Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
56 results about "CD52" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
CAMPATH-1 antigen, also known as cluster of differentiation 52 (CD52), is a glycoprotein that in humans is encoded by the CD52 gene. CD52 is present on the surface of mature lymphocytes, but not on the stem cells from which these lymphocytes were derived. It also is found on monocytes and dendritic cells. Further, it is found within the male genital tract and is present on the surface of mature sperm cells.
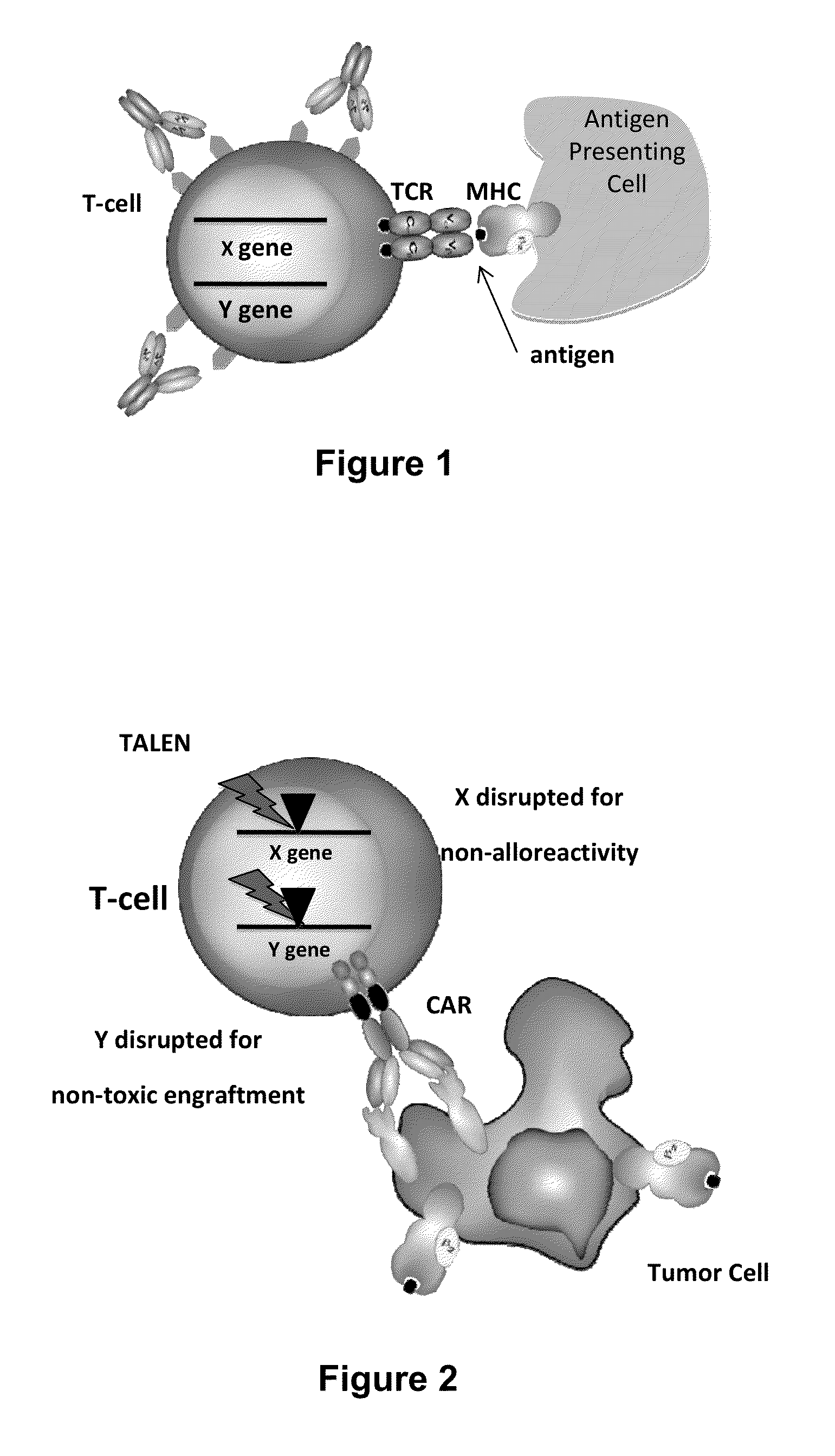
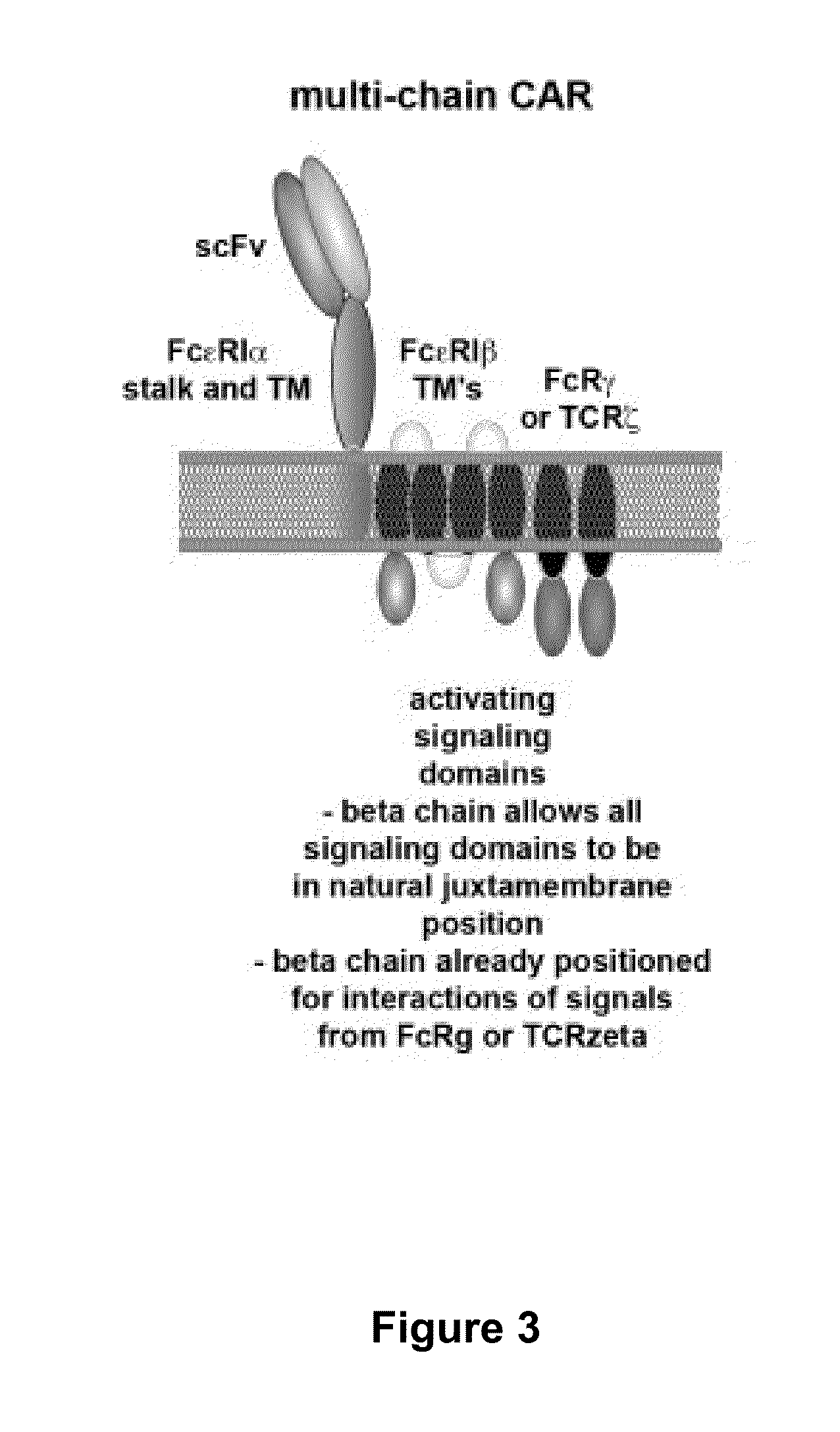
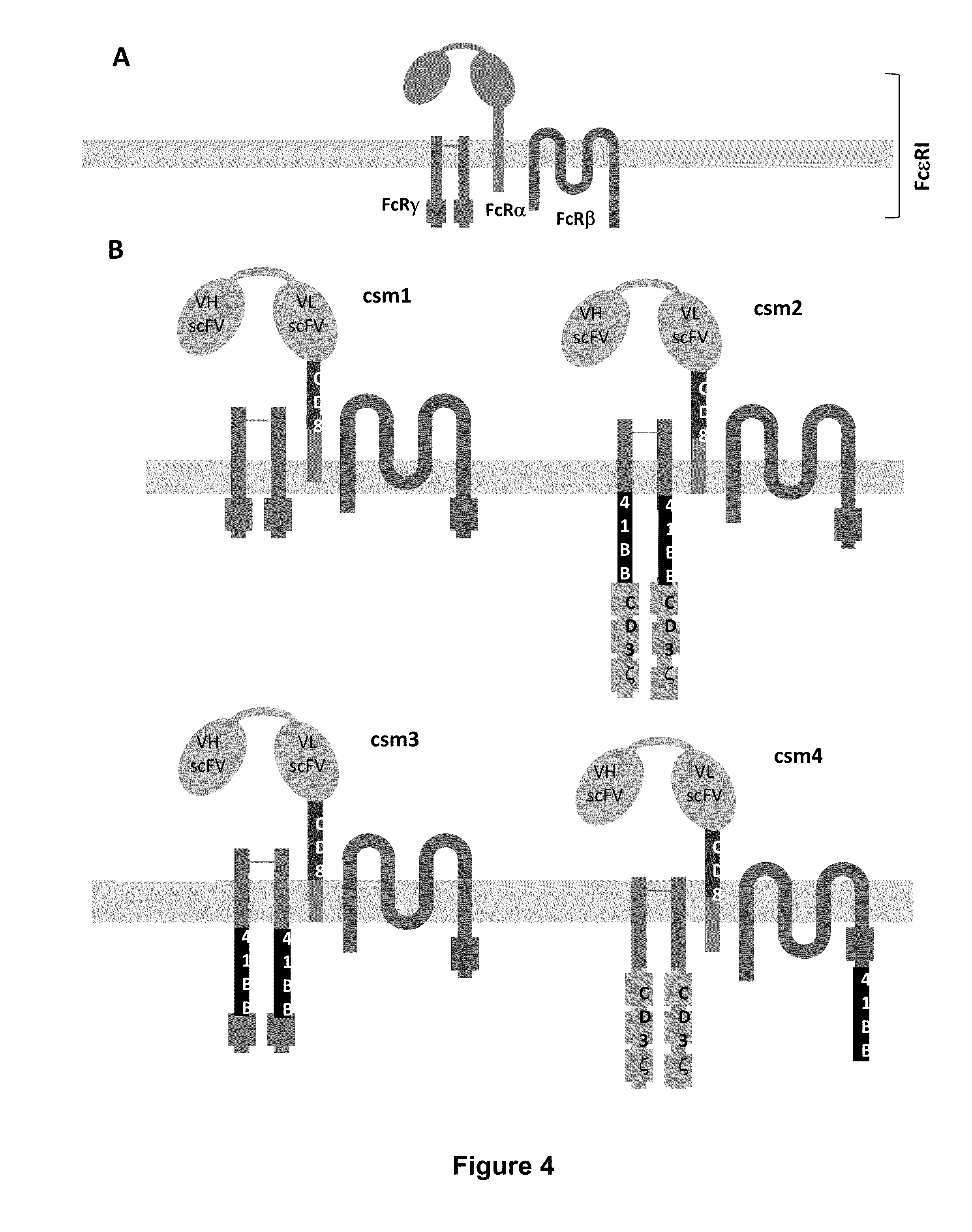
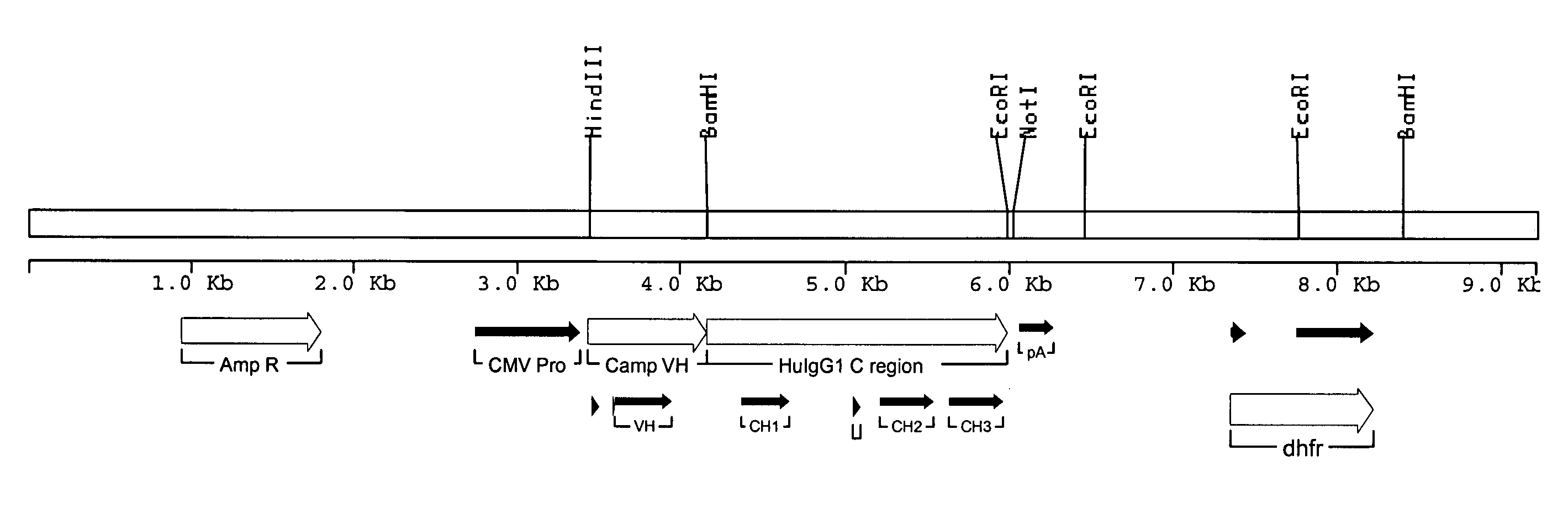
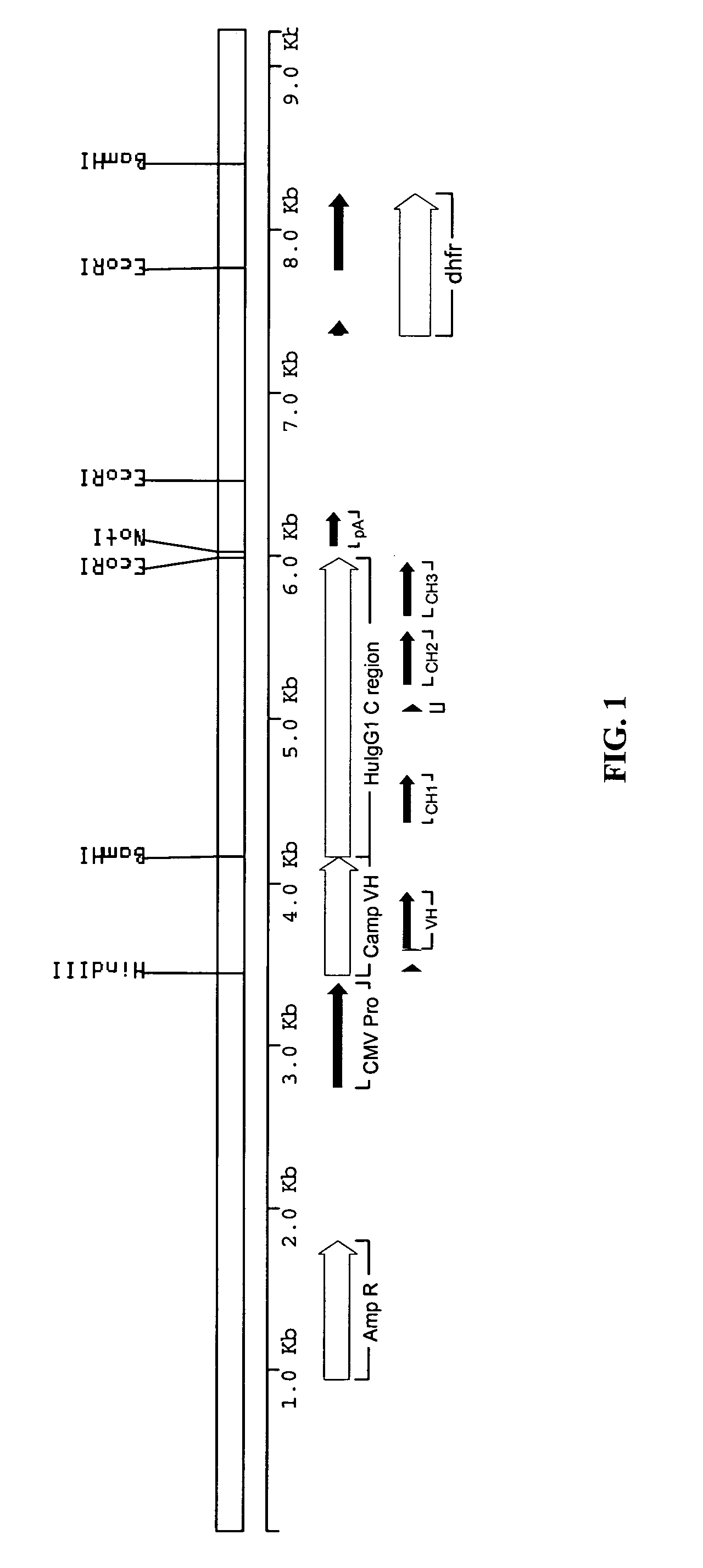
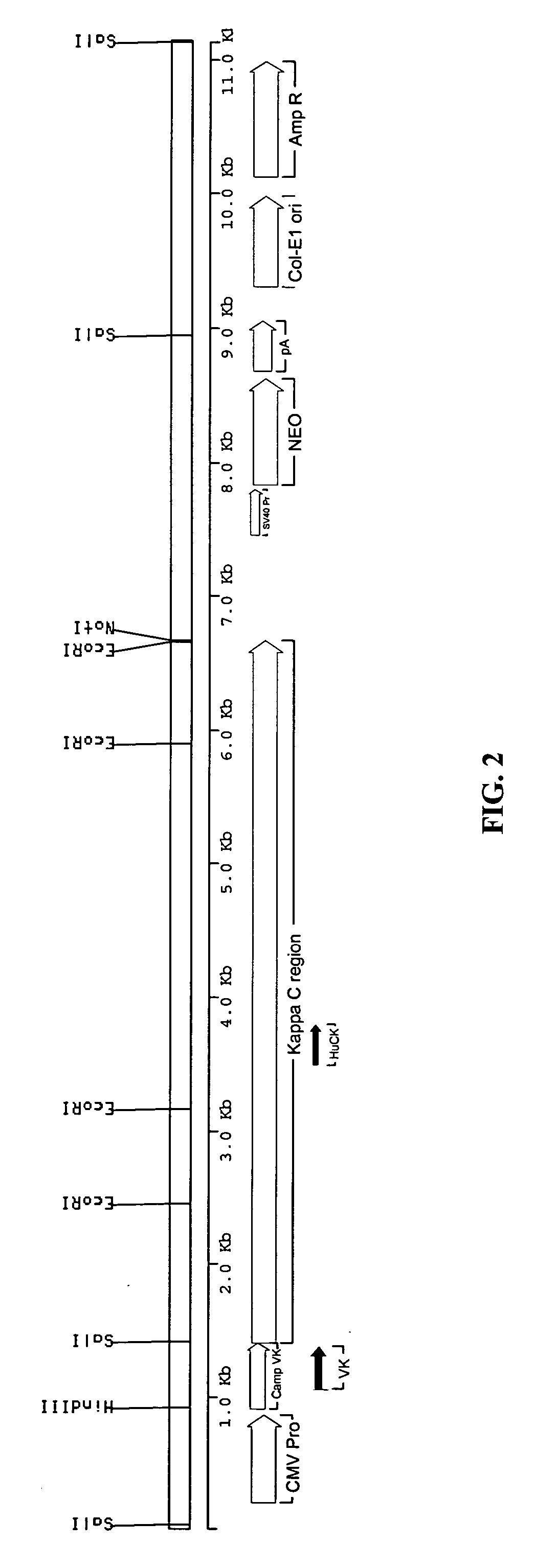
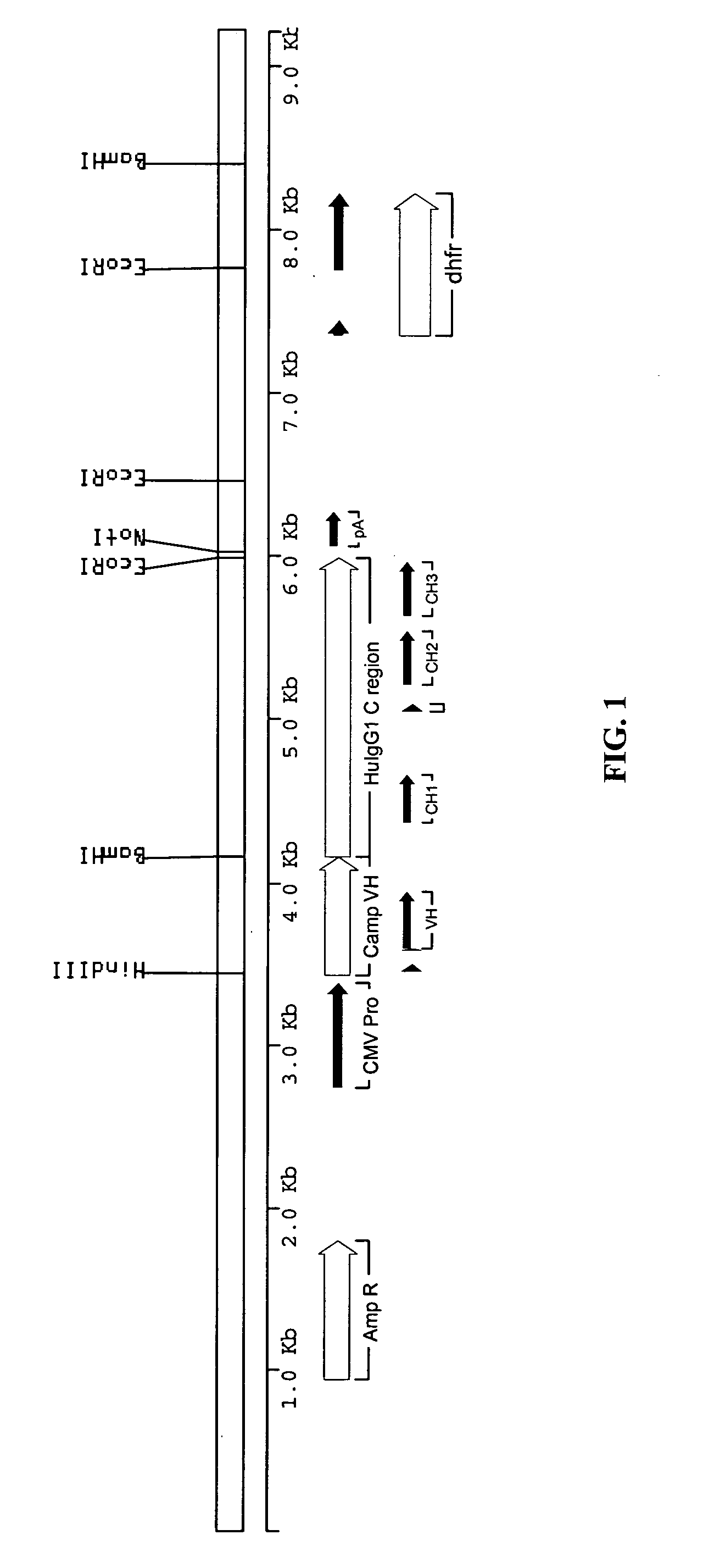
Methods for engineering allogeneic and immunosuppressive resistant t cell for immunotherapy
ActiveUS20130315884A1Precise positioningPeptide/protein ingredientsAntibody mimetics/scaffoldsImmunosuppressive drugPrimary cell
Methods for developing engineered T-cells for immunotherapy that are both non-alloreactive and resistant to immunosuppressive drugs. The present invention relates to methods for modifying T-cells by inactivating both genes encoding target for an immunosuppressive agent and T-cell receptor, in particular genes encoding CD52 and TCR. This method involves the use of specific rare cutting endonucleases, in particular TALE-nucleases (TAL effector endonuclease) and polynucleotides encoding such polypeptides, to precisely target a selection of key genes in T-cells, which are available from donors or from culture of primary cells. The invention opens the way to standard and affordable adoptive immunotherapy strategies for treating cancer and viral infections.
