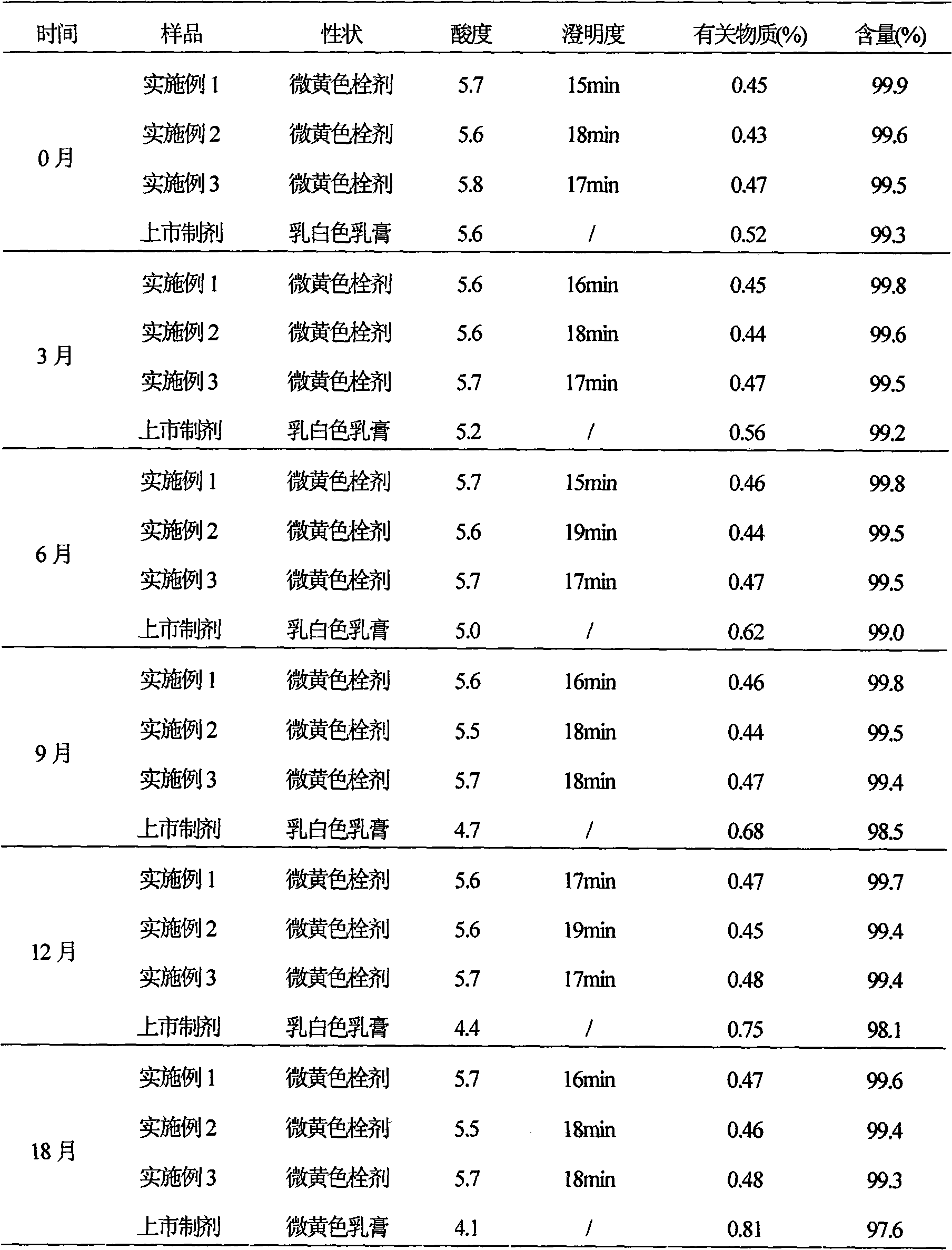
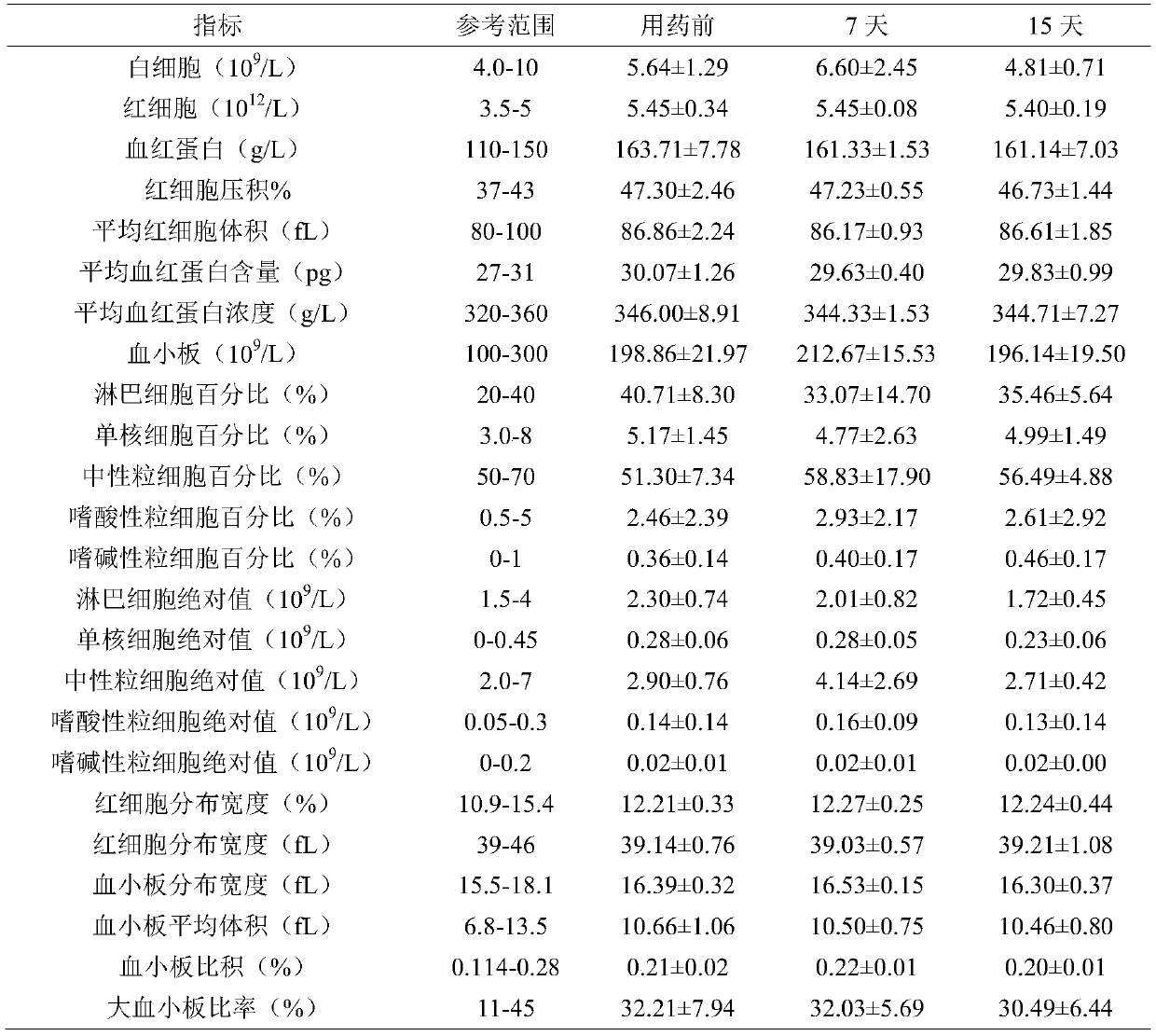
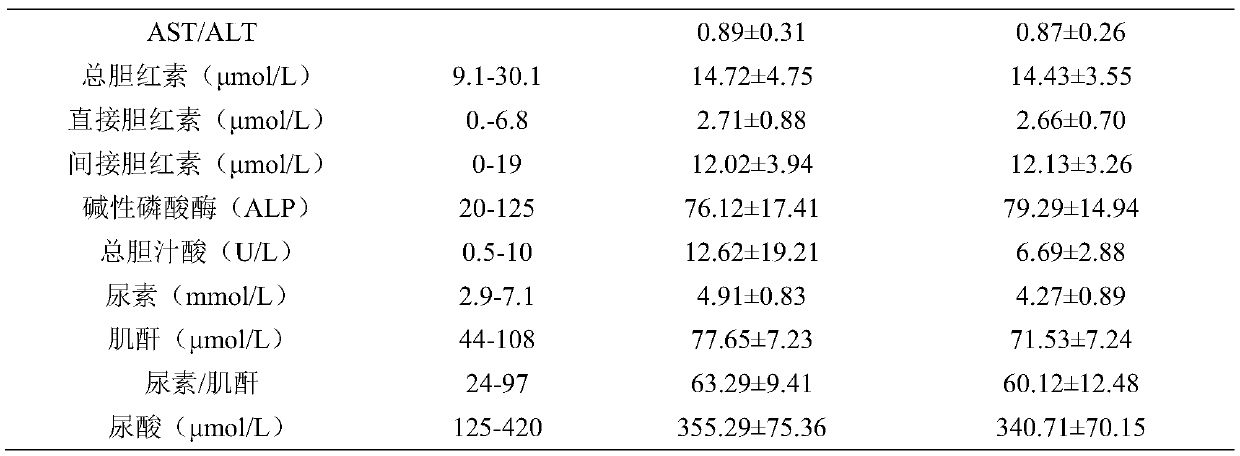
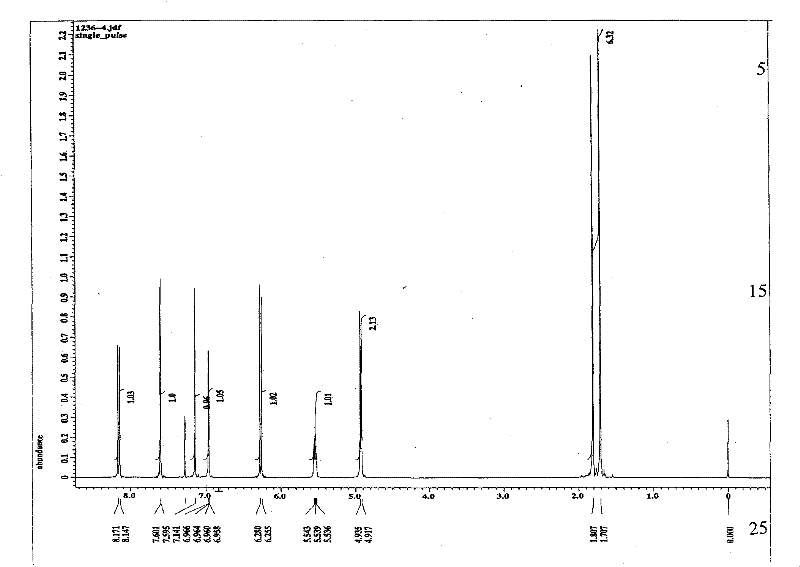
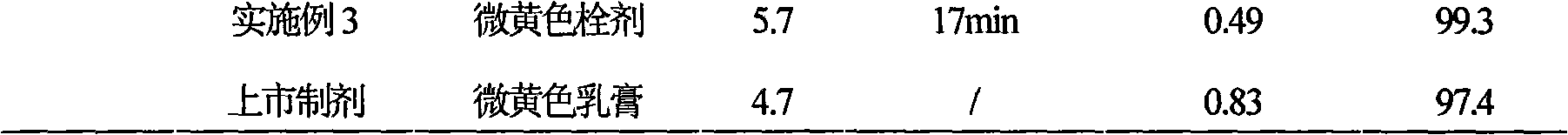Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
82results about How to "Fast drug effect" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
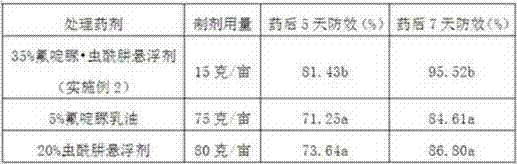
Pesticide suspension concentrate containing pleocidin and preparation method thereof
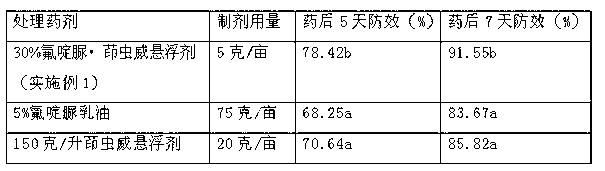
The invention provides a pesticide suspension concentrate containing pleocidin and a preparation method thereof. The suspension concentrate comprises active ingredients such as pleocidin and abamectin, emamectin benzoate, lambda-cyhalothrin, beta-cypermethrin, chlorfenapyr, chlorfluazuron, diafenthiuron, lufenuron, thiamethoxam or butene-fipronil, as well as dispersing agent, wetting agent, functional aids, other aids and water. The preparation method is as follows: mixing the active ingredients, aids and water to a homogeneous pasty material, and then feeding into a sand mill to obtain a mean grain size of 2-4mu m (4.3) by the sand milling. The suspension concentrate of the invention has a small mean grain size, high suspension rate of the product, stable performance and minimal condensate rate under storage at room temperature within two years. Furthermore, as the suspension rate of the preparation is high and the added functional aids increase spreadability and permeability, the pharmacodynamic action of the product is improved.
