4-substituted 2,7-phthalazine compound, and preparation and usethereof
A technology of naphthyridines and compounds, which is applied to a class of 4-substituted 2,7-naphthyridine compounds and their preparation and application fields, and can solve problems such as harsh reaction conditions, low yields, and difficult availability of raw materials , to achieve the effect of convenient reaction, high yield and clean reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
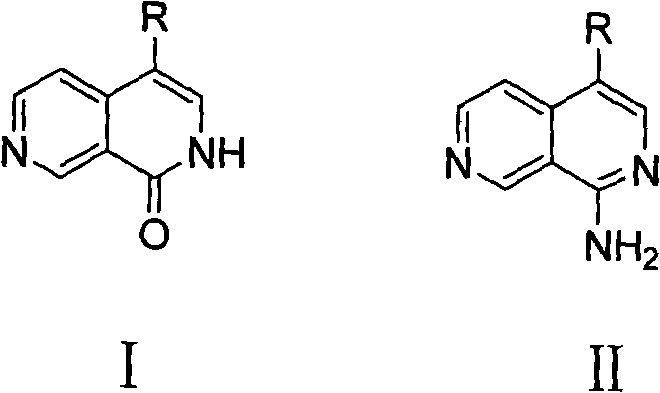
[0054] Embodiment 14-phenyl-1-carbonyl-2, the preparation of 7-naphthyridine Ia
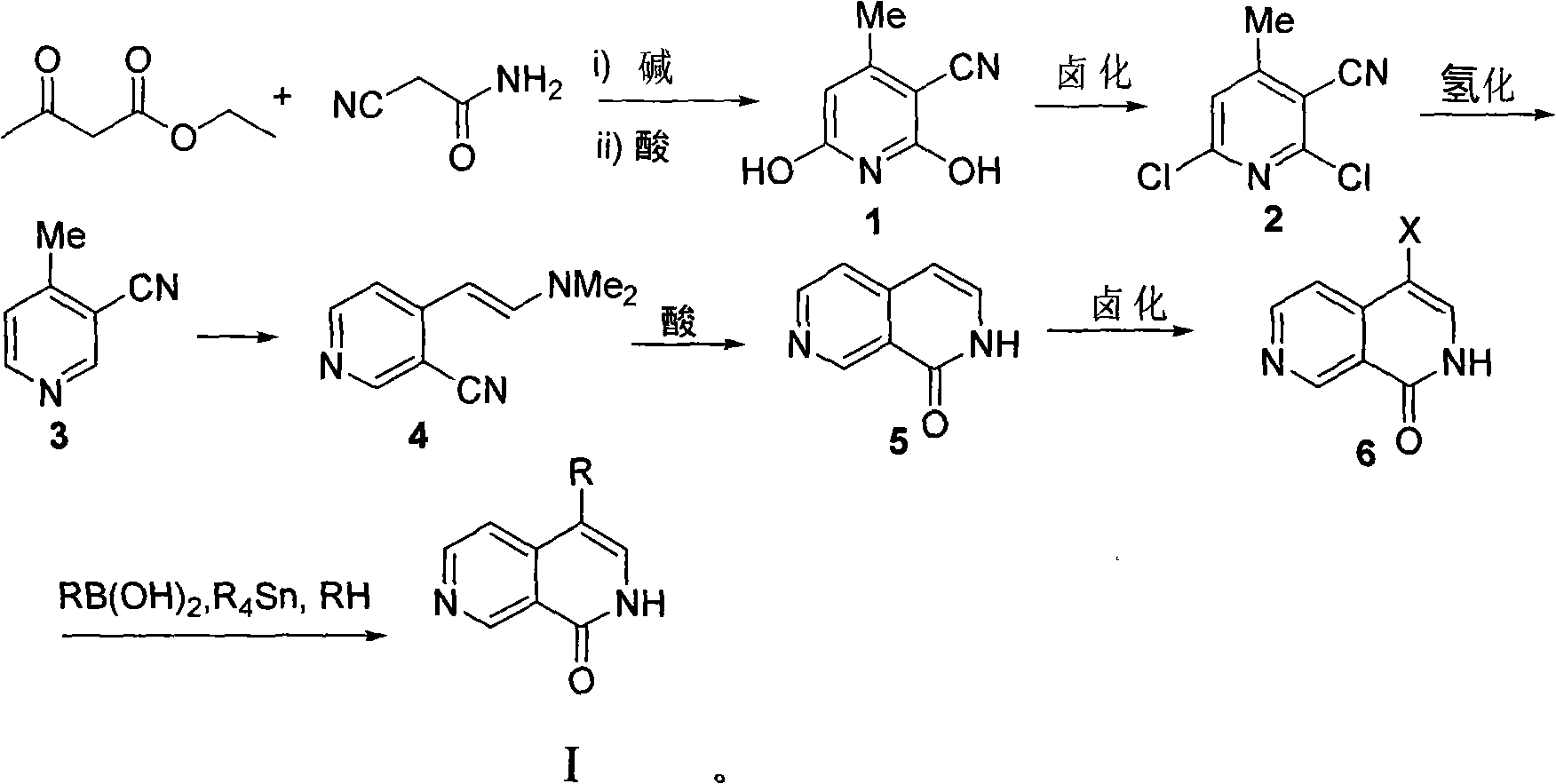
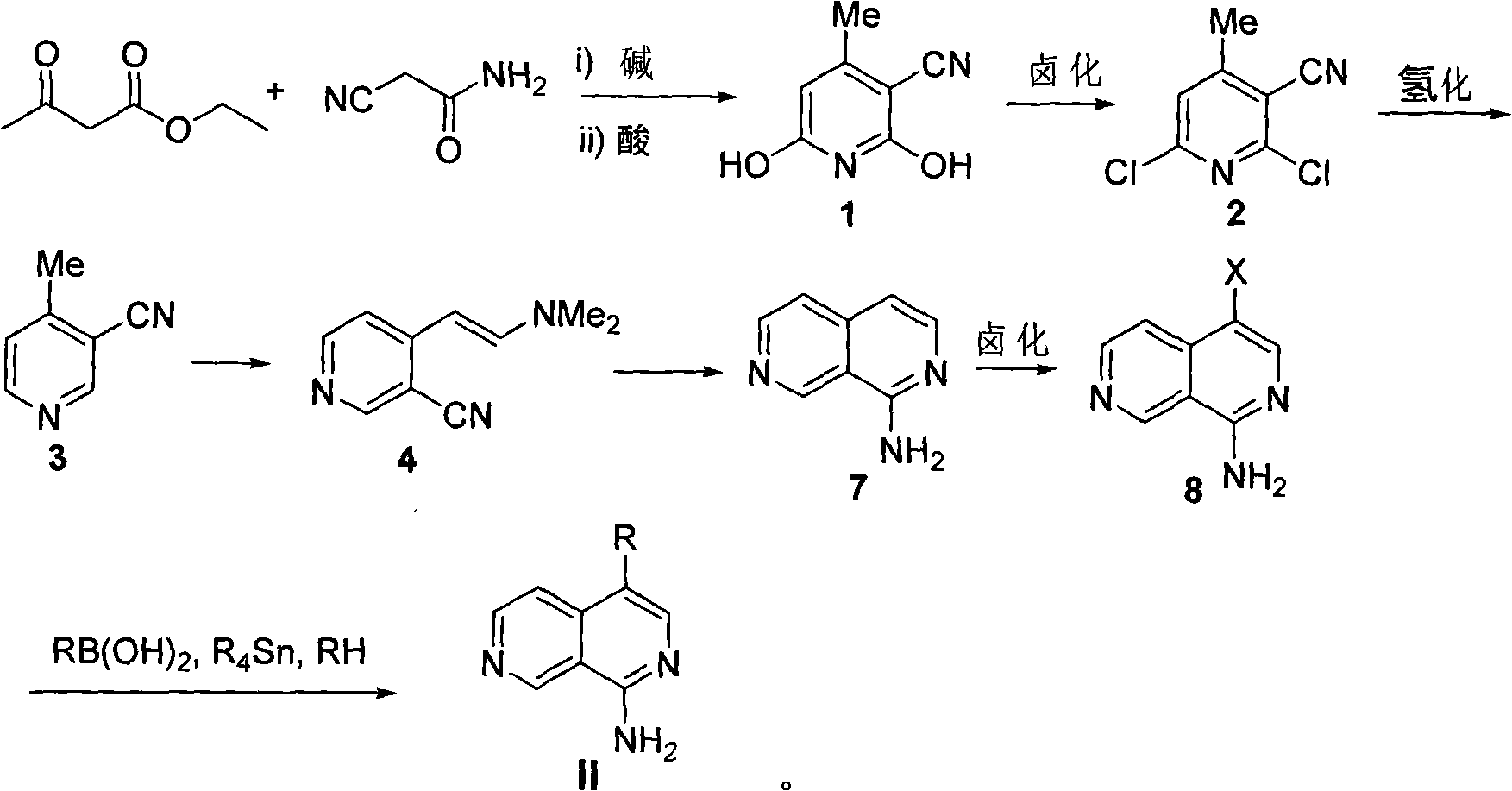
[0055] Step 1: 4-Methyl-3-cyano-2,6-dihydroxypyridine (compound 1)
[0056] Cyanoacetamide (1.0g, 11.9mmol) and ethyl acetoacetate (1.5ml, 11.9mmol) were placed in 15 ml of methanol, heated to dissolve it, and then 732.5 mg of potassium hydroxide was weighed and dissolved in 10 In the methyl alcohol of milliliter, the methanol solution