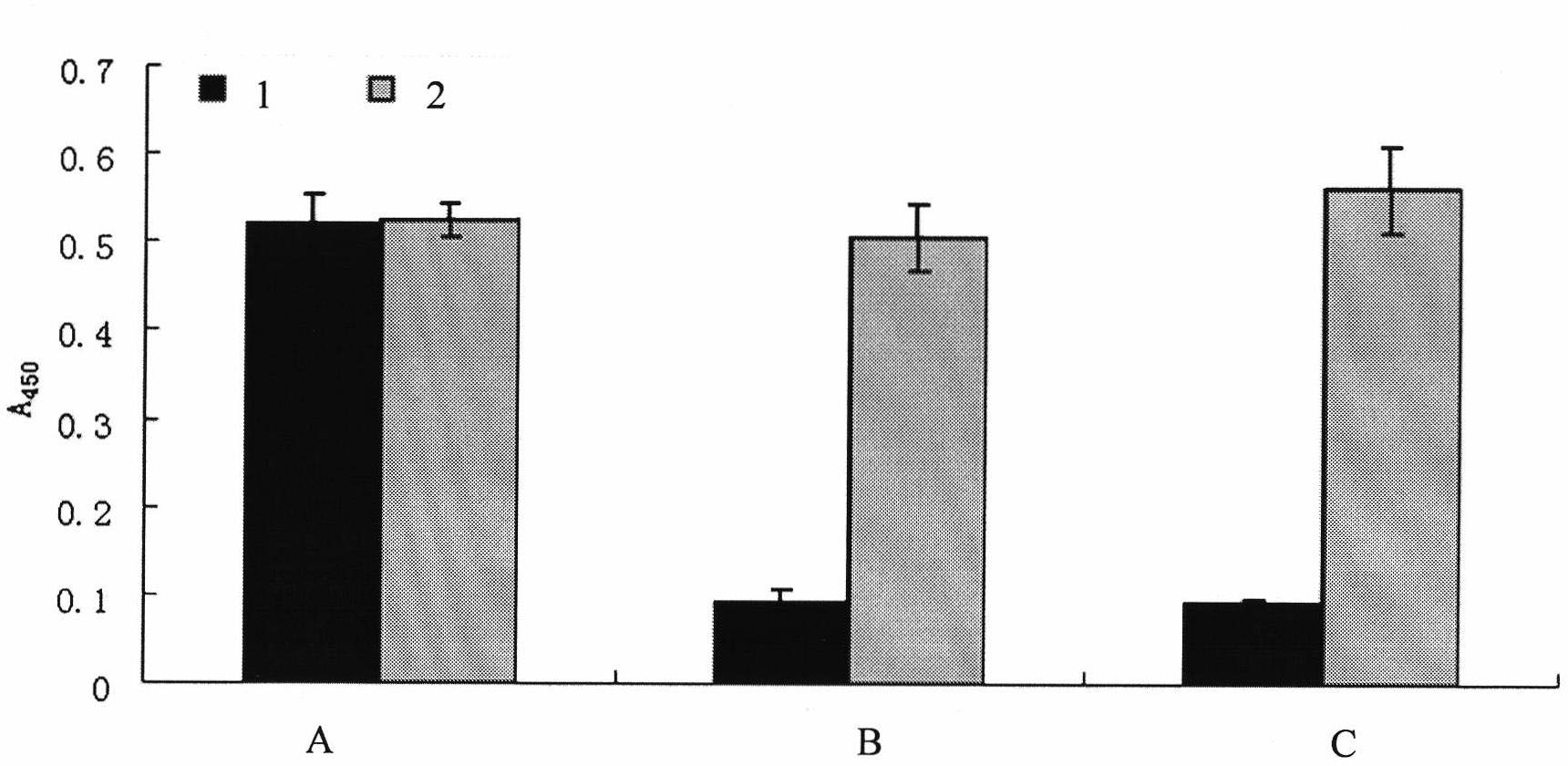
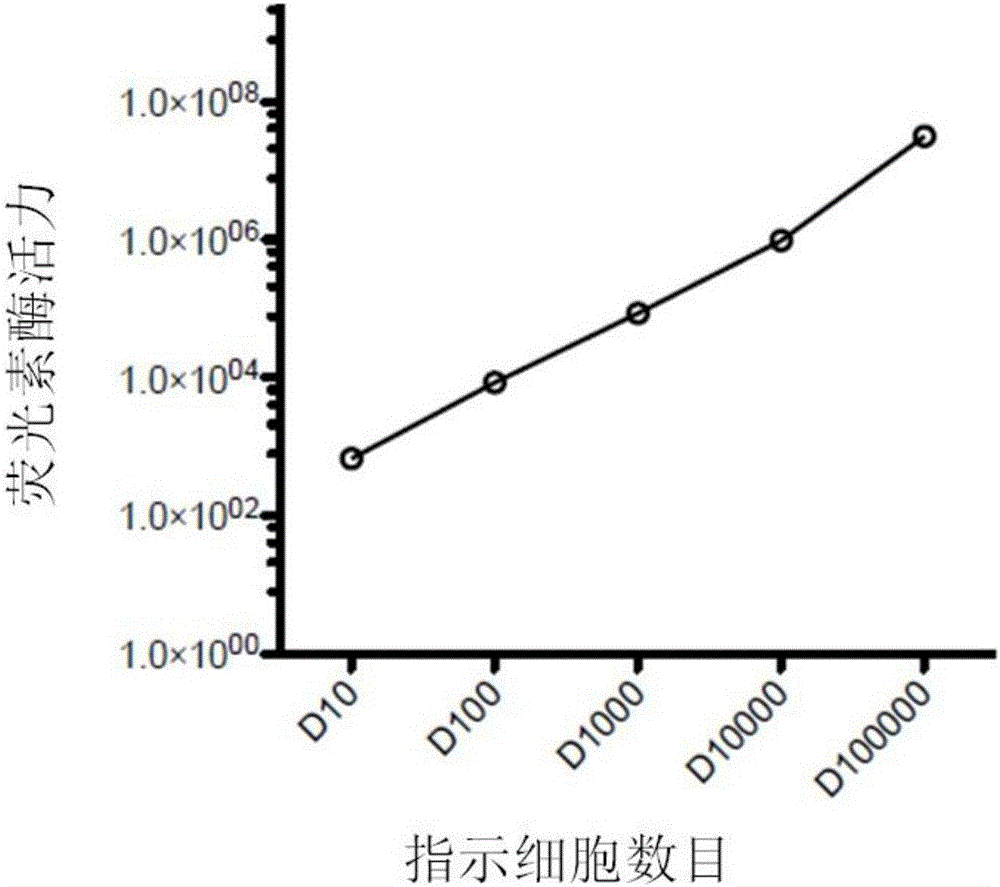
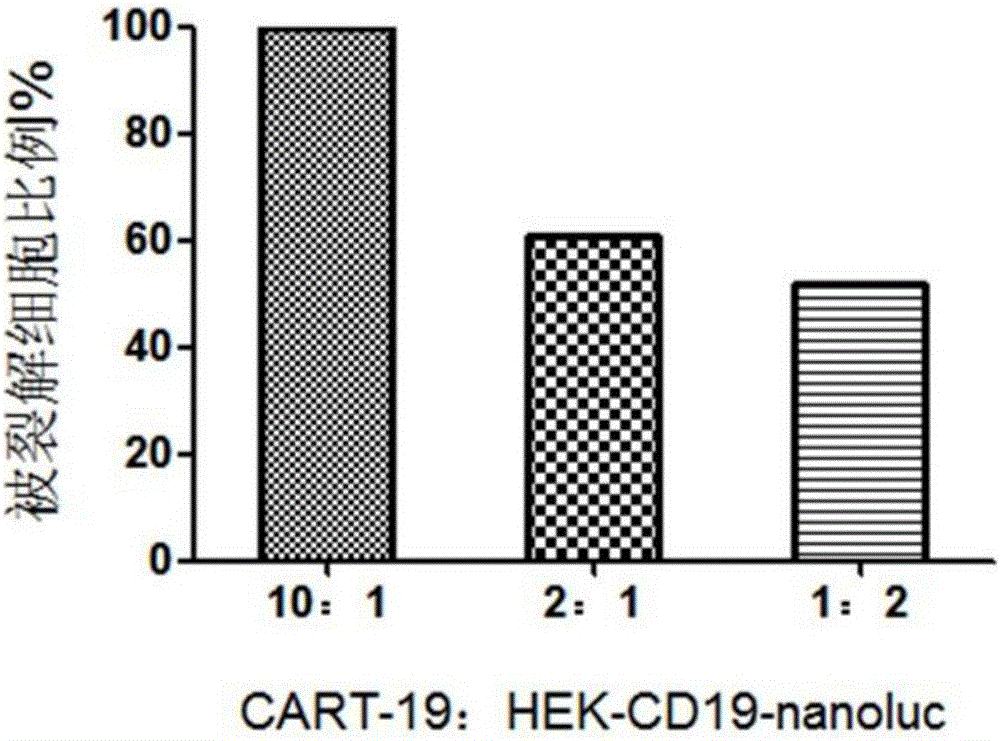
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
206 results about "Cellular immunotherapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cellular Immunotherapy. Cellular immunotherapy is an innovative treatment approach that harnesses the body’s own immune system to fight cancer. At Moffitt Cancer Center, we offer several types of cellular immunotherapy, including chimeric antigen receptor (CAR) T-cell therapy, T-cell receptor (TCR) therapy, tumor-infiltrating lymphocytes (TIL)...
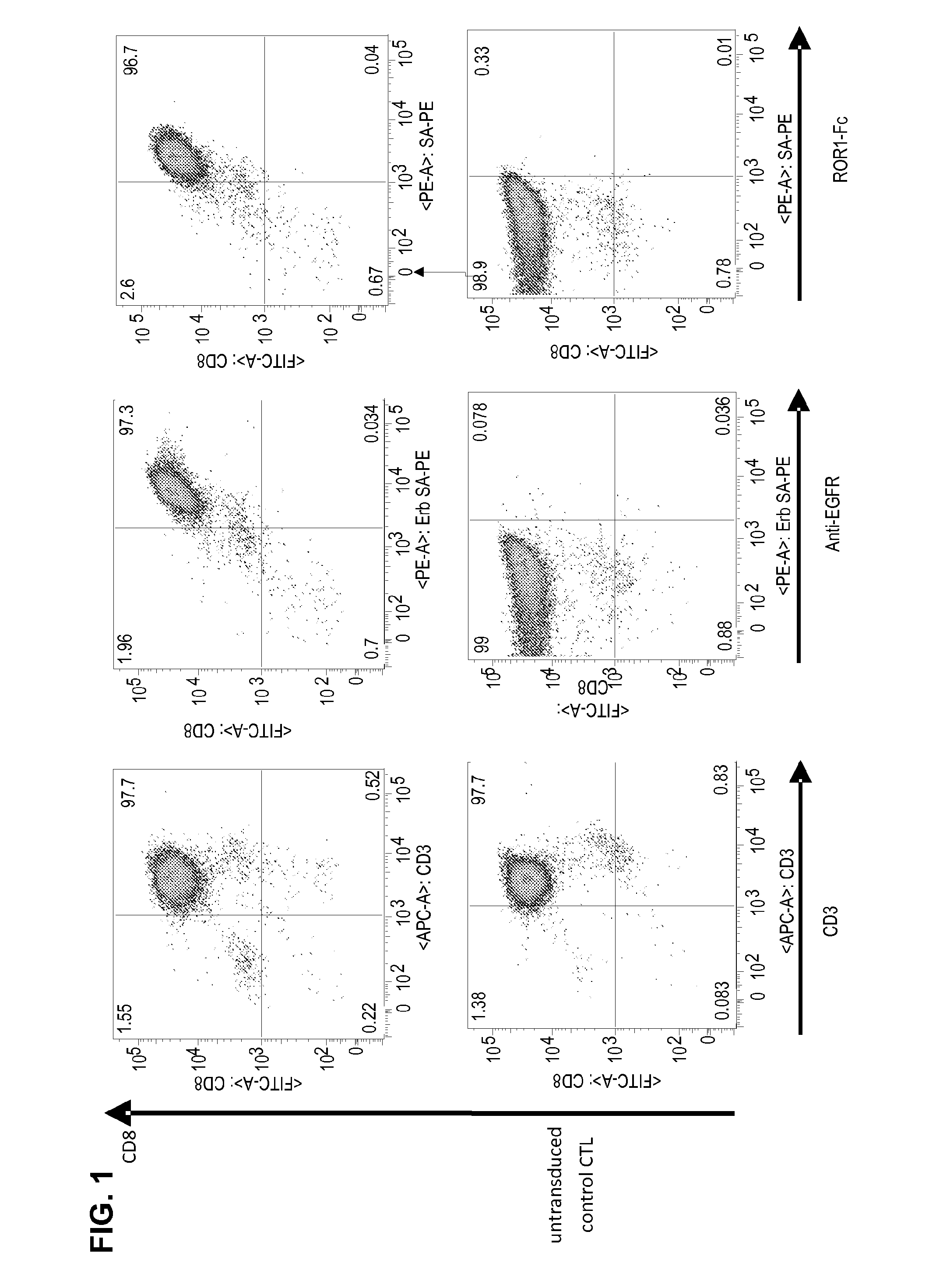
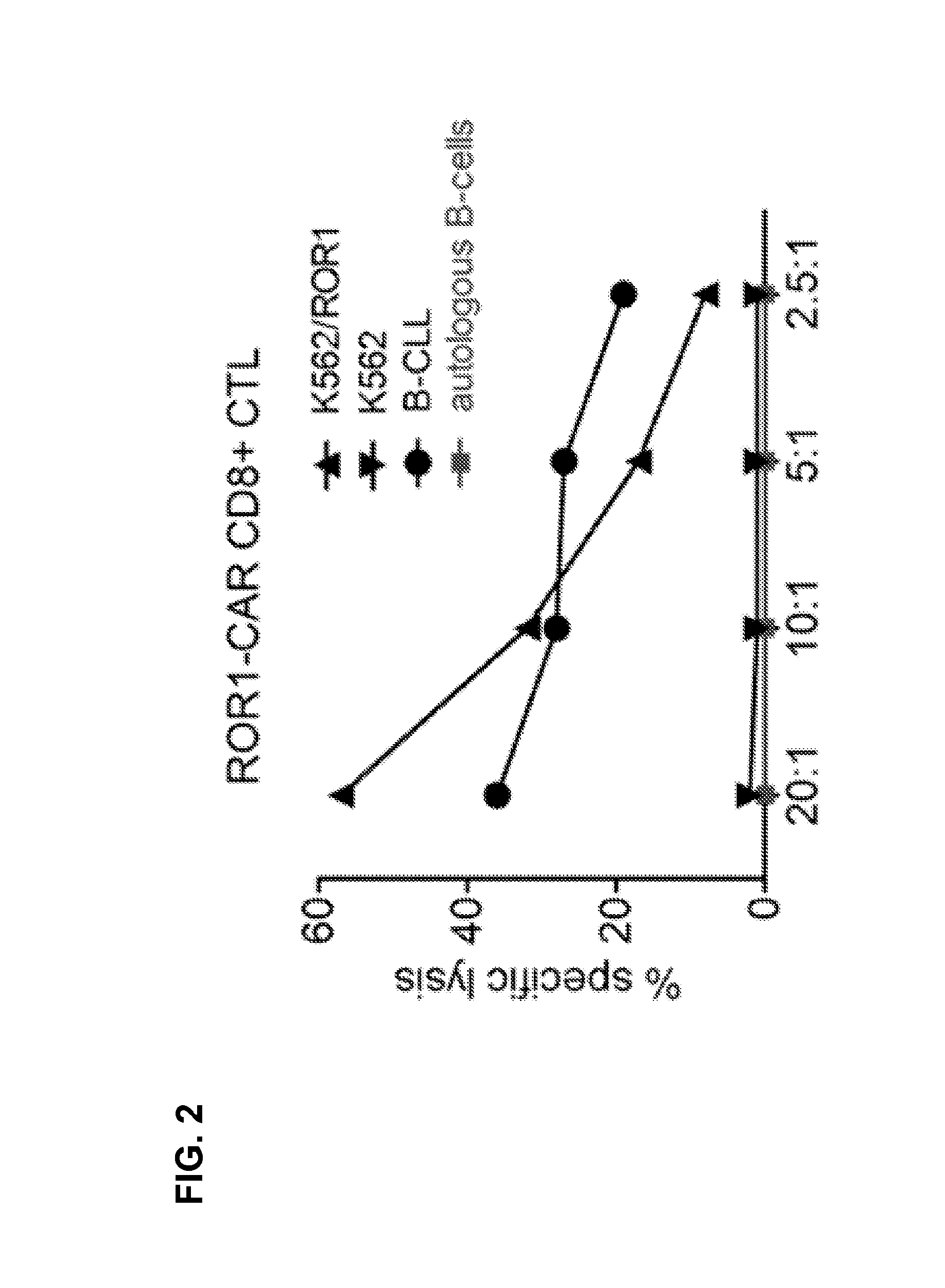
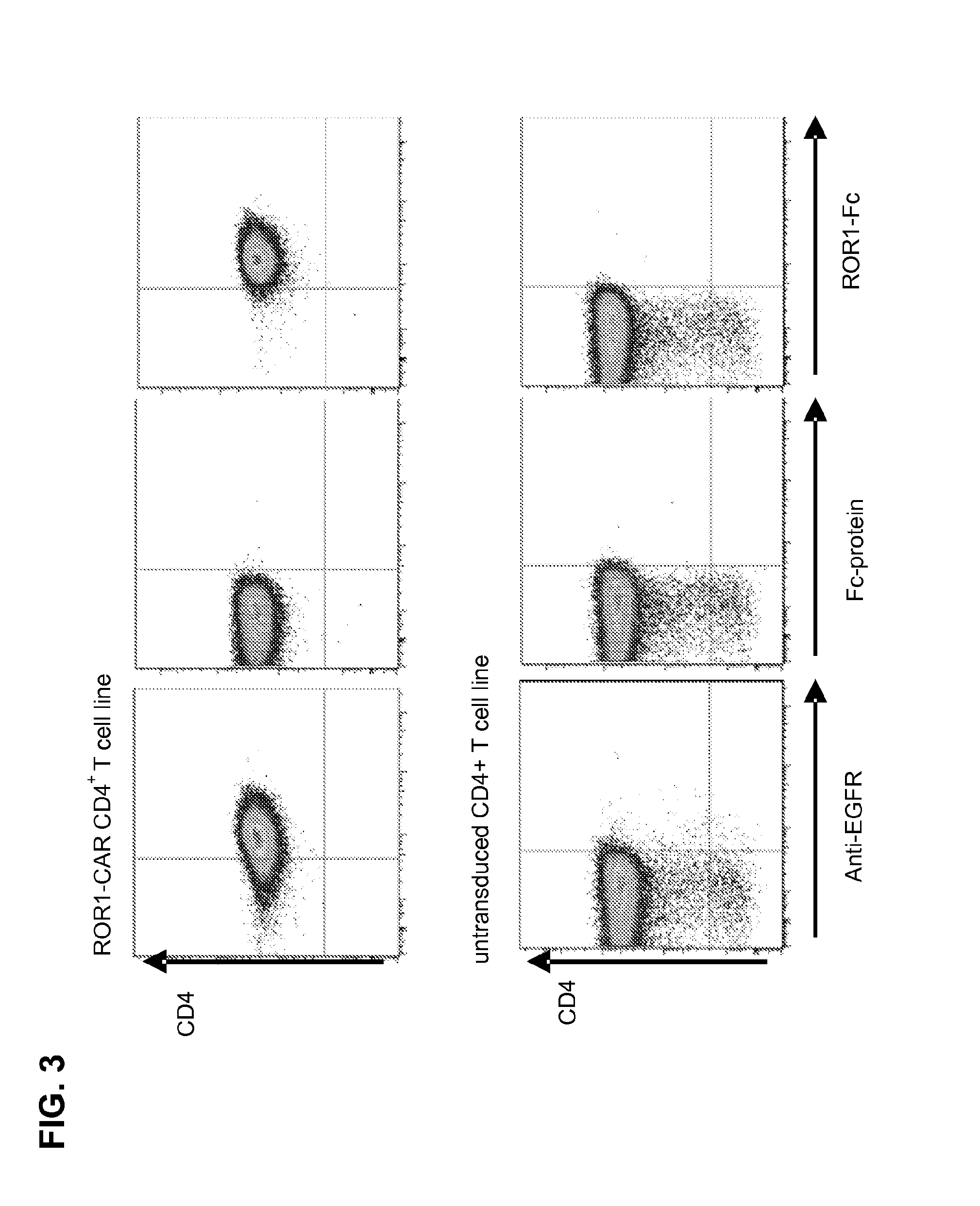
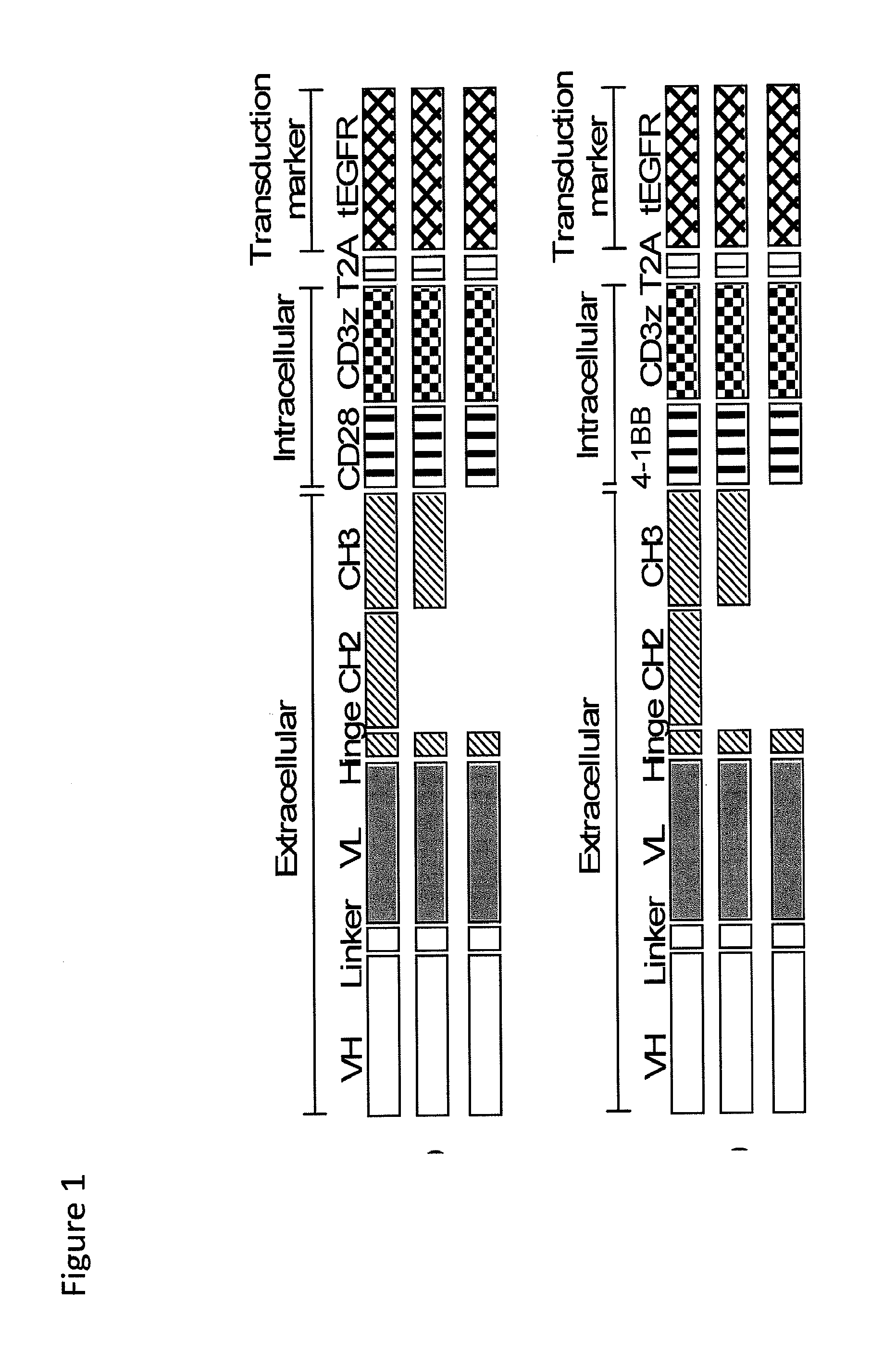
CE7-specific redirected immune cells
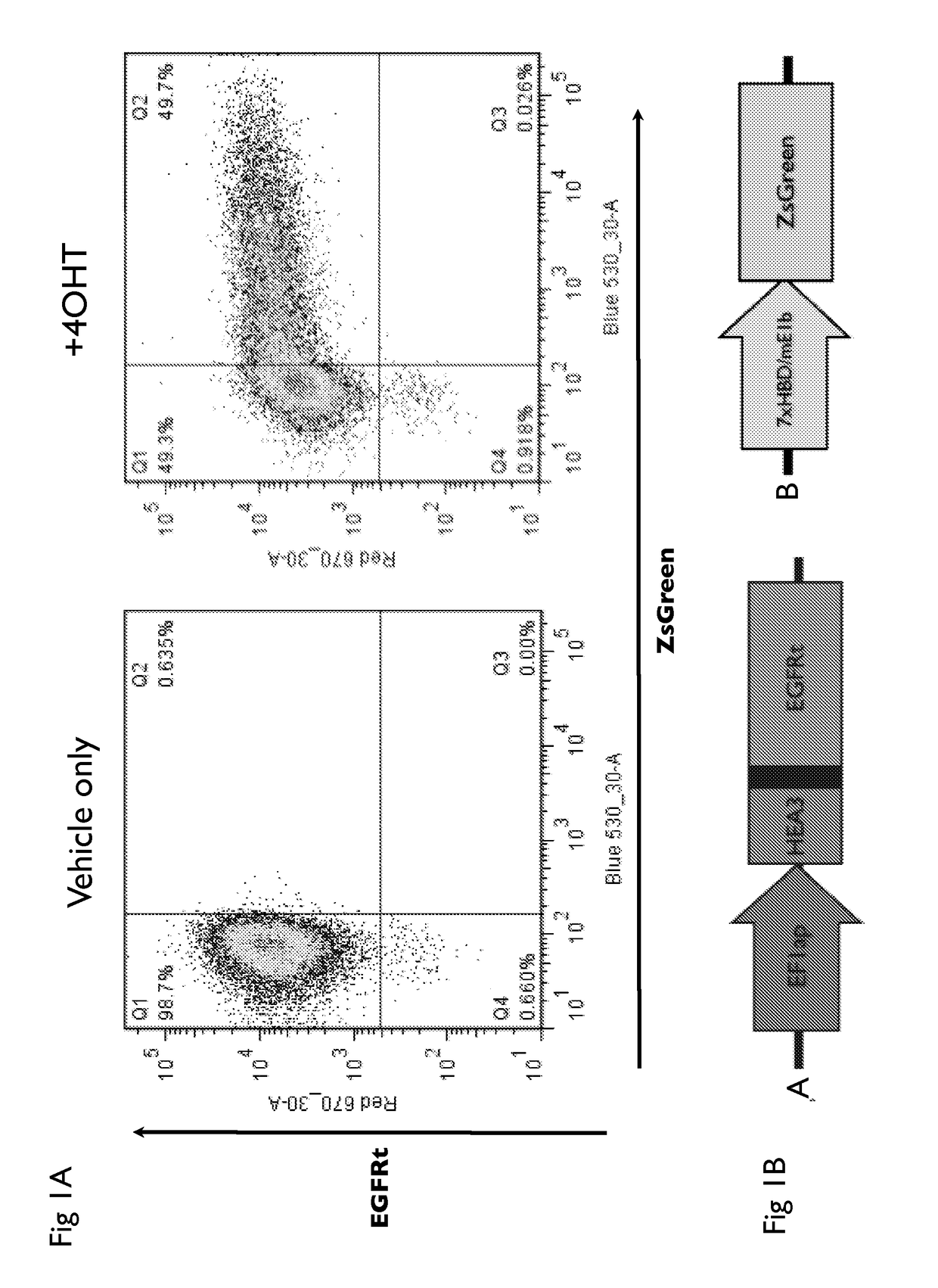
Genetically engineered, CE7-specific redirected immune cells expressing a cell surface protein having an extracellular domain comprising a receptor which is specific for CE7, an intracellular signaling domain, and a transmembrane domain, and methods of use for such cells for cellular immunotherapy of CE7+ neuroblastoma are disclosed. In one embodiment, the immune cell is a T cell and the cell surface protein is a single chain FvFc:ζ receptor where Fv designates the VH and VL chains of a single chain monoclonal antibody to CE7 linked by peptide, Fc represents a hinge —CH2—CH3 region of a human IgG1, and ζ represents the intracellular signaling domain of the zeta chain of human CD3. DNA constructs encoding a chimeric T-cell receptor and a method of making a redirected T cell expressing a chimeric T cell receptor by electroporation using naked DNA encoding the receptor are also disclosed.
Owner:CITY OF HOPE
CD19-specific chimeric T cell receptor
InactiveUS7446179B2Peptide/protein ingredientsAntibody mimetics/scaffoldsIntracellular signallingTransmembrane domain
