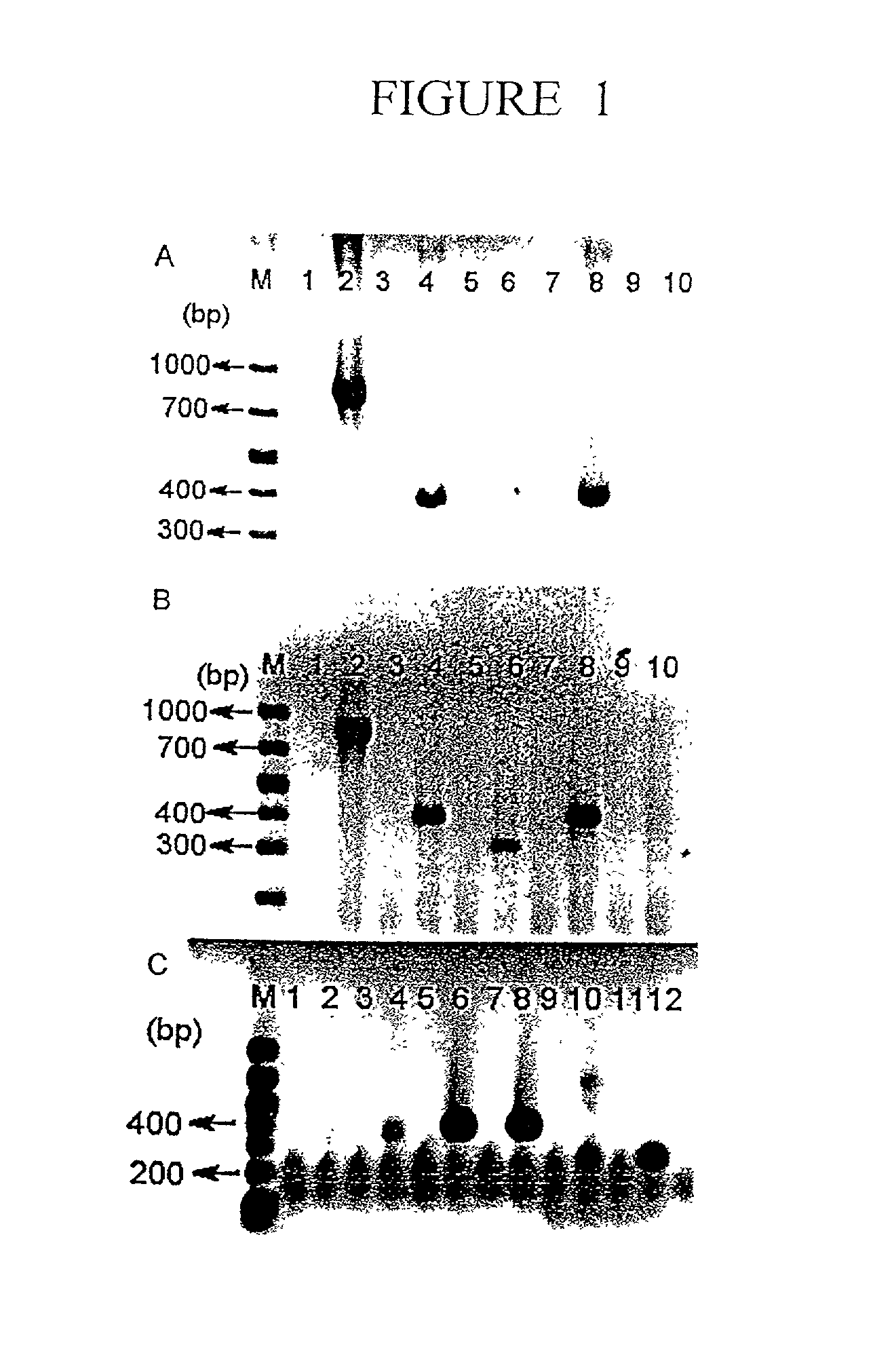
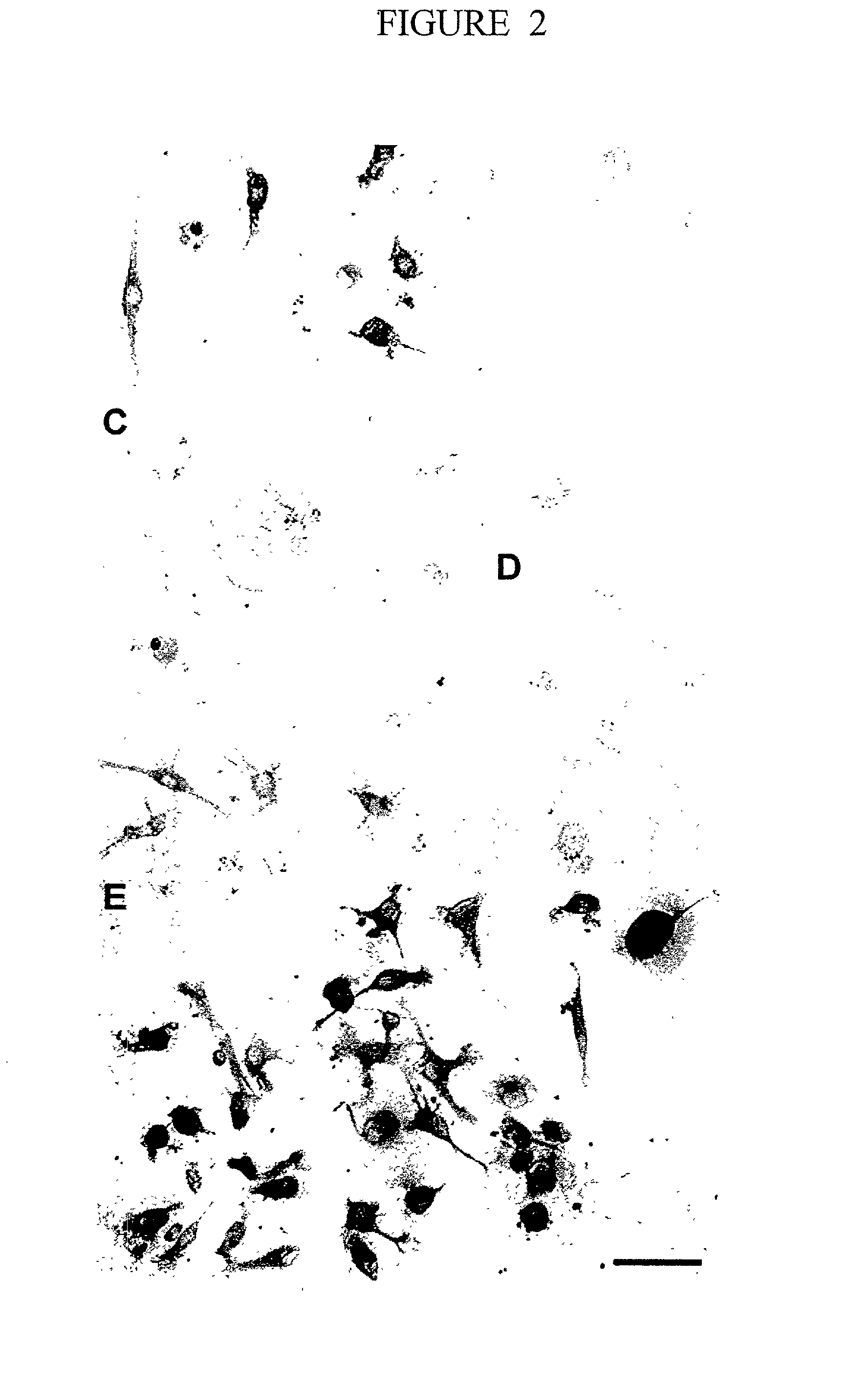
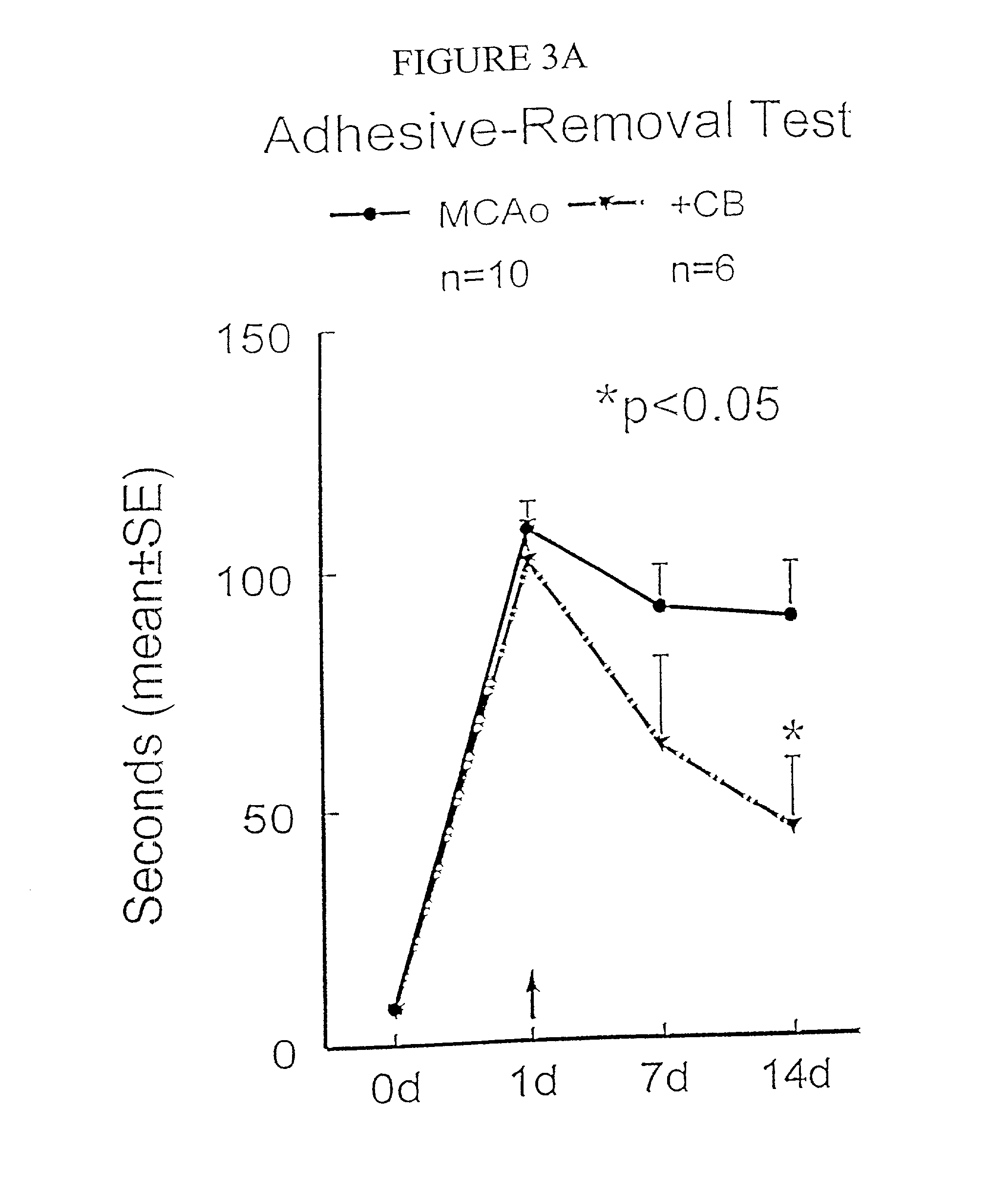
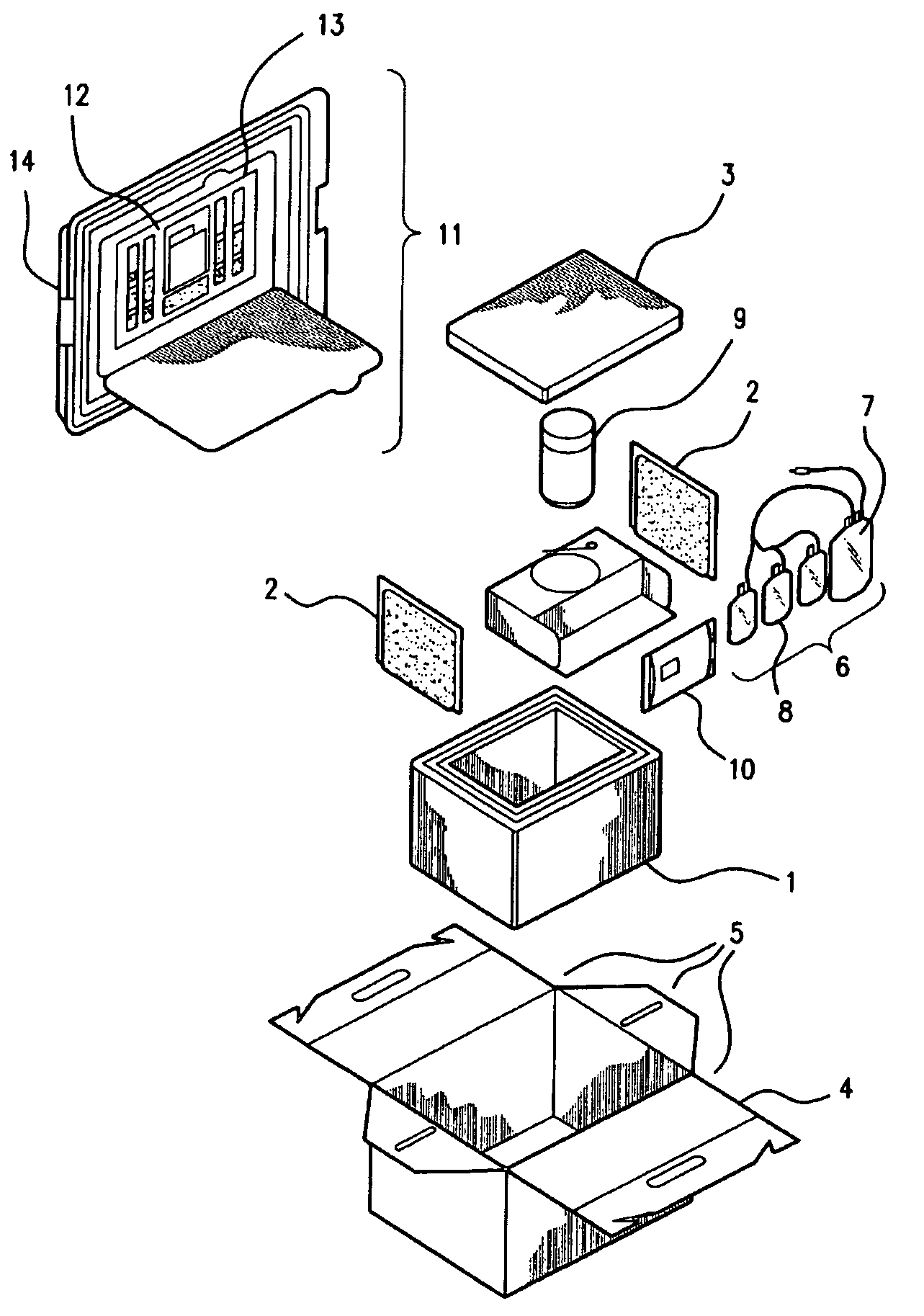
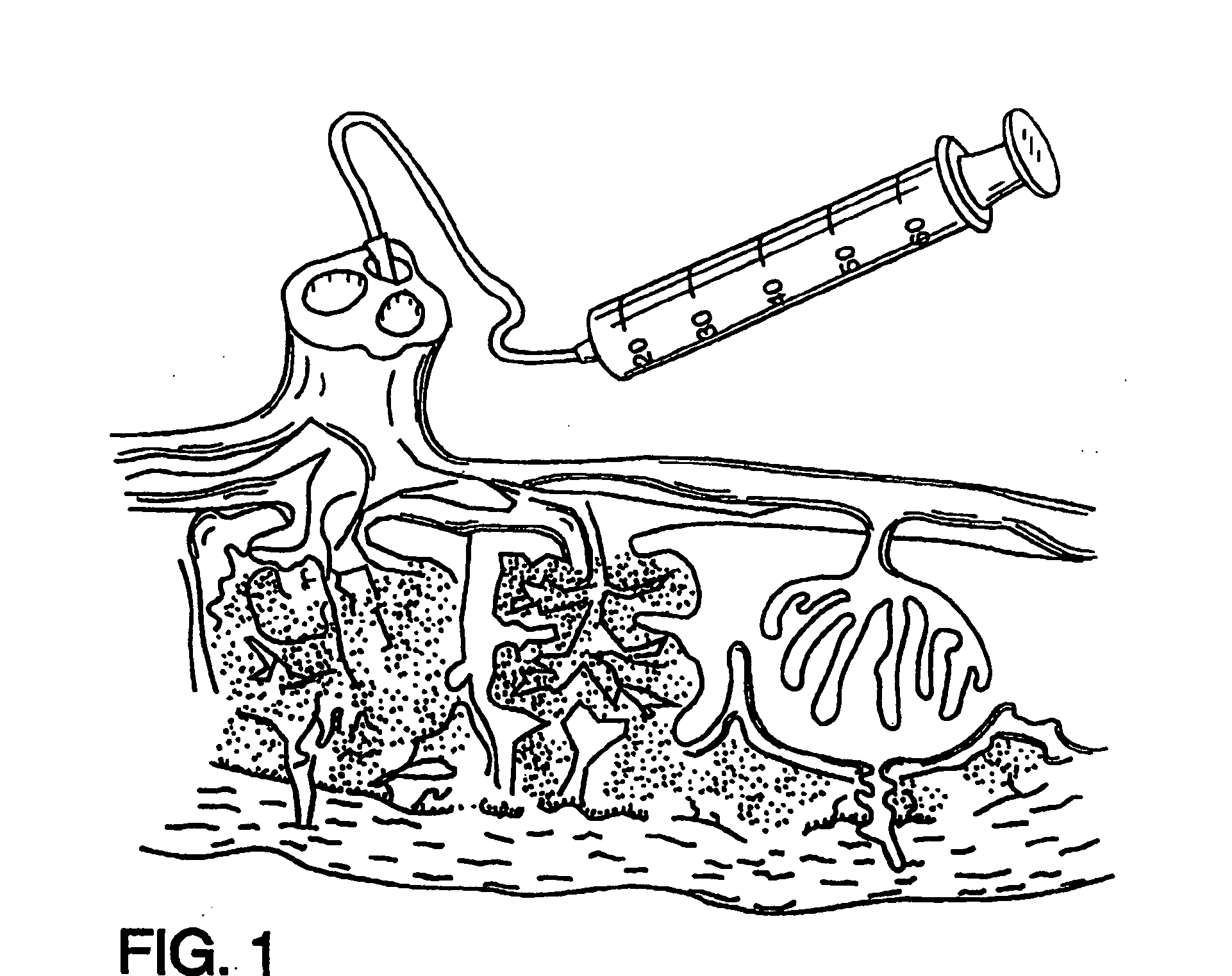Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
338 results about "Cord blood stem cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
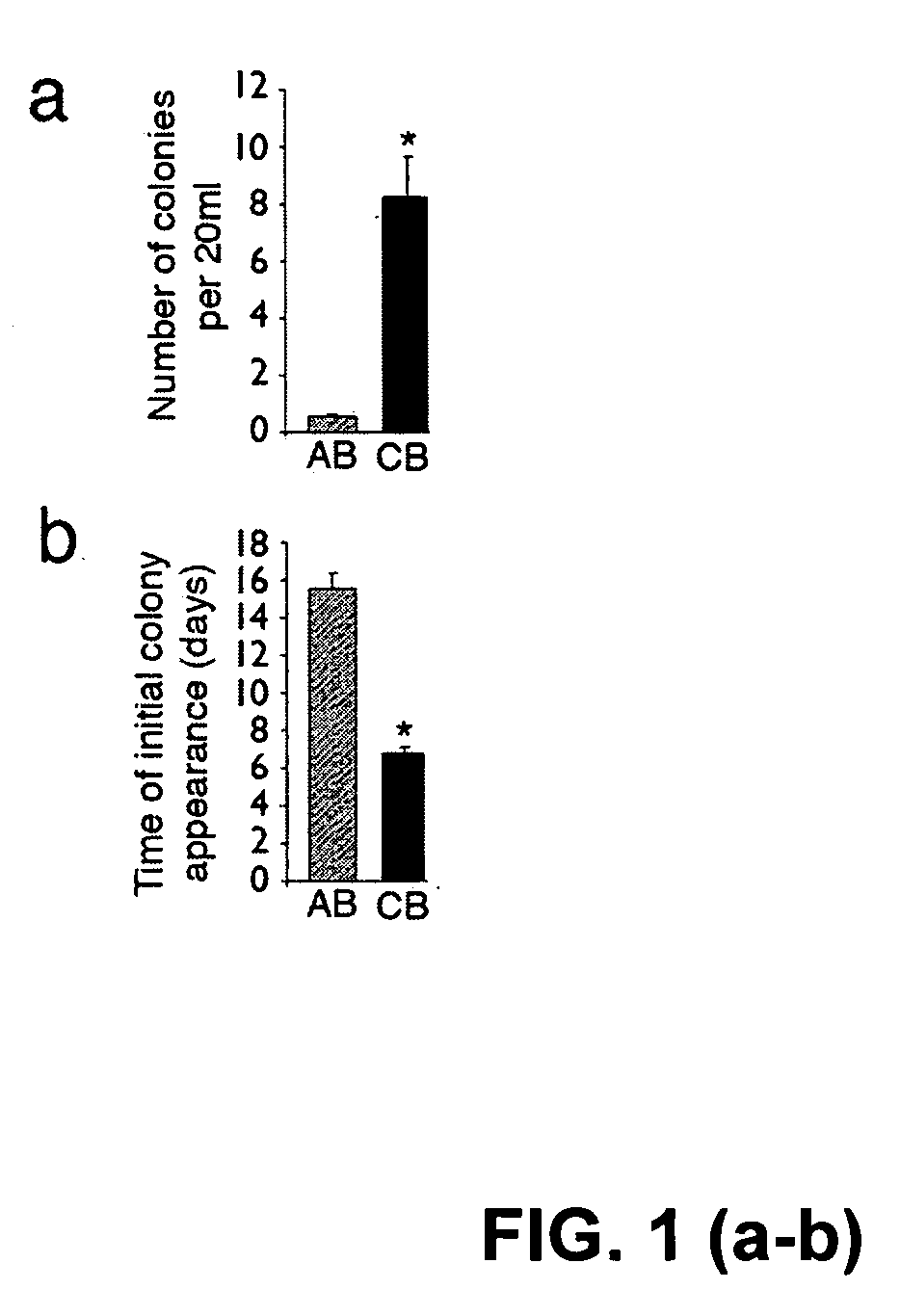
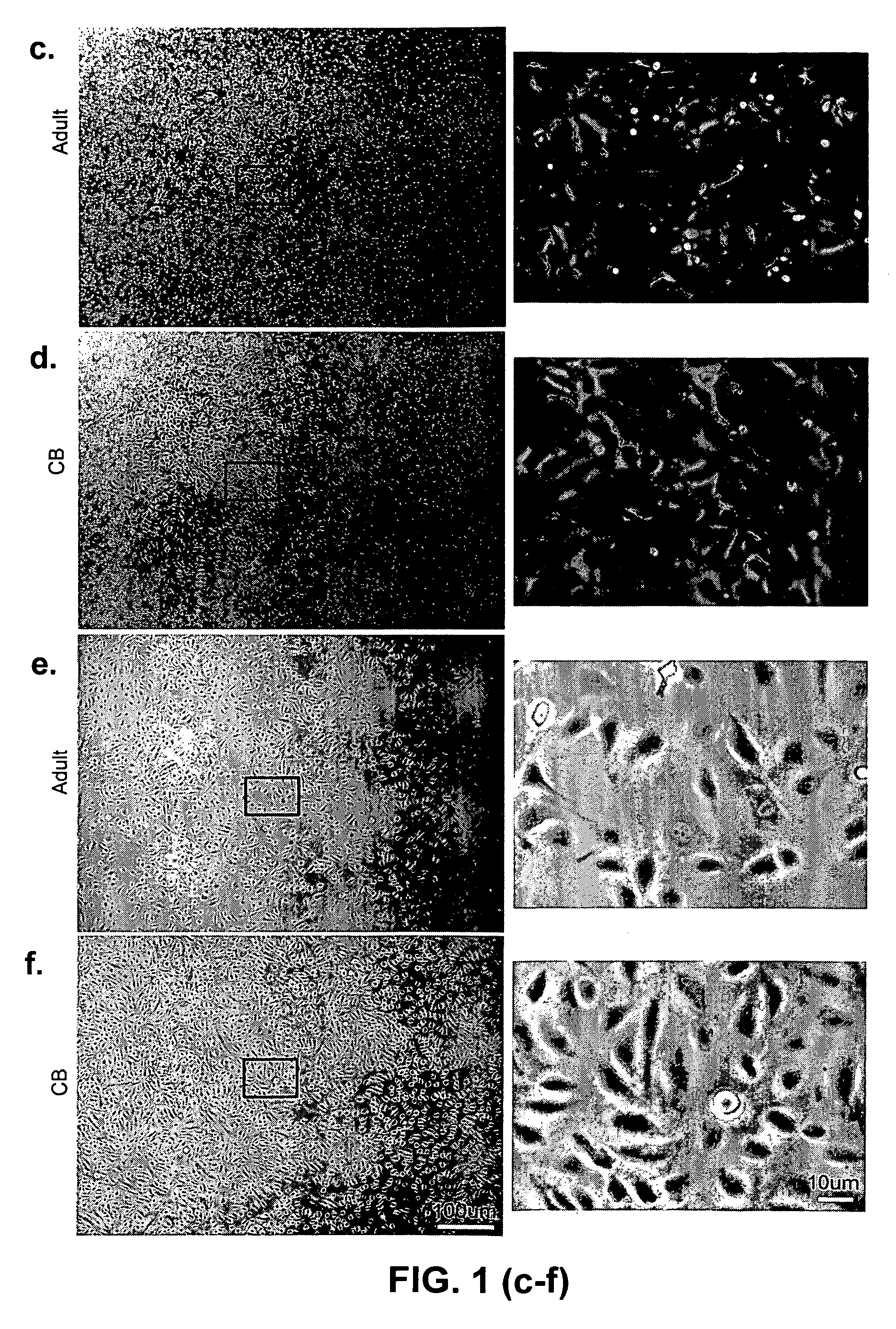
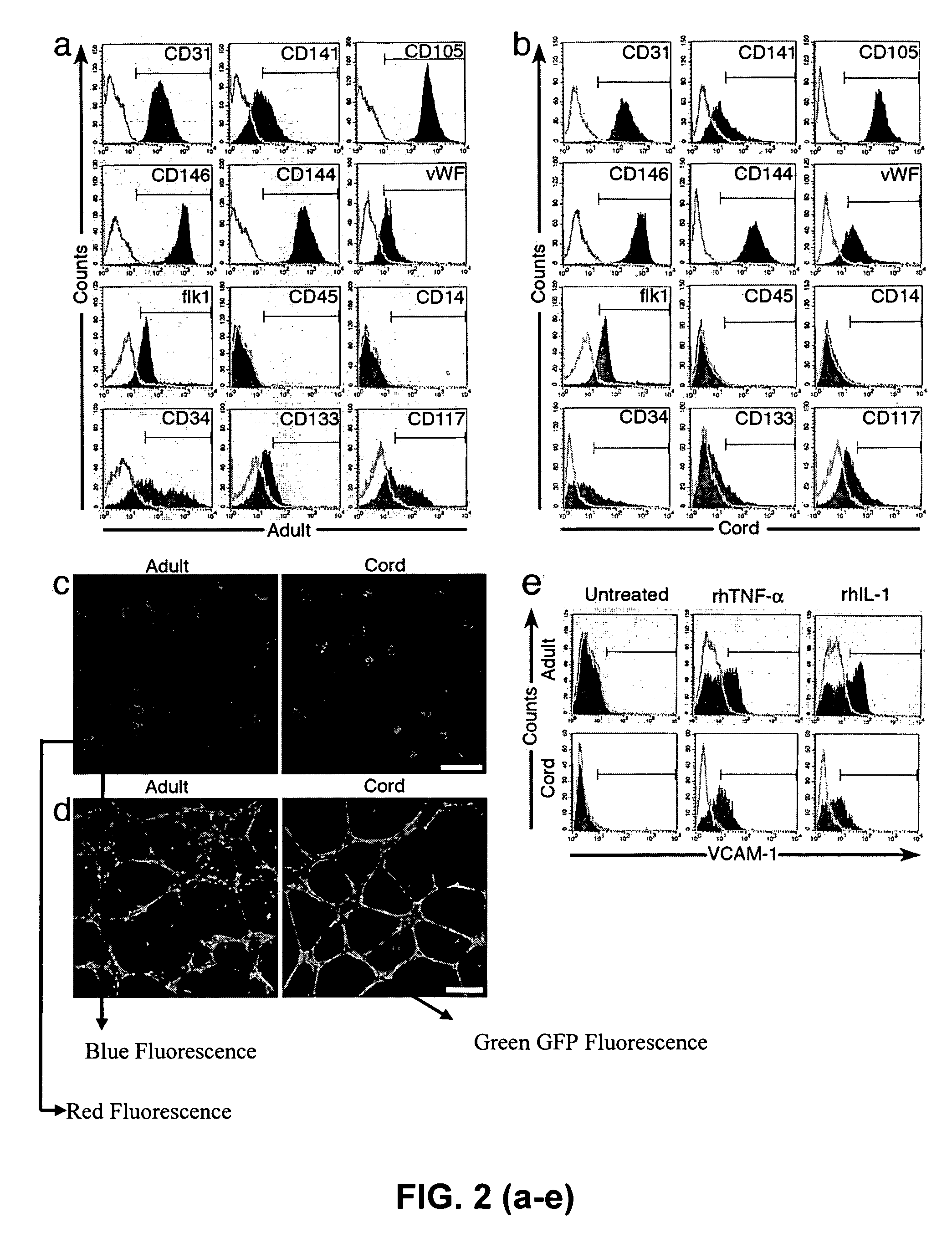
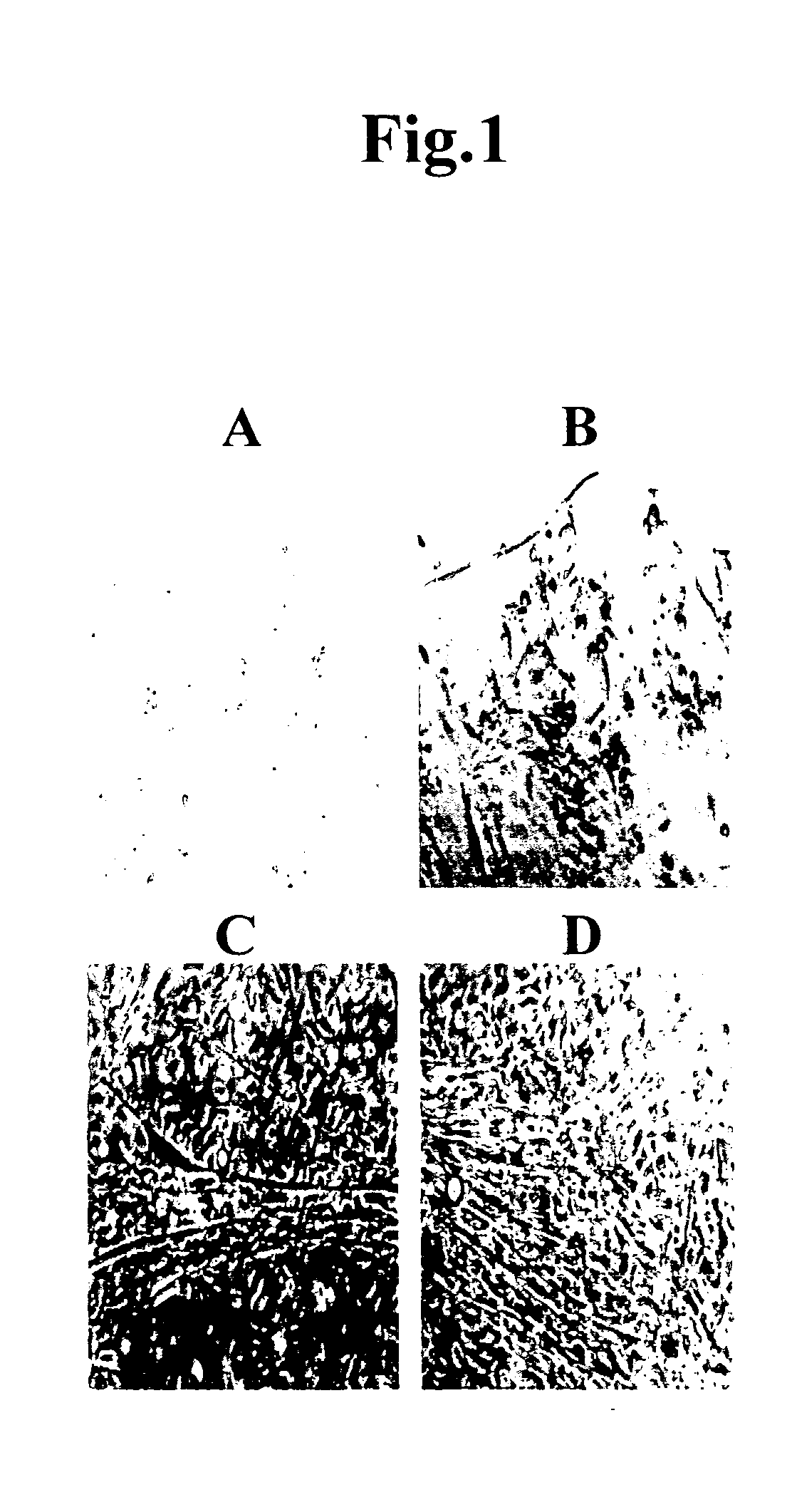
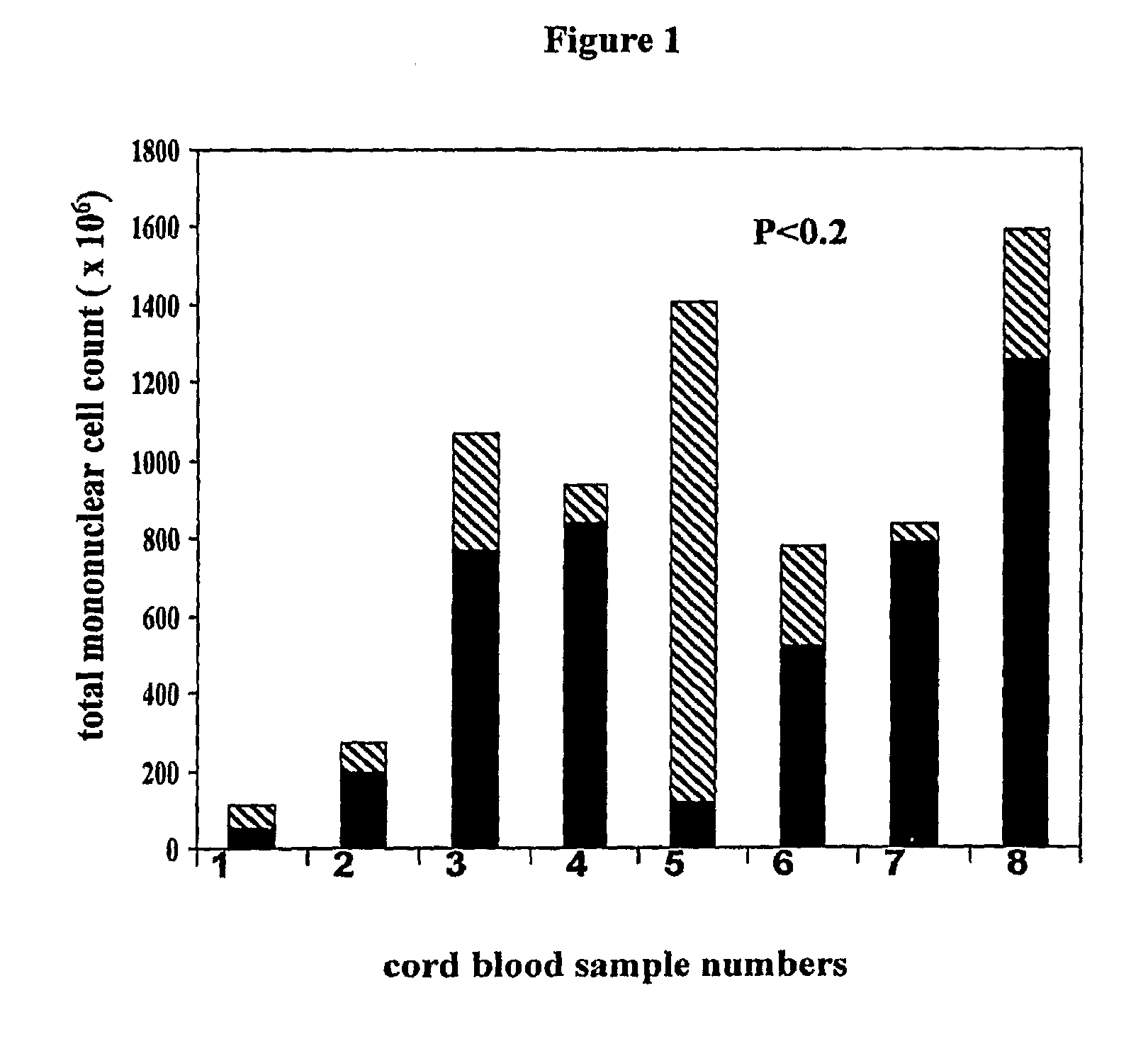
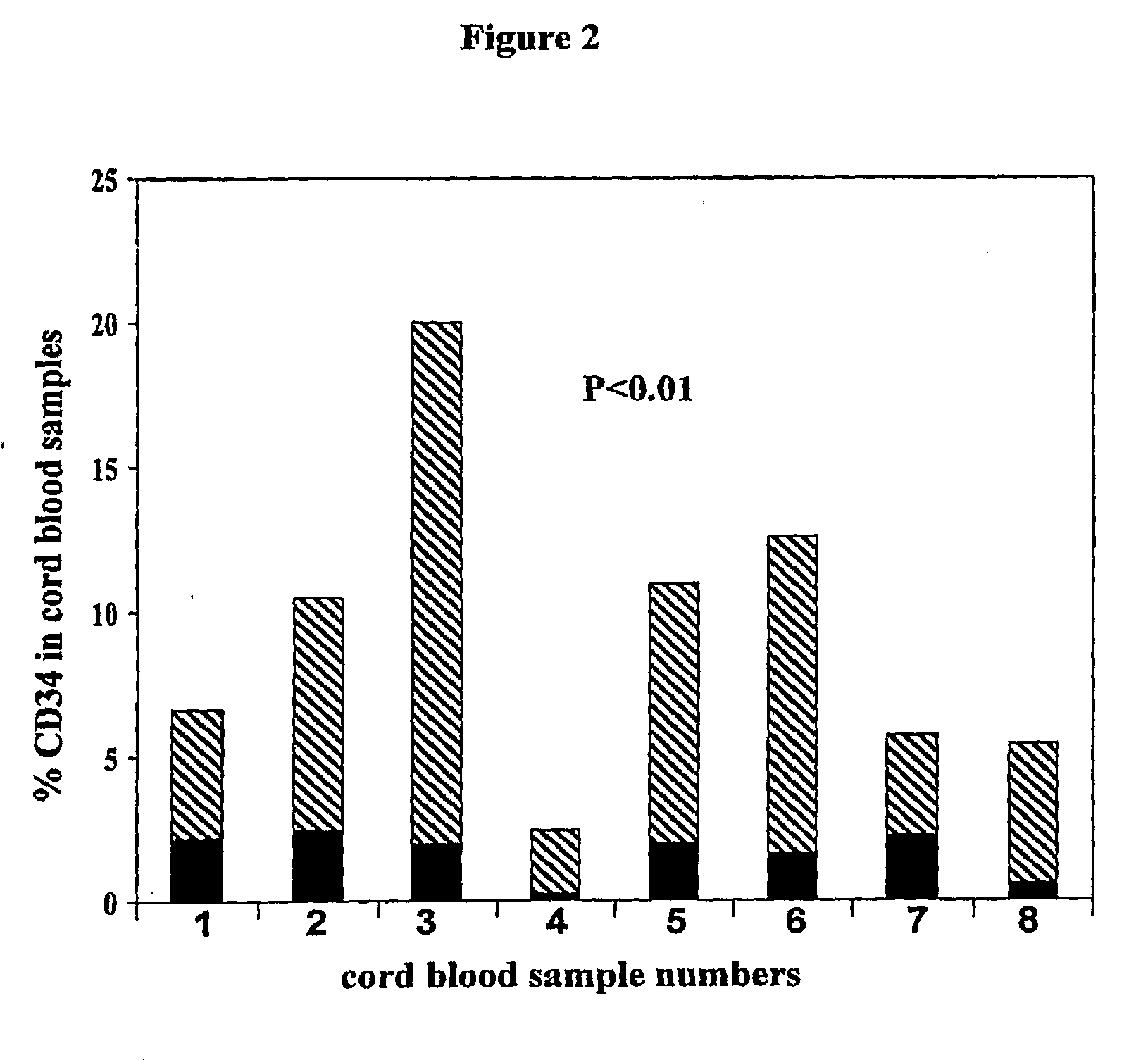
Cord blood is being used to get stem cells with which to test in people with type 1 diabetes mellitus. The stem cells from umbilical cord blood are also being used in the treatment of a number of blood diseases including blood cancers.
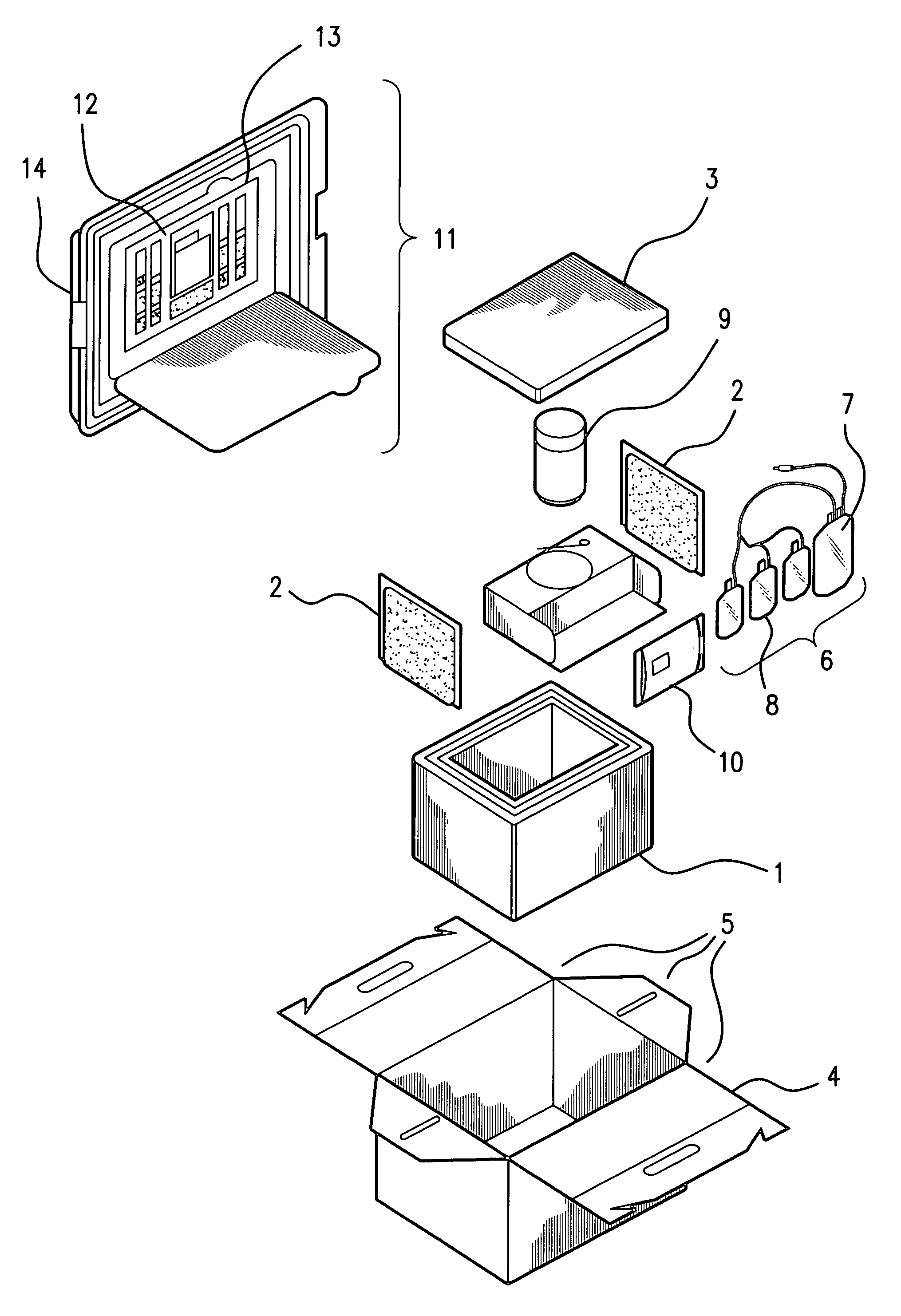
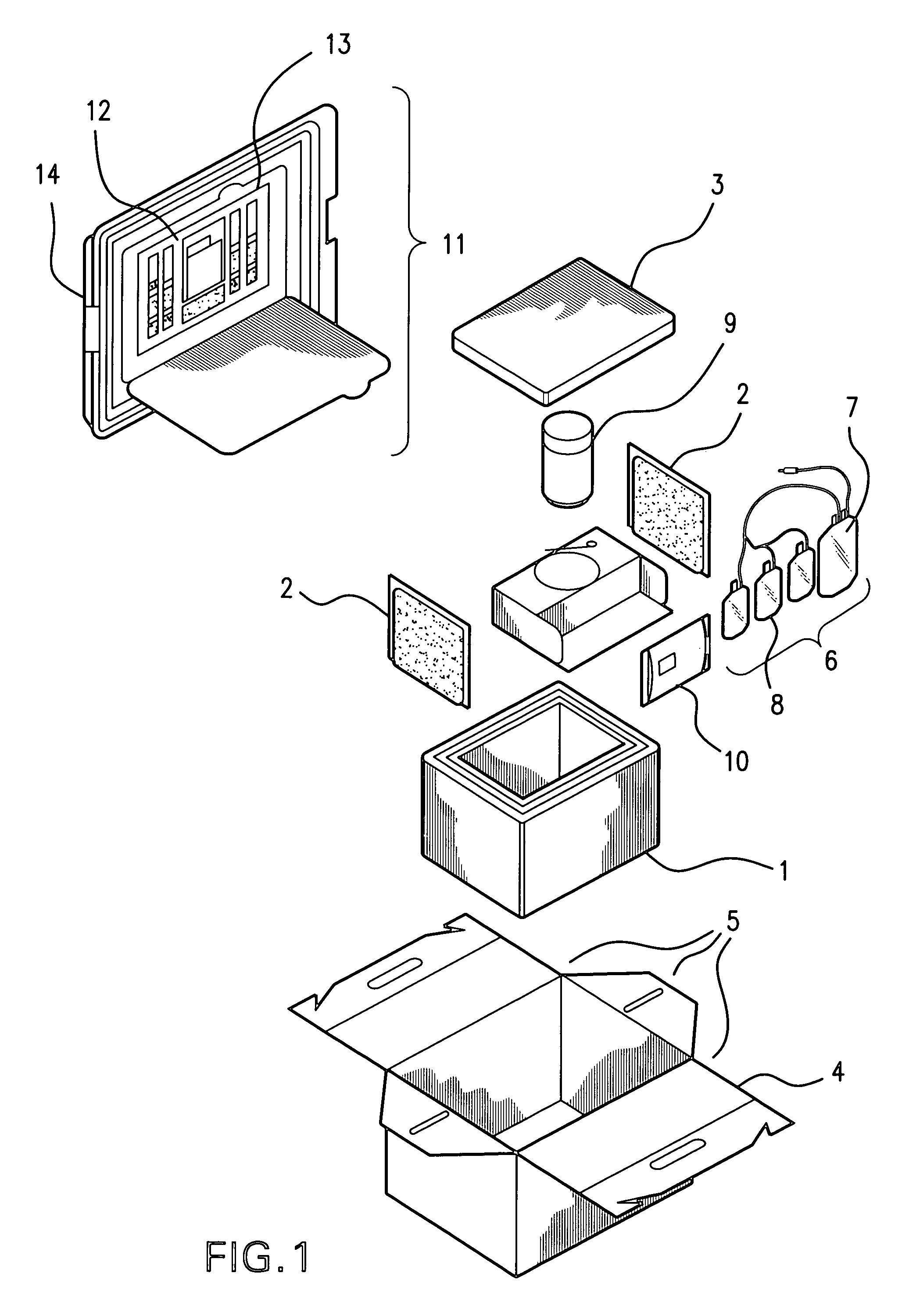
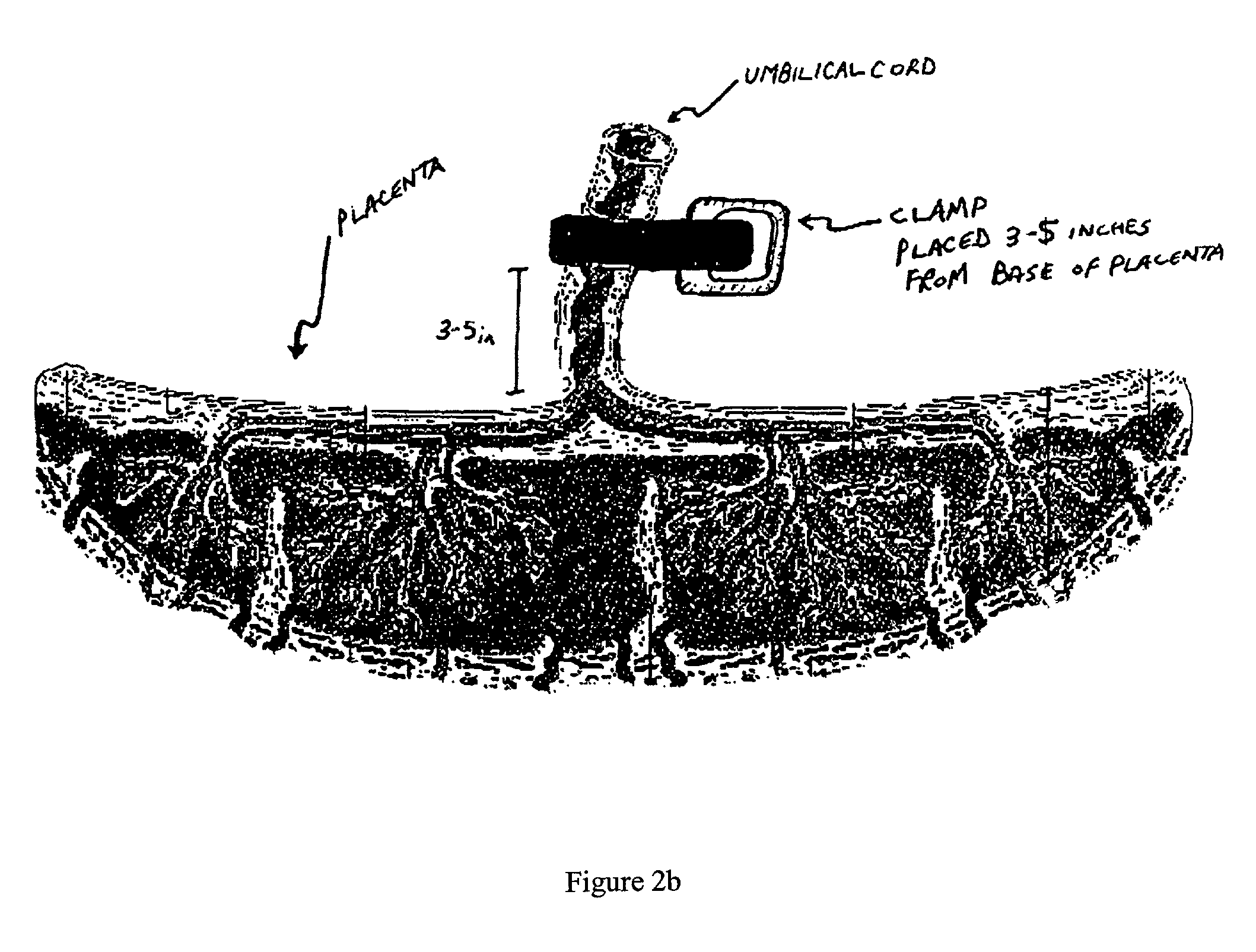
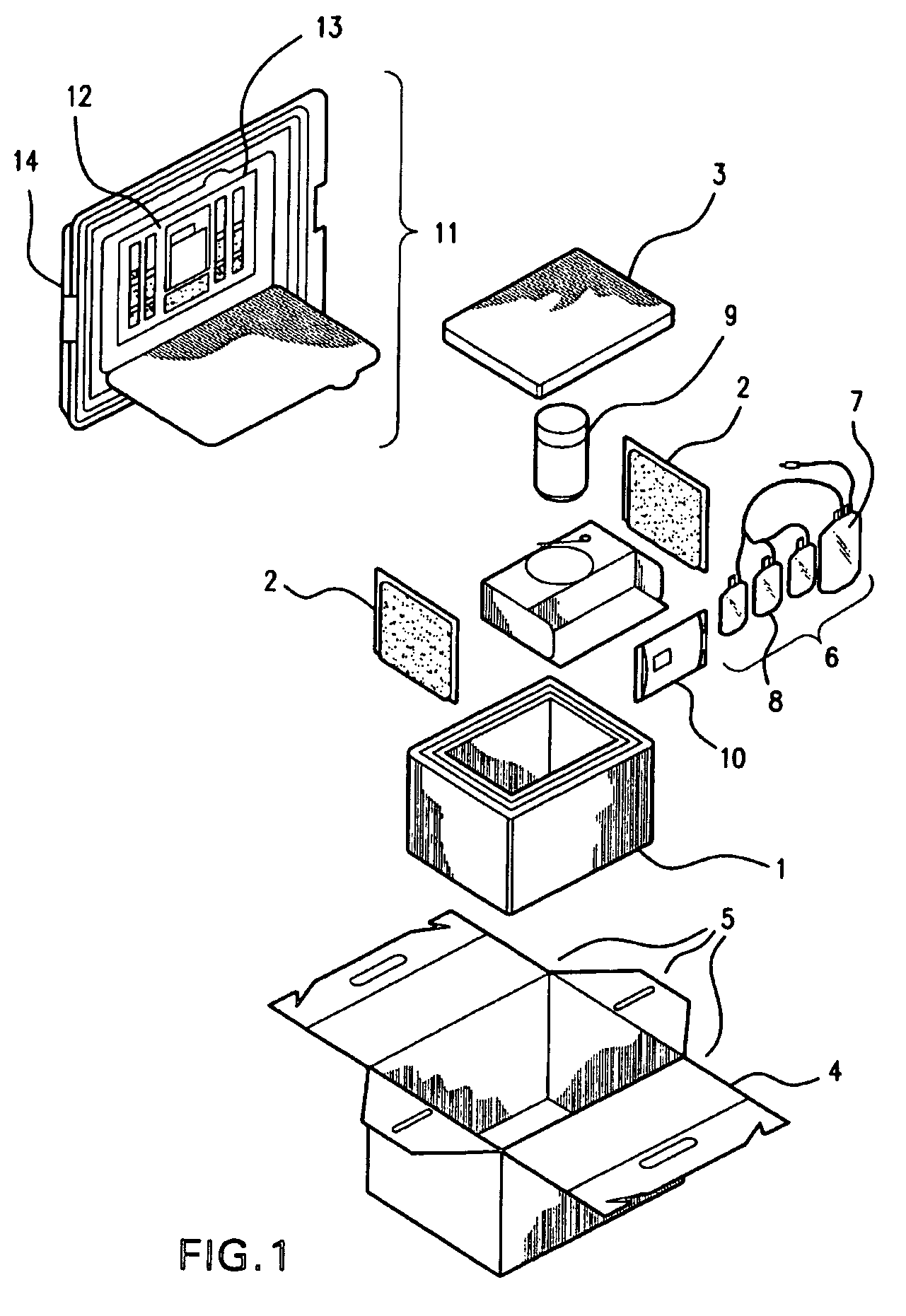
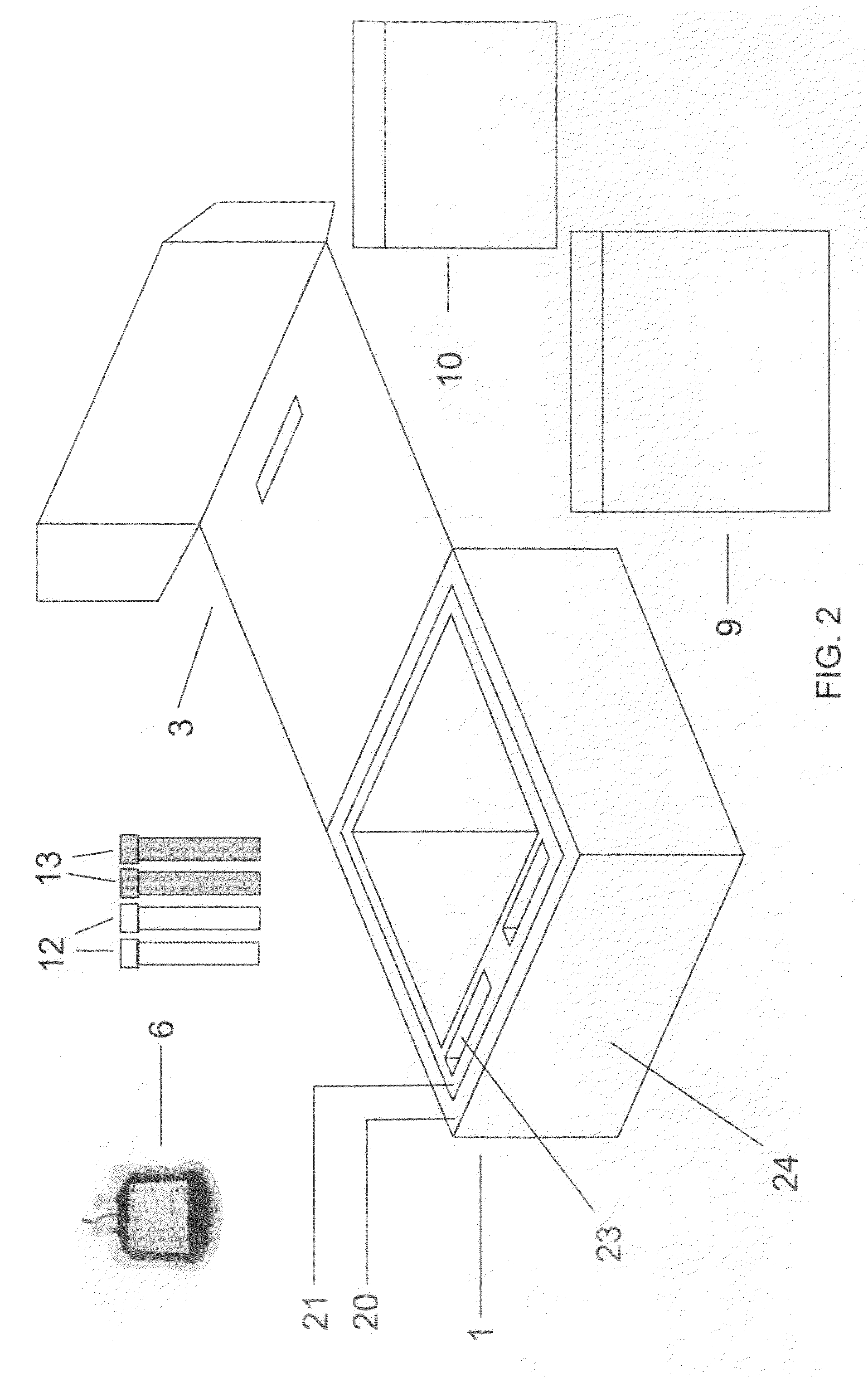
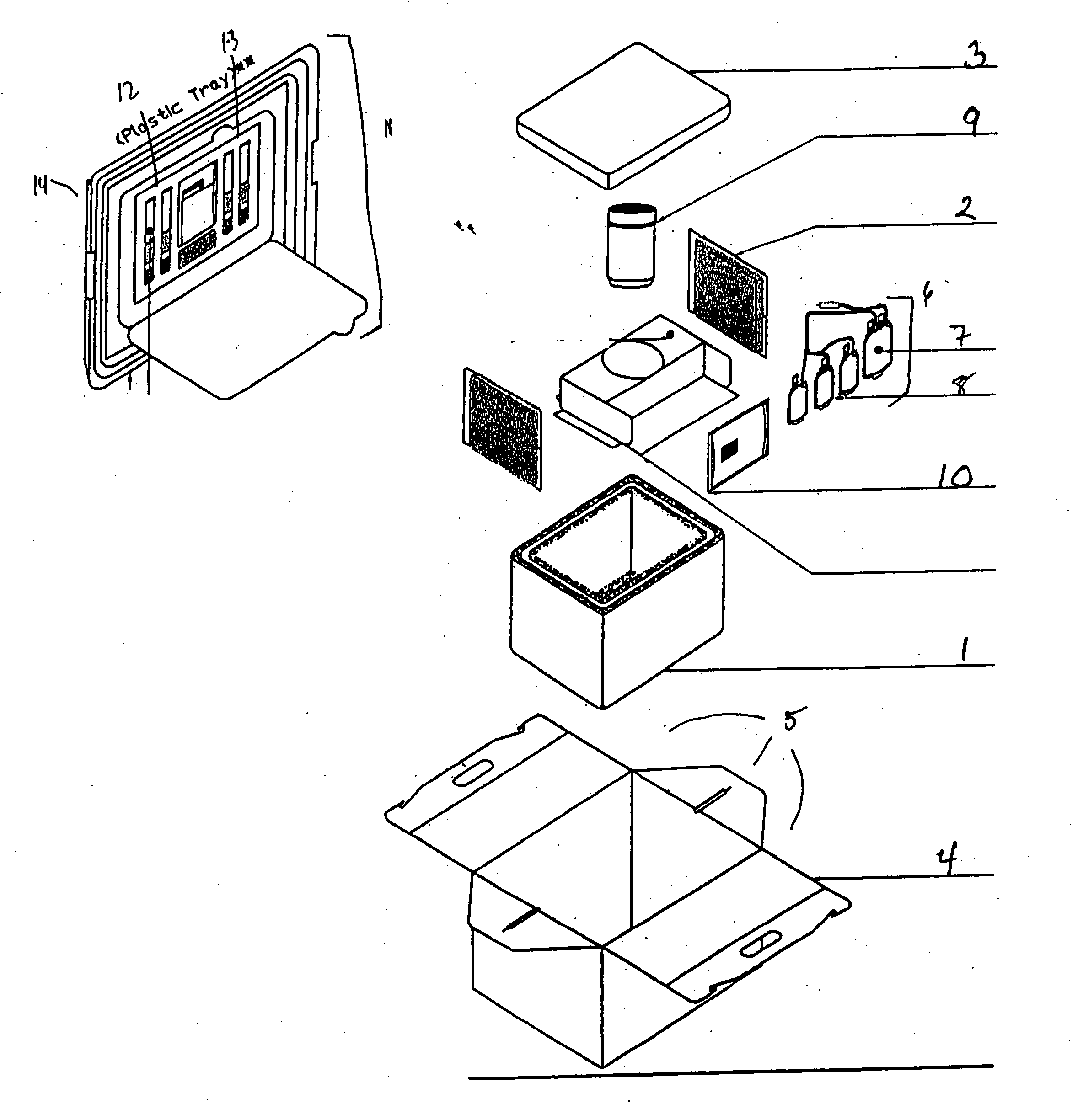
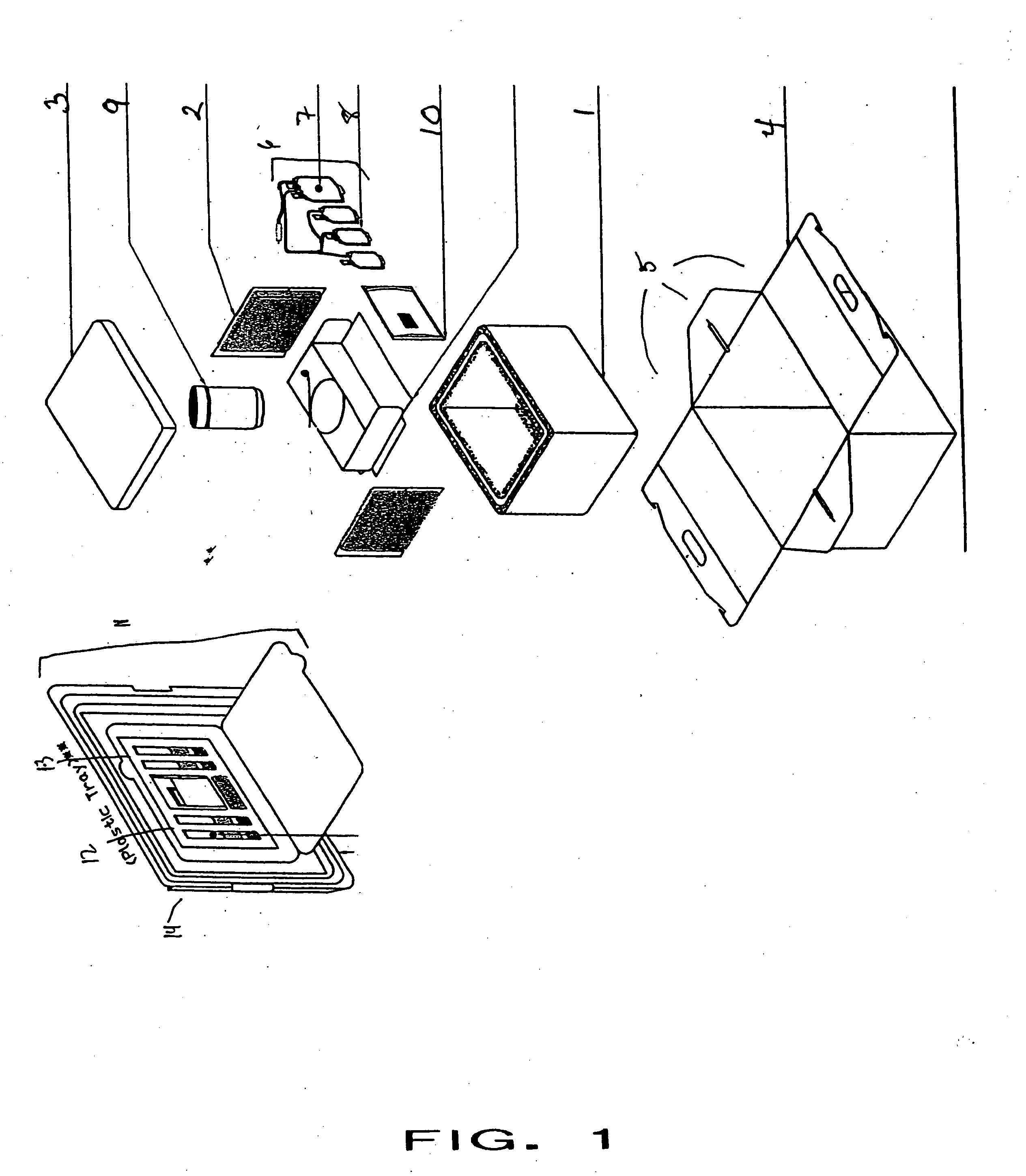
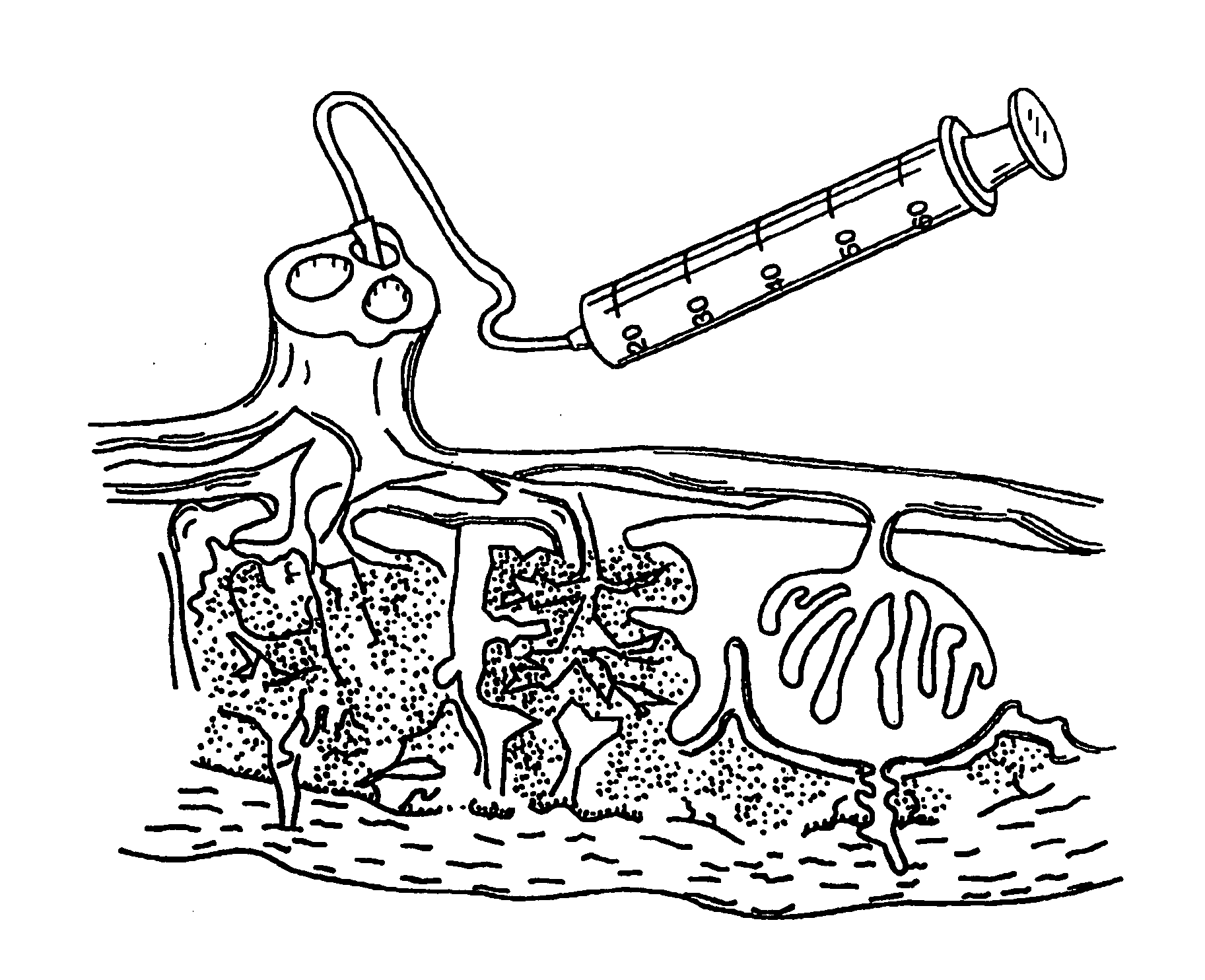
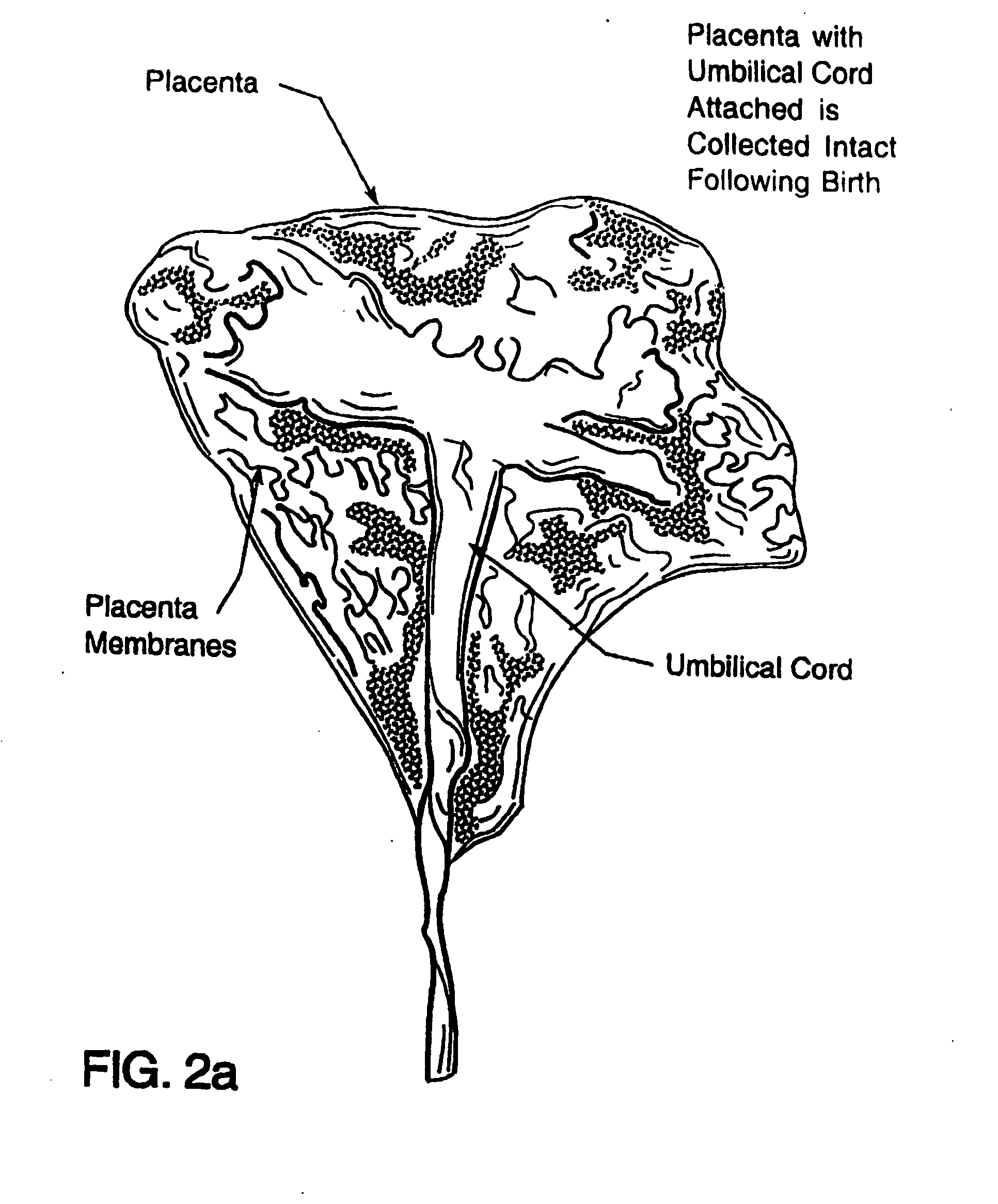
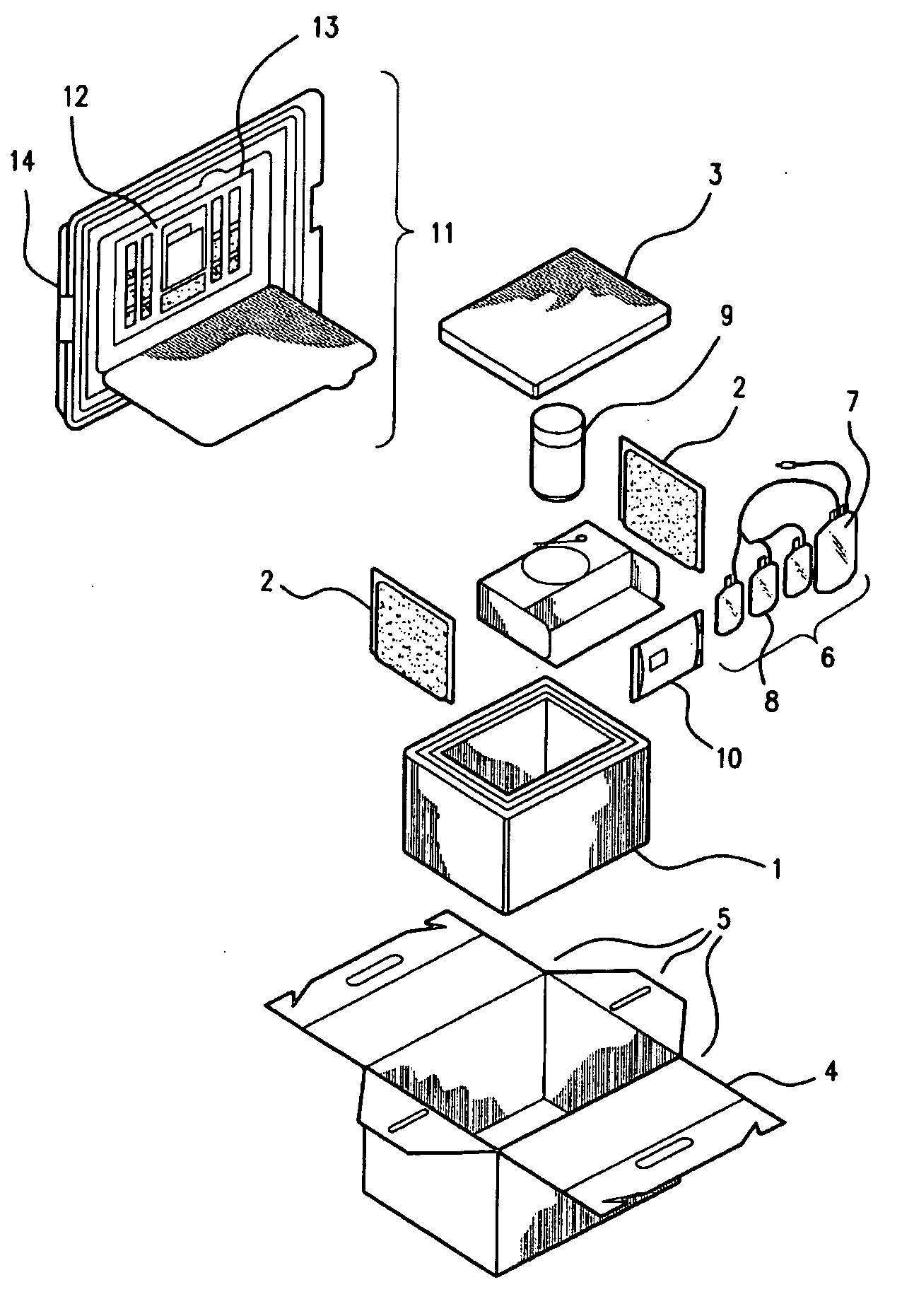
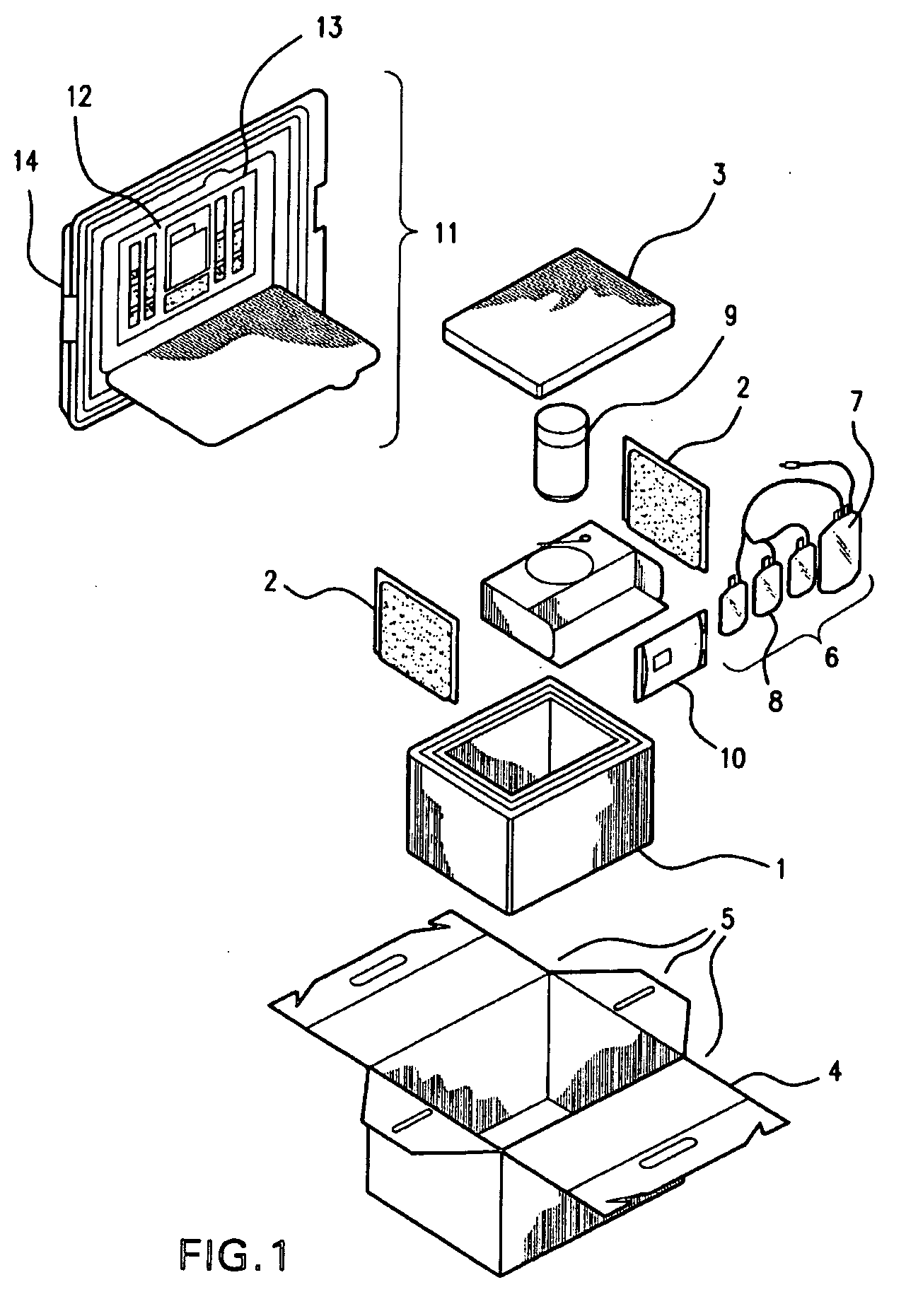
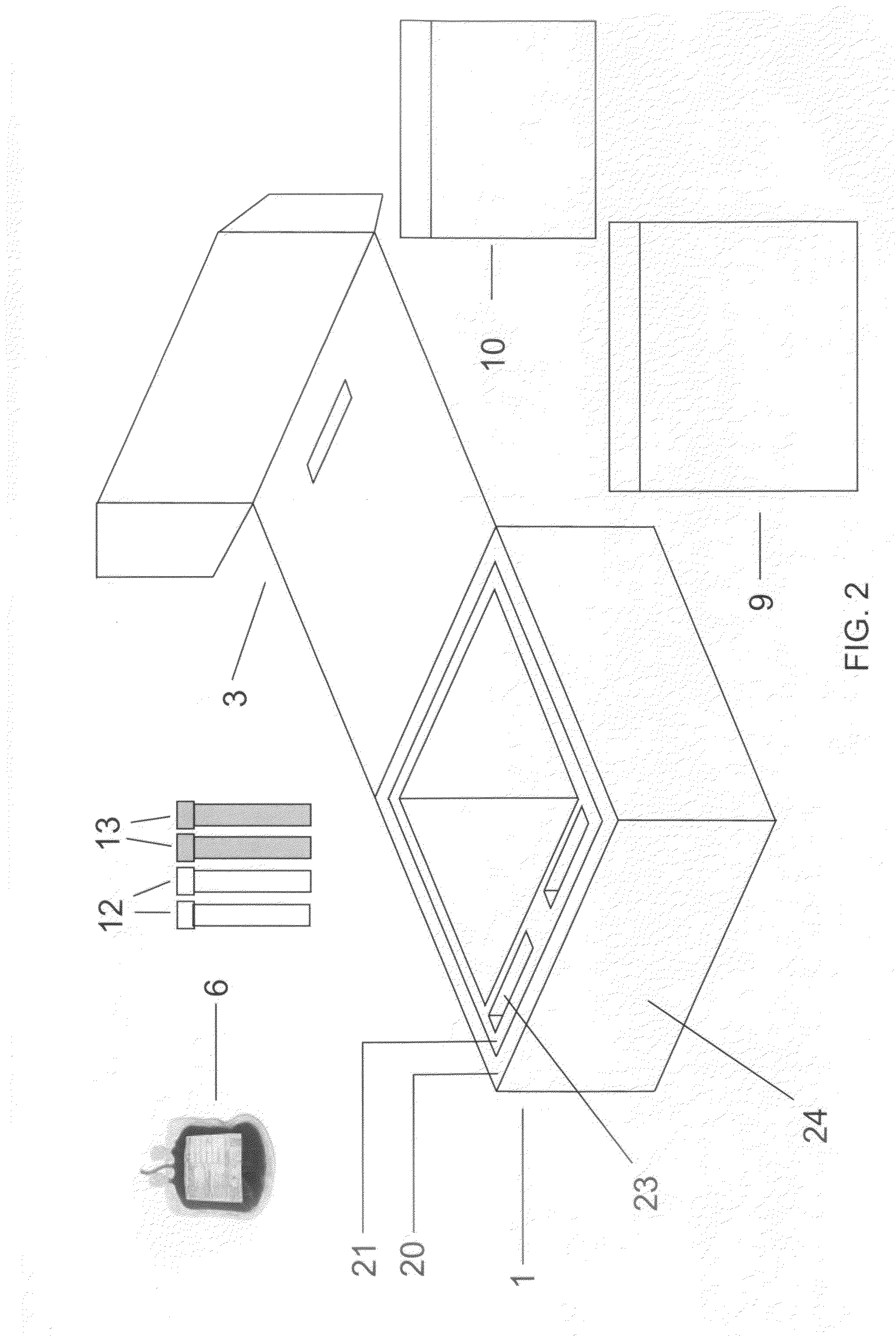
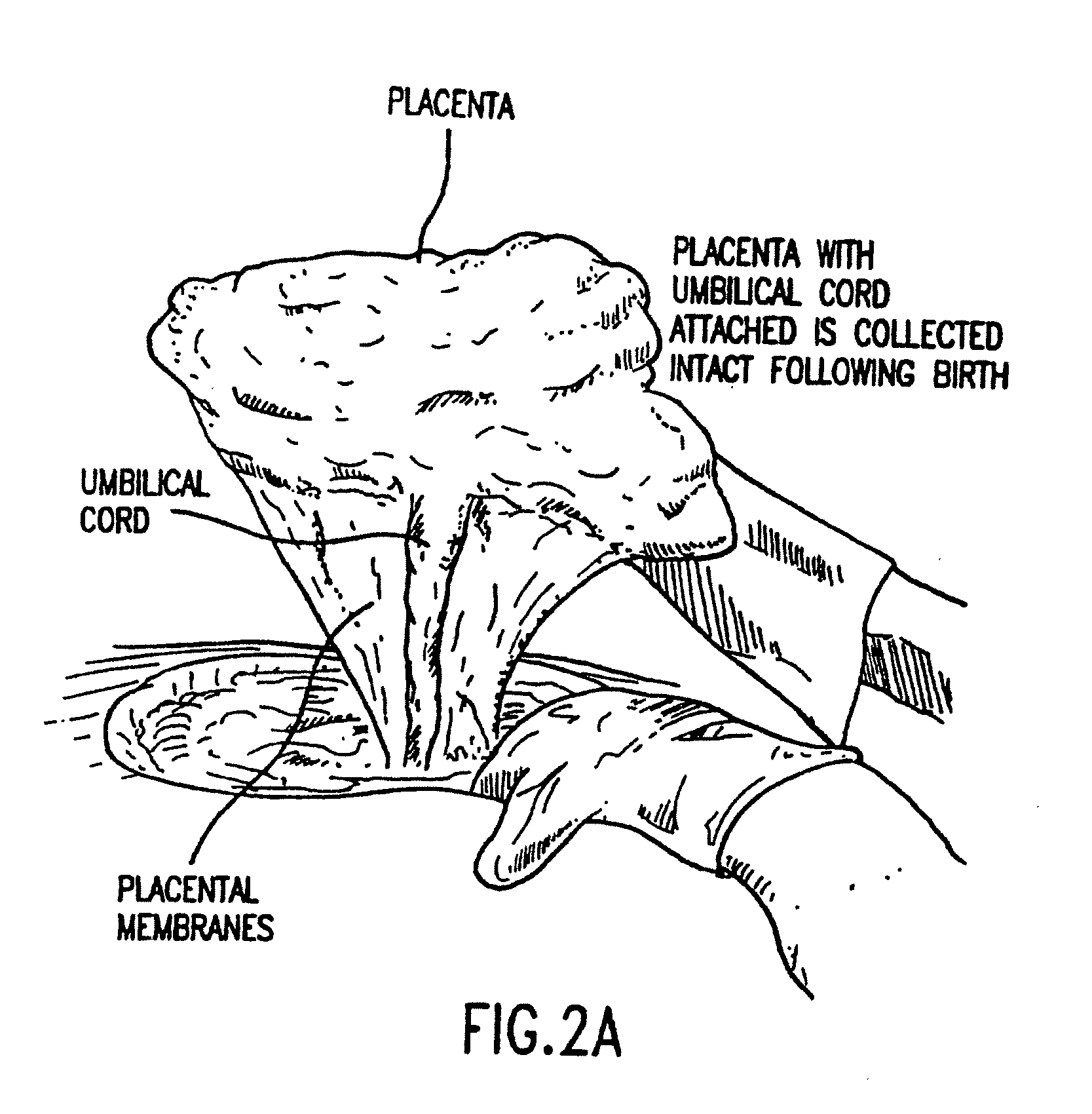
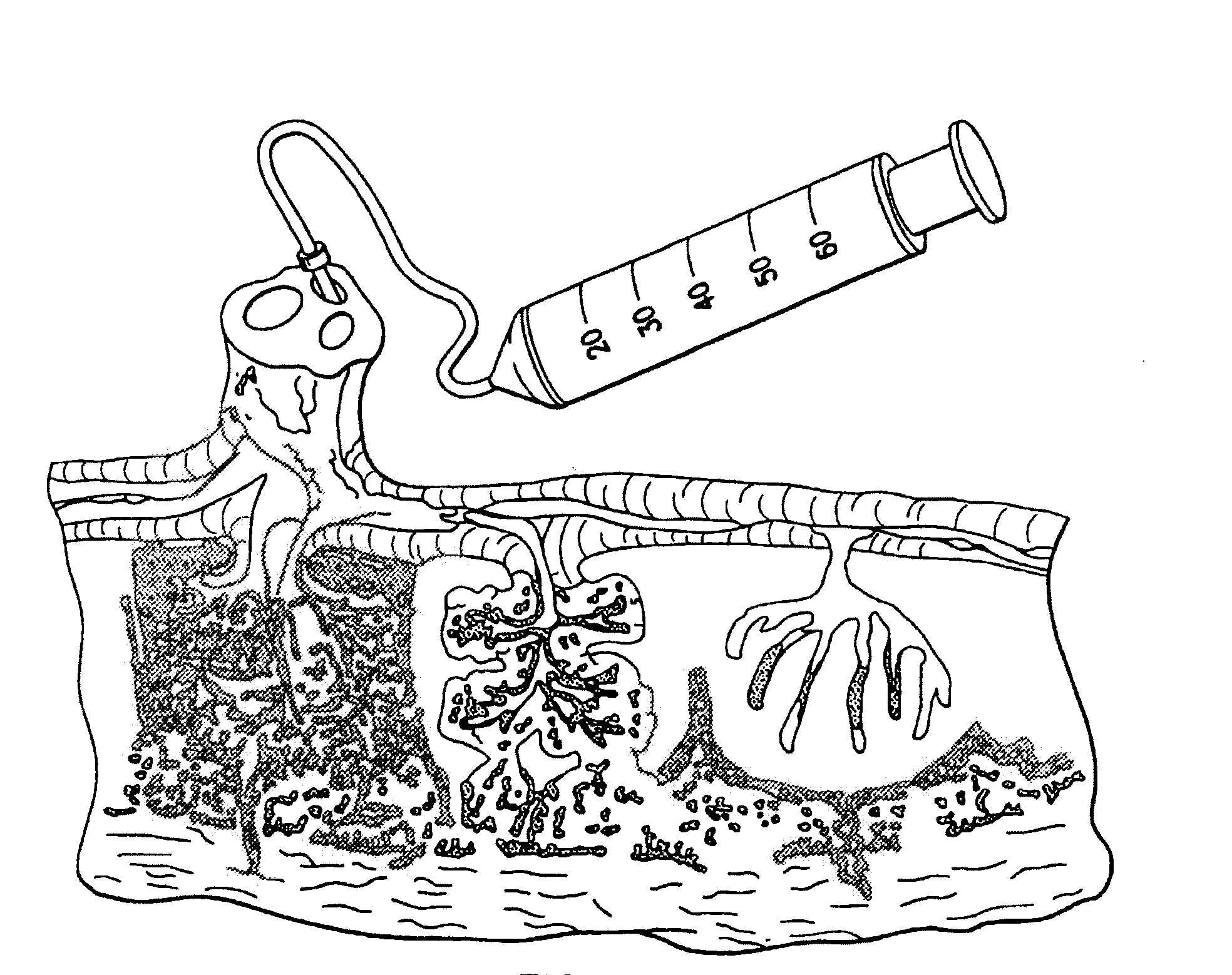
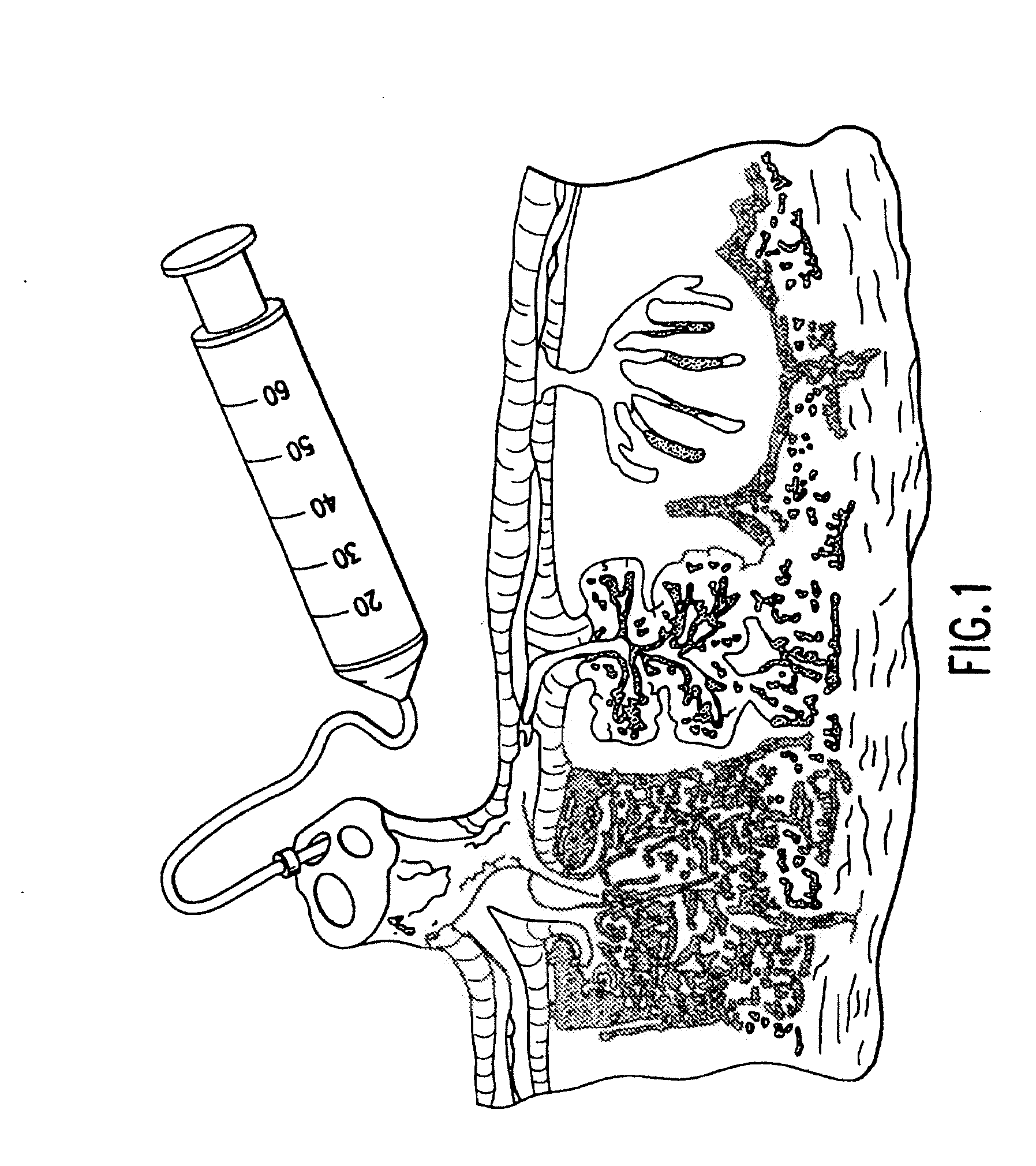
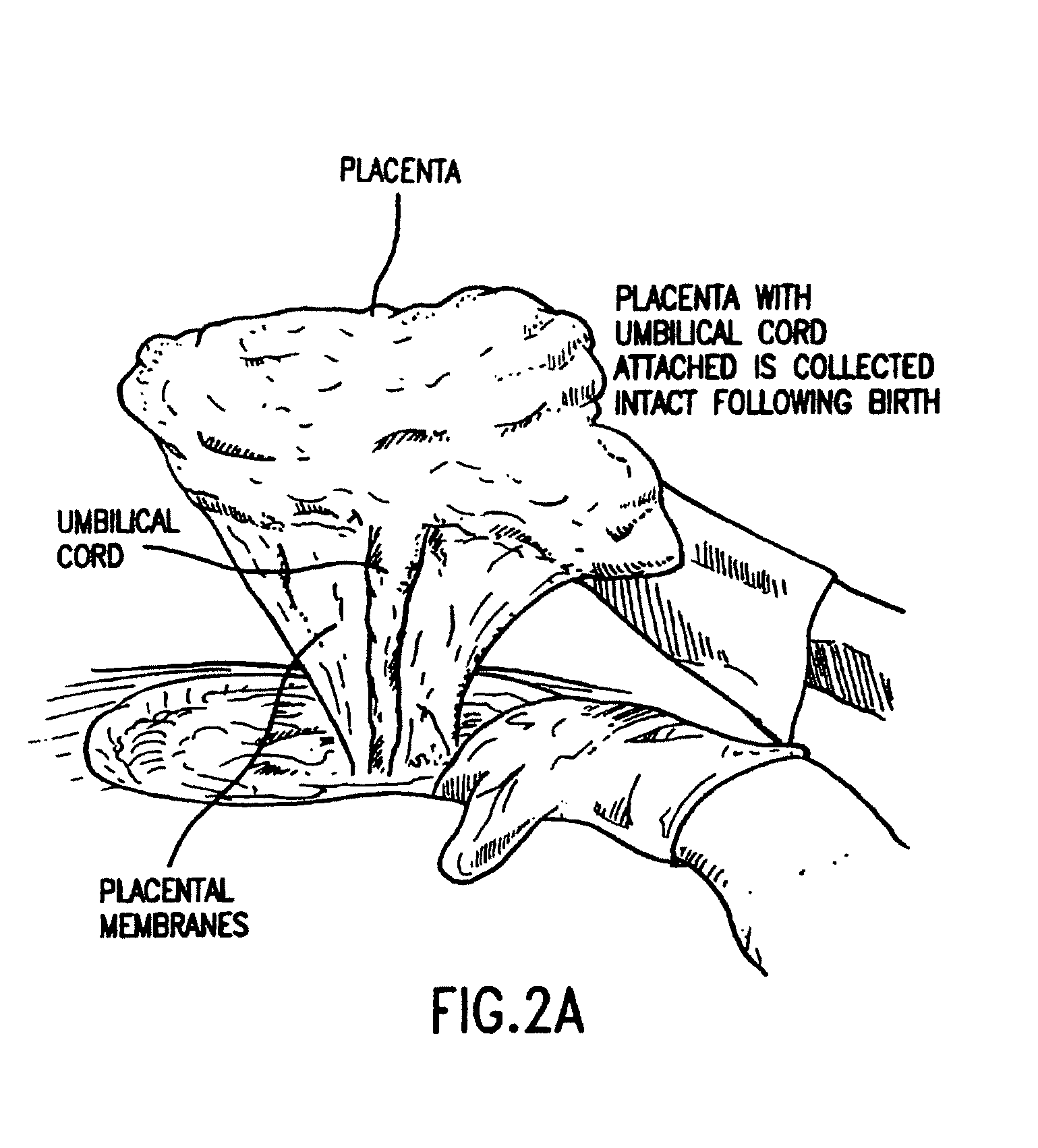
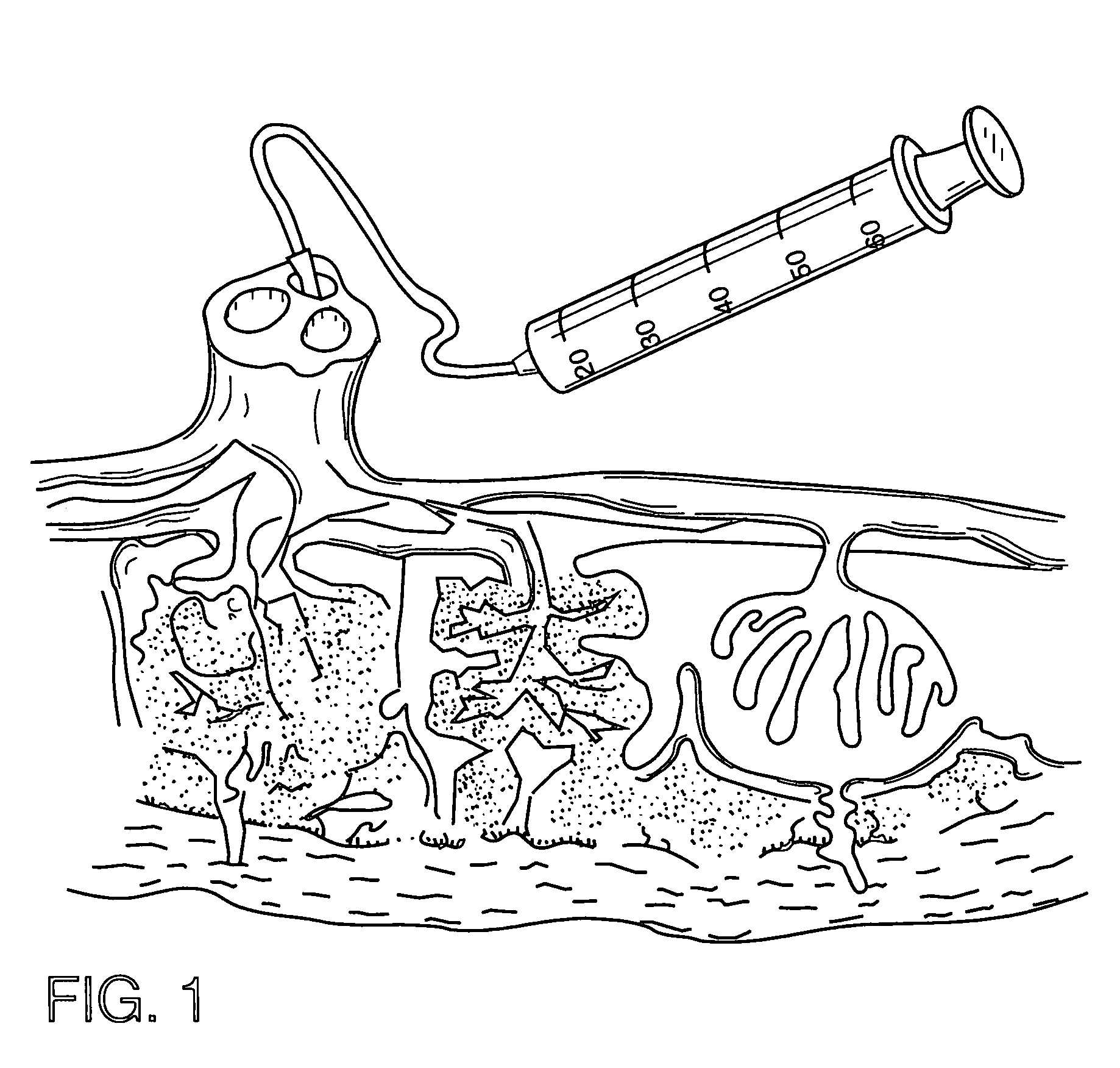
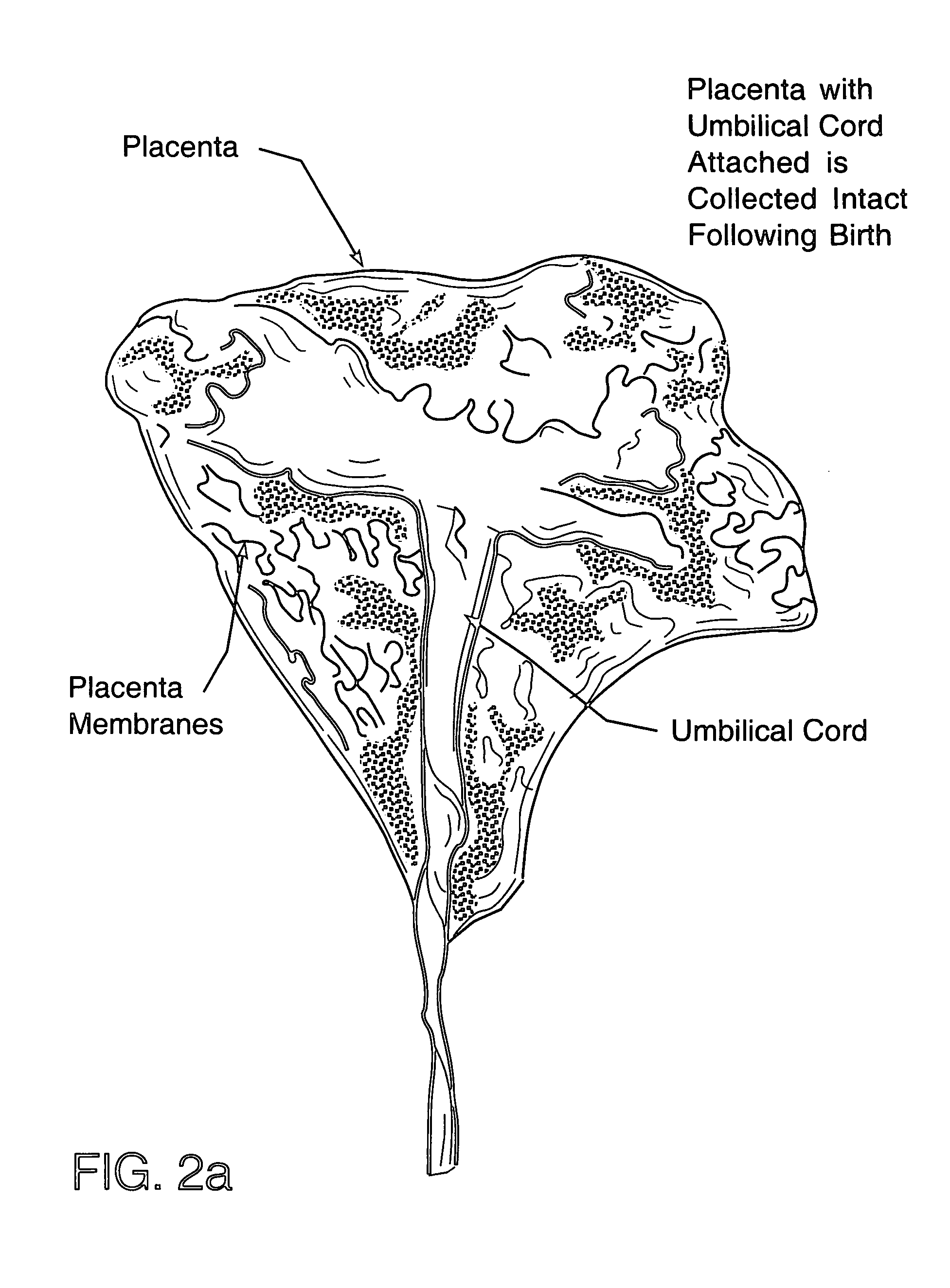
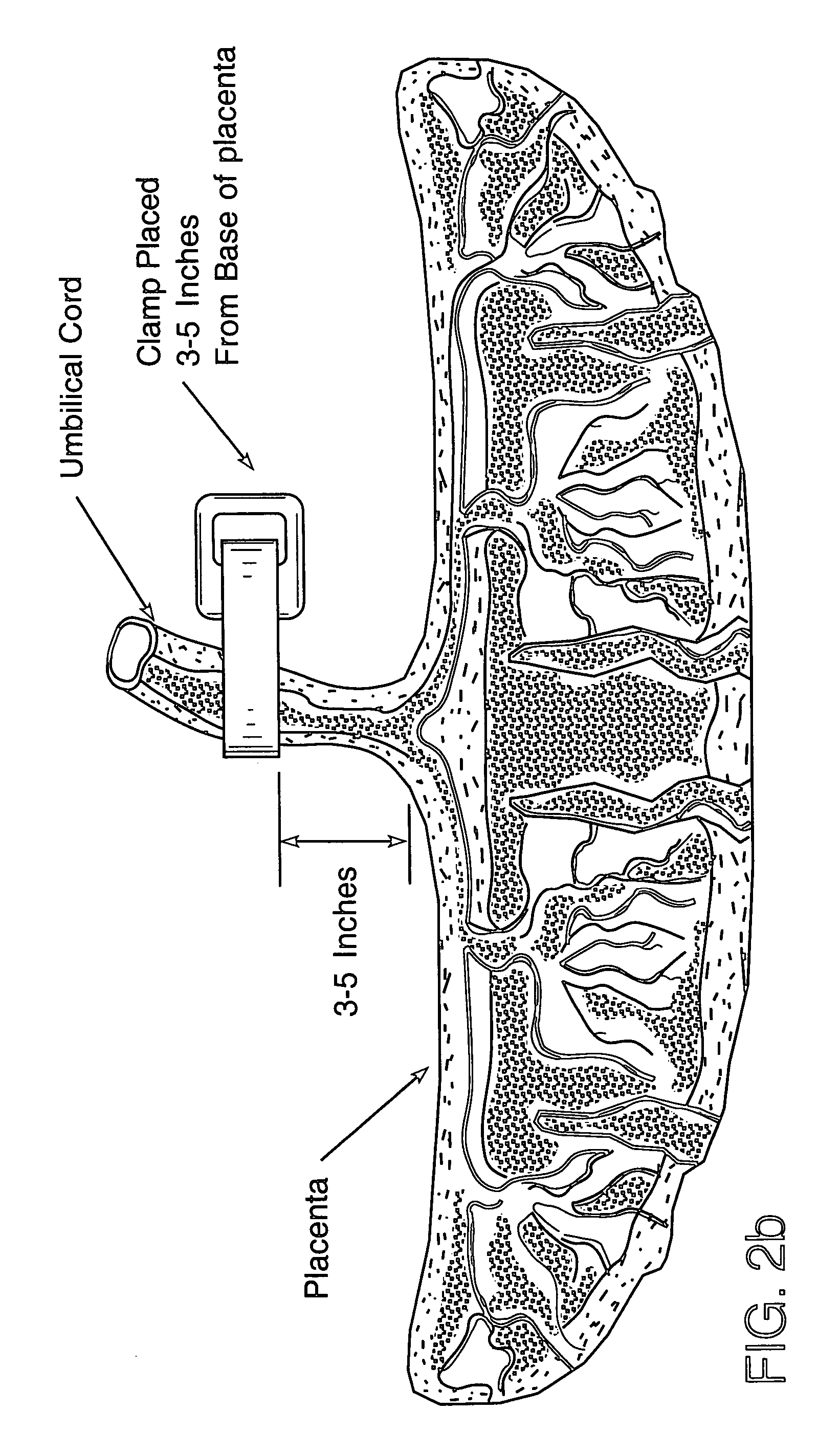
Cord blood and placenta collection kit
ActiveUS7147626B2Bioreactor/fermenter combinationsBiological substance pretreatmentsCord blood stem cellPhysiology
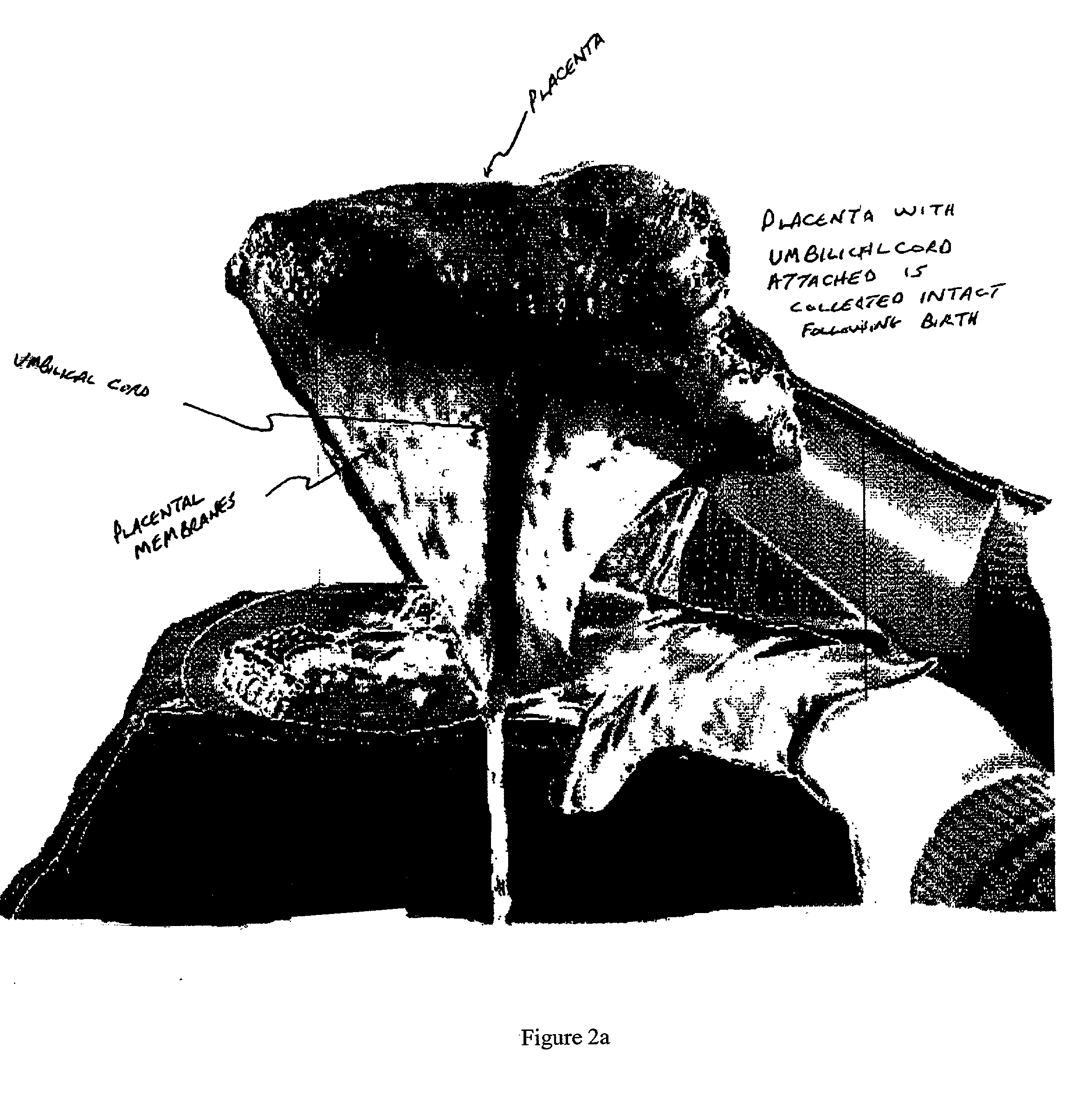
The present invention provides an improved kit for the collection of umbilical cord blood and placental blood, and collection of the placenta from which such blood is obtained. The kit improves upon existing kits in that it provides for improved user convenience, provides for the collection of the placenta itself, and better maintains the internal temperature of the container in which the collected blood and placenta are shipped to a blood bank or registry. The invention further provides a method of collecting umbilical cord and placental blood, and the placenta from which such blood is obtained, comprising using the kit described herein.
