HPLC detection method for propranolol hydrochloride genotoxic impurities
A technology of propranolol hydrochloride gene and propranolol hydrochloride, applied in the field of HPLC detection of propranolol hydrochloride genotoxic impurities, can solve the problems of unfavorable quality control of propranolol hydrochloride tablets, poor solubility of genotoxic impurities, etc. , to meet the requirements of qualitative and quantitative detection, suitable chromatographic conditions, and good durability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-5
[0021] (1) Reagent selection: acetonitrile: Merck, batch number: SBK5734; phosphoric acid: Sinopharm, batch number: 20170929.
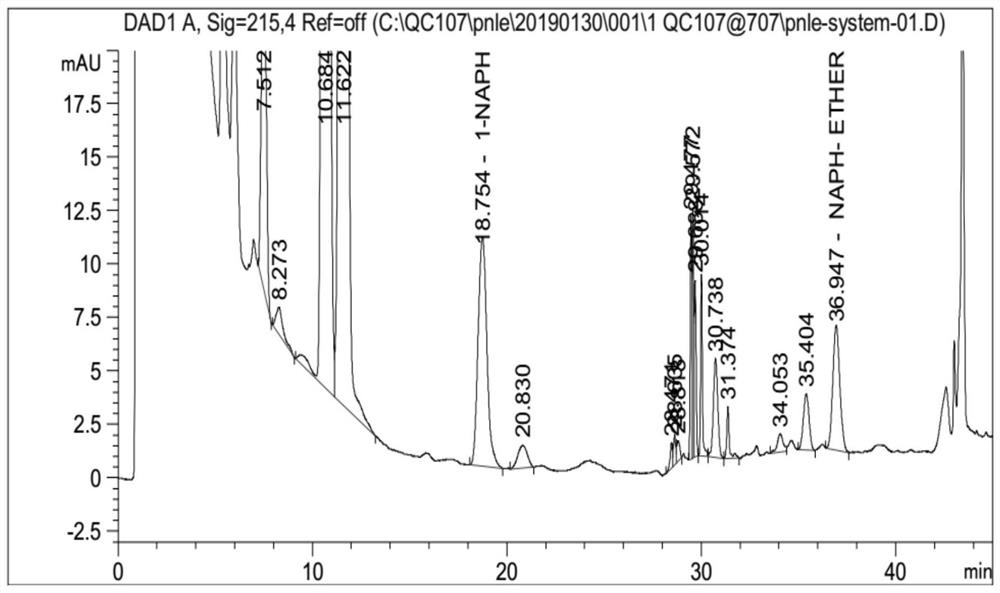
[0022] (2) Sample selection: Propranolol hydrochloride (API) provided by Yunyang (batch number 180101, 170201, 170202); 1-naphthol: Aladdin, batch number: K1717022; naphthalene glycidyl ether: LGC, batch number: 43050702, content 99.6%; Impurity A: LGC, lot number: 43010801; Impurity B: LGC, lot number: 79125; Impurity C: LGC, lot number: 66021.
Embodiment 1-4
[0024] Solution preparation: 1-naphthol and glycidyl ether are classified as Type 2 impurities by ICHM7. The maximum daily dose of propranolol hydrochloride is 240mg. The impurity limit is calculated according to the TTC method, 1.5μg / day÷240mg / day=6.25ppm, Check the quality standards of propranolol hydrochloride in various national pharmacopoeias. Only the Chinese Pharmacopoeia stipulates that 1-naphthol must not exceed 300ppm. JP17, USP41, and EP9.0 do not stipulate the limits of 1-naphthol and naphthalene glycidyl ether. According to the requirements of current regulations and Strict control strategy, the limit of 1-naphthol and glycidyl ether is proposed to be 6.25ppm, in order to make the impurity in the API controllable within 6.25ppm, the API concentration is 50mg / ml, and the impurity limit concentration is 0.3125μg / ml for analysis method development.
[0025] Diluent: The diluent consists of acetonitrile and water in a volume ratio of 1:1;
[0026] Mix the reference s...