Owner:SHENZHEN NOPOSION AGROCHEM
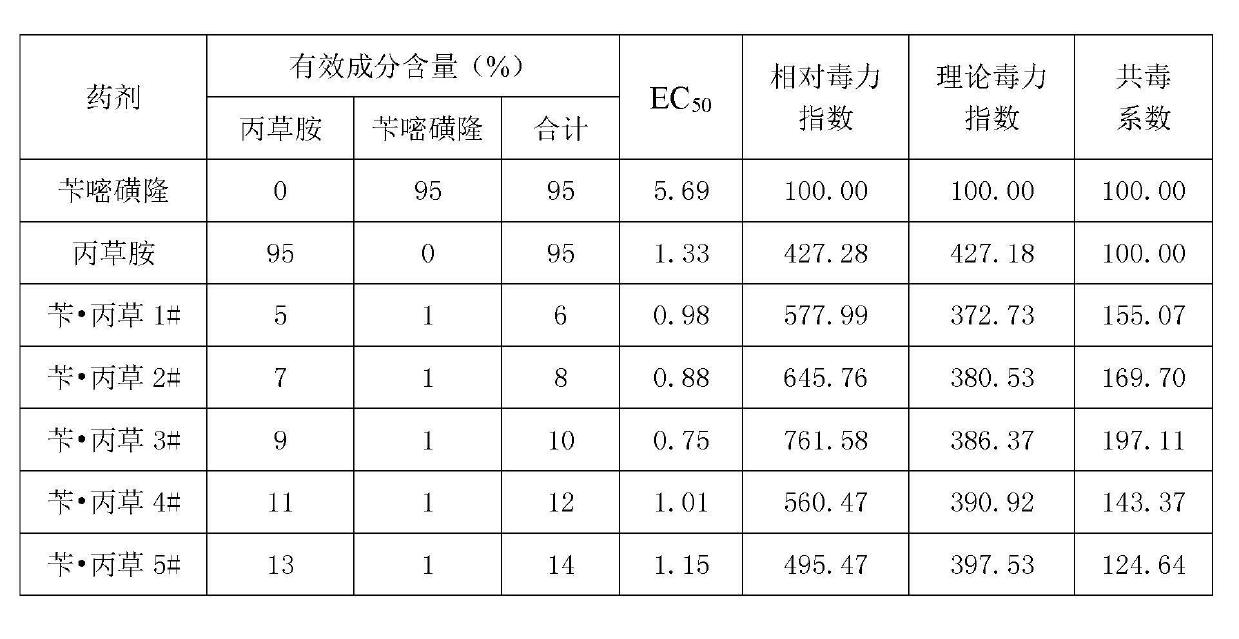
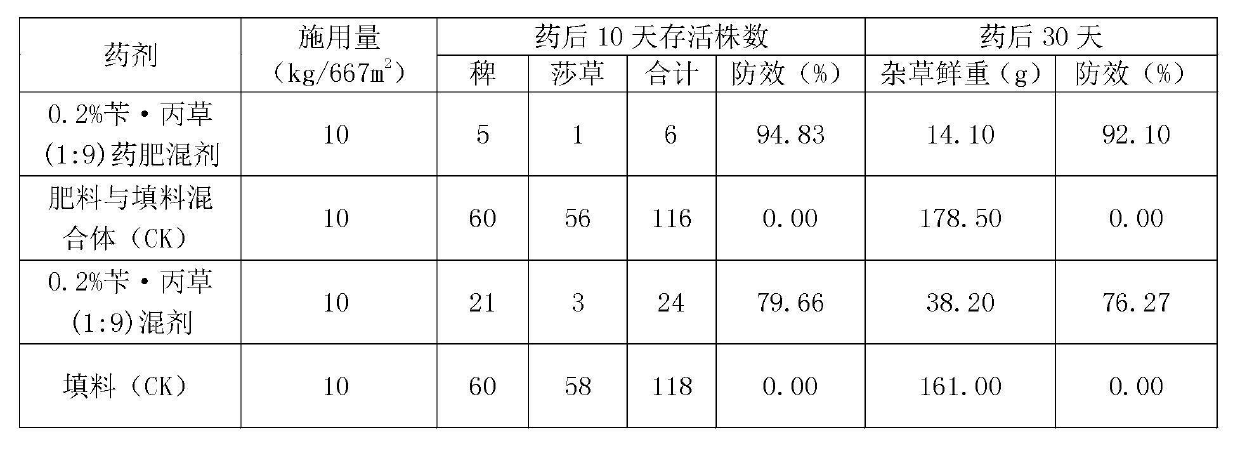
Herbicide-fertilizer for direct-seeding rice field and production method of herbicide-fertilizer
ActiveCN102674973AImprove quality stability performanceReduce pollutionFertilizer mixturesAmmonium sulfateFertilizer
The invention discloses a herbicide-fertilizer for a direct-seeding rice field. The herbicide-fertilizer contains herbicide-fertilizer mixture which is prepared by using bensulfuron-methyl with weeding activity, pretilachlor herbicide and a nitrogen-containing fertilizer, wherein the weight ratio of the bensulfuron-methyl to the pretilachlor is 1:9 and the nitrogen-containing fertilizer consists of urea and ammonium sulfate with weight ratio being 1:2. The invention additionally discloses a production method of the herbicide-fertilizer. Water is used as solvent. A spray-coating method is used for preparing herbicide-fertilizer particle mixture. The process flow is short, the quality is stable and the yield is high. The prepared herbicide-fertilizer is suitable for direct-seeding rice fields, can realize synchronous fertilization and weeding, has the characteristics of high safety, excellent stability and good efficacy, and can achieve the goal of labor saving and time saving.
