Detection method of parecoxib sodium genotoxicity impurity and application thereof
A parecoxib sodium and detection method technology, which is applied in the field of drug analysis, can solve problems such as unretrieved, and achieve the effects of simple operation, high sensitivity and specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation method of the parecoxib sodium genotoxic impurity detection solution of the present invention.
[0032] This test chooses water, acetonitrile, methanol, water: acetonitrile (1:1) to dissolve the sample, the sample is slightly soluble in water, slightly soluble in acetonitrile, and easily soluble in methanol, water: acetonitrile (1:1). Since 3 genotoxic impurities need to be added to the parecoxib sodium sample during the investigation of chromatographic conditions, the structures of impurity A, impurity B, and impurity C are all ester substances, which are easily hydrolyzed, and the solvent should not contain water, so methanol is preferred solvent for dissolving the sample.
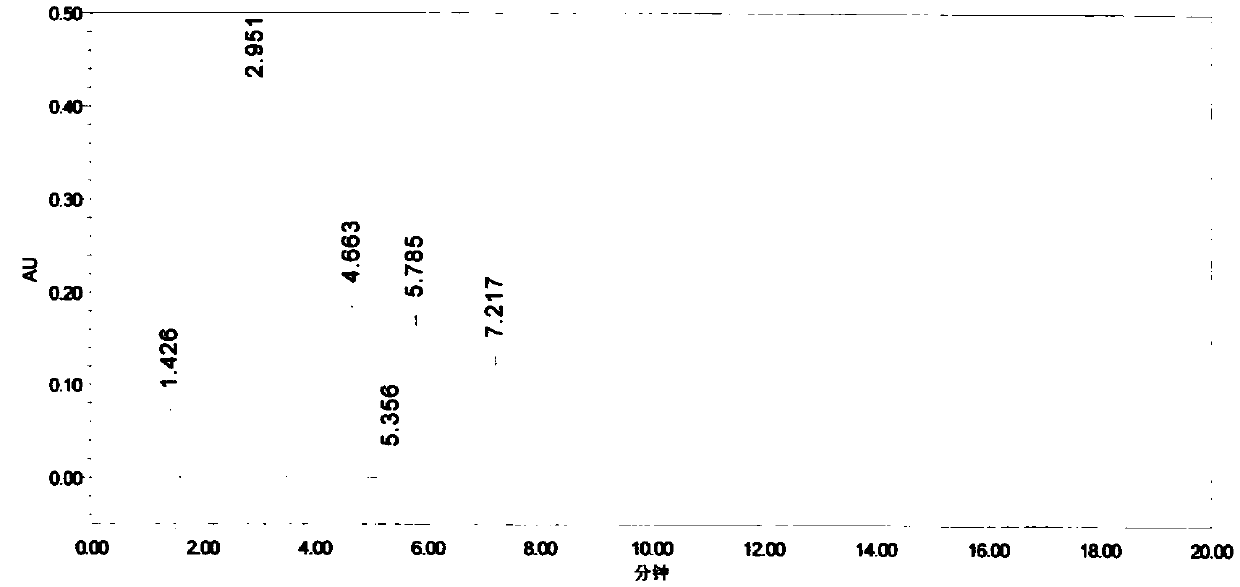
[0033] In this test, the parecoxib sodium sample was prepared into a 0.5mg / ml-4mg / ml solution for investigation. The results showed that the impurity response at 0.5mg / ml was small, and the asymmetry of the main peak at 4mg / ml was serious, which affected the detection of impurities...
Embodiment 2
[0035] The detection method of parecoxib sodium genotoxic impurity of the present invention
[0036] 1) Instruments and testing conditions
[0037] Instrument: WatersE2695 high performance liquid chromatography-ultraviolet detector;
[0038] Chromatographic column: C8 column (4.6×150mm, 5μm);
[0039] Mobile phase: water: acetonitrile (50:50);
[0040] Column temperature: 25°C; flow rate: 1.0mL / min;
[0041] Detection wavelength: 230nm; injection volume: 10μl.
