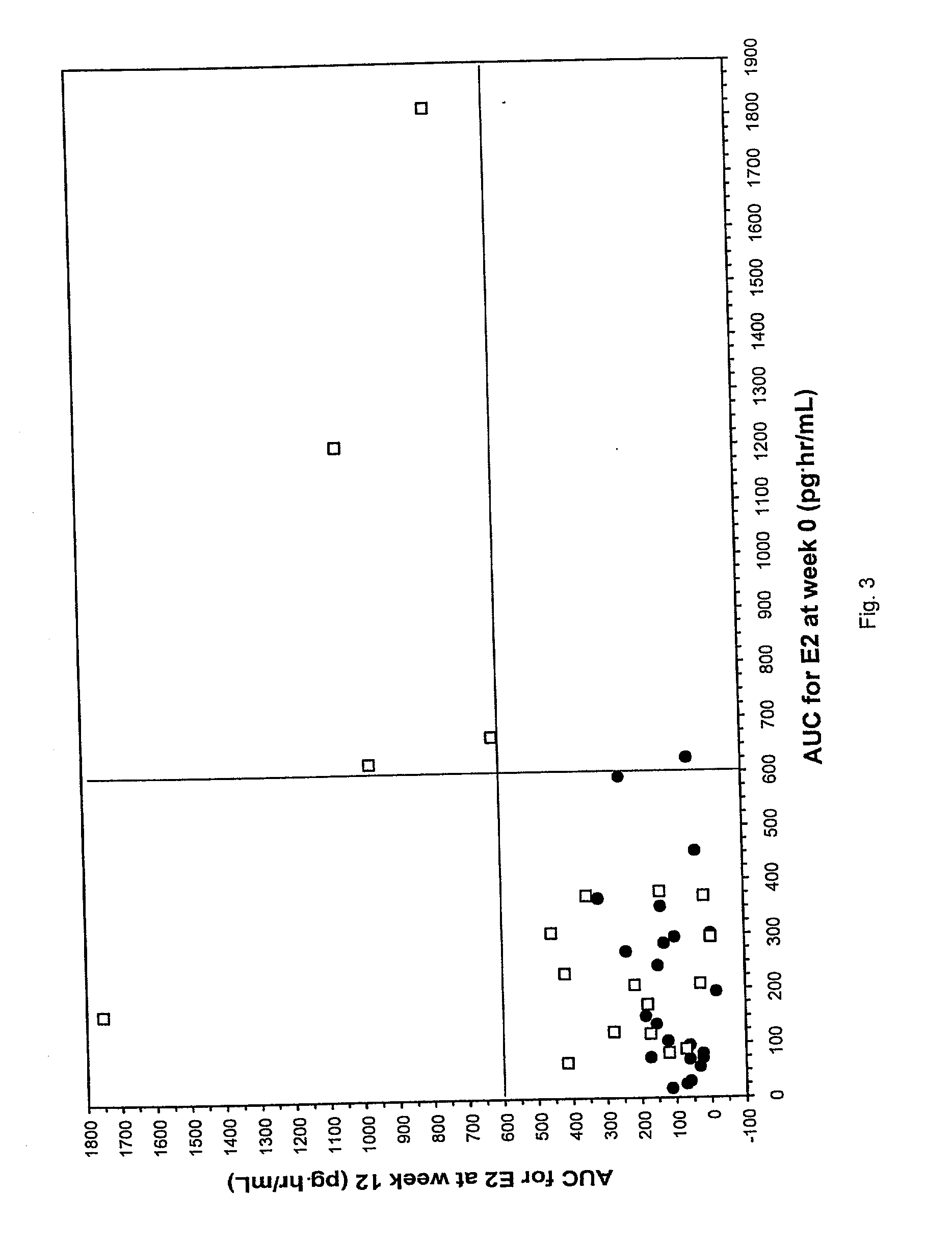
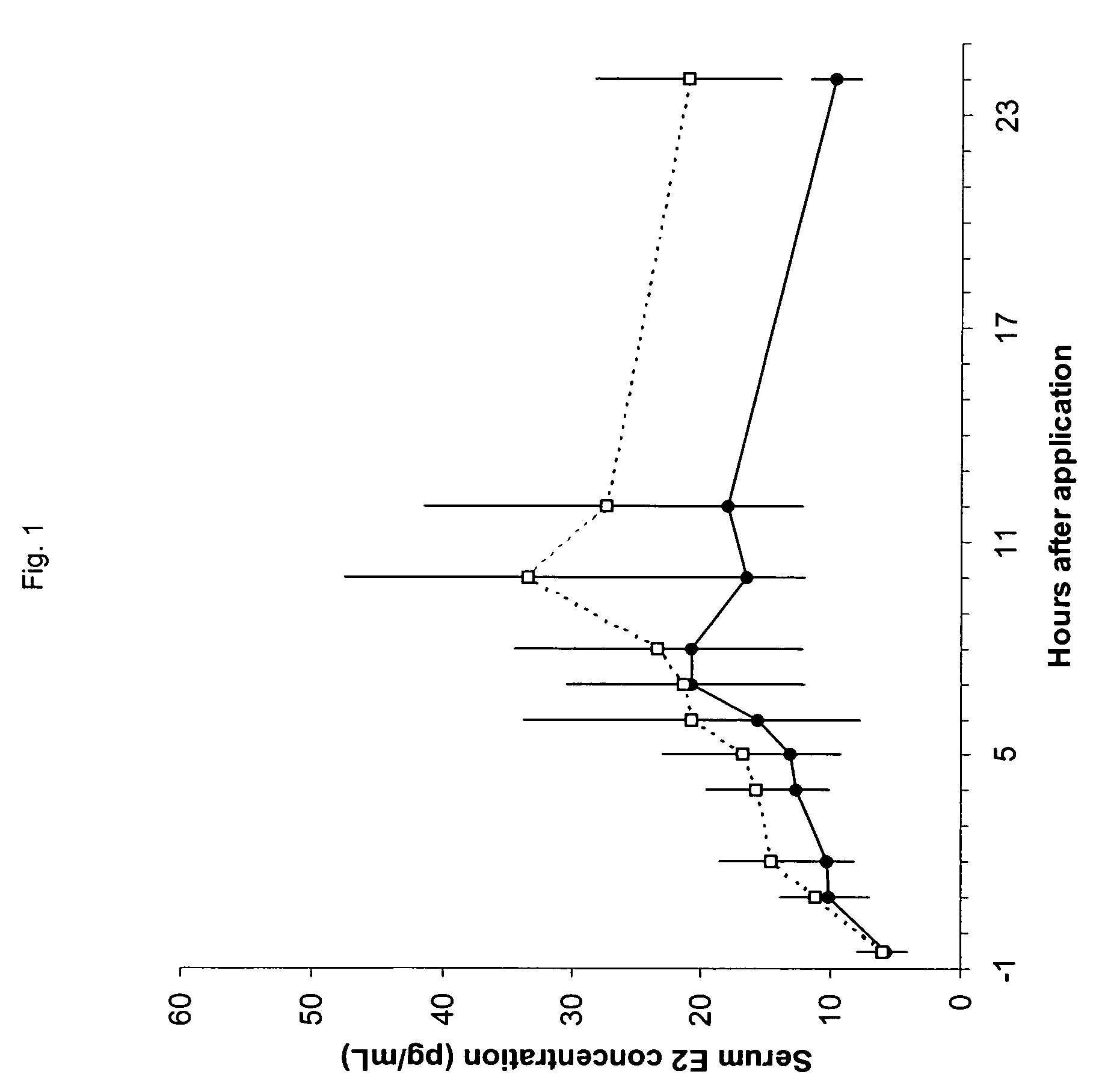
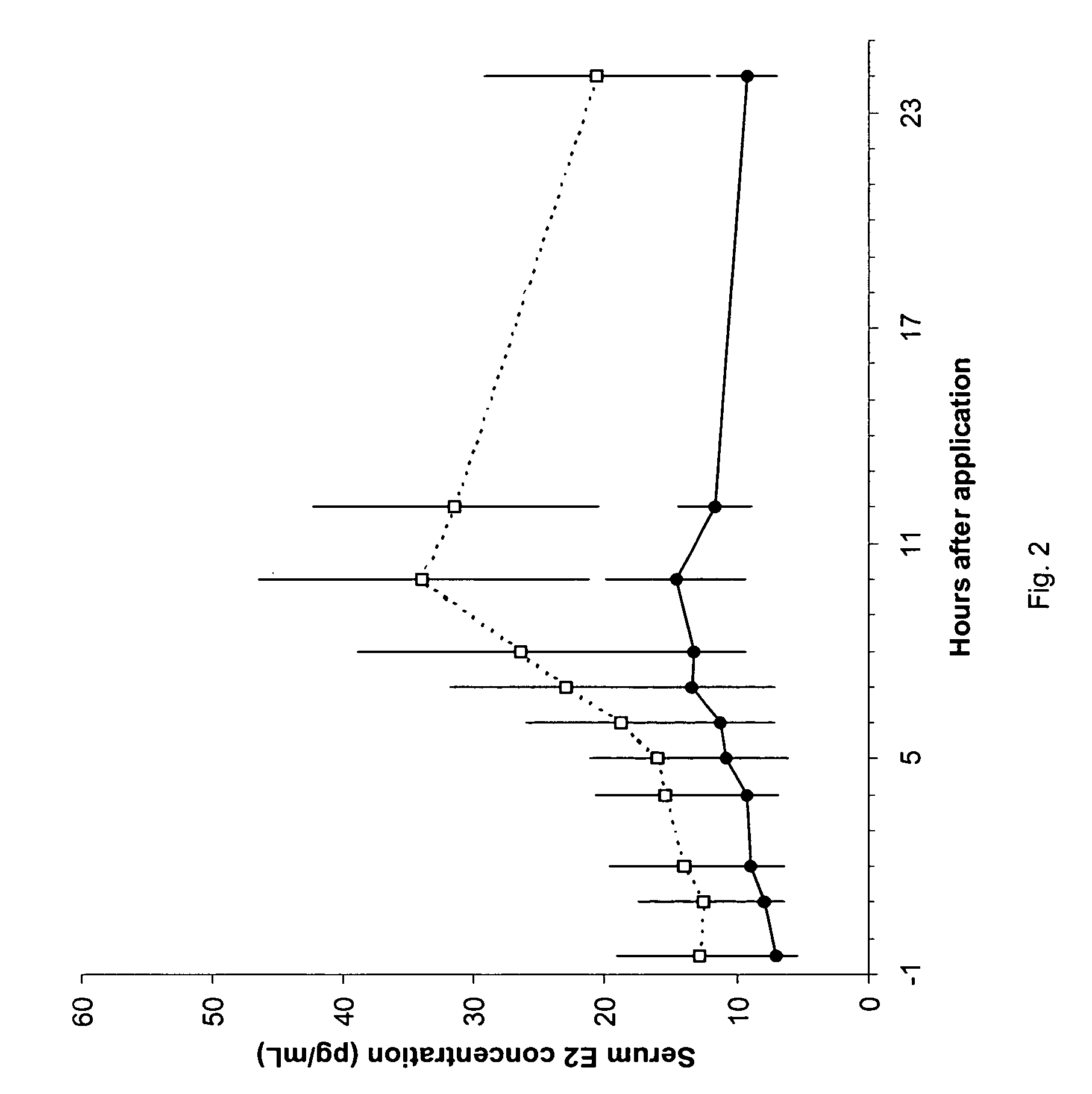
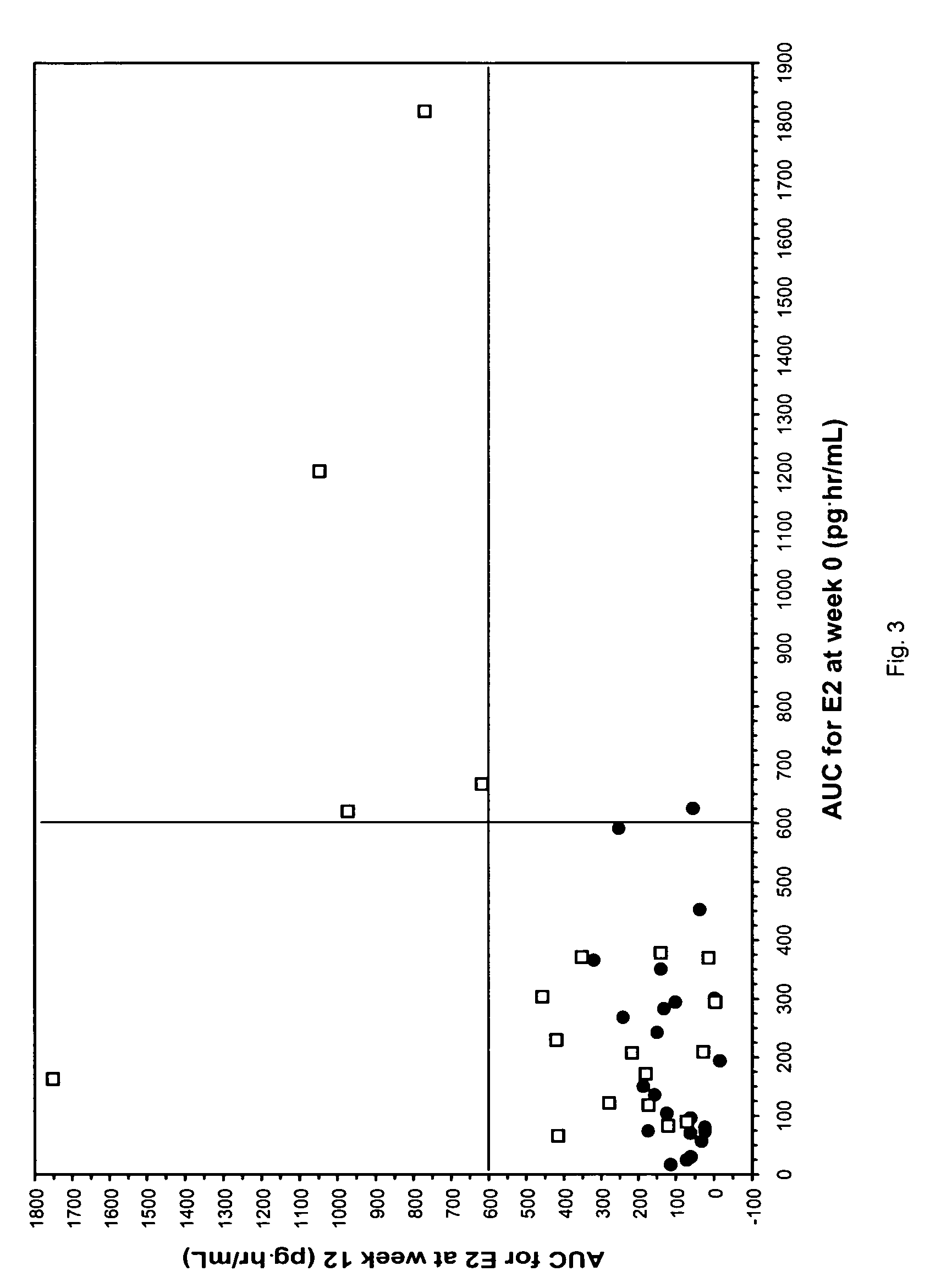
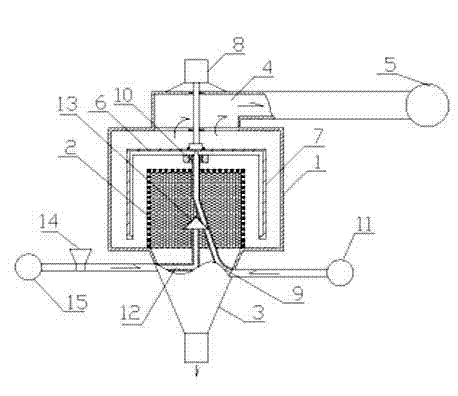
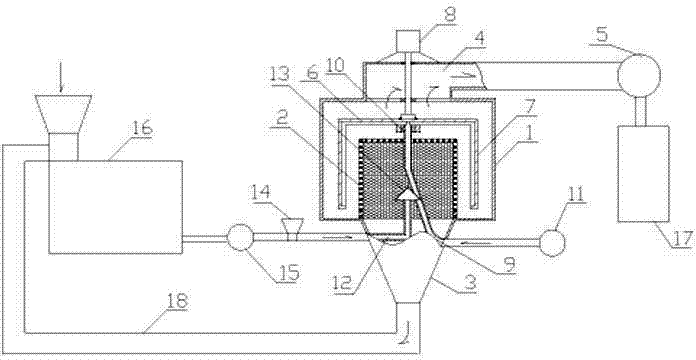
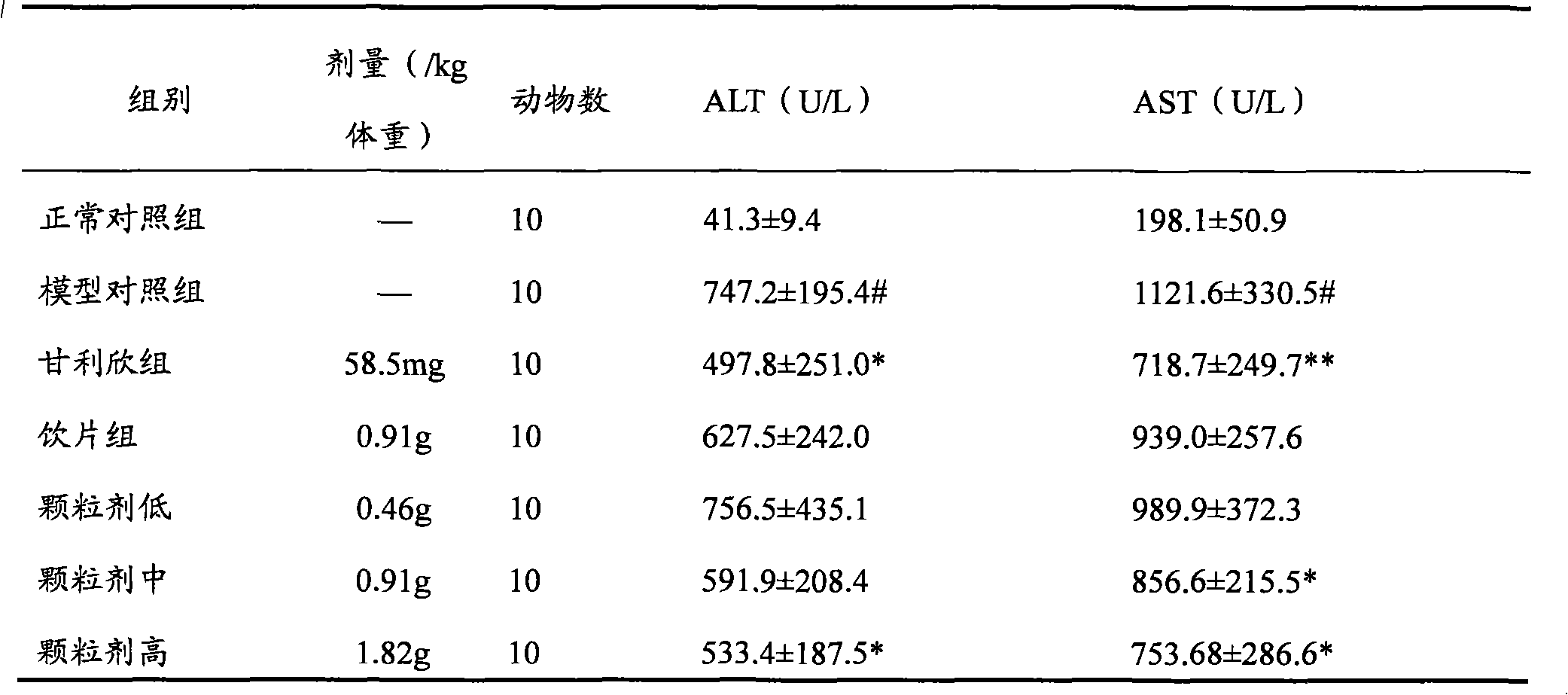
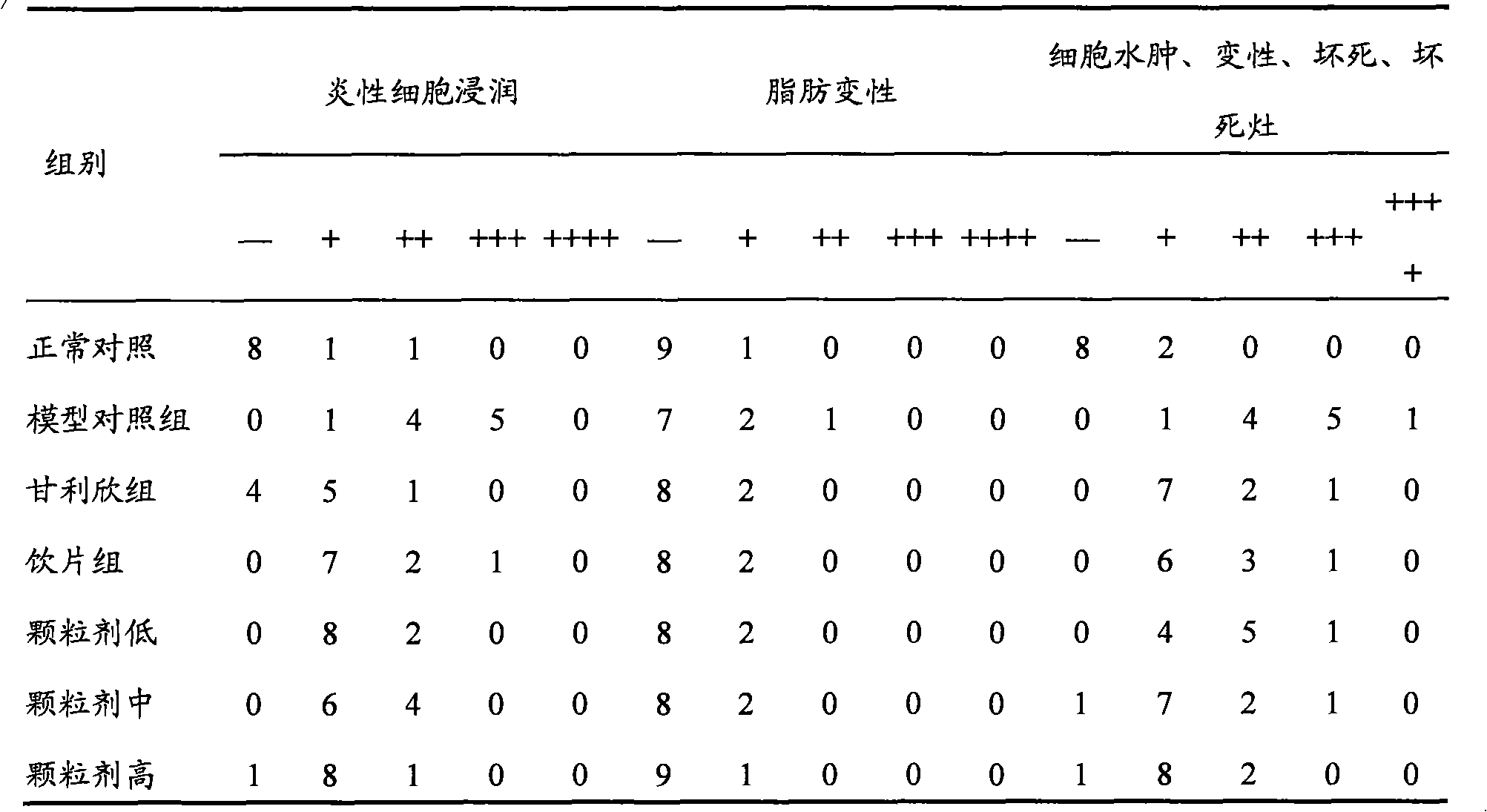
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1287results about How to "Significant clinical effect" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
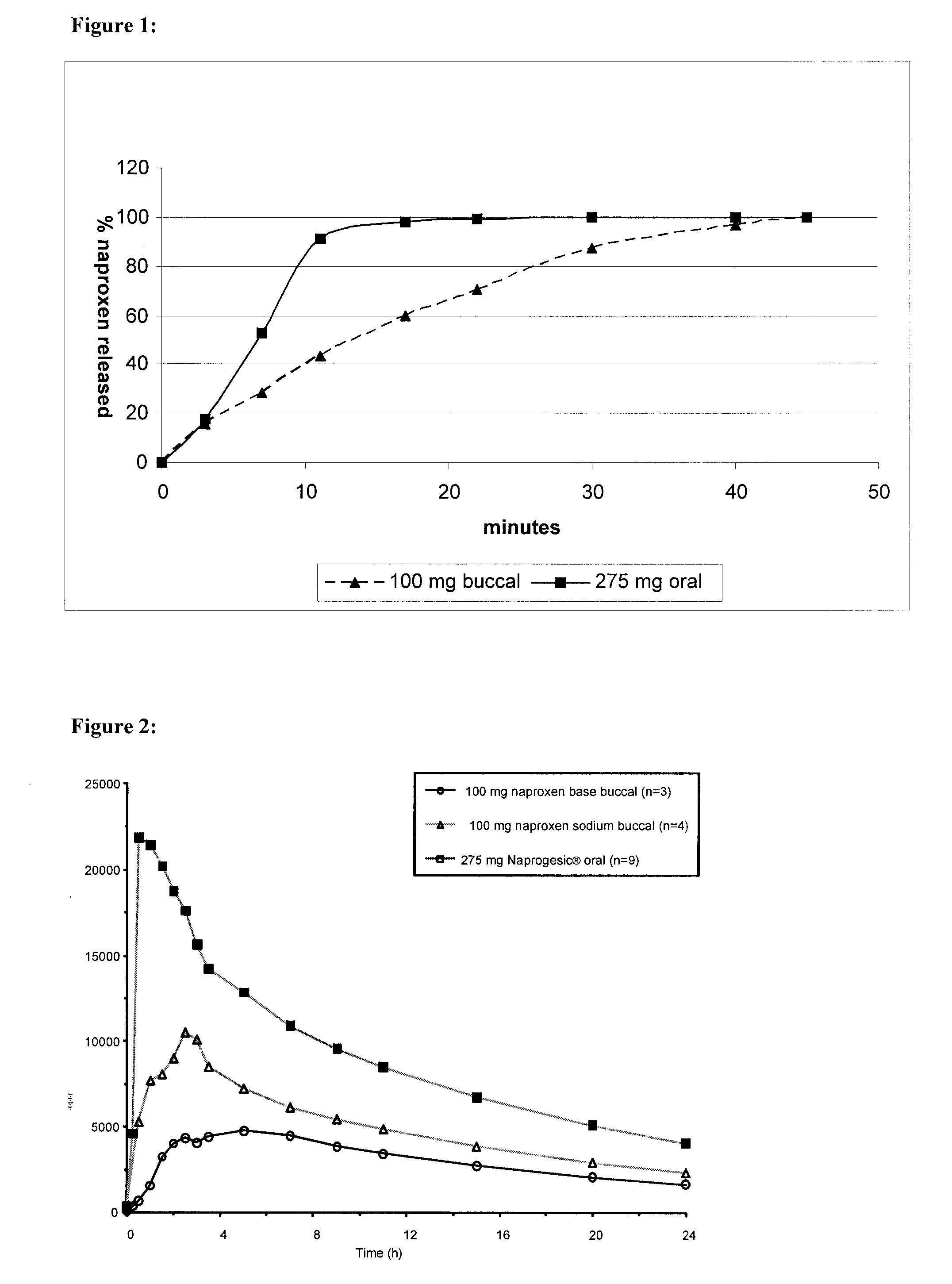
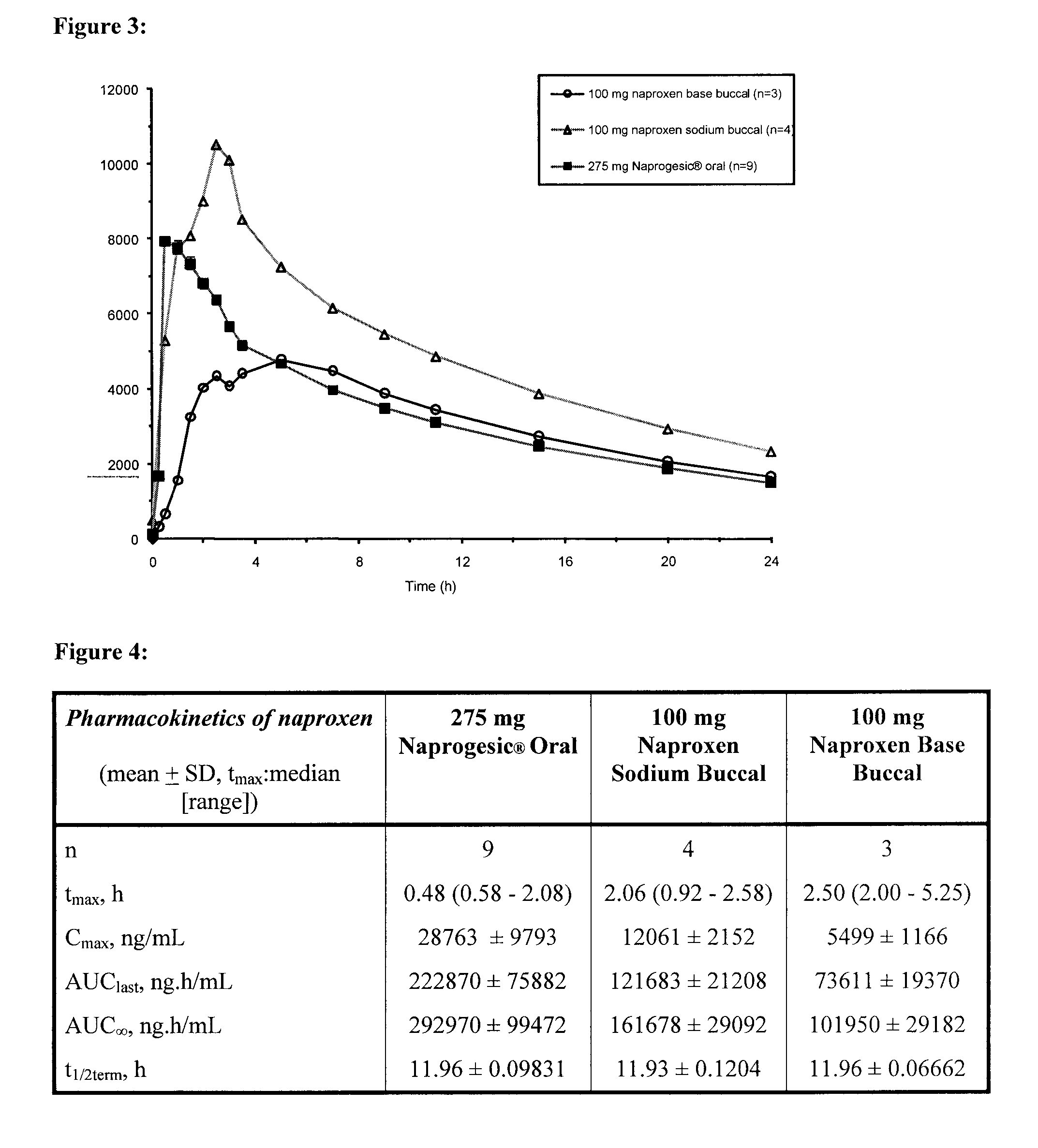
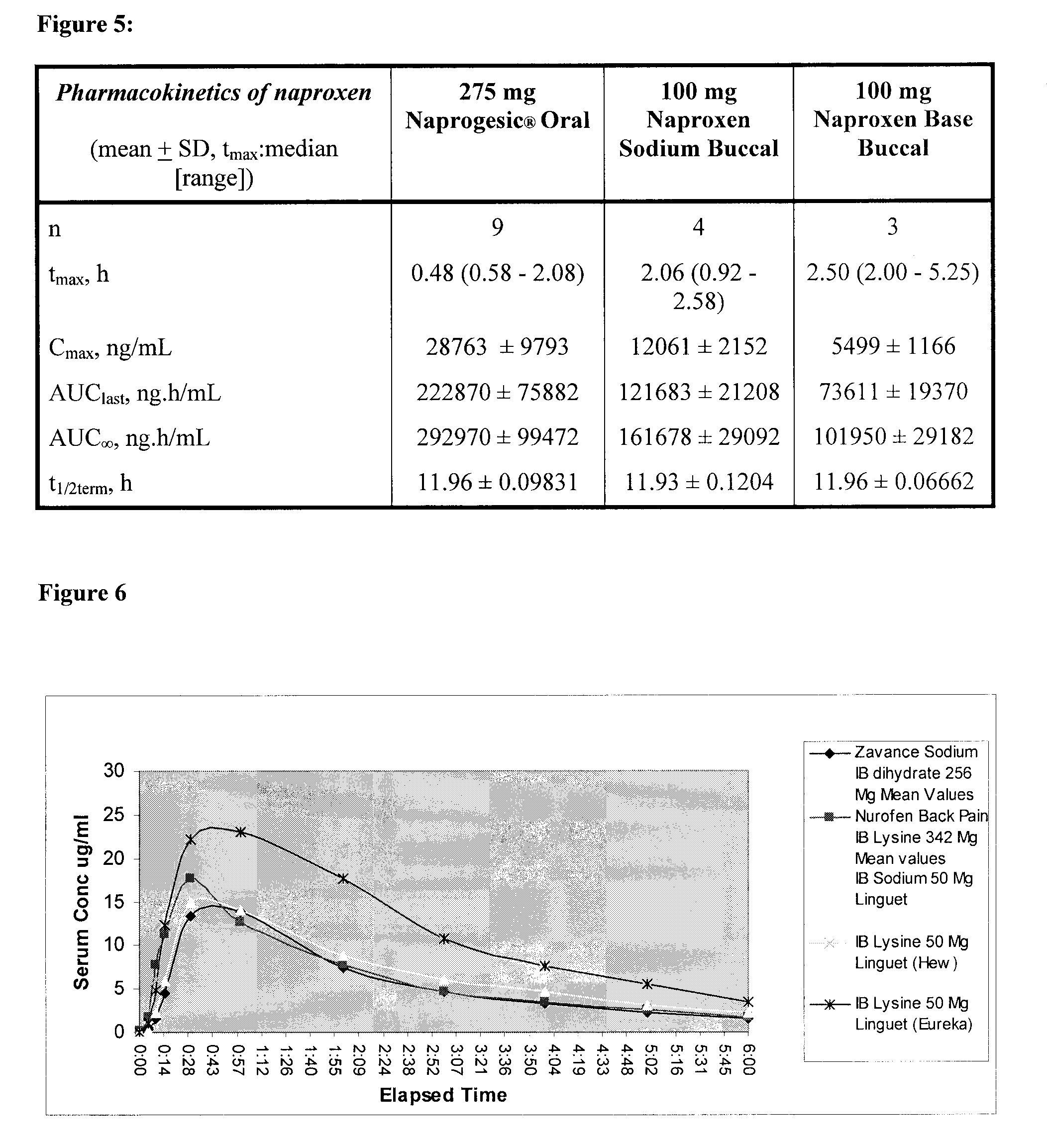
Buccal and/or sublingual therapeutic formulation
InactiveUS20120058962A1Improve release kineticsIncrease deliveryBiocideOrganic non-active ingredientsTreatment effectActive compound
Owner:LINGUAL CONSEGNA
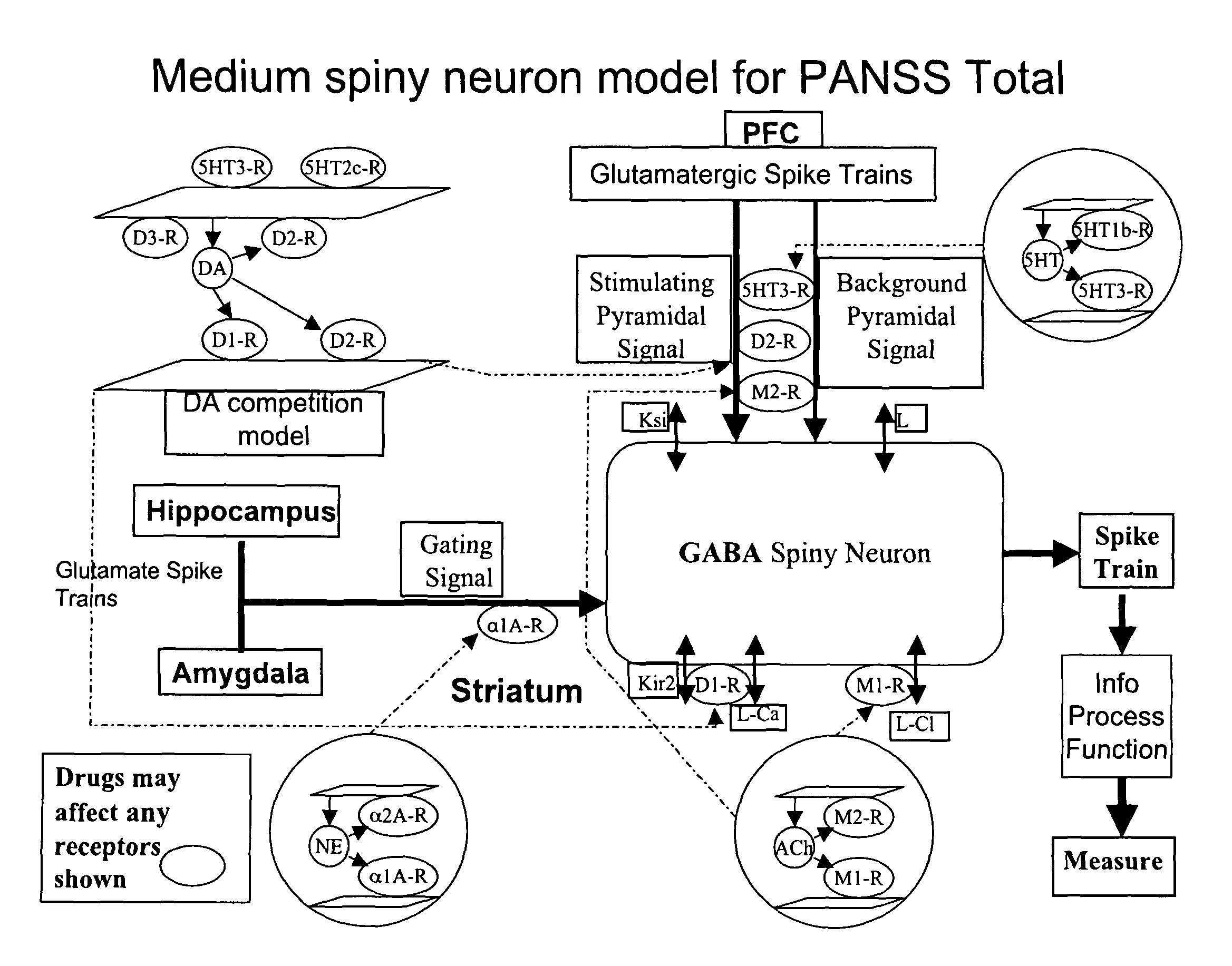
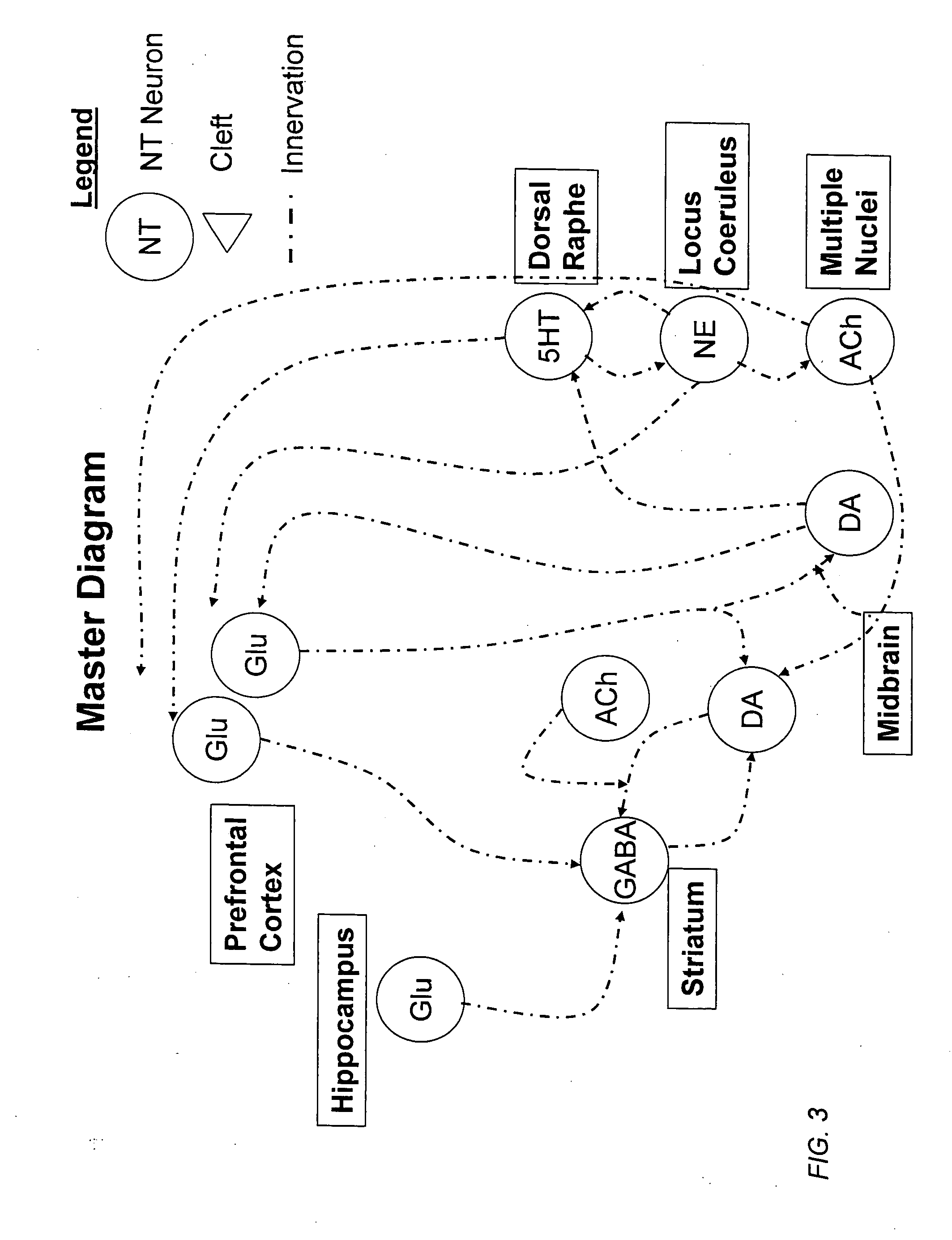
Method and apparatus for computer modeling of the interaction between and among cortical and subcortical areas in the human brain for the purpose of predicting the effect of drugs in psychiatric and cognitive diseases
ActiveUS8150629B2Easy to set upImprove clinical outcomesMedical simulationAnalogue computers for chemical processesSubstance abuserAmygdala
Computer modeling of interactions between and among cortico and subcortical areas of the human brain, for example in a normal and a pathological state resembling schizophrenia which pathological state has inputs representing the effects of a drug(s), for the purpose of using the outputs to predict the effect of drugs in psychiatric and cognitive diseases on one or more clinical scales. Diseases that can be modeled include psychiatric disorders, such as