Owner:广西乐土生物科技有限公司
Application of Dendrobium officinale polysaccharide in preparation of medicine for preventing and treating hypertension and apoplexy
ActiveCN101849957ALower blood pressureDelay damage progressionOrganic active ingredientsPowder deliveryDiseaseLarge dose
The invention relates to preparation and application of Dendrobium officinale, aiming to provide preparation of a medicine using Dendrobium officinale polysaccharide as a drug effective component, and application of the medicine for preventing or treating hypertension and apoplexy. The invention discloses the application of Dendrobium officinale in preparation of the medicine for preventing and treating hypertension or apoplexy, and extract, granules, tablets, capsules and oral liquid based on the polysaccharide and preparation methods thereof. The invention proves that the Dendrobium officinale polysaccharide is the effective component of Dendrobium officinale in preventing and treating cardiovascular and cerebrovascular diseases. By using the Dendrobium officinale polysaccharide as the special-purpose medicine, compared with Dendrobium officinale which is not extracted, the Dendrobium officinale polysaccharide has the advantages of quick medicine efficacy, large dose effect range and obvious effect.
Owner:ZHEJIANG TIANHUANG MEDICINAL PLANT PHARMA
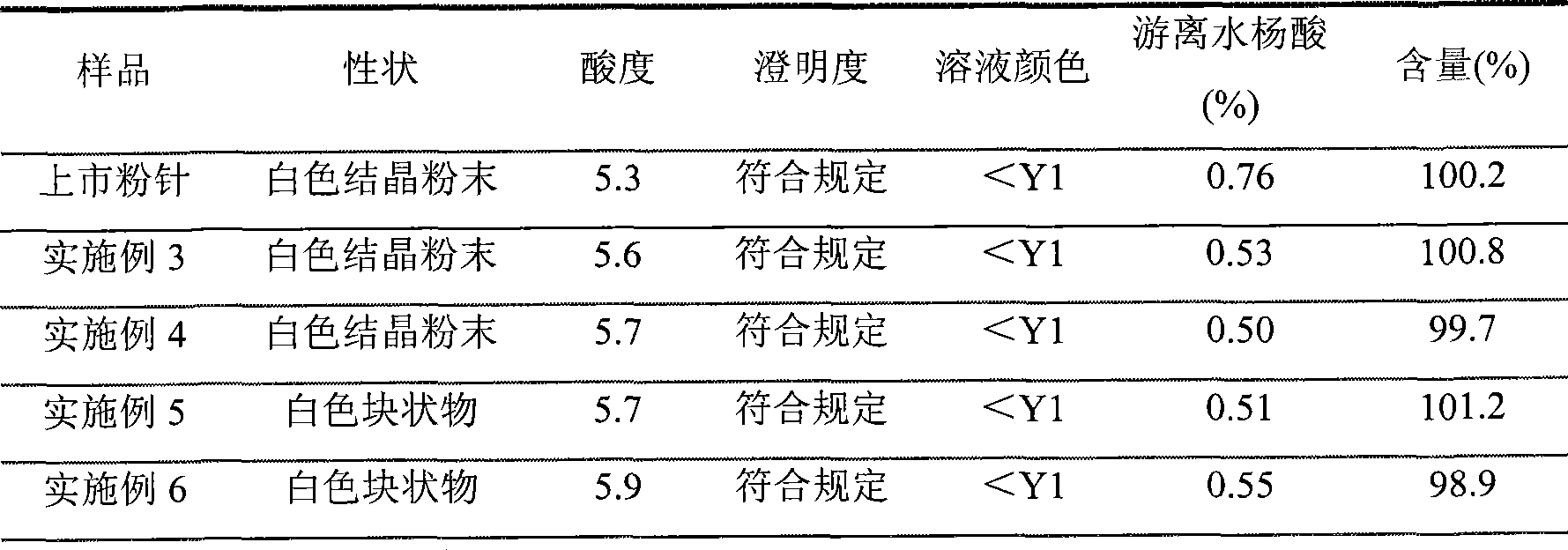
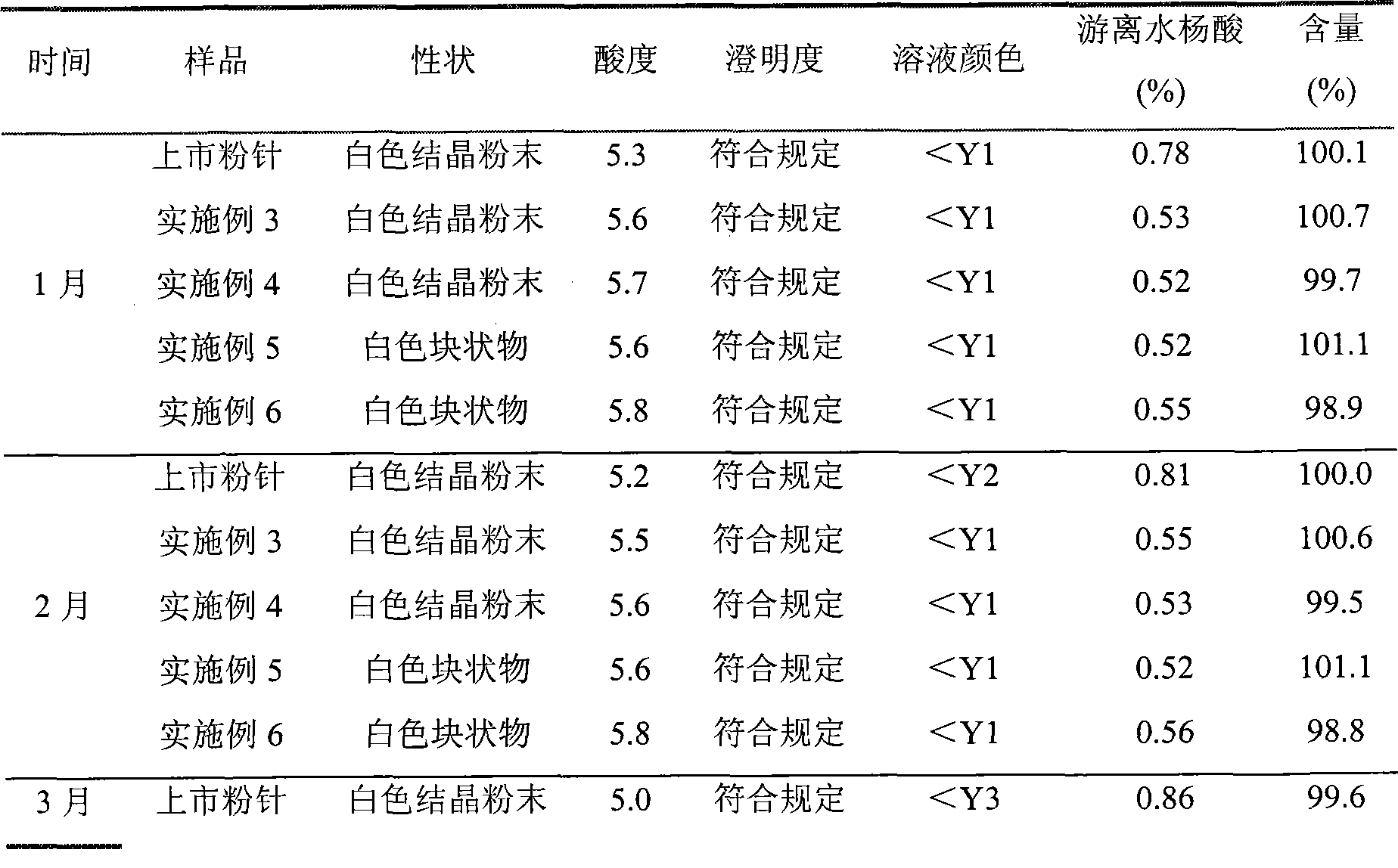
Preparation method of arginine aspirin and powder and injection preparation thereof
InactiveCN101380329AShorten the timeImprove efficiencyOrganic active ingredientsAntipyreticChemistryFreeze dry
The invention discloses an aspirin arginine and a preparation method of powder for injection thereof, the preparation method is characterized in that: arginine is dissolved in water, aspirin is further added, the arginine fully reacts with the aspirin in the water by stirring at the reaction temperature of 60-80 DEG C, chloroform is added after the reaction, the low temperature preservation, the precipitation and the filtration are carried out, sediment is washed and vacuum dried, an aspirin arginine crude product is obtained, and then the aseptic packaging or the freeze-drying after the redissolution is carried out to obtain the aspirin arginine powder for injection. The method has short reaction time and high production efficiency, and the obtained aspirin arginine powder for injection has stable product quality.