Owner:CELLECTIS SA
Camptothecin Conjugates of Anti-CD22 Antibodies for Treatment of B Cell Diseases
ActiveUS20110305631A1Increase the number ofNervous disorderPeptide/protein ingredientsCD20Autoimmune condition
Disclosed herein are compositions and methods of use comprising combinations of anti-CD22 antibodies with a therapeutic agent. The therapeutic agent may be attached to the anti-CD22 antibody or may be separately administered, either before, simultaneously with or after the anti-CD22 antibody. In preferred embodiments, the therapeutic agent is an antibody or fragment thereof that binds to an antigen different from CD22, such as CD19, CD20, CD21, CD22, CD23, CD37, CD40, CD40L, CD52, CD80 and HLA-DR. However, the therapeutic agent may an immunomodulator, a cytokine, a toxin or other therapeutic agent known in the art. More preferably, the anti-CD22 antibody is part of a DNL complex, such as a hexavalent DNL complex. Most preferably, combination therapy with the anti-CD22 antibody or fragment and the therapeutic agent is more effective than the antibody alone, the therapeutic agent alone, or the combination of anti-CD22 antibody and therapeutic agent that are not conjugated to each other. Administration of the anti-CD22 antibody and therapeutic agent induces apoptosis and cell death of target cells in diseases such as B-cell lymphomas or leukemias, autoimmune disease or immune dysfunction disease.
