Method for separating and determining dapoxetine hydrochloride and potential genetic toxicity impurities thereof
A technique for dapoxetine hydrochloride and genotoxicity, applied in the field of analytical chemistry, to achieve the effect of strong specificity, simple operation method and high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
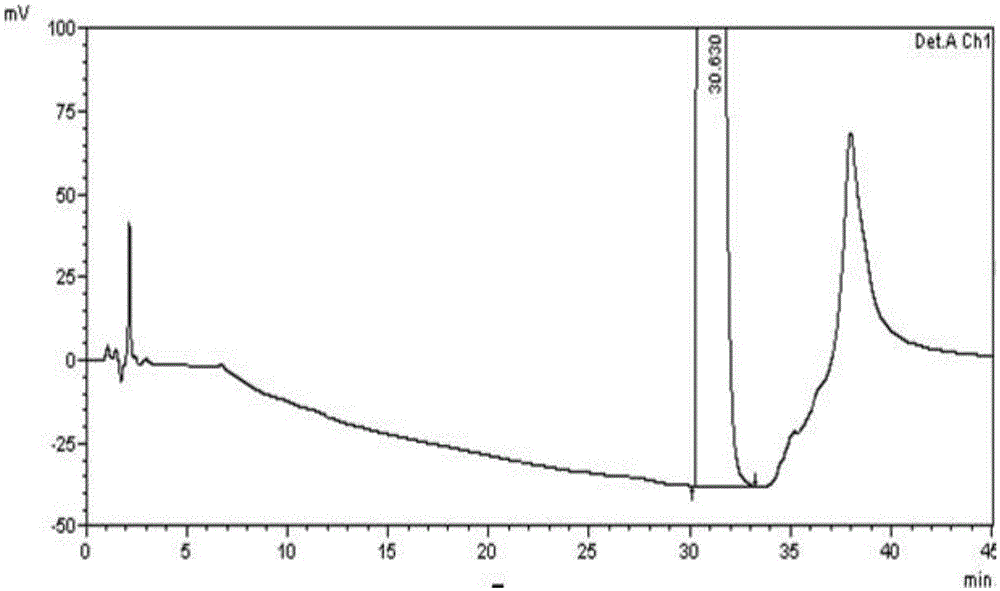
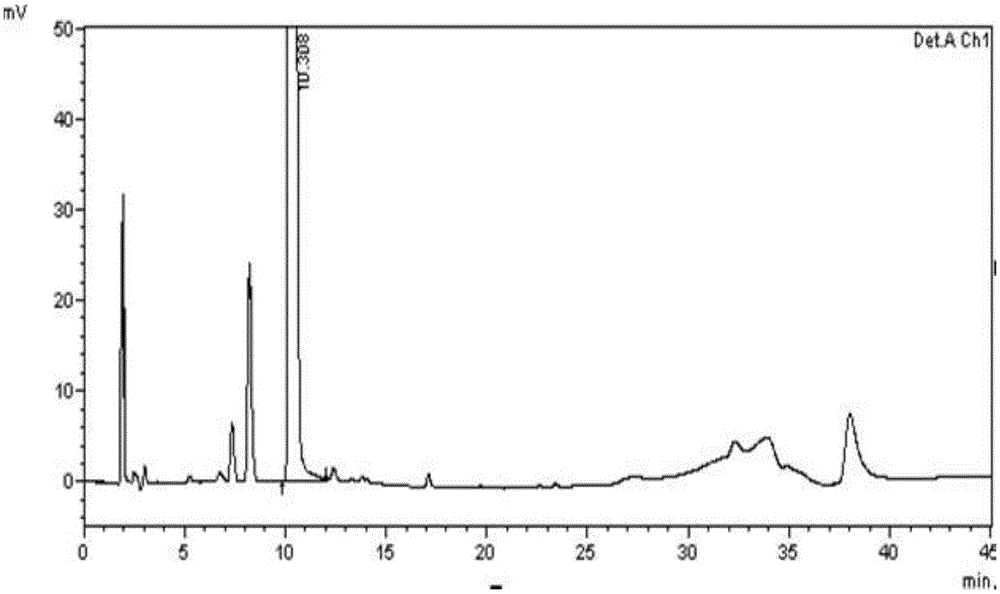
[0069] (1) Instruments and conditions
[0070] High performance liquid chromatography: SHIMADZULC-2010AHT;
[0071] Chromatographic column: VP-ODS (250×4.6mm, 5μm);
[0072] Wavelength: 210nm;
[0073] Column temperature: 30°C; flow rate: 1.0ml / min;
[0074] Injection volume: 20μl;
[0075] Mobile Phase I: Gradient Elution
[0076] time (min)
Acetonitrile (%)
water(%)
0
45
55
4
45
55
30
90
10
35
45
55
45
45
55
[0077] Diluent: the volume ratio of acetonitrile and water is 45:55
[0078] (2) Experimental steps
[0079] UI-1 Positioning Solution: Accurately weigh 20.15mg of the sample, put it in a 50ml measuring bottle, add diluent to dissolve and dilute to the mark, and mix well.