Owner:HAINAN LINGKANG PHARMA CO LTD
Dextro lipoic amidate and its prepn
InactiveCN1887882AImprove solubilityGood water solubilityOrganic active ingredientsNervous disorderMedicineHeat stability
The present invention relates to one kind of medicine, dextro lipoic amidate and its preparation process. The general expression of dextro lipoic amidate is shown. The present invention changes dextro lipoic with easy oxidation and polymerization, poor heat stability and water insolubility into stable dextro lipoic amidate with raised smelting point and raised medicinal effect. The dextro lipoic amidate may be further used to prepare other medicine preparation.
Owner:GRAND PHARM (CHINA) CO LTD
Medicine for gynecoiatry and health care and preparation method thereof
InactiveCN101569737AIntegrity guaranteedFast drug effectSexual disorderPlant ingredientsBarbed Skullcap HerbSalvia miltiorrhiza
The invention relates to a medicine for gynecoiatry and health care and a preparation method thereof. The medicine is made of the formulation based on the weight percentage: 2-12% of rhizoma atractylodis, 3-15% of bletilla striata, 2-10% of angelica dahurica, 3-12% of platycodon root, 2-10% of phyllanthus urinaria, 2-10% of saffron, 4-13% of tuckahoe, 4-10% of asiatic plantain seed, 2-10% of radix paeoniae alba, 2-10% of baikal skullcap root, 2-10% of rhizoma coptidis, 3-10% of gentianella, 3-12% of achyranthes, 3-10% of barbed skullcap herb, 3-8% of hibiscus, 2-10% of dictamnus dasycarpus, 2-15% of motherwort, 3-8% of forsythia, 1-10% of angelica, 2-8% of common cnidium fruit, 3-7% of cistanche salsa, 2-8% of salvia chinensis, 1-6% of salvia miltiorrhiza, 1-4% of nut grass and 2-8% of radix curcumae; the total content of the twenty-five drugs is 100%. The medicine has special effects on expulsion of toxin, antiphlogosis and sterilization of gynae, has the functions of stanching, astringing and healing wound, and has the remarkable advantages of no side effect and rapid treatment.
Owner:杨国俊
Niraparib granules with high utilization efficiency and preparation method thereof
ActiveCN110314094AStrong reductionPromote absorptionPharmaceutical product form changeSolid materialVitamin CD-Glucose
The invention discloses niraparib granules with a high utilization efficiency and a preparation method thereof, and belongs to the technical field of medicine production. The components of the niraparib granules include niraparib, glucose, vitamin C and a carboxylesterase inhibitor. A granule packaging machine adopted in the preparation method comprises a storage bin and a transparent upper discharge pipe, wherein the transparent upper discharge pipe penetrates through the storage bin, the two sides of the upper discharge pipe are provided with an infrared emitter and an infrared receiver respectively, the infrared emitter and the infrared receiver are capable of lifting and lowering, a turntable is arranged below the upper discharge pipe, a curved through hole is formed in the turntable,a retractable stop block with a proximity switch is arranged on one side of the turntable, and a curved groove is formed in the turntable. By adopting the niraparib granules, the amount of niraparib discharged along with excretion can be reduced, and the pharmacodynamic effect of the niraparib can be maintained; valve-related components of the granule packaging machine adopted in the preparation process of the niraparib granules have longer service life, it can be ensured that the amount of the material discharged each time is sufficient, the remaining granules with a remaining amount less than a set value are remained in the upper discharge pipe, and the application is convenient.
Owner:ANHUI HAIKANG PHARMA
Ciclopirox olamine pessary and preparation method thereof
InactiveCN101579306AImprove stabilityFast drug effectOrganic active ingredientsAntimycoticsSide effectWhole body
The invention relates to a ciclopirox olamine pessary capable of reducing toxic and side effects of a medicament caused by systemic delivery, increasing local concentration of the medicament, fully playing the therapeutic effectiveness of the medicament and improving the adaptability of patients. The pessary can realize zero order release and is prepared by the following materials by weight portion: 1 portion of ciclopirox olamine, 65 to 90 portions of substrate, 0.2 to 3 portions of stearic acid, 0.1 to 5 portions of hydrogenated castor oil and 0.05 to 2 portions of sodium benzoate.
Owner:HAINAN YONGTIAN PHARMA INST
Traditional Chinese medicine composition with efficacy of strengthening and consolidating body resistance, tonifying liver and kidney and enhancing immunity
InactiveCN111375010AImprove immunityAvoid infectionSenses disorderNervous disorderLiver and kidneyEfficacy
The invention discloses a traditional Chinese medicine composition with efficacy of strengthening and consolidating body resistance, tonifying liver and kidney and enhancing immunity. The traditionalChinese medicine composition is prepared from the following raw material medicines in parts by weight: 3-300 parts of herba epimedii, 3-300 parts of fructus ligustri lucidi, 3-300 parts of radix astragali, 3-300 parts of radix codonopsis, 3-300 parts of radix angelicae sinensis, 3-300 parts of prepared polygoni multiflori radix, 3-300 parts of radix salviae miltiorrhizae, 3-300 parts of glycyrrhizae radix, 3-300 parts of poria and 3-300 parts of fructus lycii. The traditional Chinese medicine composition disclosed by the invention can supplement deficiency of qi and blood of the human body, improve organism immunity, eliminate weak symptoms and treat symptoms, including asthenia of essence and blood, soreness and weakness of waist and knees, insomnia, forgetfulness, dizziness, tinnitus andthe like due to the surgery, the radiotherapy and the chemotherapy of a tumor patient. The traditional Chinese medicine composition can prevent COVID-19 infection.
Owner:THE FIRST HOSPITAL OF LANZHOU UNIV
Organic vegetable pest control medicine and preparation method thereof
InactiveCN107006535AActivin activityAvoid damageBiocideMagnesium fertilisersBiotechnologyPumpkin seed
The invention relates to an organic vegetable pest control medicine. The organic vegetable pest control medicine is prepared from the following raw materials in parts by weight: 120-160 parts of plant ash, 105-135 parts of white radish, 105-135 parts of onions, 90-120 parts of garlic, 90-120 parts of fresh ginger, 85-105 parts of pepper, 85-105 parts of Sichuan pepper, 70-90 parts of pumpkin seeds, 70-90 parts of calamine, 55-75 parts of wormwood, 55-75 parts of mulberry leaves, 50-70 parts of golden cypress, 50-70 parts of gynostemma pentaphyllum, 50-70 parts of piper sarmentosum, 35-50 parts of Chinese honeylocust fruit, 35-55 parts of aqueous sodium chloride solution, 30-50 parts of aqueous magnesium chloride solution, 30-50 parts of aqueous sodium carboxymethylcellulose solution and 5-15 parts of potassium fulvate powder. The invention also discloses a preparation method of the organic vegetable pest control medicine. The organic vegetable pest control medicine has obvious killing effect on ova and larvae; and pharmacodynamical effect is quick, lasting period is long, and multiple pests on fruit trees and vegetables can be effectively controlled.
