Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
180 results about "Genotoxic impurities" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparation method of remdesivir
InactiveCN111116656AReduce usageReduced risk of genotoxic impuritiesGroup 5/15 element organic compoundsAcid hydrolysisOrganic base
The invention discloses a preparation method of remdesivir, and belongs to the field of pharmaceutical chemicals; the preparation method comprises the steps: carrying out a reaction on a compound V with pentafluorophenol under the action of an alkali to obtain a compound IV; further splitting the compound IV to obtain a compound III; carrying out a reaction on the compound III with a compound II under the action of organic base with large steric hindrance, and carrying out acid hydrolysis to obtain a compound I. According to the method disclosed by the invention, the use of genotoxic nitro substitutes is avoided, the risk of genotoxic impurities is reduced, and the method is suitable for industrial large-scale production.
Owner:江苏阿尔法集团福瑞药业(宿迁)有限公司
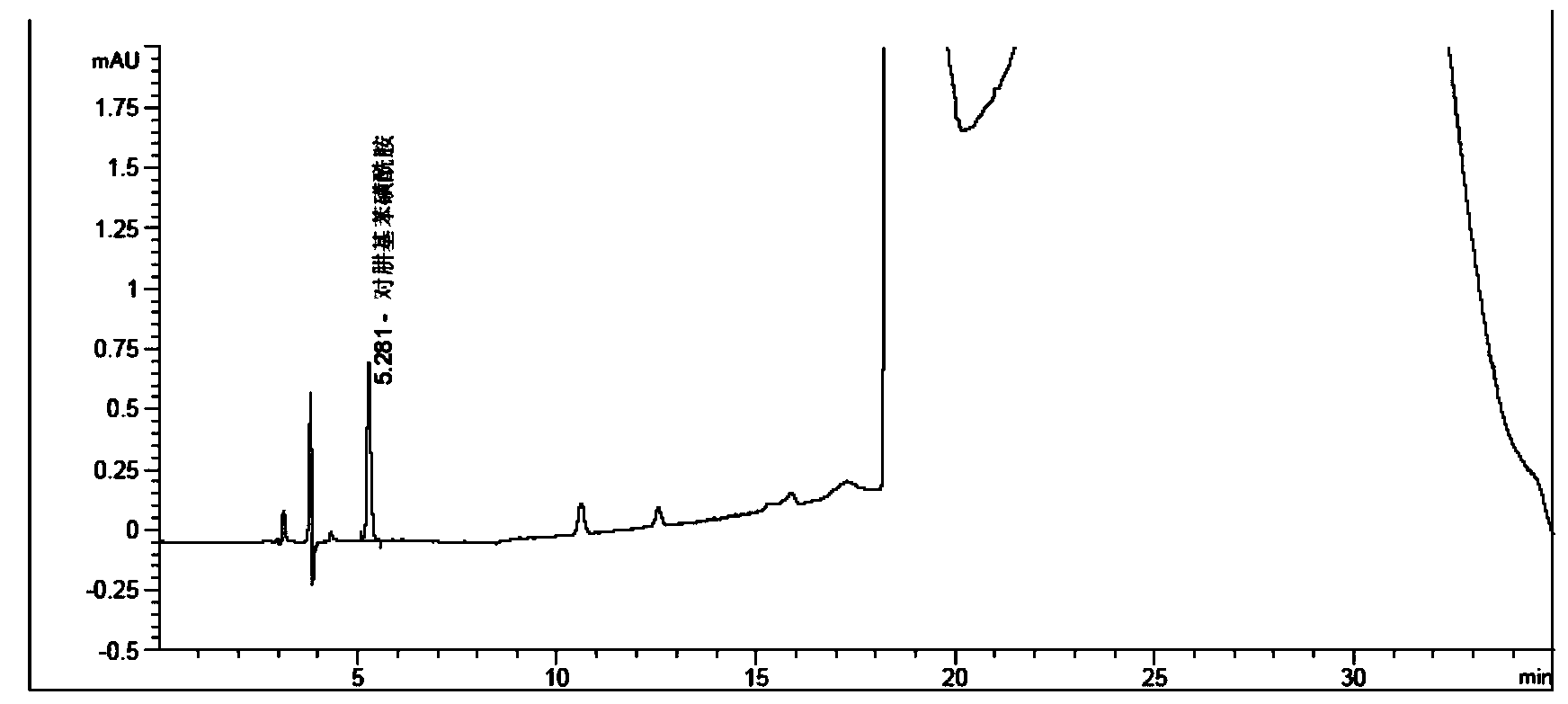
Method for measuring phenylhydrazine compound residues in crude drugs through HPLC (high performance liquid chromatography)
The invention discloses a method for measuring genotoxic impurities (or doubtful genotoxicity), namely phenylhydrazine compound residues, in crude drugs through the HPLC (high performance liquid chromatography). The detection is directly implemented by taking phenyl bonded silica gel as a chromatographic column of a solid phase and organic phase and buffer solution mixed solvent gradient elution as a mobile phase. The detection method is high in detection sensitivity, strong in specificity, high in precision, high in accuracy, convenient to operate and strong in adaptability and can be used for detecting phenylhydrazine compounds in various crude drugs, and the quality of the crude drugs can be effectively controlled.
Owner:WATERSTONE PHARMA WUHAN
Detection method of parecoxib sodium genotoxicity impurity and application thereof
ActiveCN105372376AAchieve separationHigh sensitivityComponent separationPhosphateReversed-Phase Liquid Chromatography
The present invention provides a detection method of parecoxib sodium genotoxicity impurity and application thereof. The method uses a reversed-phase liquid chromatography method. The chromatography conditions are as follows: the chromatographic column comprise a C18 column, a C8 column, a phenyl column and a Hilic column; a mobile phase consists of water-acetonitrile, dilute phosphoric acid-acetonitrile, and phosphate-acetonitrile; a flow phase comprises an aqueous phase and an organic phase in the ratio of 90:10-10:90; the column temperature is 25-40 DEG C; the flow rate of the mobile phase is 0.2-2ml / min; detection wavelength is 205-290nm; a detector is a UV detector or a photodiode array (PDA) detector; and the sample size is 0.1-40 mul. The detection method of parecoxib sodium genotoxicity impurity achieves the separation of parecoxib sodium and three genotoxicity impurities in a short period of time, has high sensitivity and specificity, and simple operation, reaches the separation rate of the main component and genotoxicity impurities, and the separation rate of genotoxicity impurities both greater than 1.5; and the method can be used for quality control of parecoxib sodium, and has practical value.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Method for synthesizing mometasone furoate or monohydrate of mometasone furoate
ActiveCN106279340AThe generation of solutionNo generationSteroidsAfter treatmentMometasone furoate monohydrate
The invention belongs to a synthesizing method for medicine, and particularly relates to a method for synthesizing mometasone furoate or a monohydrate of the mometasone furoate. The method includes the steps that 8-DM serving as a first compound is used as an initial material, and the first compound and paratoluensulfonyl chloride are subjected to a sulfonylation reaction to generate a second compound; the second compound is not subjected to after-treatment and is subjected to a chlorination reaction with RCl (R is Li, Na, K and Et3N) to generate a third compound; the third compound is not subjected to after-treatment, a part of organic alkali is replenished, and the mixture and furoyl chloride are subjected to an esterification reaction to generate a fourth compound; the fourth compound is not subjected to after-treatment, acid adjustment is carried out, a large quantity of chlorine elements existing in a reaction system are used for a ring-opening reaction, and the mometasone furoate or the mometasone-furoate monohydrate is obtained. The method is simple in technology, mild in reaction condition, high in yield, low in cost, high in quality and raw-auxiliary-material using rate, free of genetic toxicity impurity generation and suitable for industrial production.
Owner:山东锐顺药业有限公司
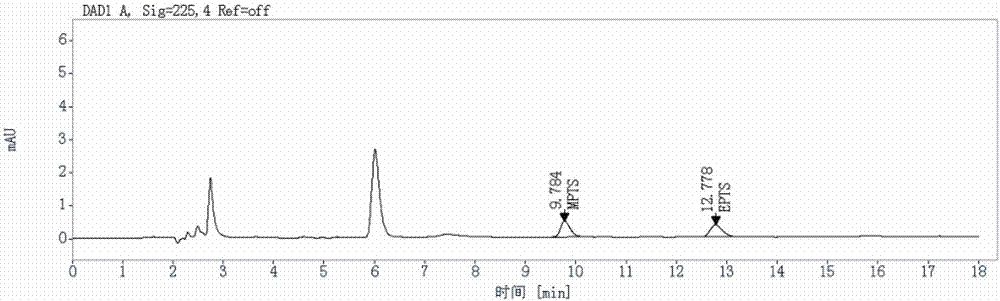
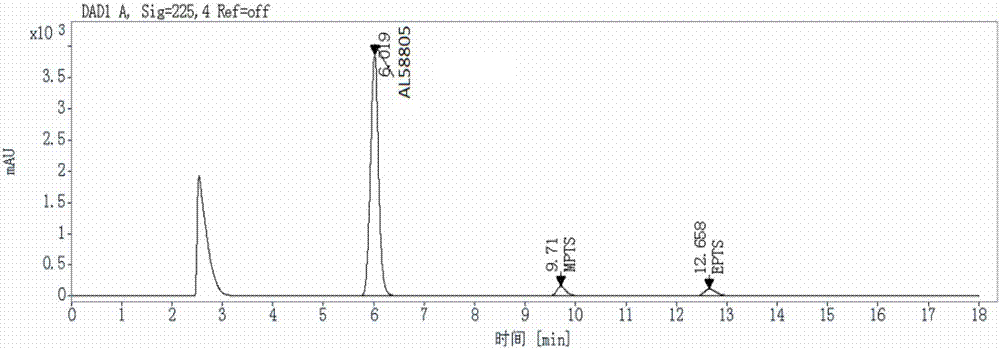
Method for detecting genotoxic impurities in AL58805 bulk drug or medicinal preparation by using high-performance liquid chromatography
ActiveCN107037153AEfficient separationDo not interfere with detectionComponent separationPhosphoric acidColumn temperature
The invention relates to a method for detecting genotoxic impurities in an AL58805 bulk drug or medicinal preparation by using high-performance liquid chromatography. The method is directed at methyl p-toluenesulfonate (MPTS) and ethyl p-toluenesulfonate (EPTS); a sample undergoes pre-treatment at first; and then high-performance liquid chromatography is employed for determination, wherein a C18 chromatographic column is employed; detection wavelength is 225 nm; an aqueous acetonitrile-phosphoric acid solution is used as a mobile phase; flow velocity is 0.8 to 1.2 mL / min; column temperature is 30 to 40 DEG C; a sample size is 20 [mu]L; and elution time is 18 min. Results show that the detection limit of MPTS is 0.3 ppm and the limit of quantitation of MPTS is 1 ppm; and the detection limit of EPTS is 0.5 ppm and the limit of quantitation of EPTS is 1.4 ppm. The method has the advantages of good specificity, simple operation, high sensitivity and accurate results and is applicable to determination of MPTS and EPTS in AL58805.