of potassium hydroxide is added dropwise in the methanol solution of reactant with dropping funnel, stirs while adding dropwise, continues 15 minutes altogether, in the process of dropping, white solid will be produced, this During this time, a sufficient amount of methanol should be added to prevent the white solid from agglomerating, and then the reaction solution was stirred and refluxed for 8 hours. After the reaction, filter out the white solid, dissolve the obtained white solid in hot water, filter out the insoluble matter, acidify the filtrate with concentr...
Embodiment 2
[0069] Example 24-(4-methylphenyl)-1-carbonyl-2,7-naphthyridine Ib
[0070] According to the method of Example 1, in step 7, phenylboronic acid was replaced with 4-methylphenylboronic acid to obtain compound 4-(4-methylphenyl)-1-carbonyl-2,7-naphthyridine Ib.
[0071] White solid, yield 52%; 1 H NMR (CDCl 3 , 300MHz) δ2.436 (s, 3H, CH 3 ), 7.29-7.32(m, 4H, H-2', H-3', H-5', H-6'), 7.38(s, 1H, H-3), 7.47(d, J=5.4Hz , 1H, H-5), 8.72 (d, J=5.7Hz, 1H, H-6), 9.68 (s, 1H, H-8), 12.17 (brs, 1H, NH); MS (ESI) m / z 236[M + ](100); HRMS Calcd for C 15 h 12 N 2 O: 236.0950, found: 236.0943.
Embodiment 3
[0072] Example 34-(4-methoxyphenyl)-1-carbonyl-2,7-naphthyridine Ic
[0073]According to the method of Example 1, in step 7, phenylboronic acid is replaced with 4-methoxyphenylboronic acid to obtain compound 4-(4-methoxyphenyl)-1-carbonyl-2,7-naphthyridine Ic.
[0074] White solid, yield 50%; 1 HNMR (DMSO-d 6 , 300MHz) δ: 3.80(s, 3H,), 7.05(d, J=8.7Hz, 2H), 7.32(s, 1H), 7.35(d, J=8.7Hz, 2H), 7.37(d, J=8.7Hz, 2H), 7.37(d, J=8.7Hz, 2H), 6.3Hz, 1H), 8.69(d, J=5.4Hz, 1H), 9.38(s, 1H); 13 C NMR (75MHz, CDCl 3 )δ: 160.8(C-4'), 159.0(C-1), 151.2(C-8), 150.3(C-6), 142.3(C-10), 132.4(C-5), 130.8(C- 2', C-6'), 126.9(C-1'), 120.6(C-9), 117.5(C-3), 115.6(C-4), 114.4(C-3', C-5') , 55.3 (O-CH 3 ); MS(EI): m / z 252 [M + ](100); HRMS Cacld.for C 15 h 12 N 2 o 2 : 252.0899, found: 252.0897.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com