Owner:GUANGXI UNIV
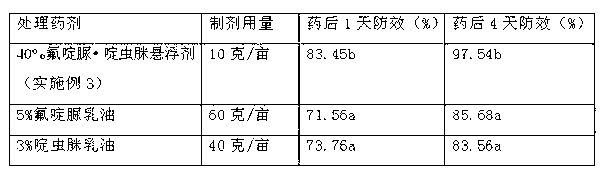
Chlorfluazuron-containing pesticide suspending agent and preparation method thereof
The invention discloses a chlorfluazuron-containing pesticide suspending agent and a preparation method thereof. The chlorfluazuron-containing pesticide suspending agent comprises: by weight, 0.1 to 40% of an active component A, 0.1 to 40% of an active component B, 2 to 10% of one or more dispersants, 1 to 5% of one or more wetting agents, 1 to 8% of one or more functional auxiliary agents, 0.1 to 4% of one or more thickening agents, 1 to 5% of one or more antifreezing agents, 0 to 1% of one or more antiseptics, 0 to 0.8% of one or more antifoaming agents, 0 to 4% of one or more PH conditioning agents, 0 to 2% of one or more anti-oxidants and 10 to 80% of water. The active component A is chlorfluazuron. The active component B is indoxacarb, tebufenozide, acetamiprid, high-efficiency cyhalothrin, thiamethoxam or butene-fipronil. Compared with the traditional pesticide powder, the chlorfluazuron-containing pesticide suspending agent has better drug effects which are the same as drug effects of missible oil, avoids a large amount of a harmful solvent used in missible oil, and has a small average particle size, a high suspension rate, stable performances and a low water separation amount at a normal temperature in two years.
Owner:江西正邦作物保护股份有限公司
Medical ray protection spray and preparation process thereof
InactiveCN105920589AHas antibacterial propertiesAnti-inflammatoryPeptide/protein ingredientsAerosol deliveryAdditive ingredientBULK ACTIVE INGREDIENT
The invention discloses medical ray protection spray. The protection spray is prepared from active ingredients including SOD (superoxide dismutase), curcumin, sodium alginate, potassium sorbate and sorbitol, and purified water. For the ingredients adopted by the medical ray protection spray, curcumin has the efficacies of resisting bacteria, resisting inflammation and easing pain, potassium sorbate can inhibit the microbial growth, sorbitol has the moisturizing function, meanwhile, sodium alginate is large in molecular weight, the interior of the space structure of sodium alginate contains many loose micropore structures which can wrap SOD in the gel form, and moreover, sodium alginate has the capacity of forming gel, thus improving the stability of SOD (superoxide dismutase). After the protection spray is sprayed to the skin surface of a patient, sodium alginate can control SOD, so that SOD is slowly released, and therefore, the drug action of the product is prolonged and improved.
Owner:福建省乐华医药科技有限公司
Medicinal composition for preventing and treating diabetic complications and preparation method thereof
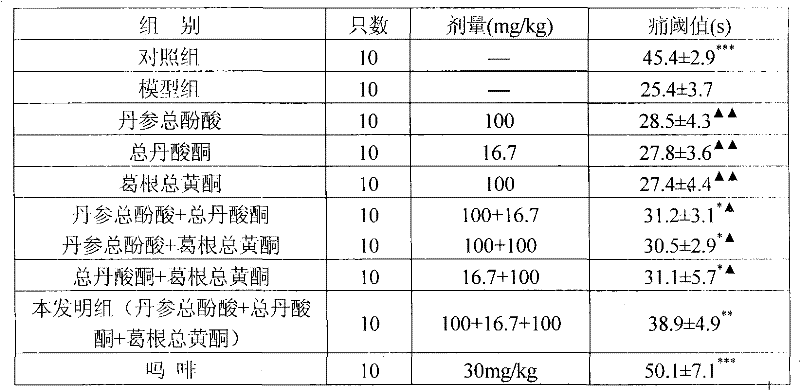
ActiveCN101953887BReduce volumeReduce dosageOrganic active ingredientsNervous disorderSalvia miltiorrhizaDiabetic complication
The invention relates to a medicinal composition for preventing and treating diabetic complications and a preparation method thereof. The medicinal composition comprises the following active ingredients in part by weight: 3 parts of total phenolic acids of root of red-rooted salvia, 0.2 to 1.5 parts of total tanshinones and 1 to 10 parts of total flavonoids of kudzuvine root, and pharmaceuticallyacceptable auxiliary materials or carriers. The preparation method comprises the following steps of: respectively extracting corresponding active ingredients from medicinal materials, namely the rootof red-rooted salvia and the kudzuvine root; and mixing the active ingredients and the pharmaceutically acceptable auxiliary materials or carriers according to the proportion to prepare a corresponding medicinal preparation. Deep studies prove that due to the compatibility of the total tanshinones in the root of red-rooted salvia, the total flavonoids of the kudzuvine root and the total phenolic acids of the root of red-rooted salvia, the medicinal composition has obvious synergistic effect, achieves unexpected obvious effect, enhances the medicinal effect of the composition of the total flavonoids of the kudzuvine root and the total phenolic acids of the root of red-rooted salvia and improves the utilization rate of the medicinal materials.
Owner:SICHUAN ACAD OF CHINESE MEDICINE SCI
Vitamin-D3-contained filling agent for preventing dairy cow mastitis and preparation method of filling agent
InactiveCN103006672AEasy to solveGood treatment effectAntibacterial agentsOrganic active ingredientsEscherichia coliAntioxidant
The invention provides a vitamin-D3-contained filling agent for preventing dairy cow mastitis and a preparation method of the filling agent. Every 10g of filling agent is prepared from the following materials by weight: 20mg-50mg of vitamin D3, 50mg-250mg of ceftiofur hydrochloride, 0.65g-2g of thickener, 50mg-100mg of antioxidant, and the balance of soybean oil or liquid paraffin. The preparation method of the vitamin-D3-contained filling agent comprises the following steps of: preparing the materials for future use; mixing the vitamin D3 and the ceftiofur hydrochloride, dispersing the obtained mixture by the soybean oil or the liquid paraffin, and ultrasonically stirring the mixture with the soybean oil or the liquid paraffin to uniformly disperse the mixture to obtain active ingredient mixed liquor; heating up residual solvent, and adding the thickener and the antioxidant into the mixture to obtain accessory mixed liquor; pouring the active ingredient mixed liquor into the accessory mixed liquor, uniformly stirring the mixture to obtain the vitamin-D3-contained filling agent for preventing the dairy cow mastitis. The vitamin-D3-contained filling agent is used for preventing and curing the dairy cow mastitis caused by pathogenic bacteria such as staphylococcus aureus, streptococcus, escherichia coli and salmonella.
Owner:QILU ANIMAL HEALTH PROD
Quality control method of four-time treated rhizoma cyperi decoction pieces
ActiveCN104155399AImproved anti-dysmenorrhea pharmacological effectsSimple and easy quality control methodComponent separationFingerprintHplc fingerprint
The invention discloses a quality control method of four-time treated rhizoma cyperi decoction pieces. The quality control method comprises the steps: establishing a four-time treated rhizoma cyperi HPLC fingerprint spectrum, and with an active ingredient alpha-cyperone as a benchmark peak, calculating the content of alpha-cyperone in the benchmark peak; and allowing fingerprint peaks of other main active ingredients to take alpha-cyperone as a reference, calculating the relative contents of the fingerprint peaks of the active ingredients according to alpha-cyperone. The method has operability, can control the relative contents of other unknown main effective ingredients only with the known reference substance alpha-cyperone ingredient content, and is a scientific method achieving control of the quality of the four-time treated rhizoma cyperi decoction pieces. The method is simple, is easy to popularize, reduces the use amount of the reference substance, solves the problems that a market monomeric material reference substance is insufficient and expensive, makes the quality of the four-time treated rhizoma cyperi decoction pieces stable, enhances the pharmacological function, and is conducive to promote rapid development of traditional Chinese medicine industries.