Owner:常州佳德医药科技有限公司
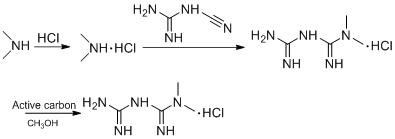
Method for treating azide ions, non-genotoxic impurity Sartan raw material medicine and immediate thereof
The invention discloses a method for treating azide ions in a system and application thereof to the preparation of a compound with a tetrazolium group and without genotoxic impurities. The method is that the azide ions contained in the hydrogen peroxide treatment system are used. The method is used for preparing the compound with the tetrazolium group and comprises the following preparation steps:enabling a compound containing a cyano group to react with an azide, adding hydrogen peroxide after the reaction to quench and remove excessive sodium azide and further obtaining the compound with the tetrazolium group. The compound prepared by the method does not contain the genotoxic impurities. The method is simple in operation, mild in reaction conditions and suitable for industrial production.
Owner:珠海润都制药股份有限公司
Linezolid injection and preparation method thereof
ActiveCN111686072AImprove quality and safetyImprove medication safetyAntibacterial agentsOrganic active ingredientsUse medicationMorpholine
The invention provides a linezolid injection and a preparation method thereof. The preparation method of the linezolid injection comprises the steps of weighing, preparation, filtration, encapsulationand sterilization, and nitrogen filling protection is carried out on liquid medicine in the steps of preparation and / or encapsulation. The content of 3-fluoro-4-(4-morpholinyl) aniline in the linezolid injection product can be obviously reduced by performing nitrogen charging protection on the liquid medicine in the steps of preparation and / or encapsulation, so that the product quality and the medication safety of the linezolid injection are favorably improved, and the medication risk of the variety is also obviously reduced by reducing the content of genotoxic impurities.
Owner:SUZHOU SIXTH PHARMA PLANT OF JIANGSU WUZHONG PHARMA GROUP
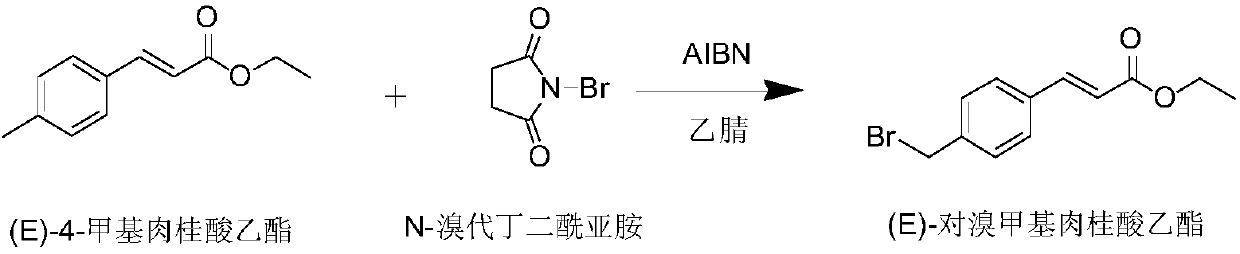
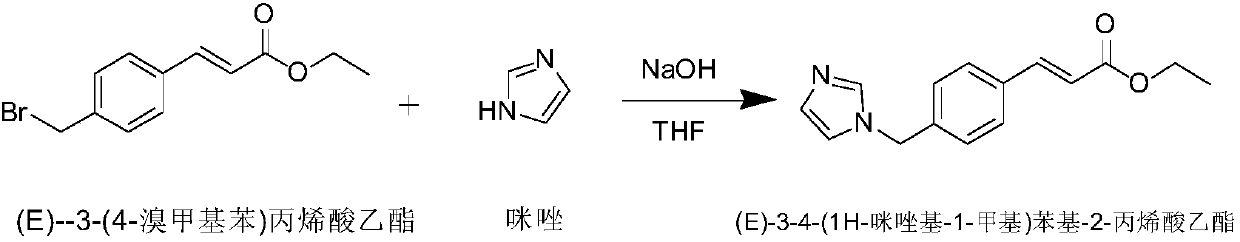
Preparation method of Ozagrel sodium
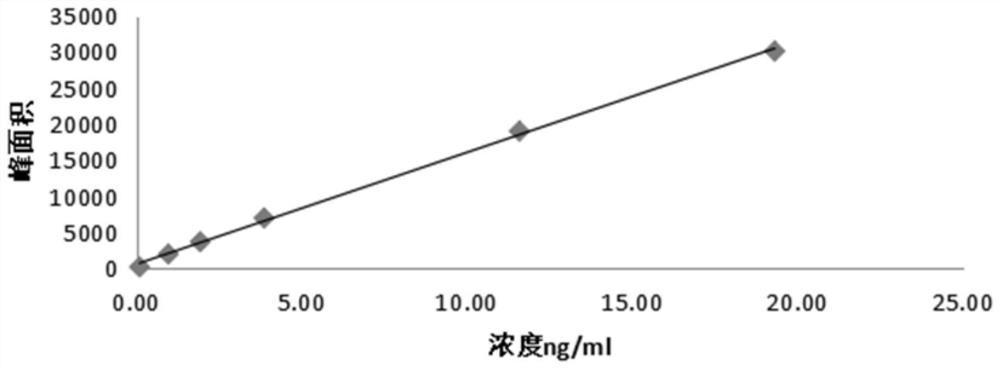
The invention discloses a preparation method of Ozagrel sodium. The method comprises the following steps: performing a bromination reaction on ethyl 4-methylcinnamate and N-bromo-succinimide by takingacetonitrile as a solvent under the triggering of azodiisobutyronitrile, so as to obtain ethyl 4-bromomethylcinnamate; performing a condensation cyclization reaction on the ethyl 4-bromomethylcinnamate and imidazole by taking sodium hydroxide as an acid-binding agent and taking tetrahydrofuran as a solvent, so as to obtain imidazole ethyl 4-methylcinnamate; performing alkali hydrolysis on the imidazole ethyl 4-methylcinnamate, so as to obtain the Ozagrel sodium. According to the preparation method, the condensation cyclization reaction is performed by taking the sodium hydroxide as the acid-binding agent and taking the tetrahydrofuran as the solvent, so that the finally obtained product does not contain toxic components, the yield and the purity of the product can be effectively improved,and the content of genotoxic impurities (the ethyl 4-bromomethylcinnamate and ethyl 4-dibromomethylcinnamate) in the product is zero.
Owner:浙江科瑞医药科技有限公司
Method for measuring ethyl p-toluenesulfonate as genetic toxicity impurities in ibuprofen
InactiveCN107782820AStrong specificityImprove linearityComponent separationGas chromatography–mass spectrometryVapor phase chromatography
The invention provides a method for detecting ethyl p-toluenesulfonate as genetic toxicity impurities in ibuprofen by gas chromatography-mass spectrometry. The method comprises the following main steps: taking 5% of phenyl-arylidene and 95% of dimethyl polysiloxane as a fixed-phase capillary column; preparing a reference substance linear solution and a sample solution; carrying out temperature programming; after samples are separated by a gas chromatograph, detecting the samples by a mass spectrometry detector, wherein a mass spectrum is an EI source; quantifying selected ions in a scanning mode, wherein the selected ions are m / z: 91, 92, 155; calculating an equation of linear regression by the concentration of a reference substance and the corresponding peak area; and calculating the content of the ethyl p-toluenesulfonate in the ibuprofen by the equation of linear regression. The method is simple, speedy and accurate, and is high in sensitivity; the detection method is scientific andobjective; and the method can be used for controlling the quality of an ibuprofen sample, and has practical value.
Owner:JIANGSU CHIA TAI FENGHAI PHARMA
Method for separating and determining dapoxetine hydrochloride and potential genetic toxicity impurities thereof
ActiveCN105548396AAchieve quality controlAchieving Security GuaranteesComponent separationStationary phaseAcetonitrile
The invention relates to the field of analysis chemistry, in particular to a method for separating and determining dapoxetine hydrochloride and potential genetic toxicity impurities thereof. The method adopts octadecyl silane bonded silica gel as a stationary phase for performing solid and liquid separation, a moving phase is a moving phase I or a moving phase II, the moving phase I is a water solution of acetonitrile, and the moving phase II is a water solution of acetonitrile, ammonium bicarbonate and diethylamine. The potential genetic toxicity impurities comprise 3-chloropropiophenone, UI-1, SM1e and Z1b. The method can realize effective separating of dapoxetine hydrochloride and potential genetic toxicity impurities thereof, can accurately detect whether the potential genetic toxicity impurities exceed the limit, and is high in specificity and high in accuracy. The operating method is simple, has the advantages of simplicity and rapidness, and has very important significance in quality control and safety guarantee of the dapoxetine hydrochloride.
