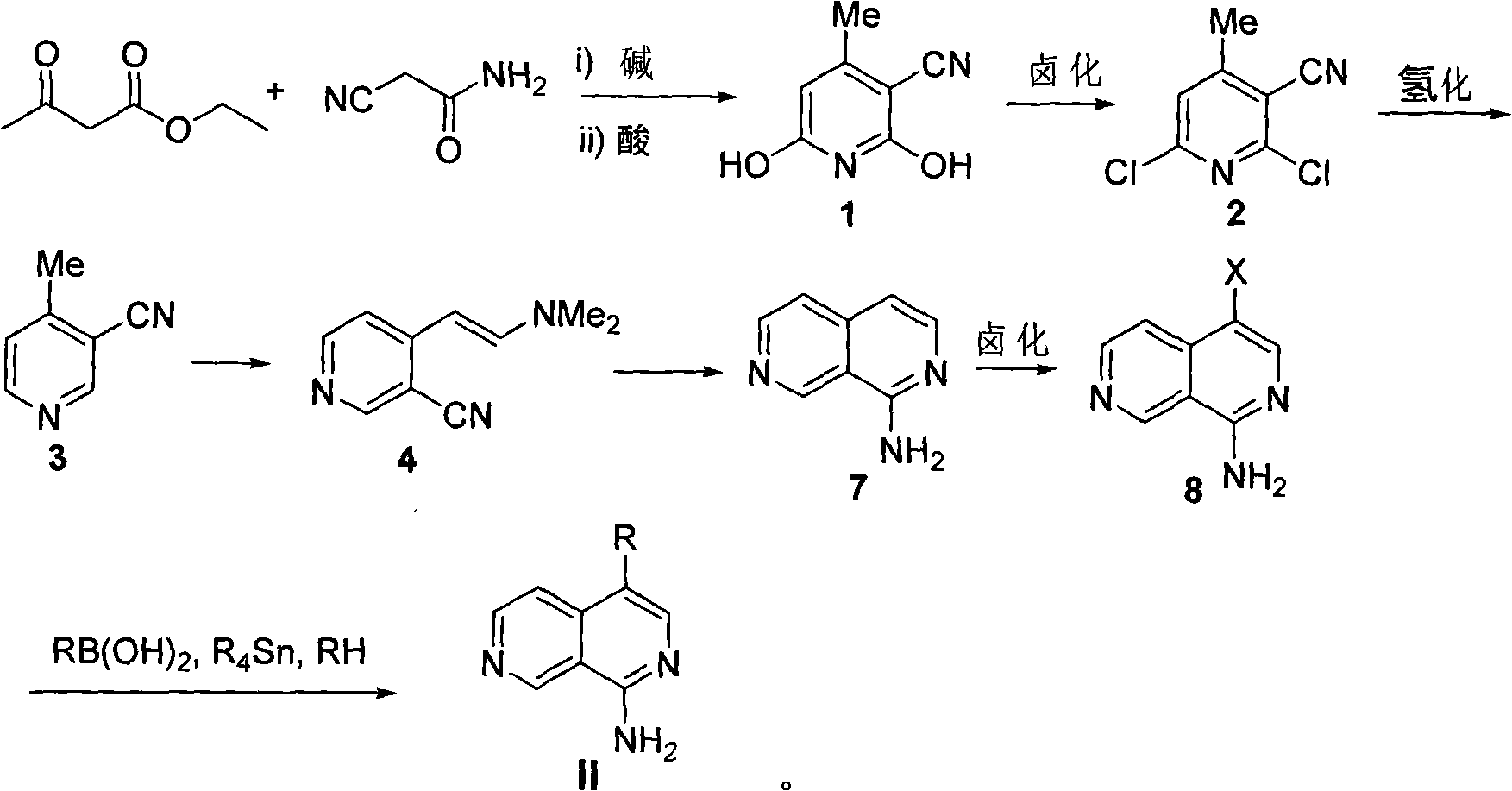
The invention relates to a 4-substituted 2,7-2,7-naphthyridine compound, a method for preparing the same and an application of the same in the preparation of mixed agonist or antagonist drugs or in prohibiting cytotoxicity, and resisting tumors and bacteria as a protease inhibitor. The compound has a structure shown in the genetic formula I or II, wherein R is a halogen, an alkyl, aryl or heteroaryl of a C1-C10 straight chain or side chain.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Method for synthesizing 4-cyclohexyl morpholine
ActiveCN102229582AReact cleanReduce pollutionOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsMorpholineCyclohexylamines
The invention discloses a method for synthesizing 4-cyclohexyl morpholine. The method comprises the following steps of: mixing diethylene glycol and cyclohexylamine in a mixing tank; allowing the mixture to pass through a fixed bed containing a supported catalyst; performing catalytic amination reaction under the hydrogen condition; condensing