Owner:CELULARITY INC
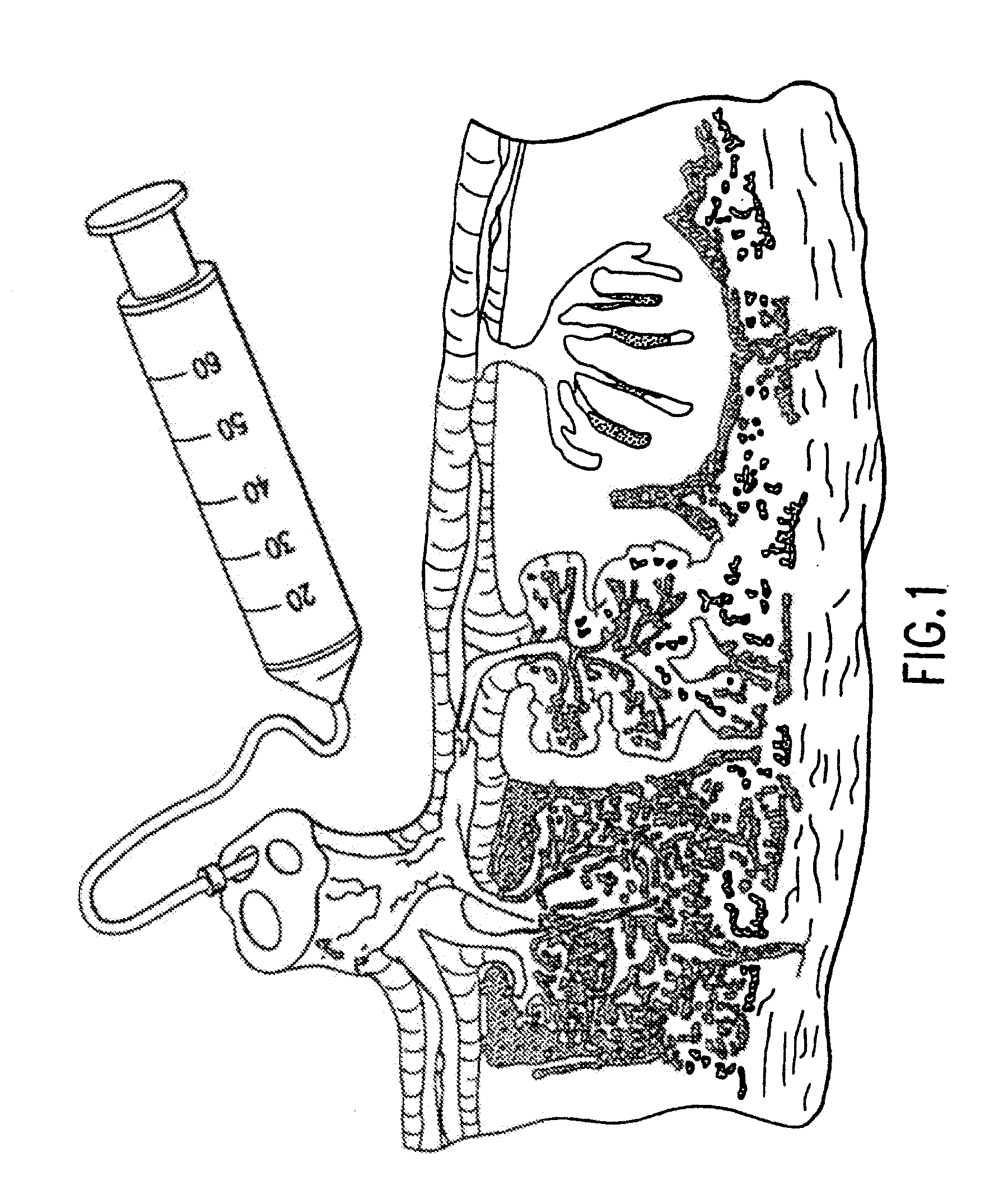
Method of collecting placental stem cells
InactiveUS20020123141A1Increase concentrationImprove the environmentSenses disorderAntipyreticCord blood stem cellEmbryo
A method of collecting embryonic-like stem cells from a placenta which has been treated to remove residual cord blood by perfusing the drained placenta with an anticoagulant solution to flush out residual cells, collecting the residual cells and perfusion liquid from the drained placenta, and separating the embryonic-like cells from the residual cells and perfusion liquid. Exogenous cells can be propagated in the placental bioreactor and bioactive molecules collected therefrom.
Owner:CELULARITY INC
Embryonic-like stem cells derived from post-partum mammalian placenta, and uses and methods of treatment using said cells
Owner:CELULARITY INC
Methods of using JNK or MKK inhibitors to modulate cell differentiation and to treat myeloproliferative disorders and myelodysplastic syndromes
InactiveUS20040028660A1Increase productionPromote recoveryBiocideGenetic material ingredientsCord blood stem cellMyeloproliferative Disorders
The present invention provides methods of modulating mammalian, particularly human, stem cell and progenitor cell differentiation to regulate and control the differentiation and maturation of these cells along specific cell and tissue lineages. The methods of the invention relate to the use of certain small organic molecules to modulate the differentiation of stem cell populations along specific cell and tissue lineages, particularly embryonic-like stem cells originating from a postpartum placenta or stem cells isolated form sources such as cord blood. The invention also relates to the treatment or prevention of myelodysplastic syndrome or myeloproliferative syndrome, or