Owner:IMMUNOMEDICS INC
Synergistic Anti-CD47 Therapy for Hematologic Cancers
ActiveUS20120282174A1Immunoglobulins against cell receptors/antigens/surface-determinantsRadioactive preparation carriersSurface markerCD20
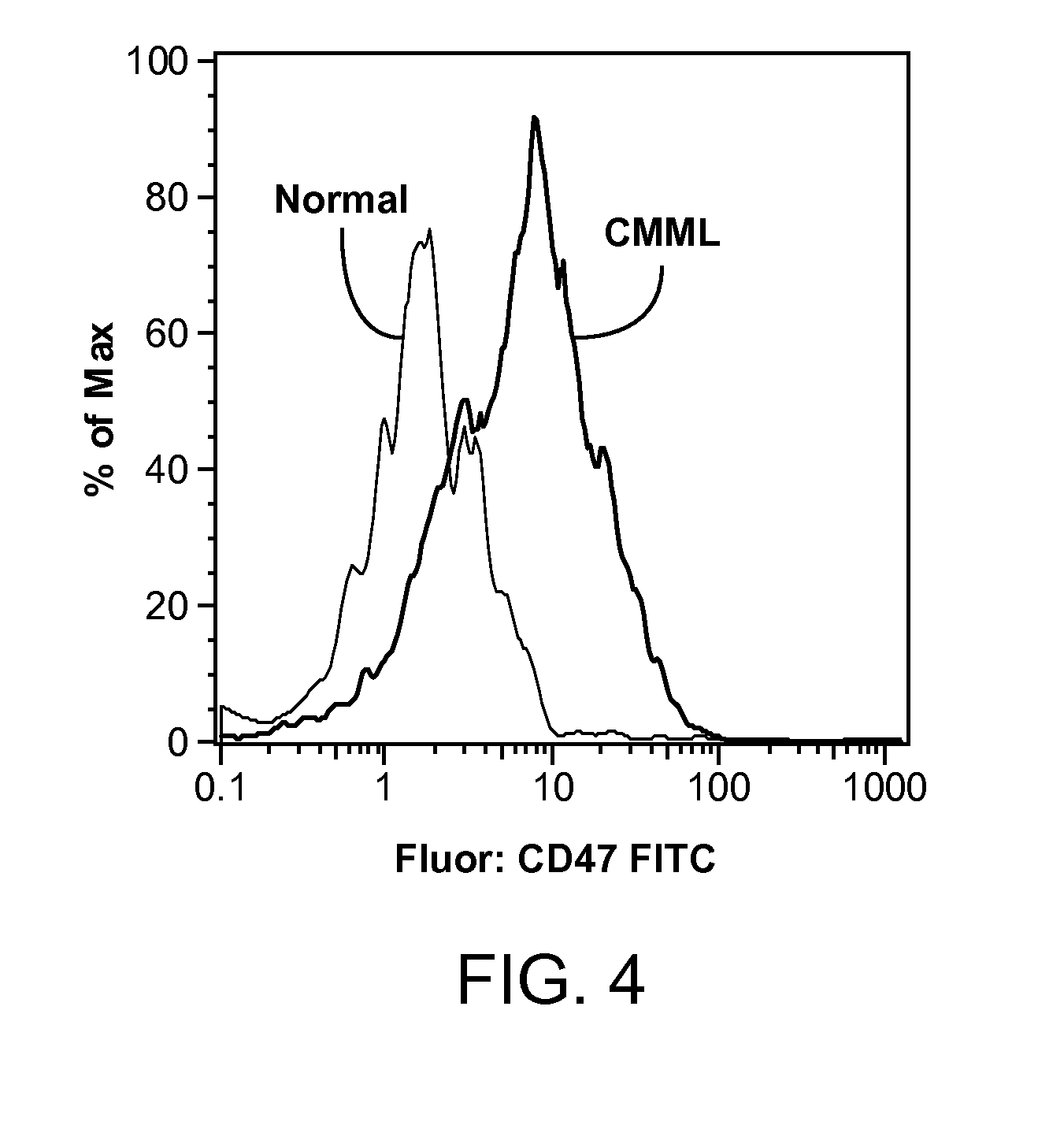
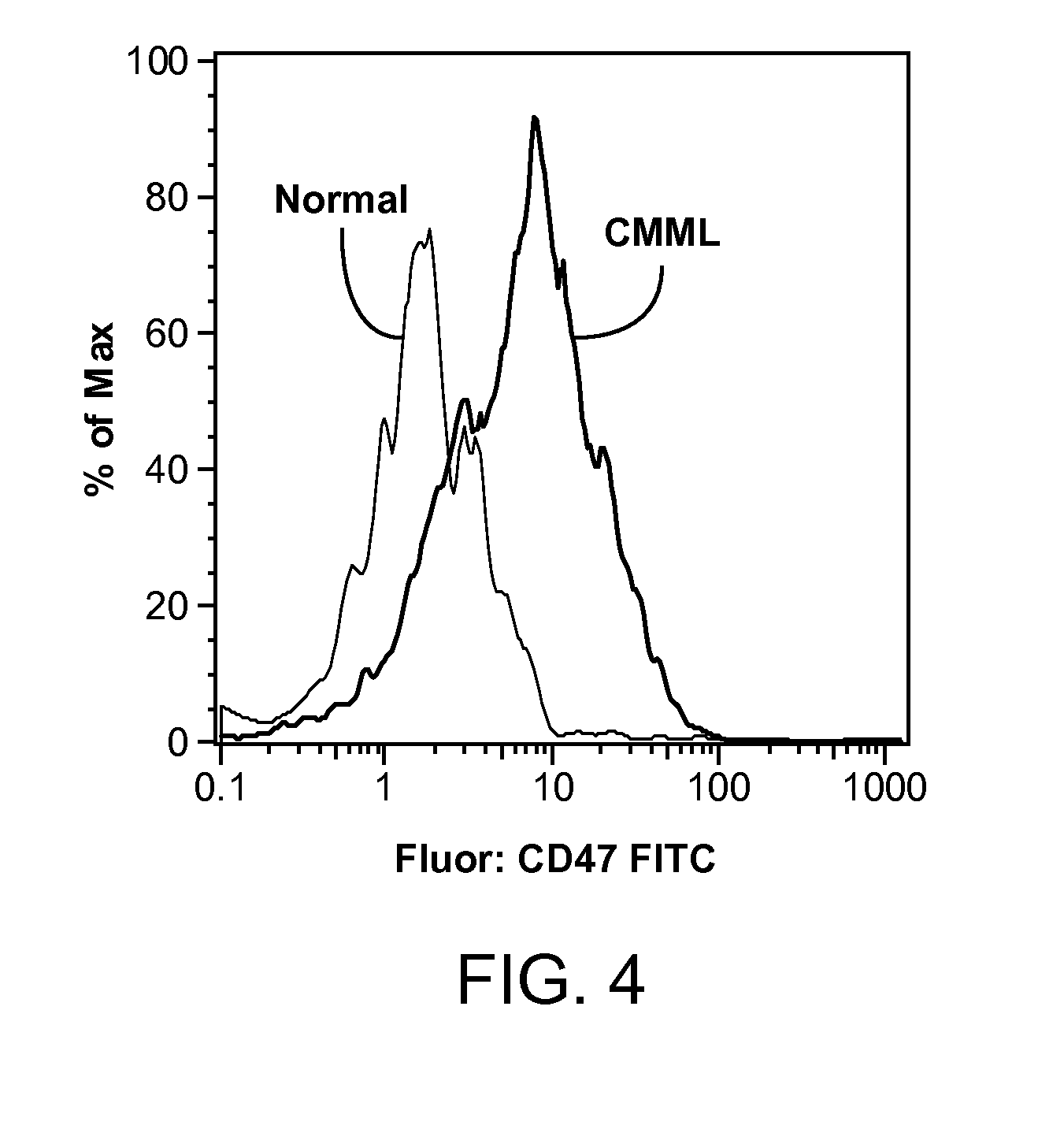
Methods are provided for treatment of hematologic cancers, particularly lymphomas and leukemias, including without limitation myelogenous and lymphocytic leukemias. A combination of antibodies specific for CD47; and specific for a cancer associated cell surface marker are administered to the patient, and provide for a synergistic decrease in cancer cell burden. The combination of antibodies may comprise a plurality of monospecific antibodies, or a bispecific or multispecific antibody. Markers of interest include without limitation, CD20, CD22, CD52, CD33; CD96; CD44; CD123; CD97; CD99; PTHR2; and HAVCR2.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Synergistic anti-CD47 therapy for hematologic cancers
Methods are provided for treatment of hematologic cancers, particularly lymphomas and leukemias, including without limitation myelogenous and lymphocytic leukemias. A combination of antibodies specific for CD47; and specific for a cancer associated cell surface marker are administered to the patient, and provide for a synergistic decrease in cancer cell burden. The combination of antibodies may comprise a plurality of monospecific antibodies, or a bispecific or multispecific antibody. Markers of interest include without limitation, CD20, CD22, CD52, CD33; CD96; CD44; CD123; CD97; CD99; PTHR2; and HAVCR2.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
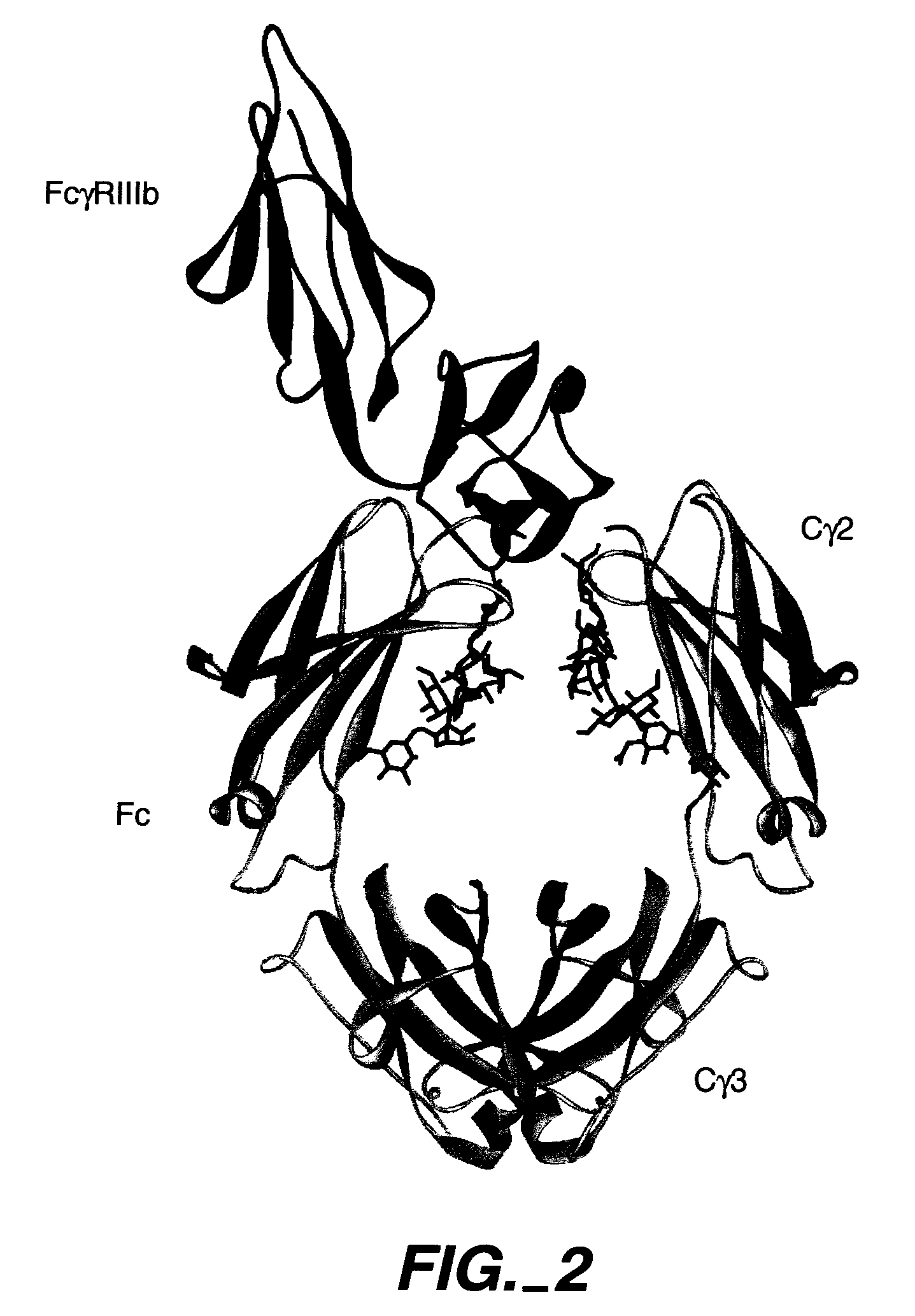
CD52 OPTIMIZED Fc VARIANTS AND METHODS FOR THEIR GENERATION
Owner:XENCOR INC
Monoclonal antibodies
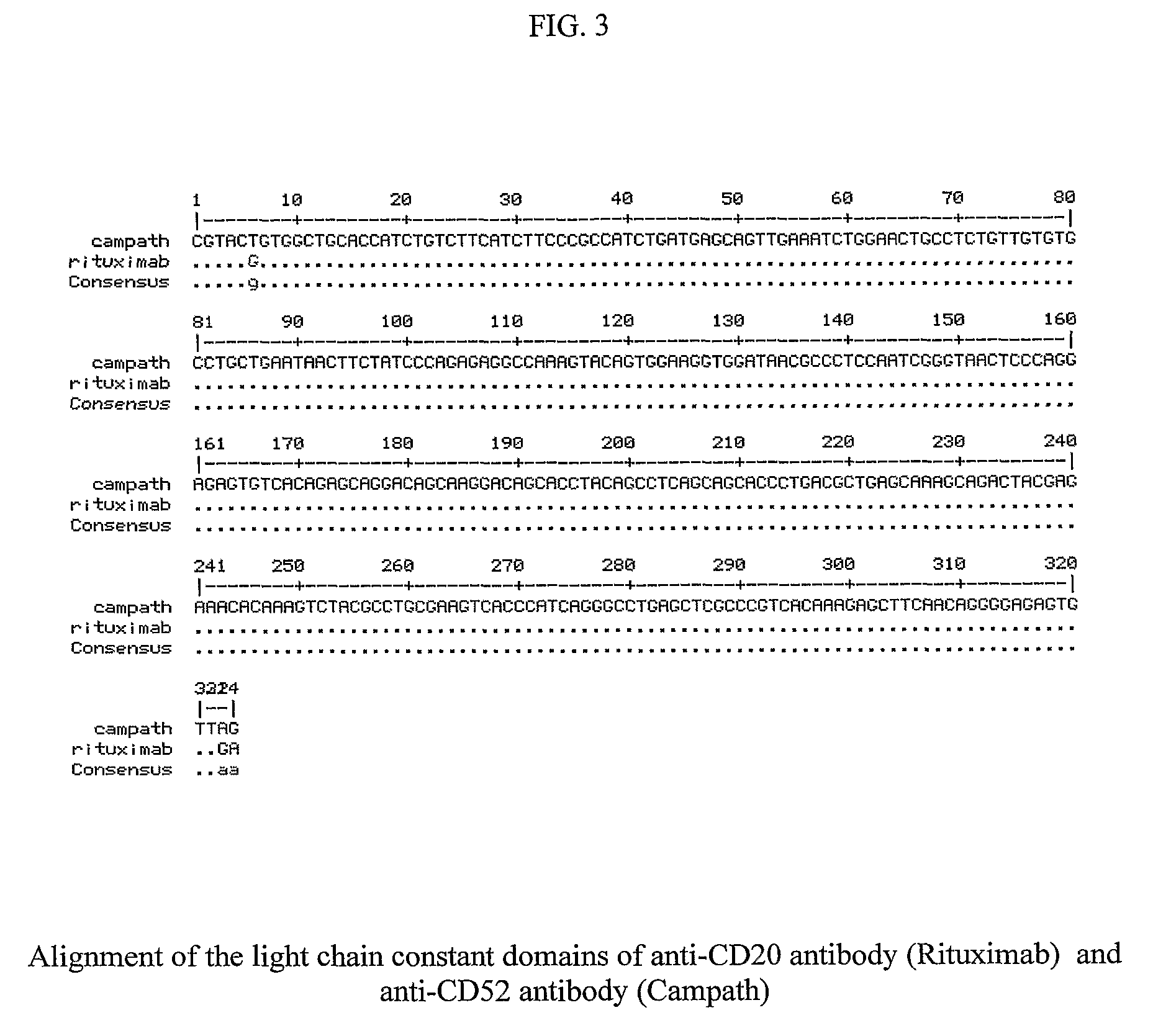
The invention provides heterochimeric antibodies and / or fragments thereof comprising (i) hypervariable region sequences wholly or substantially corresponding to sequences found in antibodies from a donor species; (ii) constant region sequences wholly or substantially corresponding to sequences found in antibodies from a target species which is different from the donor species; and (iii) heavy and / or light chain variable framework sequences which contain at least three non-CDR residues corresponding to sequences found in antibodies from a target species and at least three contiguous non-CDR residues corresponding to sequences found in antibodies from a donor species. The invention further provides antibody to canine or feline or equine antigens, e.g., CD20 or CD52, and methods of making and using antibodies as described.