[0080] 3-Chloropropiophenone positioning solution: Accurately weigh 20.32mg of the sample, put it in a 50ml measuring bottle, add diluent to dissolve and dilute to the mark, and mix well.
[0...
Embodiment 2
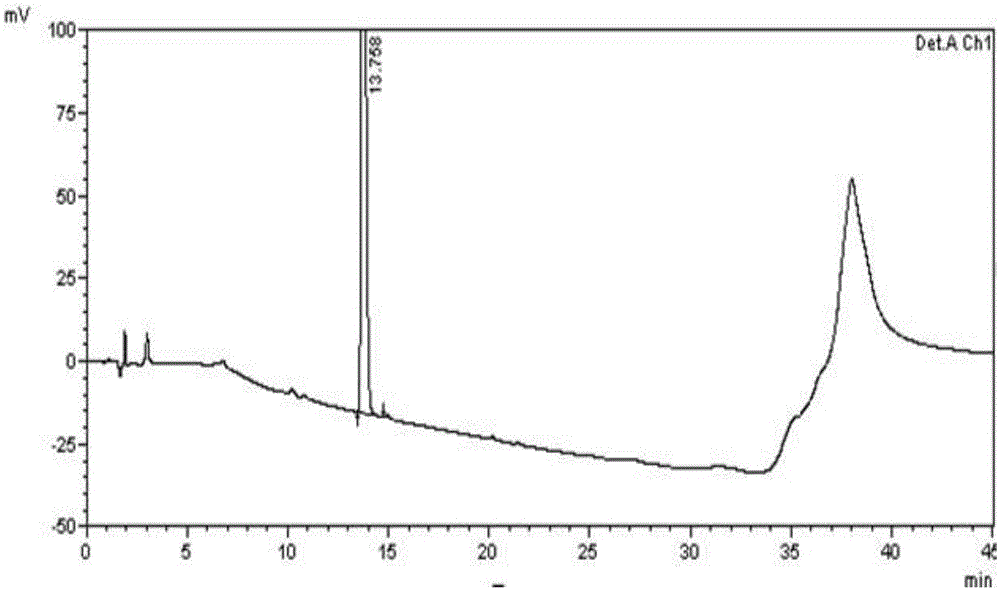
[0090] 1) Instruments and conditions
[0091] High performance liquid chromatography: SHIMADZULC-2010AHT;
[0092] Chromatographic column: WatersXTerraRP18 (3.0×150mm, 3.5μm);
[0093] Detection wavelength: 225nm Flow rate: 0.6ml / min
[0094] Column temperature: 35°C Injection volume: 10μl
[0095] Mobile phase II:
[0096] Mobile phase A: 10mmol / L ammonium bicarbonate and 6mmol / L diethylamine aqueous solution
[0097] Mobile Phase B: Acetonitrile
[0098] time (min)
Flow A(%)
Mobile phase B(%)
0
55
45
20
5
95
22
55
45
30
55
45
[0099] Diluent: Acetonitrile
[0100] (2) Experimental steps
[0101] UI-1 Positioning Solution: Accurately weigh 20.15mg of the sample, put it in a 50ml measuring bottle, add diluent to dissolve and dilute to the mark, and mix well.
[0102] 3-Chloropropiophenone positioning solution: Accurately weigh 20.32mg of the sample, put it in a 50ml measuring bottle, add...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com