symptoms thereof, comprising administration of JNK or MKK inhibitors, alone or in combination, as well as with or without the use of unconditioned cells or cells conditioned in accordance with other aspects of the invention. Finally, the invention relates to the use of such differentiated stem cells in transplantation and other medical treatments.
Owner:ANTHROGENESIS CORP +1
Human cord blood as a source of neural tissue for repair of the brain and spinal cord
InactiveUS20020028510A1Easy to distinguishImprove neurological dysfunctionNervous disorderCell differentiationDiseaseCord blood stem cell
The present invention relates to the use of umbilical cord blood cells from a donor or patient to provide neural cells which may be used in transplantation. The isolated cells according to the present invention may be used to effect autologous and allogeneic transplantation and repair of neural tissue, in particular, tissue of the brain and spinal cord and to treat neurodegenerative diseases of the brain and spinal cord.
Owner:SANERON CCEL THERAPEUTICS +1
Cord blood and placenta collection kit
ActiveUS7909806B2Bioreactor/fermenter combinationsBiological substance pretreatmentsCord blood stem cellObstetrics
The present invention provides an improved kit for the collection of umbilical cord blood and placental blood, and collection of the placenta from which such blood is obtained. The kit improves upon existing kits in that it provides for improved user convenience, provides for the collection of the placenta itself, and better maintains the internal temperature of the container in which the collected blood and placenta are shipped to a blood bank or registry. The invention further provides a method of collecting umbilical cord and placental blood, and the placenta from which such blood is obtained, comprising using the kit described herein.
Owner:CELULARITY INC
Use of umbilical cord blood to treat individuals having a disease, disorder or condition