The present invention relates to a genetically engineered, CD19-specific chimeric T cell receptor and to immune cells expressing the chimeric receptor The present invention also relates to the use of such cells for cellular immunotherapy of CD9+ malignancies and for abrogating any untoward B cell function. The chimeric receptor is a single chain scFvFc:ζ receptor where scFvFc designates the extracellular domain, scFv designates the VH and VL chains of a single chain monoclonal antibody to CD19, Fc represents at least part of a constant region of an IgG1, and ζ represents the intracellular signaling domain of the zeta chain of human CD3. The extracellular domain scFvFc and the intracellular domain ζ are linked by a transmembrane domain such as the transmembrane domain of CD4. In one aspect, the chimeric receptor comprises amino acids 23-634 of SEQ I DNO:2. The present invention further relates to a method of making a redirected T cell expressing a chimeric T cell receptor by electroporation using naked DNA encoding the receptor.
Owner:CITY OF HOPE
Expansion of lymphocytes with a cytokine composition for active cellular immunotherapy
PendingUS20170107490A1Increase stimulationImprove scalabilityPeptide/protein ingredientsBiological material analysisTissue sampleLymphocyte
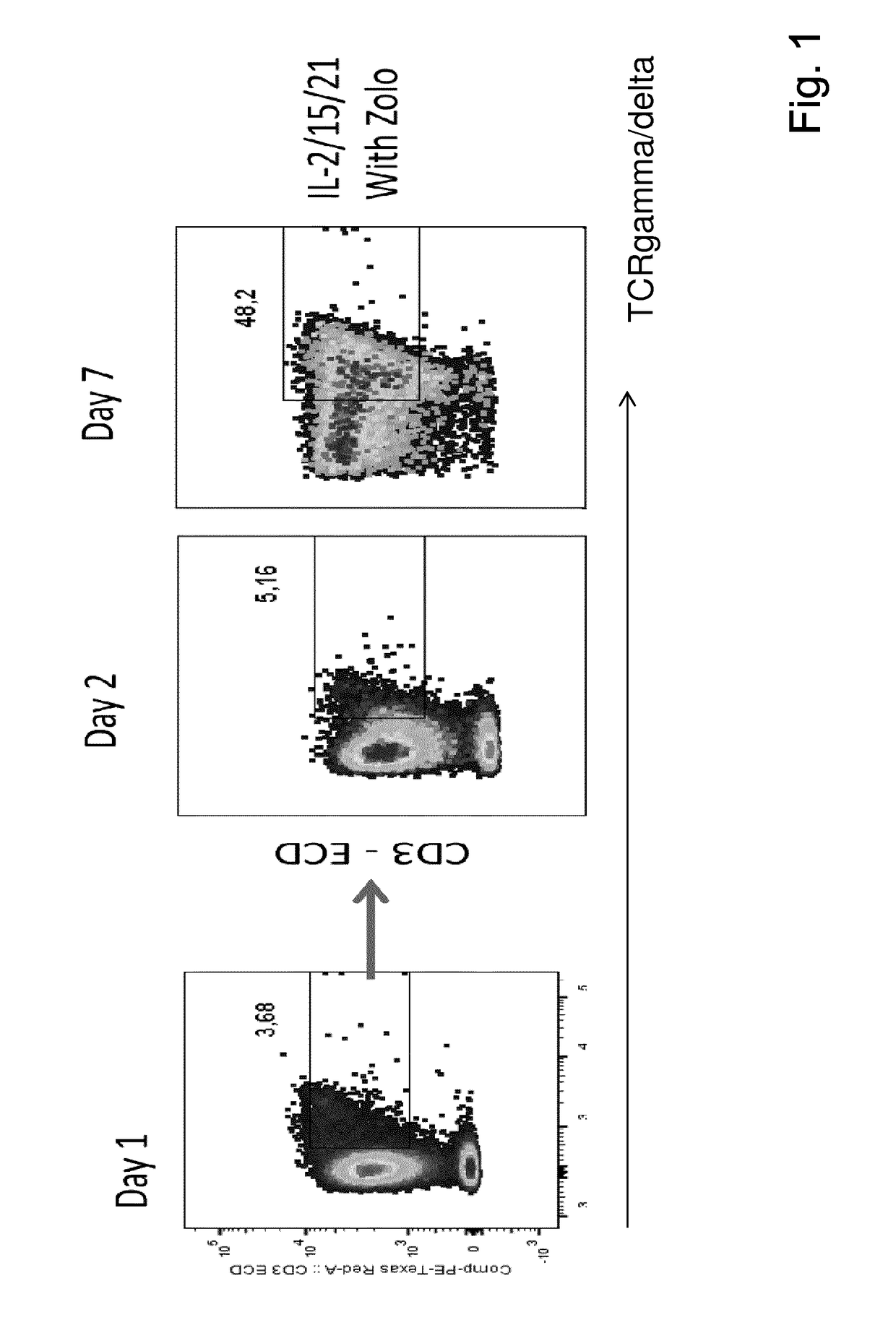
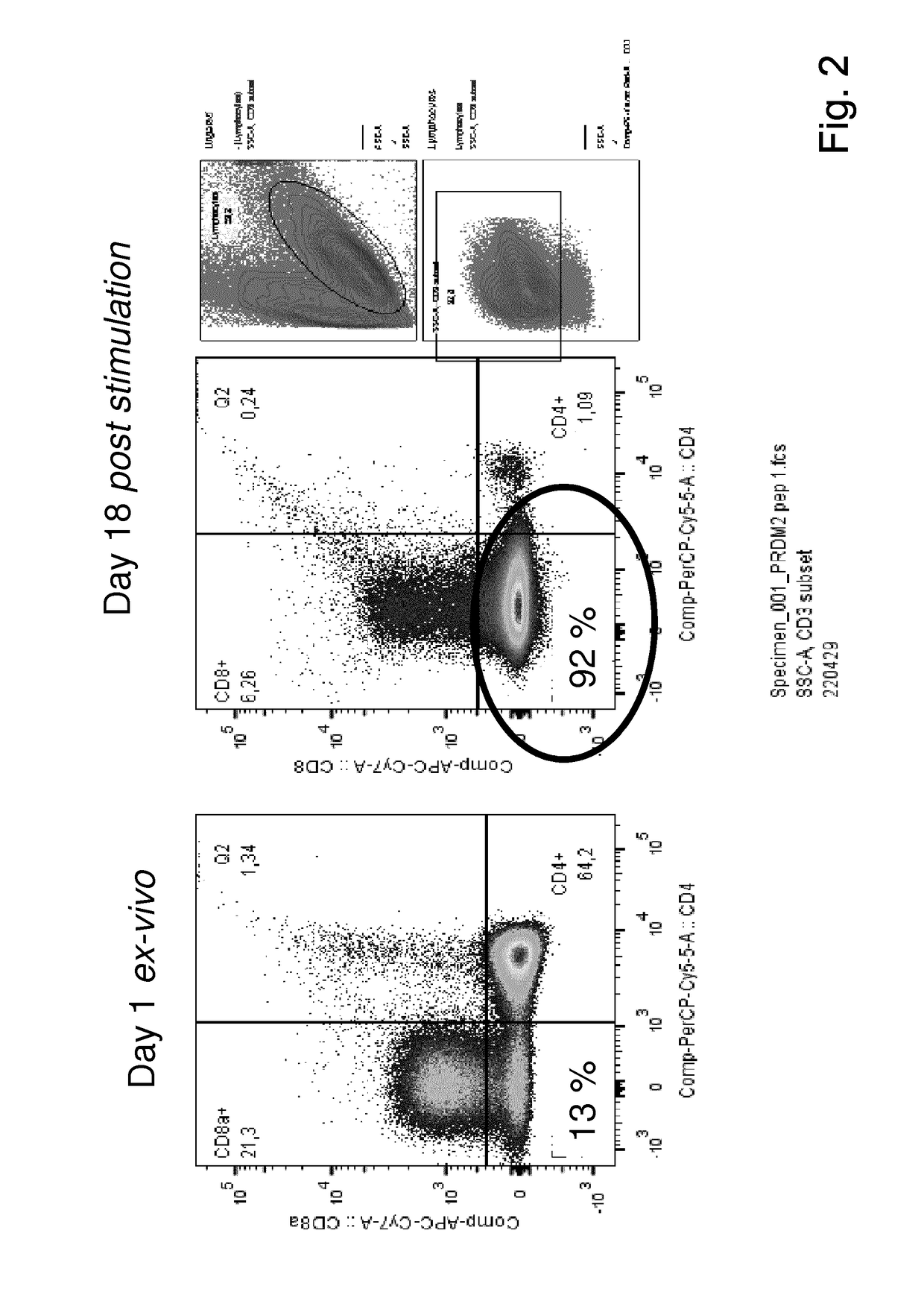
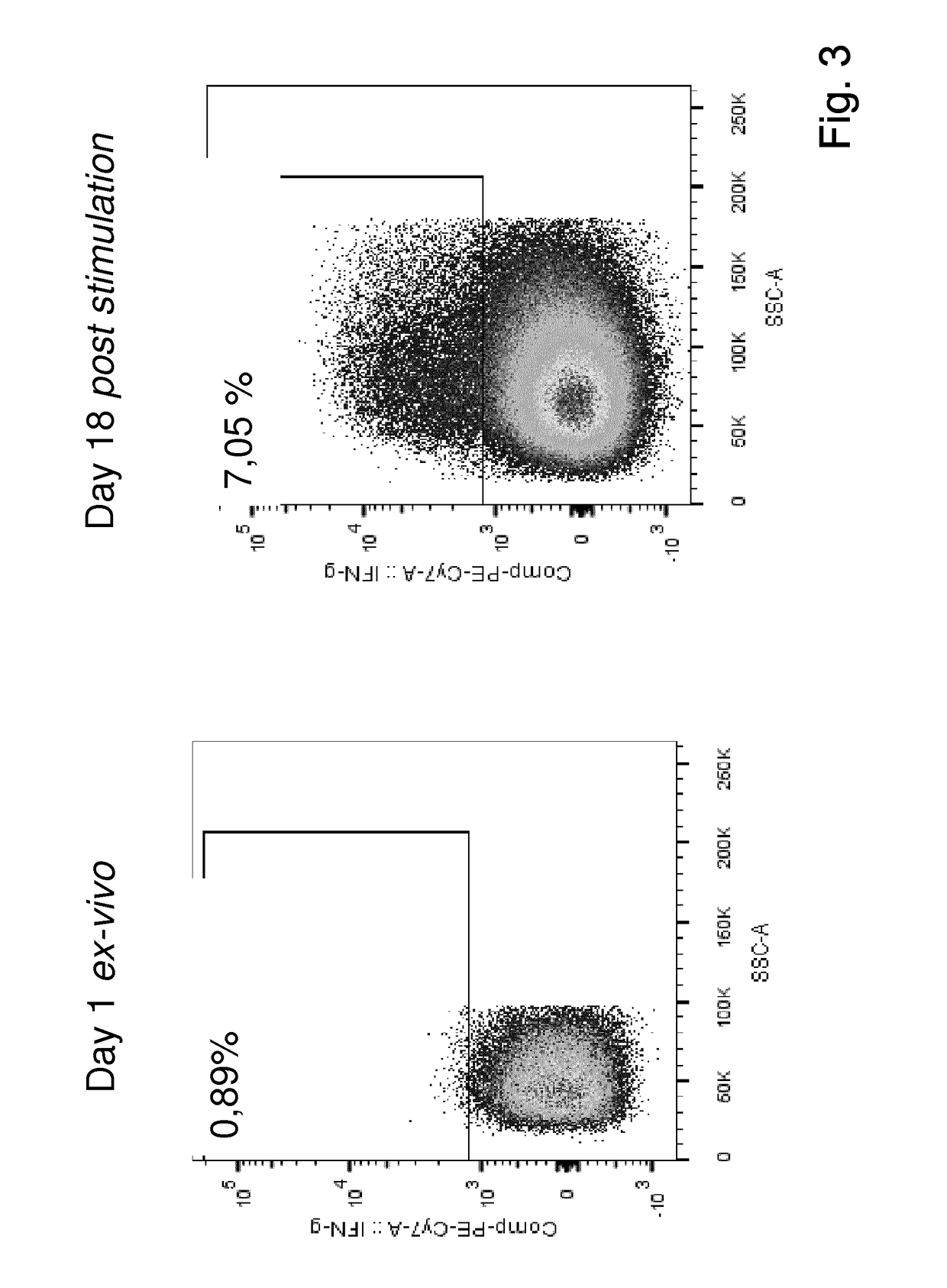
The present invention relates to a composition for expanding lymphocytes comprising at least two types of cytokines selected from interleukin 2 (IL-2), interleukin 15 (IL-15) and interleukin 21 (IL-21). It further relates to a Method of preparing a population of clinically relevant lymphocytes, comprising the steps of: obtaining a body sample from a mammal in particular a tissue sample or body liquid sample, comprising at least one lymphocyte and optionally separating the cells in the body sample, culturing the body sample in-vitro to expand and / or stimulate lymphocytes in the sample wherein the culturing comprises using IL-2, IL-15 and / or IL-21, and optionally determining the presence of clinically relevant lymphocyte in the cultured sample. The present invention also relates to an immunotherapy and the population of clinically relevant lymphocytes.