schizophrenia, bipolar disorder, major depression, ADHD, autism, obsessive-compulsive disorder, substance abuse and cognitive deficits therein and neurological disorders such as Alzheimer's disease, Mild Cognitive impairment, Parkinson's disease, stroke, vascular dementia, Huntington's disease, epilepsy and Down syndrome. The computer model preferably uses the biological state of interactions between and among cortico and subcortical areas of the human brain, to define the biological processes related to the biological state of the generic synapse model, the striatum, Locus Coeruleus, Dorsal raphe, hippocampus, amygdala and cortex, as well as certain mathematical relationships related to interactions among biological variables associated with the biological processes.
Owner:CERTARA USA INC
Hormone composition
InactiveUS20030064975A1Relief of vaginal symptomDecreased vaginal pHOrganic active ingredientsHormones regulationSelf-administration
Owner:NOVO NORDISK AS
Hormone composition
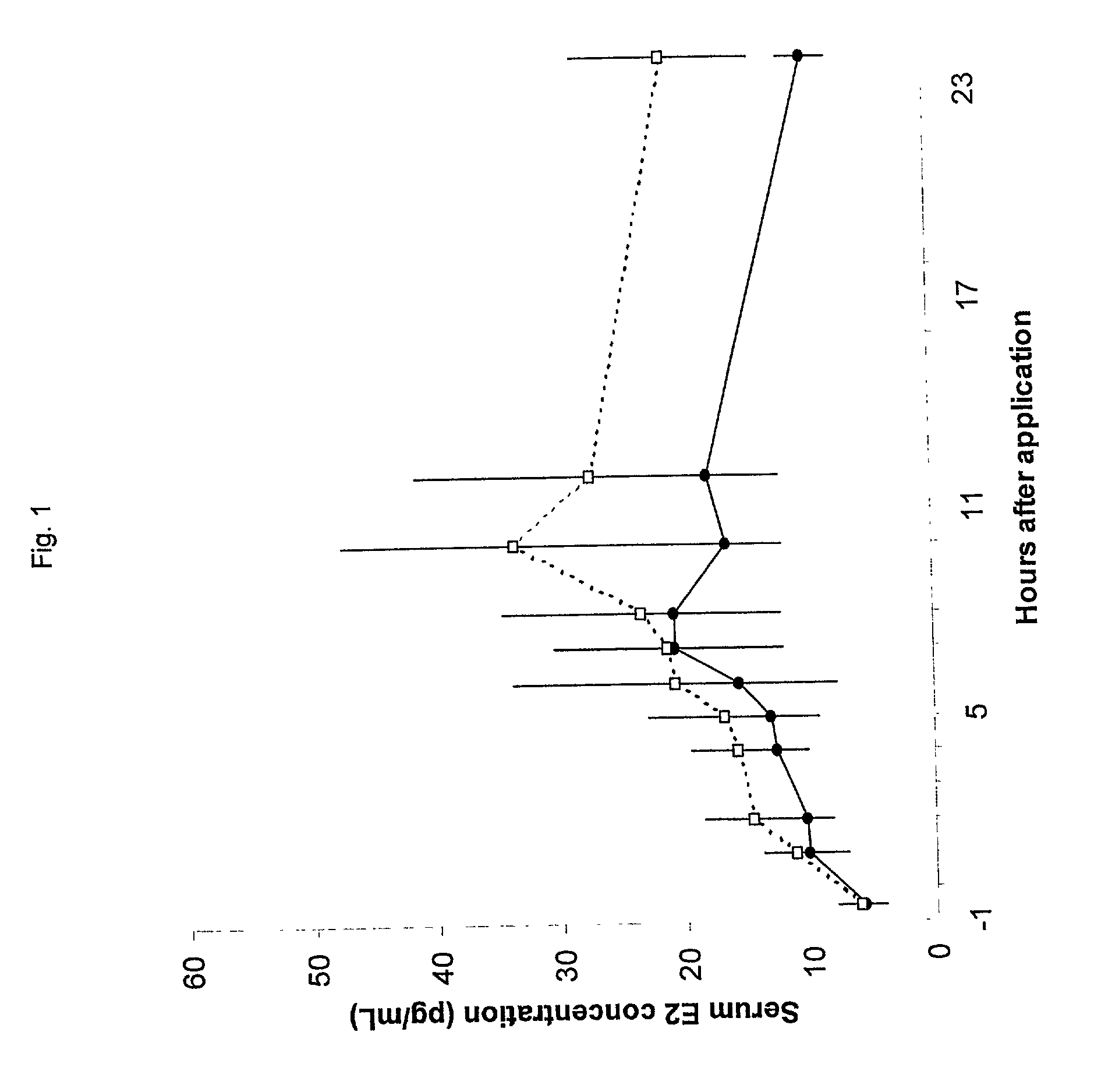
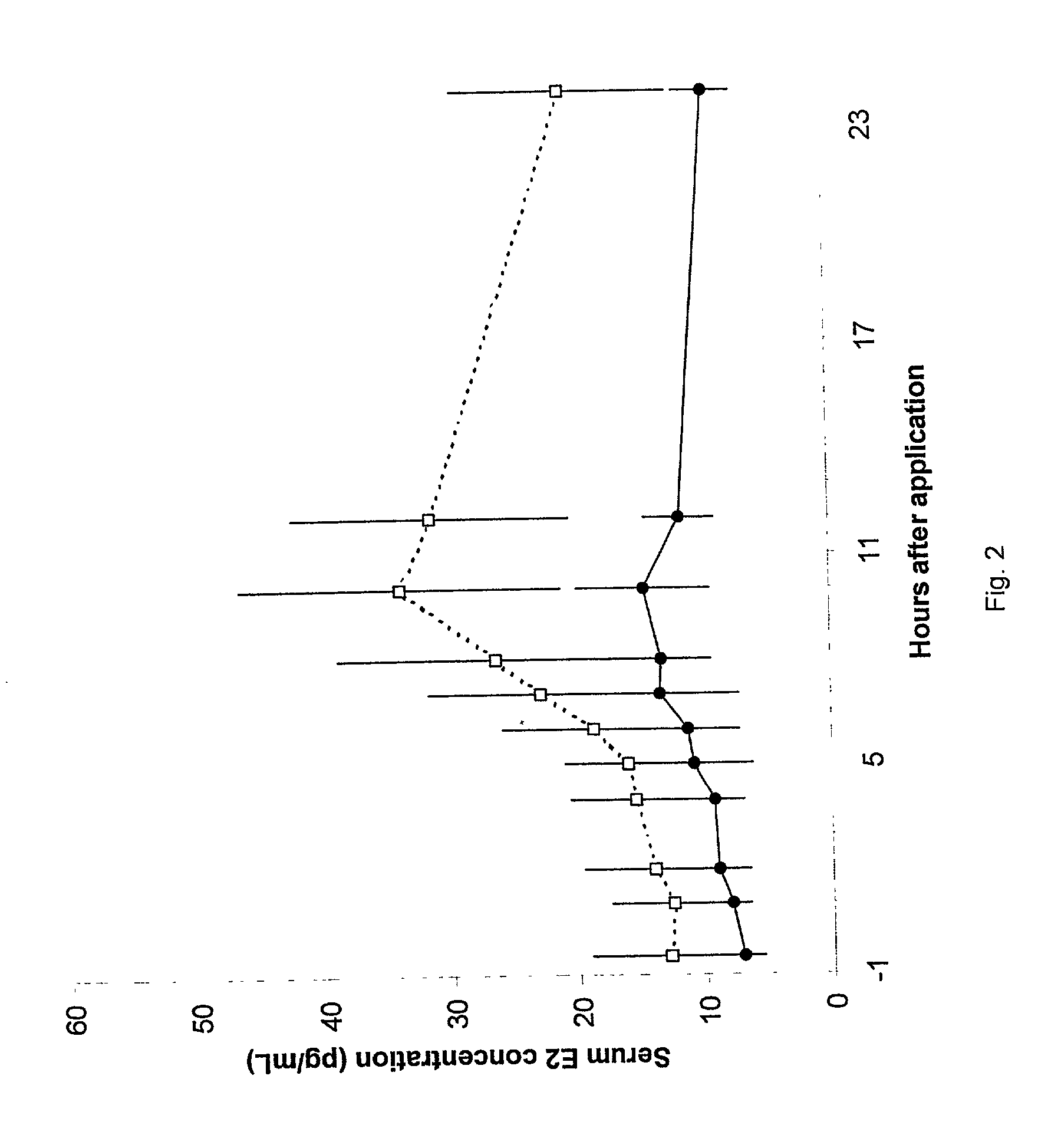
InactiveUS20050209209A1Significant clinical effectReduce absorptionOrganic active ingredientsHormones regulation
Owner:NOVO NORDISK FERNCARE A G
Process for producing immunoglobulins for intravenous administration and other immunoglobulin products
InactiveUS7138120B2Improve administeringAdminister intravenouslySerum immunoglobulinsAntiviralsChemistryIntrathecal
The present invention relates to a process for purifying immunoglobulin G from a crude immunoglobulin-containing plasma protein fraction. Said process includes a number of steps of which the anion exchange chromatography and the cation exchange chromatography are preferably connected in