and collecting products; and rectifying to obtain the4-cyclohexyl morpholine, wherein the molar ratio of the diethylene glycol to the cyclohexylamine is 1:(2-10); the aminolysis reaction temperature is between 140 and 240 DEG C; the reaction pressure is 0.6 to 1.8 Mpa; and the volume space velocity of the mixed solution is 0.1 to 0.4 h<-1>. The method has the advantages that: (1) the method is implemented without a solvent, so the reaction is clean, pollution is low and pressure for separating and purifying the products is reduced; (2) the method is high in utilization ratio of raw materials, short in flow and easy to operate, and cost is greatly reduced compared with that of other processes; and (3) the method is wide in raw material sources, low in price, mild in reaction condition, high in yield, simple in aftertreatment and applicable to industrialized production.
Owner:ZHEJIANG JIANYE CHEM
A kind of method of solid-state ball milling synthetic Sorafenib
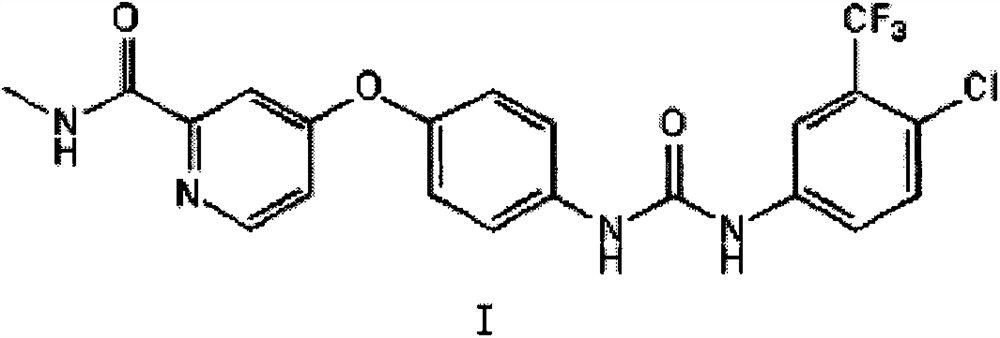
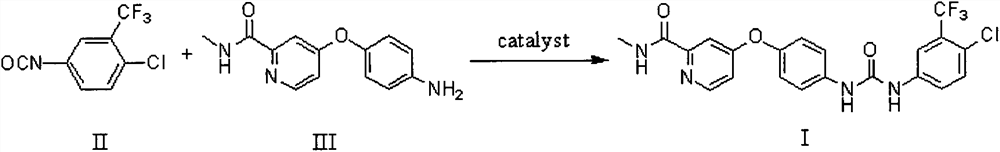
