Diphenylmethane compound and preparation method thereof
A technology of diphenylmethane and diphenylmethanol, which is applied in the field of diphenylmethane compounds and their preparation, can solve the problems of dangerous operation process, complicated reaction process, narrow application range and the like, and achieves expansion of use range and simple operation. , the effect of high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036]
[0037] The preparation method of diphenylmethane compound of the present invention comprises the steps:
[0038] (1), iodine (I 2 , 1eq), (1mmol) diphenylmethanol compound and (6mL) acetic acid (AcOH) were stirred evenly under nitrogen protection in a flask equipped with a condenser to obtain the first mixed solution, which was a black solution; then A reducing agent (H 3 PO 2 ) (50% aq., 0.5mL; 5mmol), the color of the solution gradually becomes lighter, and the second mixed solution is obtained, which is finally a yellow-brown solution;
[0039] (2) After dropping, the second mixed solution was heated, and then stirred. At this time, the solution was light yellow and clear, and the reaction was detected by thin layer chromatography (TLC) (TLC: PE / EA=10). Add water to dilute, and the solution becomes cloudy at this time, and it is a white suspension. Extract with 10mL of petroleum ether (PE), wash the aqueous phase with 3mL of PE, and wash the combined organic ...
Embodiment 1
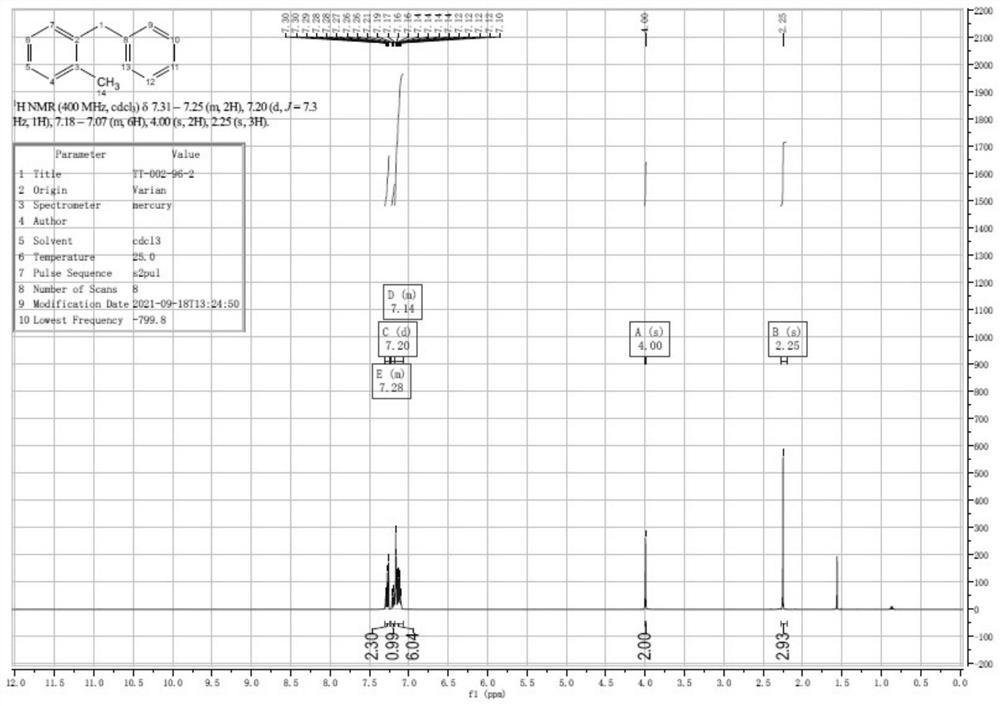
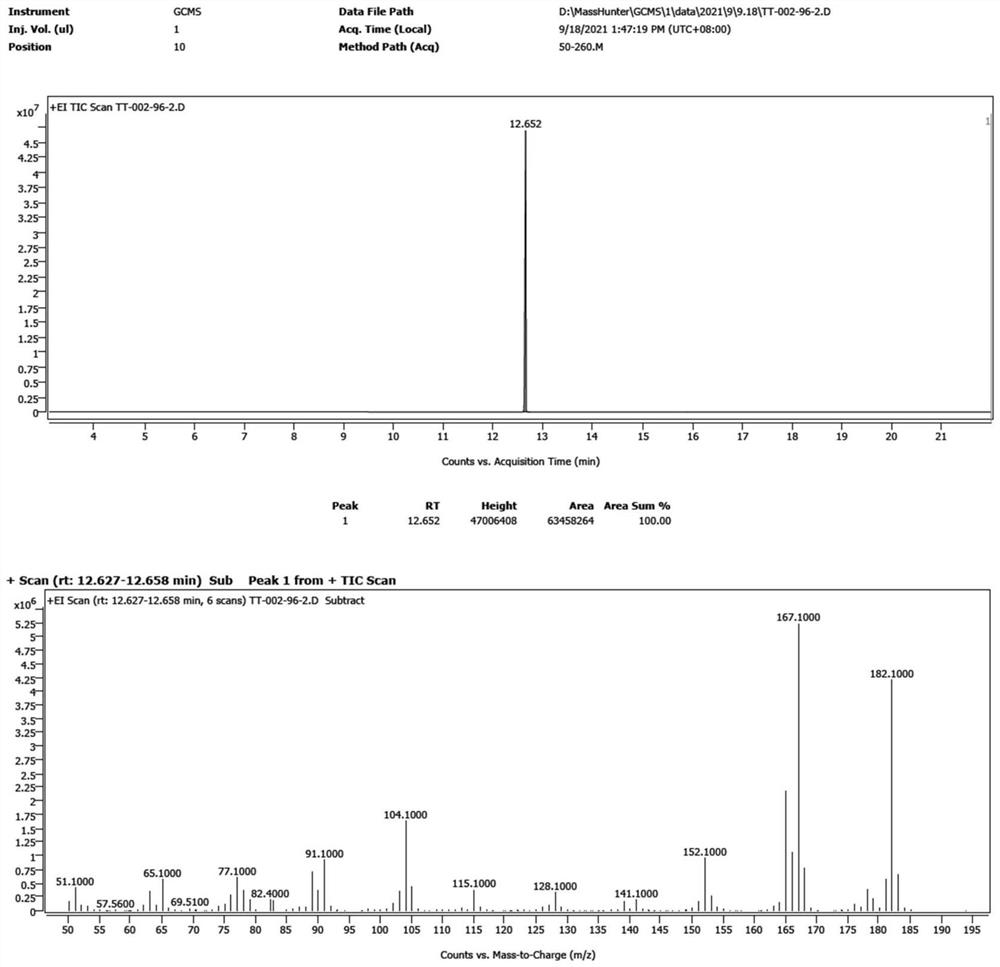
[0056] The preparation method of the 2-methyldiphenylmethane of the present embodiment comprises the steps:
[0057] (1), iodine (7.5g, 30mmol), 2-methyldiphenylcarbinol (5.99g, 30mmol) and acetic acid (190mL) were stirred evenly under nitrogen protection in a flask equipped with a condenser to obtain the first mixture liquid, which is a black solution; wherein, the stirring temperature is 25° C., and the stirring time is 5 minutes. Then, hypophosphorous acid (50% aq., 15 mL; 145 mmol) was added to the first mixed solution, and the color of the solution gradually became lighter to obtain the second mixed solution, which was finally a yellow-brown solution.
[0058] (2) After dropping, the second mixed solution was heated to 60° C. for 2 hours, and then stirred for 2 hours. At this time, the solution was light yellow and clear, and the reaction was detected by TLC. Add 200mL of water to dilute, then the solution becomes cloudy and becomes a white suspension. Extracted with PE...
Embodiment 2
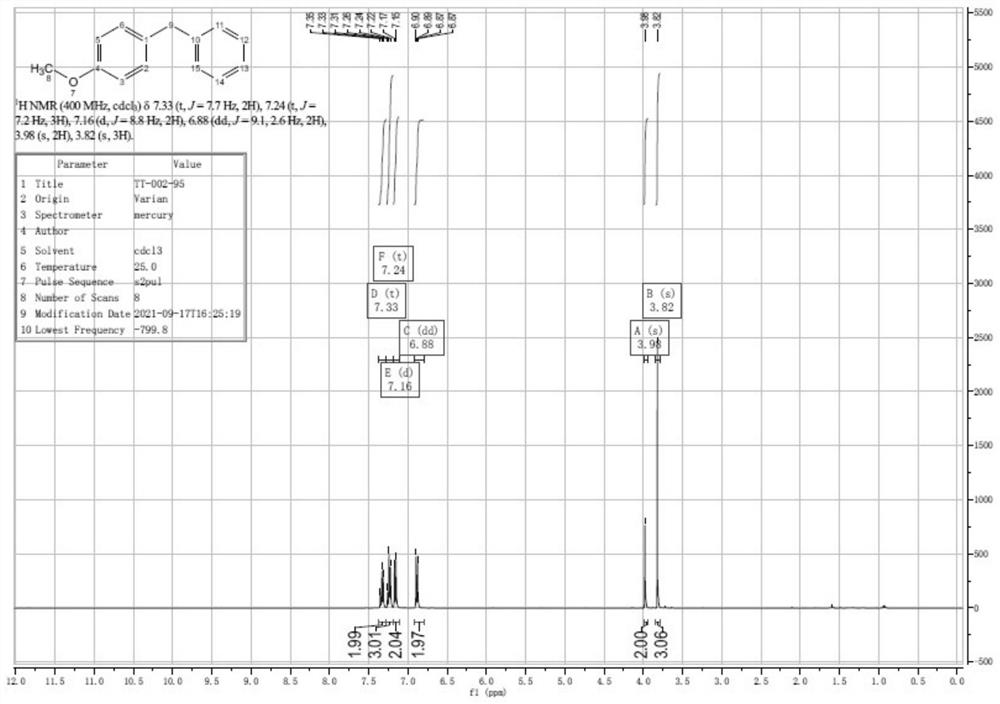
[0064] The preparation method of the 4-methoxydiphenylmethane of the present embodiment comprises the steps:
[0065] (1), iodine (7.5g, 30mmol), 4-methoxydiphenylcarbinol (6.5g, 30mmol) and acetic acid (190mL) were stirred uniformly under nitrogen protection in a flask equipped with a condenser to obtain the first The mixed solution is a black solution; wherein, the stirring temperature is 20° C., and the stirring time is 3 minutes. Then, hypophosphorous acid (50% aq., 15 mL; 145 mmol) was added to the first mixed solution, and the color of the solution gradually became lighter to obtain the second mixed solution, which was finally a yellow-brown solution.
[0066] (2) After dropping, the second mixed solution was heated to 50° C. for 2.5 hours, and then stirred for 1 hour. At this time, the solution was light yellow and clear, and the reaction was detected by TLC. Add 200mL of water to dilute, then the solution becomes cloudy and becomes a white suspension. Extracted with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com