series. An acetate buffer having a pH of about 5.0-6.0 and having a molarity of about 5-25 mM is preferably used throughout the purification process. The invention further comprises an immunoglobulin product which is obtainable by this process. The invention also relates to an immunoglobulin product which has a purity of more than 98%, has a content of IgG monomers and dimers of more than 98.5%, has a content of IgA less than 4 mg of IgA / l, and contains less than 0.5% polymers and aggregates. Said product does not comprise detergent, PEG or albumin as a stabilizer. The product is stable, virus-safe, liquid and ready for instant intravenous administration.
Owner:CSL BEHRING AG
Multi-chamber, Multi-formulation Fluid Delivery System
ActiveUS20150128873A1Reduced squeeze-strength requirementShorten the timeLiquid surface applicatorsAutomatic syringesMedicineActive ingredient
The application discloses a multi-chamber, multi-formulation fluid delivery system, comprising a multi-chamber applicator in fluid communication with multi-chamber packaging. Applicators according to the instant disclosure have reduced squeeze-strength requirements, and are useful for dispensing fluids, including medicaments, to animals, including livestock animals. The multi-chamber packaging provides separate storage for formulations containing incompatible active ingredients, and is suitable for use with the multi-chamber applicator. The application also discloses methods for using the system to simultaneously deliver multiple active ingredients, at least some of which are not suitable for co-formulation, thus reducing the time, economic burden and animal stress involved with applying multiple, separate formulations to animals.
