A kind of total synthesis method of amide alkaloid
A technology of alkaloids and amides, which is applied in the field of total synthesis of amide alkaloids, can solve the problems of unsatisfactory research needs, limited sources of Taibai Aconitum, high cost requirements, etc., to overcome the cumbersome extraction and separation process, high yield, Simple and easy to operate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
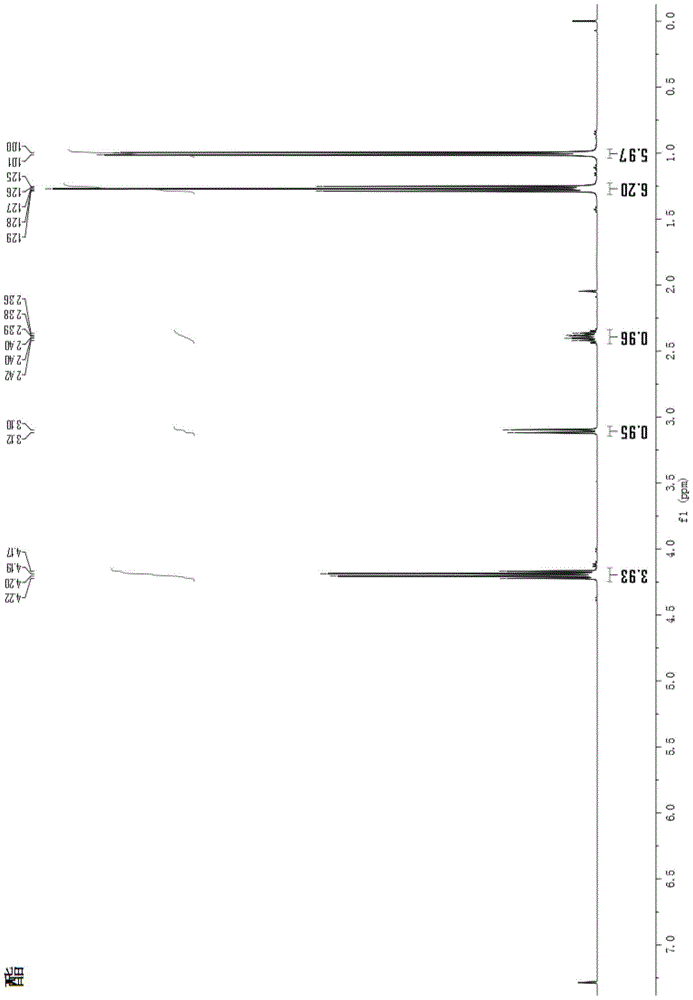
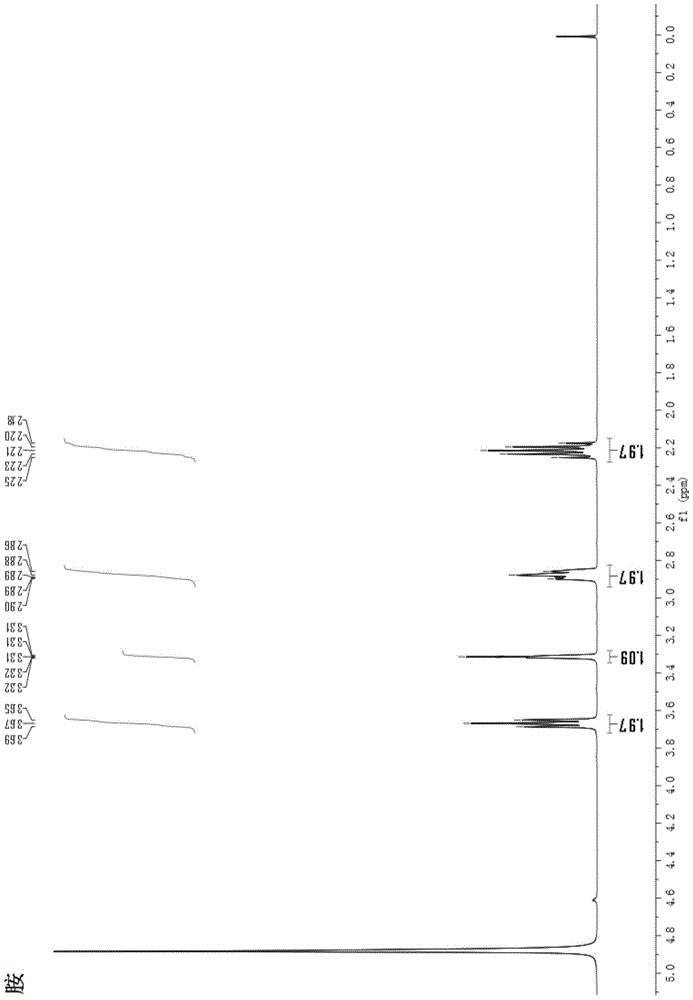
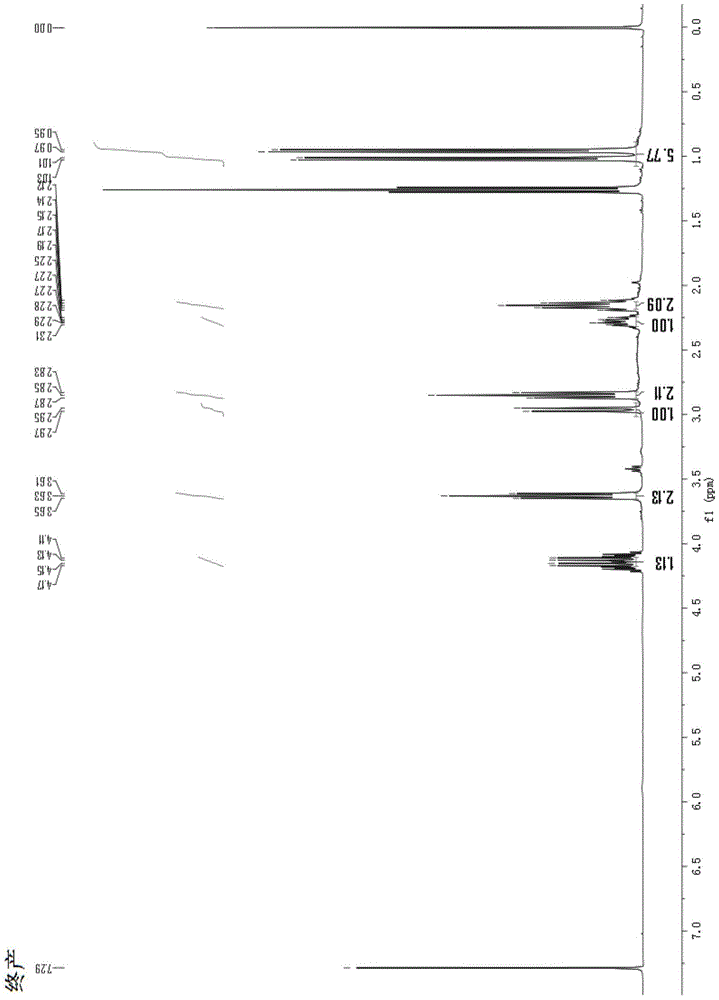
Image
Examples
Embodiment 1
[0038] The total synthesis method of 3-isopropyl-tetrahydropyrrole [1,2-a] pyrimidine-2,4 (1H, 3H)-dione comprises the following steps:
[0039] 1. Preparation of diethyl isopropylmalonate
[0040] Sodium ethoxide (5.61g, 82.5mmoL) was dissolved in 80mL of absolute ethanol, and diethyl malonate (12g, 75mmoL) was added dropwise thereto under constant stirring, and after the addition was completed, the temperature was raised and refluxed for 2 hours; the temperature was lowered and stirred continuously Isopropyl bromide (13.84 g, 112.5 mmol) was added dropwise, and after the addition was completed, the temperature was raised and the mixture was refluxed for 6 hours. TLC tracking, after the completion of the reaction, the temperature was lowered, filtered with suction, and the filtrate was collected. The pH was adjusted down to 5-6 in ice bath, and the solvent was removed by rotary evaporation. Dissolve in 100mL ethyl acetate, wash with 100mL saturated sodium chloride three tim...
Embodiment 2
[0055] The total synthesis method of 3-tert-butyl-tetrahydropyrrole [1,2-a] pyrimidine-2,4 (1H, 3H)-dione comprises the following steps:
[0056] 1. Preparation of diethyl tert-butylmalonate