Owner:江西龙开河中药饮片有限公司
Metformin hydrochloride gastric floating tablet and preparation method thereof
InactiveCN103110600AControl releaseFast drug effectOrganic active ingredientsMetabolism disorderWaxWater soluble
The invention relates to a metformin hydrochloride gastric floating tablet. The metformin hydrochloride gastric floating tablet contains metformin hydrochloride, a polymer gel material, a wax material and a foaming material, wherein the polymer gel material is a mixture of water-soluble resin and lactose; the wax material is at least one of octodecyl alcohol and stearic acid; and the foaming material is a mixture of sodium bicarbonate and magnesium stearate. A preparation method comprises wet process pelletization and powder direct tabletting, wherein the wet process pelletization comprises the following steps: A1) preparing the materials; A2) mixing the materials; A3) pelletizing; and A4) tabletting; and the powder direct tabletting comprises the following steps: B1) preparing the materials; B2) mixing the materials; and B3) tabletting. Each metformin hydrochloride gastric floating tablet contains 0.25-0.75g of metformin hydrochloride. The metformin hydrochloride gastric floating tablet disclosed by the invention has the advantages that after the tablets are taken by a patient, hydrophilic gel is hydrated and expanded, a medicament is simultaneously controlled to be released slowly, the onset of action of the medicament is fast, the medicament effect is lasting and the bioavailability of the medicament is high; and the frequency of taking the tablets of the patient can be reduced, the side effects are reduced, and the incidence rate of poor reactions is also reduced.
Owner:CHENGDU HENGRUI PHARMA
A pharmaceutical composition containing an analgesic and fospropofol disodium
InactiveCN103816542AGood analgesic effectReduce adverse reactionsOrganic active ingredientsNervous disorderNefopam HydrochlorideSedation
The invention discloses a pharmaceutical composition containing a non-narcotic analgesic or a narcotic analgesic and pharmaceutically acceptable salts thereof and fospropofol disodium. The non-narcotic analgesic is nefopam hydrochloride or nefopam. The narcotic analgesic is selected from fentanyl, remifentanil, alfentanil and sufentanil. The pharmaceutical composition can be directly used for clinic comprising anesthesia induction, maintenance of anesthesia, post operation or ICU analgesia, and the like. The pharmaceutical composition has functions of analgesia and sedation. Through addition or synergistic effects of medicines, the pharmaceutical effects are enhanced, the dosage is reduced, untoward effects in pain treatment is prevented and relieved, the number of times of administration by an anesthetist is reduced, and therefore an anesthesia induction process is more convenient and an ideal anesthesia effect can be achieved.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Preparation method of Trichosanthes kirilowii Maxim oral liquid
InactiveCN110898120AGood for treating constipationQuick Fill and SaveThreaded caps applicationDispersion deliveryAngelica Sinensis RootTrichosanthes kirilowii
The invention discloses a preparation method of Trichosanthes kirilowii Maxim oral liquid, comprising the following steps: step 1, weighing raw materials: weighing 19-21 parts of Agastache rugosus, 56-64 parts of Mangnolia officinalis, 19-21 parts of white paeony root, 9-10 parts of delavay soapberry pericarp seed, 9-11 parts of Platycodon grandiflorum, 14-16 parts of apricot kernel, 60-80 parts of Trichosanthes kirilowii Maxim, 9-13 parts of Angelica sinensis, and 19-21 parts of peach kernel. Trichosanthes kirilowii Maxim added in the invention has the effects of moistening lung for removingphlegm, smoothing intestine and relaxing the bowels and is mainly used for cough caused by dryness and phlegm and constipation due to intestinal dryness; Mangnolia officinalis has therapeutical effecton diseases such as dyspepsia and qi stagnation, abdomen distension and constipation, damp retention in middle-jiao and the like; Angelica sinensis has effects of invigorating qi and blood, regulating menstruation and relieving pain, moistening dryness and smoothing intestine; white paeony root has efficacy of warming yang and eliminating dampness, tonifying body deficiency, strengthening spleenand stomach, etc.; and peach kernel has the effect of promoting digestion and removing food retention. By combining the various medicines to achieve the effects of tonifying middle-Jiao and qi, tonifying spleen and promoting circulation, the oral liquid of the invention is used for treating constipation.
Owner:ANHUI ZANJIATIAN ECOLOGICAL AGRI CO LTD
Aromatic turmeric oil extracts and its preparing process and medicinal composition containing the same
InactiveCN1557387AClear ingredientsLess irritatingKetone active ingredientsAntiviralsVascular diseaseSide effect
The zedoary extractive is prepared with zedoary oil as material and through chromatography with silica gel column, and contains curdione 30-60 wt%, elemenone 15-40 wt%, neo-curdione 1-15 wt%, curcumenol1-20 wt% and no instable benzofuran derivative. The zedoary extractive has high effect, less side effect and high stability, and may be mixed with different medicine carrier to prepare various medicine preparations, such as capsule and injection, for treating viral diseases, cardiac and cerebral vascular diseases and tumor.
Owner:李绍平
Method for preparing tripterygium wilfordii extract by using triterpenes as main components and pharmaceutical application of tripterygium wilfordii extract
The invention discloses a method for preparing a tripterygium wilfordii extract by using triterpenes as main components and a pharmaceutical application of the triterpenes as main components. The method for preparing the tripterygium wilfordii extract by using triterpenes as main components comprises the following steps: (1) crushing a dried tripterygium wilfordii root medicinal material, screening through a screen of 10-40 meshes, and weighing 150-200g of powder; (2) performing ultrasonic extraction with an extracting solvent, wherein the usage ratio of the extracting solvent to the medicinal materials is (8-16)ml:1g; filtering the extract, reclaiming the filtrate at reduced pressure to extract the solvent to obtain concentrate, and drying to obtain a coarse product of tripterygium wilfordii extract; and (3) adding 5.0-15.0g of the coarse product of tripterygium wilfordii extract into a solvent for re-dissolving, wherein the usage ratio of the solvent to the coarse product of tripterygium wilfordii extract is (10-15)ml:1g, filtering, and reclaiming the solvent at reduced pressure to obtain the tripterygium wilfordii extract. The invention also discloses a medicament prepared from the tripterygium wilfordii extract for treating rheumatoid arthritis. The content of total triterpenes of the prepared tripterygium wilfordii extract is 50-75 percent, so that the utilization of a tripterygium wilfordii medicinal material is improved, and the toxicity of the tripterygium wilfordii medicinal material is reduced.
Owner:ZHEJIANG CHINESE MEDICAL UNIVERSITY +2
Compound injection of Xylazine, and preparation technique
InactiveCN1957918AIdeal anesthesia brake effectEasy to usePharmaceutical delivery mechanismAnaestheticsAcetic acidDihydroetorphine hydrochloride
An injection of compound xylazine for the anesthesia of wild animal is prepared through dissolving xylazine and EDTA in distilled water, adding the solution of dihydroetorphine hydrochloride, stirring, filling it in ampules, and sterilizing.
Owner:闫章年
Method for preparing lipid-lowering chewable tablet of four leaves
InactiveCN103877161AFast drug effectHigh purityMetabolism disorderPill deliveryFiltrationGinkgo biloba
The invention discloses a method for preparing a lipid-lowering chewable tablet of four leaves. The method comprises the following steps: firstly, crushing lotus leaf, folium mori, ginkgo leaf and chrysanthemum leaf to obtain powder a; carrying out supercritical CO2 extraction on the powder a to obtain an extract solution; refrigerating at 0-5 DEG C for 12 hours; carrying out suction filtration, extraction, vacuum concentration and vacuum drying to obtain a dry extract; crushing to obtain extract powder; then drying the lotus leaf and smashing by using an ultrafine grinder to obtain lotus leaf superfine powder; putting skim milk powder and microcrystalline cellulose into a mortar to grind and evenly mix, so as to obtain powder b; evenly mixing the extract powder with the powder b; adding the lotus leaf superfine powder, and evenly mixing to obtain a mixture; adding polyvinylpyrrolidone to the mixture to prepare a soft material; adding superfine silica powder after pelletizing and straightening, and then tabletting, so as to obtain the lipid-lowering chewable tablet of the four leaves. The lipid-lowering chewable tablet of the four leaves disclosed by the invention can be used for effectively reducing the weight, the fat and the liver weight of mice, and has latent effects of reducing weight and lowering lipid.