Owner:CHONGQING HUABANGSHENGKAI PHARM
Solvent system capable of effectively dissolving ornidazole or levorotatory ornidazole and injection thereof
ActiveCN110917135AAvoid defectsReduce dosageAntibacterial agentsOrganic active ingredientsIrritationPolythylene glycol
The invention discloses a solvent system capable of effectively dissolving ornidazole or levorotatory ornidazole and an injection thereof, the active component is ornidazole or levorotatory ornidazole, and the solvent system is an organic solvent formed by mixing short-chain polyethylene glycol and ethanol; the short-chain polyethylene glycol is preferably polyethylene glycol 300 or / and polyethylene glycol 400. Wherein the mixed mass fraction ratio of the short-chain polyethylene glycol to the ethanol in the solvent system is (30-100): (0-70). The method has the technical advantages which cannot be achieved by the prior art; the ornidazole injection or levorotatory ornidazole injection has the unique preparation advantages of simpler prescription, safer production, more stable preparation,less solvent amount, lower impurity (particularly genotoxic impurities) content, fewer impurities, low-temperature crystallization difficulty, low phlebitis occurrence rate, no low-pH infusion irritation, no propylene glycol and the like; the clinical medication safety is ensured, and the medication compliance of a patient is better.
Owner:SOUTHWEST UNIVERSITY +1
Preparation method of retegravir
ActiveCN111233930AReduce usageReduced risk of genotoxic impuritiesGroup 5/15 element organic compoundsAcid hydrolysisOrganic base
The invention discloses a preparation method of retegravir, which belongs to the field of pharmaceutical chemicals, and comprises the following steps: reacting a compound V with 4-trifluoromethoxyphenol under the action of alkali to obtain a compound IV; further performing chiral resolution on the compound IV to obtain a compound III; and carrying out an acid hydrolysis reaction on the compound III and the compound II under the action of organic base with large steric hindrance to obtain a compound I. According to the method disclosed by the invention, the use of genotoxic nitro substitutes isavoided, the risk of genotoxic impurities is reduced, and the method is suitable for industrial large-scale production.
Owner:JIANGSU ALPHA PHARM CO LTD
Separation and determination method of apremilast and potential genotoxic impurities thereof
ActiveCN107014910AEfficient separationAccurate determination of contentComponent separationElutionSilica gel
The invention belongs to the field of analytic chemistry, and concretely relates to a separation and determination method of apremilast and potential genotoxic impurities thereof. The method comprises the steps of adopting a chromatographic column taking octadecyl silane bonded silica gel as filler, and concretely comprises the steps of adding a diluting agent for dissolving each potential genotoxic impurity reference substance so as to prepare a reference substance solution with known concentration; adding a diluting agent for dissolving a sample for test so as to prepare a solution of the sample for test; taking the reference substance solution and the solution of the sample for test respectively for sample injection; adopting a moving phase for carrying out high performance liquid chromatography elution analysis, and recording a chromatogram map; comparing peak areas of impurities, corresponding to peak appearance times, in the reference substance solution and the solution of the sample for test, and calculating the contents of the apremilast and the potential genotoxic impurities contained in the sample for test. The method is high in specificity, high in sensitivity (the limit of detection of the potential genotoxic impurities of SM1 is 0.0004 percent), and simple to operate, and has the advantages of convenience and quickness.
Owner:CHONGQING HUAPONT PHARMA
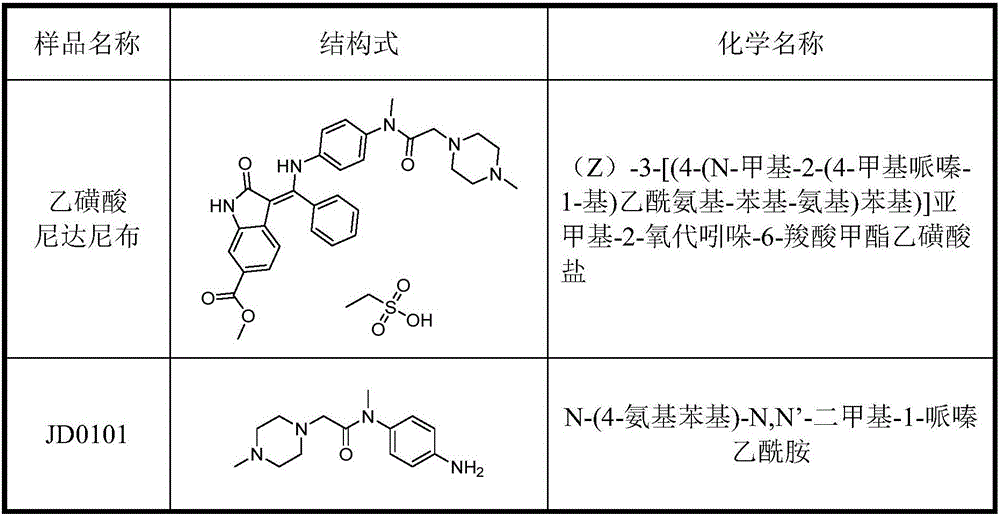
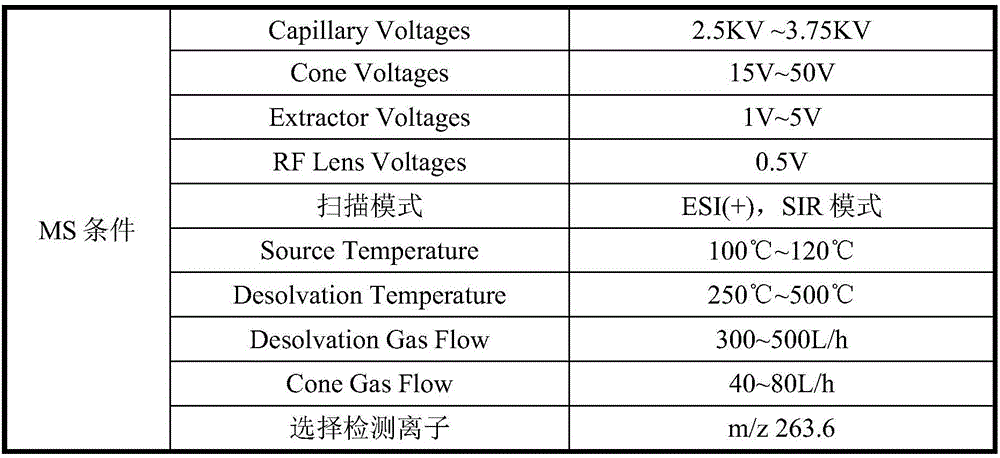
High sensitivity analysis method of genotoxic impurities in nintedanib ethanesulfonate
ActiveCN106841495AEfficient separationStrong specificityComponent separationUv detectorGradient elution
The invention discloses a high sensitivity analysis method of genotoxic impurities in nintedanib ethanesulfonate. The method is used for analysis of genotoxic impurity N-(4-aminophenyl)-N, N'-dimethyl-1-piperazine acetylamine (JD0101) in nintedanib ethanesulfonate through a high performance liquid chromatography-mass spectrometry combined analysis method. According to the method, a sample is dissolved in a mixed phase of an organic solvent and water, and the solution is subjected to gradient elution in an octadecyl-bonded silica gel chromatographic column utilizing a methanol and ammonium formate aqueous solution as a mobile phase. The method effectively solves the problem that the UV detector has poor impurity JD0101 detection sensitivity, can efficiently isolate and detect the impurity JD0101 and has the advantages of simpleness, fastness, high specificity and high sensitivity.
Owner:常州佳德医药科技有限公司
Method for detecting parecoxib sodium sulfate genotoxic impurities
PendingCN111413440AHigh detection sensitivityHigh precisionComponent separationGas liquid chromatographicDiethylsulfate
The invention relates to a method for detecting parecoxib sodium sulfate genotoxic impurities. The method comprises the following steps of dissolving a dimethyl sulfate reference substance, a diethylsulfate reference substance and a diisopropyl sulfate reference substance to obtain a sulfate impurity reference substance solution, dissolving parecoxib sodium to be detected to obtain a sample solution to be detected, and carrying out gas chromatography-mass spectrometry determination on the sulfate impurity reference substance solution and the sample solution to be detected. The chromatographicconditions are as follows: a filler of the gas chromatographic column is selected from one of a non-polar filler, a weak polar filler and a medium polar filler; and mass spectrum conditions comprisethat an electrospray ion source and a positive ion scanning mode are selected. The method is high in detection precision, has very high specificity and durability, and is simple and convenient to operate. The separation degree between genotoxic impurities is greater than 2.0, and the method can be used for quality control of parecoxib sodium bulk drugs.
Owner:SHANGHAI CHENPON PHARMA TECH
Method for determining dimethyl sulfate in medicine by derivatization gas chromatography-mass spectrometry
InactiveCN111505182AShorten the timeEasy to operateComponent separationGas liquid chromatographicSodium iodide
The invention discloses a method for determining dimethyl sulfate in a drug through derivatization gas chromatography-mass spectrometry. The method comprises the following steps of adding a derivatization solution into a drug sample, deriving dimethyl sulfate into methyl iodide, and detecting the content of methyl iodide through gas chromatography-mass spectrometry to obtain the content of dimethyl sulfate, wherein the derivatization solution is a saturated sodium iodide aqueous solution added with a trace amount of sodium thiosulfate. By adopting the method, the content of the genotoxic impurity dimethyl sulfate in the medicine can be accurately determined, and the method has the advantages of being convenient to operate, being capable of reducing the interference of a non-volatile medicine matrix and the like.
Owner:SHANGHAI SCIENPHARM CO LTD
Solvent system capable of effectively dissolving ornidazole or S-ornidazole and application thereof
ActiveCN110934824AReduce dosageImprove performanceAntibacterial agentsOrganic active ingredientsDrug utilisationOrganic solvent
Owner:CHONGQING DIANSUO MEDICAL TECH CO LTD
Method for controlling genotoxic impurities in metformin hydrochloride sustained release tablet preparation process
InactiveCN113081990ASimple control methodPrecise control methodOrganic active ingredientsMetabolism disorderNitrosoMetformin hcl
The invention relates to the technical field of preparation processes, in particular to a method for controlling genotoxic impurities in a metformin hydrochloride sustained release tablet preparation process, which comprises the following steps of: controlling the content of impurity dimethylamine in a raw material medicine metformin hydrochloride and the content of impurity nitrite in an auxiliary material hydroxypropyl methylcellulose; effective control of the genotoxic impurity N-nitrosodimethylamine is achieved, side reactions of medication of patients are reduced, and medication safety of the patients is guaranteed to a certain extent; the content of the genetic toxic impurity N-nitrosodimethylamine of the metformin hydrochloride sustained-release tablet is far lower than that of the State Food and Drug Administration and an acceptable limit specified by FDA (Food and Drug Administration).