Owner:MERIAL INC
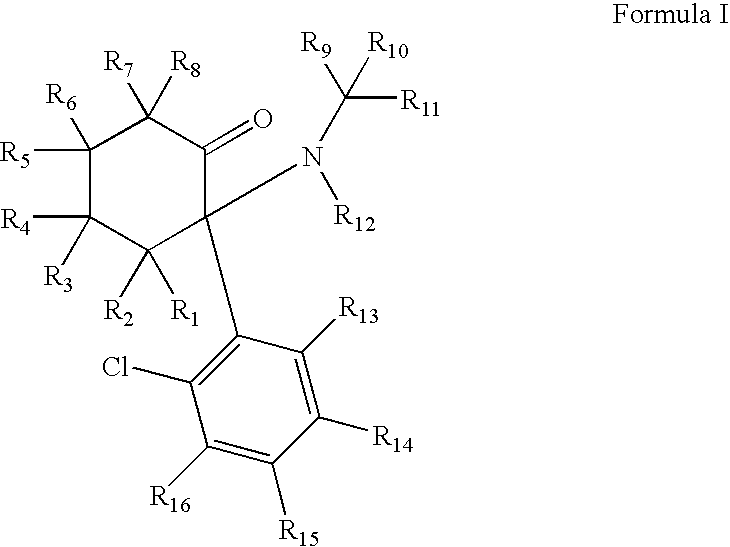
Substituted cyclohexanones
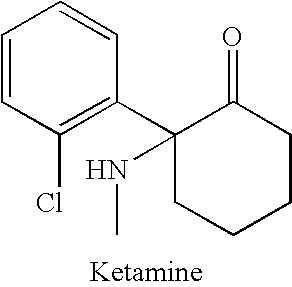
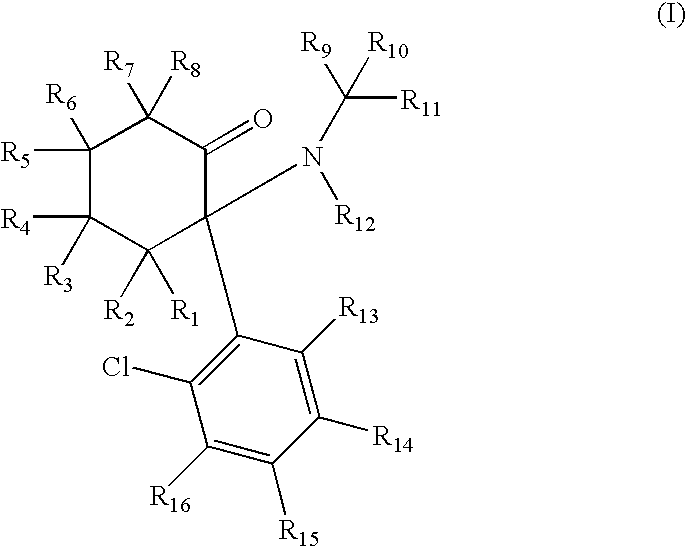
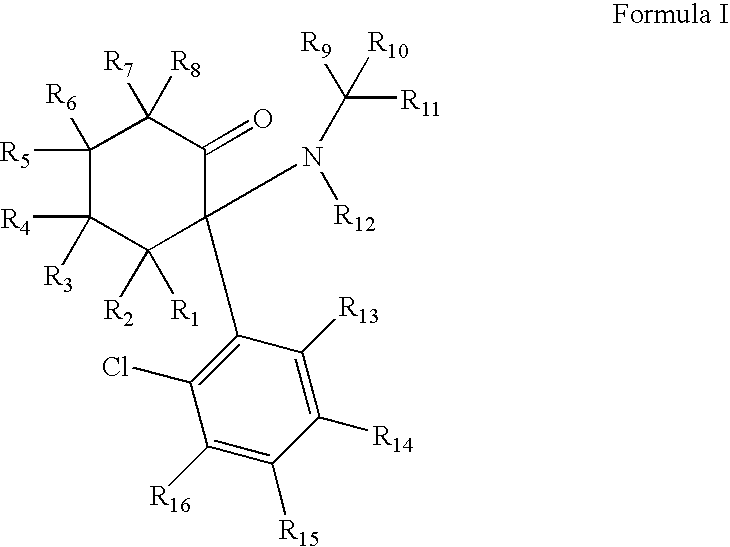
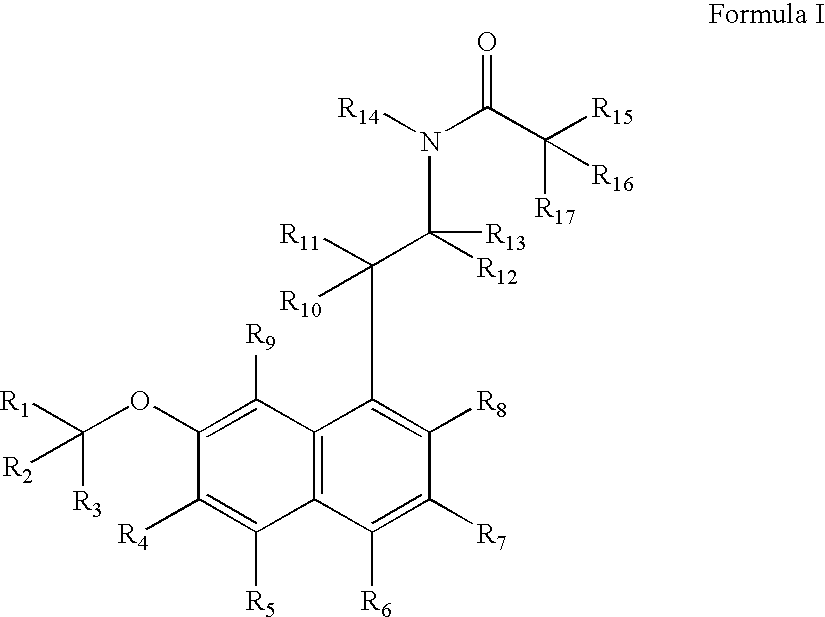
ActiveUS20080268071A1Significant clinical effectAntibacterial agentsBiocideNMDA receptor modulatorCyclohexanone
Disclosed herein are substituted cyclohexanone-based NMDA receptor modulators of Formula I, process of preparation thereof, pharmaceutical compositions thereof, and methods of use thereof.
Owner:CLEXIO BIOSCIENCES LTD
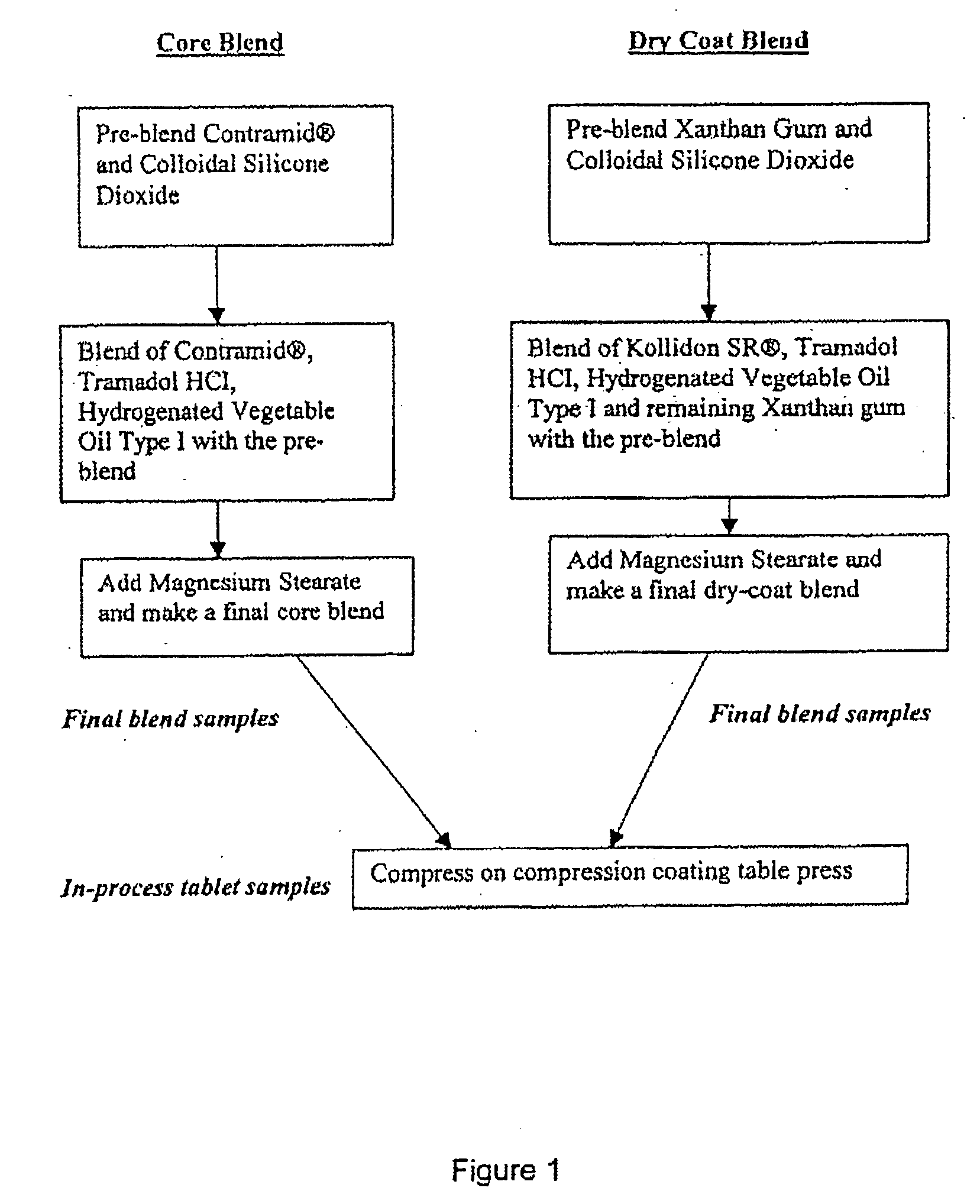
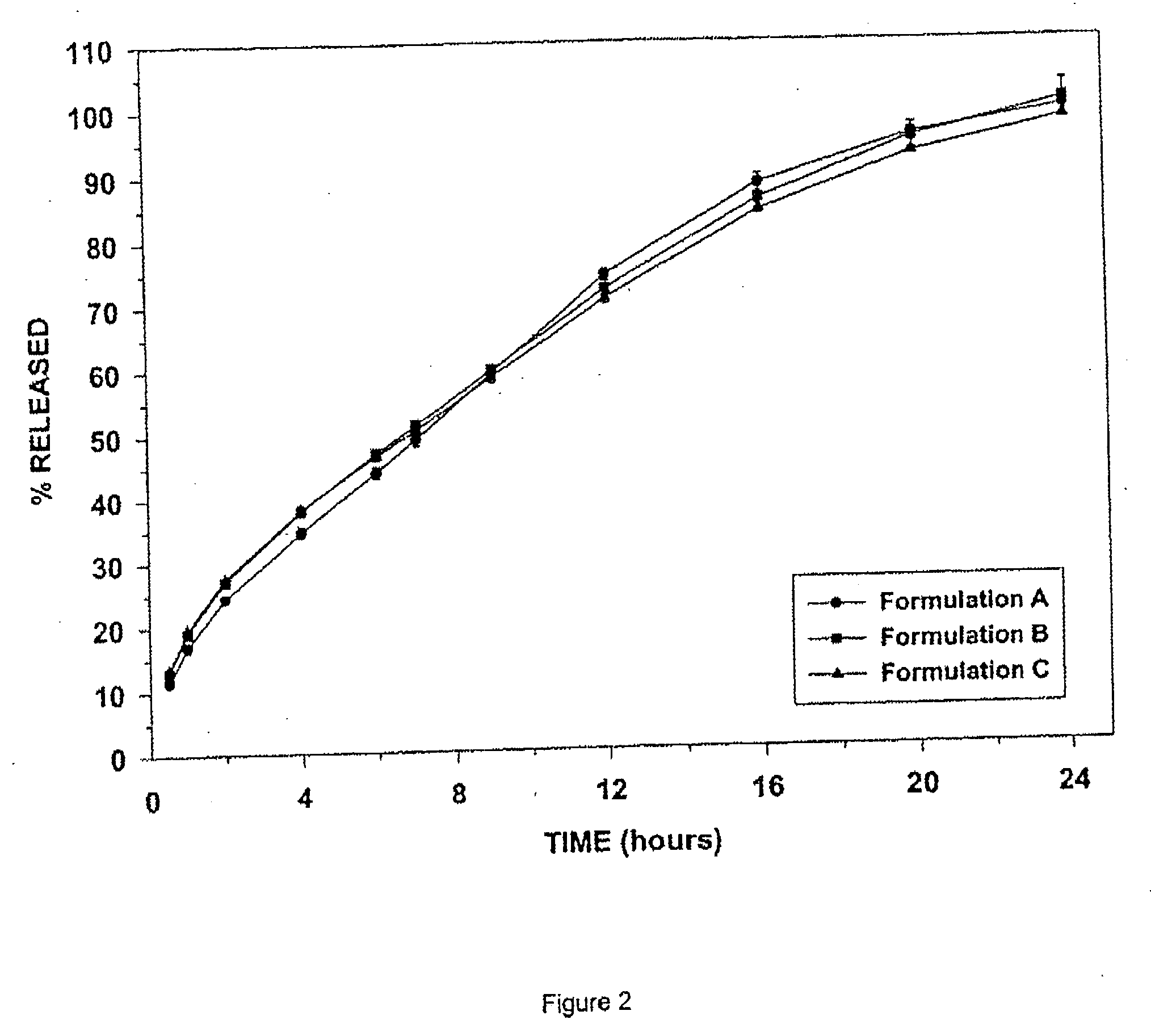
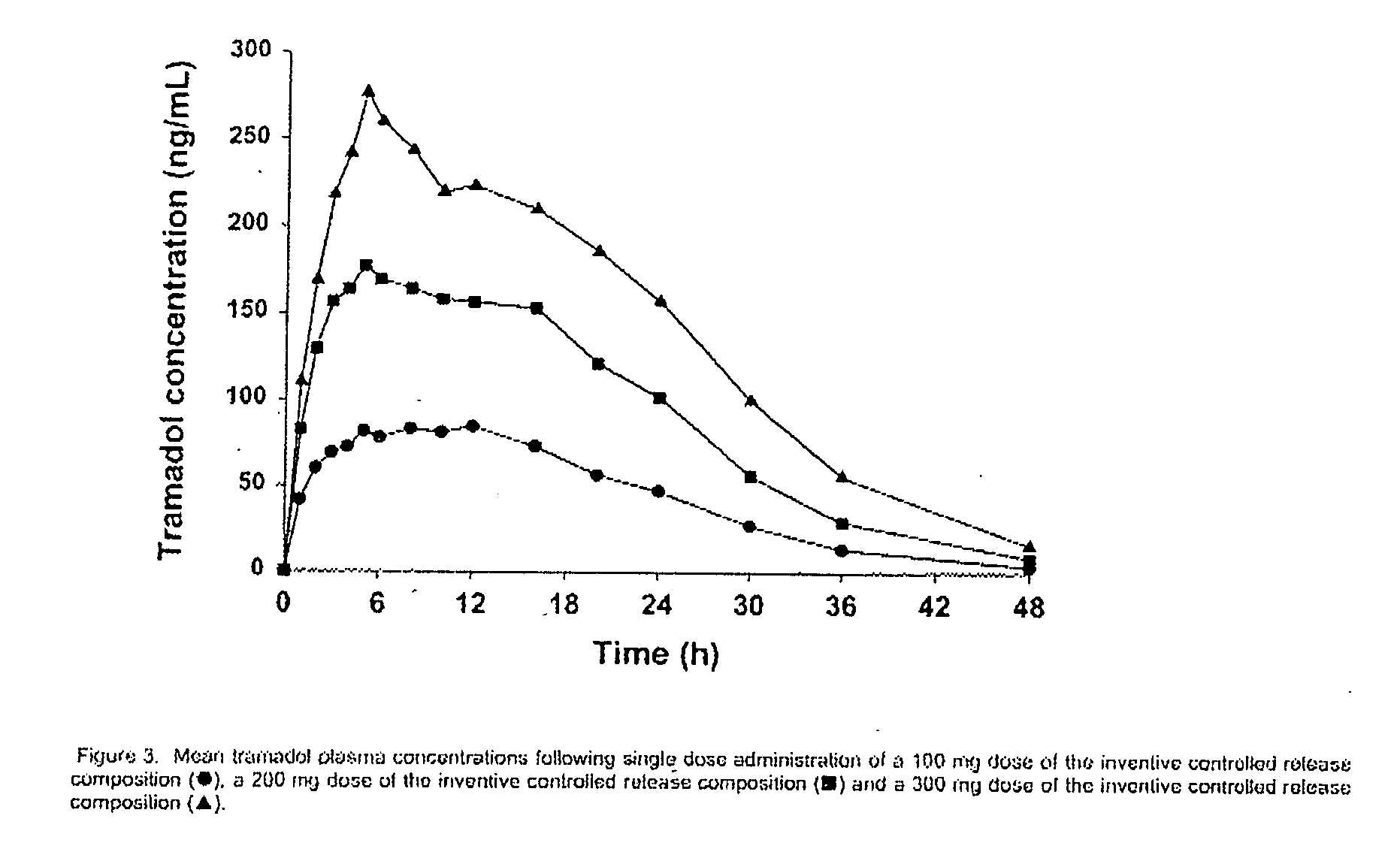
Sustained-release tramadol formulations with 24-hour clinical efficacy
InactiveUS20060172006A1Significant clinical effectOrganic active ingredientsNervous disorderControlled releaseClinical efficacy
There is disclosed a once daily oral pharmaceutical compositon for controlled release of tramadol or a salt thereof, wherein the composition, when ingested orally, provides a clinical effect over 24 hours which is a least as good as the clinical effect over 24 hours of two doses of a twice daily oral pharmaceutical composition for controlled release of tramadol, taken 12 hours apart
Owner:LABOPHARM BARBADOS
Moderate skin cream containing multiple kinds of pure natural plant extracts
ActiveCN103251530AExcitingInhibit inflammationCosmetic preparationsToilet preparationsInflammatory factorsCentella asiatica extract
The invention discloses a moderate skin cream containing multiple kinds of pure natural plant extracts. A pure natural green tea extract, a liquorice root extract, a rosmarinus officinalis leaf extract, a scutellaria baicalensis root extract, a chrysanthemum extract and a centella asiatica extract are added in raw materials of the skin cream, and all have effects of resisting inflammation and adjusting immunity; the skin cream is capable of completely inhibiting a dozen of inflammatory factors, rapidly stopping skin inflammations caused by various reasons and inhibiting malignant linkage physiological reactions in all stages of the inflammations, has an effect of sephalosprins and is safe without side effects, also has cooperative effects of resisting oxidization, soothing and preserving moisture, and especially has a remarkable clinical effect for nursing of sensitive skin and skin of infants; and the six kinds of selected pure natural plant extracts are added in the skin cream, so that the symptoms such as inflammation and allergies caused by factors such as climate, environment and stimulating substances of the infants can be greatly reduced, the tender and lovely skin of the infants is protected, and thus the moderate skin cream is suitable for being used by the infants.
Owner:FUJIAN MENGJIAOLAN DAILY CHEM
Medicament for treating gynecological inflammation and preparation method thereof
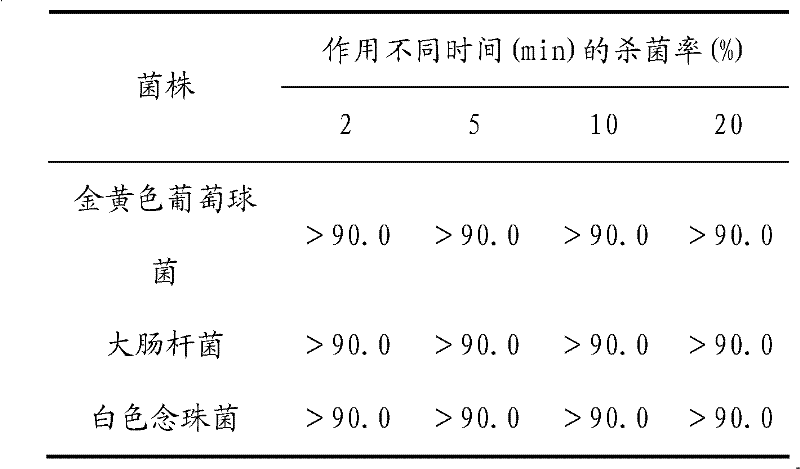
ActiveCN102160887AGood killing effectHas the effect of treating female genital itchingHydroxy compound active ingredientsSexual disorderEscherichia coliChlorhexidine Acetate
The invention provides a medicament for treating gynecological inflammation, which comprises the following components in parts by weight: 5 to 30 parts of common cnidium fruit, 4 to 30 parts of golden cypress, 4 to 30 parts of lightyellow sophora root, 5 to 30 parts of sessile stemona root, 0.1 to 2 parts of borneol, 0.1 to 2 parts of menthol, 0.1 to 2 parts of chlorhexidine acetate, and 0.1 to 2 parts of vitamin E. Compared with the prior art, the invention adopts a compound preparation consisting of classical Chinese medicaments and western medicaments, and has the characteristics of quickly killing pathogenic bacteria of western medicaments as well as diminishing inflammation and resisting bacteria of Chinese medicaments. Microbiological experiments and toxicant experiments prove that: the Chinese medicinal preparation has an excellent killing effect on pathogenic bacteria causing gynecological inflammation, such as staphylococcus aureus, candida albicans, escherichia coli and other pathogens, has the effects of treating female pruritus vulvae, has an accurate curative effect, low toxic or side effect, excellent functions of inflammation diminishing, sterilization and itching relieving.