[0042] 2) Solution preparation
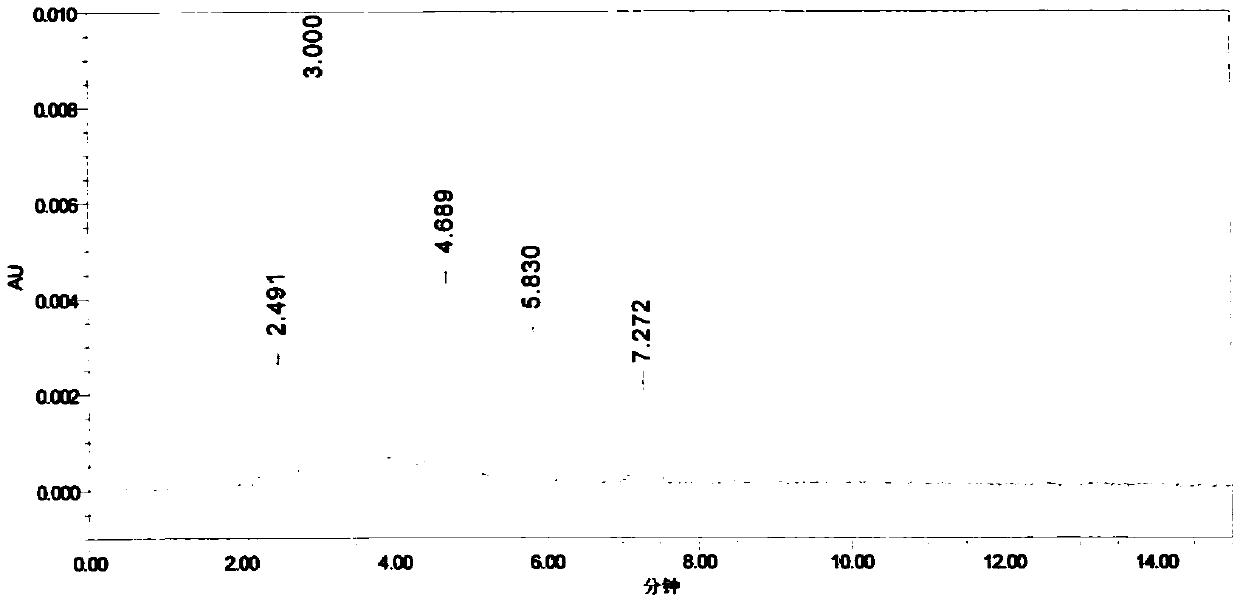
[0043] Accurately weigh an appropriate amount of impurity A, impurity B, and impurity C reference substances, add methanol to dissolve and dilute to a 0.5mg / ml solution, and use it as an impurity stock solution; accurately weigh 40mg of parecoxib sodium into a 10ml volumetric flask, and accurately weigh Measure 1ml of each impurity stock solution into the volumetric flask, add methanol to dissolve and dilute to the mark, and use it as the chromatographic condition screening solutio...
Embodiment 3
[0047] The detection method of parecoxib sodium genotoxic impurity of the present invention
[0048] 1) Instruments and testing conditions
[0049] Instrument: WatersE2695 high performance liquid chromatography-ultraviolet detector;
[0050] Chromatographic column: C8 column (4.6×150mm, 5μm);
[0051] Mobile phase: pH3.0 dilute phosphoric acid: acetonitrile (70:30);
[0052] Column temperature: 30°C; flow rate: 2mL / min;
[0053] Detection wavelength: 290nm; injection volume: 40μl.
[0054] 2) Solution preparation
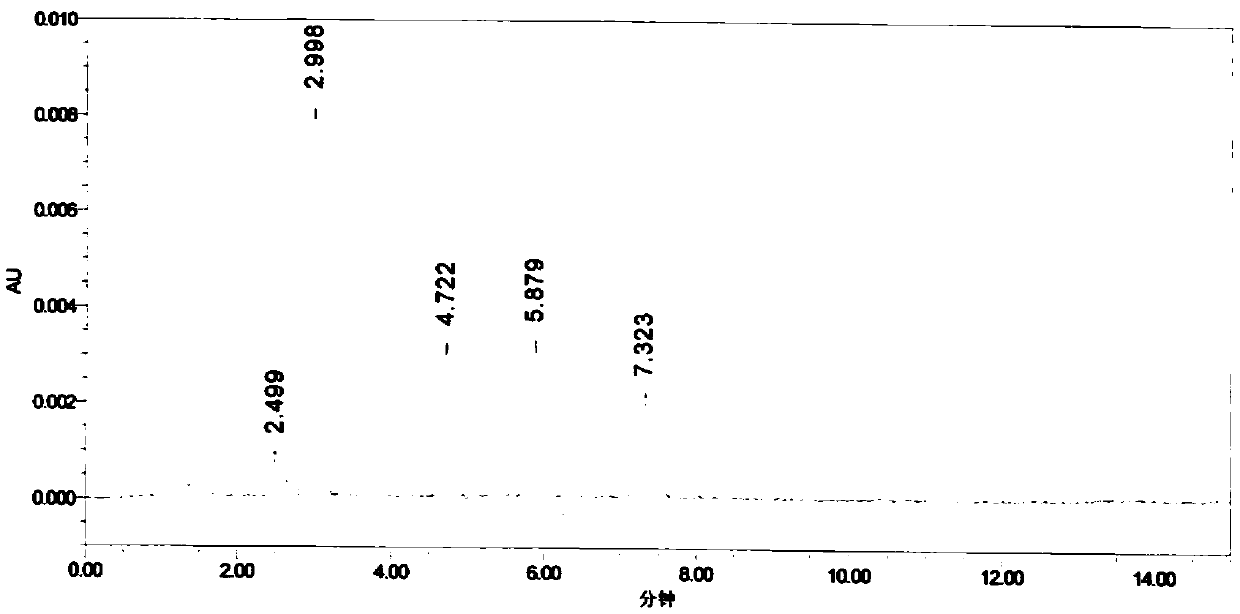
[0055] Accurately weigh an appropriate amount of impurity A, impurity B, and impurity C reference substances, add methanol to dissolve and dilute to a 0.5mg / ml solution, and use it as impurity stock solution; accurately weigh 10mg of parecoxib sodium into a 10ml volumetric flask, and accurately weigh Measure 1ml of each impurity stock solution into the volumetric flask, add methanol to dissolve and dilute to the mark, and use it as the chromatographic condition...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Flow | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com