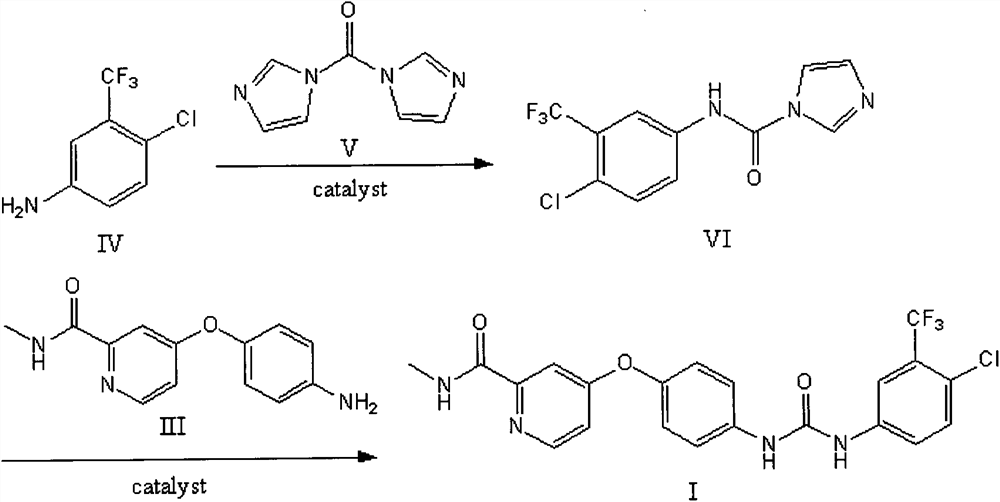
The invention relates to a method for synthesizing sorafenib by solid state ball milling. This method takes compound 4-(4-aminophenoxy)-N-picoline-2-carboxamide and compound 4-chloro-3-trifluoromethyl isocyanate as raw materials, or compound 4-chloro-3- Trifluoromethylaniline, compound N, N'-carbonyldiimidazole and compound 4-(4-aminophenoxy)-N-picoline-2-carboxamide are raw materials, under the catalysis of a small amount of liquid (and base) , Sorafenib was synthesized by solid state ball milling in a ball mill. The main innovation of the present invention is that Sorafenib is synthesized by solid ball milling in a ball mill for the first time. Compared with the method of synthesizing Sorafenib in the original liquid solvent, the method of the present invention has fast reaction speed, no dust spillage, and controllable reaction Good performance, simple operation, high reaction yield, less pollution, strong feasibility and so on.
Owner:CHINA PHARM UNIV
Prepn process of 1-chloro-3-(N-substituent)-tert-butyloxy formylamido-3-substituent-2-acetone