Owner:金日制药(中国)有限公司
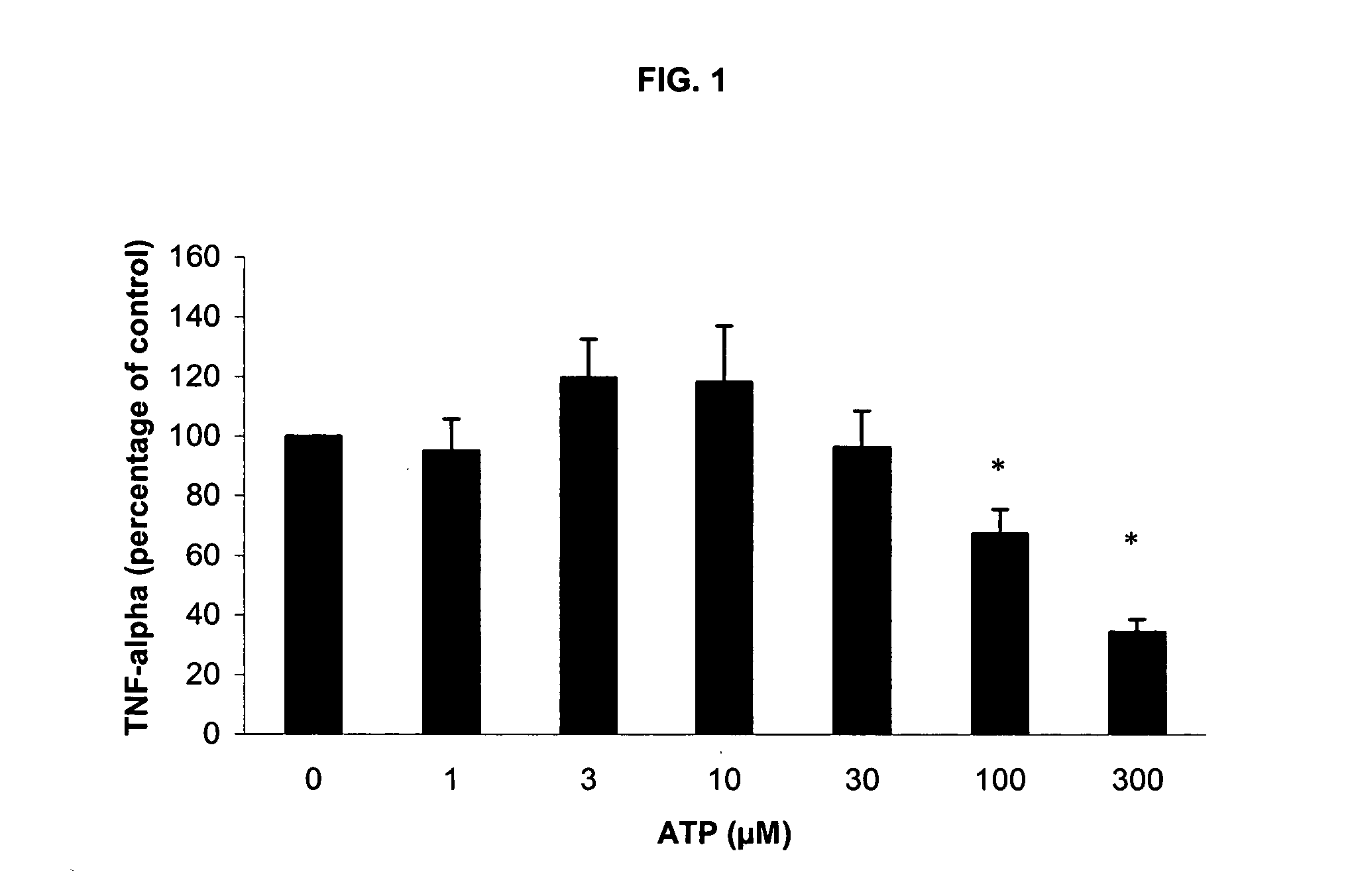
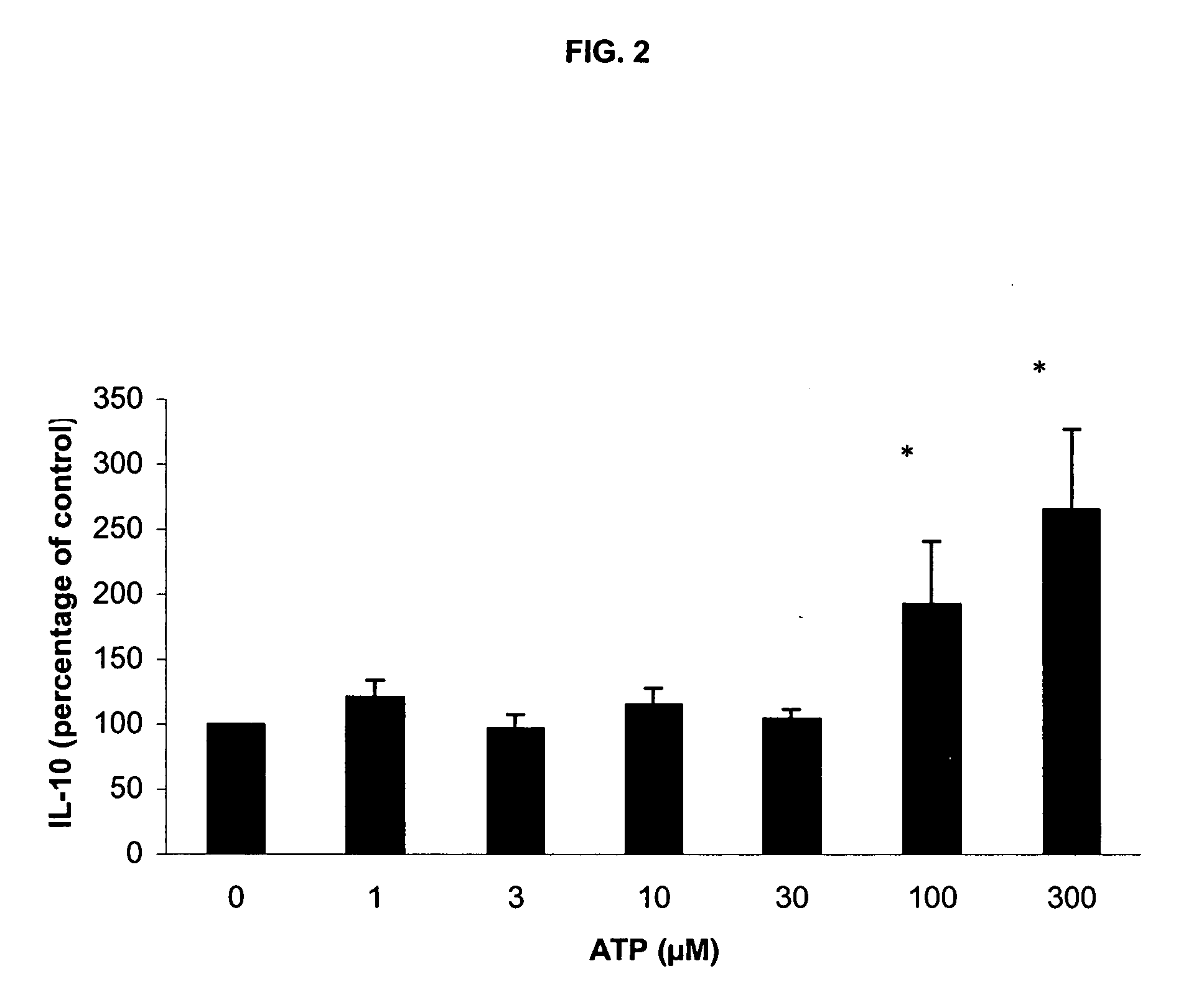
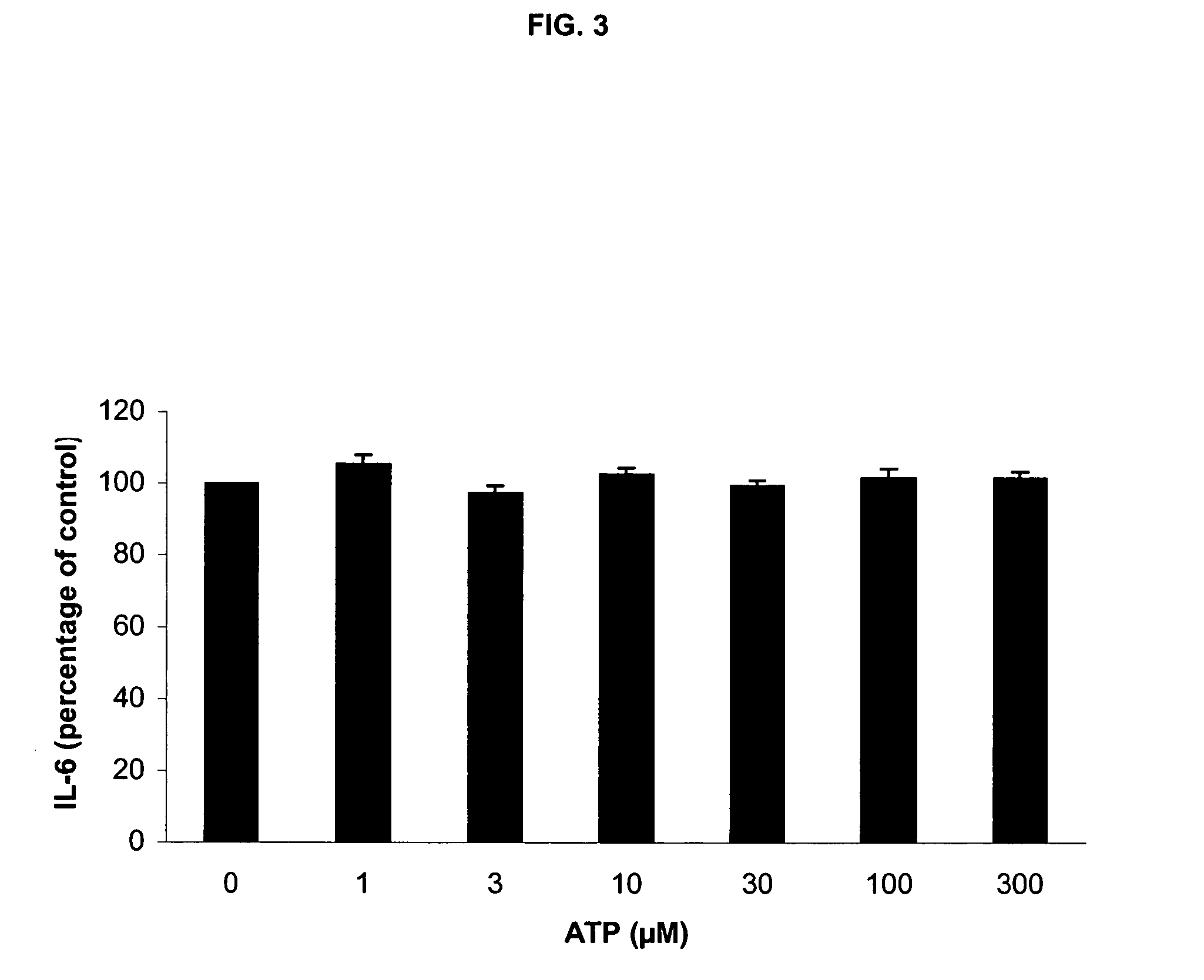
Use of ATP for the manufacture of a medicament for treating certain inflammatory conditions, oxidative stress and fatigue
InactiveUS20050261239A1Avoid problemsSignificant clinical effectBiocidePowder deliveryIntestinal structureDisease
The present invention provides the use of ATP for the manufacture of a medicine for exerting a pharmacological effect when administered to a mammal, preferably a human, selected from the group consisting of: 1°. modulating inflammation by inhibiting the inflammatory response to a strong external insult such as endotoxin (LPS) and / or phytohaemagglutinin; 2°. exerting said inhibitory effect on inflammatory response to an external stimulus even under conditions of oxidative stress, 3°. exerting a local immuno-modulating and anti-inflammatory effect in the intestine, thus preventing intestinal damage induced by a non-steroid anti-inflammatory drug (NSAIDs), 4°. exerting an immuno-modulating and anti-inflammatory effect in human intestinal cells in vitro, 5°. alleviating pulmonary symptoms, such as shortness of breath and dyspnoea, in patients suffering from an obstructive pulmonary disease, and 6°. exerting favorable clinical effects with respect to certain mental and neurological disorders and aberrant conditions. The medicine is preferably manufactured in lyophilized form.
Owner:MAASTRICHT UNIVERSITY
Method and apparatus for computer modeling of the interaction between and among cortical and subcortical areas in the human brain for the purpose of predicting the effect of drugs in psychiatric & cognitive diseases
ActiveUS20070106479A1Easy to set upImprove clinical outcomesMedical simulationAnalogue computers for chemical processesSubstance abuserHuntingtons chorea
Computer modeling of interactions between and among cortico and subcortical areas of the human brain, for example in a normal and a pathological state resembling schizophrenia which pathological state has inputs representing the effects of a drug(s), for the purpose of using the outputs to predict the effect of drugs in psychiatric and cognitive diseases. A method is provided for developing a computer model of interactions between and among cortico and subcortical areas of the human brain which comprises the steps of identifying data relating to a biological state of a generic synapse model, the striatum, Locus Coeruleus, Dorsal raphe, hippocampus, amygdala and cortex; identifying biological processes related to the data, these identified biological processes defining at least one portion of the biological state of the generic synapse model, the striatum, Locus Coeruleus, Dorsal raphe, hippocampus, amygdala, and cortex; and combining the biological processes to form a simulation of the biological state of interactions between and among cortico and subcortical areas of the human brain. Diseases that can be modeled include psychiatric disorders, such as schizophrenia, bipolar disorder, major depression, ADHD, autism, obsessive-compulsive disorder, substance abuse and cognitive deficits therein and neurological disorders such as Alzheimer's disease, Mild Cognitive impairment, Parkinson's disease, stroke, vascular dementia, Huntington's disease, epilepsy and Down syndrome. A resulting computer model is of the biological state of interactions between and among cortico and subcortical areas of the human brain, comprising code to define the biological processes related to the biological state of the generic synapse model, the striatum, Locus Coeruleus, Dorsal raphe, hippocampus, amygdala and cortex, and code to define the mathematical relationships related to interactions among biological variables associated with the biological processes. At least two of the biological processes are associated with the mathematical relationships. A combination of the code to define the biological processes and the code to define the mathematical relationships define a simulation of the biological state of the interactions between and among cortico and subcortical areas of the human brain. Computer executable software code is provided comprised of code to define biological processes related to a biological state of interactions between and among cortico and subcortical areas of the human brain including code to define mathematical relations associated with the biological processes. A computer model of interactions between and among cortico and subcortical areas of the human brain is provided, comprising a computer-readable memory storing codes and a processor coupled to the computer-readable memory, the processor configured to execute the codes. The memory comprises code to define biological processes related to the biological state of interactions between and among cortico and subcortical areas of the human brain, and code to define mathematical relationships related to interactions among biological variables associated with the biological processes.
Owner:CERTARA USA INC
Substituted cyclohexanones
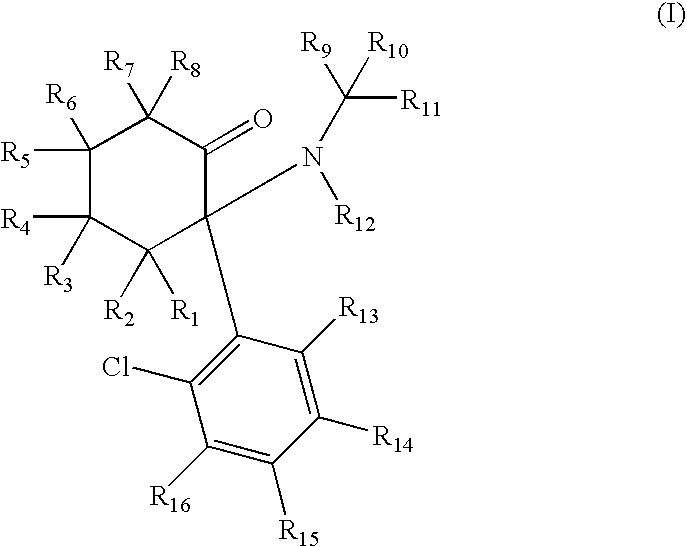
ActiveUS7638651B2Significant clinical effectAntibacterial agentsBiocideCyclohexanoneNR1 NMDA receptor
Disclosed herein are substituted cyclohexanone-based NMDA receptor modulators of Formula I, process of preparation thereof, pharmaceutical compositions thereof, and methods of use thereof.