The present invention provides methods of using cord blood and cord blood-derived stem cells in high doses to treat various conditions, diseases and disorders. The high-dose cord blood and cord blood-derived stem cells have a multitude of uses and applications, including but not limited to, therapeutic uses for transplantation and treatment and prevention of disease, and diagnostic and research uses. In particular, the cord blood or cord blood-derived stem cells are delivered in high doses, e.g., at least 3 billion nucleated cells per treatment, where treatment may comprise a single or multiple infusions. The invention also provides for the use of cord blood or cord blood-derived stem cells from multiple donors without the need for HLA typing.
Owner:CELULARITY INC
Cord blood and placenta collection kit
ActiveUS20060060494A1Solve insufficient capacitySufficient volumeBioreactor/fermenter combinationsBiological substance pretreatmentsCord blood stem cellObstetrics
The present invention provides an improved kit for the collection of umbilical cord blood and placental blood, and collection of the placenta from which such blood is obtained. The kit improves upon existing kits in that it provides for improved user convenience, provides for the collection of the placenta itself, and better maintains the internal temperature of the container in which the collected blood and placenta are shipped to a blood bank or registry. The invention further provides a method of collecting umbilical cord and placental blood, and the placenta from which such blood is obtained, comprising using the kit described herein.
Owner:CELULARITY INC
Renovation and repopulation of decellularized tissues and cadaveric organs by stem cells
A method of manufacturing a tissue matrix for implantation into a patient is disclosed. The method sets forth collecting embryonic stem cells from a placenta which has been treated to remove residual cord blood and seeding the collected stem cells onto or into a tissue matrix. The seeded tissue matrix is then implanted on or into a patient. The seeded tissue matrix made by the method of the present invention is also disclosed.
Owner:CELULARITY INC
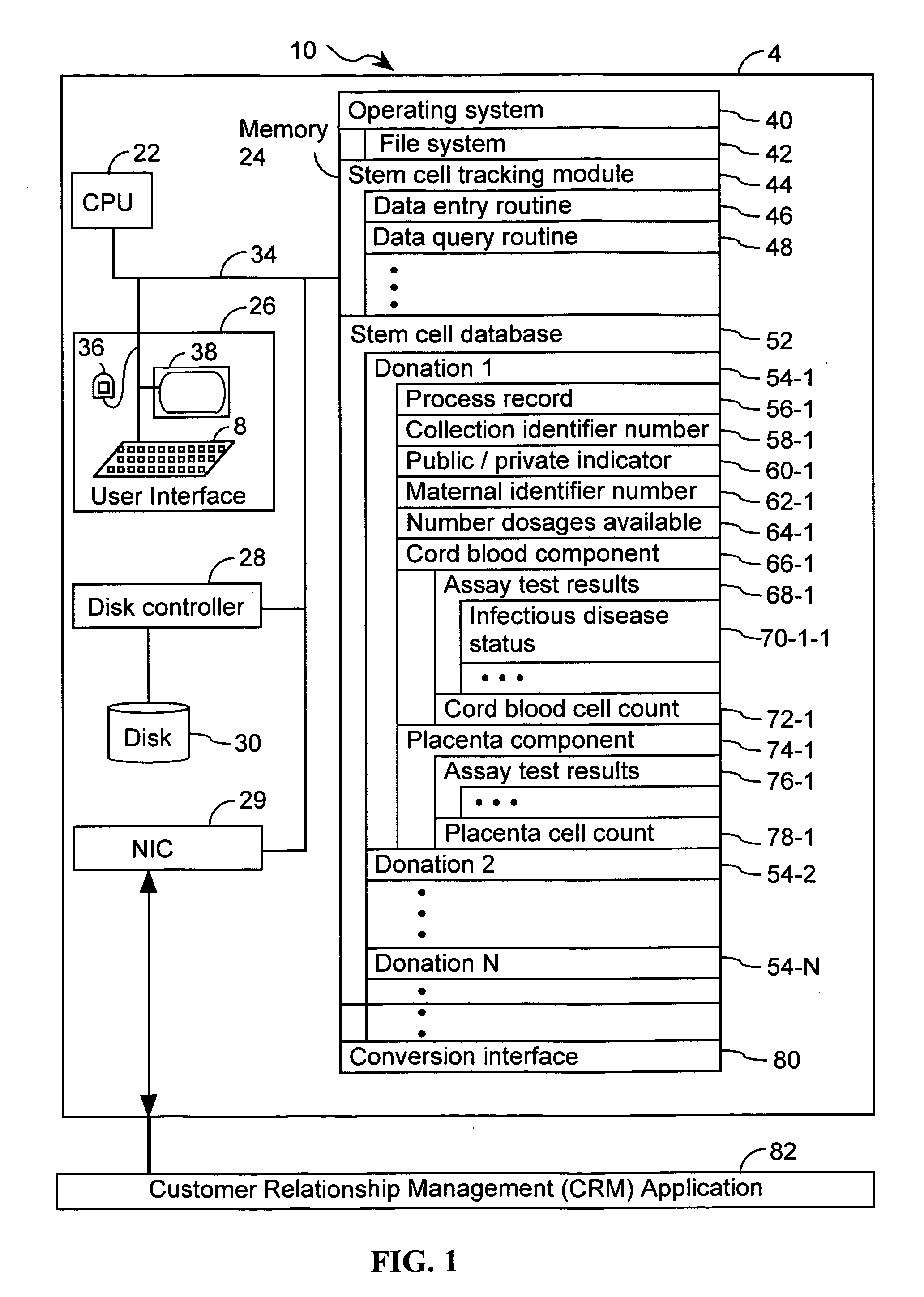
Systems and methods for providing a stem cell bank
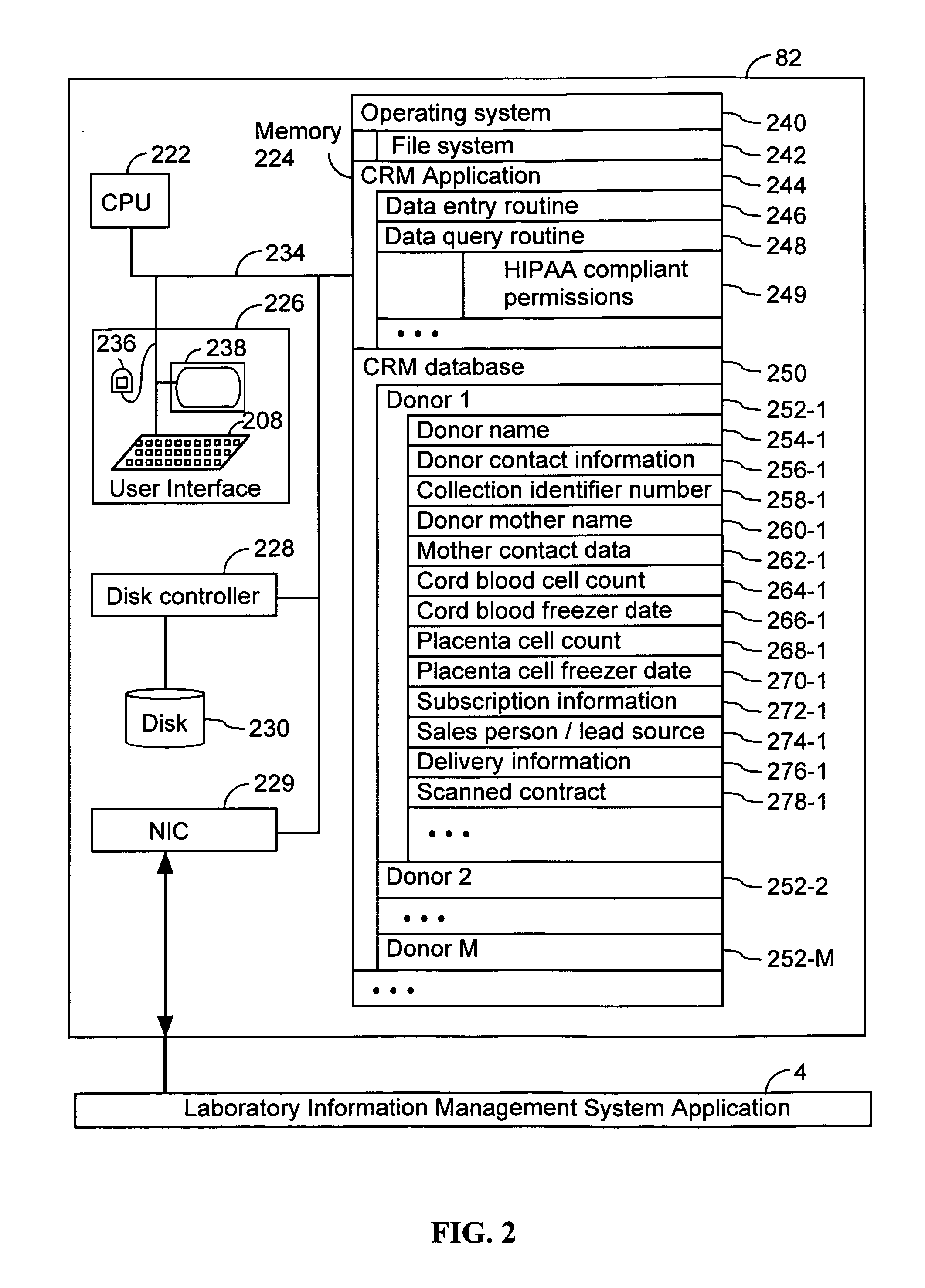
