NK cell in-vitro amplification system and culture method
An in vitro expansion and NK cell technology, which is applied in the direction of cell culture active agent, cell culture support/coating, animal cells, etc., can solve the problems of difficult quality control of product stability and high economic cost of NK cell culture medium, and achieve activation The effect of fewer components, less manpower, and simplified culture methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
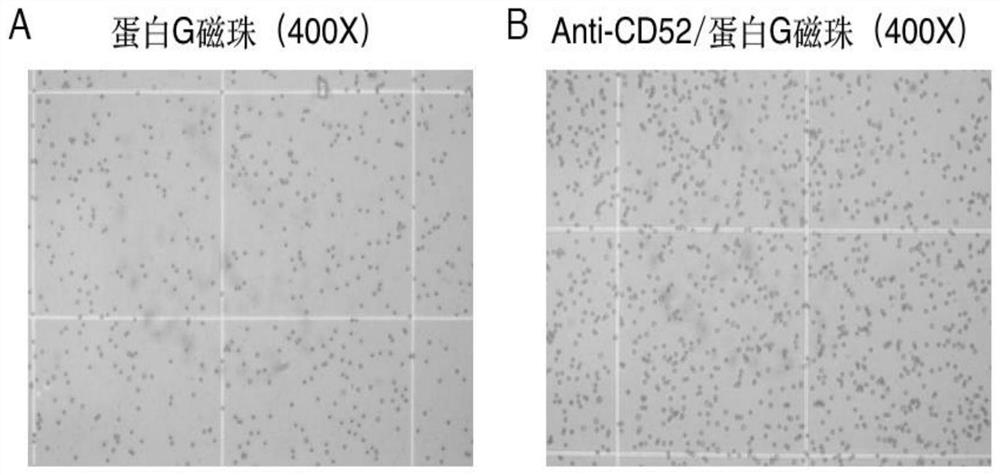
[0069] Example 1 Preparation method of anti-CD52 immunomagnetic beads
[0070] 1. Place the tube containing the magnetic microspheres on the vortex shaker, turn on the instrument, shake, and mix the magnetic microspheres thoroughly.
[0071] 2. Pipette 100 μl of magnetic microspheres into a sterile EP tube.
[0072] 3. Add 100 μl of pH7.4 PBS buffer to the EP tube, and vortex to resuspend. The EP tube was placed in the magnet, and stood for 30 Sec, so that the magnetic beads were adsorbed on the magnet side of the EP tube. Aspirate the supernatant. PBS was added and the washing was repeated 3 times. Go to supernatant.
[0073] 4. Add 100 μl of pH7.4 PBS buffer and 30 μl of purified Anti-CD52 (IgG, 10-30ug) to the EP tube.
[0074] 5. Mix well and incubate at 4°C for 30 minutes with stirring.
[0075] 6. Place the EP tube in the magnet, and after the magnetic beads are completely adsorbed to one side of the magnet, remove the supernatant. Additional PBS was added and the...
Embodiment 2
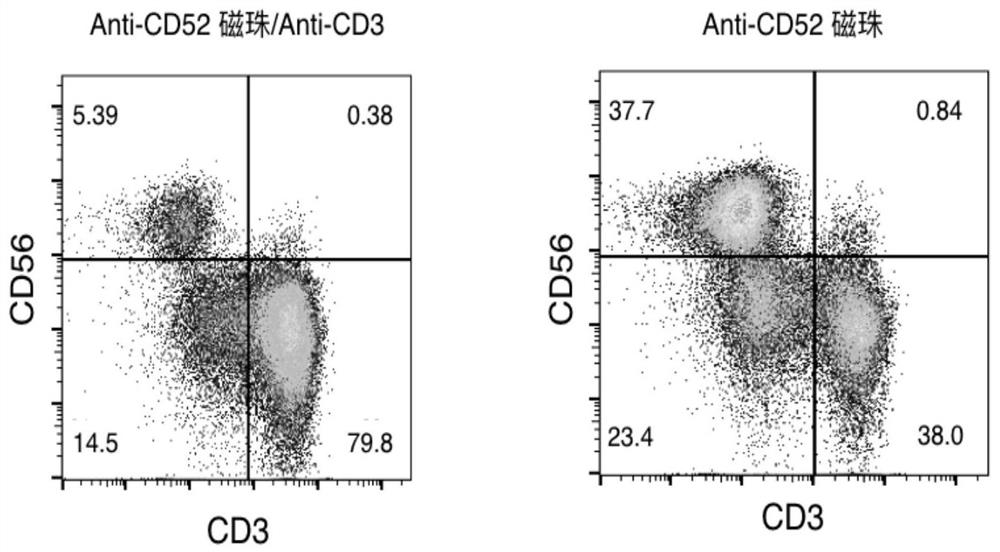
[0084] Example 2 Anti-CD52 Immunomagnetic Bead Amplification, Culture and Flow Analysis of NK Cells
[0085] 1. Preparation of PBMC cells. Peripheral blood was collected from healthy volunteers, anticoagulated with heparin, and human peripheral blood mononuclear cells (PBMC) were separated by Ficoll density centrifugation.