Owner:CLEXIO BIOSCIENCES LTD
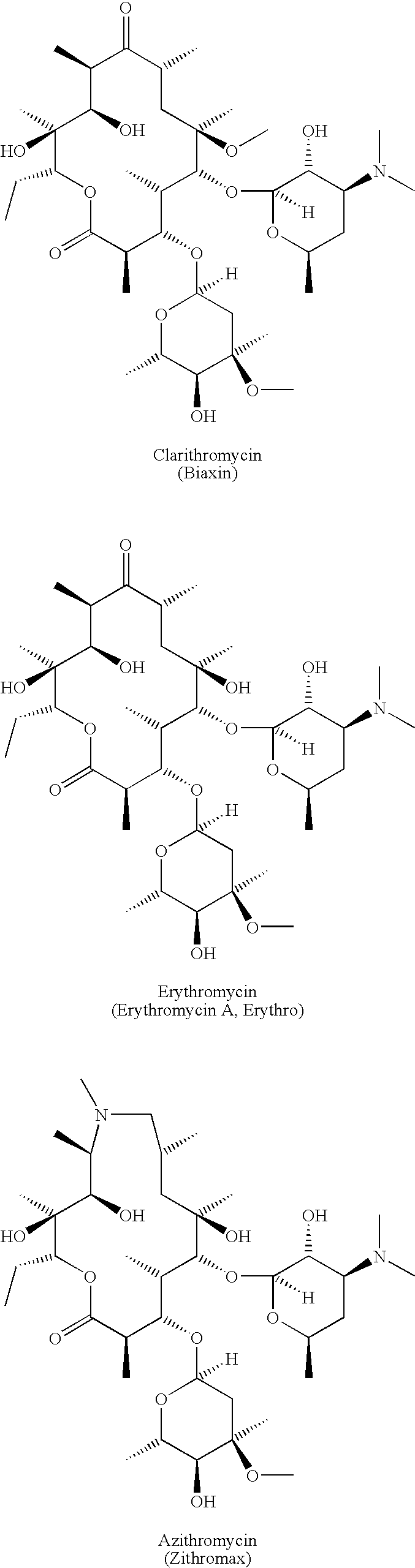
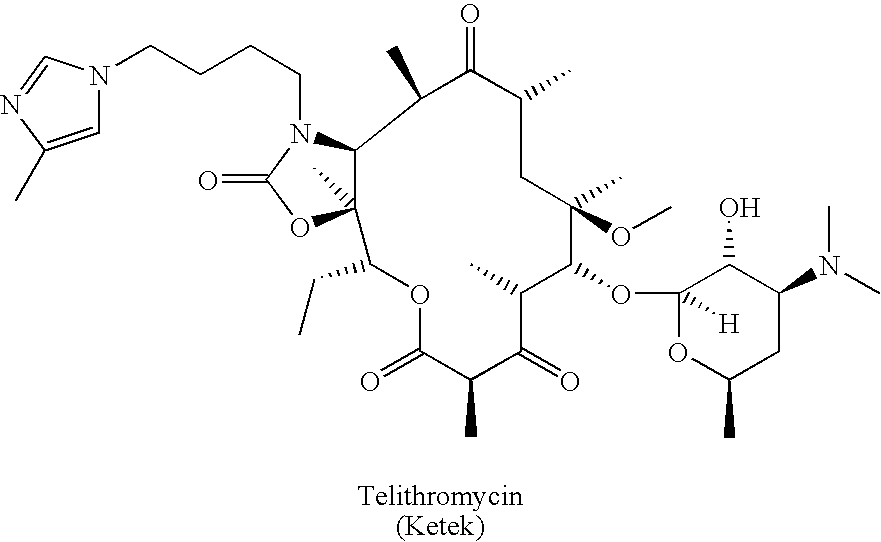
Preparation and utility of substituted erythromycin analogs
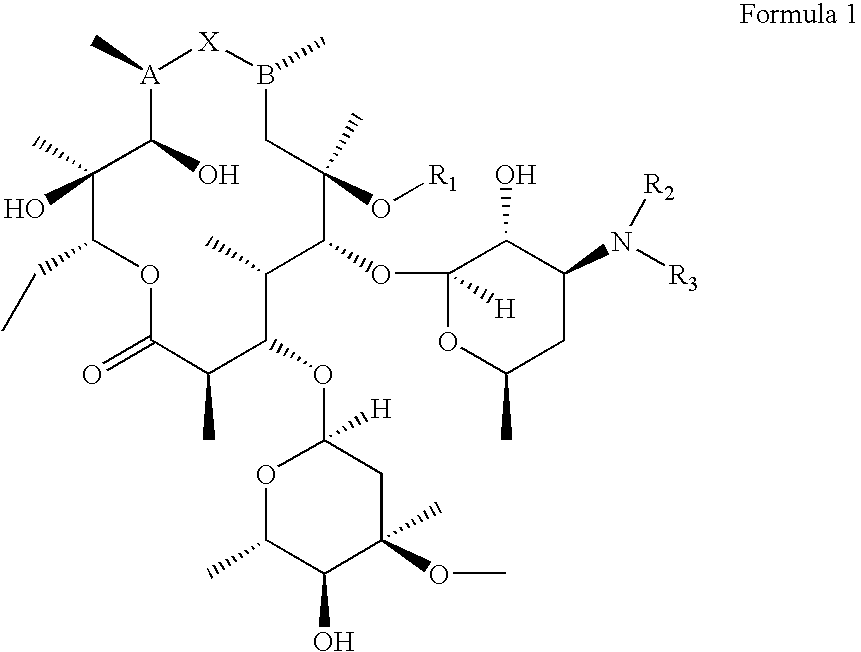
InactiveUS20070281894A1Decreased inter-individual variation in plasma levelsSignificant clinical effectAntibacterial agentsBiocideChemical synthesisMicroorganism
The present disclosure is directed to novel macrolide antibiotics of Formula 1 and pharmaceutically acceptable salts and prodrugs thereof; and the chemical syntheses and medical uses of these novel macrolide antibiotics for the treatment and / or management of infections caused by various aerobic and anaerobic gram-positive and gram-negative microorganisms as well as various mycobacteria.
Owner:AUSPEX PHARMA INC
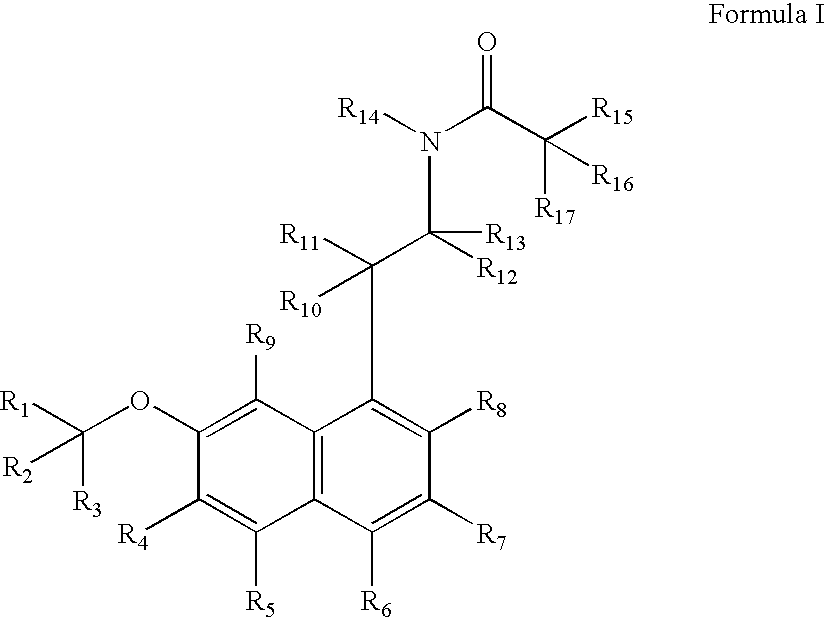
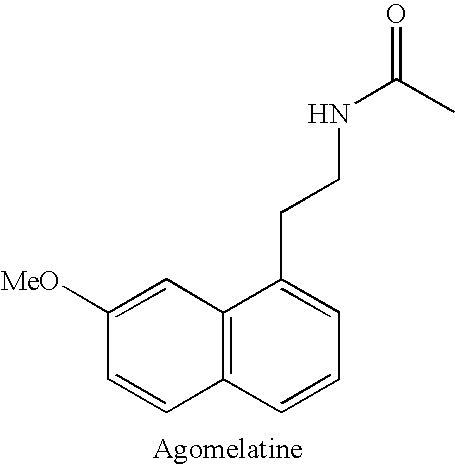
Substituted naphthalenes
Disclosed herein are substituted naphthalene-based melatonin (MT) receptor modulators and / or 5-HT receptor modulators of Formula I, process of preparation thereof, pharmaceutical compositions thereof, and methods of use thereof.
Owner:AUSPEX PHARMA INC
Chinese traditional medicine composition for treating arthritis hyperosteogeny intervertebral disc hernia ion
InactiveCN101095785AQuick resultsGood curative effectAnthropod material medical ingredientsSkeletal disorderArthritisANGELICA ROOT
The invention discloses a Chinese medicinal composition for treating arthritis, osteoproliferation and disc protrusion, which comprises the following raw material constituents (by weight ratio): large-leaf gentian root, ledebouriella root, Ligusticum wallichii, Clematis chinensis, achyranthes and cyathula root, root of herbaceous peony, prepared rehmannia root, Chinese angelica root, eucommia bark, Cinnamomum cassia, wolferry fruit, morinda root, dipsacus root, poria cocos, Chinese ephedra, Loranthus mulberry mistletoe, cassia twig, corydalis tuber, notoginseng, notopterygium root, root of red rooted saliva, tetrandra root, spatholobus stem, dried orange peel, licorice root, pangolin scales, buthus martensi karsch, earthworm, wood louse, dragon's blood resin, wasp's nest and drynaria.
Owner:韩世昌
Novel medical pH (potential of Hydrogen) electrode catheter
PendingCN107752983AImprove the stability of useImprove the quality of useCatheterDiagnostic recording/measuringHydrogenBiomedical engineering
The invention discloses a novel medical pH (potential of hydrogen) electrode catheter, which comprises a catheter, an impedance ring and conducting wires, wherein the catheter is a hollow pipe body; further, the conducting wires are arranged in the pipe body; the impedance ring is mounted at a preset position of the pipe body; the preset position where the impedance ring is mounted is provided with a wire leading hole; further, the impedance ring is connected with the conducting wires led out from the wire leading hole in a butting manner. When the novel medical pH electrode catheter is applied, the conducting wires are firstly completely threaded into the catheter; the single conducting wire is taken out from a corresponding mounting vacancy of the impedance ring; the conducting wire is wound on the catheter, and afterwards, is threaded into the impedance ring; the impedance ring is fixedly arranged at the position of the conducting wire; finally, the closing-in location is carried out on the impedance ring by adopting a necking process; thus, the assembly of the whole pH electrode catheter is completed. In comparison with a segment-by-segment connection method adopted in the prior art, the novel medical pH electrode catheter adopts the integrated assembly for the catheter and the impedance ring; the water-leakage problem is thoroughly solved and meanwhile, the pH electrode catheter is enhanced in use stability and quality and has a better clinical use effect.
Owner:重庆金山医疗技术研究院有限公司
Tibetan medicine composite for treating diabetes and preparation method of preparation thereof
InactiveCN101700397AGood curative effectSmall side effectsMetabolism disorderUnknown materialsDiabetes mellitusSide effect
The invention relates to a Tibetan medicine composite for treating diabetes, comprising the following components: 4.8-7.2g of medicine terminalia fruit, 2.4-3.6g of Chaxun ointment, 4-6g of long pepper extract, 3.2-4.8g of bright salt, 4.8-7.2g of trona, 2.4-3.6g of costustoot, 4-6g of fructus coriandri sativi, 2.4-3.6g of Tibet inula root, 4.8-7.2g of round cardamom, 4-6g of dried ginger, 2.4-3.6 cortex cinnamomi, 4-6g of red flower, 4-6g of savoury rhododendron leaf, 1.6-2.4g of pomegranate seed, 2.4-3.6g of rhubarb, 0.8-1.2g of purpurea halitium, 0.8-1.2g of flos caryophyllata, 4-6g of fructus terminaliae billericae, 1.6-2.4g of Shaji ointment, 2.4-3.6g of emblic leafflower fruit, 0.8-1.2g of crocus sativus, 0.8-1.2g of bear gall, 4-6g of fructus tsaoko, 4-6g of siberian solomonseal rhizome, 2.4-3.6g of puncturevine caltrop fruit, 4.8-7.2g of embelia laeta, 7.4-9.6g of herba dracocephali tangutici, and 7.4-9.6g of tinospora sinensis. The Tibetan medicine composite of the invention has significant clinical efficacy, little toxic and side effects and simple and convenient preparation.
Owner:尼玛次仁
Normal-temperature preparation method for ultrafine powder hawthorn and special bilateral airflow sieving machine thereof
ActiveCN102512520AImprove broken rateImprove bioavailabilityAntibacterial agentsPowder deliveryGranularityMetallurgy
The invention discloses a normal-temperature preparation method for ultrafine powder hawthorn. The method comprises the following steps of: cleaning hawthorn, and drying till the water content is less than or equal to 10 percent; smashing the hawthorn at the normal temperature to obtain coarse powder of which the granularity is 60-80 meshes; putting the coarse powder into a rod mill for smashing,conveying into the special bilateral airflow sieving machine through wind for sieving with a 500-mesh sieve, sieving to obtain powder of which the particle diameter is less than or equal to 25 micrometers, and conveying to a cyclone aggregator through airflow generated by a draught fan for collecting to obtain finished ultrafine powder coccidia powder; and collecting powder which is not sieved with the 500-mesh sieve with a funnel, and conveying to the rod mill through a pipeline for smashing circularly once again. The method has the advantages that: the entire preparation process is performed at the normal temperature without low temperature or special additional conditions; the particle diameter of prepared finished hawthorn micro powder is less than or equal to 25 micrometers, the cell-wall breaking rate and biological availability are greatly increased, and the pharmacological action of a medicament is enhanced; and due to the adoption of the bilateral airflow sieving machine, thepreparation process of ultrafine powder hawthorn is simplified, and the aim of controlling the quality standard of Chinese medicinal powder is fulfilled.
Owner:河南省康星生物科技有限公司 +1
Chinese medicinal composition granules and preparation method thereof
ActiveCN101850066AGood effectSafe to takeAntinoxious agentsGranular deliveryDiseaseSalvia miltiorrhiza
The invention relates to Chinese medicinal composition granules and a preparation method thereof. The Chinese medicinal composition granules comprise the following ruptured powder in part by weight: 3 to 18 parts of American ginseng ruptured powder, 1 to 24 parts of pseudo-ginseng ruptured powder, 6 to 36 parts of dendrobium ruptured powder, and 9 to 45 parts of root of red-rooted salvia ruptured powder, wherein the granularity D90 of the ruptured powder is between 5 and 75 mu m. The invention also provides a method for preparing the Chinese medicinal composition granules. The method comprises the following steps of: uniformly mixing the American ginseng ruptured powder, pseudo-ginseng ruptured powder, dendrobium ruptured powder, and root of red-rooted salvia ruptured powder of which the D90 is between 5 and 75 mu m; preparing a soft material by adopting aqueous ethanol at the concentration of over 20 vol percent; and after granulating by using a granulator with 10 to 30 meshes, drying and finishing the granules to obtain the Chinese medicinal composition granules. The Chinese medicinal composition granules are applied to preventing and regulating human cardiac-cerebral vascular system diseases and sub-health state such as weak immunity and fatigability, have the obvious advantages of high medical effect, high quality uniformity, convenient carrying and administration, safety, reliability, and the like, and can meet the requirement on modern fast-paced lifestyle.