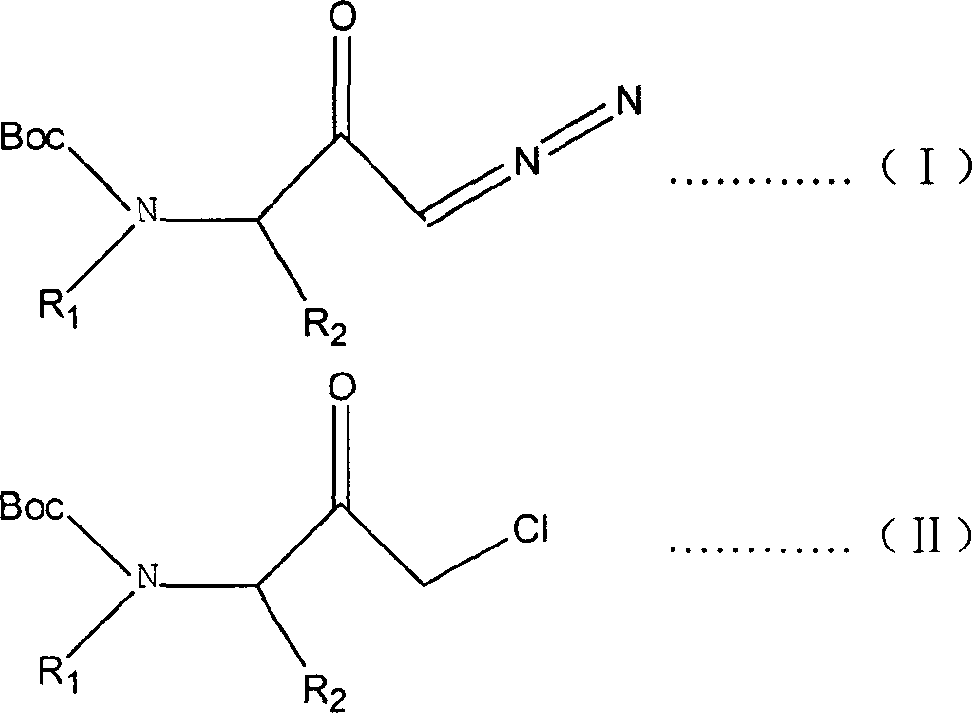
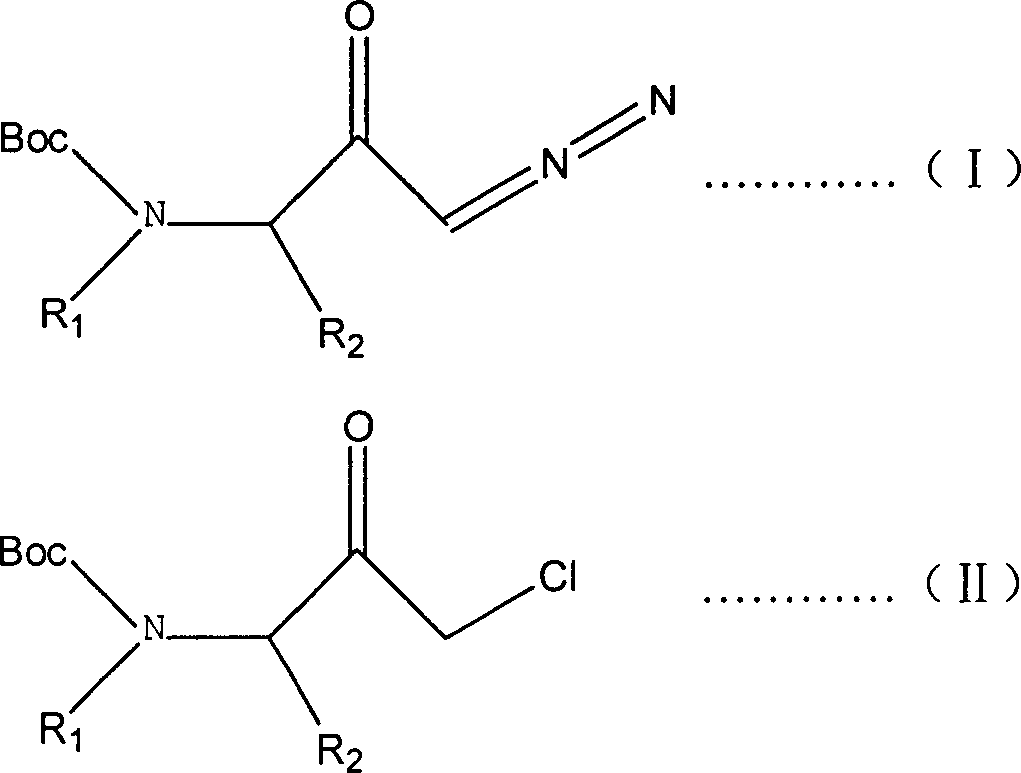
ActiveCN1277812CConvenient sourceLow priceCarbamic acid derivatives preparationOrganic compound preparationReaction temperatureSolvent
The present invention is preparation process of 1-chloro-3-(N-substituent)-tert-butyloxy formylamido-3-substituent-2-acetone. The preparation process has 1-diazo-3-(N-substituent)-tert-butyloxy formylamido-3-substituent-2-acetone as main material and HCl gas as chlorinating reagent, and includes dissolving the main material in dry solvent, introducing dry HCl gas into the solution under the protection of inert gas to react at the temperature from -40 deg.c to +40 deg.c and final decompression drying to eliminate solvent, with the molar ratio between the main material and HCl gas being 1 to 1-10. The preparation process realizes the reaction through controlling the amount of added HCl gas and the reaction temperature, and has low production cost and no industrial pollution caused by metal cation.