Owner:POLYBIOCEPT GMBH
Method and compositions for cellular immunotherapy
ActiveUS20140314795A1Maintain normalEnhance immune responsePeptide/protein ingredientsAntibody mimetics/scaffoldsPharmaceutical formulationWilms' tumor
The present invention provides methods and compositions to confer and / or augment immune responses mediated by cellular immunotherapy, such as by adoptively transferring genetically modified tumor specific CD8+ T cells in the presence of tumor-specific, subset specific genetically modified CD4+ T cells, wherein the CD4+ T cells confer and / or augment a CD8+ T cells ability to sustain anti-tumor reactivity and increase and / or maximize tumor-specific proliferation of the tumor-specific CD8+ T cells of interest. Pharmaceutical formulations produced by the method, and methods of using the same, are also described.
Owner:FRED HUTCHINSON CANCER CENT
Method and compositions for cellular immunotherapy
ActiveUS20150306141A1Enhanced cytokine productionIncreased proliferationPeptide/protein ingredientsAntibody mimetics/scaffoldsIn vivoTransmembrane domain
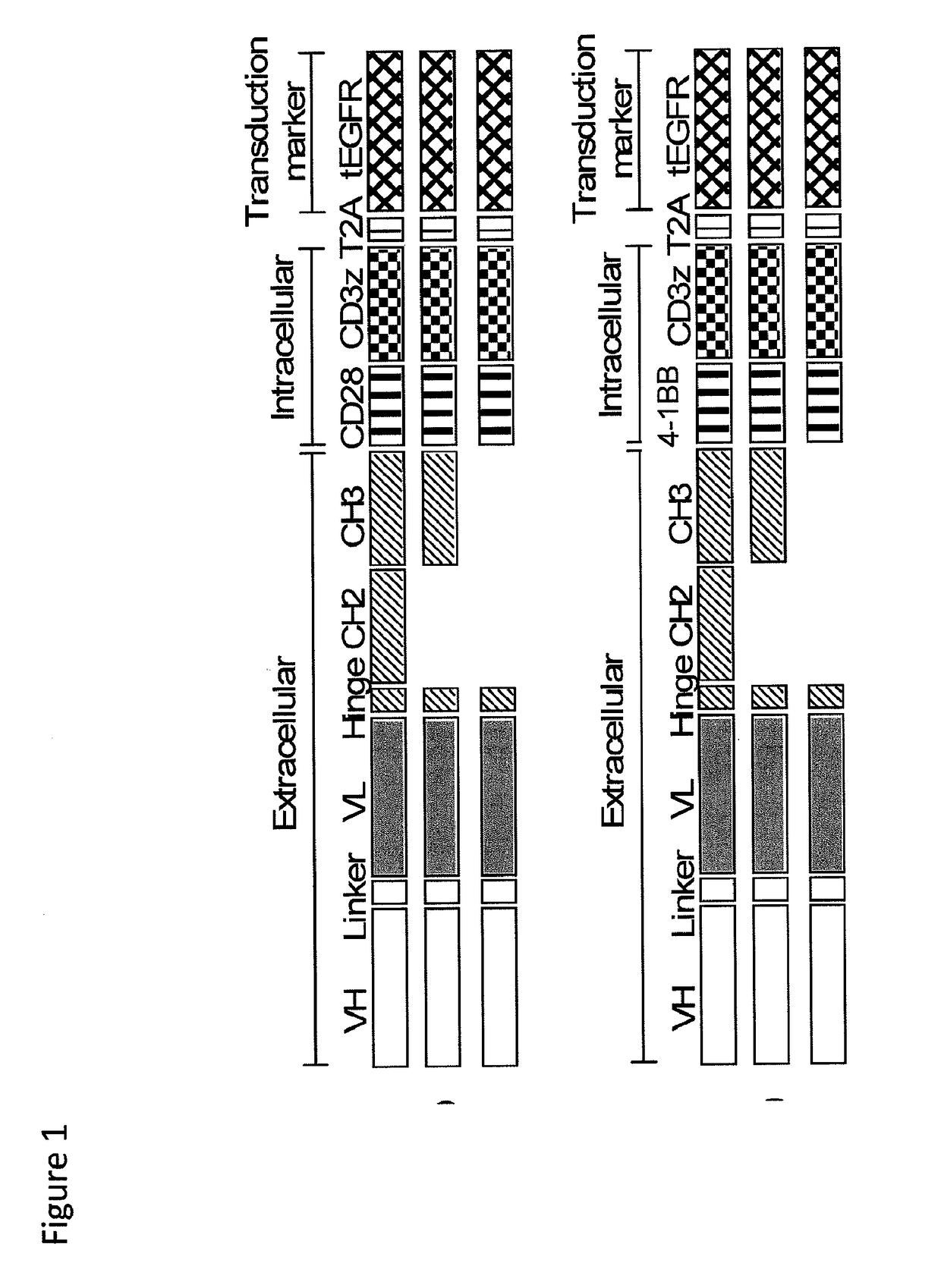
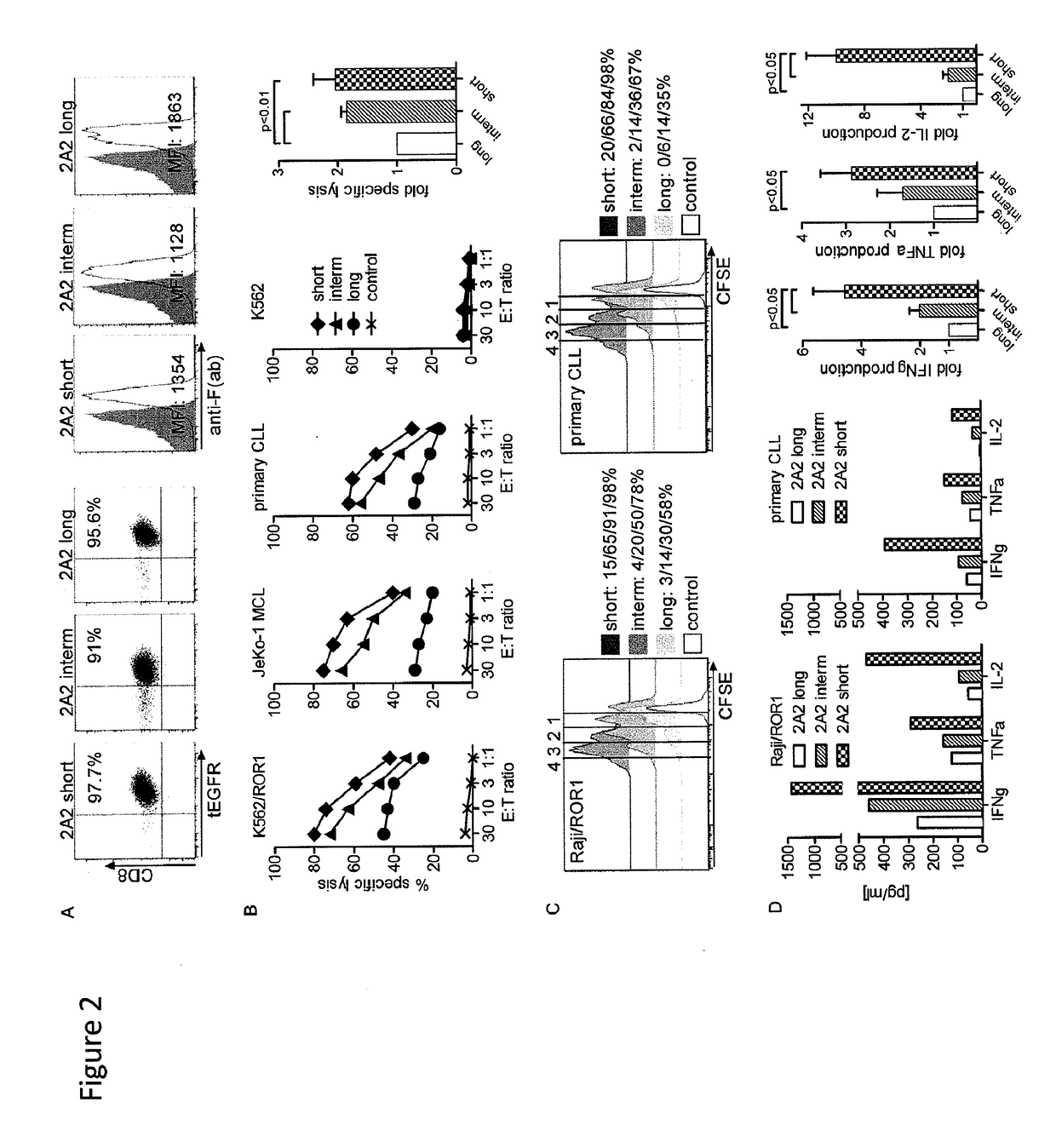
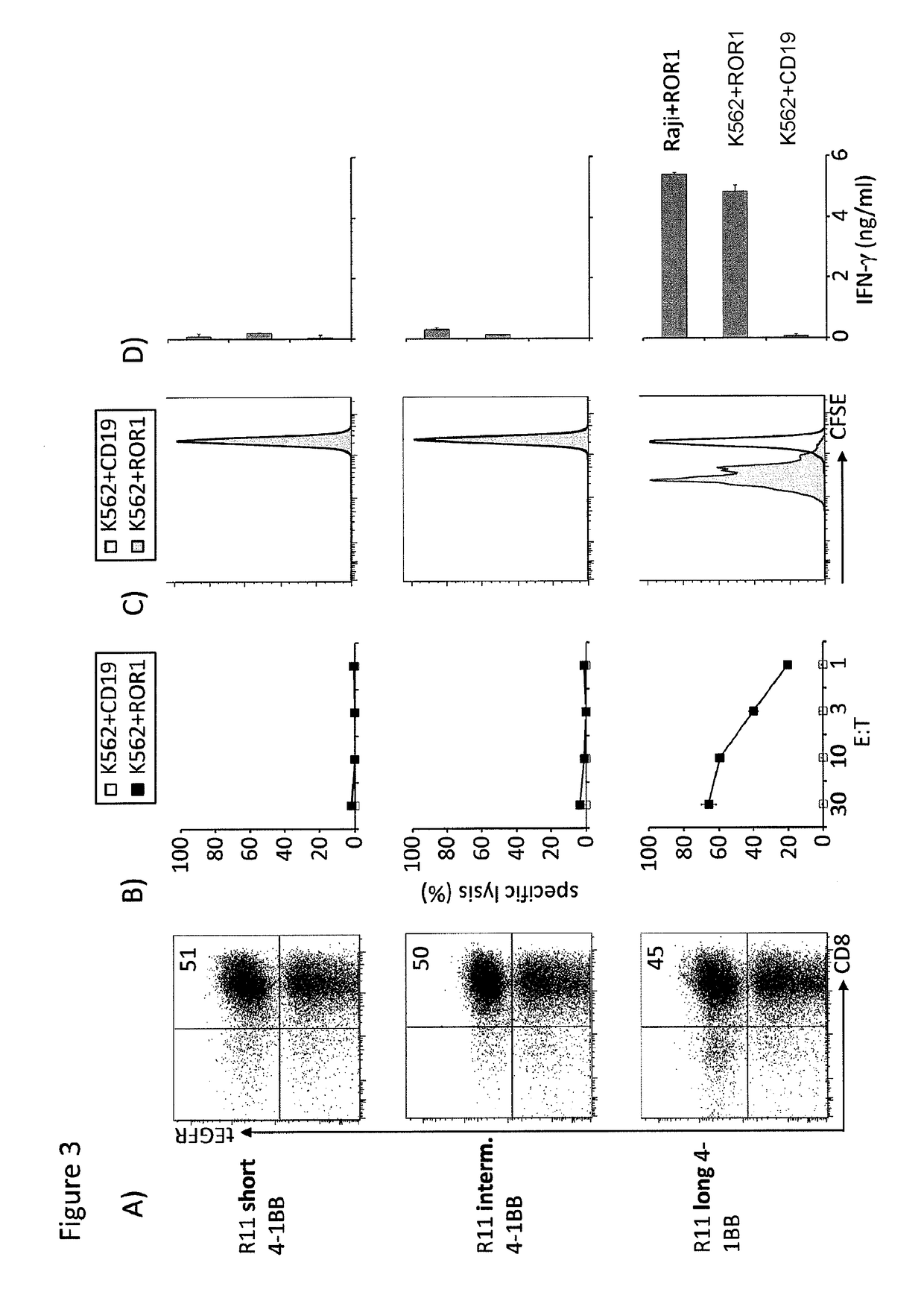
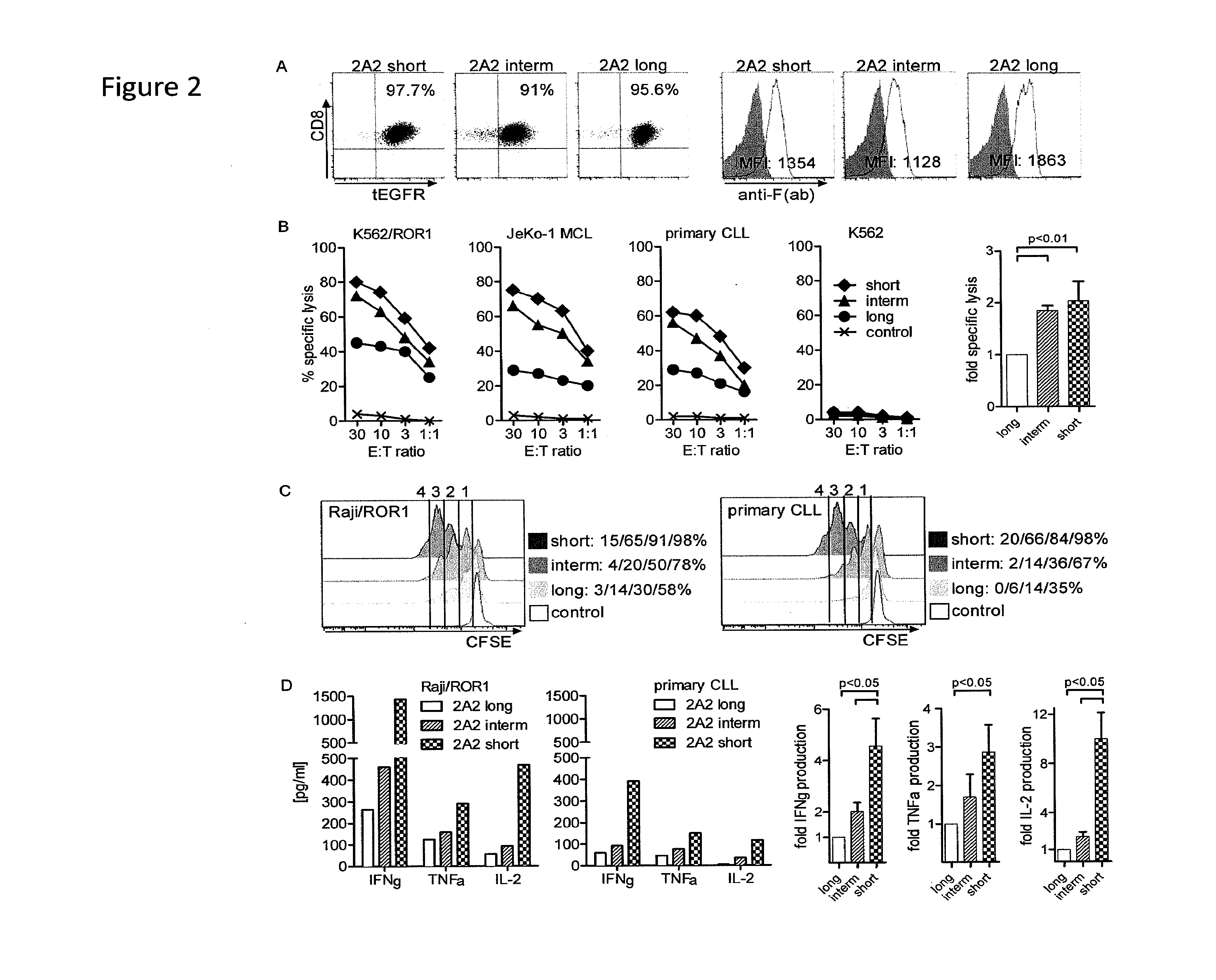
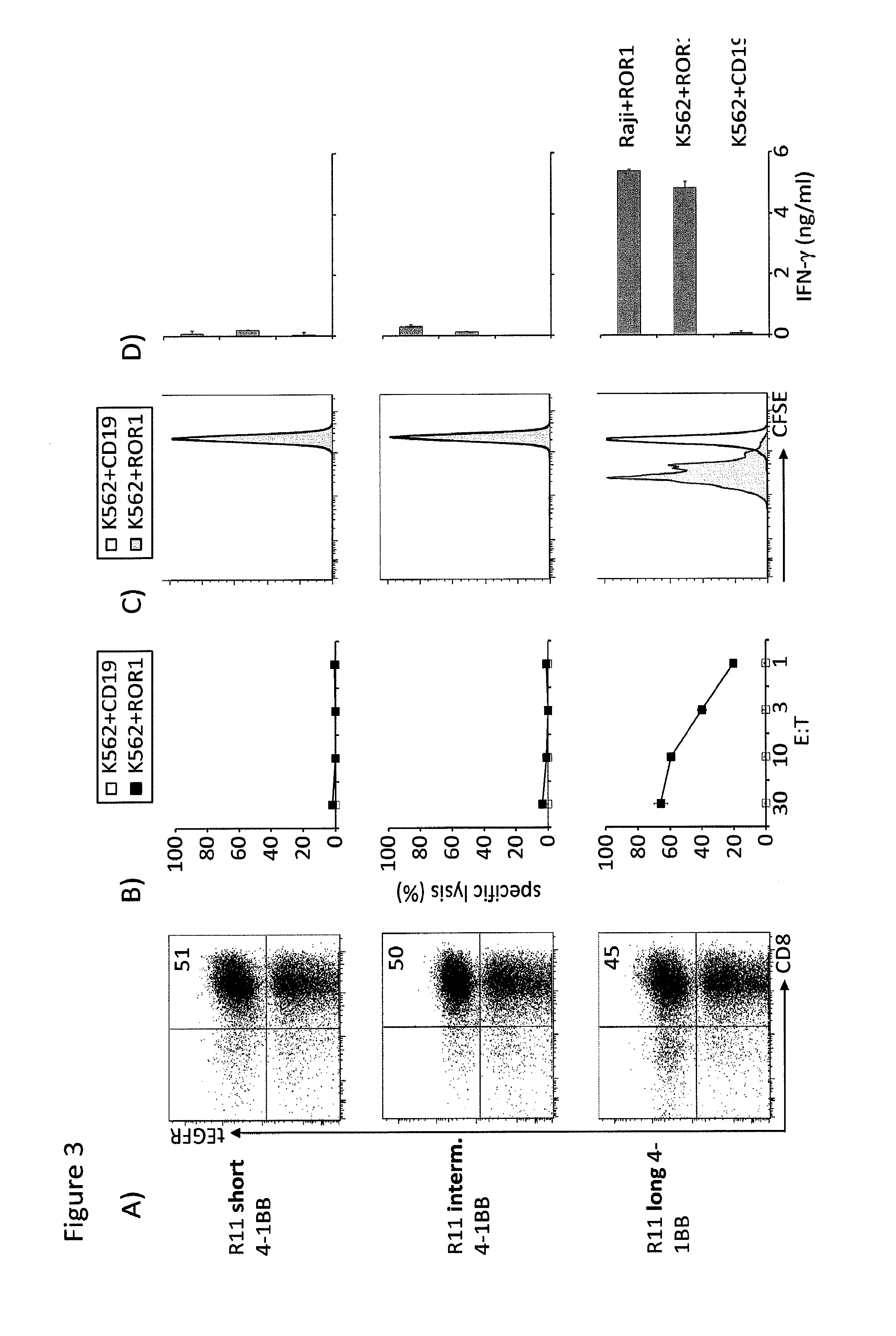
The present invention provides nucleic acids, vectors, host cells, methods and compositions to confer and / or augment immune responses mediated by cellular immunotherapy, such as by adoptively transferring CD8+ central memory T cells or combinations of central memory T cells with CD4+ T cells that are genetically modified to express a chimeric receptor. In embodiments the genetically modified host cell comprises a nucleic acid comprising a polynucleotide coding for a ligand binding domain, a polynucleotide comprising a customized spacer region, a polynucleotide comprising a transmembrane domain, and a polynucleotide comprising an intracellular signaling domain. It has been surprisingly found that the length of the spacer region can affects the ability of chimeric receptor modified T cells to recognize target cells in vitro and affects in vivo efficacy of the chimeric receptor modified T cells. Pharmaceutical formulations produced by the method, and methods of using the same, are also described.