Owner:ARATANA THERAPEUTICS
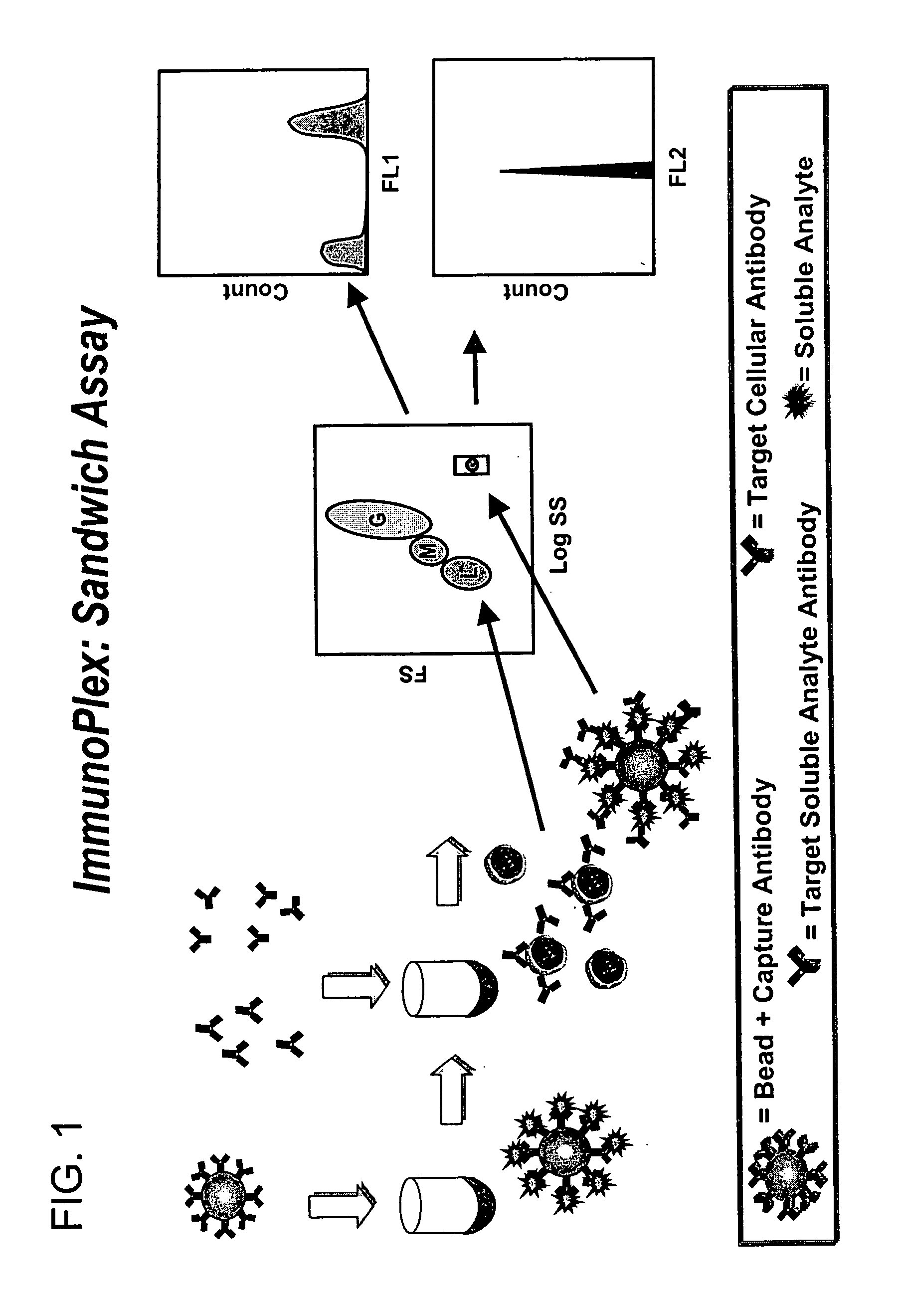
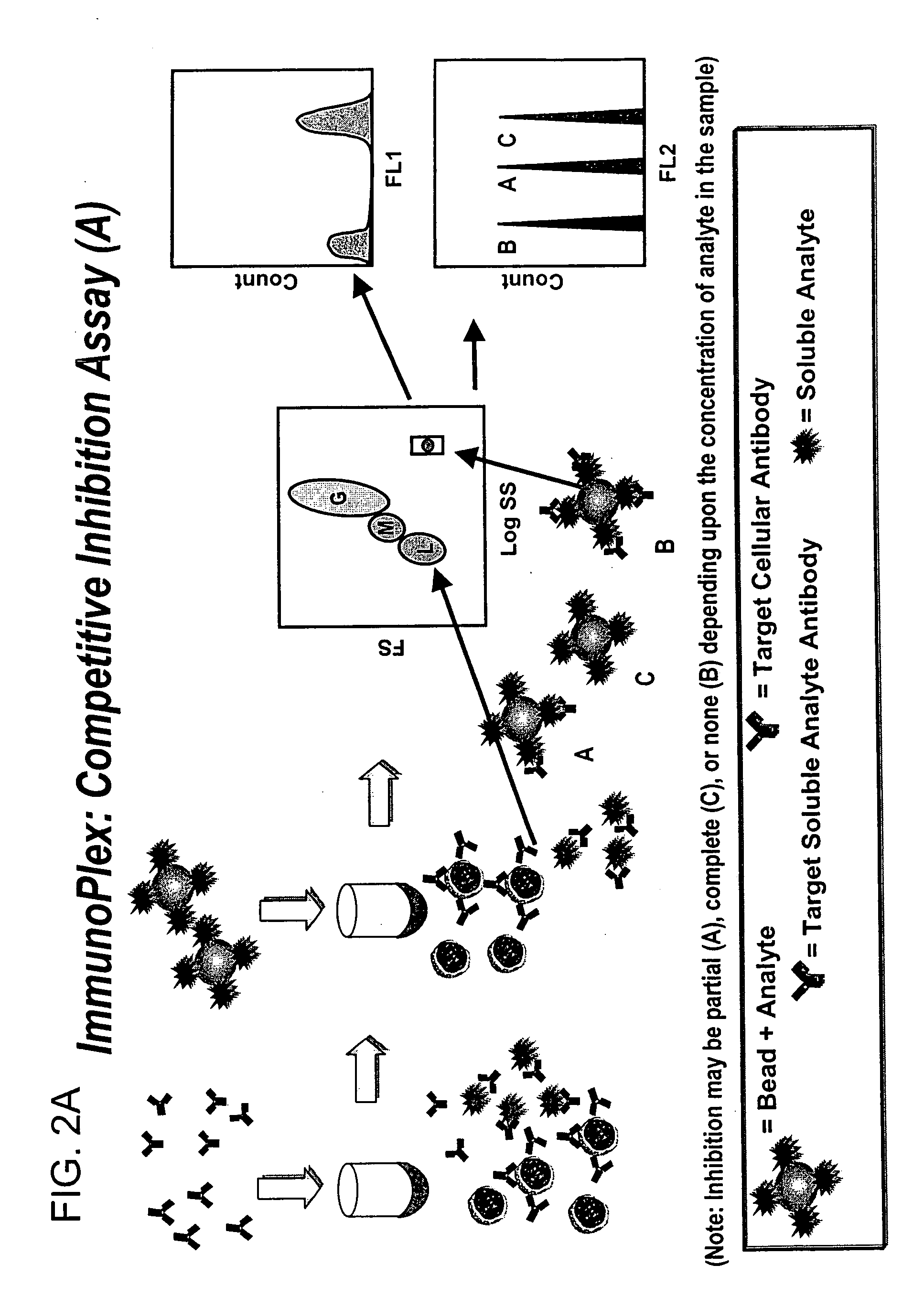
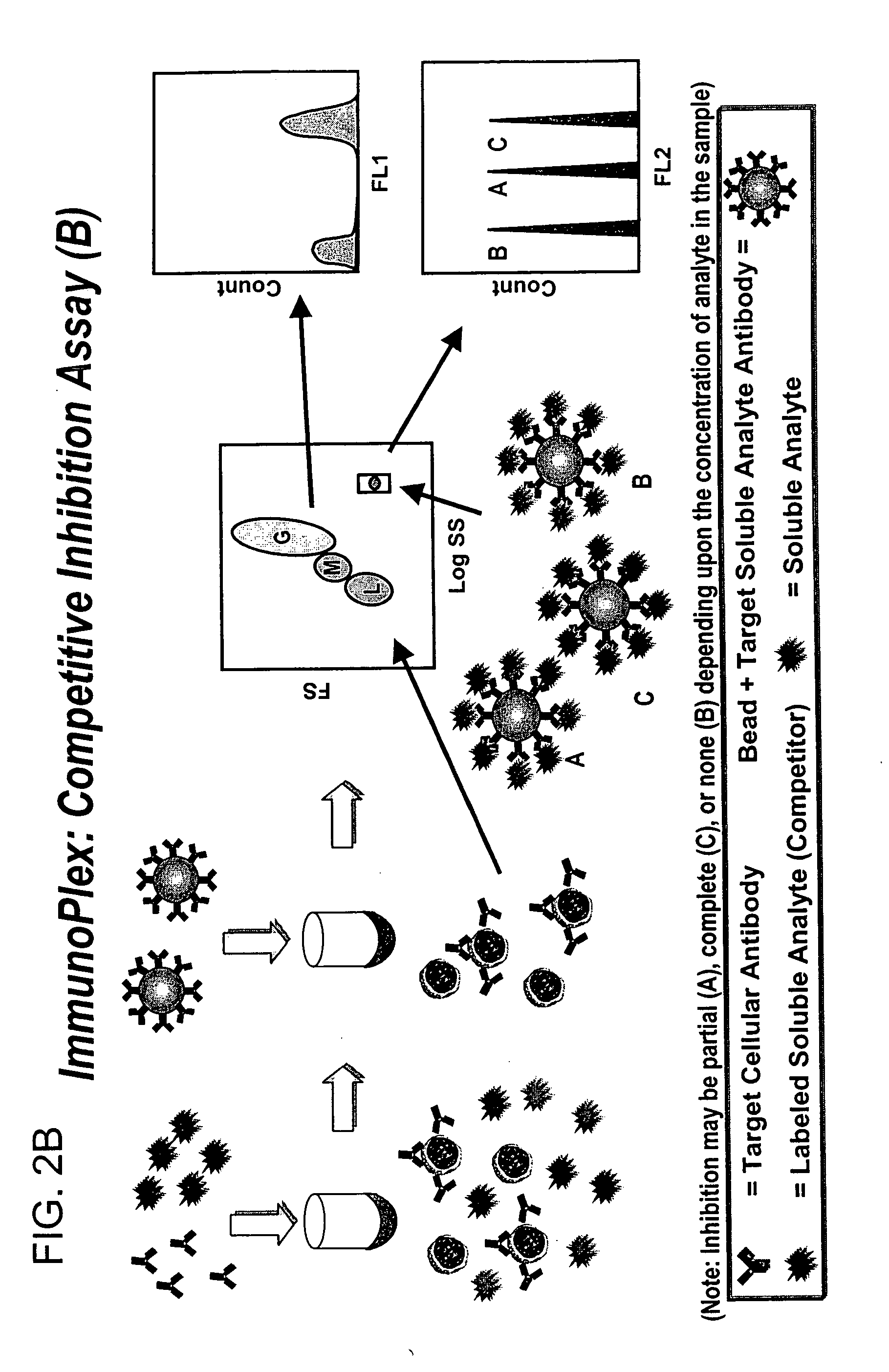
Methods for substantially simultaneous evaluation of a sample containing a cellular target and a soluble analyte
Methods are provided for monitoring treatment of a subject using heparin therapy or a ligand, such as an CD20 or CD52 antibody, that binds to a cellular marker and to which the subject may develop masking reactions or auto-antibodies that inhibit evaluation of treatment. The methods involve adding to a single container a sample containing cells that express the cellular target with (i) a soluble ligand that binds the cellular target, (ii) a soluble ligand that binds the soluble analyte or a competing soluble analyte; and (iii) a capture medium that binds directly to the soluble analyte, indirectly to the soluble analyte, or to the soluble ligand that binds to the soluble analyte. Complexes formed in the container by interaction of these components are substantially simultaneously analyzed and quantitated without physically separating the complexes. Kits for performing the assays are also provided.
Owner:BECKMAN COULTER INC
Modified anti-CD52 antibody
ActiveUS7264806B2Extended circulation timeReduce the numberSenses disorderNervous disorderImmunogenicityCD52
The present invention provides for modified forms of anti-CD52 antibodies with reduced numbers of potential T-cell epitopes that are expected to display decreased immunogenicity.
Owner:MERCK PATENT GMBH
Anti-Cd52 Antibody Treatment for Diabetes
InactiveUS20070286857A1Metabolism disorderImmunoglobulins against cell receptors/antigens/surface-determinantsType 1 diabetesAnti-CEA Antibody
The present invention provides for the prevention and / or treatment of Type 1 diabetes mellitus with CD52 specific antibodies, e.g. CAMPATH-1H.
Owner:GENZYME CORP
Monoclonal antibodies
The invention provides heterochimeric antibodies and / or fragments thereof comprising (i) hypervariable region sequences wholly or substantially corresponding to sequences found in antibodies from a donor species; (ii) constant region sequences wholly or substantially corresponding to sequences found in antibodies from a target species which is different from the donor species; and (iii) heavy and / or light chain variable framework sequences which contain at least three non-CDR residues corresponding to sequences found in antibodies from a target species and at least three contiguous non-CDR residues corresponding to sequences found in antibodies from a donor species. The invention further provides antibody to canine or feline or equine antigens, e.g., CD20 or CD52, and methods of making and using antibodies as described.
Owner:ARATANA THERAPEUTICS
Anti CD52 monoclonal antibody, coding sequence and use thereof
The present invention provides an anti-CD52 specific monoclonal antibody. Said monoclonal antibody has good stability and strong specificity, and is specially applicable for preventing and curing graft rejection reaction and curing chronic lymphocyte leukosis. Said invention also provides the preparation method of said monoclonal antibody and medicine composition containing said monoclonal antibody.
Owner:SHANGHAI ZHANGJIANG BIOTECH CO LTD
Modified anti-CD52 antibody
ActiveUS20050152898A1Extended circulation timeLow immunogenicitySenses disorderNervous disorderAnti-CEA AntibodyVaccine Immunogenicity
The present invention provides for modified forms of anti-CD52 antibodies with reduced numbers of potential T-cell epitopes that are expected to display decreased immunogenicity.
Owner:MERCK PATENT GMBH
Monoclonal antibodies directed to CD52
The invention provides antibody to canine or feline or equine antigens, e.g., canine CDS2, and methods of making and using antibodies as described.
Owner:ARATANA THERAPEUTICS
Methods and compositions for treatment
InactiveUS20120058082A1Eliminate side effectsReduces effective amountPeptide/protein ingredientsAntipyreticSide effectLymphocyte depletion
The invention provides methods for improving the efficacy and reducing side effects of anti-CD52 antibody treatment. The methods can be used to treat patients who are in need of immunoregulation such as lymphocyte depletion and patients who have cancer. Also included are compositions useful for these methods.