InactiveUS20050276792A1Sufficient amountEfficiently procure and process and bank and dispenseBiocideEmbryonic cellsCord blood stem cellMedicine
Methods, computer systems, and computer program products for maintaining a stem cell registry comprising information about a plurality of stem cell units. A donor is enrolled in the stem cell registry. A stem cell unit from the donor is characterized. Information about the stem cell unit, obtained by the characterizing, is recorded in the stem cell registry. Computer readable media comprising a plurality of data records. One or more respective data records in the plurality of data records comprises (i) a collection identifier number that uniquely corresponds to a stem cell donation, (ii) a cord blood cell count associated with the stem cell donation, and (iii) a placenta blood cell count associated with the stem cell donation. Additional computer readable media comprising a plurality of data records. One or more respective data records in the plurality of data records comprises (i) a cord blood cell count associated with a stem cell donation, (ii) a placenta blood cell count associated with the stem cell donation, and (iii) an indication of at least two stem cell transplant units in the stem cell donation.
Owner:CELULARITY INC
Use of umbilical cord blood to treat individuals having a disease, disorder or condition
The present invention provides methods of using cord blood and cord blood-derived stem cells in high doses to treat various conditions, diseases and disorders. The high-dose cord blood and cord blood-derived stem cells have a multitude of uses and applications, including but not limited to, therapeutic uses for transplantation and treatment and prevention of disease, and diagnostic and research uses. In particular, the cord blood or cord blood-derived stem cells are delivered in high doses, e.g., at least 3 billion nucleated cells per treatment, where treatment may comprise a single or multiple infusions. The invention also provides for the use of cord blood or cord blood-derived stem cells from multiple donors without the need for HLA typing.