Owner:SHANDONG INST FOR FOOD & DRUG CONTROL +1
Method for measuring genotoxic impurities in pradaxa
The invention discloses a method for measuring genotoxic impurities in pradaxa. According to the method, octadecylsilane chemically bonded silica is taken as a chromatographic column of a stationary phase, and a mixed solvent of an organic phase and a buffer is performed with gradient elution to be taken as a mobile phase for direct detection. The detection method has the advantages of high detection sensitivity, strong specialization, high precision, strong accuracy and convenient operation; has wide applicability, and can effectively control the quality of bulk drug.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Synthetic drug genotoxic impurity analysis method based on solid phase microextraction
ActiveCN111266094AWide detection rangeStable structureOther chemical processesComponent separationFiberMicrosphere
The invention relates to a synthetic drug genotoxic impurity analysis method based on solid phase microextraction. The solid-phase microextraction fiber comprises covalent organic framework nano microspheres and a fiber carrier, wherein the covalent organic framework nano microspheres are loaded on the surface of the fiber carrier; wherein the covalent organic framework nano microsphere is formedby combining two ligands tri-(4-aminophenyl)-amine and tri-(4-formylphenyl)-amine. The determination of the genotoxic impurities in the raw material medicine is achieved. The solid-phase microextraction fiber has the characteristics of high temperature resistance, high repeated use frequency and high adsorption capacity.
Owner:SHANDONG ANALYSIS & TEST CENT
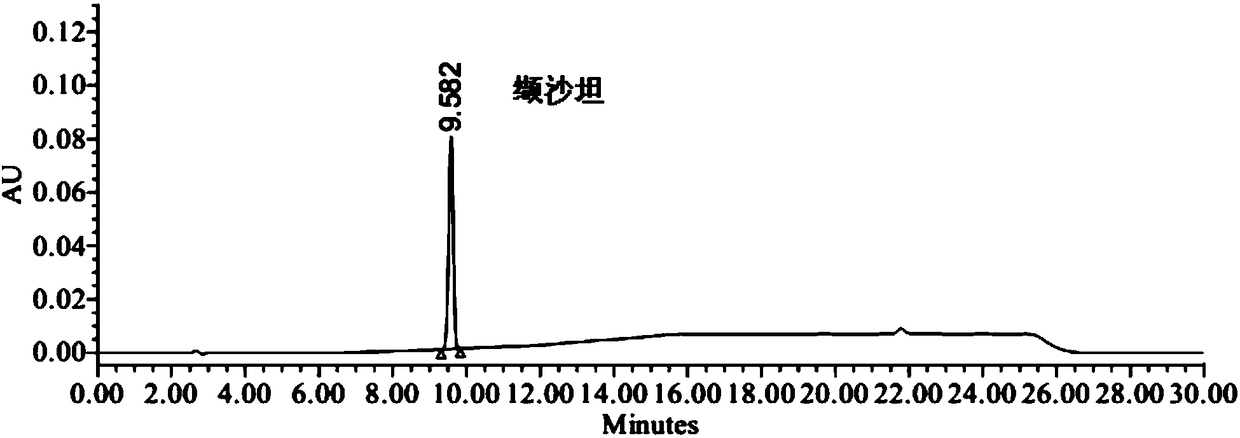
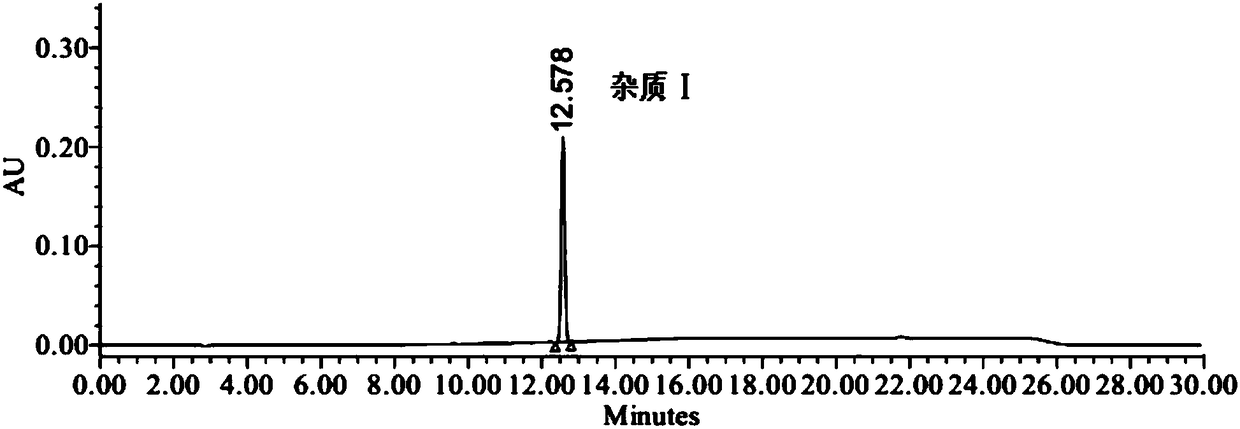
Method of analyzing and determining genotoxic impurities in valsartan by HPLC
The invention provides a method of analyzing and determining genotoxic impurities in valsartan by HPLC (high performance liquid chromatography) and belongs to the technical field of pharmaceutical analysis. The method takes phenylsilane bonded silica gel as a stationary phase and a trifluoroacetic acid aqueous solution and an acetonitrile trifluoroacetate solution as a mobile phase and adopts a gradient elution manner; and the established high performance liquid phase analysis method can accurately determine the two potential genotoxic impurities in the valsartan, namely 4'-bromoethyl-2-cyanobiphenyl (the impurity I) and 4',4'-bisbromomethy-2-cyanobiphenyl (the impurity II). The HPLC-UV (ultraviolet) method is simple to operate and short in analysis time; the sensitivity can reach 0.5ppm;a reliable basis is provided for improvement of valsartan quality standards and research of other sartan genotoxic impurities.
Owner:QILU PHARMA CO LTD
Preparation method of 1-(1-methoxy pyran glucosyl)-4-methyl-3-[5-(4-fluorophenyl)-2-thienyl methyl] benzene
InactiveCN103936800AWon't happenEasy to produceSugar derivativesSugar derivatives preparationOrganic layerMethyl group
The invention discloses a preparation method of 1-(1-methoxy pyran glucosyl)-4-methyl-3-[5-(4-fluorophenyl)-2-thienyl methyl] benzene. The method comprises the steps of adding tetrahydrofuran, toluene and 2-(5-bromine-2-methyl benzyl)-5-(4-fluorophenyl) thiophene in a reaction kettle, protecting by using N2 gas, cooling, and dropping a n-butyllithium solution; then dropping a toluene solution of 2,3,4,6-tetra-O-trimethylsilyl-D-glucolactone; finally, dropping an acidic methanol solution; adjusting the pH value to be 7-8, adding toluene, stirring, standing, collecting an organic layer, firstly washing the organic layer by using saturated salt water, and then concentrating and drying to obtain remainders; adding toluene into the remainders, heating and dissolving, cooling, then slowly adding n-hexane, stirring, filtering, and drying to obtain a product. The process disclosed by the invention is simple; genotoxic impurities can not be generated in a production process, so that side effects to a human body can be avoided.
Owner:安徽联创生物医药股份有限公司
Method for detecting 2,2,6,6-tetramethylpiperidinooxy with high performance liquid chromatography-mass spectrometry
InactiveCN109632981AEasy to operateThe test result is accurateComponent separationConcentration gradientLinear regression
The invention discloses a method for detecting 2,2,6,6-tetramethylpiperidinooxy with high performance liquid chromatography-mass spectrometry. The method comprises the steps that 1, a test solution and a reference stock solution is prepared; 2, the test solution and the reference stock solution with a certain concentration gradient are subjected to sample introduction, a high performance liquid chromatograph mass spectrometer is used for detecting and recording a chromatogram; and 3, linear regression analysis is performed on the mass concentration of the reference stock solution and chromatogram peak area, a regression equation and correlation coefficients are obtained, and a standard curve is manufactured; and the peak area of 2,2,6,6-tetramethylpiperidinooxy in the chromatogram of the test solution is utilized to calculate the content of 2,2,6,6-tetramethylpiperidinooxy by an external standard method. The method provided by the invention is the first method for detecting 2,2,6,6-tetramethylpiperidinooxy developed in the field. The method has accurate detection result, high sensitivity, good stability, and low detection limit, and totally meets detection requirements for genotoxic impurities in the field.
Owner:CHANGZHOU HEQUAN PHARMA CO LTD
HPLC method for detecting genotoxic impurities in candesartan cilexetil
The invention relates to a method for detecting genotoxic impurities in candesartan cilexetil, and belongs to the technical field of medicine quality control. The detection method comprises the stepsof 1, preparing a reference substance solution; 2, preparing a test solution; 3, preparing a sample adding test solution; 4, taking Thermo Gold C18 as a chromatographic column, taking a methanol-watermixed solution with a volume ratio of 63:37 as a mobile phase A, and taking acetonitrile as a mobile phase B, wherein the detection wavelength is 240nm; introducing a sample and carrying out gradientelution according to a table 1, wherein the flow rate is 1.0 ml / min, the column temperature is 30 DEG C, and the sample size is 80[mu]l; and recording a chromatogram, and calculating the content of the genotoxic impurity NDBA. The invention provides an HPLC / UV detection method of genotoxic impurity NDBA in candesartan cilexetil.