Owner:PORTON FINE CHEM
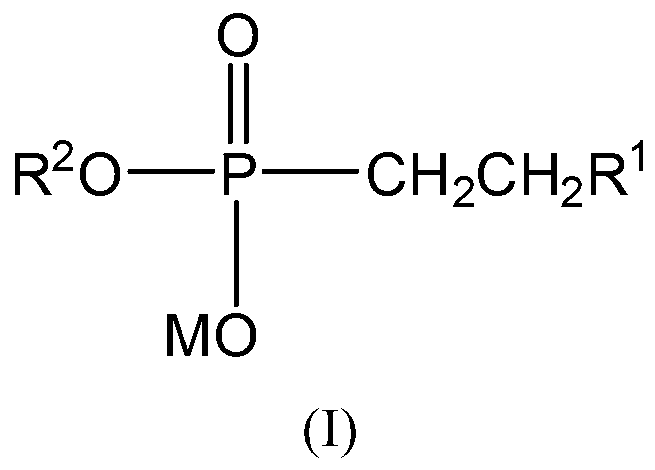
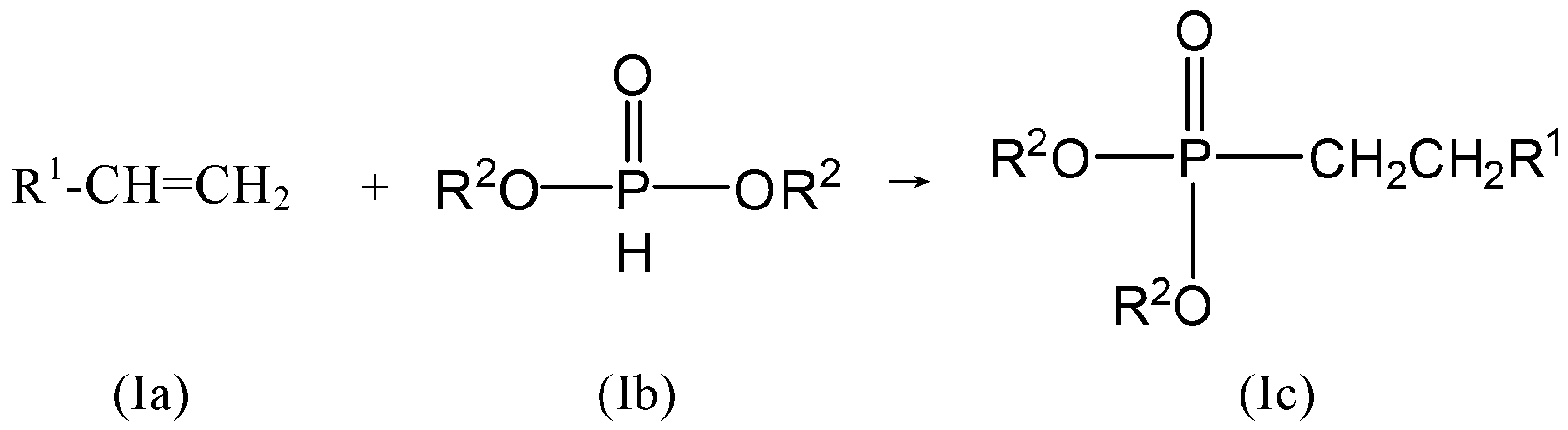
Synthesis method of monoalkyl hydrocarbyl phosphonate
InactiveCN103073582ASimple reaction conditionsEasy post-processingGroup 5/15 element organic compoundsRecovering materialsSynthesis methodsCombinatorial chemistry
The invention provides a synthesis method of monoalkyl hydrocarbyl phosphonate. Particularly, the invention provides a compound shown as a formula I and a preparation method thereof. In the formula, definitions of R1, R2 and M are defined as the specification. The invention further discloses an application of taking the compound shown as the formula I as an extracting agent, and an extracting agent component prepared from the compound shown as the formula I.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Synthesizing method for 1,2-dimethoxy benzene
InactiveCN101811942BReact cleanReduce pollutionEther preparation by ester reactionsBenzeneReaction temperature
The invention discloses a synthesizing method for 1,2-dimethoxy benzene, which utilizes pyrocatechol, strong base and chloromethane as raw materials and water as solvent to react in a high pressure autoclave; wherein the mol ratio of strong base to pyrocatechol is 2-3:1, the mole ratio of chloromethane to pyrocatechol is 2-3:1, the reaction temperature is 50-100 DEG C, the reaction pressure is 0.3-0.5 MPa, the reaction time is 4-10h, and the 1,2-dimethoxy benzene is obtained through carrying out liquid separating and rectification on reaction liquid. The method of the invention is adopted to synthesize 1,2-dimethoxy benzene, thus having the characteristics of environment friendless, simple process and high yield.