Owner:SEATTLE CHILDRENS HOSPITAL +1
gRNA for knockout of wild type T cell TCR [beta] strand and method
ActiveCN107354156AHigh knockout efficiencySimple manufacturing methodImmunoglobulin superfamilyStable introduction of DNAT cellCellular immunity
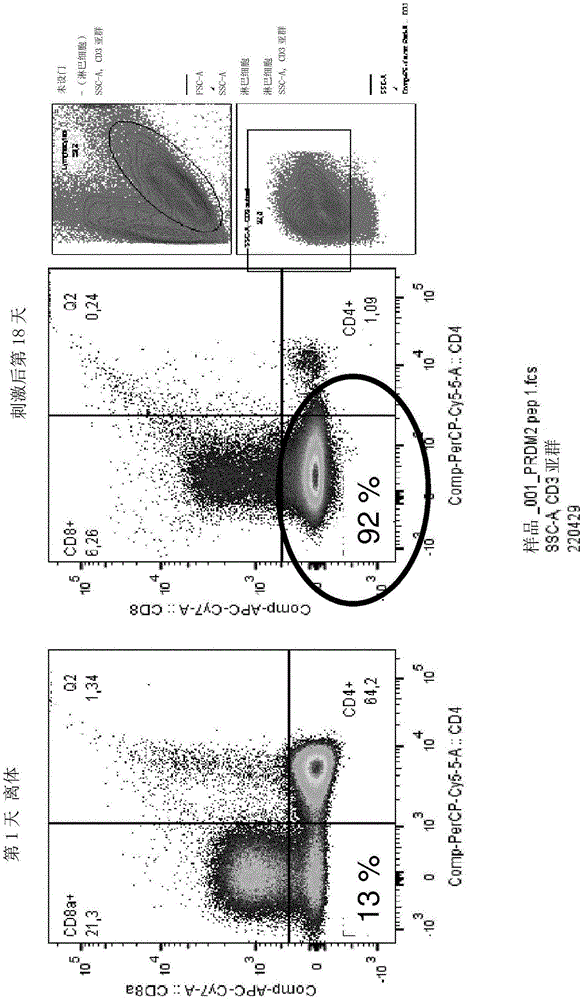
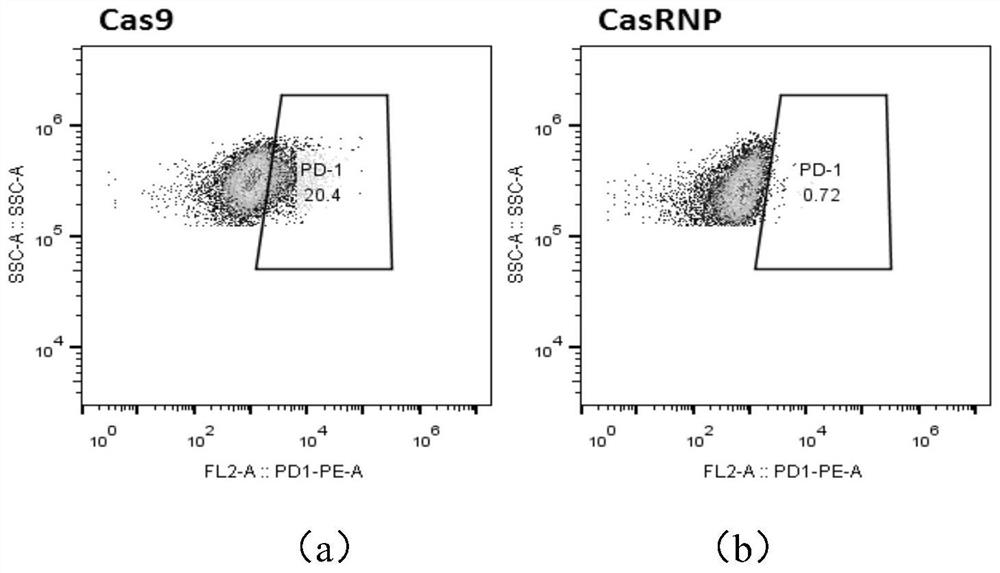
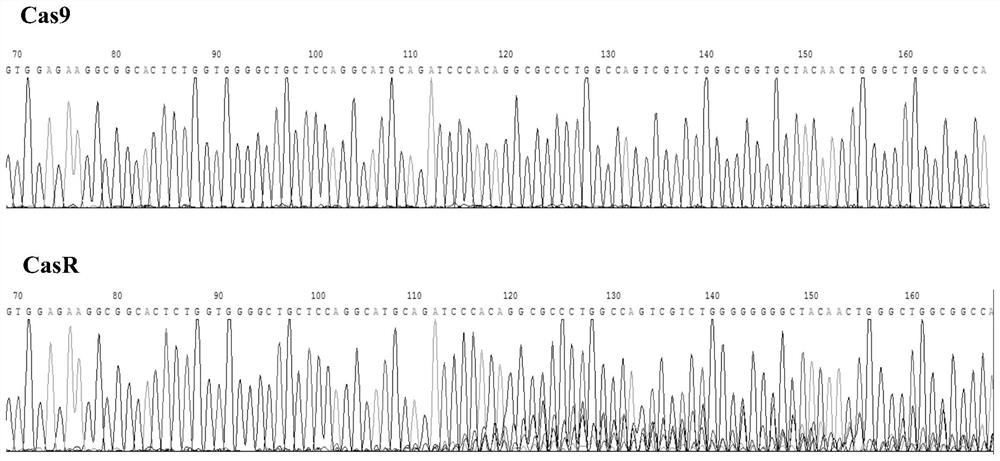
The invention discloses gRNA for knockout of a wild type T cell TCR [beta] strand and a method. The sequence of the gRNA is as shown in SEQ ID NO: 1, by utilizing a CRISPR / Cas9 technology, the gRNA and CRISPR / Cas9 jointly infect a T cell, the wild type T cell TCR [beta] strand is knocked out, and the T cell lacking the wild type TCR [beta] strand is constructed and used for CAR-T or TCR-T cellular immunotherapy. According to the gRNA for knockout of the wild type T cell TCR [beta] strand and the method, the knockout efficiency is high, the preparation method is relatively simple and easy, and T cells lacking wild type TCR [beta] strands can be provided for clinic rapidly and efficiently.
Owner:THE FIFTH AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIV
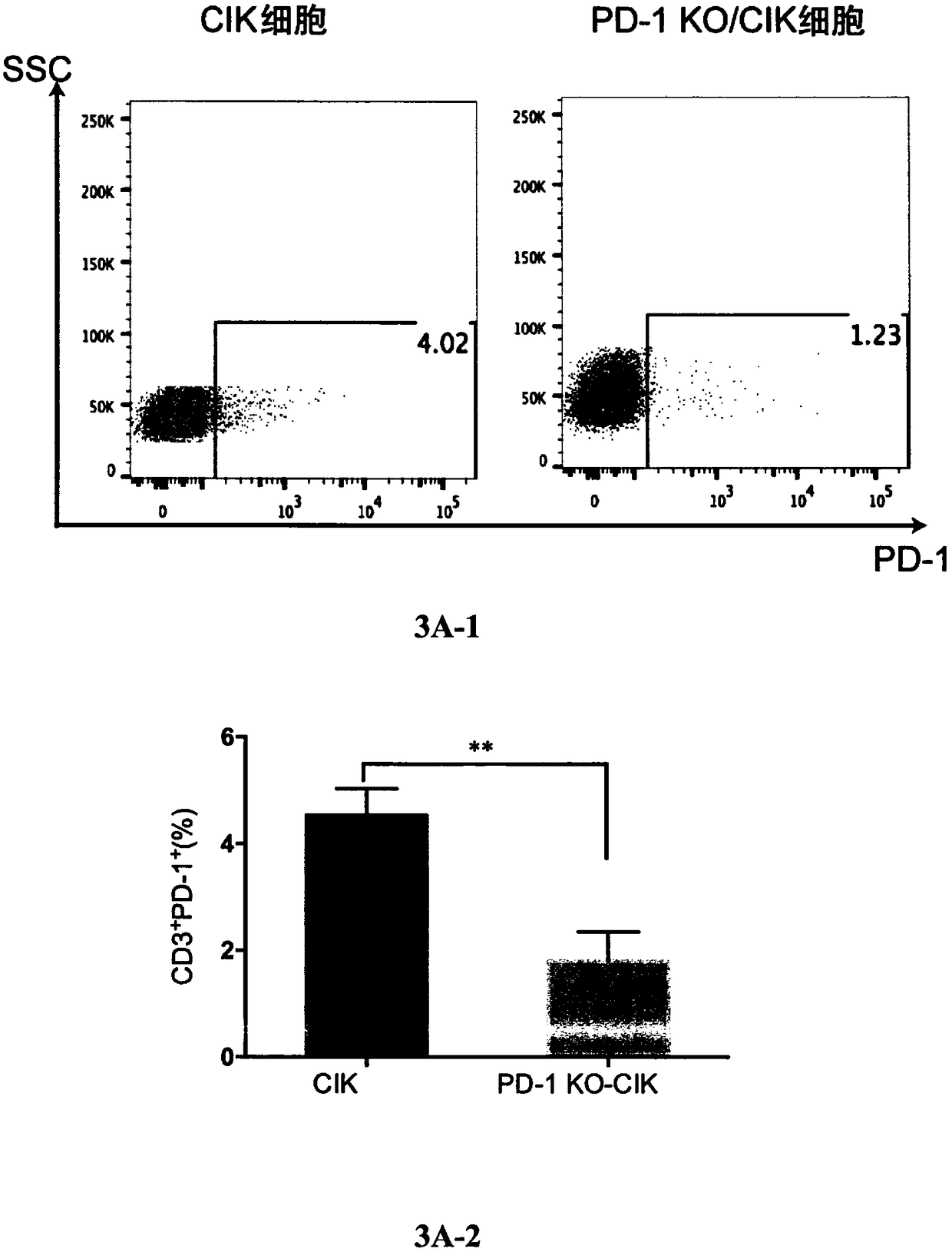
Method for enhancing anticancer capability of cells and enhanced type cells obtained by the method
InactiveCN108753817AEnhance the ability to resist liver cancer cellsIncrease lethalityGenetically modified cellsMammal material medical ingredientsEffector cellCell based
A method for enhancing anticancer capability of CIK cells is provided. The method includes knockout of a PD-1 gene of the CIK cells based on a CRISPR / Cas9 technique. The invention also provides enhanced type CIK cells obtained by the method. Experiments prove that the knockout of the PD-1 gene from the CIK cells can effectively enhance anti-hepatoma capability of the CIK cells, thus providing novel effector cells for tumor cellular immunotherapy.
Owner:北京华伟康信生物科技有限公司
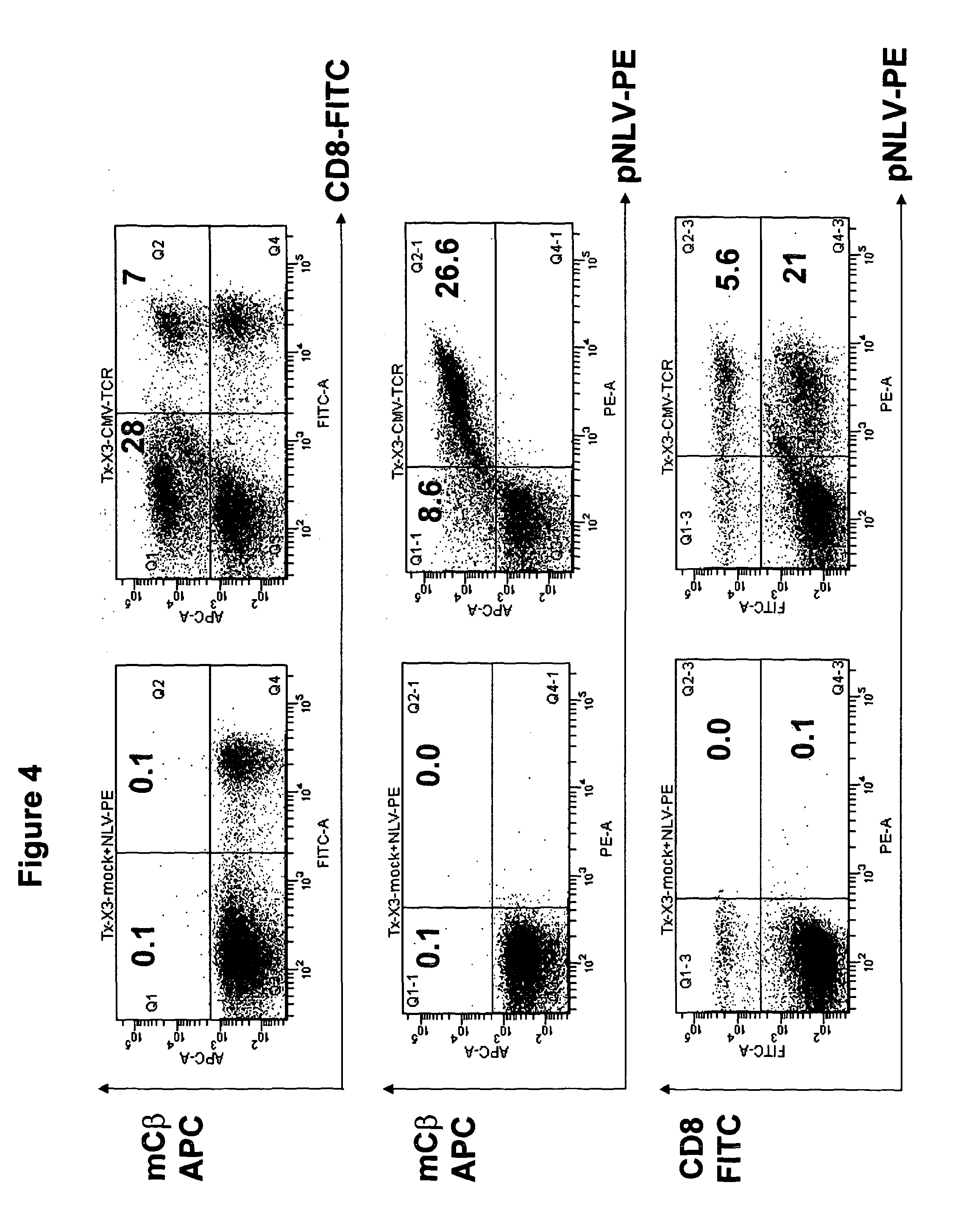
T-cell receptor capable of recognising an antigen from cytomegalovirus
The present invention provides a T-cell receptor (TCR) which binds to a peptide from the cytomegalovirus (CMV) phosphoprotein pp65 having the amino acid sequence NLVPMVATV (SEQ ID No. 1) when presented by a major histocompatability complex (MHC) molecule. The present invention also provides a nucleotide sequence encoding such a TCR, a vector comprising such a nucleotide sequence and its use to produce a CMV-specific T-cell. The present invention also provides the use of CMV-specific T-cell for cellular immunotherapy.