Owner:GENZYME CORP
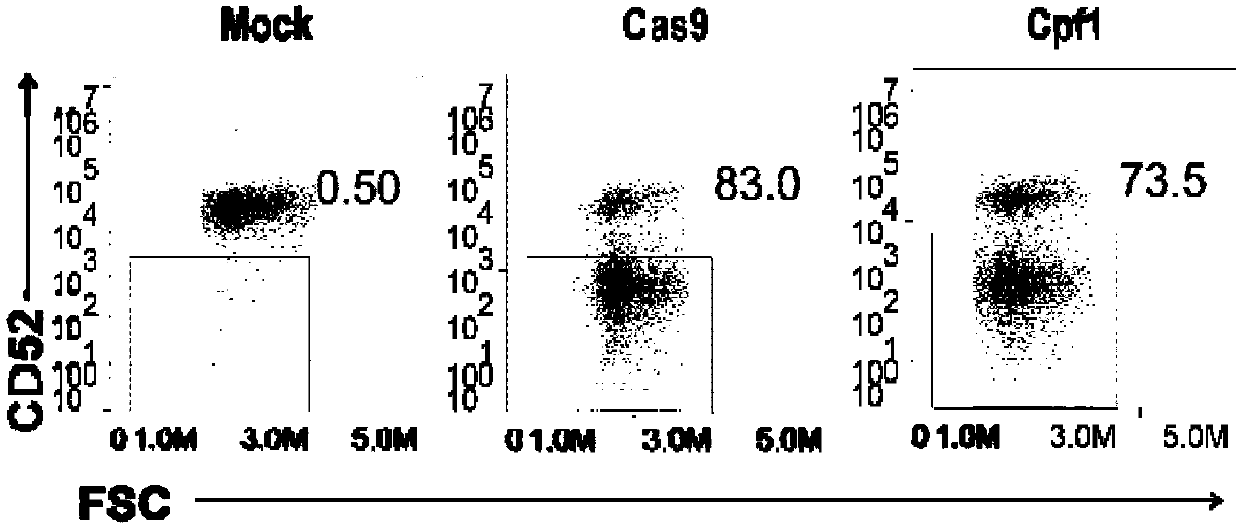
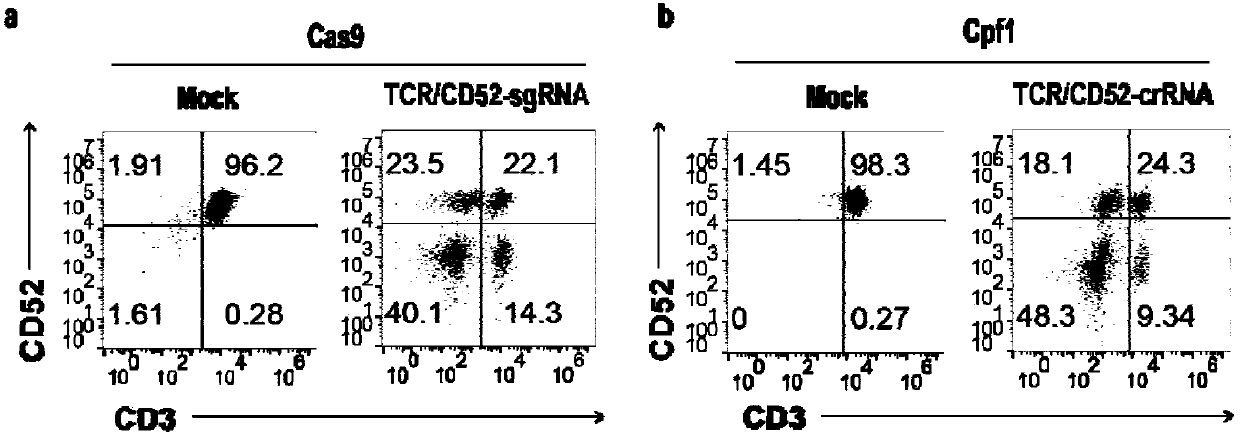
General type CART/TCRT cells with antibody drug resistance as well as construction method of general type CART/TCRT cells
ActiveCN107828730APreserve targetingAvoid exclusionImmunoglobulin superfamilyMammal material medical ingredientsAntigenAntigen receptors
The invention belongs to the fields of genetic engineering and synthetic biology, and in particular relates to general type CART / TCRT cells with antibody drug resistance as well as a construction method of the general type CART / TCRT cells. The general type CART / TCRT cells are allogeneic T cells having chimeric antigen receptors and T cell receptors, and alpha and beta chains and CD52 molecules ofthe T cell receptors are knocked out; a technology for knocking out the alpha and beta chains and the CD52 molecules of the T cell receptors is a CRISPR technology; the general type CART / TCRT cells not only retain the targeting of tumor specific antigen, but also eliminate the problems of GvHD and rejection, and weaken the sensitivity to an antibody drug Alemtuzumab; the general type CART / TCRT cells can be used as a general type simple, low-cost and high-activity CART / TCRT cell preparation.
Owner:NANJING BIOHENG BIOTECH CO LTD
Humanised anti-CD52 antibodies
Owner:ABZENA (CAMBRIDGE) LTD
Recombinant method for the production of a monoclonal antibody to CD52 for the treatment of chronic lymphocytic leukemia
InactiveUS20090220520A1Stable expressionImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDNA constructChronic lymphocytic leukemia
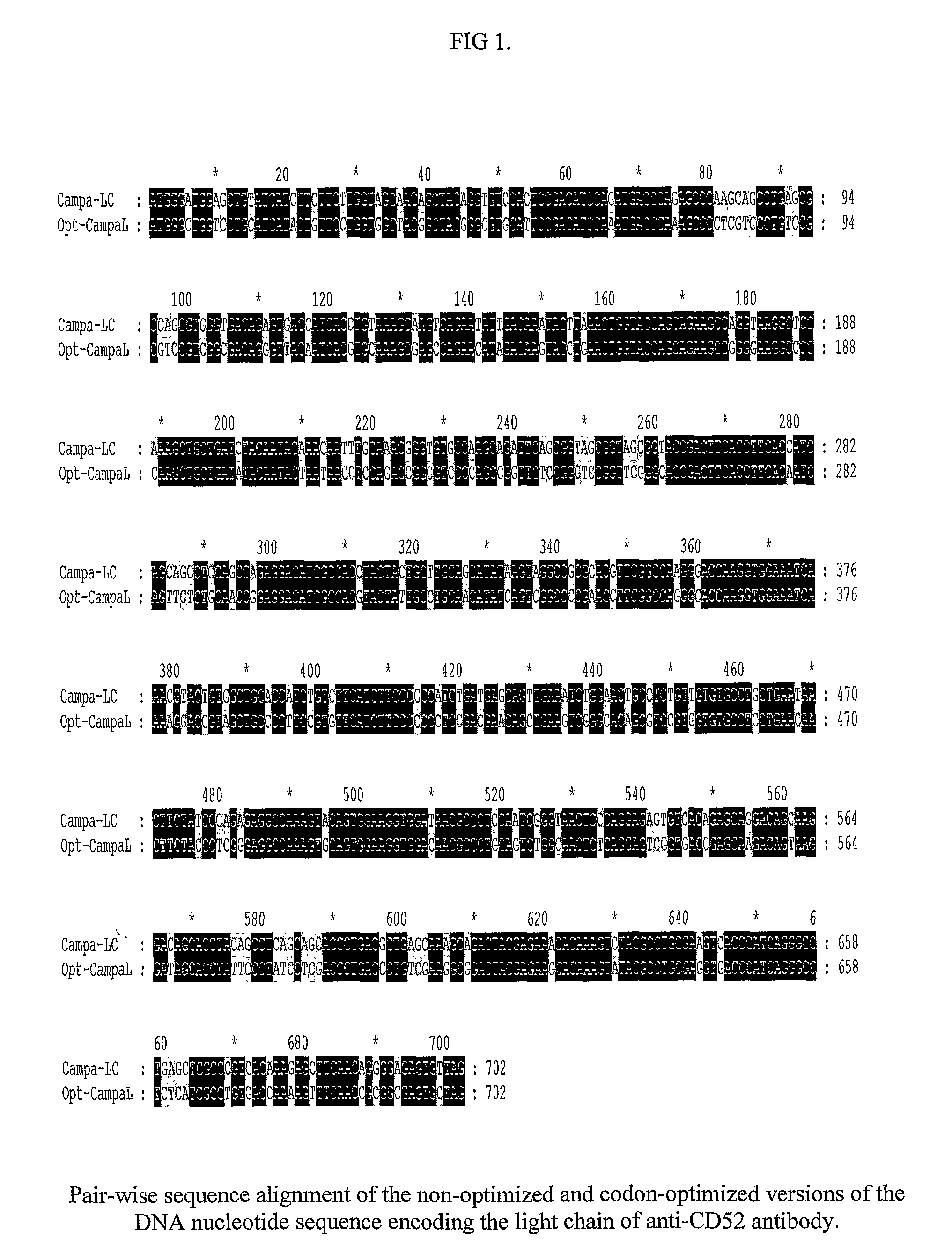
The present invention relates to the recombinant method used for the production of soluble form monoclonal antibody that binds to CD52. The procedure describes the de novo synthesis of the nucleic acid sequence encoding anti-CD 52, transformation of the constructed nucleic acid sequences into competent bacteria and the sub-cloning of the same into mammalian expression vectors for expression of the desired protein. DNA constructs comprising the control elements associated with the gene of interest has been disclosed. The nucleic acid sequence of interest has been codon optimized to permit expression in the suitable mammalian host cells.
Owner:AVESTHAGEN
Monoclonal antibodies directed to cd52
The invention provides antibody to canine or feline or equine antigens, e.g., canine CD52, and methods of making and using antibodies as described.
Owner:ARATANA THERAPEUTICS
NK cell in-vitro amplification system and culture method
PendingCN113293132AThe cultivation method is simpleLess active componentsCulture processCell culture supports/coatingNatural Killer Cell Inhibitory ReceptorsTrophoblast
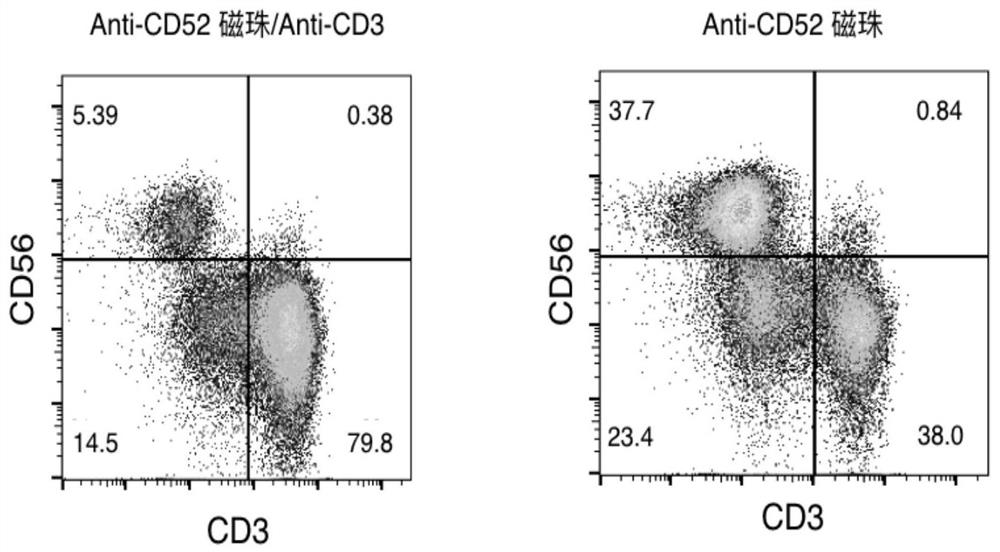
The invention provides an NK cell in-vitro amplification system. The NK cell in-vitro amplification system comprises an activation culture medium for cell activation and a proliferation culture medium for cell proliferation, the activating culture medium is composed of an activating agent and a basic culture medium, and the activating agent is a combination of solid-phase anti-CD52 and IL-2; the proliferation culture medium is composed of a proliferation agent and a basic culture medium, and the proliferation agent is IL-2. Compared with the prior art, the NK cell in-vitro amplification system disclosed by the invention has the following technical effects: 1) the culture medium contains the immobilized anti-CD52, and the NK cells can be efficiently stimulated to enter an activated and proliferated state by combining the use of IL-2, so that a large number of activated NK cells can be easily obtained; moreover, no trophoblasts are used in the culture process, and the prepared NK cells can be used for clinical research and treatment; and 2) the NK cell culture method is simplified, few NK cell activation components are used, few material resources and manpower are used, the production cost of NK cell preparation is obviously reduced, and the method is particularly suitable for large-scale production.
Owner:江苏豪科生物工程有限公司
Therapeutic agent for cancer which comprises combination of il-18 and molecule-targeting antibody
ActiveUS20170224791A1Improve anti-tumor effectHigh therapeutic effectPeptide/protein ingredientsDigestive systemCD33PD-L1
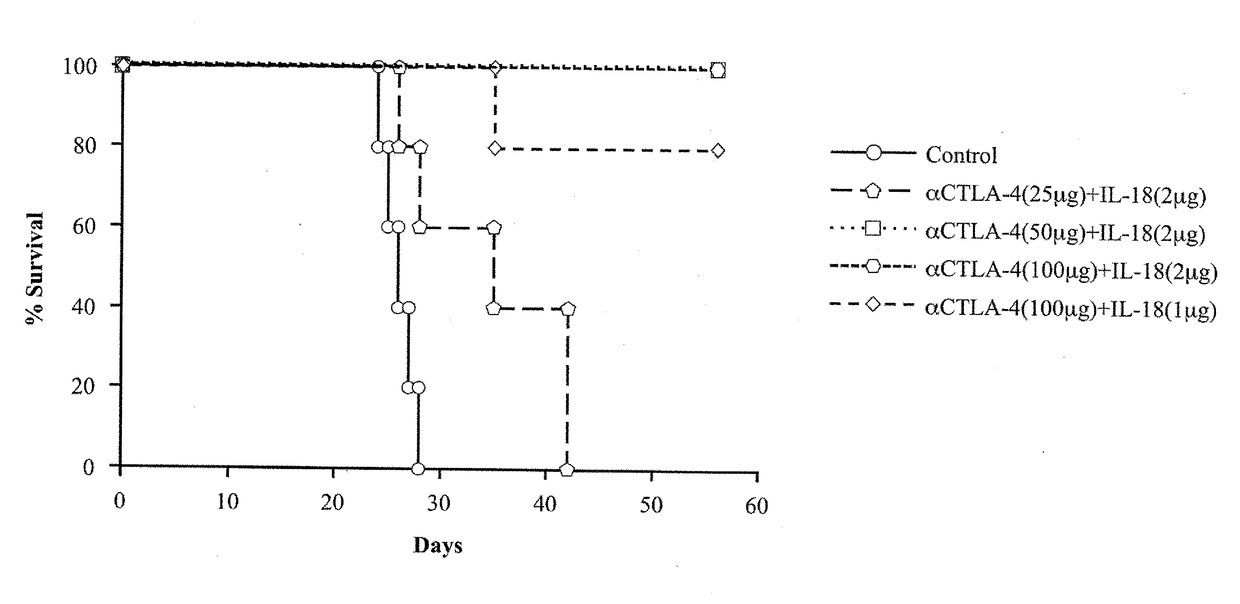
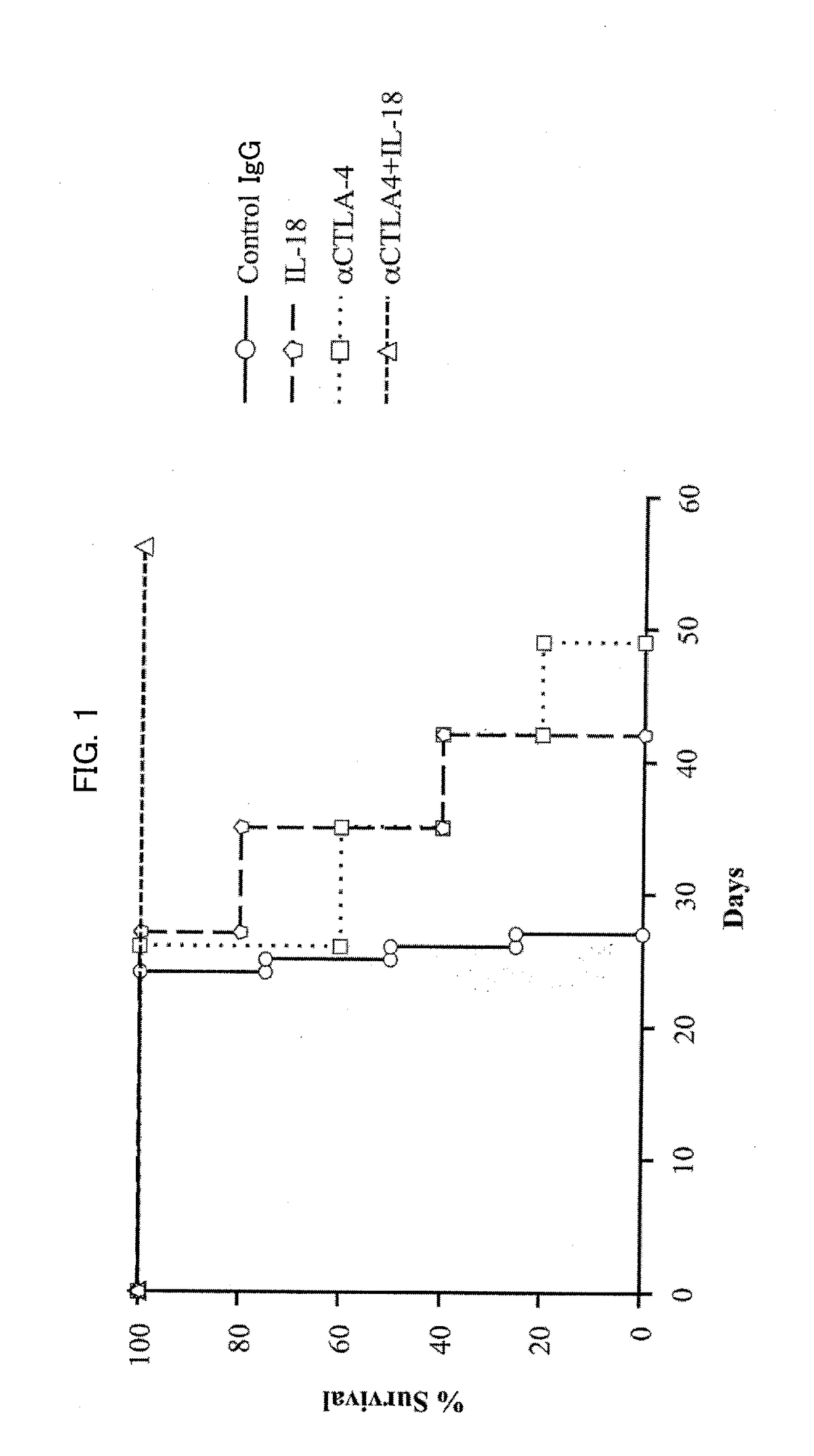
A cancer therapeutic agent according to an embodiment of the present invention contains, as active ingredients, IL-18 and one or more antibodies selected from the group consisting of an anti-PD-L1 antibody, an anti-PD-1 antibody, an anti-PD-L2 antibody, an anti-CTLA-4 antibody, an anti-CD25 antibody, an anti-CD33 antibody, and an anti-CD52 antibody.