Owner:ZHEJIANG UNIV
Synthesis method of (poly)fluorophenylpyridine compounds
InactiveCN106243018AReduce manufacturing costLower reaction costOrganic chemistryGrignard reagentSynthesis methods
The invention discloses a synthesis method of (poly)fluorophenylpyridine compounds. The method is characterized by under anhydrous and oxygen-free conditions, using tetrahydrofuran as a solvent, an alkyl Grignard reagent or lithium alkylide as an activating agent and equivalent zinc halide as an assistant agent and adopting a compound formed by a nickel source and a diphosphine ligand as a catalyst to achieve cross-coupling of polyfluorobenzene and halogenated pyridine, thus synthesizing a series of (poly)fluorophenylpyridine compounds with yield of about 90% and purify not less than 99%. The synthesis method has the beneficial effects that use of expensive (poly)fluorophenylboronic acid and palladium catalysts is avoided by adopting cheaper (poly)fluorophenyl and nickel compound as the raw material and the catalyst, so the synthesis method has obvious economic advantages; the synthesis method has the advantages of simple reaction system, mild conditions, simplicity in operation, easiness in aftertreatment, low pollution, easiness in industrialization, and the like and shows higher social value and industrial popularization prospect.
Owner:SHAANXI NORMAL UNIV
Preparation method of L-homoserine
ActiveCN110283088AReduce usageReduce dosageOrganic compound preparationOrganic chemistry methodsPulp treatmentSulfonium
The invention belongs to the technical field of organic chemistry, and discloses a preparation method of L-homoserine, which comprises the following steps of S1, regulating L-methionine to be acidic with concentrated hydrochloric acid, then reacting with slightly excessive dimethyl sulfate at room temperature to produce an L-methionine sulfonium salt aqueous solution; S2, heating the L-methionine sulfonium salt aqueous solution obtained in the step S1 to reflux, then dropwise adding a saturated potassium bicarbonate solution, performing thermal cracking to remove dimethyl sulfide, so as to obtain a crude L-homoserine aqueous solution; S3, concentrating the crude L-homoserine aqueous solution obtained in the step S2, performing pre-cooling, removing salt with cation exchange resin, concentrating and crystallizing an eluent to obtain free crude L-homoserine, then adding acetone to perform pulping treatment on the crude L-homoserine to obtain high-purity L-homoserine. The method has the advantages that the raw materials are cheap and easy to obtain, the operation is simple, the cost is low, and is suitable for industrial large-scale production.
Owner:CHENGDU BAISHIXING SCI & TECH IND
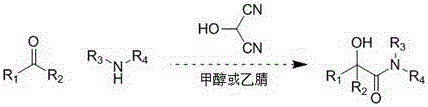
Method for synthesizing α-hydroxyamides by 2-hydroxymalonocyanide
ActiveCN103086818BSynthetic cleanSafe and efficient synthesisOrganic compound preparationCarboxylic acid amides preparationCyanideKetone
The invention relates to synthesis of alpha-oxyamide through reaction of 2-hydroxy propylene cyanide as well as corresponding aldehyde or ketone and amine, which mainly solves the problems that the preparation of common alpha-oxyamide has a plurality of reaction steps, the reaction operation is complicated, the reaction cost is high, the post treatment is a trouble, and the like. According to the method, 2-hydroxy propylene cyanide as well as corresponding aldehyde or ketone and amine are directly mixed, methanol or acetonitrile is taken as the solvent, and the product can be obtained by stirring for 10-120 minutes. The method for synthesizing alpha-oxyamide is safe and efficient.