Owner:UCL BUSINESS PLC
Expansion method of various lymphocyte subpopulations and application of expansion method
ActiveCN105624107ASuppress deathImprove developmentGenetic material ingredientsMammal material medical ingredientsWhite blood cellNatural Killer Cell Inhibitory Receptors
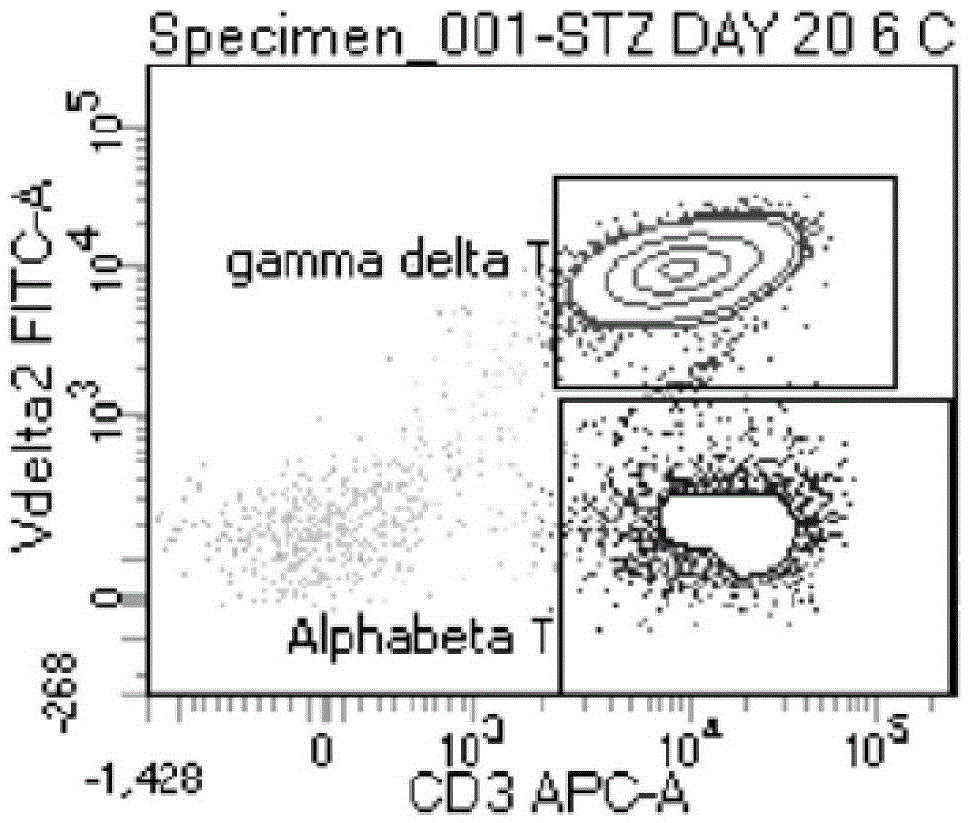
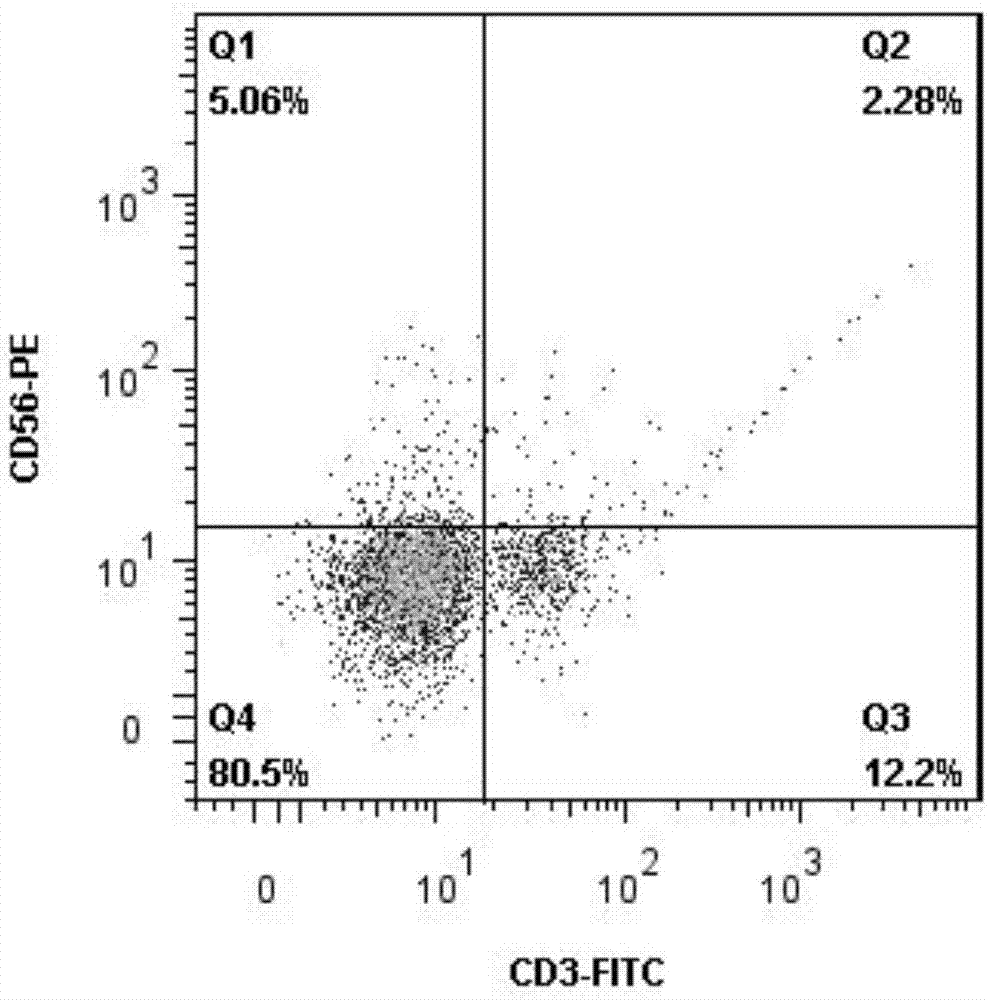
The invention discloses an expansion method of various lymphocyte subpopulations and application of the expansion method to preparation of an adoptive immunotherapy medicine for cancers. The expansion method comprises the following steps: S1, adding IFN gamma and zoledronic acid into a culture medium of PBMC collected in the blood of a tumor patient, and re-adding an OKT3 antibody and recombinant human interleukin-2 after culture to stimulate PBMC so as to finish preliminary expansion; S2, after finish of preliminary expansion, mixing immune lymphocytes with feeder layer cells, and after adding zoledronic acid, an OKT3 antibody and recombinant human interleukin-2, continually performing expansion. By adoption of the expansion method, after twice expansion, the sum of the immune cells can be 10,000 to 80,000 times by expansion, the expanded immune cells include alpha beta T cells, gamma delta T cells, NKT cells and NK cells, and tumor cells can be directly killed, or a cellular immunotherapy drug is prepared to kill the tumor cells.
Owner:杭州朔溪生物医药有限公司
Medicine composition and application thereof
The invention relates to the field of cellular immunotherapy of tumor and in particular to a medicine composition and application thereof. The medicine composition comprises a medicine specifically targeting kidney tumor GPC3, wherein the medicine specifically targeting kidney tumor GPC3 comprises any one or a combination of at least two of CAR-T (Chimeric Antigen Receptor T-Cell Immunotherapy) cells, an anti-GPC3 antibody, TCR-T (T-lymphocyte Cell Receptor) cells targeting GPC3, and siRNA of DC-CIK (Dendritic Cell-Cytokine-Induced Killer) cells targeting GPC3 or targeting silenced GPC3. Testsshow that GPC3 can be new targeting antigen of nephroblastoma, and by adopting the medicine composition, nephroblastoma can be effectively killed and inhibited.
Owner:SHENZHEN IN VIVO BIOMEDICINE TECH LTD
Amplification method for NK cell without trophocyte
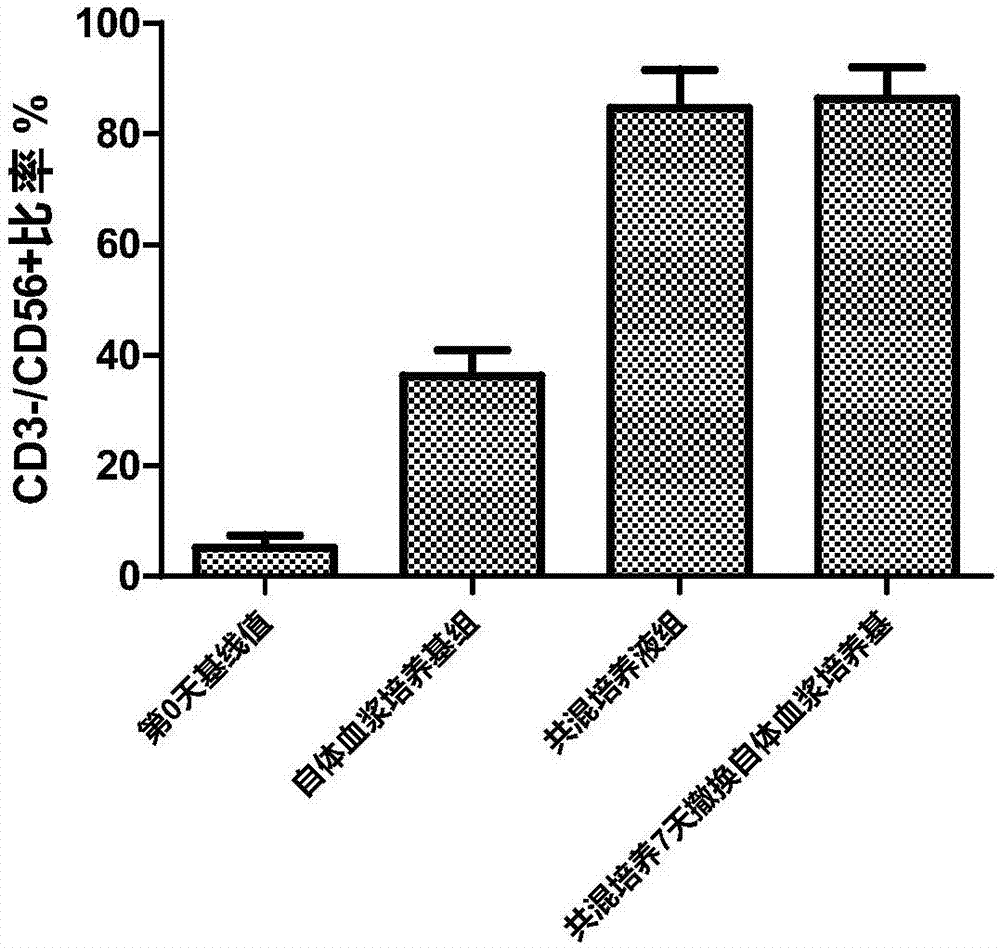
ActiveCN107460167AHigh purityLow costBlood/immune system cellsCell culture active agentsSerum free mediaLymphocyte culture
The invention discloses an amplification method for an NK cell without trophocyte. The method comprises the following steps: adopting a blending culture solution for culturing the NK cell and culturing for 7 days, and then using a self-plasma culture medium for culturing, supplying the fluid per 2-3 days in the culturing process, maintaining the cell density of 2.0*106 / mL and culturing for more than 20 days. The blending culture solution is composed of 1-10% of self-plasma, 200-1500 IU / mL IL-2, 10-50ng / mL IL-15 and 10-50ng / mL IL-21 added into a lymphocyte culture medium. The self-plasma culture medium is composed of 1-10% of self-plasma and 200 IU / mL IL-2 added into a serum-free medium. According to the amplification method for the NK cell without trophocyte disclosed by the invention, cord blood or autologous peripheral blood is taken as a cell source, no trophocyte is required in the culturing process, the amplification efficiency is high and the purity of the acquired NK cell is high. The NK cell acquired according to the method can be used as a medicine compound for cell immunization therapy and is mainly used for treating and / or preventing infectious diseases and / or cancer.
Owner:山东省齐鲁细胞治疗工程技术有限公司 +1
Allogeneic cellular immunotherapy for invasive pulmonary aspergillosis (IPA)
ActiveUS20060115487A1Snake antigen ingredientsVertebrate antigen ingredientsAllogeneic cellGraft versus host disease induction