Owner:ANHUI XINHUA UNIV
Coumarin and phenylchromone extract from angelica polymorpha maxim and preparation method and use thereof
InactiveCN102188464AQuality is easy to controlActive ingredients are clearAntipyreticAnalgesicsSide effectPharmacodynamics
The invention relates to an angelica polymorpha maxim extract, which contains coumarin and phenylchromone components, wherein the coumarins include one or more of oxypeucedanin hydrate, pabulenol, isoimperatorin, osthol, bergapten, imperatorin, 22,23-dihydroavermectin, isopimpinellin and oxypeucedanin ethylether; and the phenylchromones include one or more of 3'R-(+)hamaudol, 3',-O-acetylhamaudol, angeliticin and ledebouriellol. The invention also relates to a preparation method, a quality control method and a medicinal composition of angelica polymorpha maxim extract and use of the angelica polymorpha maxim extract in the preparation of medicines or products for treating pain and / or inflammatory disorders. The experiments on the pharmacodynamic and acute toxicity of the extract indicate that the extract has the advantages that: the active ingredients are clear; the medicinal effect is remarkable; the toxic and side effects are small; the product quality is high; the dosage is small; the quality is controllable; and the like.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
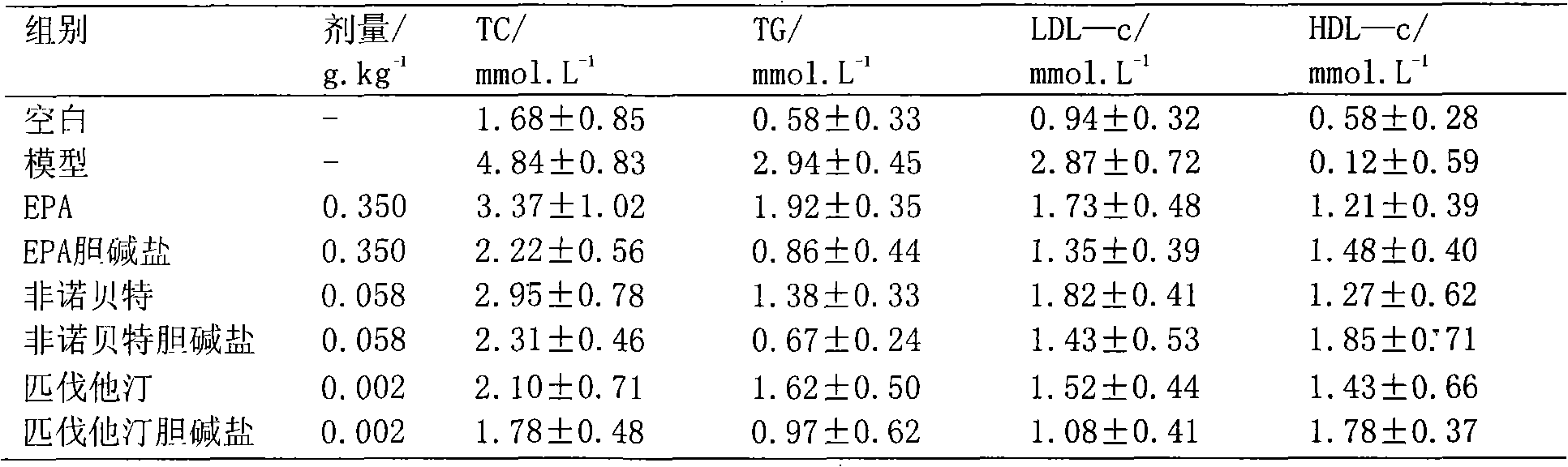
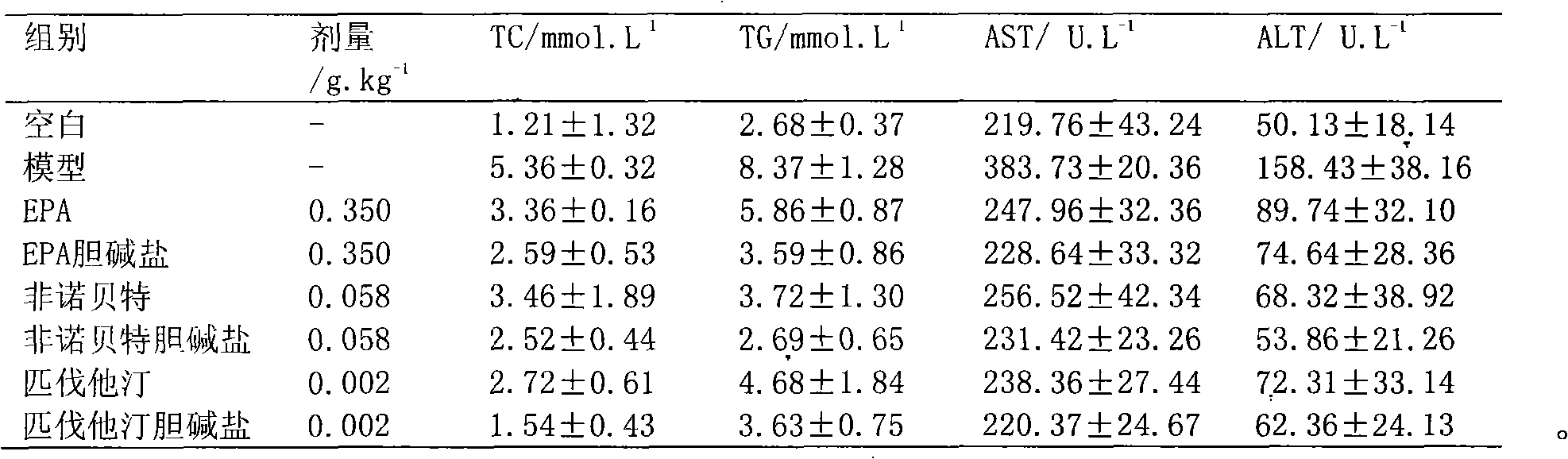
Choline salt of hypolipidemic drug and preparation method and pharmaceutical use thereof
InactiveCN101863780APromote absorptionEfficient use ofOrganic chemistryElcosanoid active ingredientsDocosahexaenoic acidPitavastatin
The invention relates to a choline salt of a hypolipidemic drug and a preparation method and a pharmaceutical use thereof. The invention provides a choline salt of a class of hypolipidemic drugs, and the hypolipidemic drugs include but not limited to clofibrate, libet, fenofibrate, ciprofibrate, gemfibrozil, acipimox, niacin, lovastatin, simvastatin, pravastatin, fluvastatin, atorvastatin, rosuvastatin, pitavastatin, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) and other unsaturated fatty acids. The choline salt of the hypolipidemic drug can be used for treating hyperlipidemia and other cardiovascular diseases. The invention also provides a preparation method of the choline salt of the hypolipidemic drug.
Owner:北京利乐生制药科技有限公司
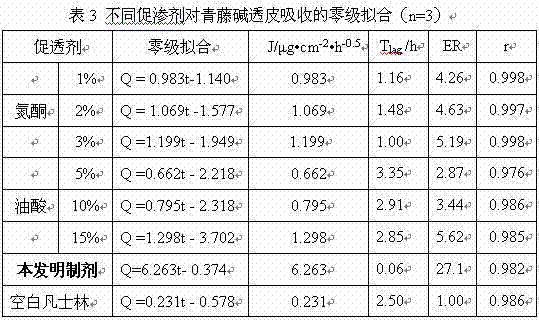
Sinomenine preparation and preparation method thereof
ActiveCN103690473AGood transdermal effectFast drug effectOrganic active ingredientsAntipyreticEucalyptus oilMedicine
The invention aims to provide a sinomenine preparation. The preparation is prepared from sinomenine with a proper concentration, ostrich oil, vitamin E with a proper concentration and eucalyptus oil. The preparation method comprises the following steps: heating and melting the ostrich oil into liquid; adding sinomenine powder, and uniformly stirring and dispersing; adding eucalyptus oil and vitamin E, stirring and cooling. According to the preparation, the transdermal effect of sinomenine can be improved and the drug effect of the medicine can be improved; ostrich oil is a pure natural raw material, is capable of reducing skin irritation when used as a drug-loading matrix; in addition, ostrich oil has arthritis resistance and can realize arthritis treatment when used together with sinomenine.