Owner:ZHONGSHAN ZHONGZHI PHARMA GRP +1
Method of using small diameter intracorneal inlays to treat visual impairment
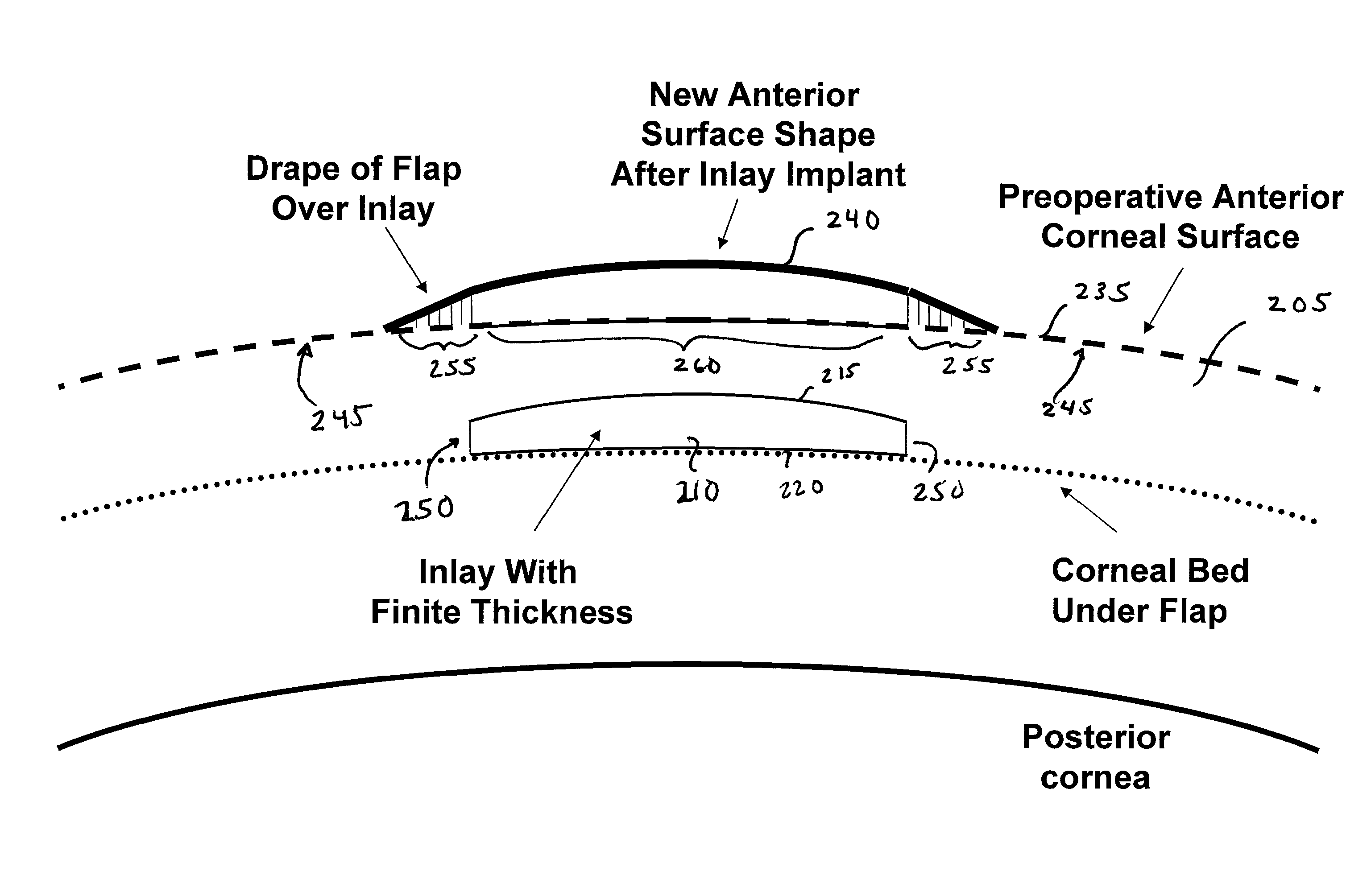
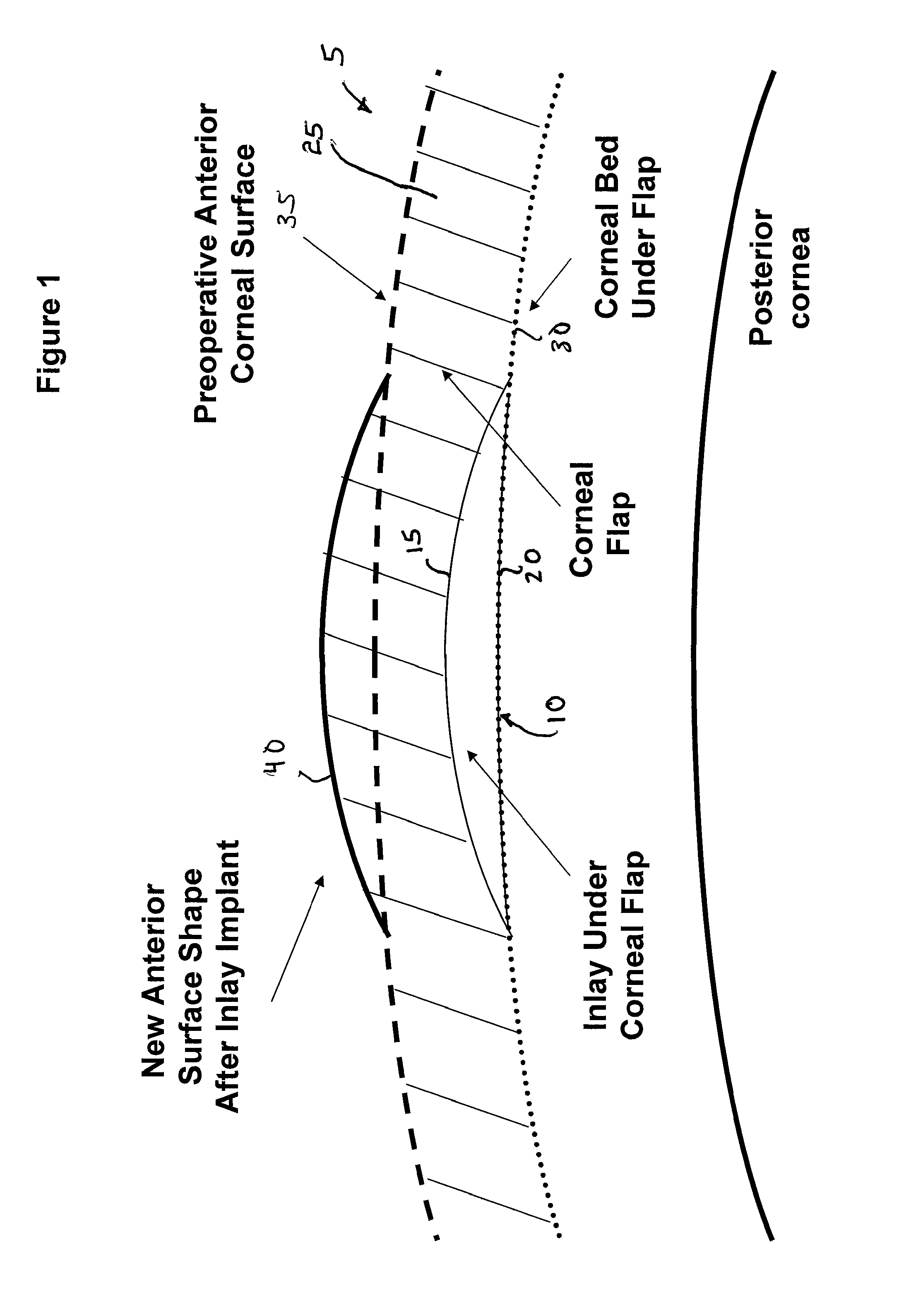
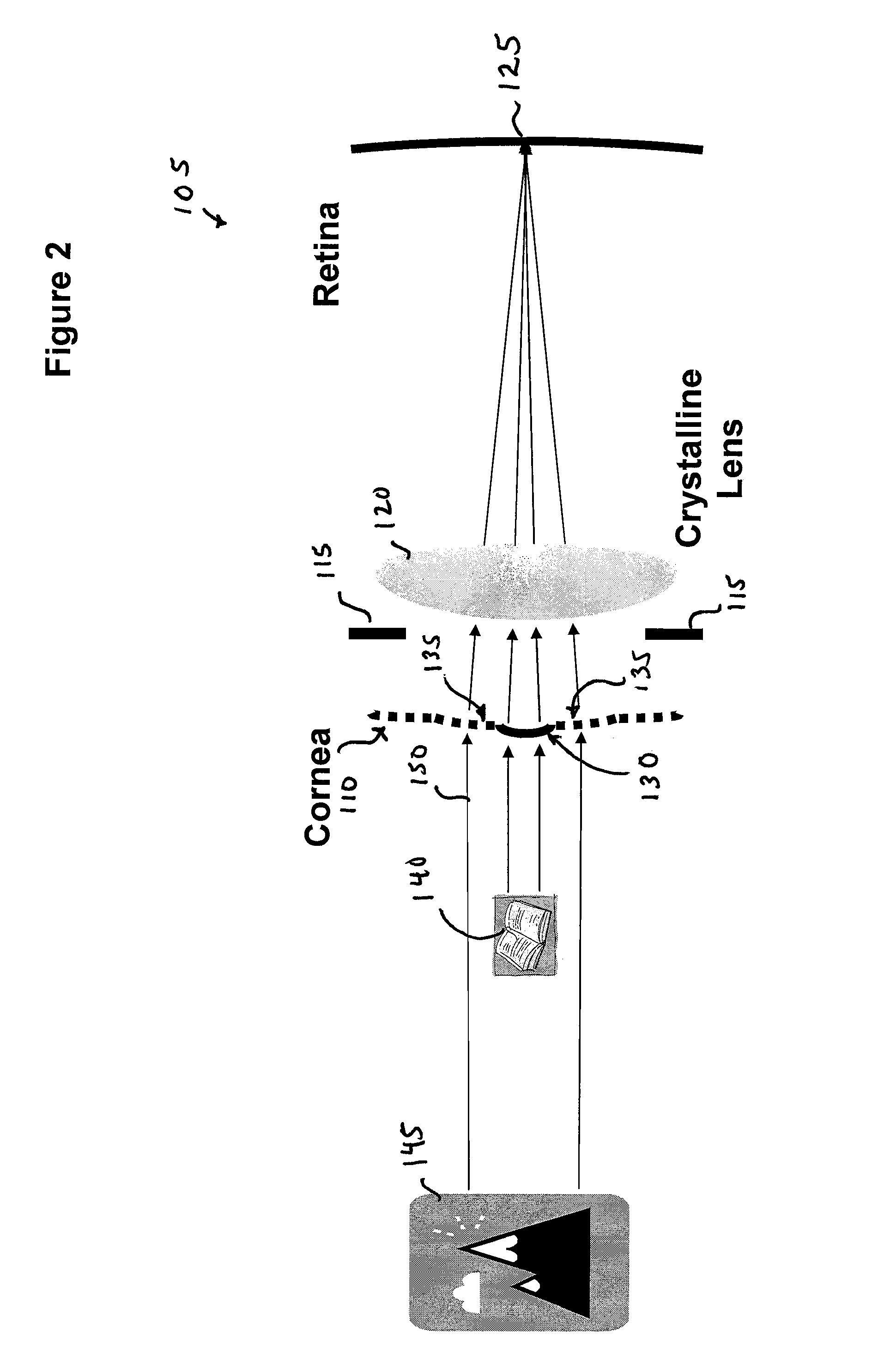
ActiveUS8057541B2Increase powerFine surfaceLaser surgeryIntraocular lensCorneal surfaceImpaired visual acuity
Provided herein are small diameter inlays for correcting vision impairments by altering the shape of the anterior corneal surface. In an embodiment, inlays having diameters smaller than the pupil are provided for correcting presbyopia. To provide near vision, an inlay is implanted centrally in the cornea to induce an “effective” zone on the anterior corneal surface, within which diopter power is increased. Distance vision is provided by a region of the cornea peripheral to the “effect” zone. In another embodiment, small diameter inlays are provided that induce effective optical zones on the anterior corneal surface that are much larger in diameter than the inlays. The increase in the effective optical zone, due at least in part to a draping effect, allows an inlay to produce a much larger clinical effect on a patient's vision than the diameter of the inlay.
Owner:REVISION OPTICS
Chinese medicine whitening and spot-removing facemask powder and preparation method thereof
InactiveCN101411681ASmall side effectsGood clinical effectCosmetic preparationsToilet preparationsLicorice rootsAdemetionine
The invention discloses traditional Chinese medicine mask powder for whitening and dispelling freckle, which has the advantages of little toxicity and capability of effectively achieving whitening, dispelling freckle and the like. A formulation for the mask powder comprises 3 to 10 grams of ginseng, 3 to 15 grams of rhizome polygonati, 6 to 15 grams of radix asparagi, 3 to 10 grams of rhizome typhonii, 3 to 10 grams of angelica dahurica, 6 to 15 grams of angelica, 3 to 10 grams of white stiff silkworm, 6 to 15 grams of fructus xanthil, 3 to 10 grams of bletilla, 6 to 15 grams of white gourd seed, 3 to 15 grams of gingko leaf, 3 to 10 grams of fragrant solomonseal rhizome, 3 to 10 grams of puncturevine, 3 to 10 grams of euphorbia lathyris, 1 to 6 grams of pearl powder, 1 to 6 grams of litharge, 3 to 10 grams of mint, 1 to 3 grams of human placenta, 3 to 6 grams of cinnamon, 3 to 10 grams of tasselblower, 3 to 10 grams of leucophanite, 1 to 3 grams of borneol, 10 to 30 grams of green bean powder, and 3 to 10 grams of honey-fried licorice root.
Owner:赵献民
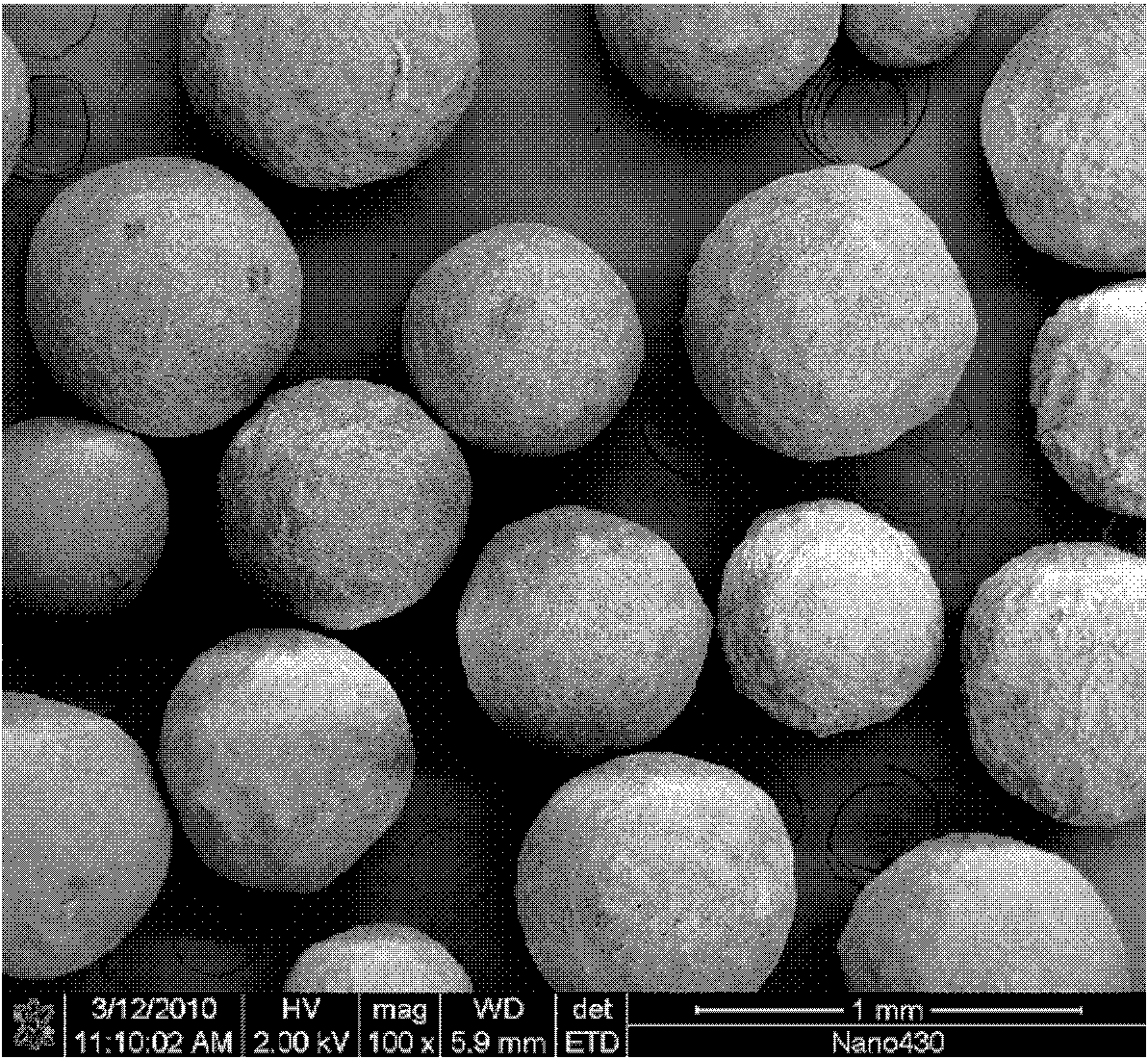
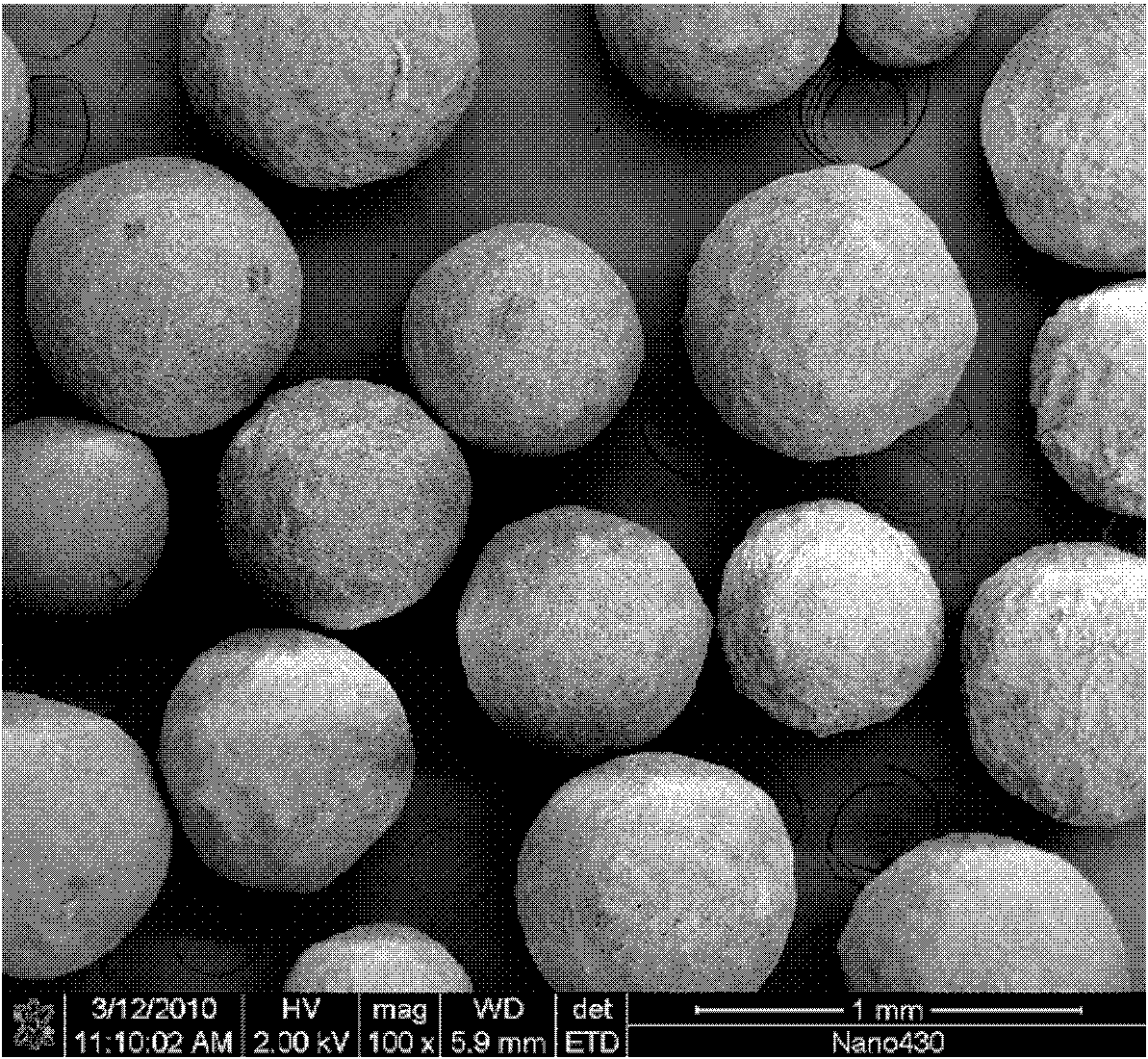
Self-setting calcium phosphate micro spheres, method for preparing same and application thereof
InactiveCN101850133ASelf-curingFree formabilityImpression capsDentistry preparationsFiltrationSlurry
The invention discloses self-setting calcium phosphate micro spheres, a method for preparing the same and application thereof. The preparation method comprises the following steps of: mixing glutin and deionized water to obtain solution of glutin; mixing polysaccharide gum and deionized water to obtain solution of polysaccharide gum; mixing completely dissolved solution of glutin and solution of polysaccharide gum to obtain solution of glutin / polysaccharide gum; fully mixing the solution of glutin / polysaccharide gum and calcium phosphate cement by stirring to obtain pasty self-setting calciumphosphate slurry, fully dispersing the self-setting calcium phosphate slurry into soybean oil by stirring, removing the soybean oil by filtration, and repeatedly washing the micro spheres by acetone and alcohol to obtain the wet micro spheres; and performing curing hydration and drying of the wet micro spheres to obtain the self-setting calcium phosphate micro spheres. The method has the advantages that: the sintering treatment of the micro spheres is unnecessary, medicaments, growth factors or other active (functional) components can be synchronously loaded in the preparation process, the grain size of the obtained self-setting calcium phosphate micro spheres is 0.12 to 2.61mm, and the obtained self-setting calcium phosphate micro spheres are not disintegrated in 6 to 32 hours.
Owner:SOUTH CHINA UNIV OF TECH
Traditional Chinese medicine composition for treating infantile indigestion with food retention and preparation method and quality control method thereof
ActiveCN101455690AEffective quality controlDistinguishing color effect is obviousComponent separationDigestive systemActive componentCurative effect
The invention relates to a Chinese traditional medicine composition treating infantile indigestion with food retention, a preparation method thereof and a quality control method. The composition is composed of Corium stomachichum galli, medicated leaven, hawthorns, lagehead atractylodes and other raw materials as active components or water extracts of the raw materials as active components and pharmaceutically acceptable additives. The preparation method thereof is simple and feasible, and can maximally maintain effective components in herbs to take curative effect of the herbs. The quality control method can effectively control quality of medicine and guarantee stability of the curative effect of the medicine through thin-layer identification for the hawthorns and the lagehead atractylodes and content identification for the Corium stomachichum galli.