A method for stimulating the immune system in immunocompromised patients in order to treat opportunistic infection. The method involves the infusion of intentionally mismatched allogeneic cells. In order to prevent graft vs. host disease complications, the allogeneic cells can be irradiated prior to infusion.
Owner:MIRROR BIOLOGICS INC +1
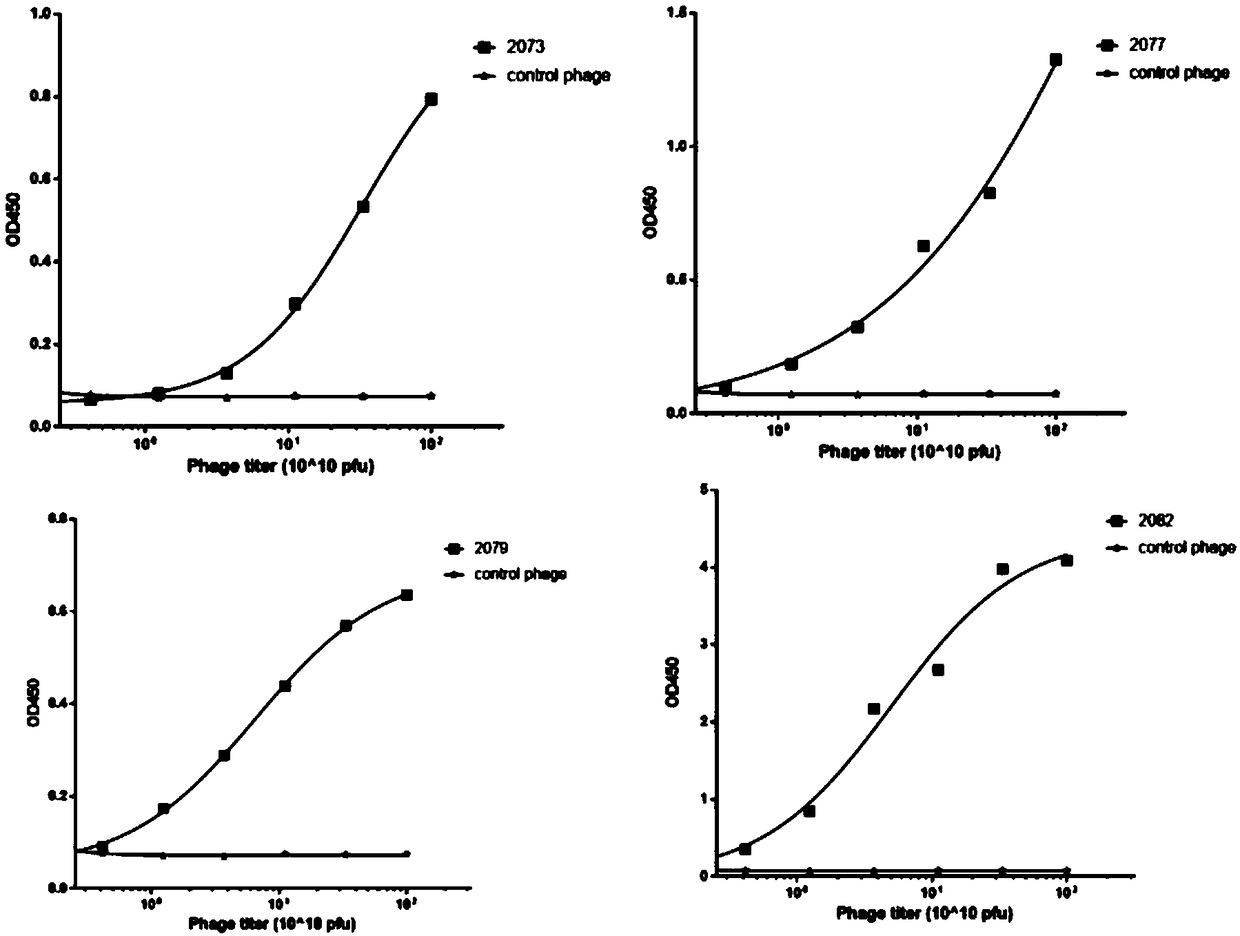
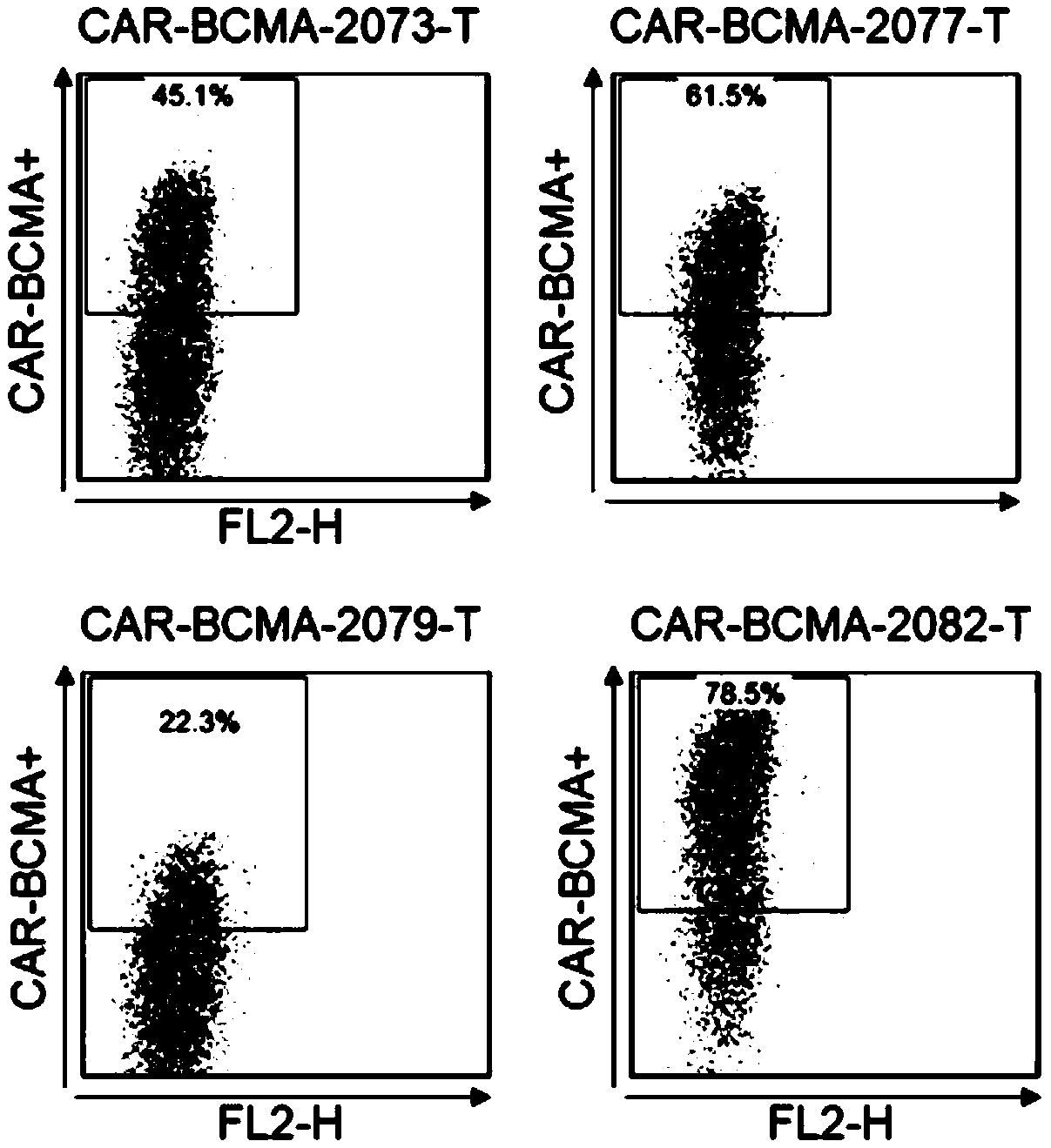
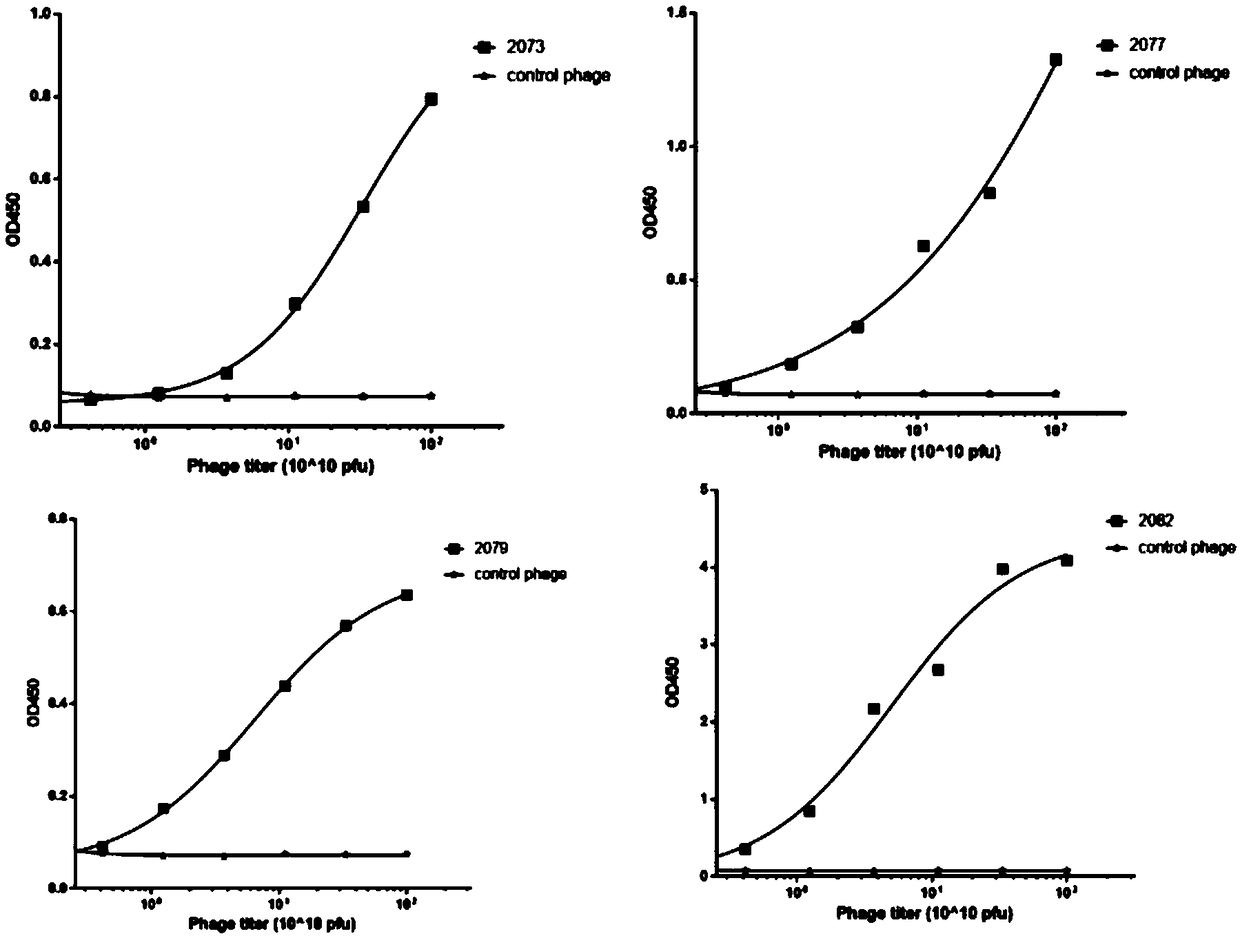
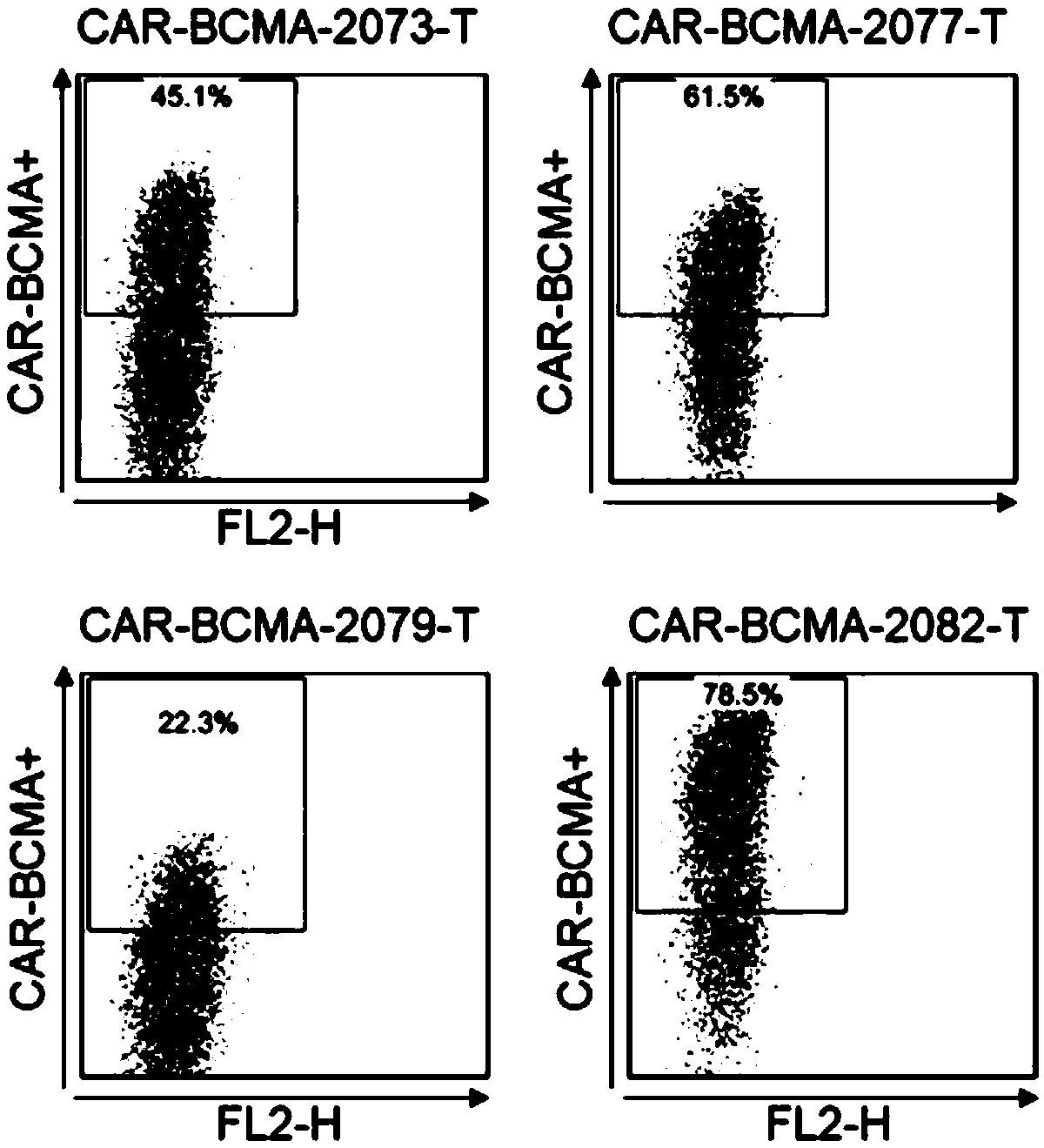
Antibody and chimeric antigen receptor for targeting BCMA protein, and drug
The invention discloses an antibody and a chimeric antigen receptor for targeting a BCMA protein, and a drug, and relates to the technical field of cellular immunotherapy. The antibody comprises a heavy chain CDR region represented by SEQ ID NO.22-24 and a light chain CDR region represented by SEQ ID NO.30-32, or comprises a heavy chain CDR region represented by SEQ ID NO.54-56 and a light chain CDR region represented by SEQ ID NO.62-64. The antibody has the ability to specifically bind to the BCMA protein, and the chimeric antigen receptor T cell prepared by using the antibody has a specifickilling effect on target cells positive for the BCMA protein, and can be used for preparing the drug for treating or preventing tumors.
Owner:BIORAY LABORATORIES INC
Expansion of lymphocytes with a cytokine composition for active cellular immunotherapy
The present invention relates to a composition for expanding lymphocytes comprising at least two types of cytokines selected from interleukin 2 (IL-2), interleukin 15 (IL-15) and interleukin 21 (IL-21). It further relates to a Method of preparing a population of clinically relevant lymphocytes, comprising the steps of: obtaining a body sample from a mammal in particular a tissue sample or body liquid sample, comprising at least one lymphocyte and optionally separating the cells in the body sample, culturing the body sample in-vitroto expand and / or stimulate lymphocytes in the sample wherein the culturing comprises using IL-2, IL-15 and / or IL-21, and optionally determining the presence of clinically relevant lymphocyte in the cultured sample.The present invention also relates to an immunotherapy and the population of clinically relevant lymphocytes.
Owner:保利比奥斯博特有限责任公司
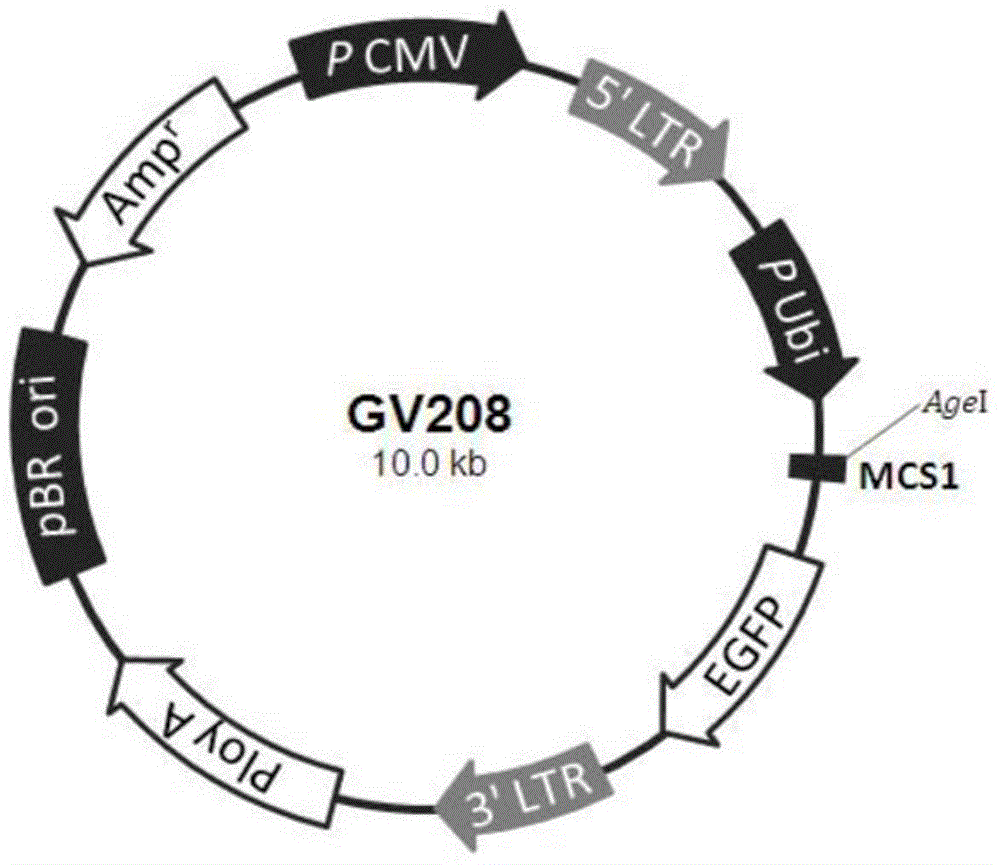
Recombinant lentivirus and application thereof
ActiveCN106749675ASignificant in vivo and in vitro amplificationSignificant tumor killing effectMammal material medical ingredientsImmunoglobulinsAbnormal tissue growthMicro environment
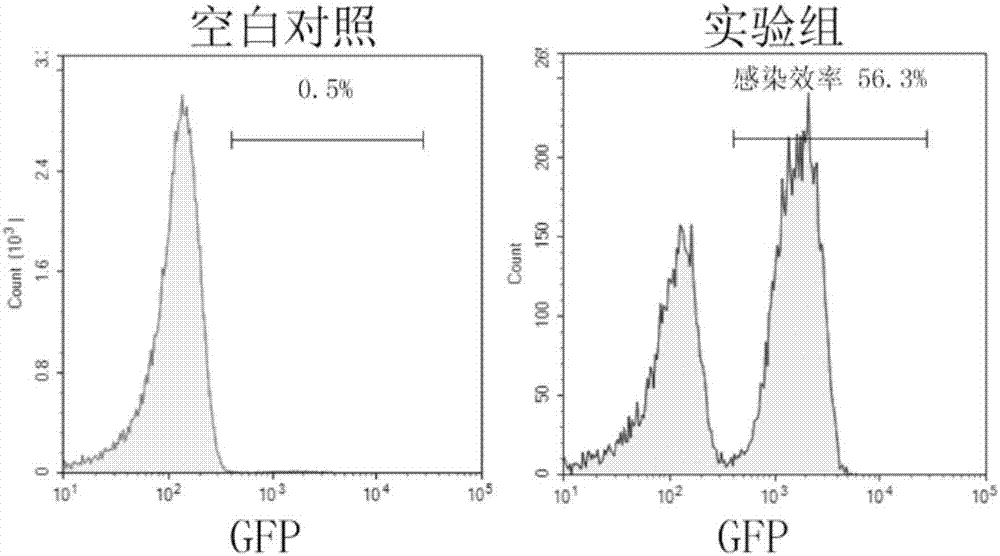
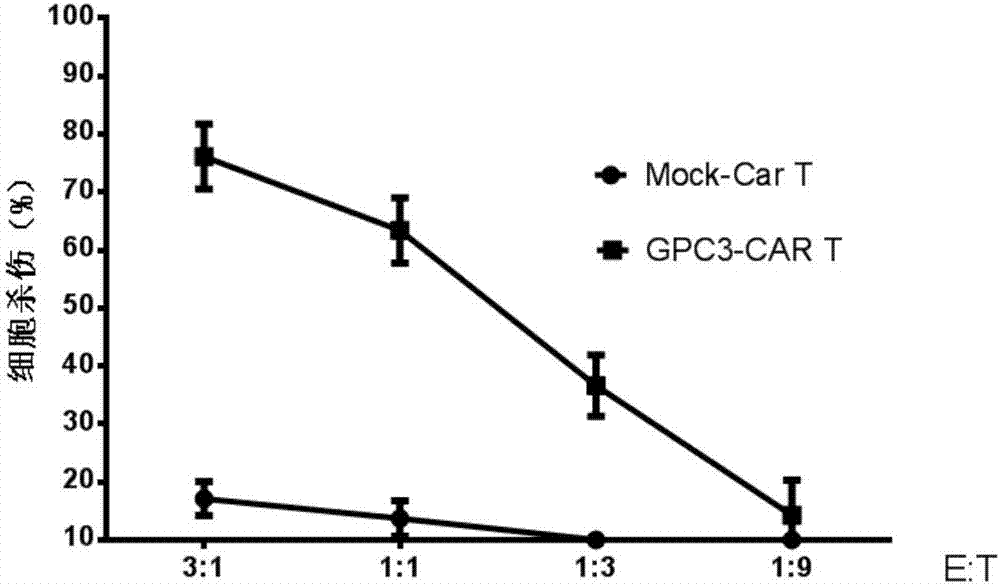
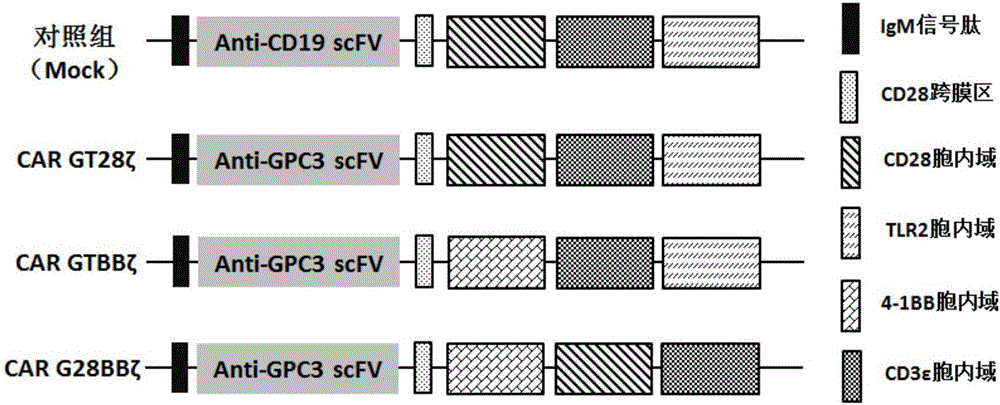
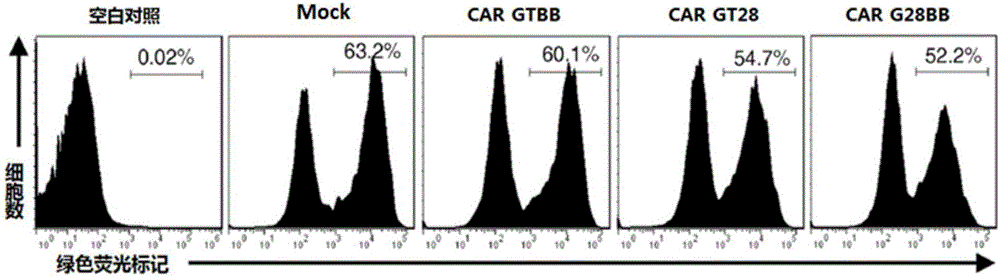
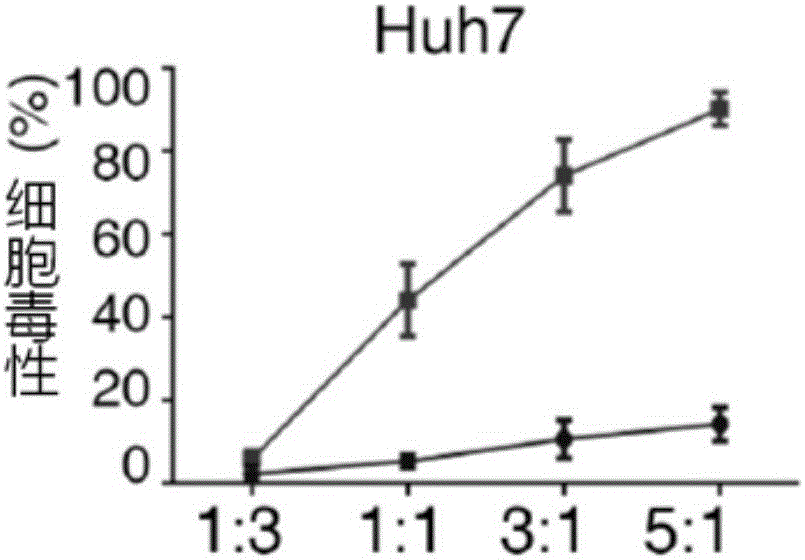
The invention relates to the field of tumor cellular immunotherapy, and in particular relates to a recombinant lentivirus and application thereof. The recombinant lentivirus comprises a chimeric antigen receptor, wherein the chimeric antigen receptor mainly comprises signal peptide, an antigen recognition domain, a transmembrane domain, an intracellular co-stimulation signal transduction domain and a CD3 zeta signal transduction domain which are serially connected; the intracellular co-stimulation signal transduction domain mainly comprises a human TLR2 (Toll Like Receptor 2) intracellular domain. A GPC3 CAT T (Glypican 3 CAT T) cell prepared from the recombinant lentivirus has an intense cell killing effect on liver cancer cells, a Th1 cell factor can be highly expressed, a tumor killing effect caused by non-CAR T (Chimeric Antigen Receptor T) cell can be stimulated to the maximum extent, escape and potential reoccurrence risk of GPC 3-tumor cells can be effectively prevented, the tumor cells can be killed by T cells expressing the chimeric antigen receptor, normal tissue can be slightly damaged, a tumor immunosuppression micro environment can be broken through, and thus a relatively good treatment effect on solid tumor can be achieved.
