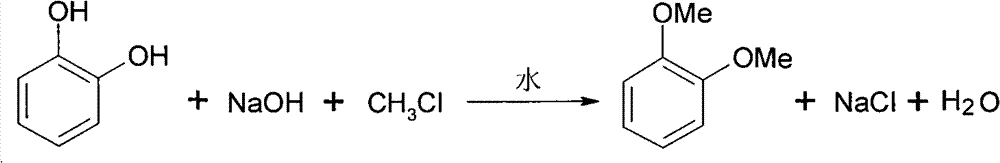
Synthesizing method for 1,2-dimethoxy benzene
A technology of phthalate and a synthesis method, which is applied in the directions of ether preparation, ester reaction preparation of ether, organic chemistry, etc., can solve the problems of increased production cost, cumbersome treatment process, human harm from toluene, etc., and achieves increased cost and simple operation. , The effect of concise production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1, a kind of synthetic method of phthalic dimethyl ether:
[0018] Add 22.0 g (0.2 mol) of catechol, 16 g (0.4 mol) of sodium hydroxide, and 130 ml of water into a high-pressure reaction kettle with a stirring temperature measuring device, and close the lid of the kettle. use N 2 After checking for leaks and replacing the air several times, 20.2 g (0.4 mol) of methyl chloride was added, the temperature was raised to 50° C., and the pressure was 0.3 MPa. Keep the temperature and pressure for 4h and finish the reaction.
[0019] The reaction solution was left to separate liquids to obtain the lower oil phase, and the atmospheric pressure intermittent distillation was carried out to collect fractions at 216.5-217.7°C to obtain 24.8g of the product with a yield of 90.0% and a purity of 99%. The structure of the obtained product was characterized by infrared spectroscopy. .
Embodiment 2
[0020] Embodiment 2, a kind of synthetic method of phthalic dimethyl ether:
[0021] Add 22.0 g (0.2 mol) of catechol, 20 g (0.5 mol) of sodium hydroxide, and 150 ml of water into a high-pressure reaction kettle with a stirring temperature measuring device, and close the lid of the kettle. use N 2 After checking for leaks and replacing the air several times, 25.2 g (0.5 mol) of methyl chloride was added, the temperature was raised to 60° C., and the pressure was 0.38 MPa. Keep the temperature and pressure for 6h and then finish the reaction.
[0022] The reaction solution was left to separate liquids to obtain the lower oil phase, which was subjected to batch rectification at atmospheric pressure, and the fraction at 216.5-217.7°C was collected to obtain 25.5 g of the product with a yield of 92.5% and a purity of 99%. The structure of the obtained product was characterized by infrared spectroscopy. .
Embodiment 3
[0023] Embodiment 3, a kind of synthetic method of phthalic dimethyl ether:
[0024] Add 22.0 g (0.2 mol) of catechol, 24 g (0.6 mol) of sodium hydroxide, and 180 ml of water into a high-pressure reaction kettle with a stirring temperature measuring device, and close the lid of the kettle. use N 2 After checking for leaks and replacing the air several times, 30.3 g (0.6 mol) of methyl chloride was added, the temperature was raised to 80° C., and the pressure was 0.43 MPa. Keep the temperature and pressure to react for 8h and finish the reaction.
[0025] The reaction solution was left to separate liquids to obtain the lower oil phase, and the batch rectification was carried out at atmospheric pressure, and the fraction at 216.5-217.7°C was collected to obtain 26.3g of the product with a yield of 95.2% and a purity of 99%. The structure of the obtained product was characterized by infrared spectroscopy. .
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com