Synthesis process of novel green naphthol chromophore type products
A synthetic process and green technology, which is applied in the field of synthetic process of new-type green naphthol color-based products, can solve problems such as difficult recycling, and achieve the effects of increased yield, good reproducibility, and improved purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
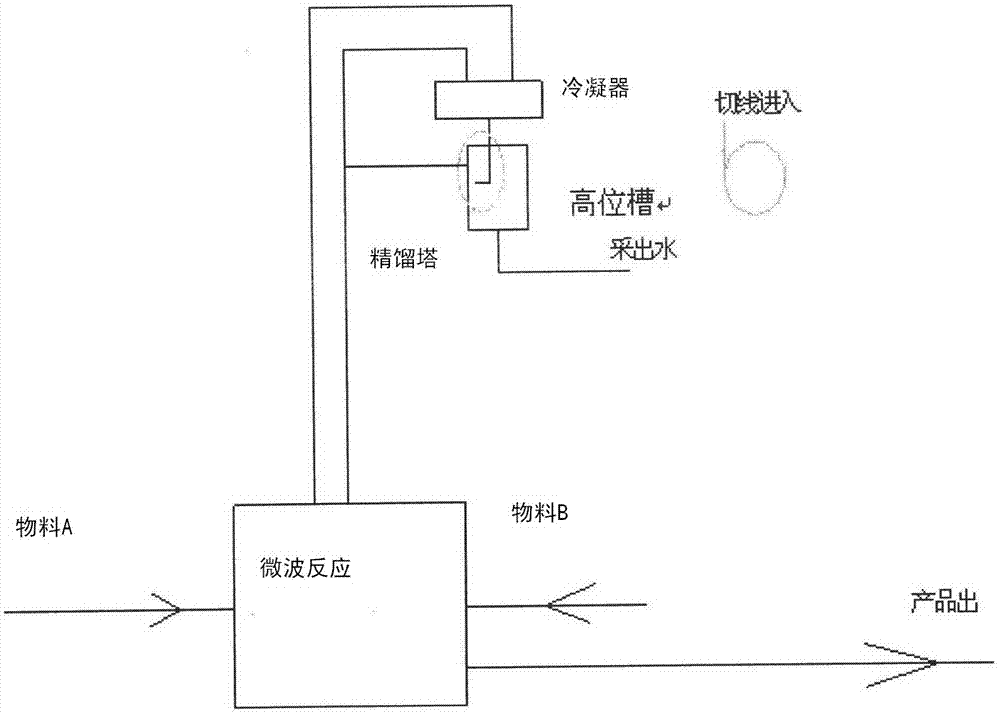
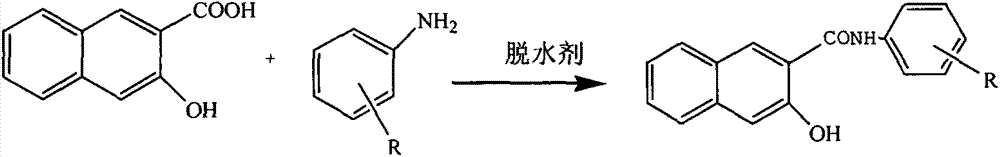
[0027] A novel green naphthenol color-based product synthesis process is characterized in that it comprises the following steps:
[0028] 1) The input material 2,3-acid and aniline compound are dissolved in a suitable solvent, and the solvent selection is generally a solvent that does not react and azeotrope with the initial raw materials and products, and forms an azeotrope with water and is immiscible with water, such as Toluene, chlorobenzene, etc.;
[0029] 2) Pass into a microwave reactor for reaction, the temperature in the microwave reactor is generally controlled at 90-150 degrees, and the reaction time is 0.5-5h;
[0030] 3) Since 1 equivalent of water will be released during the reaction process, it must be transferred in time. The coupling process of reaction and azeotropic rectification is used to continuously separate the produced water. First, the azeotropic solvent produced by rectification enters the high-level In the tank, the rapid separation of water and so...
Embodiment 2
[0034]
[0035] Dissolve 1 equivalent of 2,3-acid and 1 equivalent of 4,-chloro-2,5-dimethoxyaniline in 10 equivalents of chlorobenzene, control the reaction temperature to 110 degrees, and set the reaction time to 1h. During the process, the rectification reflux ratio R=1.2. After the reaction is completed, the bottom temperature is controlled to 150 degrees, the reflux ratio is 0.8, and the chlorobenzene is rectified. After the chlorobenzene is removed, the product is released, and the product yield is 99%. , The product purity is 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com