Owner:武汉药明康德新药开发有限公司
A kind of low-temperature plasma-treated solar cell back film and preparation method thereof
ActiveCN103367490BHigh bonding strengthIncrease surface tensionFinal product manufactureSynthetic resin layered productsWeather resistanceWater vapor
The invention belongs to the technical field of solar battery packaging assemblies and particularly relates to a solar battery back film subject to low-temperature plasma treatment. The solar battery back film is mainly formed by adhering a base layer and a fluorine-based film layer, wherein the surface of the base layer is provided with a plasma-treated film layer II or a plasma treatment surface grafting copolymer layer II, the surface of the fluorine-based film layer is provided with a plasma-treated film layer I, and the surface of the plasma-treated film layer I is provided with a plasma treatment surface grafting copolymer layer I. The invention further provides a preparation method of the solar battery back film. As the plasma-treated film layers and the plasma treatment surface grafting copolymer layers assist in blocking water vapor, the solar battery back film has the characteristics of better weather resistance, good chemical resistance and excellent electrical insulation. The preparation method is very quick in response, can achieve an expected purpose in a few seconds, is very suitable for a circulation system and facilitates continuous production of the solar battery back film.
Owner:HENGDIAN GRP DMEGC MAGNETICS CO LTD
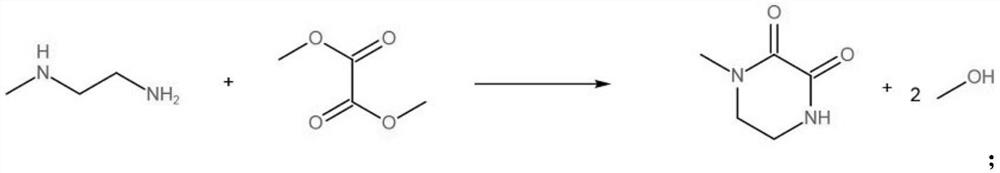
A kind of method for preparing n-methylpiperazine
The invention provides a method for preparing N-methylpiperazine, which solves the problem that the existing method for preparing N-methylpiperazine uses diethanolamine, a dangerous product, as a raw material, which is harmful to human health and the environment, and the raw material for preparing diethanolamine is not easy. Technical problems such as obtaining, the preparation method includes ester aminolysis reaction and hydrogenation reduction reaction, namely, dimethyl oxalate and N-methylethylenediamine undergo aminolysis reaction to generate lactam; and then use the lactam as a reactant , adding catalyst Raney nickel, at a temperature of 150-200 ° C and a pressure of 3.0-5.0 MPa, hydrogen is introduced to carry out hydrogenation reduction reaction, and finally the N-methylpiperazine is obtained, which can be widely used in chemical materials technical field.
Owner:SHANDONG GUOBANG PHARMA +1
Method for combining di-n-propylamine through n-propylamine disproportionated reaction and used catalyst
ActiveCN102614895BReact cleanReduce pollutionMetal/metal-oxides/metal-hydroxide catalystsAmino compound preparation by disproportionationHydrogenActive component
Owner:ZHEJIANG JIANYE CHEM
Diphenylmethane compound and preparation method thereof
PendingCN114292154AHigh purityHigh yieldOrganic compound preparationHydrocarbon from oxygen organic compoundsDiphenylmethaneDiphenylmethanol
The invention provides a diphenylmethane compound and a preparation method thereof.The preparation method comprises the steps that iodine, a diphenylmethanol compound and acetic acid are stirred under nitrogen protection, then a reducing agent is added, a mixed solution is obtained, and the reducing agent is hypophosphorous acid; the mixed solution is heated and then stirred, thin layer chromatography is used for detecting that the reaction is finished, extraction, washing, drying and concentration are carried out, and functional groups in the diphenyl methanol compound are selected from one or more of chlorine, bromine, methyl, methoxyl or hydrogen; in the prior art, the metal reduction reaction carried out by using hydrazine hydrate often needs high temperature, but the preparation method disclosed by the invention can efficiently and completely react within 8 hours at the temperature of not more than 70 DEG C; according to the method, the diphenylmethane compound can be synthesized from the benzhydrol compound safely, reliably and mildly, no hazardous gas and other hazardous products are generated, impurities are easy to remove, the reaction is clean, the obtained product is high in purity, column chromatography purification is not needed, and the yield is high.