Owner:迪嘉药业集团股份有限公司
HPLC detection method for propranolol hydrochloride genotoxic impurities
ActiveCN111929372AImprove quality controlMonitor qualityComponent separationO-Phosphoric AcidGradient elution
The invention relates to an HPLC (High Performance Liquid Chromatography) detection method for propranolol hydrochloride genotoxic impurities. The method comprises the following steps of: preparing amixed reference substance mother solution from 1-naphthol, naphthyl glycidyl ether and a diluent in a constant-volume manner; preparing a sample solution from propranolol hydrochloride and a diluent in a constant-volume mode; and detecting the mixed reference substance mother solution and the sample solution by adopting a high performance liquid chromatograph to obtain a qualitative and quantitative detection result of propranolol hydrochloride genotoxic impurities 1-naphthol and naphthyl glycidyl ether, wherein the conditions of high performance liquid chromatography as follows: a C18 chromatographic column is adopted; and gradient elution is carried out by taking a phosphoric acid aqueous solution as a mobile phase A and acetonitrile as a mobile phase B at the column temperature of 20-30DEG C and the detection wavelength of 215-220nm. The method has the advantages of good specificity, solution stability, sensitivity, linearity, accuracy, precision and durability, appropriate chromatographic conditions and good separation effect, can meet the requirements of qualitative and quantitative detection, and is beneficial to quality control of genotoxic impurities 1-naphthol and naphthyl glycidyl ether in API so as to monitor the quality of drugs.
Owner:JIANGSU YUNYANG PHARMA GRP
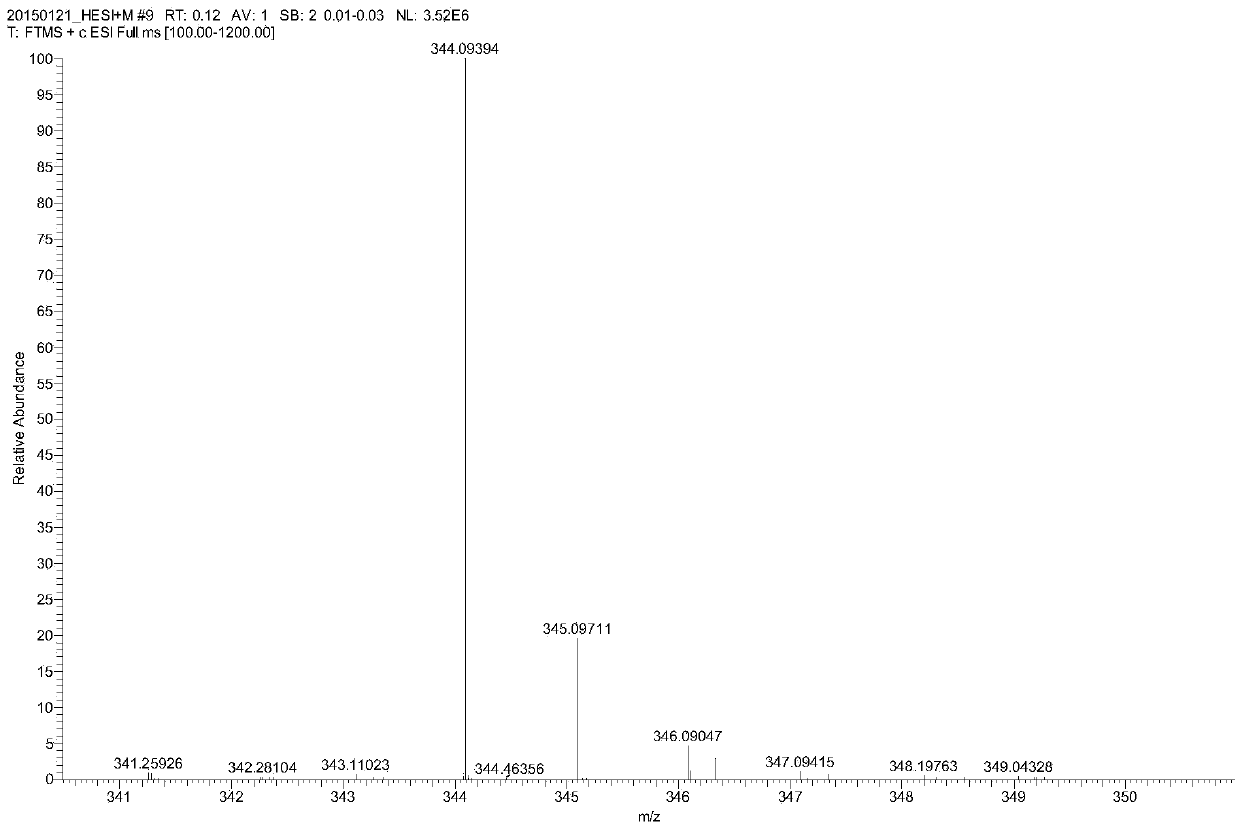
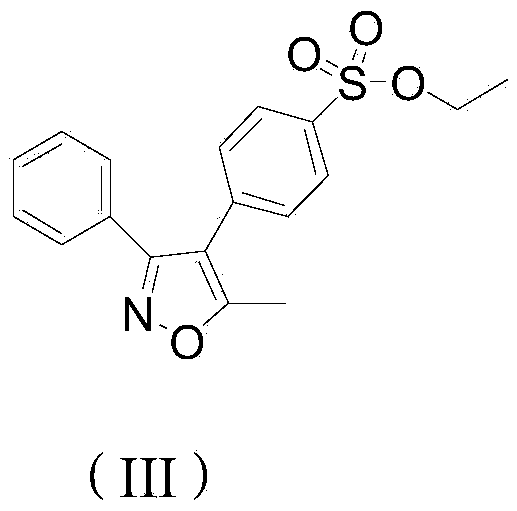
Preparation method of parecoxib sodium synthesis technology impurities
ActiveCN105367508AEffectively control the quality of finished productsEffective quality controlOrganic chemistryComponent separationParecoxib sodiumMass spectrometry
The present invention provides a preparation method of parecoxib sodium synthesis technology impurities. The method comprises the step of using 5-methyl-3,4-diphenylisoxazole as a raw material to synthesize a target product 4-(5-methyl-3-phenyl-isoxazolyl) ethyl benzenesulphonate through sulfonation reaction and esterification reaction. The impurities are genotoxic impurities of parecoxib sodium, and the study of a synthesizing method of the impurities is beneficial to the study of an impurity profile of parecoxib sodium and quality control of a parecoxib sodium product. The structure of the impurities is as shown in a formula in the specification.
Owner:BENGBU BBCA MEDICINE SCI DEV
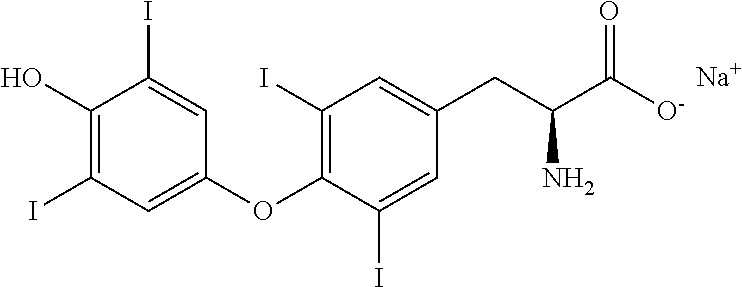
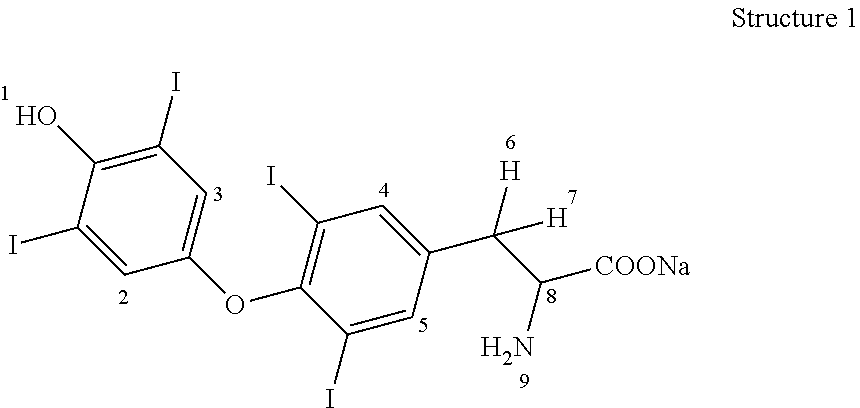
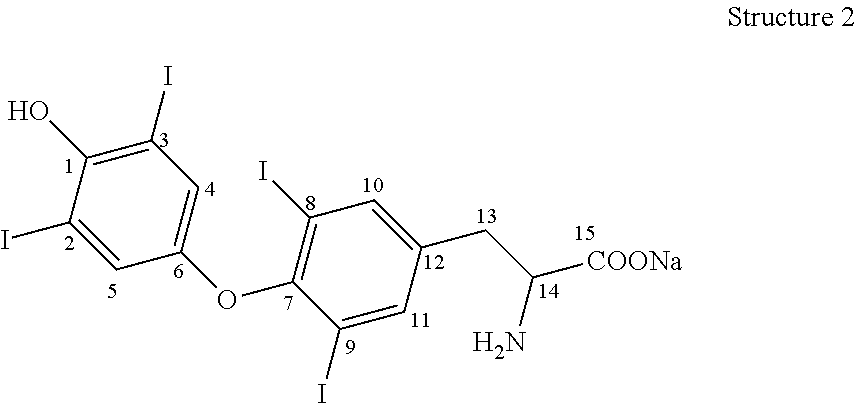
Process for the preparation of levothyroxine sodium
ActiveUS9428444B2Simple and convenient and economical with commercial feasibilitySpeed up the processOrganic compound preparationAmino-carboxyl compound preparationPropanoic acidIodide
The present invention provides a novel process for the preparation of highly pure Levothyroxine Sodium, i.e., (S)-2-amino-3-[4-(4-hydroxy-3, 5-diiodophenoxy)-3,5-diiodophenyl] propanoic acid sodium salt via two process intermediates viz 3,5-Diiodo L-Tyrosine copper complex and novel Bis (p-anisyl) iodonium Iodide. The invention also provides levothyroxine pentahydrate free from genotoxic impurities and liothyronine levels below 0.04% wt / wt.