Owner:CELULARITY INC
Cord blood and placenta collection kit
ActiveUS20080208158A1Small volumeBioreactor/fermenter combinationsBiological substance pretreatmentsCord blood stem cellPhysiology
The present invention provides an improved kit for the collection of umbilical cord blood and placental blood, and collection of the placenta from which such blood is obtained. The kit improves upon existing kits in that it provides for improved user convenience, provides for the collection of the placenta itself, and better maintains the internal temperature of the container in which the collected blood and placenta are shipped to a blood bank or registry. The invention further provides a method of collecting umbilical cord and placental blood, and the placenta from which such blood is obtained, comprising using the kit described herein.
Owner:CELULARITY INC
Tissue matrices comprising placental stem cells
A method of manufacturing a tissue matrix for implantation into a patient is disclosed. The method sets forth collecting embryonic stem cells from a placenta which has been treated to remove residual cord blood and seeding the collected stem cells onto or into a tissue matrix. The seeded tissue matrix is then implanted on or into a patient. The seeded tissue matrix made by the method of the present invention is also disclosed.
Owner:CELULARITY INC
Treatment of premature birth complications
InactiveUS20090136471A1Promote formationMany formatsAntibacterial agentsOrganic active ingredientsPremature thelarcheCord blood stem cell
The present invention provides methods of treating one or more complications of premature birth suffered by premature infants, comprising administering to the premature infant umbilical cord blood stem cells and, optionally, placental stem cells. The present invention also provides methods of combining and administering, and compositions comprising, umbilical cord blood stem cells, particularly autologous cord blood cells, and placental stem cells for the treatment of premature infants.
Owner:CELULARITY INC
Method of collecting placental stem cells
A method of collecting embryonic-like stem cells from a placenta which has been treated to remove residual cord blood by perfusing the drained placenta with an anticoagulant solution to flush out residual cells, collecting the residual cells and perfusion liquid from the drained placenta, and separating the embryonic-like cells from the residual cells and perfusion liquid. Exogenous cells can be propagated in the placental bioreactor and bioactive molecules collected therefrom.
Owner:CELULARITY INC
Separation method of buffering stem cell in human placenta
InactiveCN1548529AMicrobiological testing/measurementSkeletal/connective tissue cellsAnatomical structuresCord blood stem cell
The present invention discloses the separation method of mesenchymal stem cell in human placenta. On the basis of past separation of tissue cell, the present inventor separates from placenta mesenchymal stem cell with high purity via perfusion process based on the special anatomical structure of placenta. Identification result shows that the mesenchymal stem cell separated from placenta has the biological characteristic and polydirectional differentiation capacity as the reported mesenchymal stem cell of marrow. Owing to the infantile cell component and wide source of placenta, like cord blood, the present invention will have wide clinical application foreground.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
Isolation, expansion and use of clonogenic endothelial progenitor cells
InactiveUS20050266556A1Increase in HSCHigh activityBiological material analysisMammal material medical ingredientsCord blood stem cellFeeder Layer
A hierarchy of endothelial colony forming cells (EPCs) was identified from mammalian cord blood, umbilical vein and aorta. A newly isolated cell named high proliferative potential—endothelial colony forming cell (HPP-ECFC) was isolated and characterized. Single cell assays were developed that test the proliferative and clonogenic potential of endothelial cells derived from cord blood, or from HUVECs and HAECs. EPCs were found to reside in vessel walls. Use of a feeder layer of cells derived from high proliferative potential-endothelial colony forming cells (HPP-ECPCS) from human umbilical cord blood, stimulates growth and survival of repopulating hematopoietic stem and progenitor cells. Stimulation of growth and survival was determined by increased numbers of progenitor cells in in vitro cultures and increased levels of human cell engraftment in the NOD / SCID immunodeficient mouse transplant system.
Owner:INDIANA UNIV RES & TECH CORP
Induction of Myocardial Cell From Mammalian Bone Marrow Cell or Cord Blood-Derived Cell and Fat Tissue
InactiveUS20070212676A1Improve securityEasy to getCell differentiationMicrobiological testing/measurementCord blood stem cellCardiac muscle
This invention provides a method for differentiating mammalian bone marrow cells or cord blood-derived cells into myocardial precursor cells and / or myocardial cells by culturing said bone marrow cells or cord blood-derived cells with cells isolated from mammalian fat tissues or a culture supernatant thereof.
Owner:JAPAN SCI & TECH CORP
Compositions and methods for enhanced generation of hematopoietic stem/progenitor cells
The present invention relates to methods, kits and compositions for expansion of hematopoietic stem / progenitor cells and providing hematopoietic function to human patients in need thereof. In one aspect, it relates to kits and compositions comprising a Notch agonist and an aryl hydrocarbon receptor antagonist. Also provided herein are methods for expanding the hematopoietic stem / progenitor cells using kits and compositions comprising a Notch agonist and an aryl hydrocarbon receptor antagonist. The hematopoietic stem / progenitor cells expanded using the disclosed kits, compositions and methods include human umbilical cord blood stem / progenitor cells, placental cord blood stem / progenitor cells and peripheral blood stem cells. The present invention also relates to administering hematopoietic stem / progenitor cells expanded using a combination of a Notch agonist and an aryl hydrocarbon receptor antagonist to a patient for short-term and / or long-term in vivo repopulation benefits.
Owner:NOVARTIS AG +1
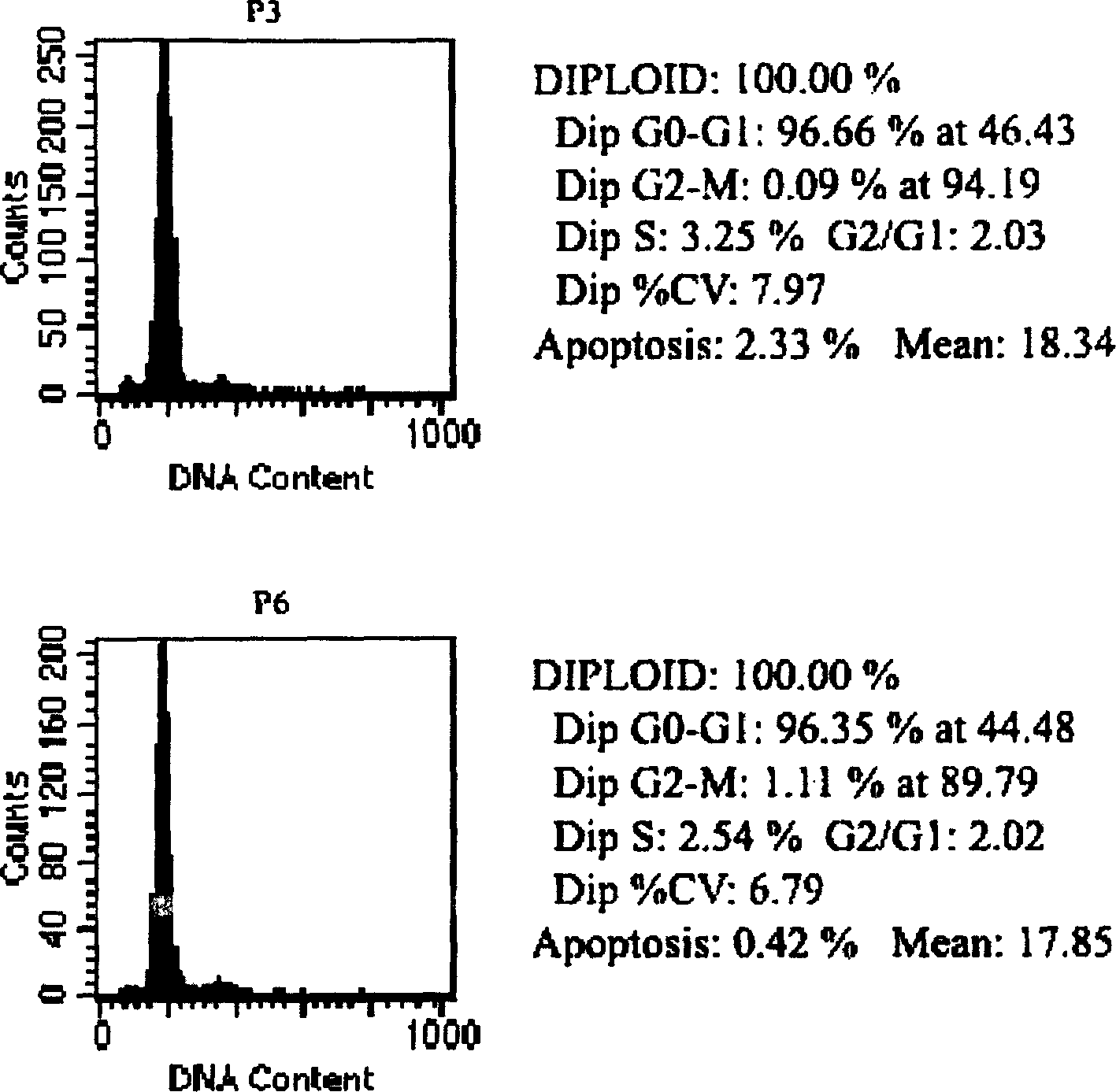
In vitro culture of mesenchymal stem cells (MSC) and a process for the preparation thereof for therapeutic use
InactiveUS20050059152A1Promote growthEliminate riskCulture processMammal material medical ingredientsCord blood stem cellMesenchymal stem cell
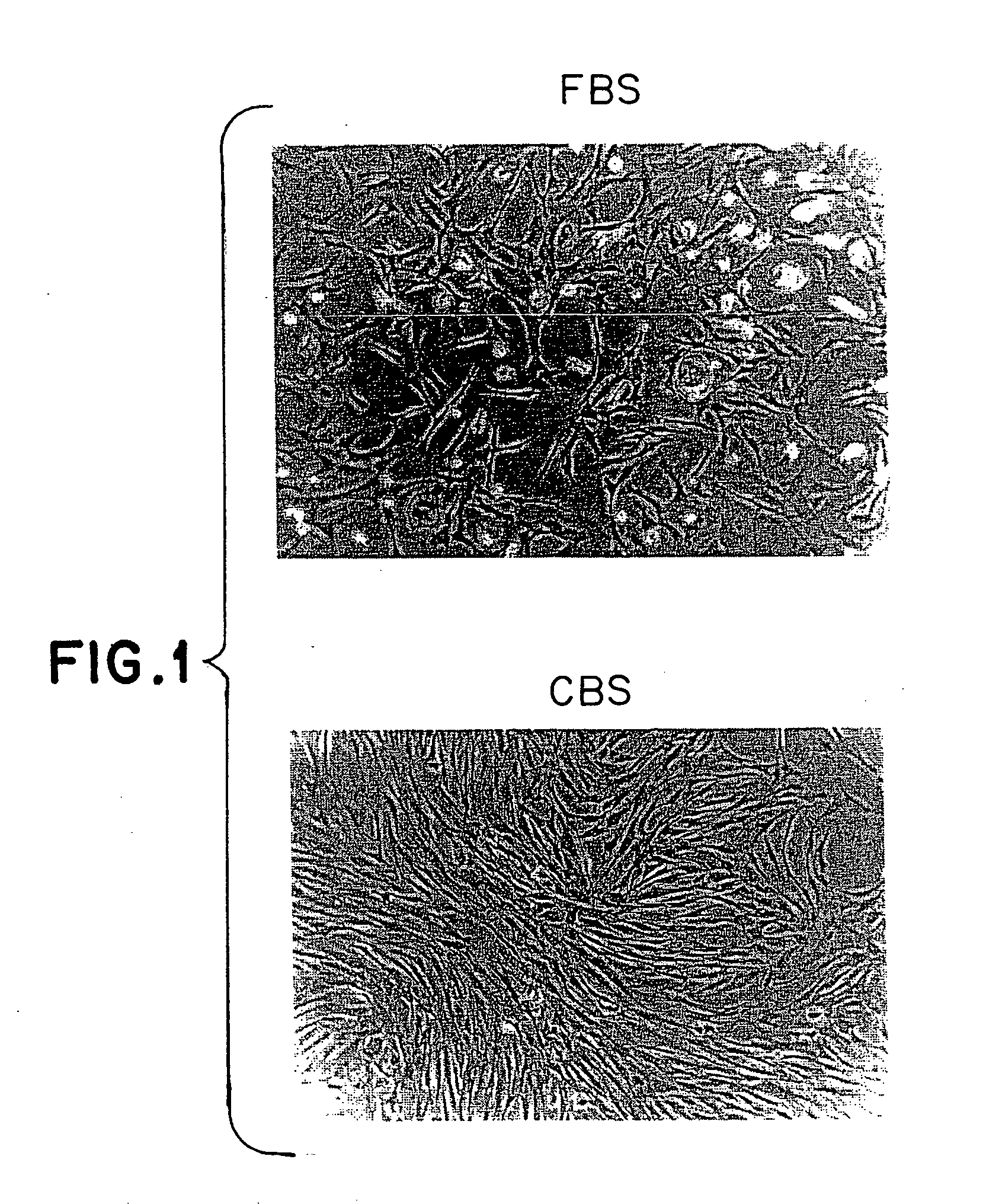
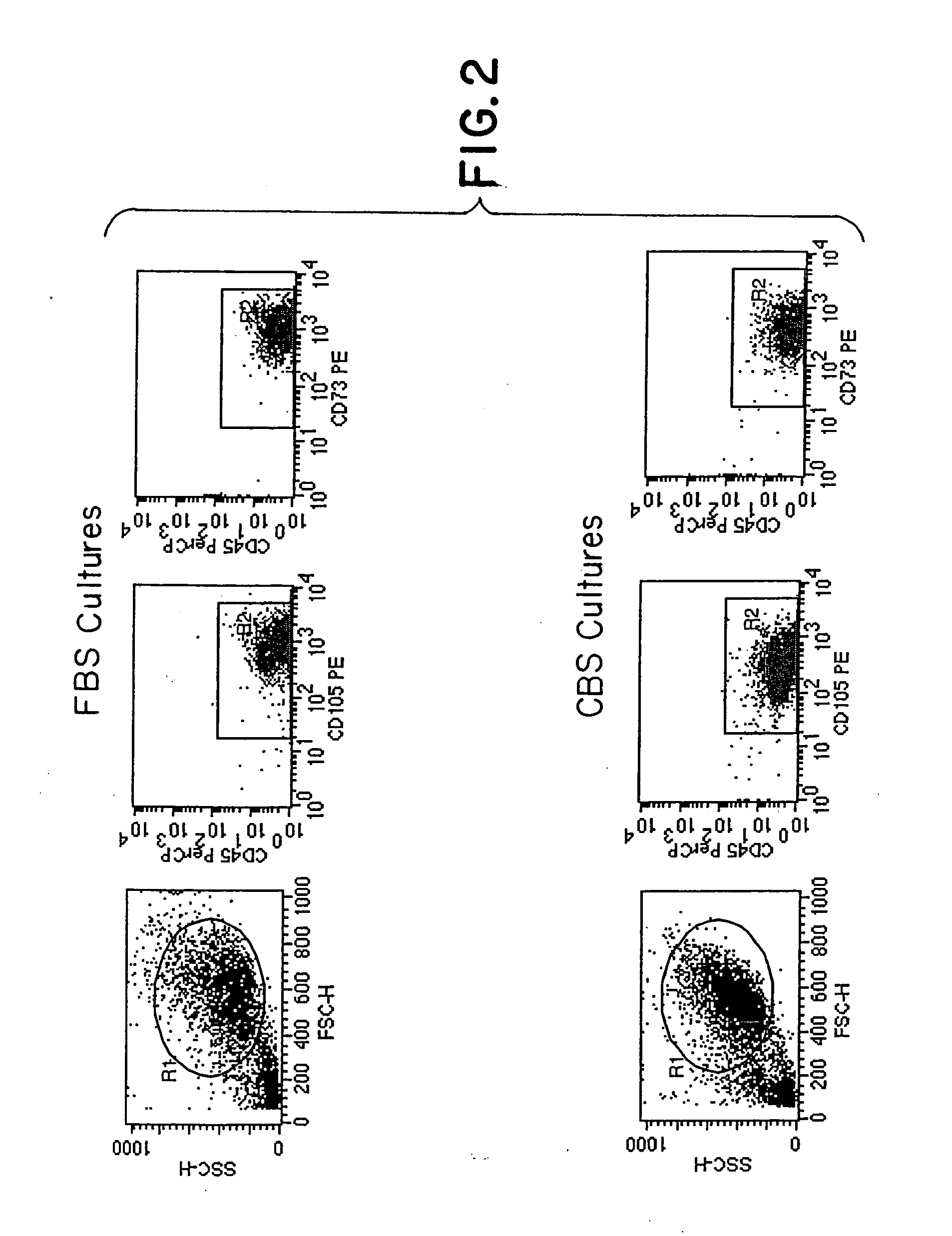
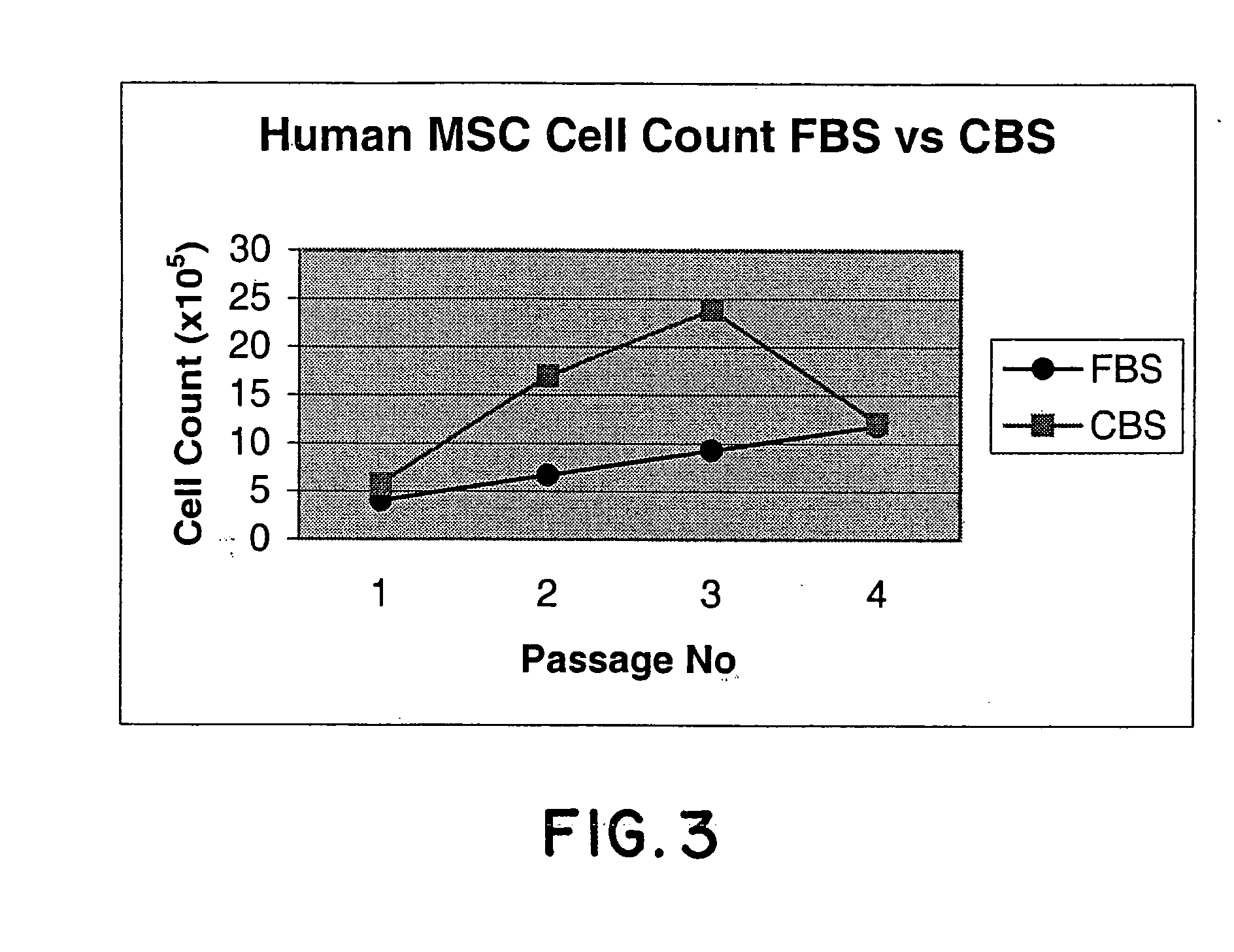
Method of culturing mesenchymal stem cells (hMSC) by culturing the stem cells in a culture medium containing Cord Blood Serum, and a process for preparation thereof for therapeutic use.
Owner:RELIANCE LIFE SCI PVT
Growth of neural precursor cells using umbilical cord blood serum and a process for the preparation thereof for therapeutic purposes
InactiveUS20040203142A1Promote growthArtificial cell constructsBlood/immune system cellsCord blood stem cellCell culture media
This invention is concerned with stem cells derived from umbilical cord blood serum and a method for growing human embryonic stem cells and adult cells comprising sera separated from clotted umbilical cord blood, including growing and differentiating cord blood stem cells into neural precursors, comprising transdifferentiating CD34+ stem cells from mononuclear cells derived from umbilical cord blood to neural precursors. The stem cells obtained from the umbilical cord include pluripotent stem and progenitor cell population of mononuclear cells, and separating pluripotent stem and progenitor cell population of mononuclear cells obtained from the umbilical cord blood. A magnetic cell separator is used to separate out cells which contain a CD marker and then expanding the cells in a growth medium containing retinoic acid and one or more growth factors BDNF, GDNF, NGF and FGF as a differentiating agent. The invention is also concerned with the transplantation and repair of nerve damage, stokes, spinal injury, Parkinson's and Alzheimer's, prepared in accordance with the aforesaid method and a media for culturing umbilical cord blood stem calls consisting essentially of cord blood stem cells derived from umbilical cord blood serum.
Owner:RELIANCE LIFE SCI PYT
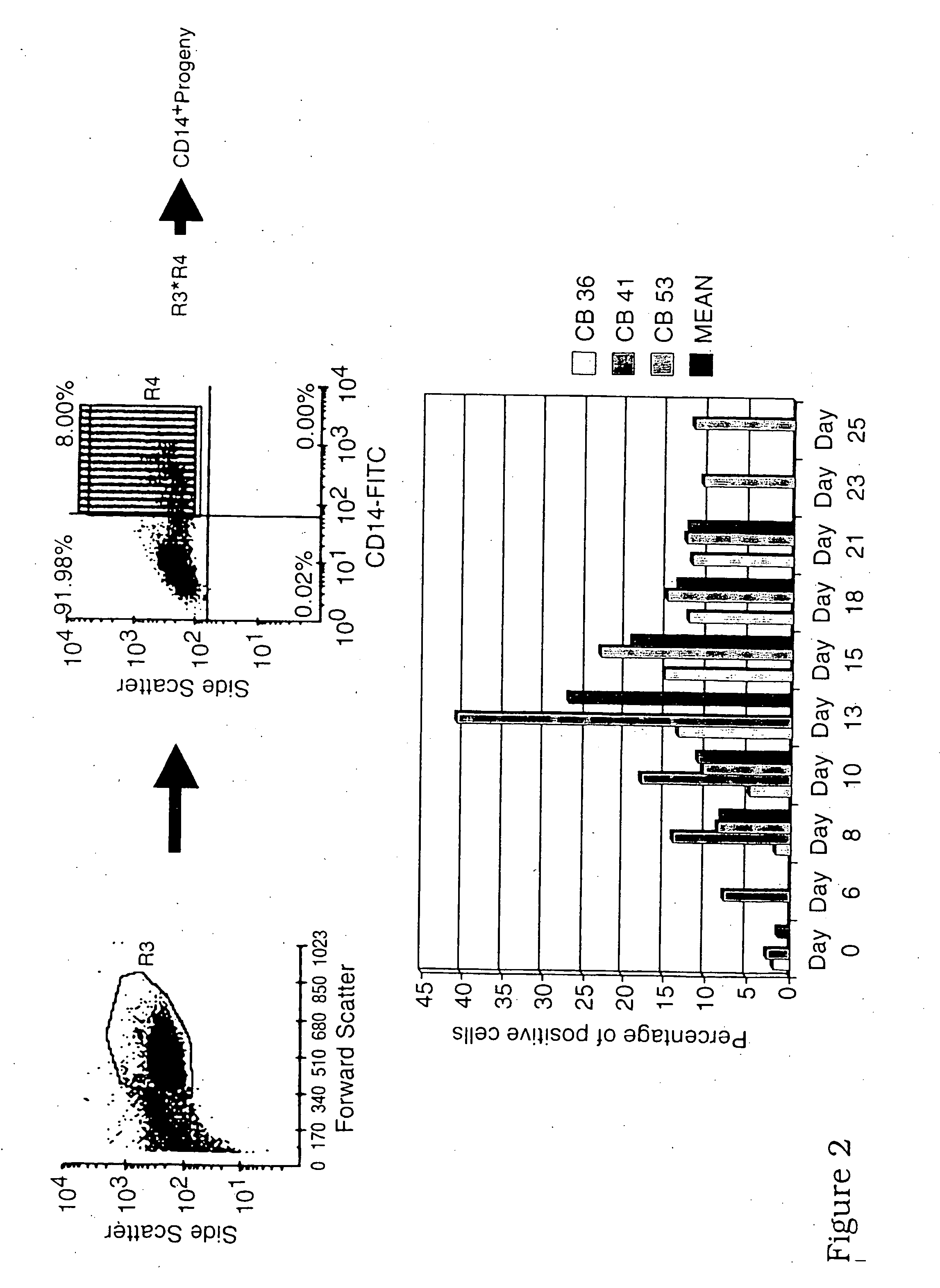
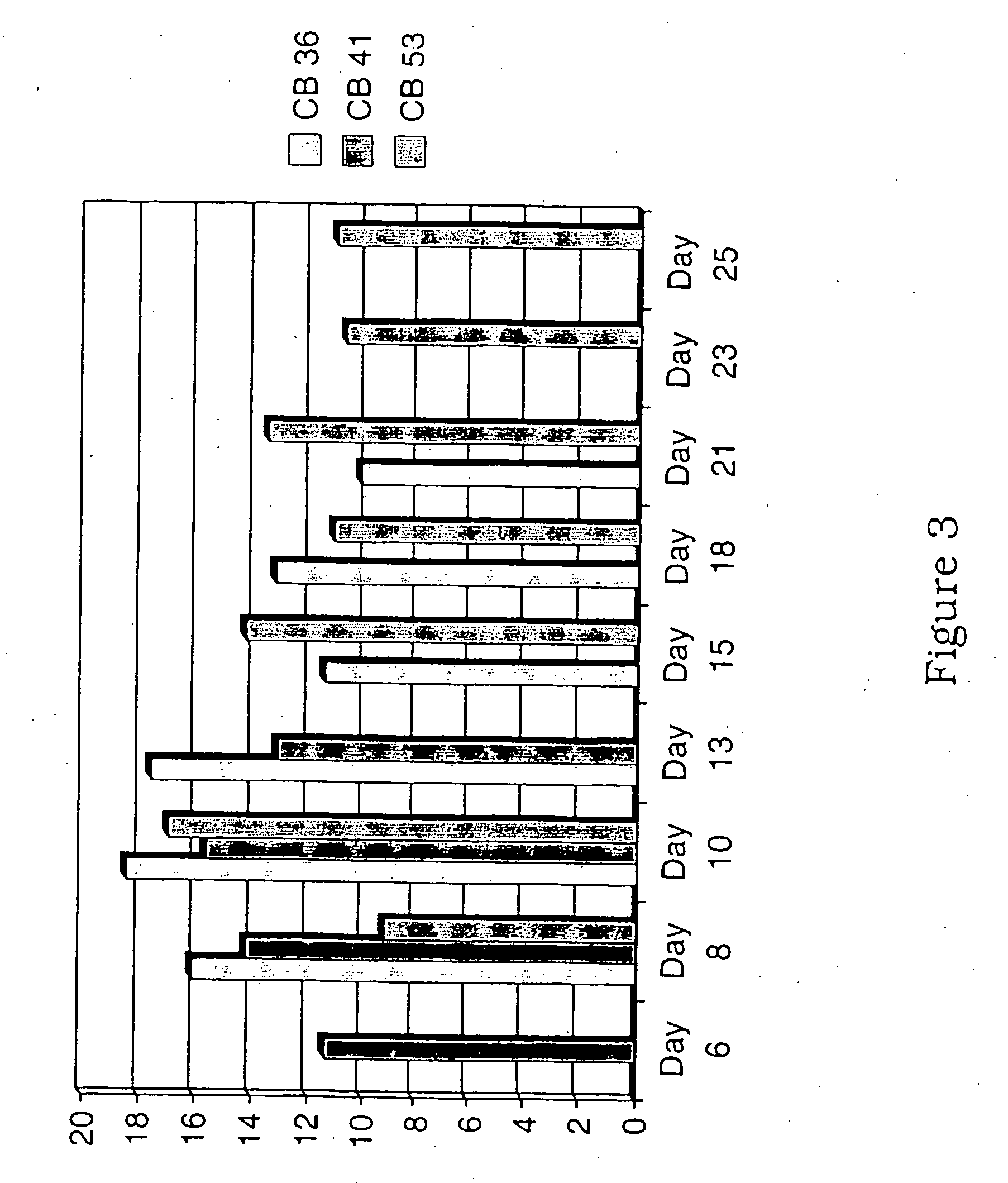
Generation of dendritic cells from cd34+precursors
The present invention relates to a method for inducing development of dendritic cells from CD34+ precursor cells. More particularly, the present invention relates to a method of differentiating CD34+ precursor cells into myeloid- and or lymphoid-dendritic cells. The present invention provides a protocol for the development of dendritic cells from a biological sample inter alia cord blood, bone marrow or peripheral blood CD34+ stem cells. The dendritic cells of the present invention are useful as therapeutic cellular agents such as in the development of vaccines and in modulating immunological responsiveness. More particularly, the present invention provides compositions which can be used for inducing a protective immune response in a subject against inter alia pathogenic infections, autoimmune diseases and cancer.