Owner:ZHEJIANG UNIV
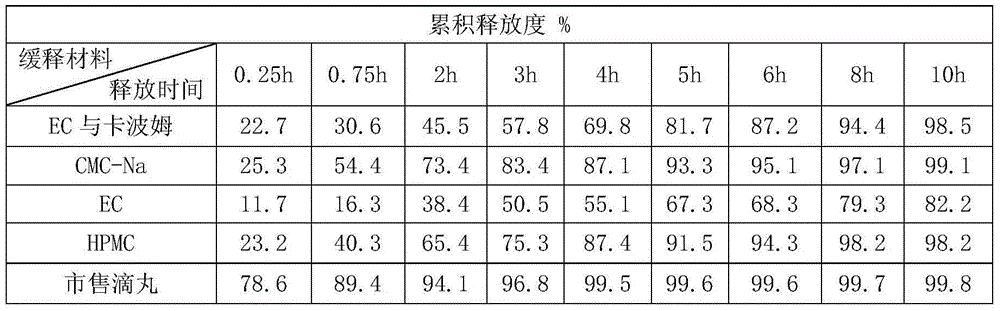
Gynecological menstruation-regulating slow-release dropping pills and preparation method thereof
ActiveCN106466396AFully extractedGood sustained release effectPharmaceutical delivery mechanismPharmaceutical non-active ingredientsAlcoholActive component
The invention provides gynecological menstruation-regulating slow-release dropping pills and a preparation method thereof. According to the present invention, by optimizing the original herb extraction process and using the double extraction method of alcohol extraction and water extraction, the active components of the Chinese herbs are completely extracted; in order to overcome the defects of the gynecological menstruation-regulating dropping pills in the prior art, a lot of test screening researches are performed; and the ethyl cellulose is used to compound so as to provide the good biodegradability, the carbomer has the good slow release effect is adopted as the skeleton material, and a proper amount of polyethylene glycol is added to prepare the slow release dropping pills, such that the product quality is stable, the number of medication can be reduced, the stable plasma concentration can be maintained, and the efficacy can be enhanced.
Owner:GUIZHOU YIBAI WOMAN BIG PHARMA FACTORY
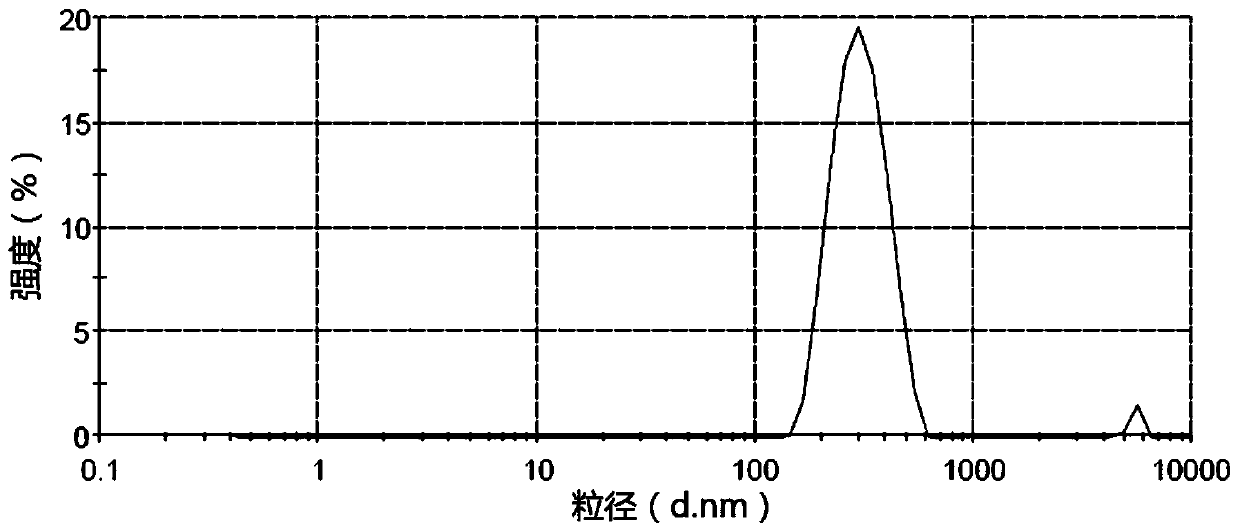
Aprepitant nanocrystal suspension and preparation method and application thereof
InactiveCN110478316AOvercome the defect of mixing and easy flocculation and sedimentationLower requirementOrganic active ingredientsDigestive systemCelluloseSuspending Agents
The invention provides an aprepitant nanocrystal suspension and a preparation method and application thereof. The preparation method of the aprepitant nanocrystal suspension comprises the following steps that an aprepitant nanocrystal solution is added into blank material fluid for mixing, and the aprepitant nanocrystal suspension is obtained; the aprepitant nanocrystal solution is prepared from aprepitant nanocrystals and a stabilizer; and the blank material fluid comprises a thickening suspending agent, and the thickening suspending agent is one or more of syrup, sorbitol, xanthan gum, Arabic gum, starch slurry, cellulose class, povidone class, Tween 80 and poloxamer. According to the provided aprepitant nanocrystal suspension, the particle diameter of the aprepitant nanocrystal suspension has no obvious difference before and after being frozen, the stability is good, and the storage condition adaptability is good; and meanwhile the dissolving-out speed of the aprepitant nanocrystalsuspension is high, the oral bioavailability is higher than that of other aprepitant bulk drugs.
Owner:JIANGXI UNIVERSITY OF TRADITIONAL CHINESE MEDICINE +1
Method for rapid growth of fish
InactiveCN107691301ANutritious propertiesImprove conversion rateFood processingClimate change adaptationFowlFishery
The invention relates to the technical field of agricultural production, in particular to a method for rapid growth of fish. The method comprises the steps of (1) pond treatment, wherein a pond is under the blazing sun for 7 days before fry are put in the pond, then quicklime is added to sludge on the bottom of the pond for pond disinfection, before water introduction, a mixture of fermented fowlmanure and straw is added into the pond, and the mixture of fermented fowl manure and straw is mixed with the topsoil on the bottom of the pond; (2) fry treatment, wherein healthy and undamaged fry are selected, immersed in 100-200 ppm of Formalin solution and subjected to dipping bath for 1 hour; (3) fry feeding, wherein the fry are fed with fattening Chinese herbal medicine feed every day afterput in the pond, and feeding is conducted at three times every day. By means of the method, fish can rapidly grow and get fattened.
Owner:东兰县安篓东二养殖场(微型企业)
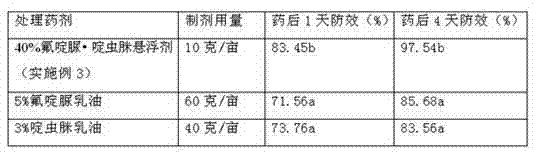
Clothianidin and beta-cyfluthrin suspending agent and preparation method thereof
ActiveCN111084191AImprove wettability and dispersibilityFast drug effectBiocideAnimal repellantsSulfonateClothianidin
The invention relates to a clothianidin and beta-cyfluthrin suspending agent and a preparation method thereof. The suspending agent is prepared from the following raw materials in parts by weight: 20-25 parts of clothianidin, 5-10 parts of beta-cyfluthrin, 4-7 parts of a composite dispersing agent, 0.2-0.6 part of a nonionic lipid emulsifier, 0.3-0.7 part of an anionic sulfonate wetting agent, 3-5parts of an anti-freezing agent, 1-1.5 parts of a thickening agent, 0.1-0.3 part of a preservative, 0.5-1 part of a regulator and 0.1-0.5 part of a defoaming agent , with the balance being water. Thesuspending agent provided by the invention is prepared by adding the composite dispersing agent, the nonionic lipid emulsifier and the anionic sulfonate wetting agent into effective components; through a compounding effect of the composite dispersing agent, the nonionic lipid emulsifier and the anionic sulfonate wetting agent, the mixing uniformity of the components of the suspending agent can besignificantly improved; the wetting and dispersing effects of the suspending agent on the surface of the root part of garlic can be enhanced; thus, the pesticide effect of the suspending agent is exerted to a maximum extent when the suspending agent is used in a low-temperature environment; and by addition of the anti-freezing agent, the storage stability of a product can be easily kept when thesuspending agent is used in the low-temperature environment.
Owner:南京保丰农药有限公司
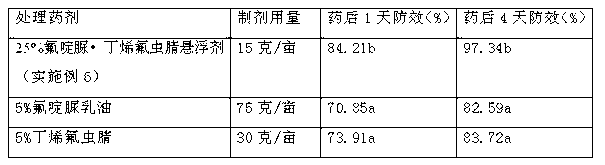
Pesticide suspending agent containing chlorfluazuron and butene-fipronil and preparation method of pesticide suspending agent
The invention provides a pesticide suspending agent containing chlorfluazuron and butene-fipronil and a preparation method of the pesticide suspending agent. The pesticide suspending agent comprises an active component A which is chlorfluazuron and an active component B which is butene-fipronil. A pesticide effect of the pesticide suspending agent provided by the invention is superior to that of conventional powder and emulsion oil, and meanwhile, a lot of harmful solvents used in the emulsion oil are avoided. The pesticide suspending agent is less in average grain size, high in suspensibility, stable in performance and extremely small in water bleeding amount when being preserved at normal temperature within two years. The pesticide effect of the compound pesticide suspending agent is remarkably superior to that of the single component chlorfluazuron or butene-fipronil.
Owner:江西正邦作物保护股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com