Owner:AZICO BIOPHORE INDIA
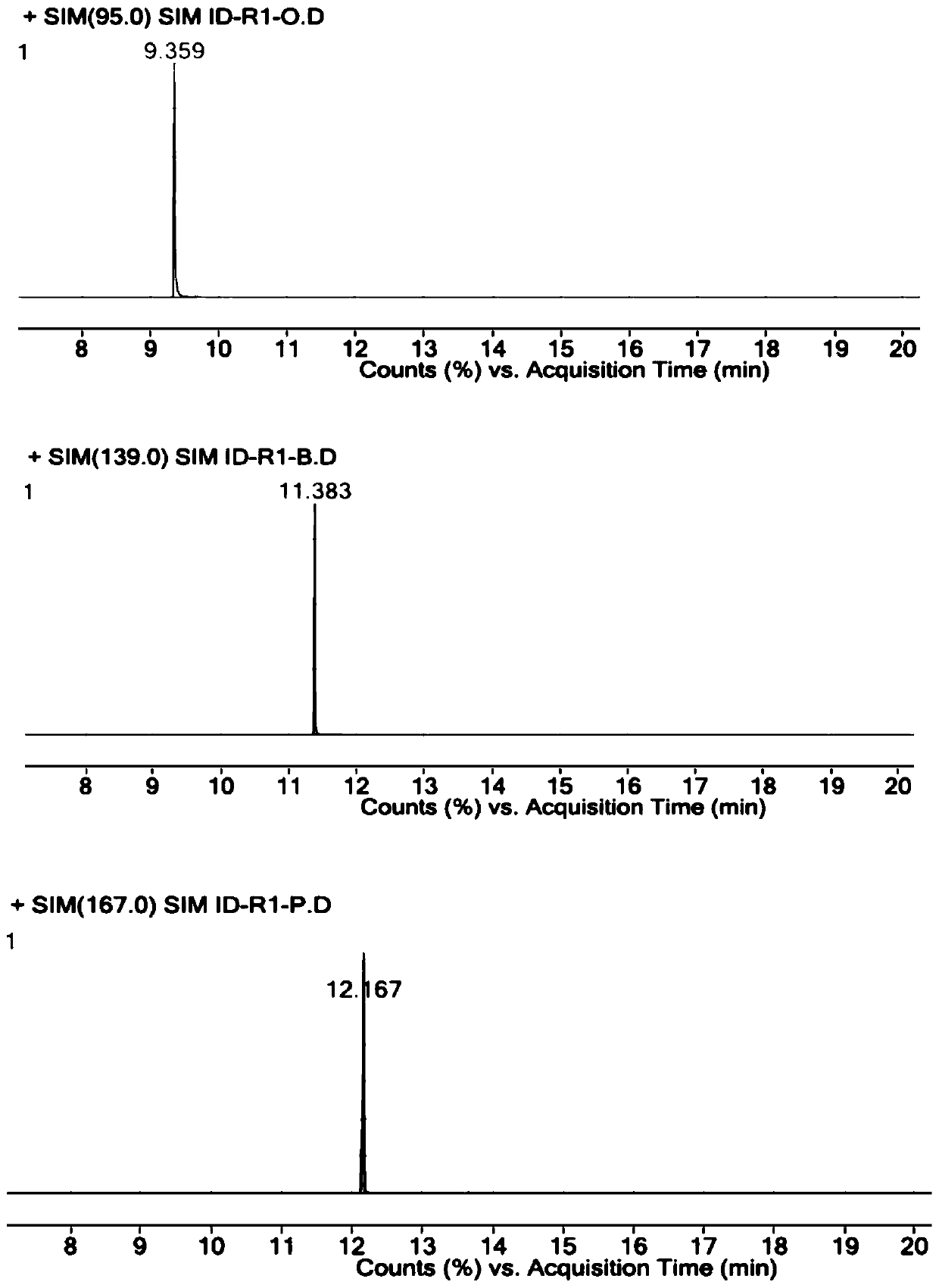
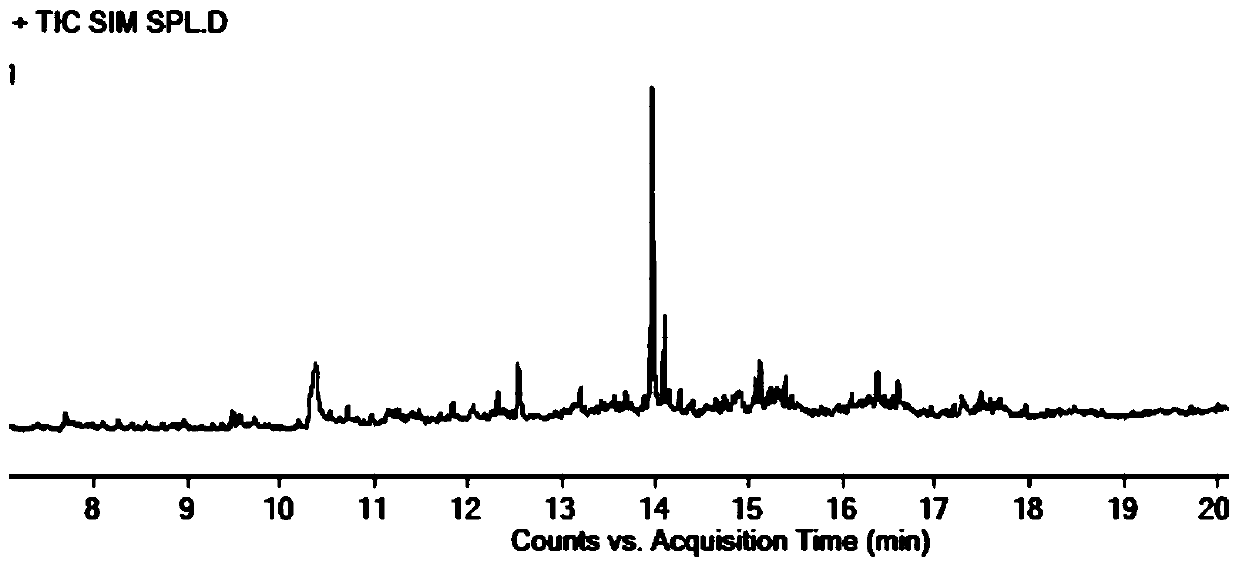
Method for detecting parecoxib sodium genotoxic impurities
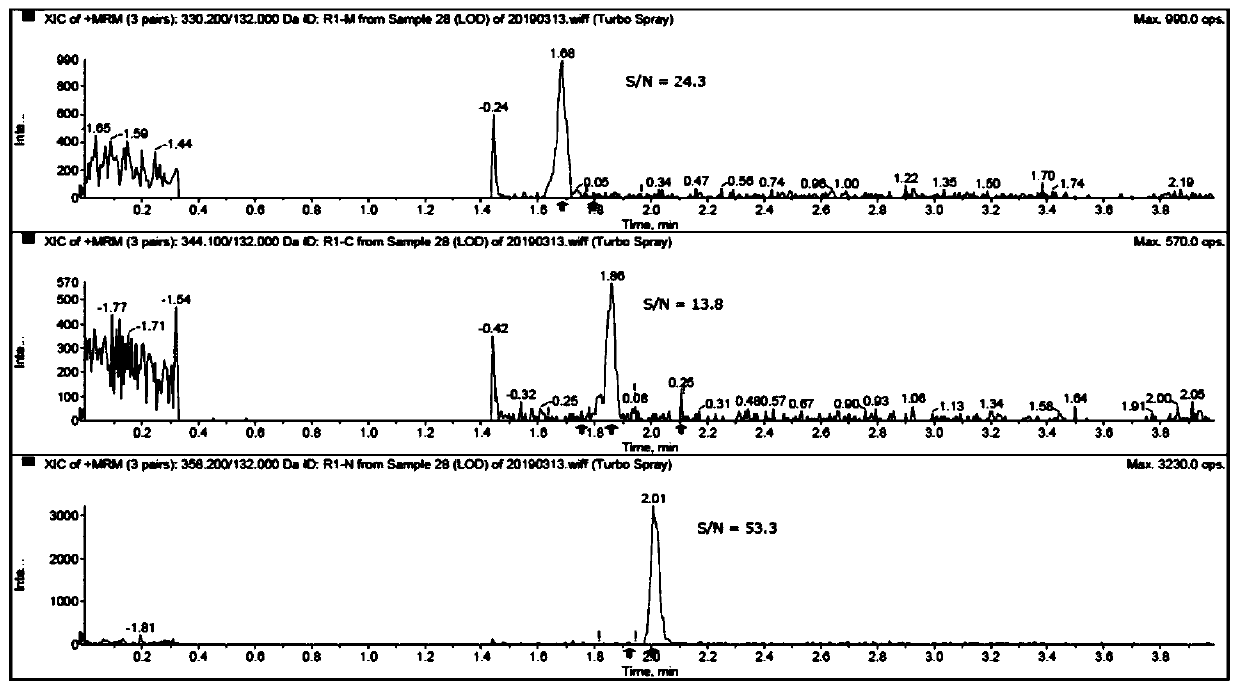
PendingCN111089931AEasy to separateHigh detection precisionComponent separationESI mass spectrometryParecoxib sodium
The invention relates to a method for detecting parecoxib sodium genotoxic impurities. The detection method comprises the following steps of dissolving an R1-M reference substance, an R1-C reference substance and an R1-N reference substance separately to obtain three kinds of impurity reference substance solutions; dissolving parecoxib sodium to be tested to obtain a sample solution to be tested;performing liquid chromatography / mass spectrometry determination on the three kinds of impurity reference substance solutions and the sample solution to be tested; the chromatographic conditions include: the mobile phase A is volatile acid or buffer solution, and the mobile phase B is acetonitrile; and the mass spectrometry conditions include: selecting an electrospray ionization, a positive ion scanning mode, the declustering potential is 25 to 200V, the collision energy is 10 to 100eV, the capillary voltage is 2000 to 6000V, the capillary temperature is 250 to 400 DEG C, and the drying gas temperature is 250 to 600 DEG C. The detection limit of the above method reaches 0.3ppm, the limit of quantification reaches 1ppm, and the quality control of pareximab sodium bulk drugs can be well carried out.