Owner:MEDICAL RES & DEV CORP +1
Method for detecting bioactivity of humanized anti-CD52 antibody
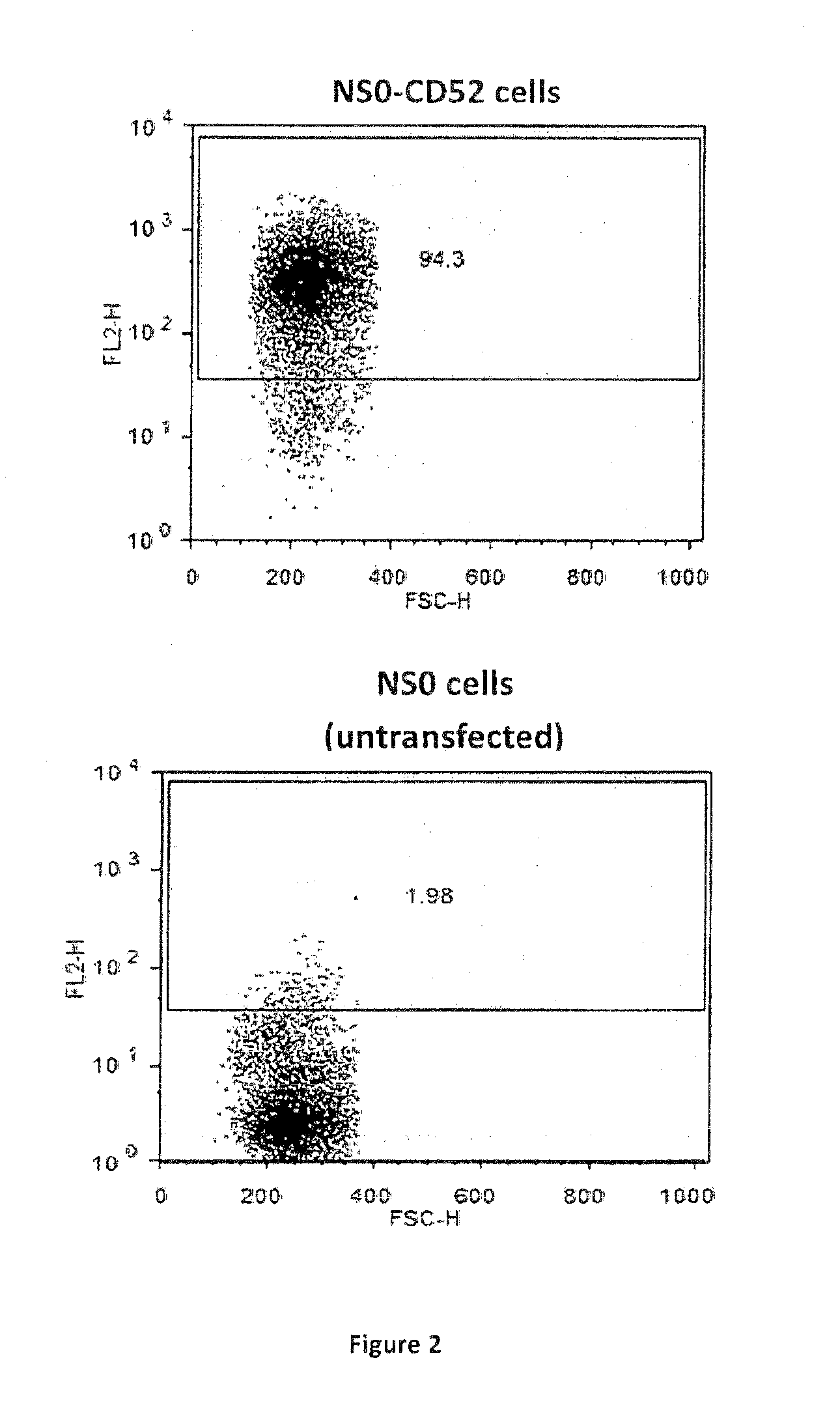
The invention discloses a method to detect the biological activity of humanized antibody CD52, which comprises dilution of specimens and a reference product having given activity, making use of CD52 positive cells for competitive binding assay and detection with a flow cytometry, and calculation of the results. The CD52 positive cells used in the competitive binding assay steps are CHO-C cells. The CHO-C cells used by the invention have the advantages of high sensitivity and high stability.
Owner:上海国健生物技术研究院
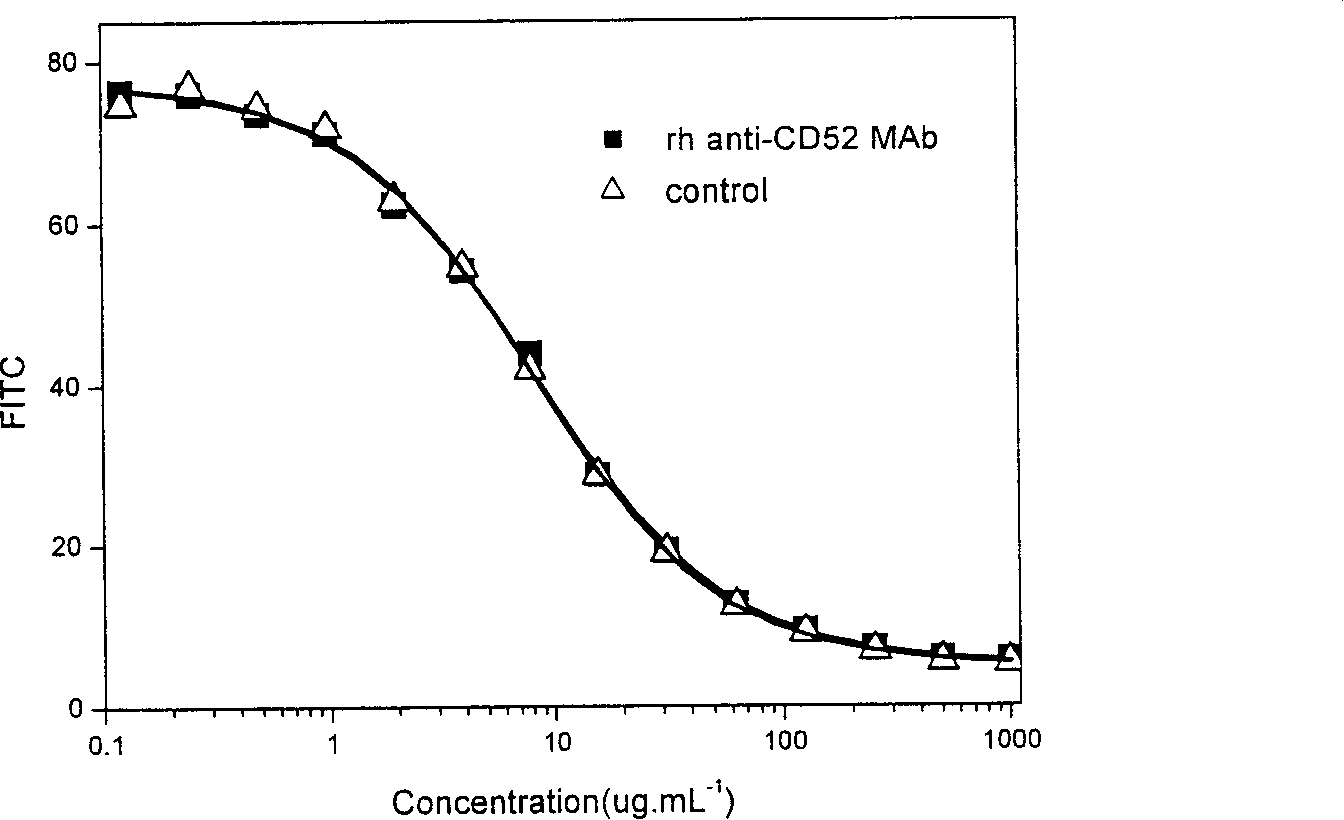
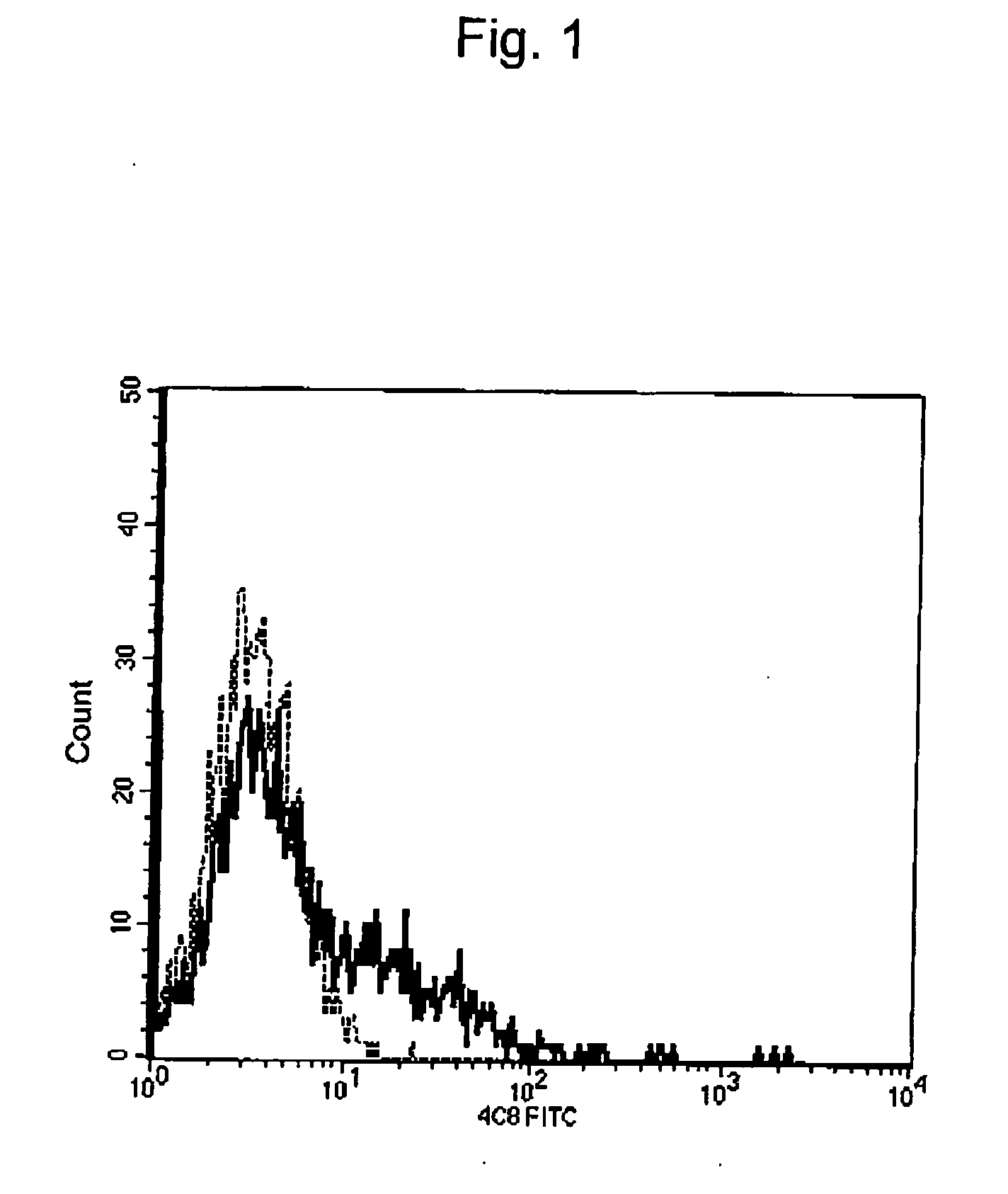
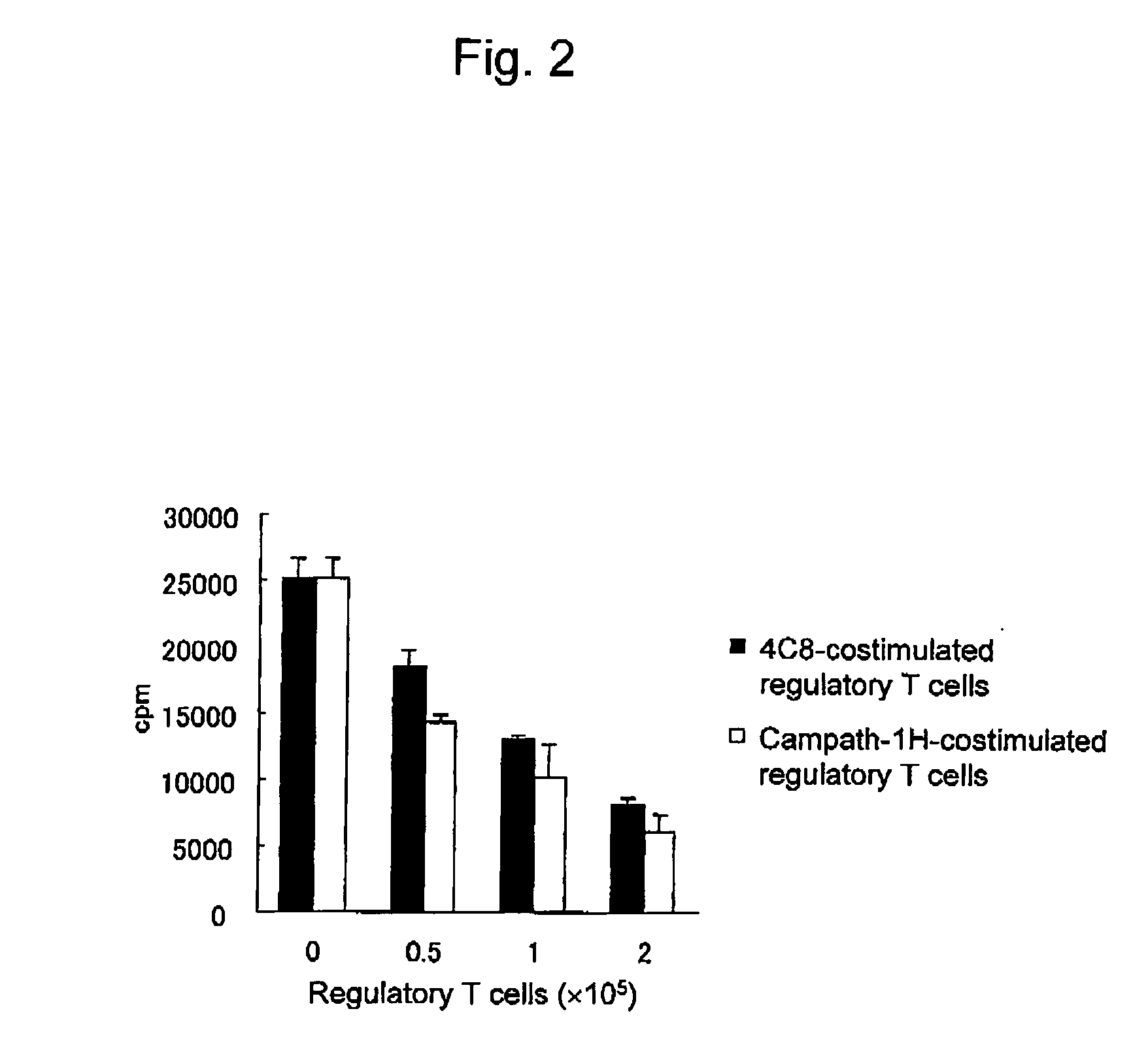
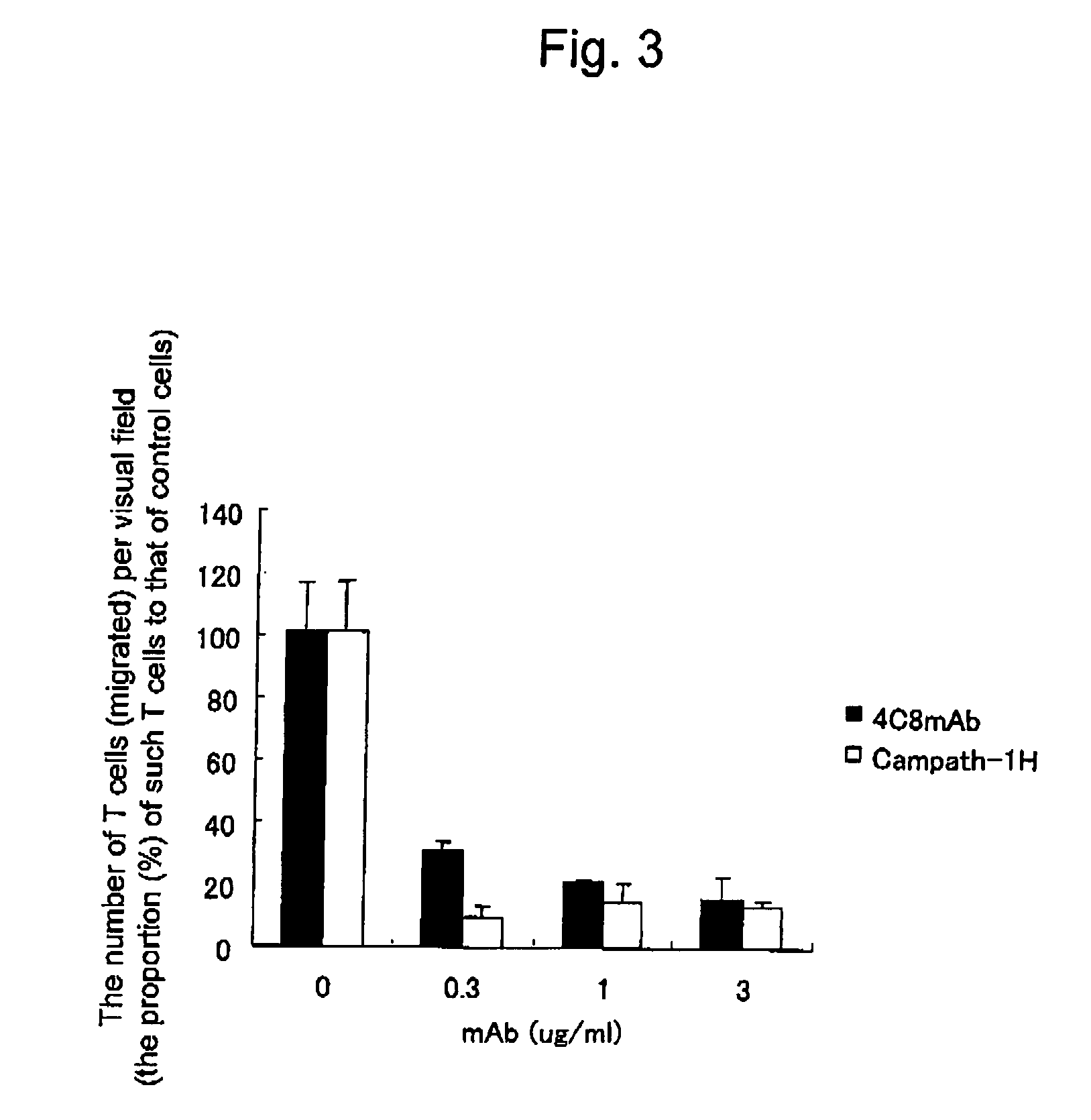
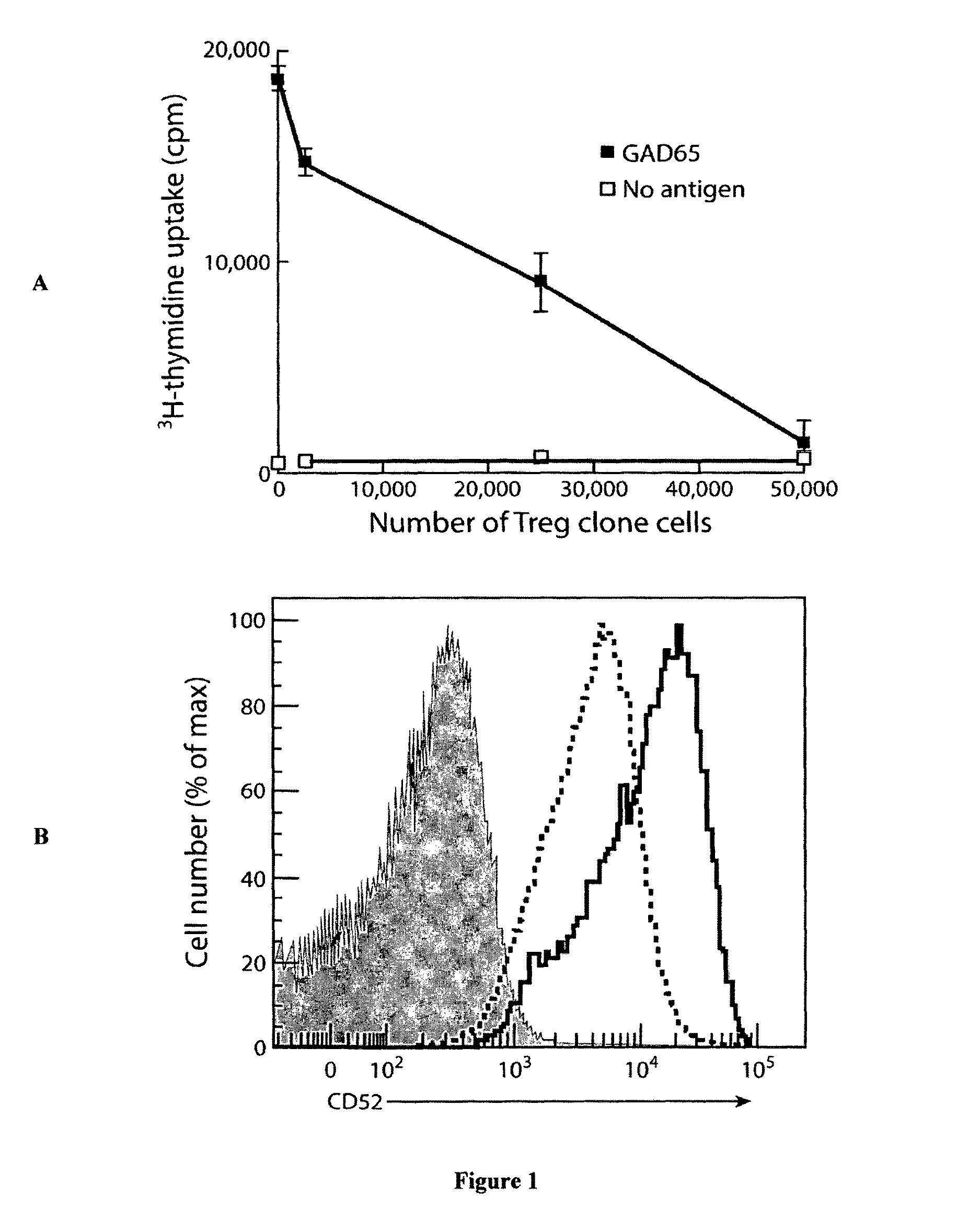
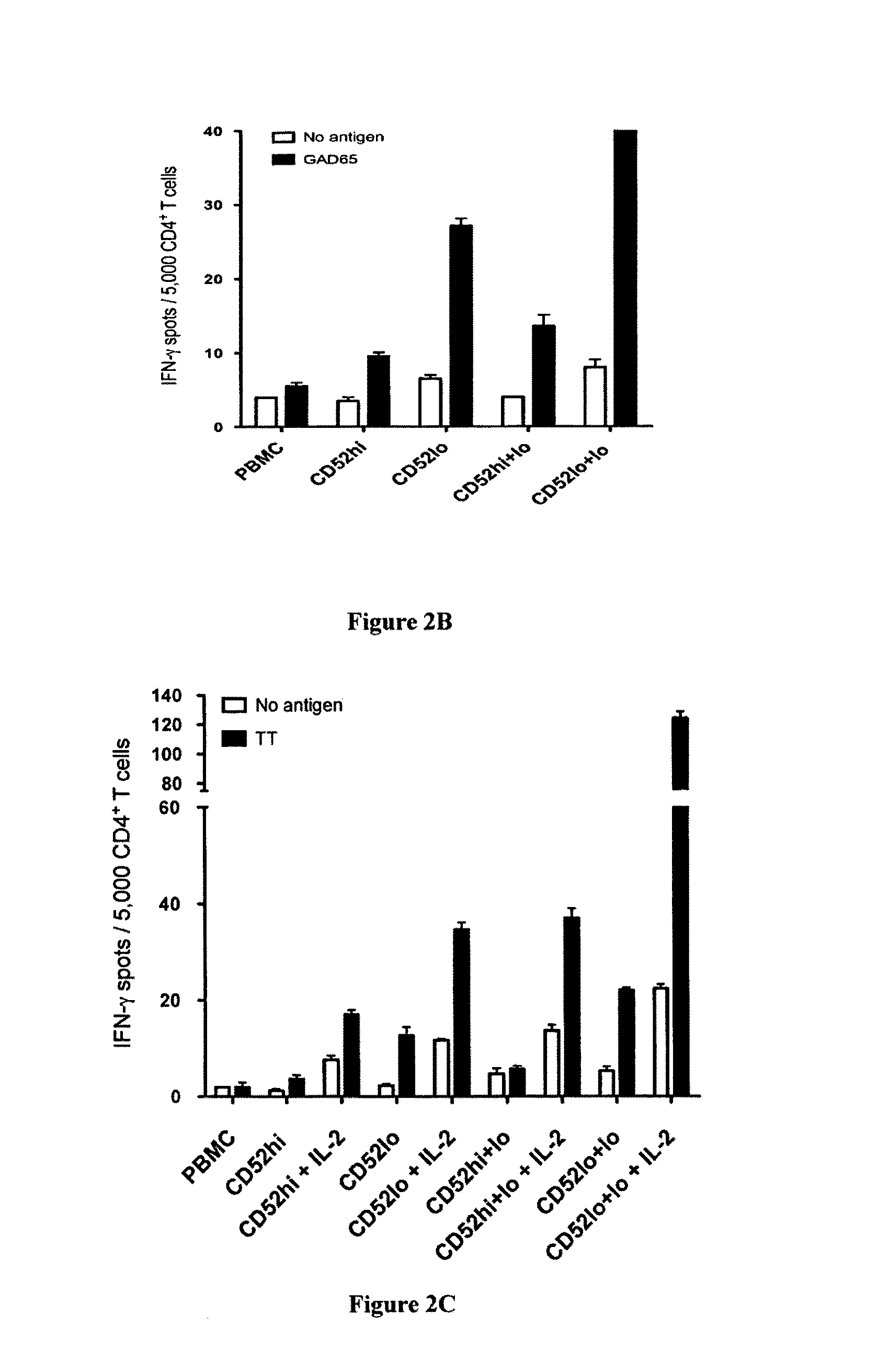
Method of inducing differentiation and proliferating regulatory t cell by anti-cd52 antibody and medicinal composition therefor
InactiveUS20060228351A1Suppressing transendothelial cell migrationFacilitated DiffusionImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsThymocyte migrationRegulatory T cell
An object of the present invention is to provide a method for inducing differentiation and / or promoting proliferation of regulatory T cells, a method for suppressing the transendothelial cell migration of immunocytes, a method for suppressing immunity using these methods, and a pharmaceutical composition to be used for these methods. Provided are a method for inducing differentiation and / or promoting proliferation of regulatory T cells by causing CD52 agonists, including anti-CD52 antibodies, to act on CD52-expressing T cells, a method for suppressing the transendothelial cell migration of immunocytes, and a method for suppressing immunity using these methods.