[0057] Sodium ethoxide (5.61g, 82.5mmoL) was dissolved in 80mL of absolute ethanol, and diethyl malonate (12g, 75mmoL) was added dropwise thereto under constant stirring, and after the addition was completed, the temperature was raised and refluxed for 2 hours; the temperature was lowered and stirred continuously Bromo-tert-butane (15.41 g, 112.5 mmol) was added dropwise, and after the dropwise addition was completed, the temperature was raised and the mixture was refluxed for 6 hours. TLC tracking, after the completion of the reaction, the temperature was lowered, filtered with suction, and the filtrate was collected. The pH was adjusted down to 5-6 in ice bath, and the solvent was removed by rotary evaporation. Dissolve in 100mL ethyl acetate, wash with 100mL saturated sodium chlorid...
Embodiment 3
[0063] The total synthesis method of 3-cyclopropyl-tetrahydropyrrole [1,2-a] pyrimidine-2,4 (1H, 3H)-dione comprises the following steps:
[0064] 1. Preparation of dimethyl cyclopropylmalonate
[0065] Sodium ethoxide (5.61g, 82.5mmoL) was dissolved in 80mL absolute ethanol, and dimethyl malonate (9.9g, 75mmoL) was added dropwise thereto under constant stirring, and the temperature was raised and refluxed for 2 hours after the dropwise addition; Bromocyclopropane (13.61 g, 112.5 mmol) was added dropwise with stirring, and after the dropwise addition was completed, the temperature was raised and the mixture was refluxed for 6 hours. TLC tracking, after the completion of the reaction, the temperature was lowered, filtered with suction, and the filtrate was collected. The pH was adjusted down to 5-6 in ice bath, and the solvent was removed by rotary evaporation. Dissolve in 100mL ethyl acetate, wash with 100mL saturated sodium chloride three times, combine the organic phases, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com