Owner:SHENZHEN IN VIVO BIOMEDICINE TECH LTD
Replacement of cytotoxic preconditioning before cellular immunotherapy
ActiveUS20180296572A1Improve patient outcomesReduce needOrganic active ingredientsMammal material medical ingredientsMedicineLymphocyte
Provided herein are novel therapeutic compositions and methods that keep cellular immunotherapies in the circulation or at the site of injection for extended periods of time without resorting to the use of cytotoxic preconditioning. More specifically the compositions and methods herein lymphodeplete and reduce or ablate sites in the secondary lymphatics where the cellular immunotherapy is bound and sequestered, without the use of cytotoxic preconditioning.
Owner:AVM BIOTECH
CE7-specific redirected immune cells
InactiveUS20060246548A1Antibody mimetics/scaffoldsGenetic material ingredientsDNA constructCell Surface Proteins
Owner:CITY OF HOPE
Drug regulated transgene expression
ActiveUS20170152297A1Proven safety recordImproved profileVirusesPeptide/protein ingredientsNucleotideTransmembrane domain
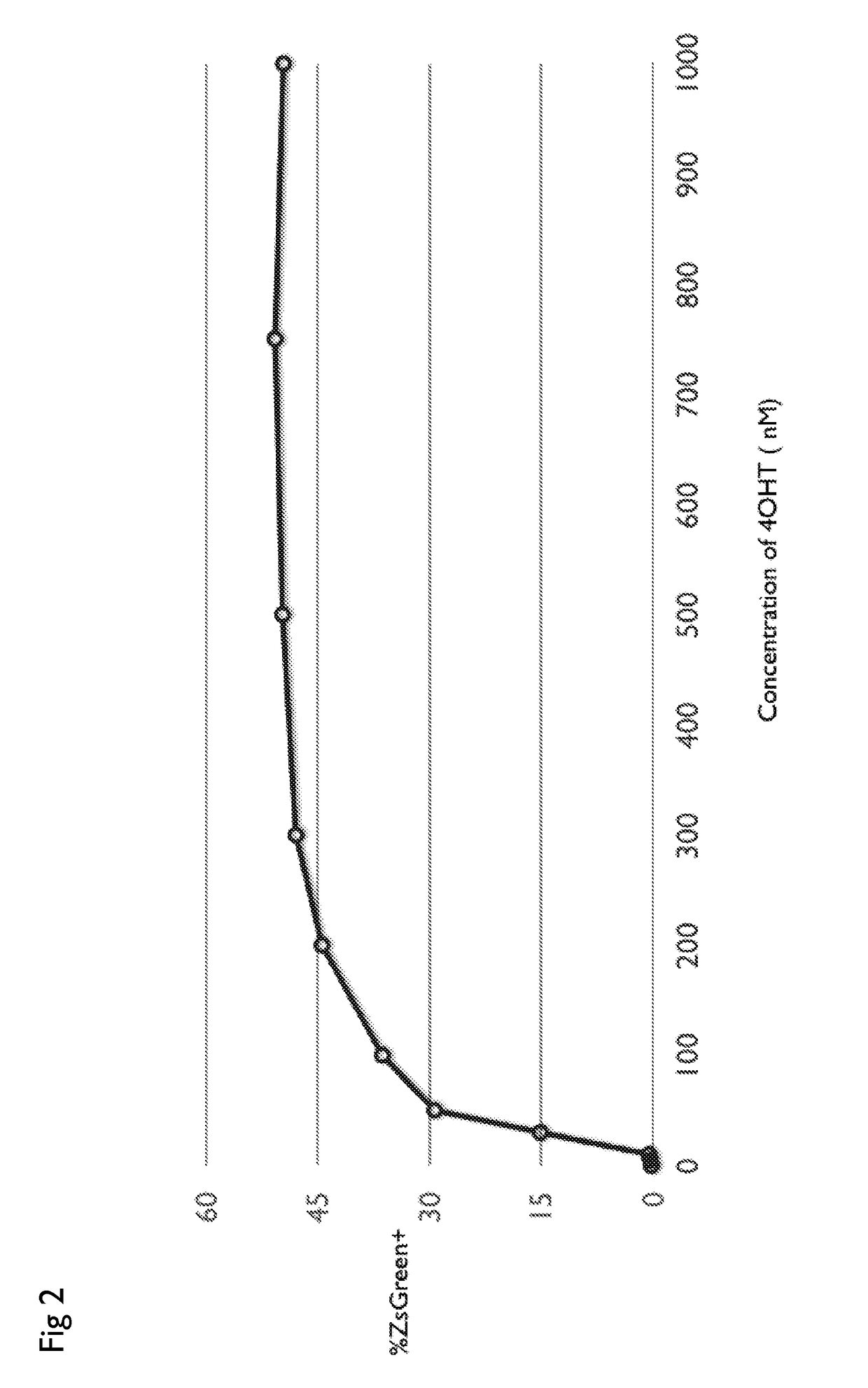
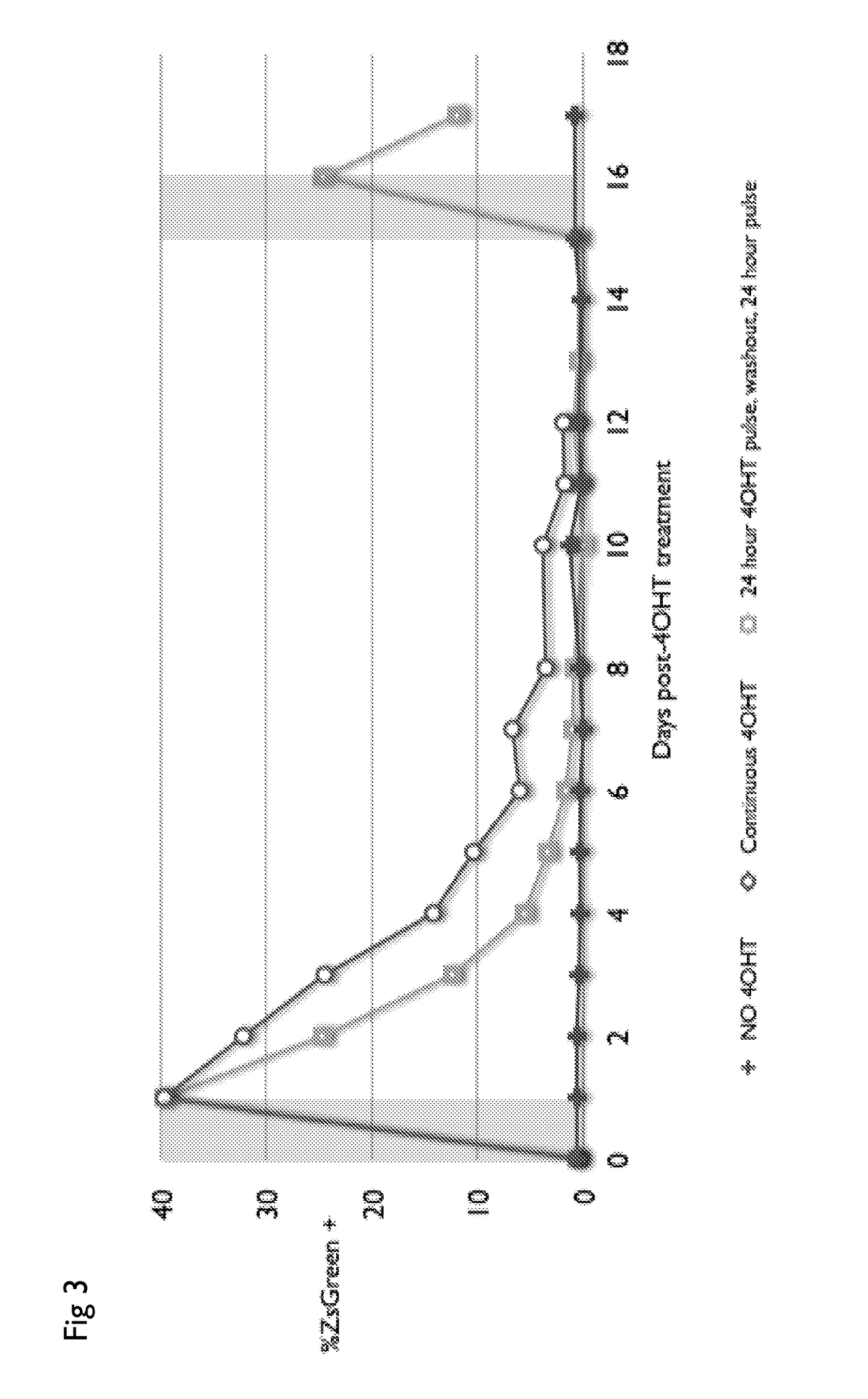
The present invention provides nucleic acids, vectors, host cells, methods and compositions to confer and / or augment immune responses mediated by cellular immunotherapy, such as by adoptively transferring CD8+ central memory T cells or combinations of central memory T cells with CD4+ T cells that are genetically modified to express a chimeric receptor under the control of an inducible promoter. In some alternatives the genetically modified host cell comprises a nucleic acid comprising a polynucleotide coding for a chimeric antigen receptor comprising a ligand binding domain, a polynucleotide comprising a spacer region, a polynucleotide comprising a transmembrane domain, and a polynucleotide comprising an intracellular signaling domain under the control of a drug inducible promoter. Controlling the expression of the chimeric receptor provides for the ability to turn expression on and off depending on the status of the patient. Pharmaceutical formulations produced by the method, and methods of using the same, are also described.
Owner:SEATTLE CHILDRENS HOSPITAL (DBA SEATTLE CHILDRENS RES INST)
Method and compositions for cellular immunotherapy
ActiveUS20180200298A1Efficient activationPeptide/protein ingredientsAntibody mimetics/scaffoldsIn vivoTransmembrane domain
The present invention provides nucleic acids, vectors, host cells, methods and compositions to confer and / or augment immune responses mediated by cellular immunotherapy, such as by adoptively transferring CD8+ central memory T cells or combinations of central memory T cells with CD4+ T cells that are genetically modified to express a chimeric receptor. In embodiments the genetically modified host cell comprises a nucleic acid comprising a polynucleotide coding for a ligand binding domain, a polynucleotide comprising a customized spacer region, a polynucleotide comprising a transmembrane domain, and a polynucleotide comprising an intracellular signaling domain. It has been surprisingly found that the length of the spacer region can affects the ability of chimeric receptor modified T cells to recognize target cells in vitro and affects in vivo efficacy of the chimeric receptor modified T cells. Pharmaceutical formulations produced by the method, and methods of using the same, are also described.
Owner:SEATTLE CHILDRENS HOSPITAL +1
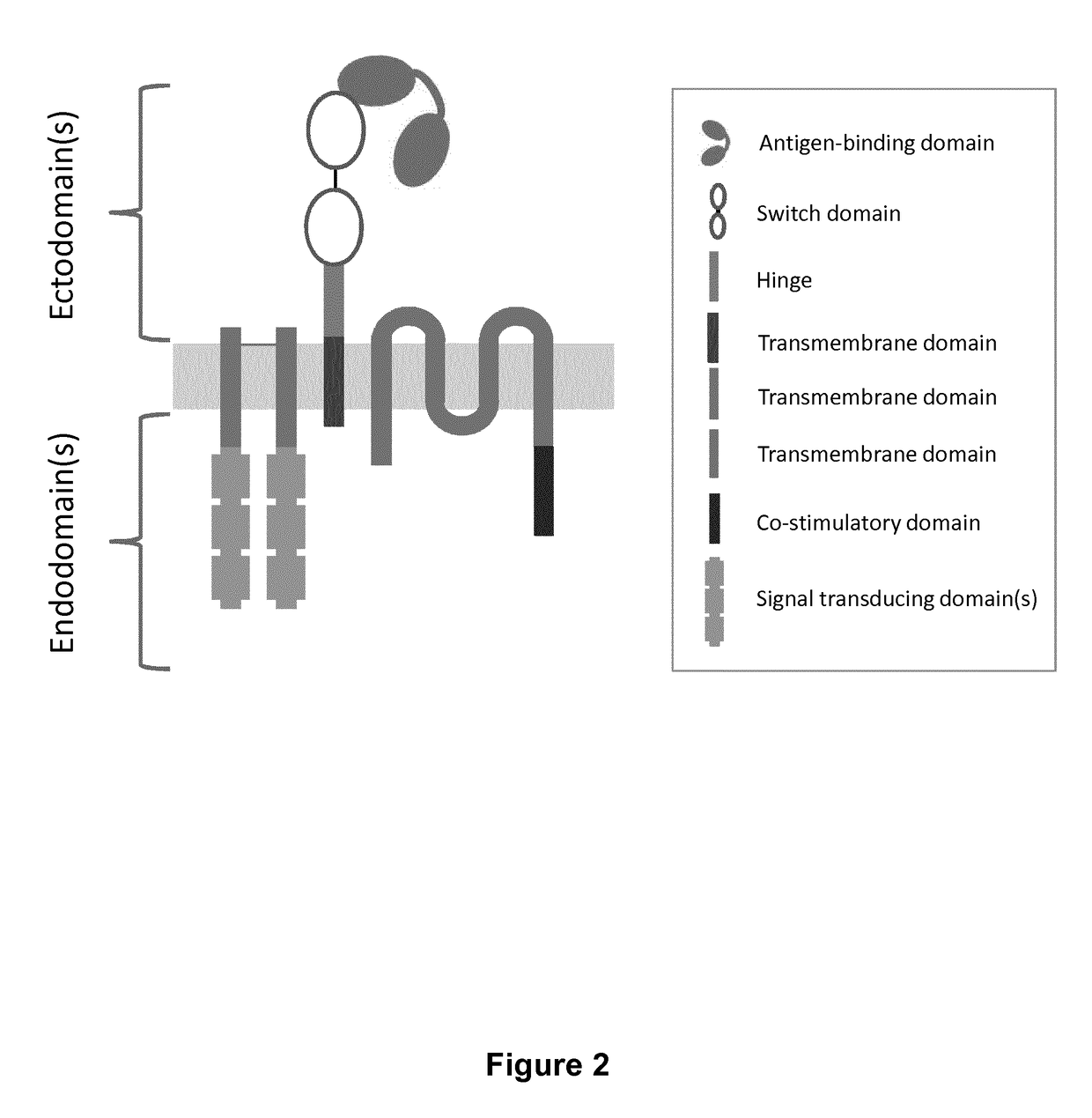
Chimeric antigen receptors with integrated controllable functions
ActiveUS20180237533A1Suppress mutationReliable functionPolypeptide with localisation/targeting motifOrganic active ingredientsAntigen receptorsT lymphocyte
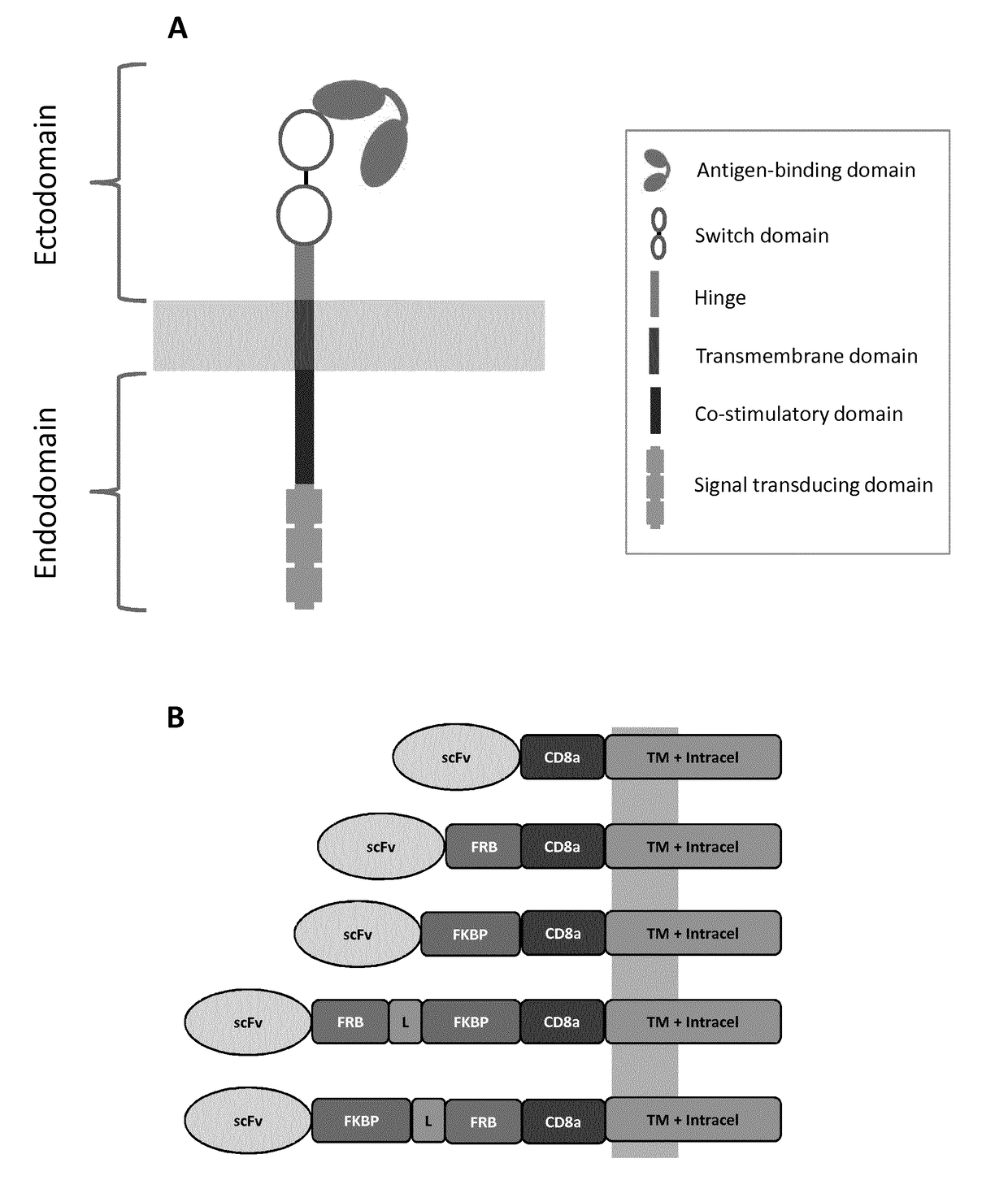
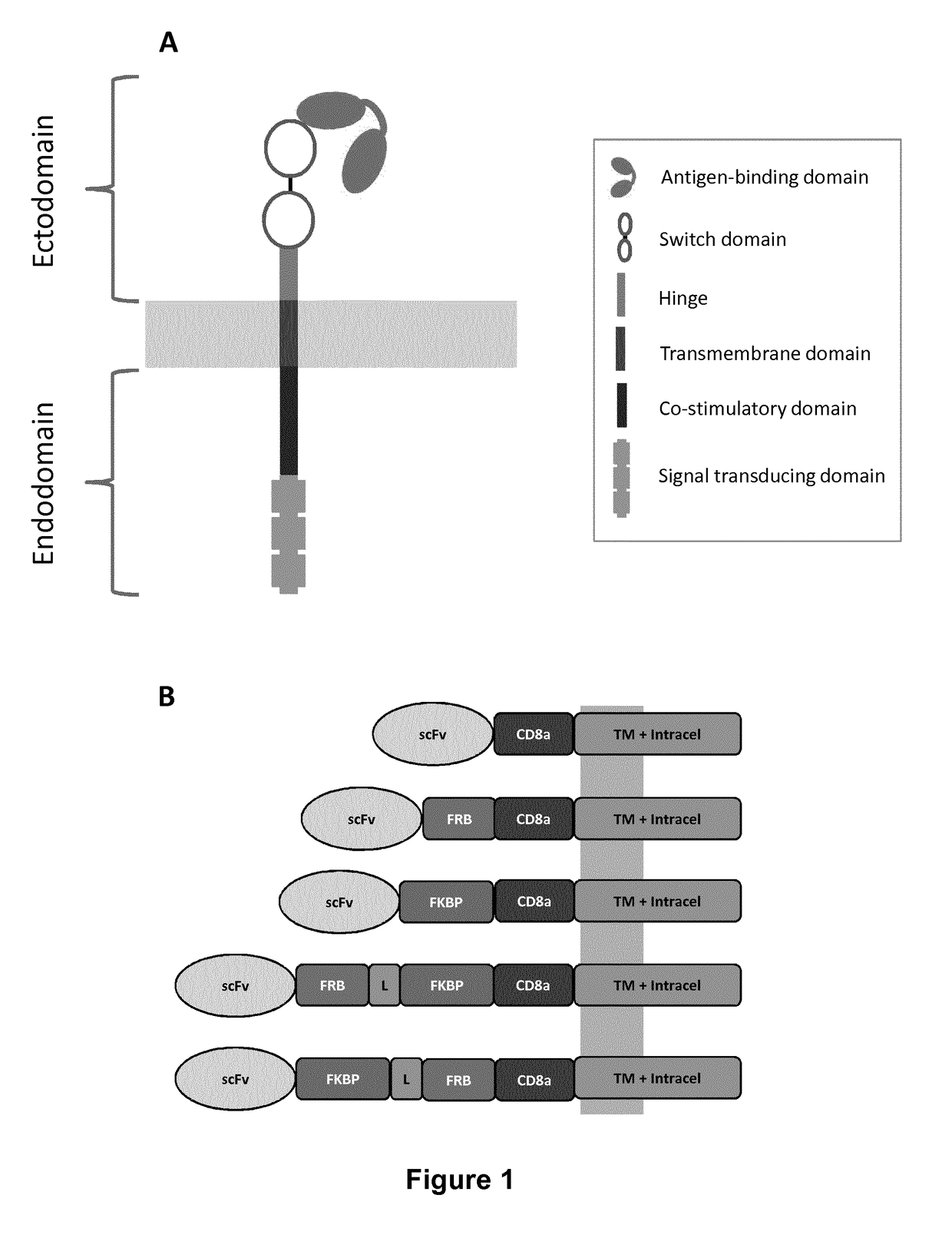
The present invention relates to the field of cell immunotherapy and more particularly to a new generation of chimeric antigen receptors (CAR), allowing the control of immune cells endowed with such CARs through the interaction with small molecules. More particularly, the present invention relates to chimeric antigen receptor which comprise in at least one ectodomain a molecular switch turning the antigen binding function of the receptor from an off to on state, and vice versa. The present invention thus provides more controlled and potentially safer engineered CAR endowed immune cells, such as T-lymphocytes.