Owner:KIRIN BREWERY CO LTD
Method Of Identifying Risk For Thyroid Disorder
A method for identifying a patient that is at risk for developing a thyroid disorder that occurs subsequent to treatment with a regimen that depletes lymphocytes, comprising determining whether antibodies directed against thyroid peroxidase or thyroid microsomes are present in the patient, wherein if the antibodies are present in the patient then the patient is at increased risk for developing a thyroid disorder. A particular embodiment is a method for identifying a patient with multiple sclerosis that is at risk for developing a thyroid disorder that occurs subsequent to treatment with a regimen that depletes CD52-positive cells, comprising determining whether antibodies directed against thyroid peroxidase or thyroid microsomes are present in the patient, wherein if the antibodies are present in the patient then the patient is at risk for developing the thyroid disorder.
Owner:GENZYME CORP
Methods and compositions for diagnosis and treatment of autoimmune disease secondary to multiple sclerosis
ActiveUS20160356788A1Enhanced Risk ManagementEliminate side effectsNervous disorderMicrobiological testing/measurementLymphocyte depletionAutoimmune disease
The invention provides methods of diagnosing and treating multiple sclerosis (MS) patients, including methods of identifying and treating multiple sclerosis patients who are at increased risk of developing a secondary autoimmune disease following lymphocyte depletion, caused, e.g., by treatment with an anti-CD52 antibody. Also embraced are methods of selecting treatment regimens for MS patients, and reagents useful in the above methods.
Owner:CAMBRIDGE ENTERPRISE LTD
Selective elimination of cd52and uses thereof
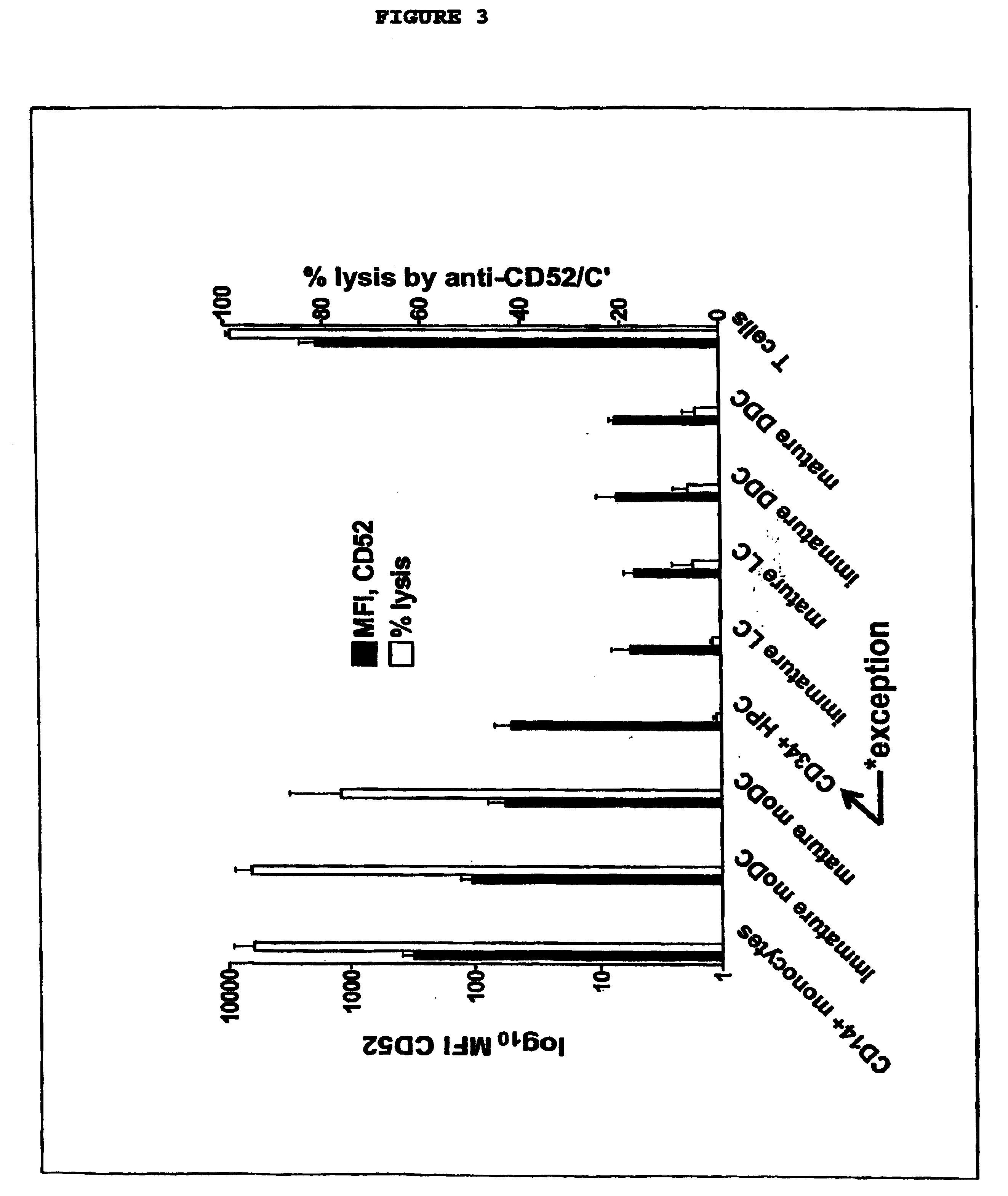
InactiveUS20050026854A1Genetic material ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsLangerhan cellInterstitial Dendritic Cells
This invention provides an agent capable of destruction of CD52+ cells, including CD52+ dendritic cells, without affecting CD52 negative dendritic cells. CD52 negative dendritic cells include hut are not limited to Langerhans cells (LCs) or dermal-interstitial dendritic cells (DDC-IDCs). This invention also provides an agent capable or selective elimination of CD52+ dendritic cells. This invention further provides an agent capable of protection of CD52 negative dendritic cells as well as a composition comprising the above described agent, a pharmaceutical composition comprising an effective amount of the above agent, and a pharmaceutically acceptable carrier. Finally, this invention provides various uses of the above agent and the above compositions.
Owner:SLOAN KETTERING INST FOR CANCER RES
Soluble mediator
ActiveUS9585969B2Suppressing effector T-cell functionLower immune responseAntibacterial agentsPeptide/protein ingredientsAutoimmune diseaseT cell
The present disclosure relates to a soluble CD52 glycoprotein and its use in treating diseases regulated by effector T-cells, for example autoimmune diseases such as type 1 diabetes. The present disclosure also relates to fusion proteins comprising the soluble glycoprotein, to cells expressing high levels of CD52, and to diagnostic methods based on the detection of CD52 expression levels in a subject.
Owner:WALTER & ELIZA HALL INST OF MEDICAL RES
Method of Identifying Risk for Thyroid Disorder
A method for identifying a patient that is at risk for developing a thyroid disorder that occurs subsequent to treatment with a regimen that depletes lymphocytes, comprising determining whether antibodies directed against thyroid peroxidase or thyroid microsomes are present in the patient, wherein if the antibodies are present in the patient then the patient is at increased risk for developing a thyroid disorder. A particular embodiment is a method for identifying a patient with multiple sclerosis that is at risk for developing a thyroid disorder that occurs subsequent to treatment with a regimen that depletes CD52-positive cells, comprising determining whether antibodies directed against thyroid peroxidase or thyroid microsomes are present in the patient, wherein if the antibodies are present in the patient then the patient is at risk for developing the thyroid disorder.
Owner:GENZYME CORP
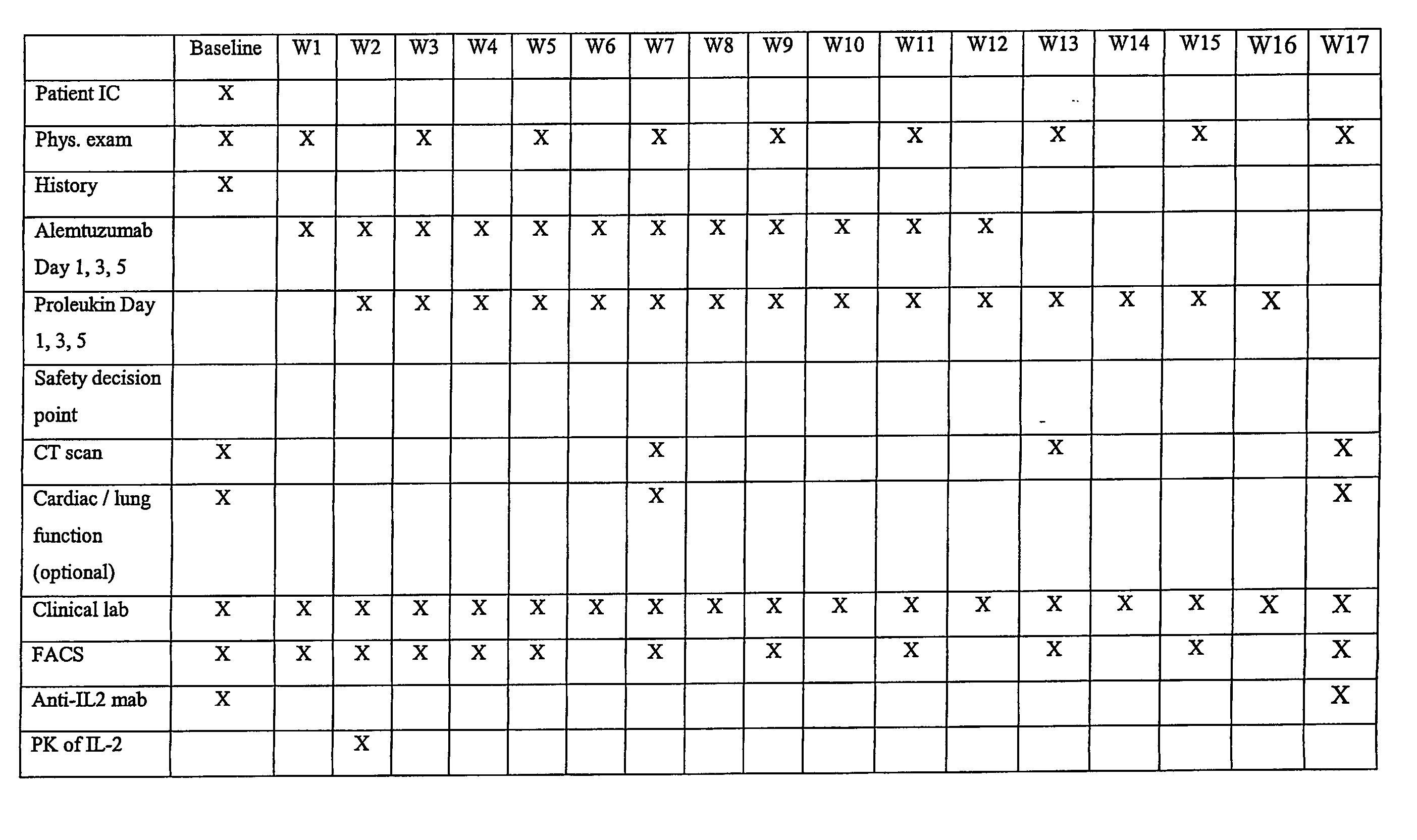
Methods of Therapy for Chronic Lymphocytic Leukemia
InactiveUS20070274948A1Eliminate side effectsImprove securityPeptide/protein ingredientsAntibody ingredientsInterleukin IITreatment response
Methods for treating a human with chronic lymphocytic leukemia using a combination of an interleukin-2 and an anti-CD52 antibody are provided. These therapeutic agents are administered as two separate pharmaceutical compositions, one containing an IL-2, the other containing an anti-CD2 antibody, according to a dosing regiment. Administering of these two therapeutic agents together results in a positive therapeutic response that is improved with respect to that observed with anti-CD52 antibody alone.
Owner:KAROLINSKA UNIV HOSPITAL +1
High-efficiency proliferation reagent for peripheral blood NK (Natural killer) cells in vitro and operation instruction
InactiveCN109486758AHigh purityHigh activityBlood/immune system cellsCell culture active agentsNatural Killer Cell Inhibitory ReceptorsCD16
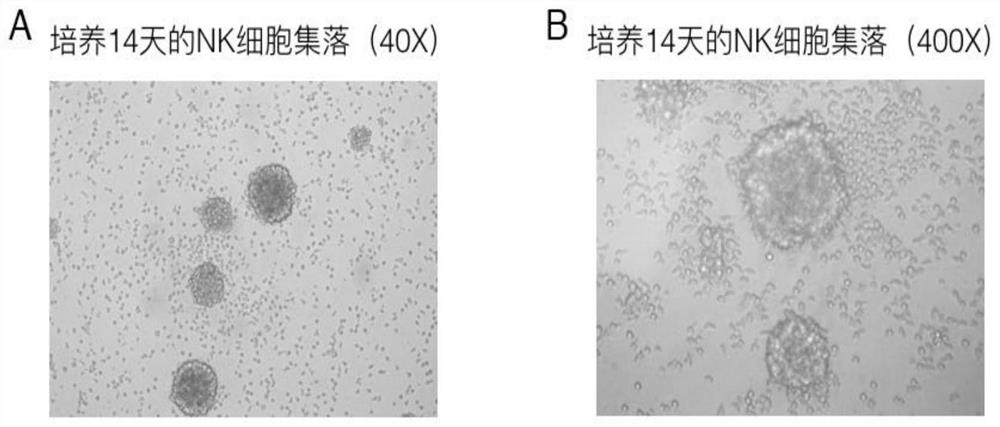
The invention relates to the technical field of cell culture, and discloses a high-efficiency proliferation reagent for peripheral blood NK (Natural killer) cells in vitro and an operation instruction. The reagent comprises the following components: an NK cell proliferation reagent: a gamma-ray inactivated recombinant K562 feeder cell, a CD52 antibody, an IL-2, a CD16 antibody, a CD3 antibody anda CD56 antibody; the gamma-ray inactivated recombinant K562 feeder cell is a K562 cell line for membrane expression of 41BBL and IL-15 which is lethally radiated by a 100G gamma-ray. According to thehigh-efficiency proliferation reagent for the peripheral blood NK cells in vitro and the operation instruction, the prepared NK cells have high purity and killing activity. Through detection, when theNK cells are cultured for 14 days, the purity of the NK cells can reach more than 90%; when the target ratio is 10:1, the killing activity of the NK cells on K562 cells can reach 80% or more; the proliferation speed of the prepared NK cells is high; when the NK cells are cultured for 14-17 days, the cell proliferation rate is more than 500; the NK cells prepared by the invention have excellent effects for targeted killing of tumor cells.
Owner:青岛麦迪赛斯生物科技有限公司
Anti CD52 monoclonal antibody, coding sequence and use thereof
The present invention provides an anti-CD52 specific monoclonal antibody. Said monoclonal antibody has good stability and strong specificity, and is specially applicable for preventing and curing graft rejection reaction and curing chronic lymphocyte leukosis. Said invention also provides the preparation method of said monoclonal antibody and medicine composition containing said monoclonal antibody.
Owner:SHANGHAI ZHANGJIANG BIOTECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com