Owner:上海泰坦科技股份有限公司
A kind of total synthesis method of amide alkaloid
ActiveCN104693205BReasonable designMeet the need for in-depth researchOrganic chemistrySynthesis methodsTetralin
Owner:XI AN JIAOTONG UNIV
Method and used catalytic agent for synthesizing N-butylethylamine
ActiveCN102614881BAvoid generatingAvoid compositionMetal/metal-oxides/metal-hydroxide catalystsAmino compound preparation by disproportionationPtru catalystFixed bed
The invention discloses a loading-type catalytic agent for synthesizing N-butylethylamine. Gamma-alumina after roasting serves as a carrier, active components composed of Cu and Ni are loaded on the carrier, sum of weight of the Cu, the Ni and the gamma-AI2O3 after roasting is total weight, sum of weight of the Cu and the Ni occupies 25%-38% of the total weight, and ratio of amount of substance of the Cu and the Ni is 0.9-1.5 / 1. The invention further provides a preparation method of the loading-type catalytic agent. A method for synthesizing the N-butylethylamine by using the loading-type catalytic agent is further provided, ethylamine and the n-butylamine are mixed in a mixing tank to obtain mixed liquor which passes through a fixed bed reactor containing activated loading-type catalyticagent after being vaporized, an amine disproportionated reaction is carried out in a hydrogenation condition, products are collected after condensation, and rectification is carried out to obtain theN-butylethylamine.
Owner:ZHEJIANG JIANYE CHEM
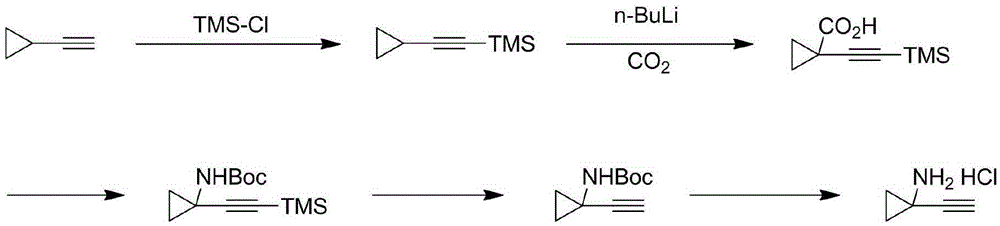
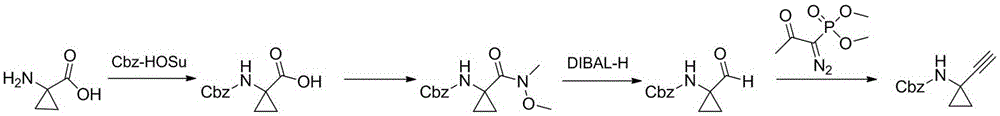
Technological synthesis method of 1-amino cyclopropyl acetylene
InactiveCN105348112AImprove responseEasy to operateCarbamic acid derivatives preparationOrganic compound preparationSynthesis methodsIndustrial scale
The invention discloses a technological synthesis method of 1-amino cyclopropyl acetylene. The method includes: taking compound 1 as the starting material, and carrying out oxidation, Corey-Fuchs reaction, and amino protection group removal so as to obtain the 1-amino cyclopropyl acetylene. The reagents and raw materials used by the method provided by the invention are cheap and easily available, and low in cost. With the advantages of high reaction safety, simple operation and high yield, the method is suitable for industrial scale-up production. (with the synthesis process shown as the specification).
Owner:SHANGHAI HAOYUAN CHEMEXPRESS
A kind of flame-retardant graft modification method of nylon 66 fabric
InactiveCN103866563BPromote combustionFacilitate the grafting reactionFibre treatmentNylon materialCombustion
The invention relates to a flame retardant graft modification method for nylon 66 fabric by a microwave irradiation method. The grafting reaction is carried out by the microwave method, and the flame retardant graft modification method has the advantages of short reaction time, even reaction, and small effect on the performance of the fabric. Allylthiourea is adopted as a grafted monomer, and thus the combustion performance of the grafted nylon 66 fabric is obviously improved; the monomer and the fabric surface are bonded through a chemical bond, the bonding effect is stable and lasting and thus the washability of the fabric is high.
Owner:BEIJING UNIV OF CHEM TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com