[0086] 2. NK cell culture. PBMCs were adjusted to 2 × 10 in serum-free medium (i.e. Lonza X-VIVO 15 serum-free medium). 6 cells / ml, the anti-CD52 immunomagnetic beads (4×10) prepared in Example 1 were added to Lonza X-VIVO 15 serum-free medium. 5 cells / ml), anti-CD3 antibody (1ng / ml) and IL-2 (200U / ml) to form an activation medium, and cultured at 37°C for 48 hours in 5% CO2.
[0087] 3. According to the cell growth state, change the medium every 2-3 days with the proliferation medium consisting of 200U / ml IL-2 and basal medium (i.e. Lonza X-VIVO 15 serum-free medium) until the 14th day. sky.
[0088] 4, flow cytometry detection. On day 14 of cell cult...
Embodiment 3
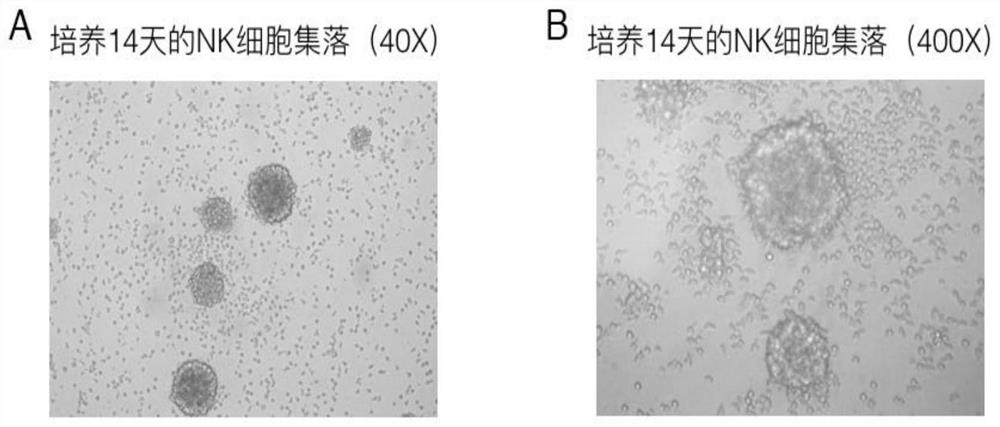
[0093] Example 3 Comparison of expansion efficiency of NK cells cultured with different Anti-CD52 combinations
[0094] 1. The peripheral blood of 2 healthy volunteers (Volunteer 1 and Volunteer 2) were collected respectively, anticoagulated with heparin, and human peripheral blood mononuclear cells (PBMC) were separated by Ficoll density centrifugation.
[0095] 2. Use serum-free medium (i.e. Lonza X-VIVO 15 serum-free medium) for PBMC to adjust the cell concentration to 2×10 6 cells / ml.
[0096] 3. Culture of NK cells.
[0097] Culture method 1. According to Example 2, anti-CD52 and anti-CD3 were pre-coated and activated in combination with IL-2 (200U / ml) to culture PBMC to induce NK cell differentiation and expansion.
[0098] Cultivation method 2, adopts the activation medium and the proliferation medium of the present invention to cultivate:
[0099] The activation medium consists of an activator and a basal medium (ie, Lonza X-VIVO 15 serum-free medium), and the activ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com