Owner:THE OF THE TRUSTEES OF THE SISTERS OF MERCY & QUEENSLAND
Methods for the isolation and expansion of cord blood derived T regulatory cells
ActiveUS20060062763A1Prevent proliferationBiocideArtificial cell constructsCord blood stem cellT-regulatory cell
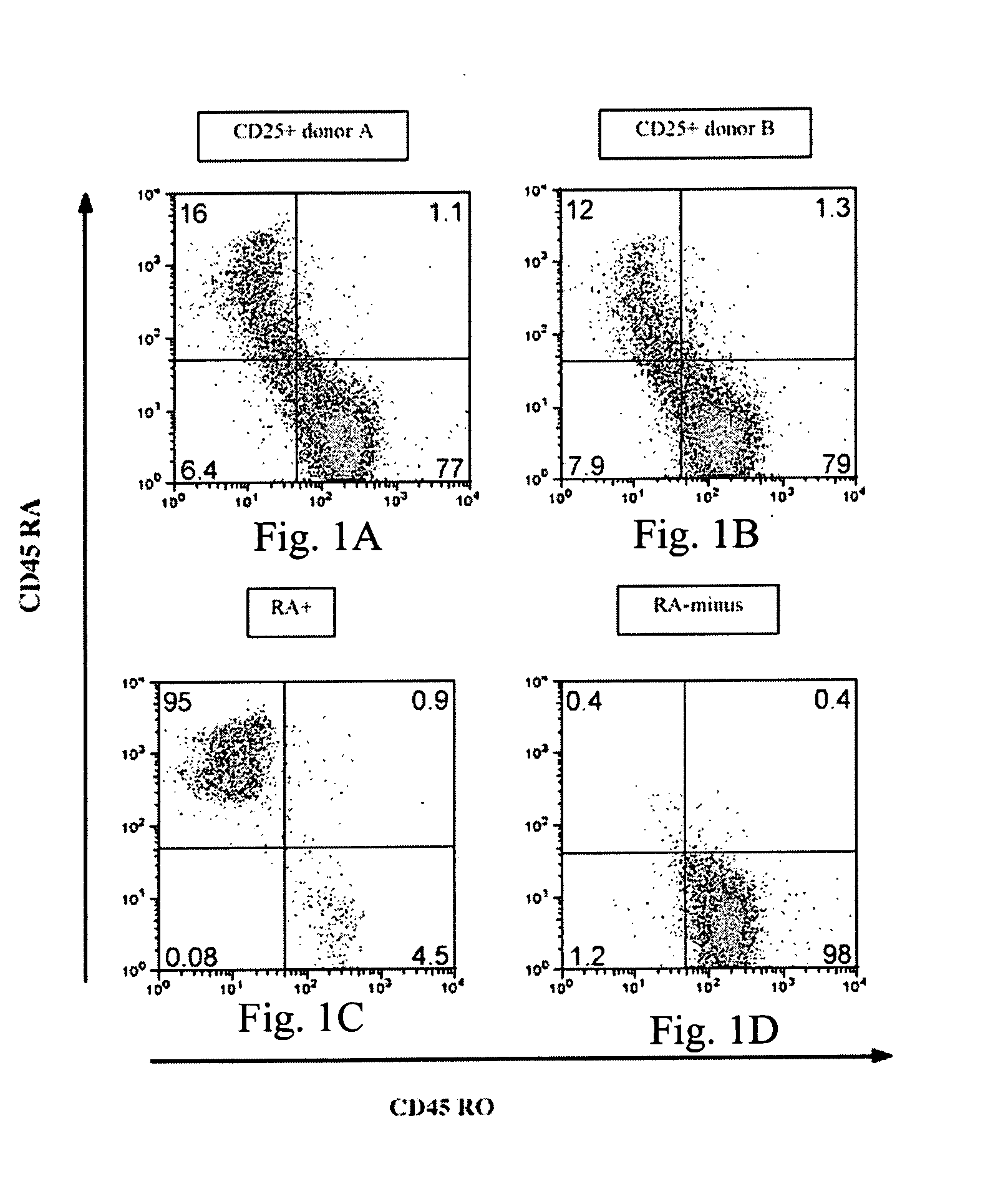
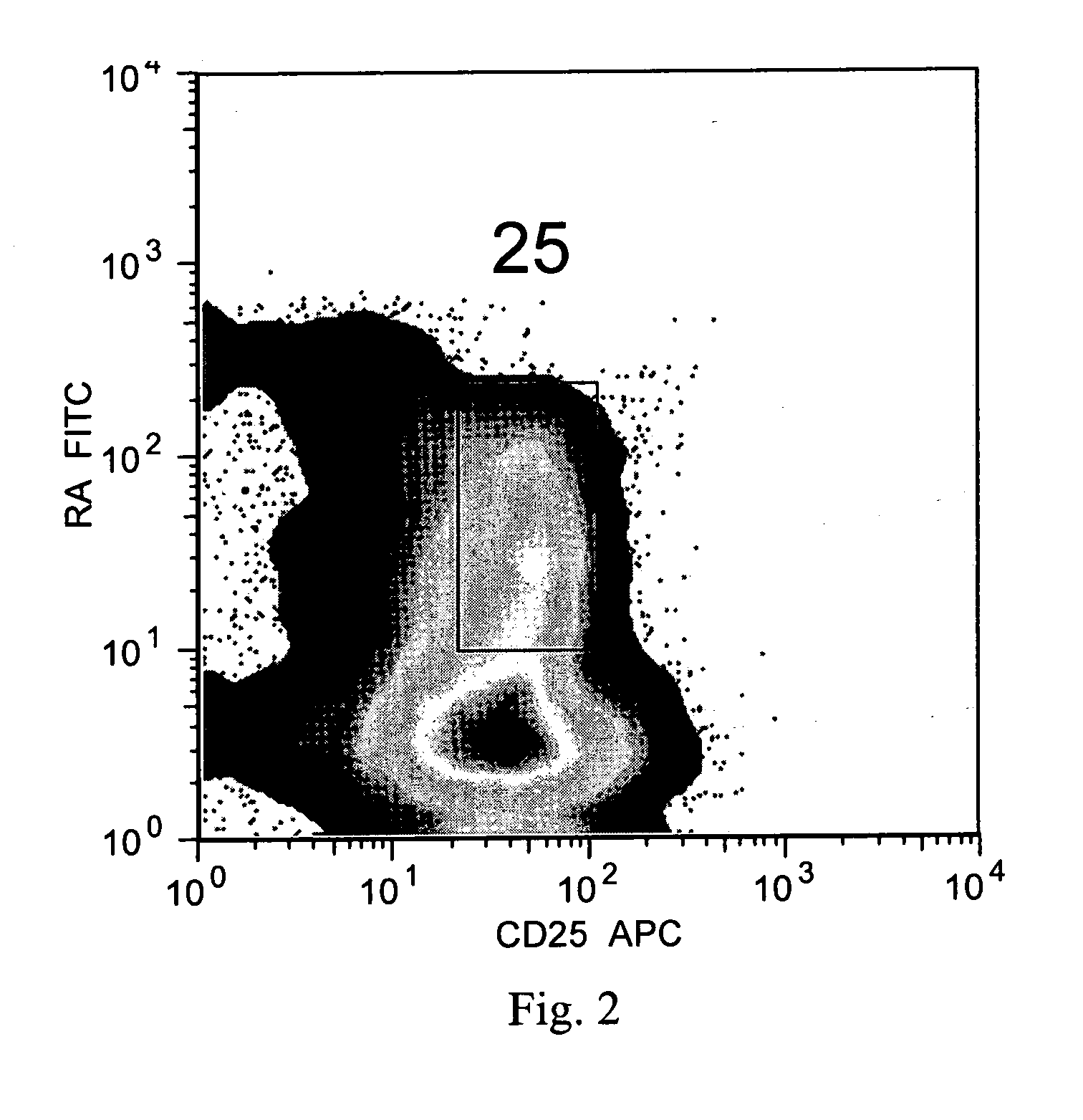
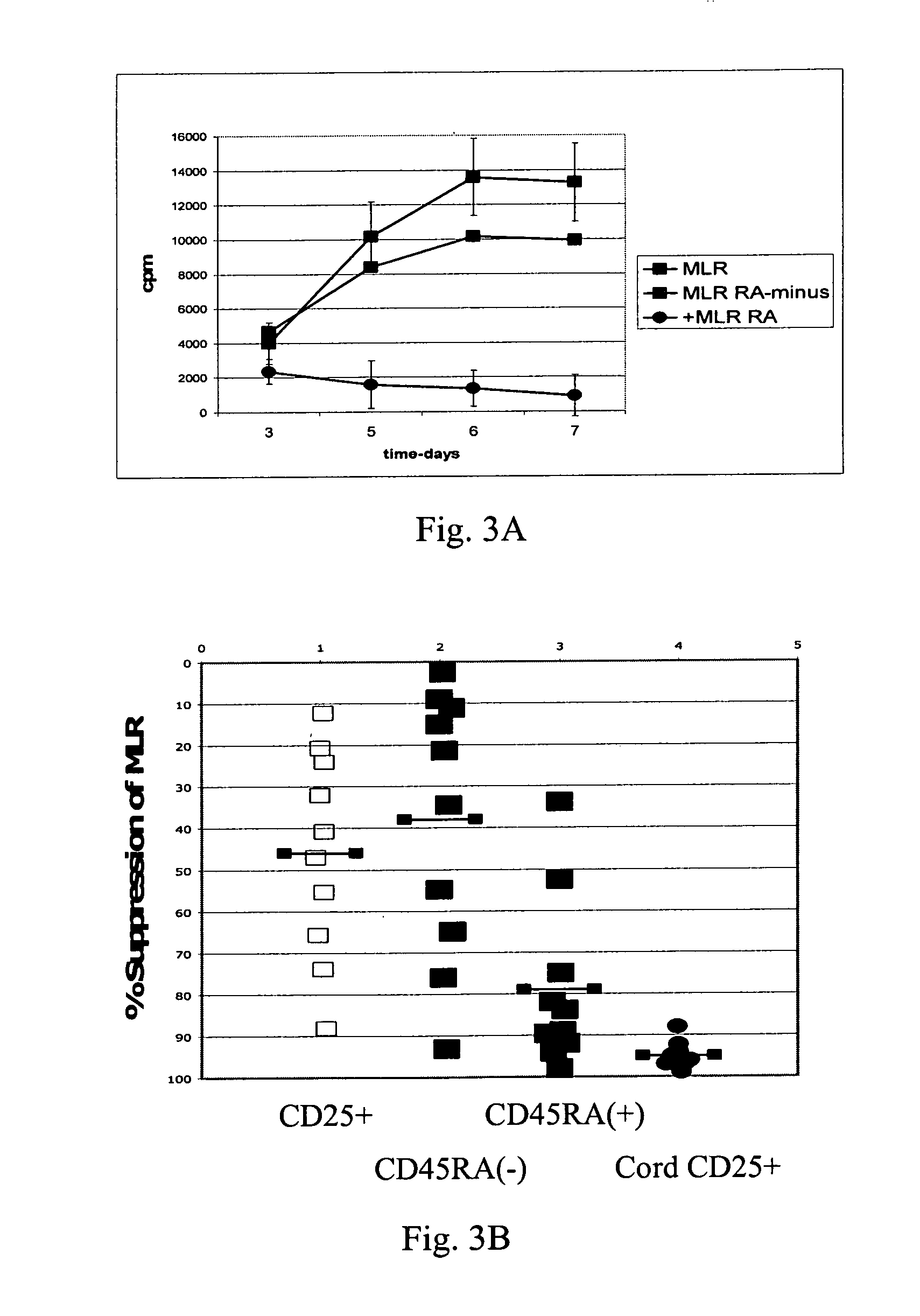
The present invention encompasses methods, and kits for the isolation and expansion of T regulatory cells having the CD45RA+ phenotype, including such cells from human umbilical cord blood.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
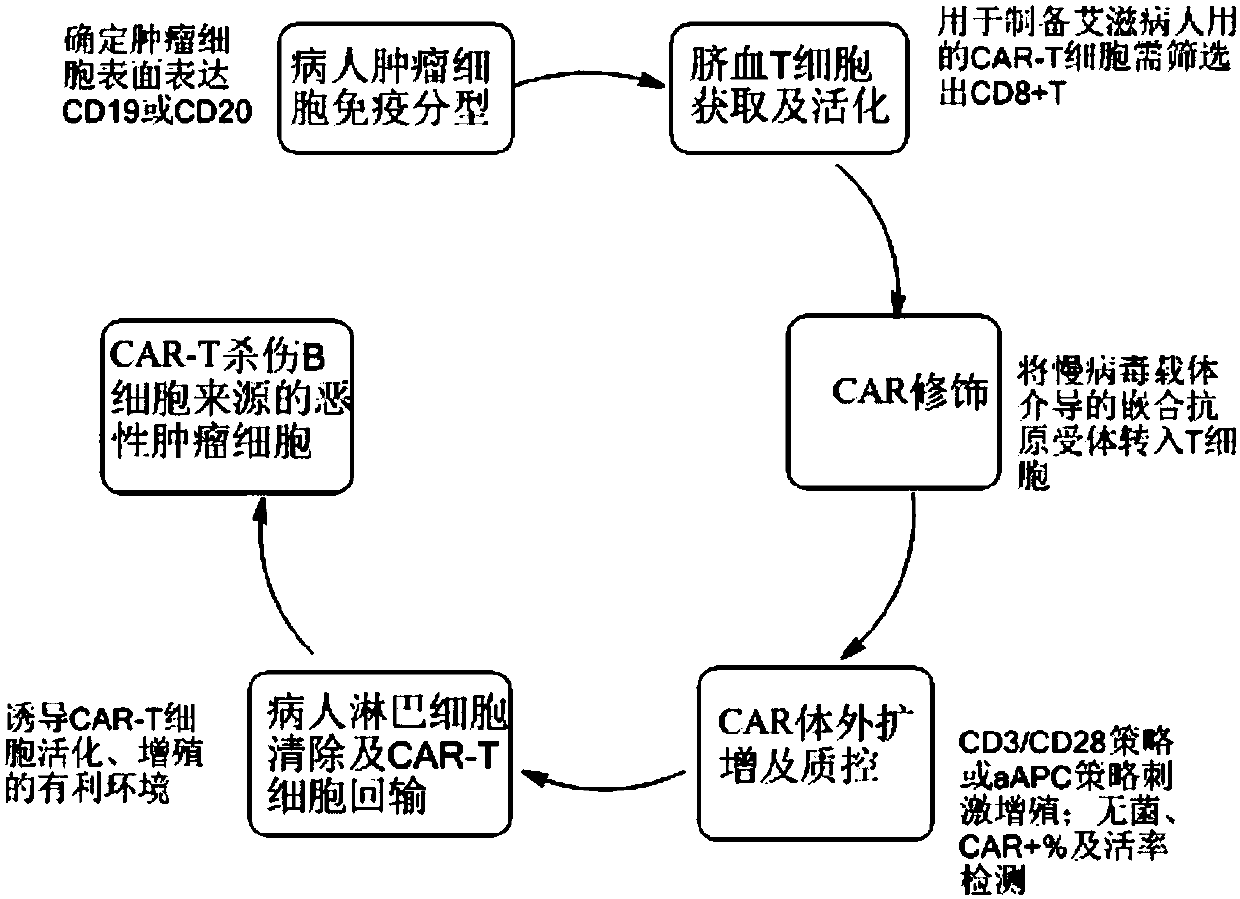
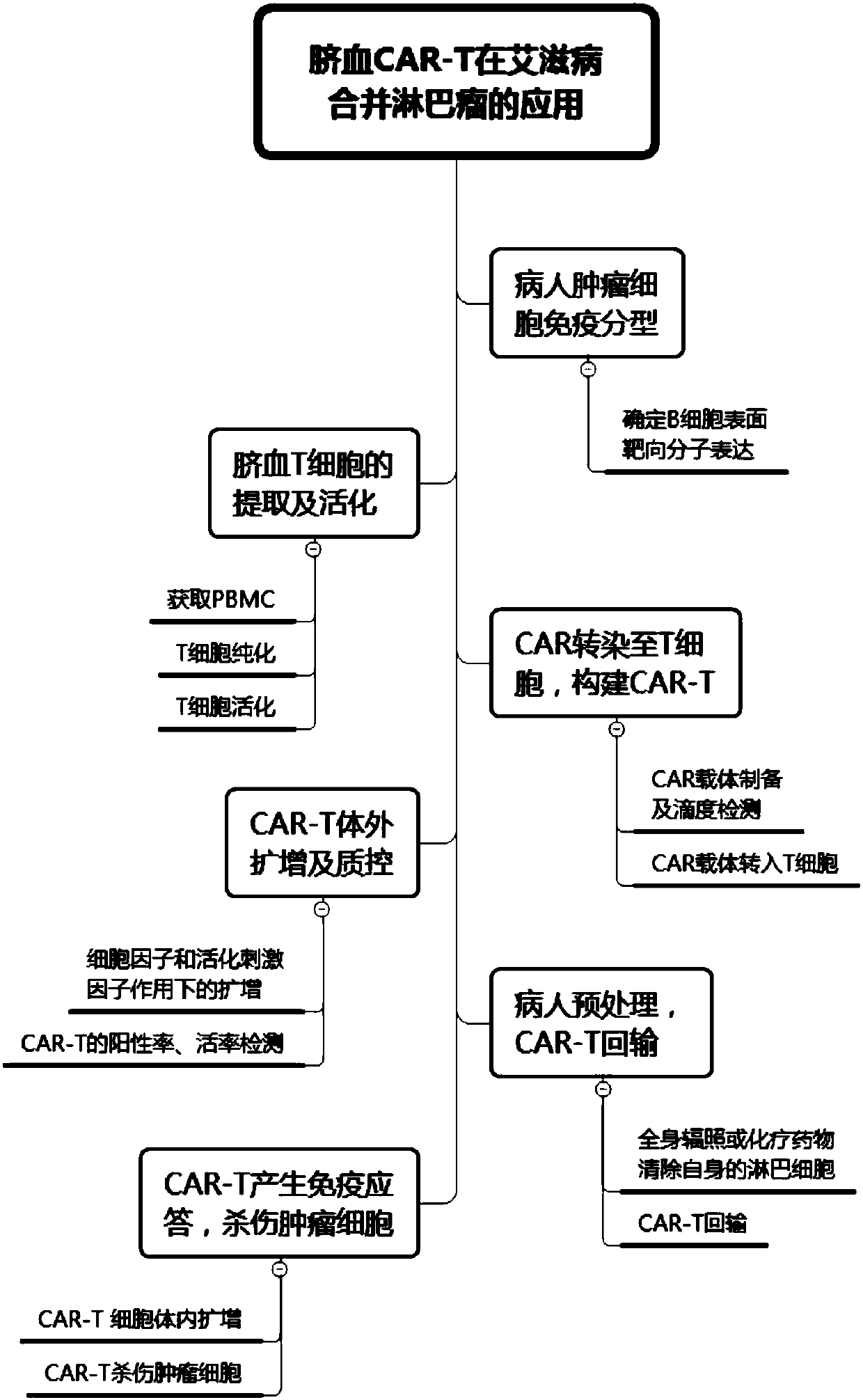
CAR-T cell for treating AIDS-associated lymphoma, and preparation method and application thereof
PendingCN107904259ALow storm riskSmall storm riskGenetically modified cellsAntiviralsAIDS-related lymphomaCord blood stem cell
The invention discloses a preparation method of a CAR-T cell for treating AIDS-related lymphoma. A CD8+ T cell is used to produce the CAR-T cell, and the CD8-T cell derives from a cord blood T cell. The method includes the following steps: preparing cord blood mononuclear cells from cord blood, removing tumors and other cells by using a human T cell purification kit to obtain T cells, and carryingout separation by using magnetic beads to obtain the CD8+ T cell; activating the CD8+ T cell by using an appropriate medium and appropriate stimulation conditions; transferring CAR to the CD8+ T cellby using lentivirus to prepare the CAR-T cell; and amplifying the CAR-T cell in vitro by using cytokines and activating stimulators to achieve the desired effective dose. The invention also relates to the CAR-T cell prepared by the method, and an application thereof. The CAR-T cell prepared in the invention has a good cell activity and a high proliferation speed, and reduces the attack risk of GVHD; and only the CD8+T cell of the umbilical blood T cells of AIDS patients is used to prepare the CAR-T cell for, so the risk of input CAR-T infected with in-vivo HIV is reduced.
Owner:SHANGHAI LONGYAO BIOTECH CO LTD
Stem and progenitor cell compositions recovered from bone marrow or cord blood; system and method for preparation thereof
InactiveUS20070269887A1Not labor intensiveEasy to implementOther blood circulation devicesBiomass after-treatmentProgenitorMicrocontroller
The invention includes compositions of stem and progenitor cells recovered from bone marrow or cord blood containing most of the viable CD34+ cells and substantially depleted of red blood cells resident in the original sample, without any xenobiotic additives to aid cell separation. The invention also includes a system and method for preparing the compositions. The system includes a bag set and a processing device, which utilizes an optical sensor, microcontroller, servo motor, accelerometer, load cell, and battery. The system and method utilize centrifugation to stratify the cells into layers and then separate and transfer the stem cells into a stem cell bag. The processing device's microcontroller receives input from the device's accelerometer, load cell and optical sensor to direct the metering valve in the bag set to open and close to permit the transfer of as many stems cells as possible with as few red cells as possible.
Owner:THERMOGENESIS
Compositions and populations of cells obtained from the umbilical cord and methods of producing the same
InactiveUS20090280093A1Extensive mechanical processingFacilitate typeBiocideMammal material medical ingredientsProgenitorCord blood stem cell
The present invention relates to populations and compositions of stem and progenitor cells derived from the umbilical cord, and methods of obtaining the same. In some embodiments, one or more entire umbilical cords or sections thereof are subjected to a process where a cell population is derived without prior removal of any blood vessel. The population may be derived using mechanical and chemical means. The presently disclosed process may be applied to a single umbilical cord or to a plurality of umbilical cords, for example, as a batch process. Optionally, this process includes removing some or all cord blood before deriving the population. In some embodiments, presently disclosed cell populations include mesenchymal stem cells derived from Wharton's jelly and endothelial progenitor cells derived from a wall of a blood vessel of an umbilical cord. Optionally, the cell population includes stem cells derived from cord blood. The presently disclosed cell populations and compositions may be banked and / or used in a number of clinical or other applications. Exemplary applications include but are not limited to applications related to regenerative medicine, for screening compounds, for research, and for gene therapy.
Owner:THE REGENERATIVE MEDICINE INST
Method of growing mesenchymal stem cells from bone marrow
InactiveUS20090004661A1Function increaseGood potential sourceMicrobiological testing/measurementCulture processCord blood stem cellMesenchymal stem cell
The present invention provides a method for culturing mesenchymal stem cells using cord blood serum, for therapeutic purposes in regenerative medicine; and in particular the present invention provides for the use of these cells in the treatment of PD, and the present invention has provided proliferation and neuronal differentiation of the MSCs in a xenofree culture system for clinical applications in a simple two step protocol, and the in vivo functional efficacy was tested in Parkinson's animal model.
