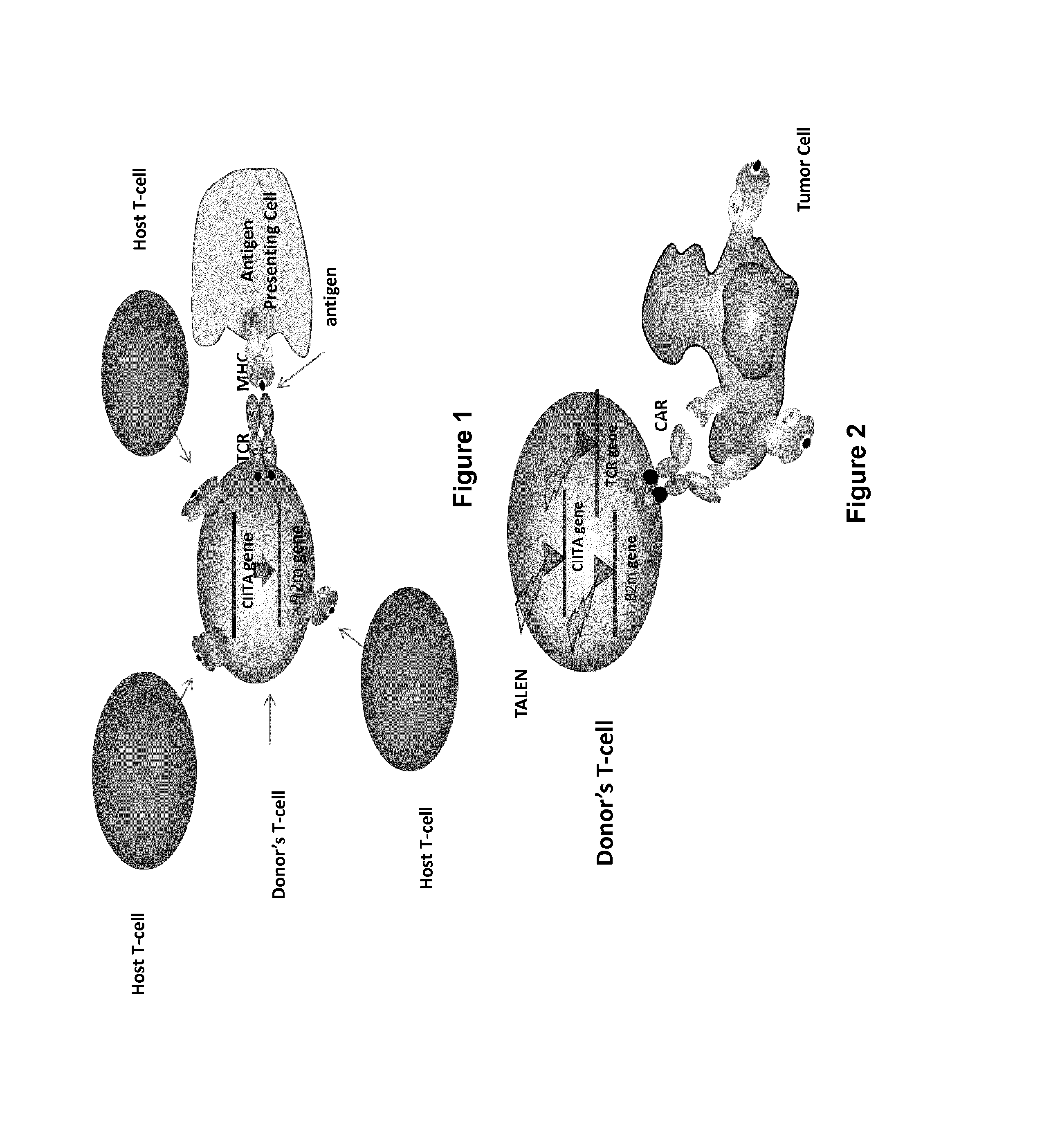
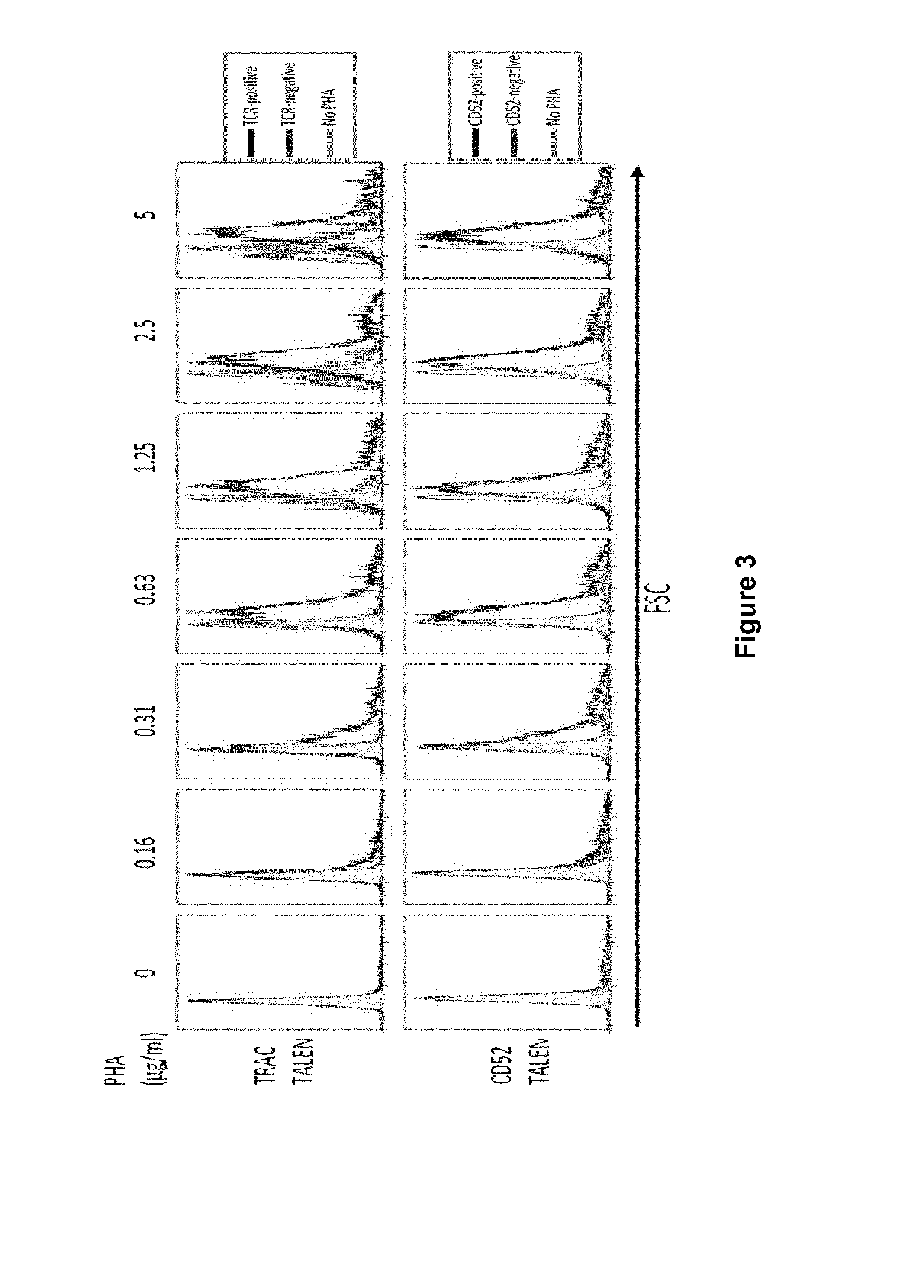
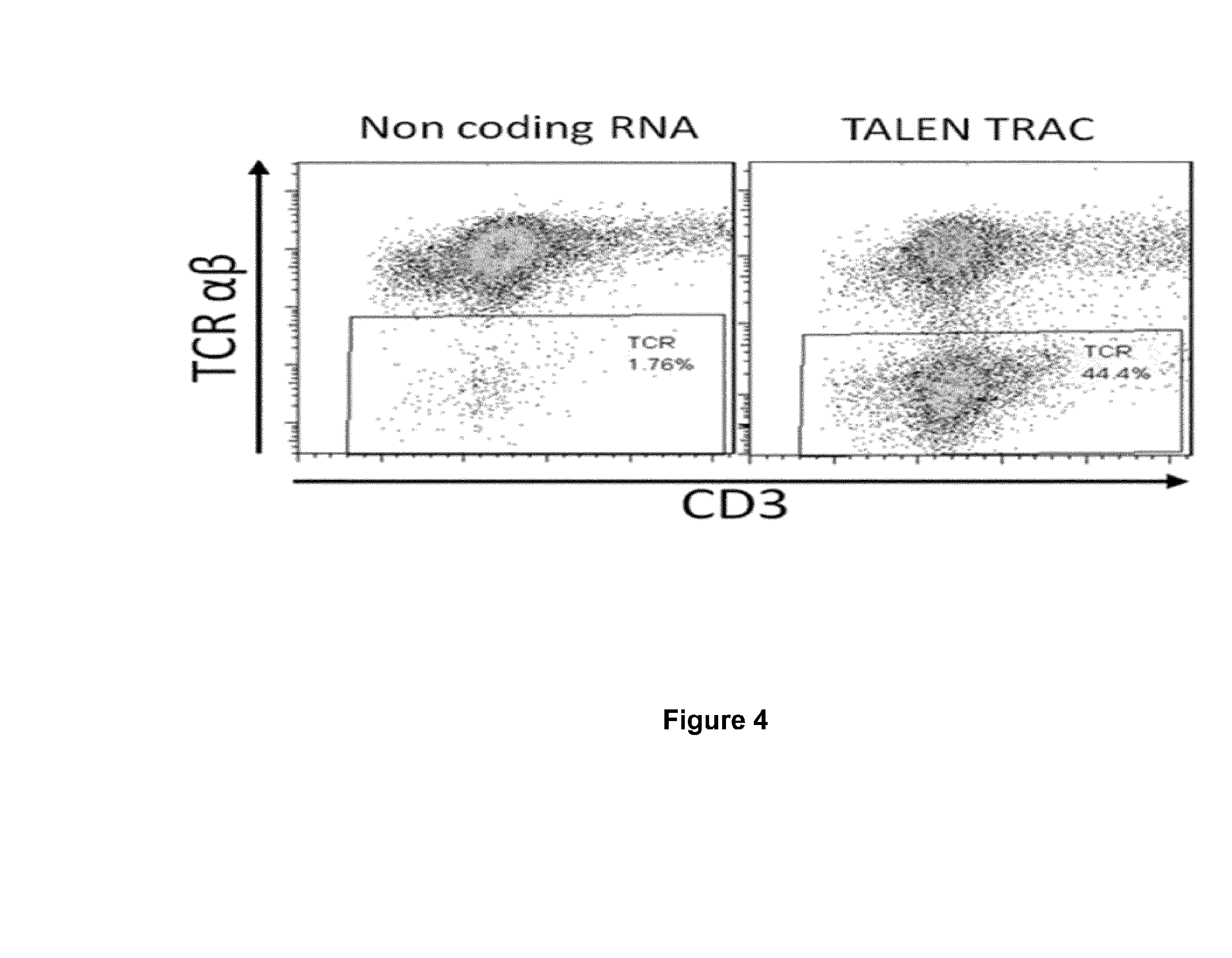
Method for generating t-cells compatible for allogenic transplantation
a technology of allogenic transplantation and t-cells, applied in the field of engineered t-cells, can solve the problems of inability to provide prolonged expansion and anti-tumor activity in vivo, degraded immune function, and low number of patient's lymphocytes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0258]TALE-Nucleases Cleaving Human CIITA
[0259]mRNA encoding the TALE-nucleases targeting exons of the human CIITA gene were ordered from Cellectis Bioresearch (8, rue de la Croix Jarry, 75013 PARIS). Table 3 below indicates the target sequences cleaved by each of the two independent entities (called half TALE-nucleases) each containing a repeat sequence engineered to bind and cleave between target sequences consisting of two 17-bp long sequences (called half targets) separated by a 15-bp spacer. Because Exon 2 and 3 are shared by all transcript variants of CIITA, two TALEN pairs were designed for Exon 2 and 3. No obvious offsite targeting in the human genome have been predicted using TALE-Nucleases targeting these sequences.
TABLE 3Description of the CIITA TALE-nucleasesand related target sequencesTarget nameTarget sequenceTALEN 1_Exon 2_CMH-II-TATTCCCTCCCAGGCAGCTCacagtgtgccaccaTGGAGTTGGGGCCCCTA(SEQ ID NO: 55)TALEN 2_Exon 2_CMH-II-TATGCCTCTACCACTTCTATgaccagatggacctGGCTGGAGAAGAAGAGA(...
PUM
| Property | Measurement | Unit |
|---|---|---|
| voltage | aaaaa | aaaaa |
| voltage | aaaaa | aaaaa |
| voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com