Owner:BEIJING ASIA EAST BIO PHARMA CO LTD
Tilmicosin solid dispersible granule as well as preparation method and application thereof
ActiveCN103830187ASimple processEasy to realize industryAntibacterial agentsOrganic active ingredientsParaffin waxFormulary
The invention discloses a tilmicosin solid dispersible granule as well as a preparation method and an application thereof. The tilmicosin solid dispersible granule consists of tilmicosin and a carrier accessory. The carrier accessory is one of glycerin monostearate, stearyl alcohol, saturated triglyceride, glycerinum simple lipid, paraffin wax, animal wax, vegetable wax or fatty powder or mixture thereof. The preparation method of the tilicosin solid dispersible granule comprises the following steps: weighing respective carrier accessories according to the formula proportion, heating, melting and mixing uniformly; adding the tilmicosin, stirring uniformly, cooling, pelleting through spraying of a fluidized bed, balling, cooling, sieving and collecting to obtain the tilmicosin solid dispersible granule. The tilmicosin solid dispersible granule solves the problem of medicine palatability, by feed mixing administration, the fluidity and dispersibility are good and medication is convenient; after animals take the granule, the medicine releases slowly, so that the safety is high; besides, the biological availability of tilmicosin medicine is enhanced, and the clinical using effect is remarkable.
Owner:SOUTH CHINA AGRI UNIV
Bone grafting device for tubular bone defect healing
The invention relates to a bone grafting device for tubular bone defect healing, and belongs to the technical field of orthopedic medical devices. The bone grafting device is used for grafting tubular bones to promote the healing of the tubular bones, and comprises an outer sleeve, an inner sleeve and a connecting rod, wherein the inner diameter of the outer sleeve is matched with the outer diameter of the two ends of a tubular bone defect part required for bone grafting, and the inner sleeve is positioned in the outer sleeve; the connecting rod is inserted in the inner sleeve, and the diameter of the connecting rod is matched with the inner diameter of the inner sleeve; and the length of the connecting rod is greater than that of the inner sleeve, and the inner sleeve and the outer sleeve are made of biodegradable magnesium alloy. In the bone grafting device, the connecting rod in the inner sleeve can firmly connect the two ends of a defective tubular bone, a high-quality autologous cancellous bone is filled between the inner sleeve and the outer sleeve to simultaneously play roles of bone grafting and reinforced fixing, and the biodegradable magnesium alloy has a function of inducing bone formation, thereby the fracture healing is promoted. The bone grafting device has the advantages of firm bone grafting, high intensity, early ambulation for patients, and good clinical effect.
Owner:张英泽
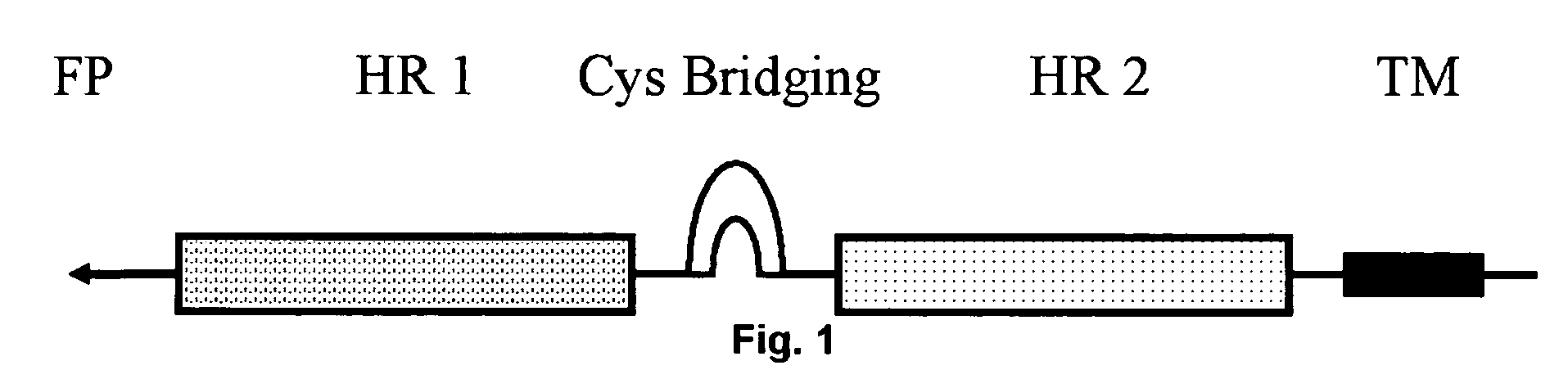
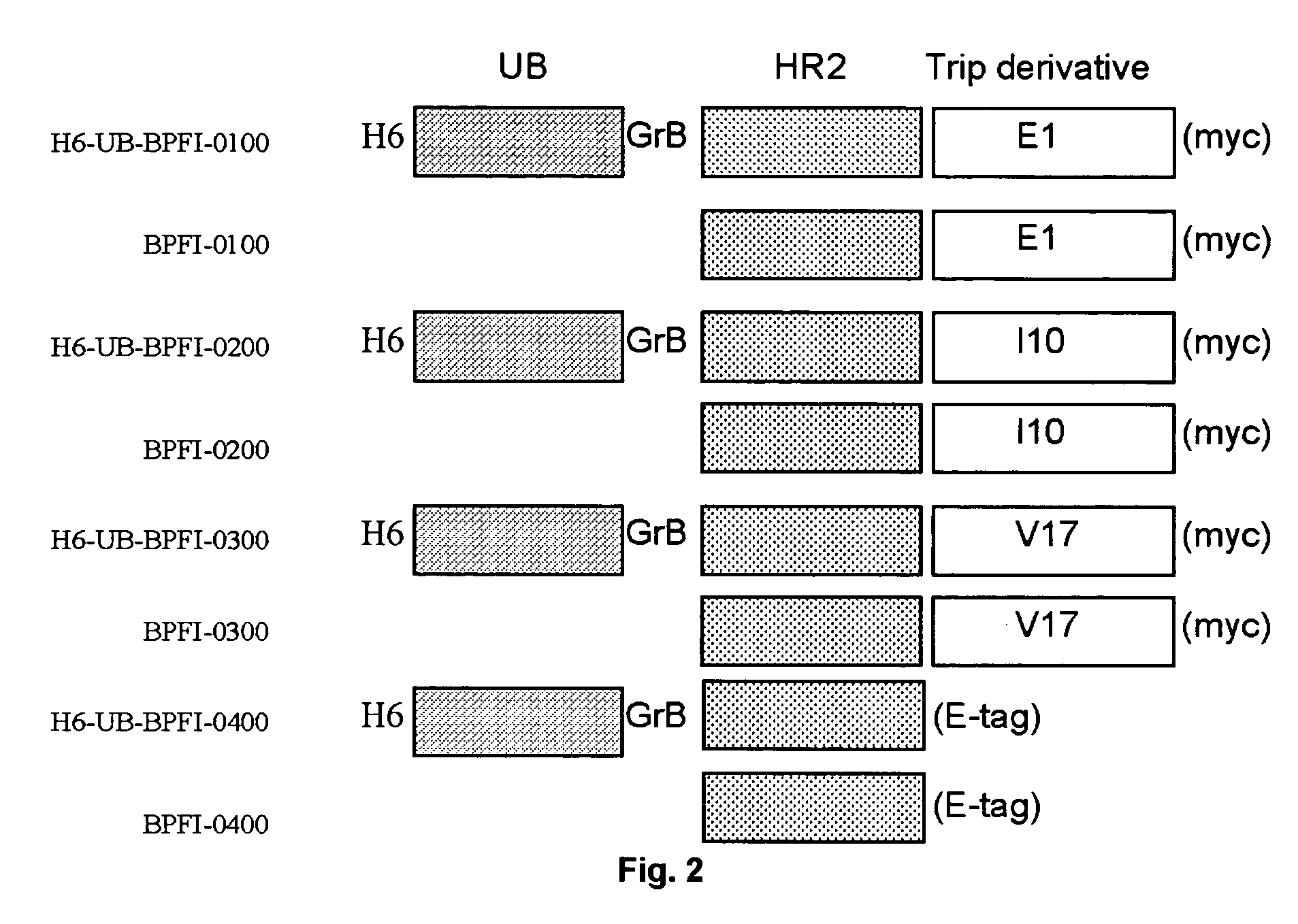
Multimerised HIV fusion inhibitors
InactiveUS20050202043A1Improve isolationHigh protein yieldPeptide/protein ingredientsAntibody mimetics/scaffoldsImmunodeficiency virusTherapy HIV
There are provided multimeric fusion proteins exhibiting anti-viral activity. The fusion proteins comprise the HR2 region of the ectodomain of the human immunodeficiency virus gp41 protein which is fused to a multimerisation domain peptide such as a trimerisation domain derived from tetranectin. The multimerised fusion proteins may be used as HIV fusion inhibitors in the treatment of AIDS.
Owner:BOREAN PHARMA APS
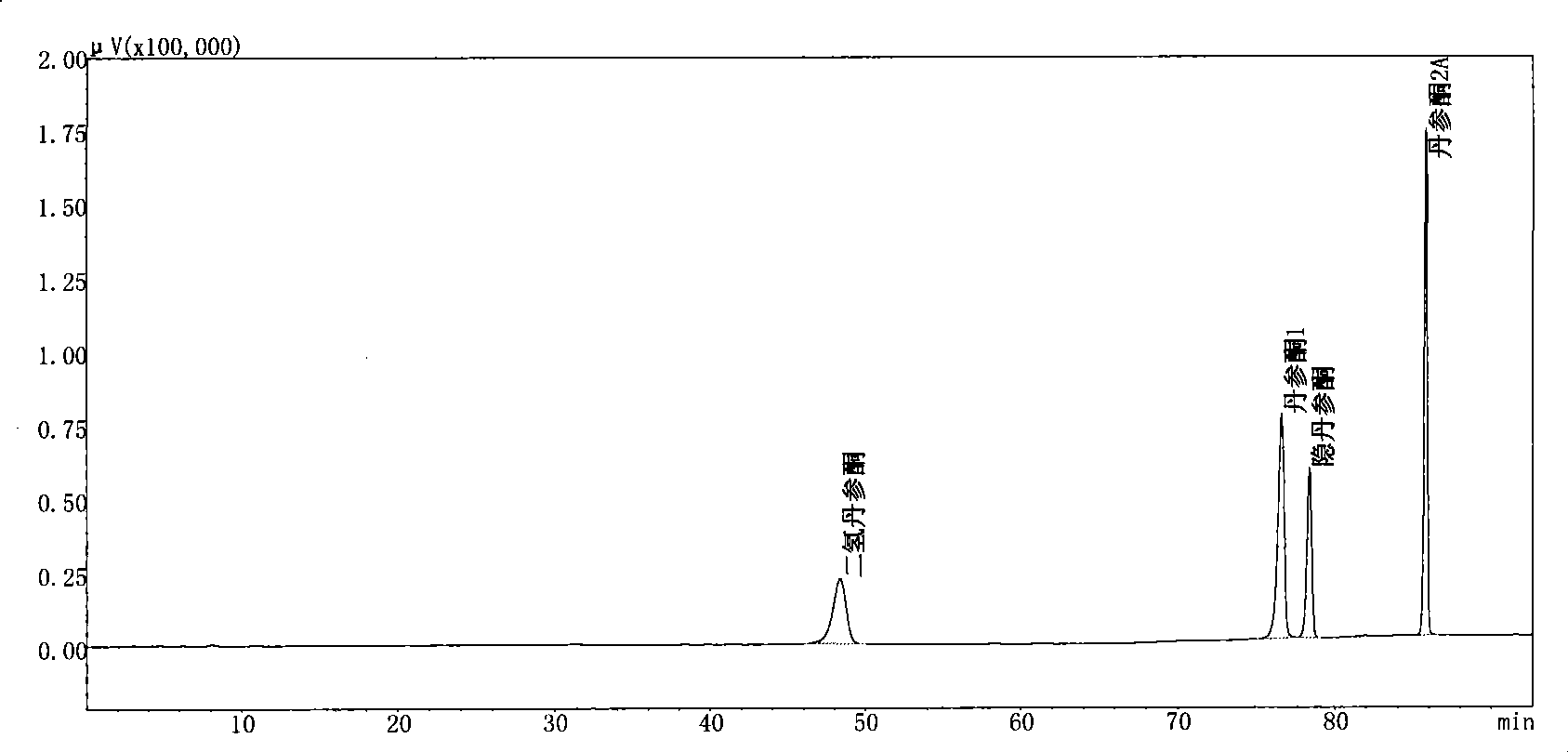
Antibiotic tanshinone extract, preparation method thereof, use and products produced thereby
InactiveCN101411745AHigh purityReasonable proportionAntibacterial agentsOrganic active ingredientsTanshinone IIAAntibiotic Y
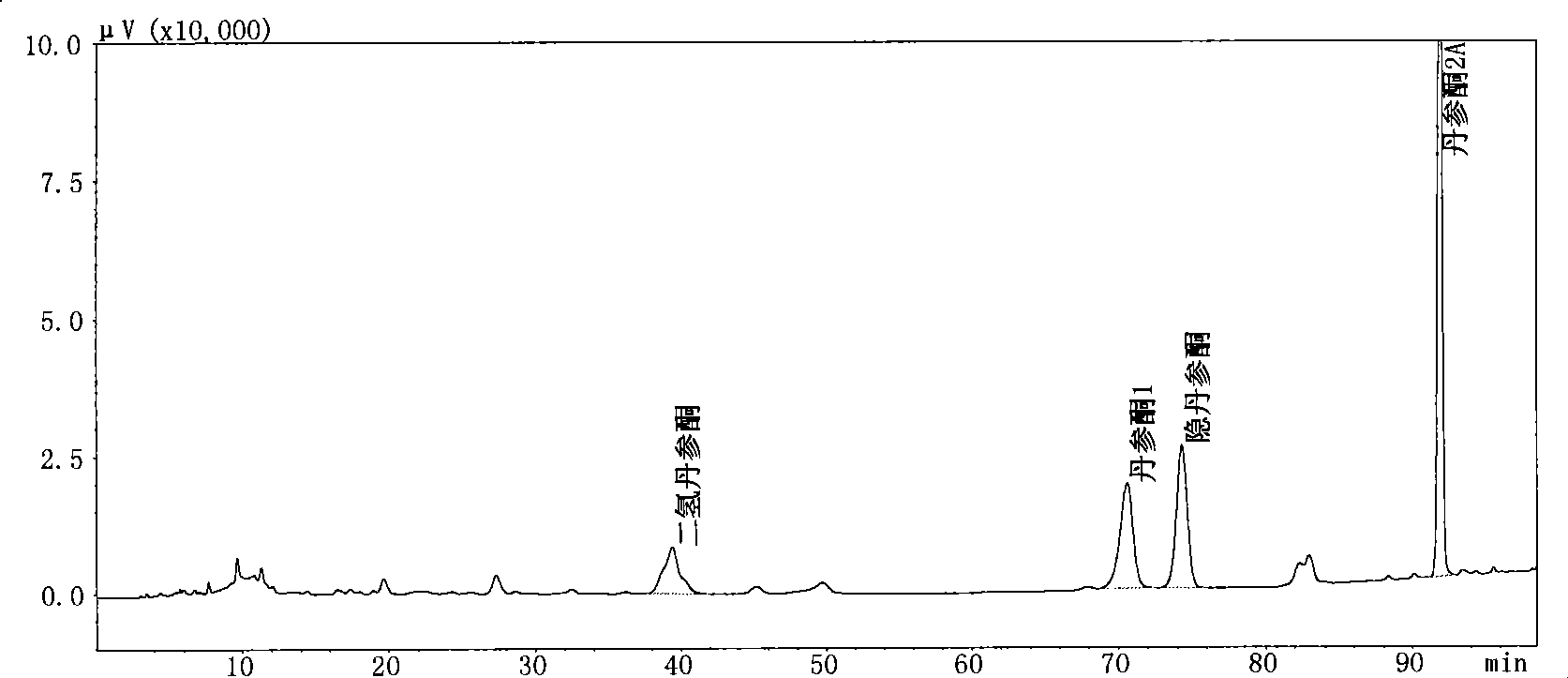
The invention relates to an antibacterial tanshinone extract extracted from Salvia spp. roots, a preparation method thereof, application of the antibacterial tanshinone extract and products of the antibacterial tanshinone extract. The extract contains cryptotanshinone, dihydro-tanshinone, tanshinone I and tanshinone IIA, wherein the total weight content of the four compounds is 40 to 99 percent of the total weight content of the extract; the weight content of the cryptotanshinone is 50 to 95 percent of the total weight of the four compounds; the weight content of the dihydro-tanshinone is 5 to 30 percent of the total weight of the four compounds; the weight content of the tanshinone I is 0 to 20 percent of the total weight of the four compounds; and the weight content of the tanshinone IIA is 0 to 40 percent of the total weight of the four compounds. The preparation method comprises the steps of diacolation extraction and purification for two times. The extract is used for preparing antibacterial medicines or other products. The tanshinone extract obtained has high purity, more reasonable proportions of various compositions, and can exert better clinic effect.
Owner:BOTANIC CENTURY BEIJING
Medicine for treating chronic hepatitis b
ActiveCN101357219AGood treatment effectGood curative effectDigestive systemAntiviralsSalvia miltiorrhizaChronic hepatitis
The invention discloses a medicine for treating chronic hepatitis B, which contains the raw materials by the mixture ratio: 6-18% of herba artemisiae capillaries, 1-7% of Chinese thorowax, 1-7% of angelica, 3-12% of radix paeoniae alba, 3-12% of salvia miltiorrhiza, 1-10% of radix curcumae, 1-7% of rhizoma corydalis, 1-7% of common burreed rhizome, 1-7% of rhizoma zedoariae, 1-7% of nutgrass galingale rhizome, 1-7% of fructus meliae toosendan, 1-10% of dangshen, 1-7% of largehead atractylodes rhizome, 6-18% of radix astragali, 3-12% of indian bread, 1-7% of immature bitter orange, 1-7% of amomum villosum, 1-7% of areca, 0.5-5% of Chinese eaglewood and 1-7% of liquorice; the medicine is prepared by adopting conventional method. The medicine has the efficacies of clearing heat and promoting diuresis, nourishing the liver and invigorating the spleen, regulating the flow of qi and removing blood stasis, and can be used for treating chronic hepatitis B with remarkable effects, short period of treatment and rapid effects.
Owner:LANZHOU FOCI PHARM CO LTD
Kit for separated culture of DC-CIK cells, and application thereof
InactiveCN102978161AHigh purityHigh activityBlood/immune system cellsLymphocyte cultureCoating system
The invention provides a kit for separated culture of DC-CIK cells, and an application thereof. The kit comprises a lymphocyte separation liquid, a peripheral blood sample treatment liquid, a CIK cell induced propagation system, a DC induced propagation system, a cell culture bottle coating system, a lymphocyte culture medium GT-T551 and a cell culture bag. The application of the kit in separating and culturing the DC-CIK cells comprises the following steps of separation of peripheral blood sample mononuclear cells, separation of the DC cells and CIK cells, induced propagation of the DC cells, the induced propagation of the CIK cells and co-culture of the DC cells and CIK cells. The lymphocytes obtained from the separation by the kit have very high purity and activity; the propagation rate of the CIK cells is fast; the proportion of CD3+CD56+ double positive cells is high; operation method is simple; and conditions are easy to control. The kit can be widely applied in the separated culture for the DC-CIK cells of human and mammals.
Owner:JIANGYIN CHI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com