Owner:RELIANCE LIFE SCI PVT
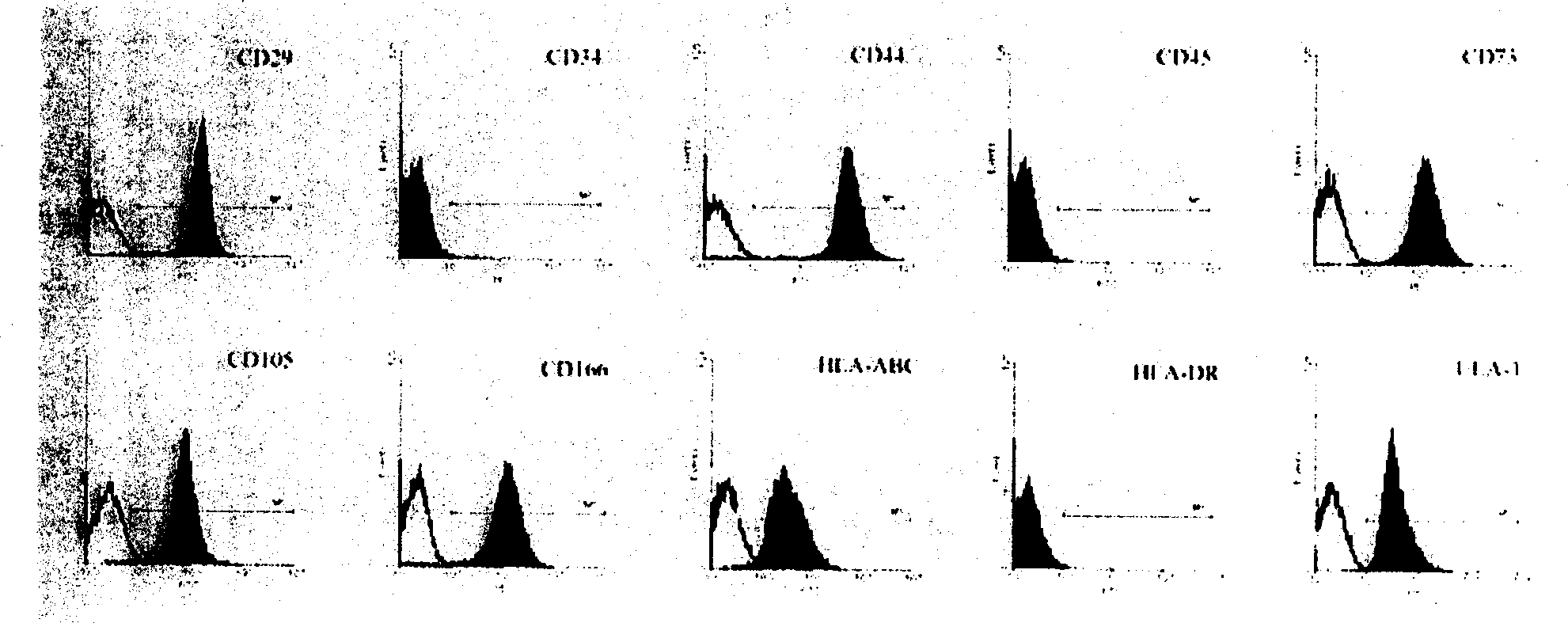
Isolated Embryonic-Like Stem Cells Derived From Human Umbilical Cord Blood
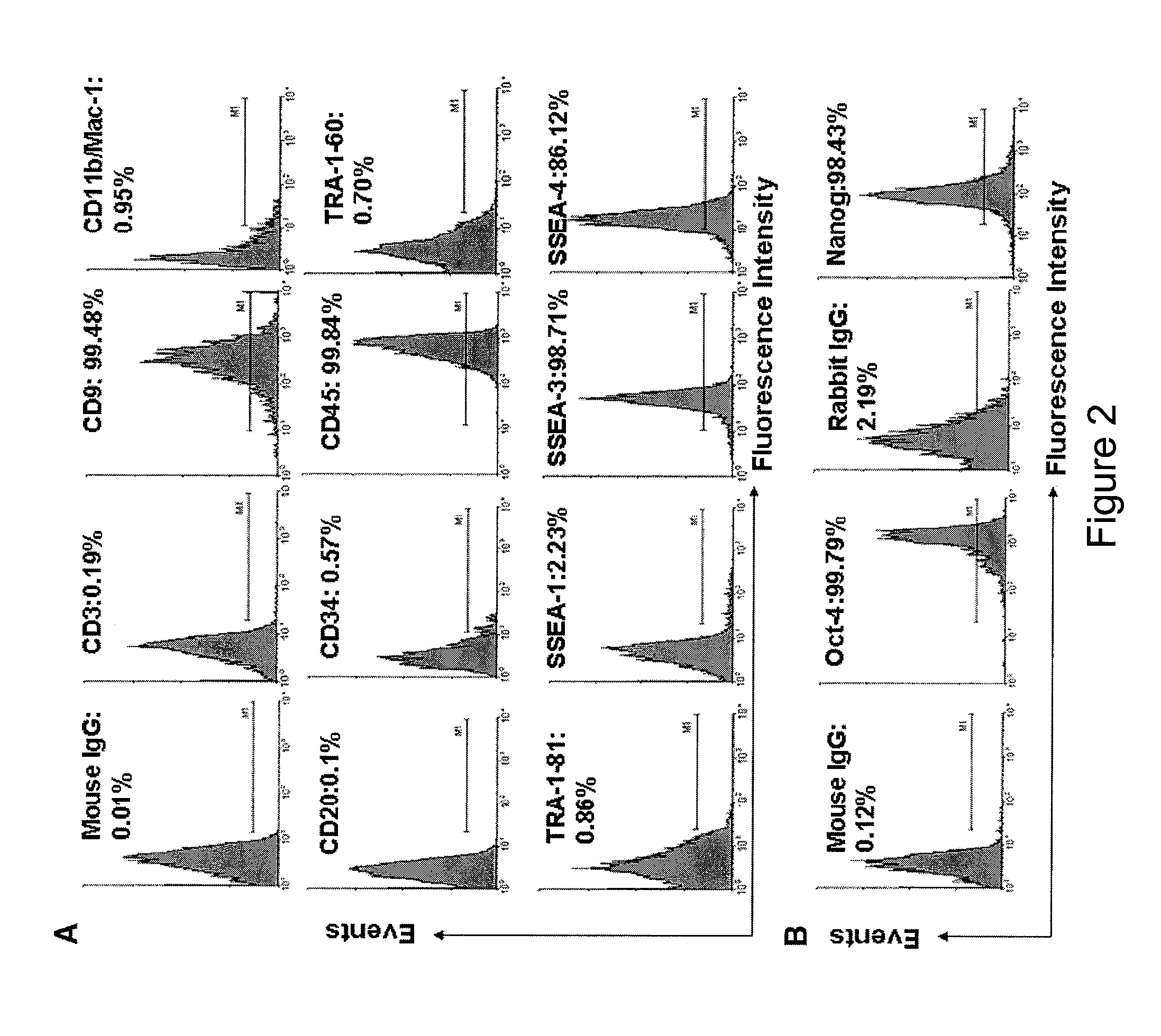
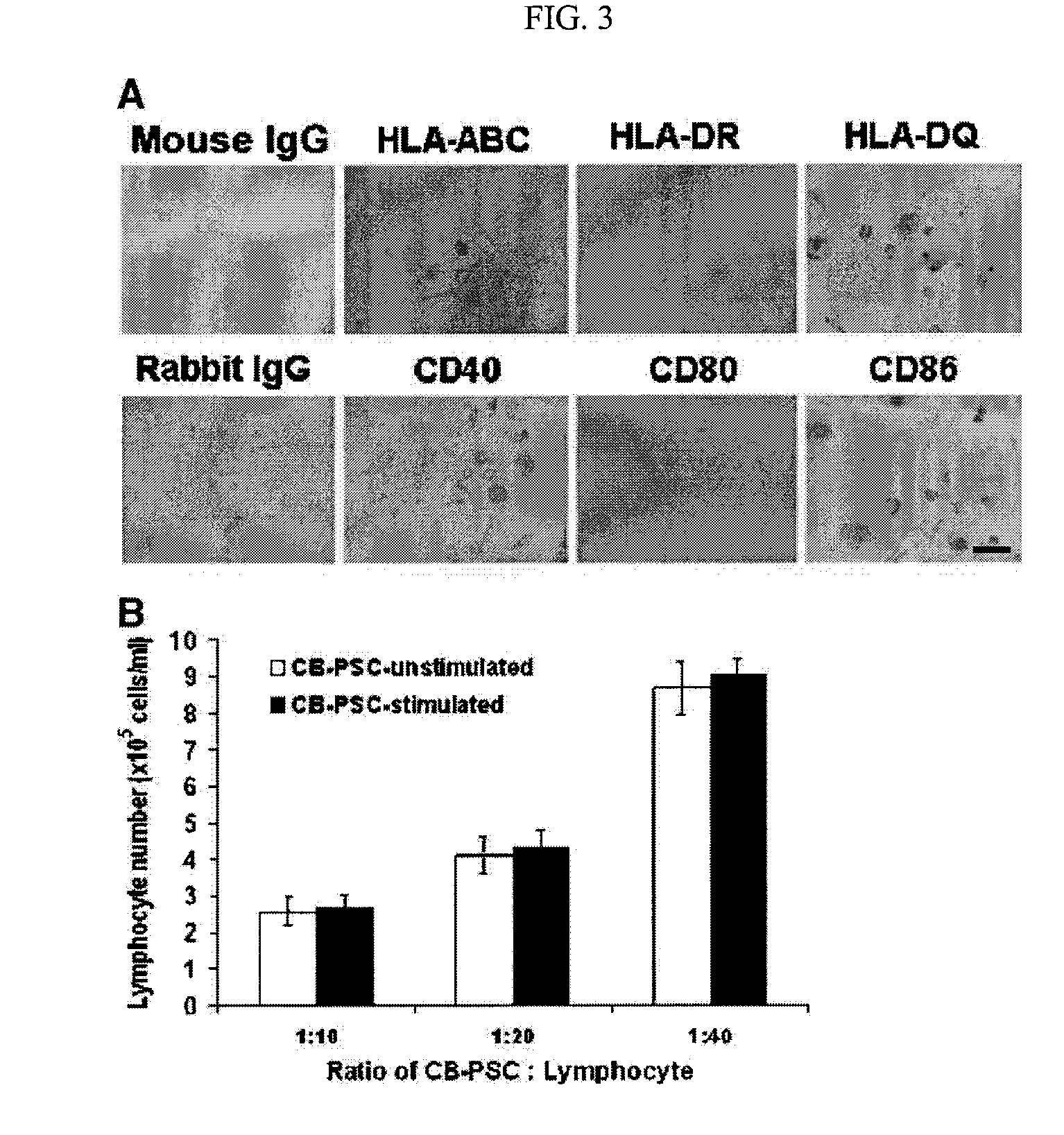
ActiveUS20090175832A1Low immunogenicityAbility to proliferateBiocideMetabolism disorderHematopoietic cellAutoimmune condition
The present invention is related generally to embryonic-like stem cells isolated from human umbilical cord blood, designated herein as cord blood-stem cells (CB-SC's), which display the characteristics of embryonic stem cells and hematopoietic cells. These cells have the capability of proliferation and are able to differentiate to multiple types of cells. In addition, the CB-SC display low immunogenicity and immune regulation. These cells are, therefore, suitable for use in stem cell-based therapies for the treatment of diseases such as Parkinson's disease, diabetes, spinal cord damage, multiple sclerosis, cardiovascular disease, stroke and birth defects, and for preventing, treating and / or reducing an autoimmune disease in a mammalian subject.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Universal donor chimeric antigen receptor cells
InactiveUS20160237407A1Good curative effectImprove localizationPolypeptide with localisation/targeting motifImmunoglobulin superfamilyProgenitorCord blood stem cell
Disclosed are allogeneic cells useful for the treatment of cancer in a universal donor, off the shelf, manner. In one embodiment of the invention cord blood derived T cell progenitors are matured with anti-CD3 and anti-CD28, interleukin-7 and transfected with a construct encoding a chimeric antigen receptor (CAR) targeting a tumor antigen or a tumor endothelial associated antigen on the antigen binding domain. The intracellular domain containing CD3 zeta chain and at least one shRNA domain encoding a transcript which generates at least one siRNA capable of inhibiting expression of HLA I and / or HLA II. In another embodiment mesenchymal stem cells are transfected with CAR to enhance migration into tumors and induce tumor death, reduction of inflammation, or immune sensitization. In another embodiment universal donor CAR-MSC are disclosed.
Owner:BATU BIOLOGICS
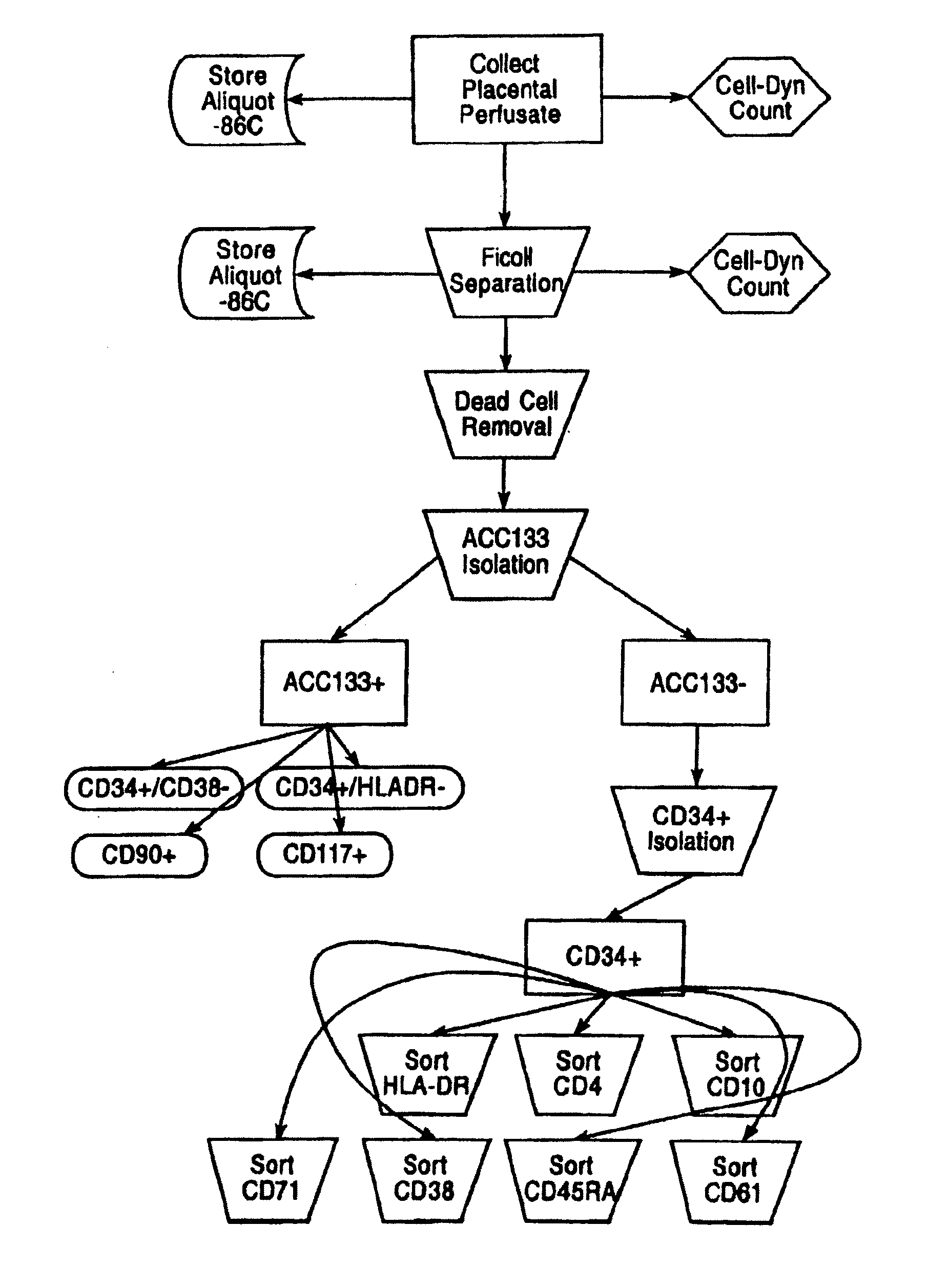
Methods for collecting and using placenta cord blood stem cells
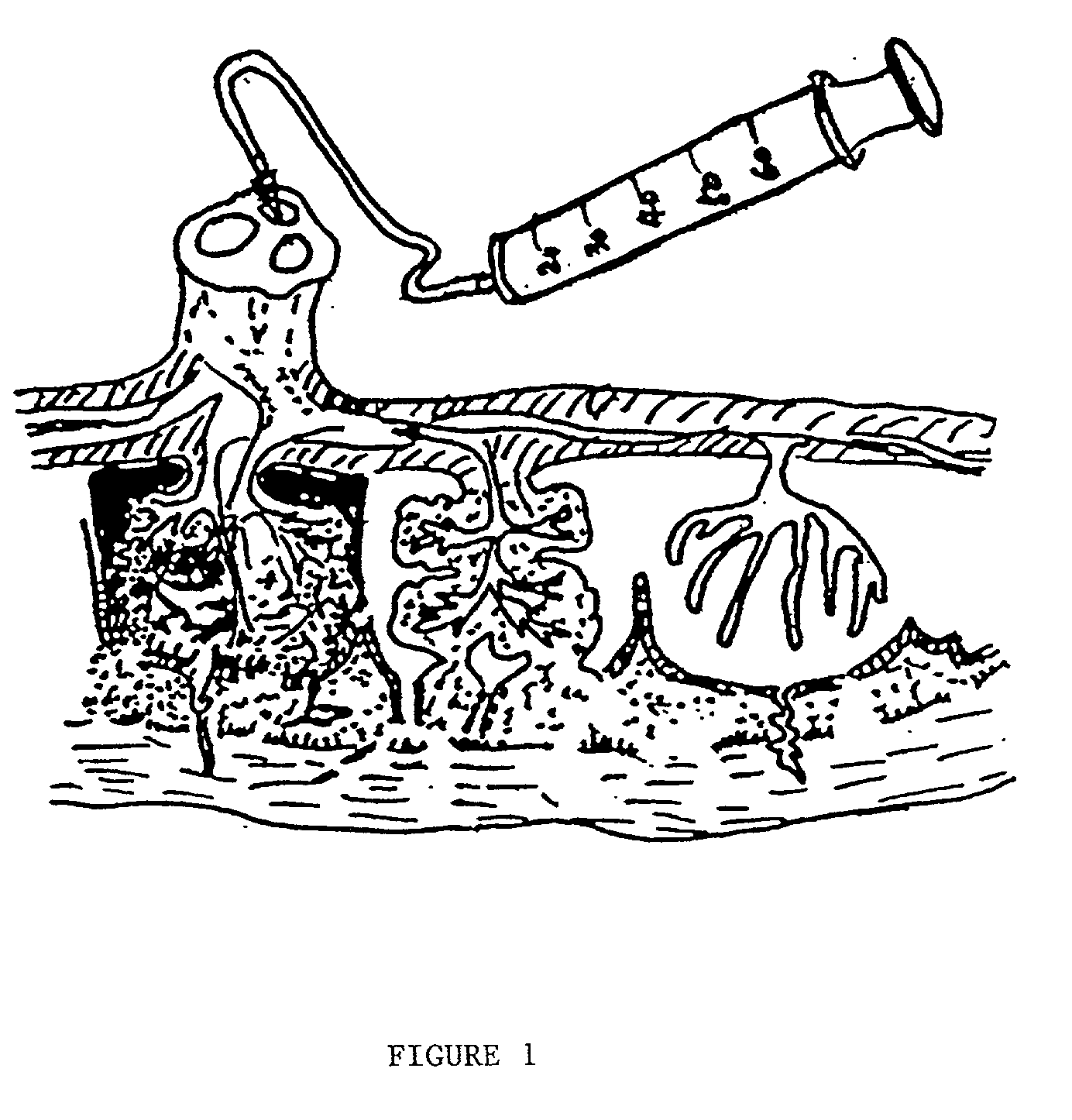
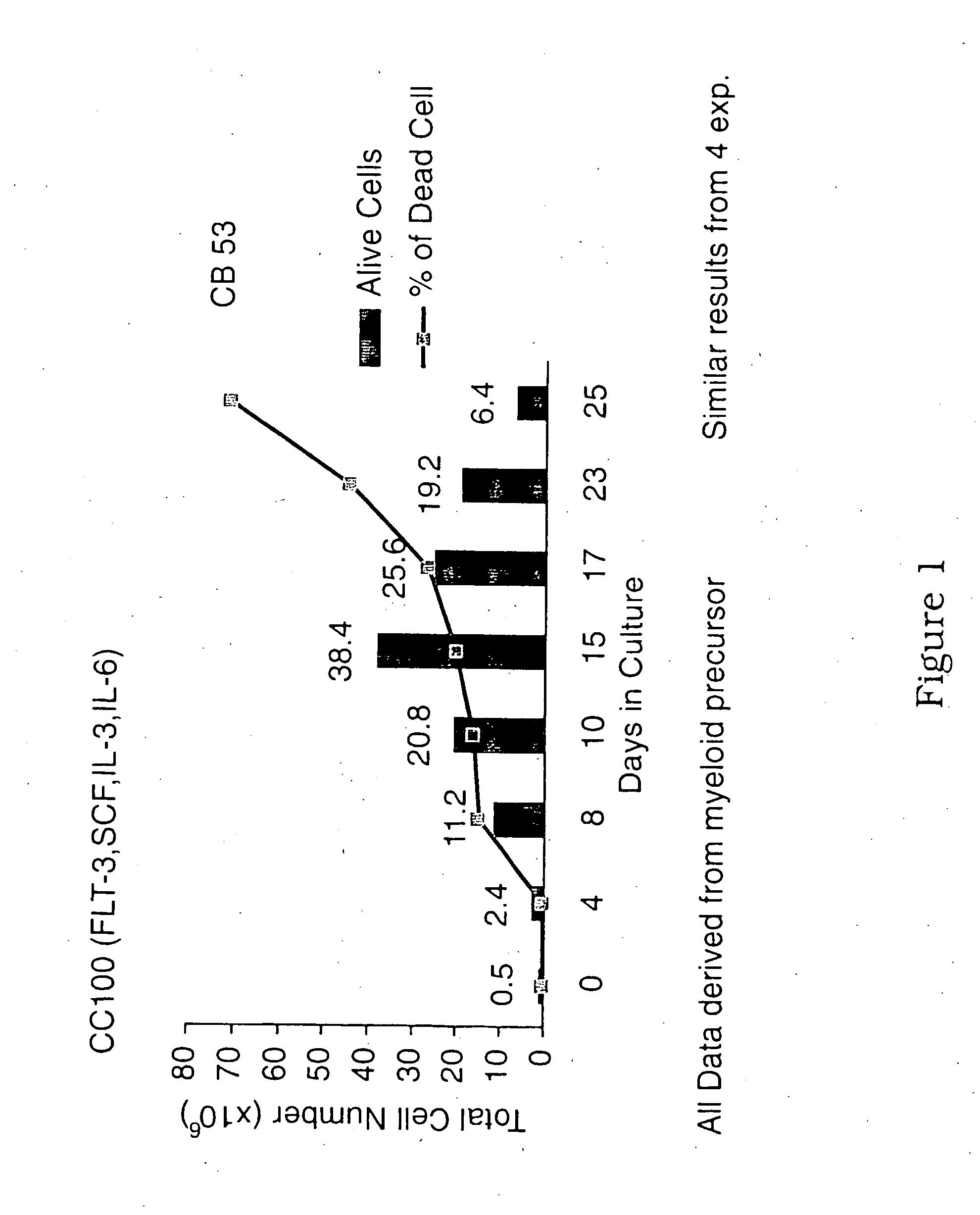
ActiveUS20090123437A1Raise countBiocideMammal material medical ingredientsPeristaltic pumpCord blood stem cell
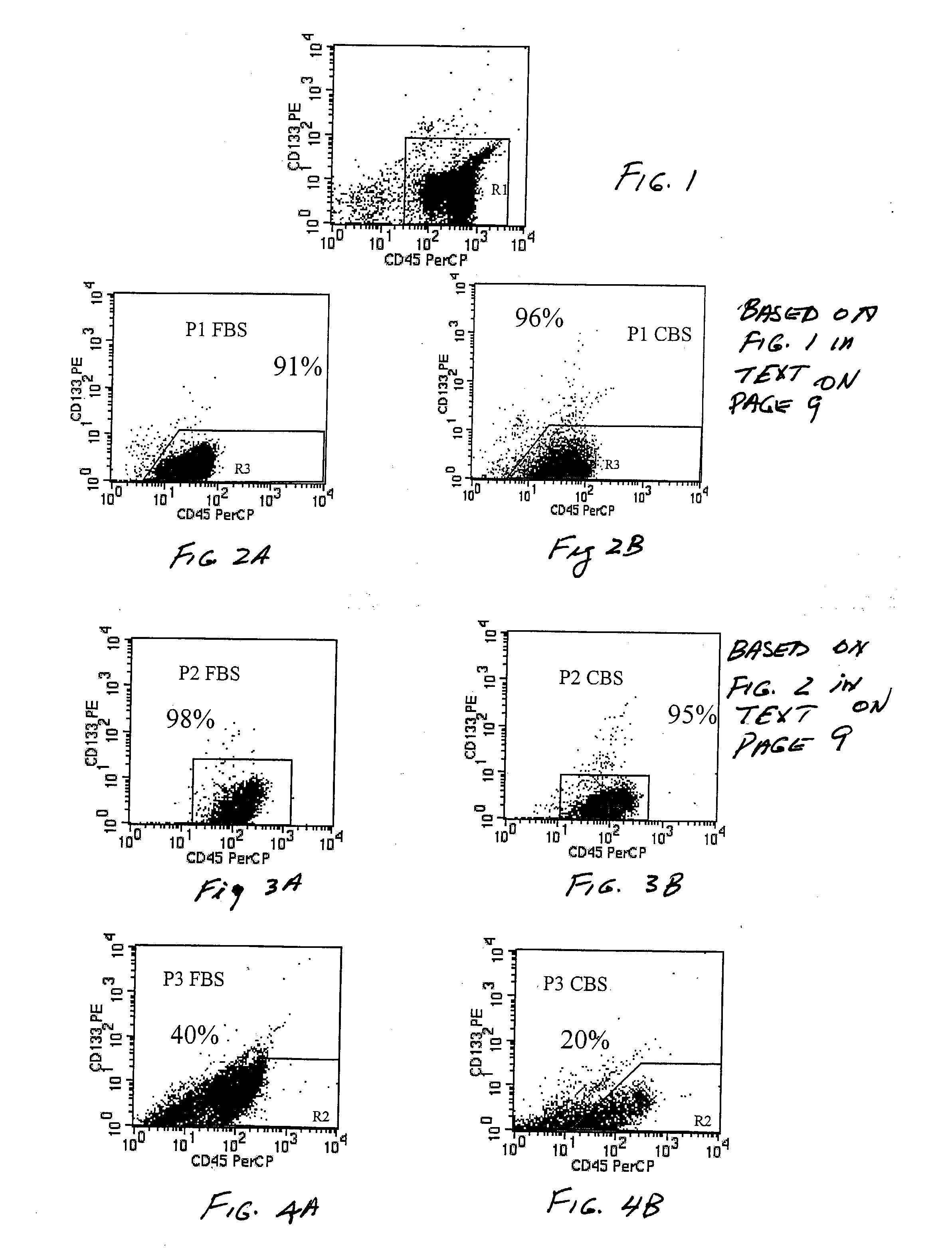
An innovative method of collecting cord blood stem cells from an isolated mammalian non-exsanguinated or partially exsanguinated placenta by placental perfusion is described and also an easy method for safe long duration cold storage of the placenta. Placental perfusion can include perfusing the isolated placenta with a pulsatile flow of perfusion solution, for example, using a pulsatile or peristaltic pump or device. The stem cells can then be isolated from the perfusate. Significantly increased amounts of CD133+ stem cells can be collected from the perfusate. The perfusion solution can include an anticoagulant. The isolated mammalian placenta need not be treated with an anticoagulant prior to perfusing. The isolated placenta can be free from an anticoagulant prior to perfusing.
Owner:CELULARITY INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com