Owner:SHANGHAI CHENPON PHARM TECH CO LTD
A mass spectrometry method for detecting sulfonate genotoxic impurities based on dielectric barrier discharge ion source
ActiveCN109192652AFast and accurate detection and analysisImprove throughputSamples introduction/extractionMaterial analysis by electric/magnetic meansP-toluenesulfonateGenotoxicity
The invention provides a mass spectrometry detection method of sulfonate genotoxic impurities based on a dielectric barrier discharge ion source, and belongs to the technical field of pharmaceutical analysis. The method comprises the following steps: a sample solution is dripped as a sample point at a certain interval in the length direction of the sample table, the sample point is dried naturally, the dielectric barrier discharge ion source is aligned with the mass spectrometer at the front end of the sample point, the dielectric barrier discharge ion source is turned on, the heating platformand the mass spectrometer are in a working state, and mass spectrometry analysis and detection are carried out. A method for directly detect genotoxic impurities in medicine by dielectric barrier discharge ionization mass spectrometry is disclosed, From the sample preparation to the result, the rapid detection and analysis of the genotoxic impurity methyl p-toluenesulfonate in the drug can be completed in a few minutes. The established method can detect and analyze the methyl p-toluenesulfonate in the drug sensitively and quickly, thus realizing the high-throughput drug screening, and has good practical application value.
Owner:SHANDONG ANALYSIS & TEST CENT
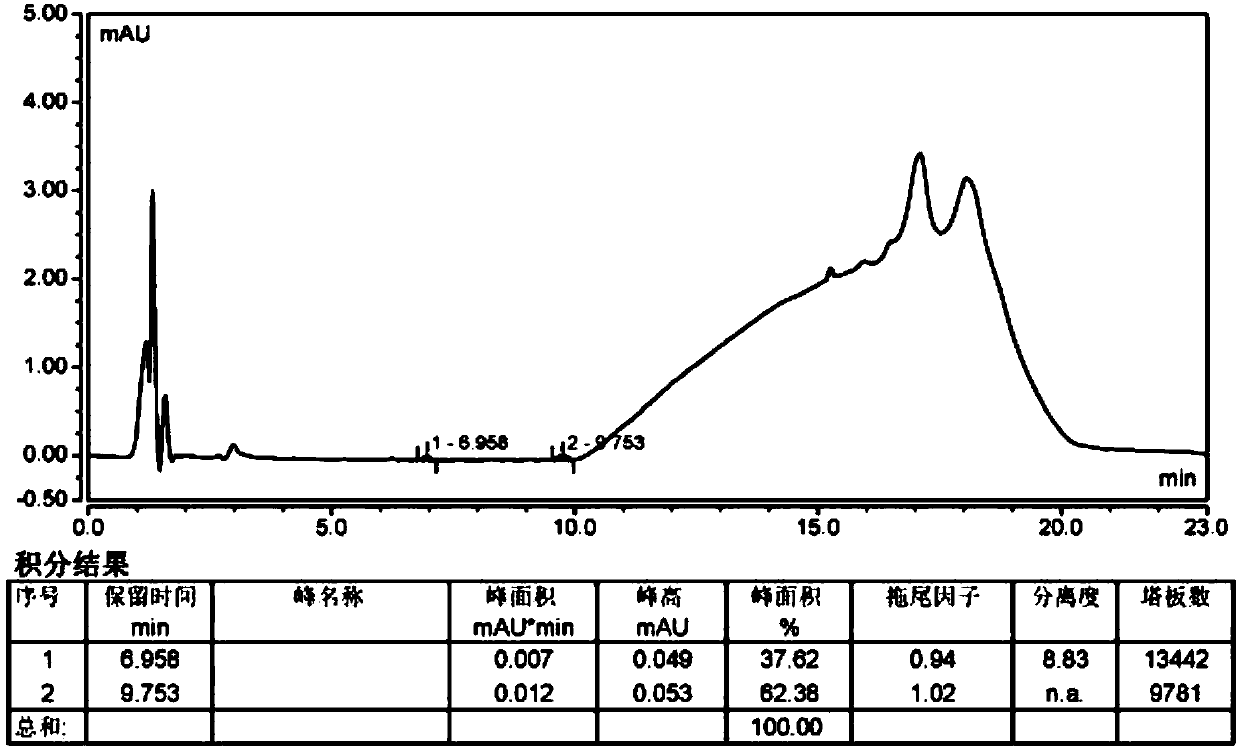
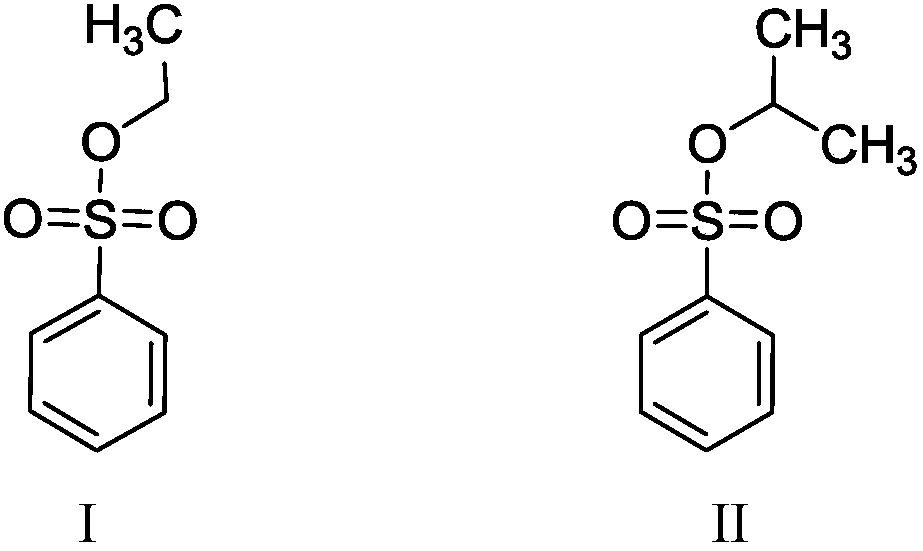
Method for separating and measuring bepotastine besilate and potential genotoxic impurities thereof with HPLC (High Performance Liquid Chromatography) method
ActiveCN107782832ASeparation assay is validHigh sensitivityComponent separationPhosphoric acidGradient elution
The invention belongs to the field of analytical chemistry, in particular to a method for separating and measuring bepotastine besilate and potential genotoxic impurities thereof with a HPLC (High Performance Liquid Chromatography) method. According to the method, an adopted chromatographic column is characterized in that octadecylsilane chemically bonded silica is taken as a filler, a flowing phase A and a flowing phase B are adopted for performing gradient elution, and enter a detector for detecting; the potential genotoxic impurities includes ethyl benzenesulfonate and isopropyl benz-enesulfonate; the flowing phase A is a phosphoric acid solution, and the flowing phase B is an acidic acetonitrile solution. By adopting the method, the contents of the bepotastine besilate and the potential genotoxic impurities thereof can be effectively separated and measured; high sensitivity, specificity and repeatability are achieved; the measurement is not interfered by a solvent peak during detection, and a detection result is accurate and reliable, so that the method has an extremely important significance in realizing quality control for the bepotastine besilate and a preparation thereof.