Embodiment 1
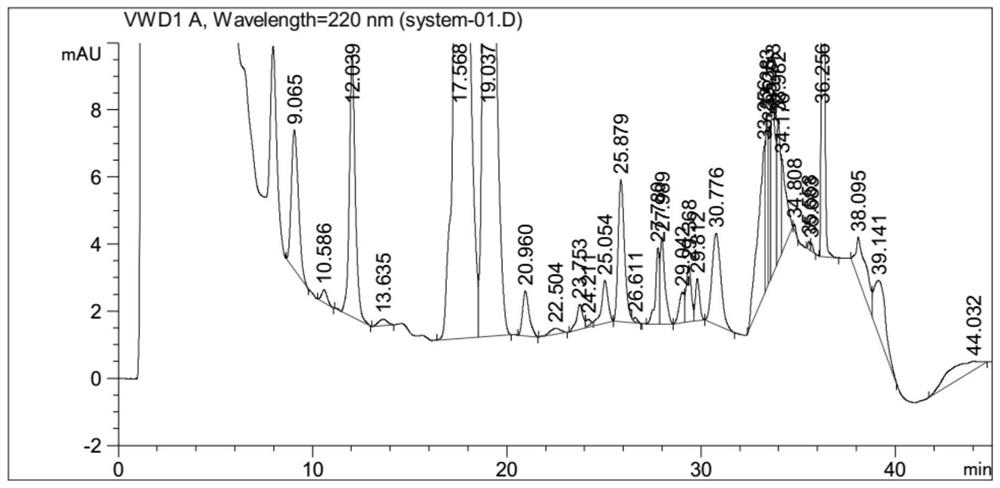
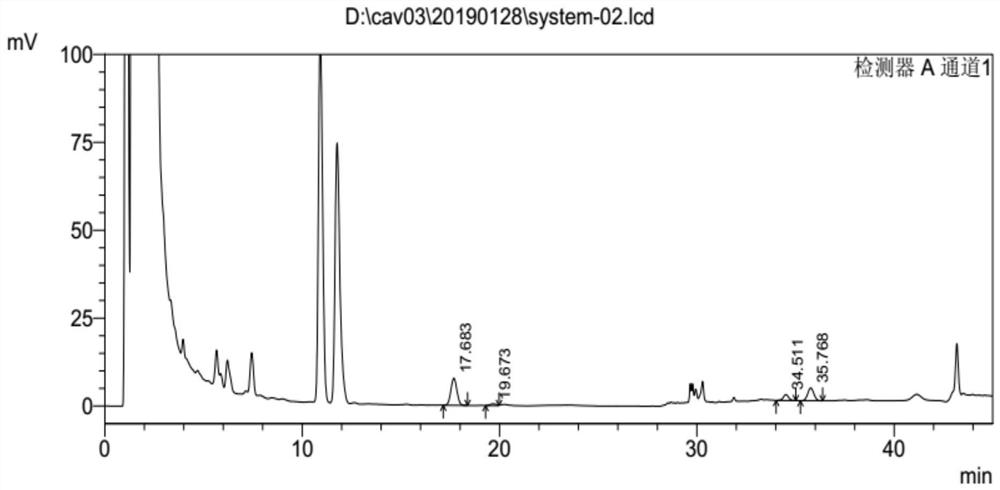
[0031] The above-mentioned system suitability solution is detected by 106 Agilent VWD high-performance liquid chromatography instruments, and the conditions of high-performance liquid chromatography are: C18 chromatographic column; with phosphoric acid aqueous solution as mobile phase A, the volume percentage of phosphoric acid in mobile phase A phosphoric acid aqueous solution is 0.1 %, with acetonitrile as mobile phase B, gradient elution was performed, the flow rate was 1.0ml / min, the injection volume was 40μl, and the column temperature was 30°C;
[0032] The chromatographic column adopts octadecylsilane bonded silica gel as filler Waters Atlantis T3 (4.6 × 150mm, 3 μ m) chromatographic column, and the gradient elution program is: time 0min: mobile phase B is 40%, time 18min flow Phase B is 40%, time 25min mobile phase B is 50%, time 35min mobile phase B is 50%, time 36min mobile phase B is 80%, time 45min mobile phase B is 80%, time 46min mobile phase B is 40% , time 55mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com