Owner:CHONGQING HUAPONT PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


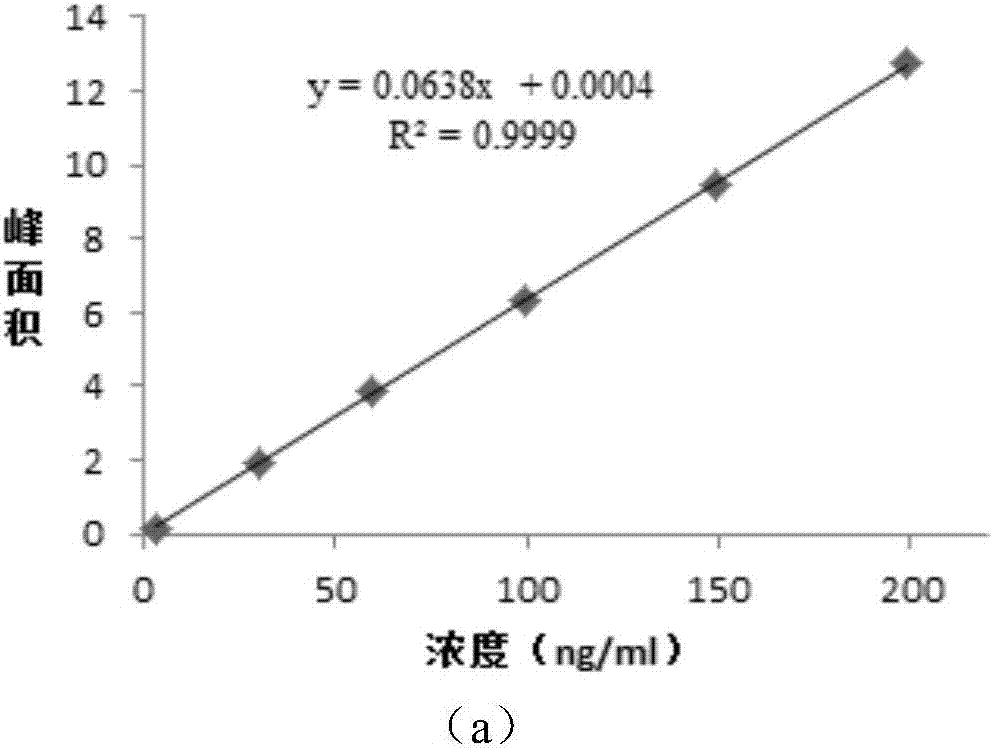
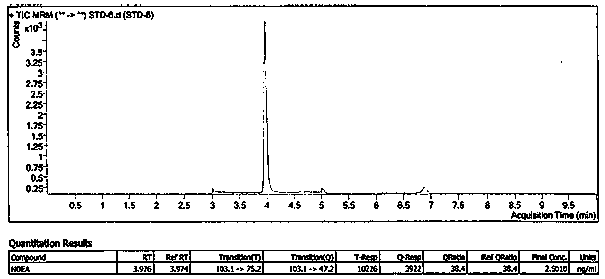
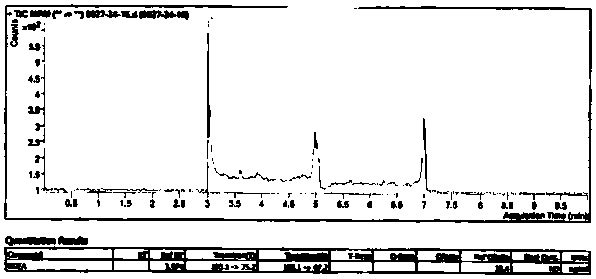
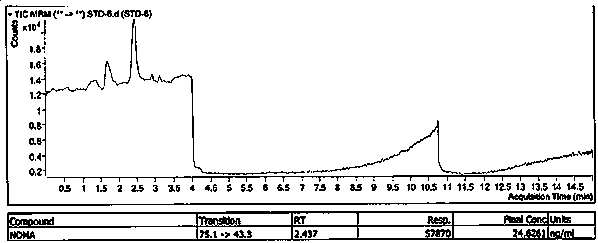

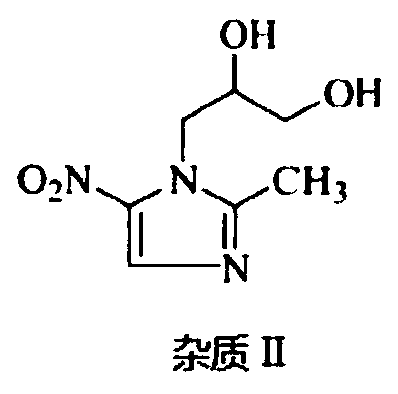
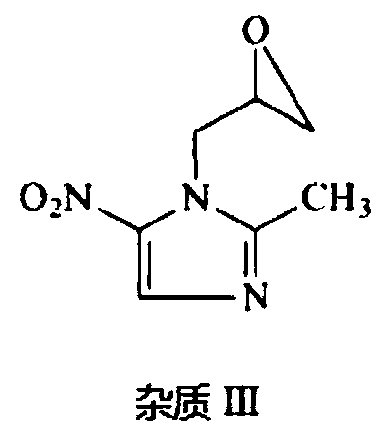
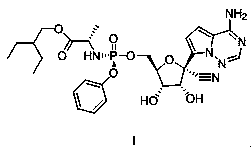
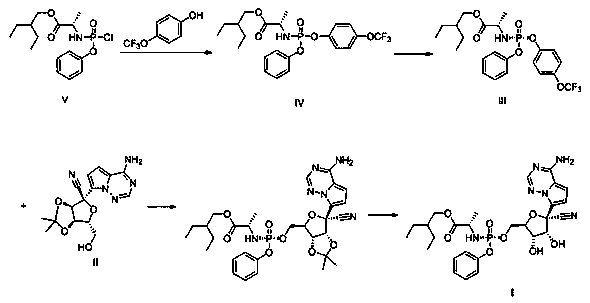
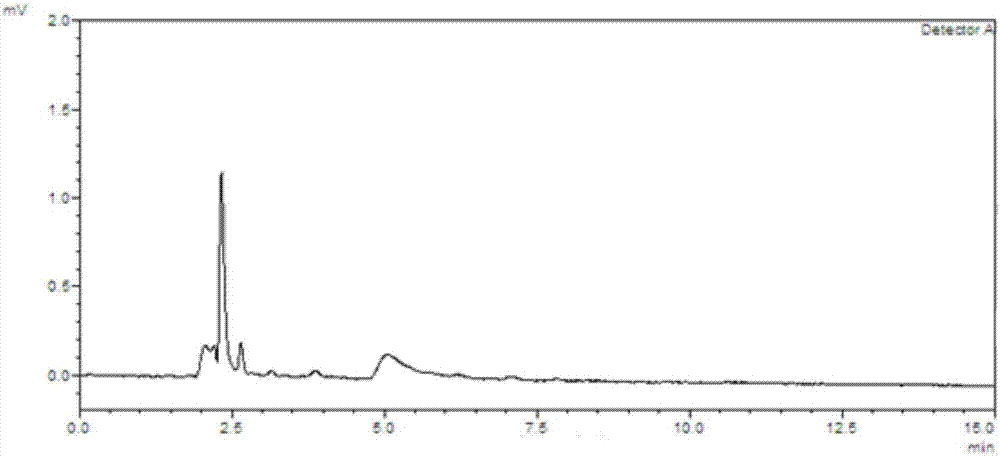
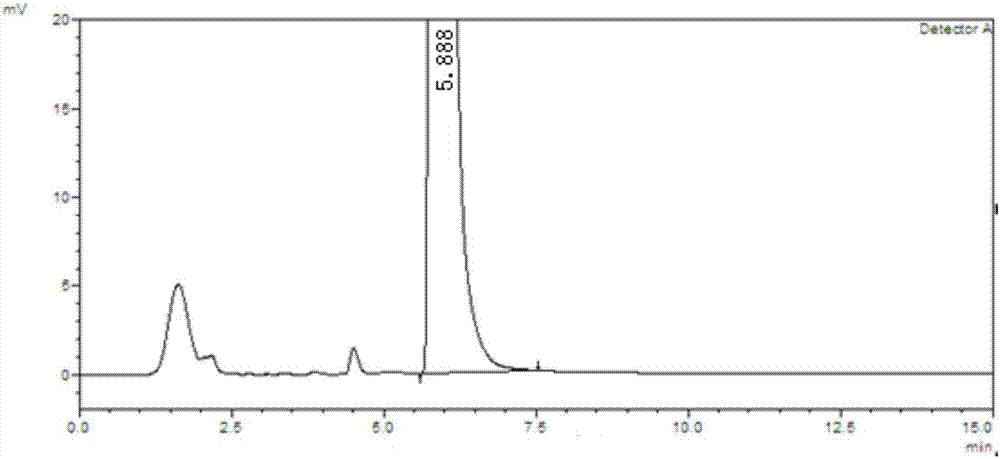
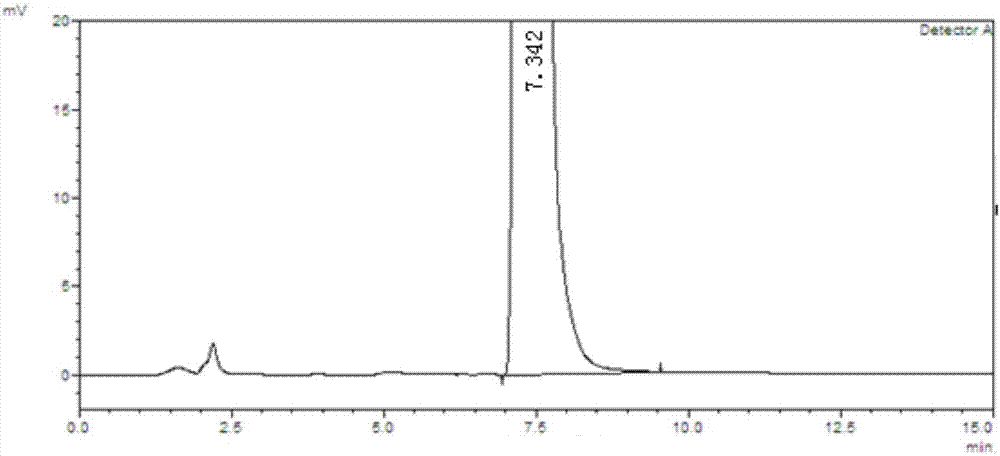
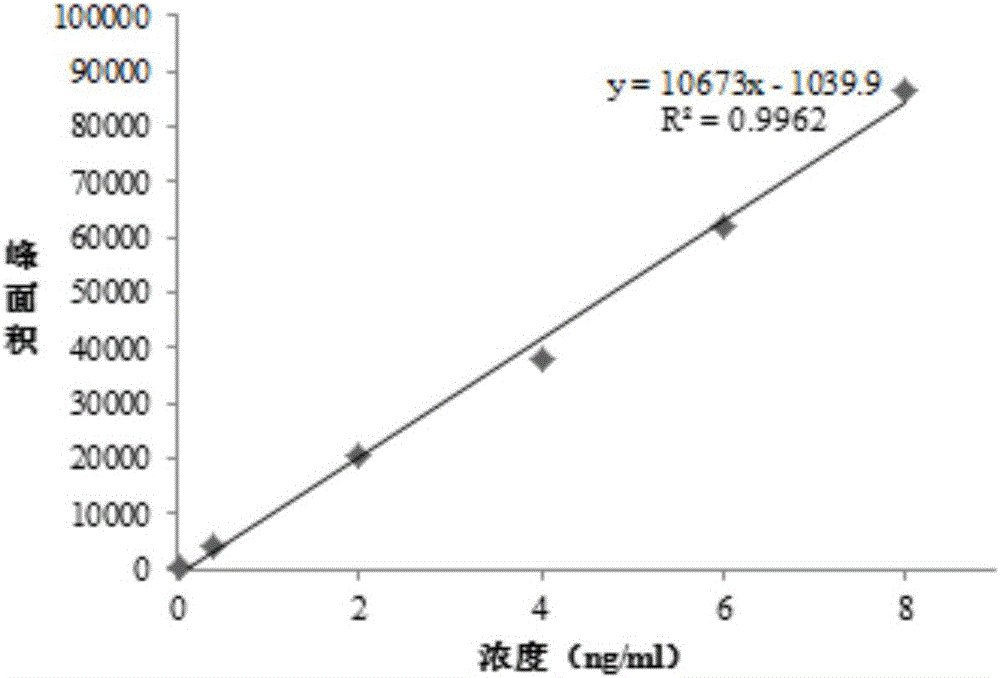



![Preparation method of 1-(1-methoxy pyran glucosyl)-4-methyl-3-[5-(4-fluorophenyl)-2-thienyl methyl] benzene Preparation method of 1-(1-methoxy pyran glucosyl)-4-methyl-3-[5-(4-fluorophenyl)-2-thienyl methyl] benzene](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dc4ccd1a-5a8f-42ae-8b36-a83536c0860d/406517dest_path_image001.PNG)