Owner:CELLECTIS SA
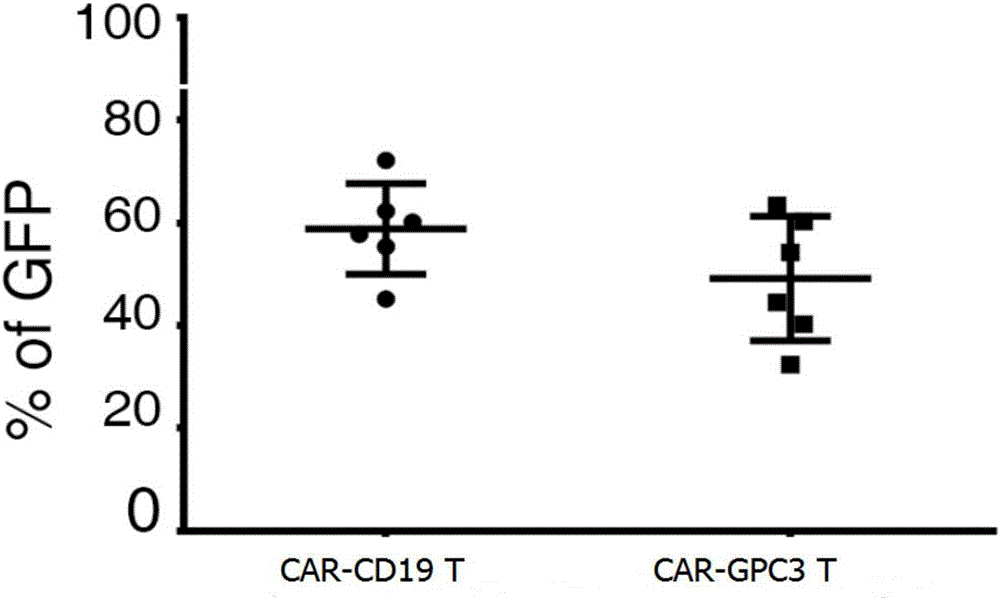
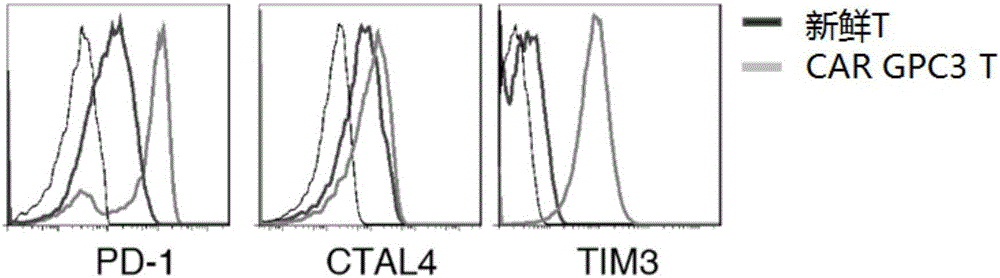
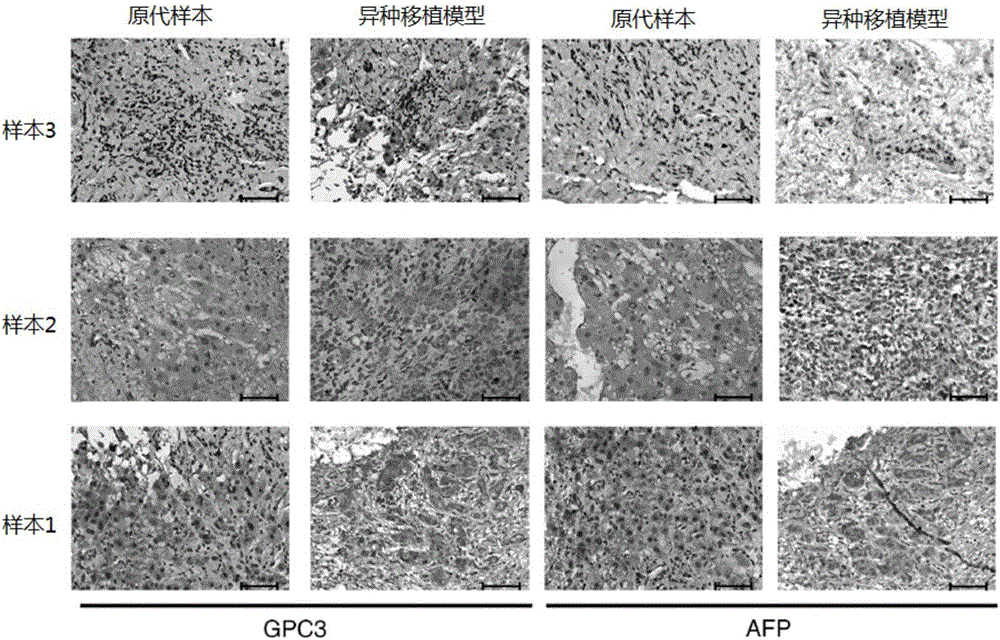
Model and method for detecting inhibiting effect of chimeric antigen receptor (CAR) T cells on hepatoma cells
InactiveCN106619719AStable supplyDetermining in vivo tumor killing abilityMammal material medical ingredientsAntineoplastic agentsCheck pointWilms' tumor
The invention relates to the field of cellular immunotherapy of tumors, in particular to a model and a method for detecting inhibiting effect of chimeric antigen receptor (CAR) T cells on hepatoma cells. The model is obtained by transplanting primary hepatoma tissues into a model living body, and transplanting the CAR T cells into the model living body. The model provided by the invention can be used for effectively simulating the tumor malignant degree of a patient, and assessing the inhibiting effect of the CAR T cells on hepatomas of different malignant degrees; the model can be used for effectively assessing the inhibiting effect of the expression of immunosuppression check point molecules in primary hepatoma cells on the CAR T cells.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
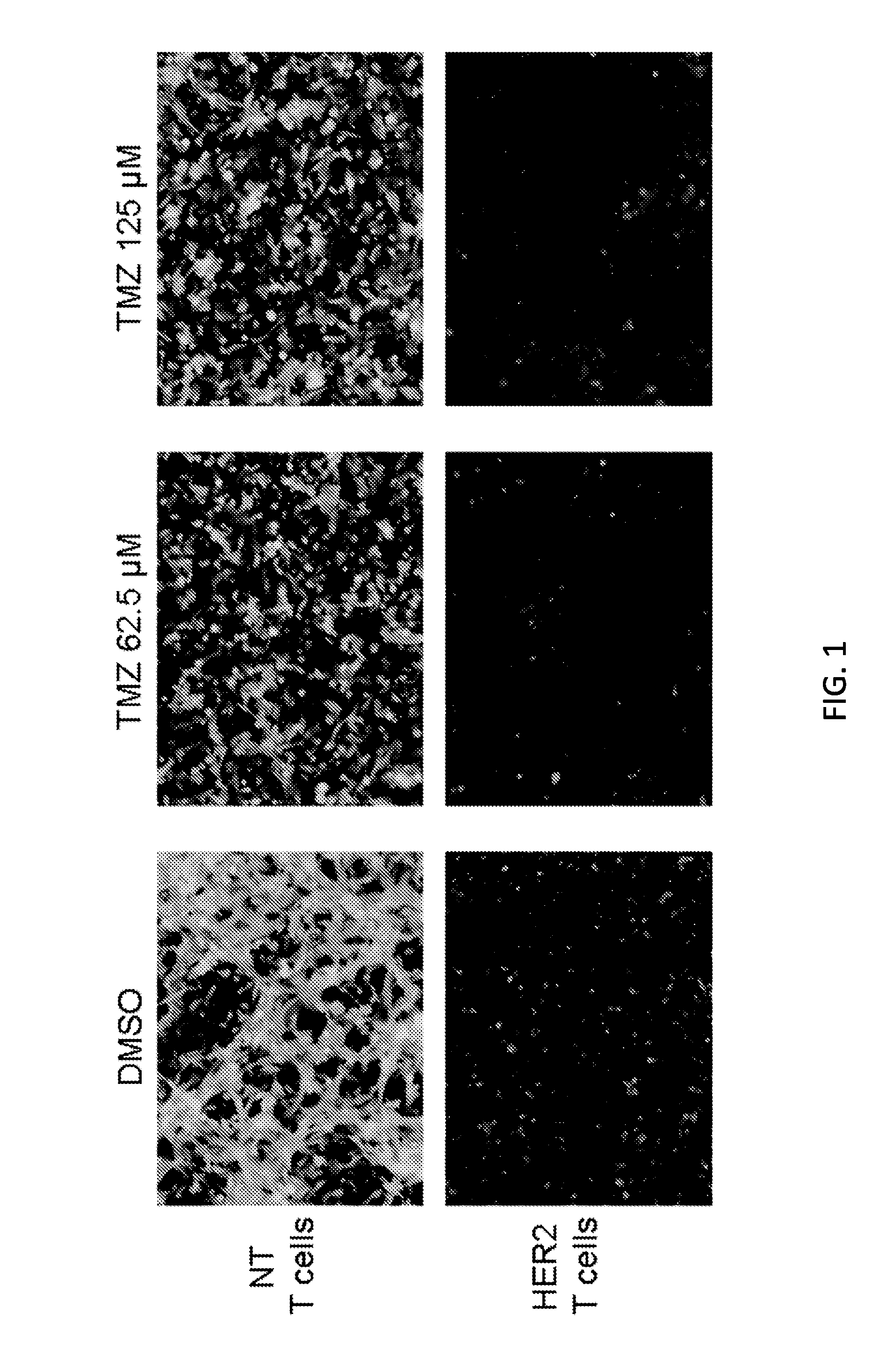
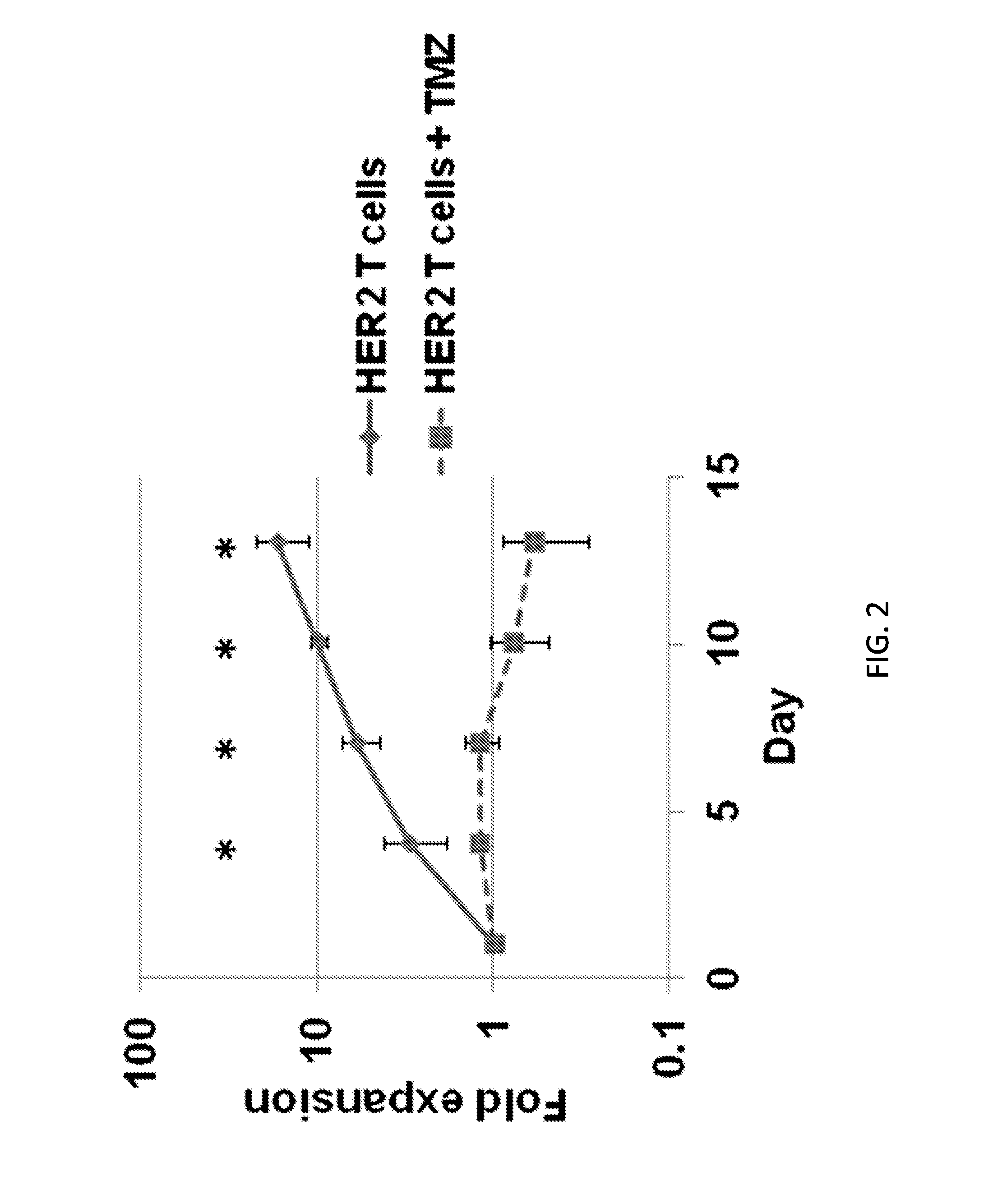
Chemotherapy-resistant immune cells
InactiveUS20160024175A1Effective immunotherapeutic responseOrganic active ingredientsVirusesTumor antigenT cell
Embodiments of the disclosure include compositions and methods useful for treating cancers that are sensitive to a chemotherapy, such as temozolomide. The methods allow effective cell immunotherapy to be used with chemotherapy when the cell immunotherapy is susceptible to being rendered ineffective by the chemotherapy. In specific aspects, the cancer is being treated by temozolomide (TMZ) and tumor antigen-specific T-cells that are resistant to TMZ.
Owner:BAYLOR COLLEGE OF MEDICINE
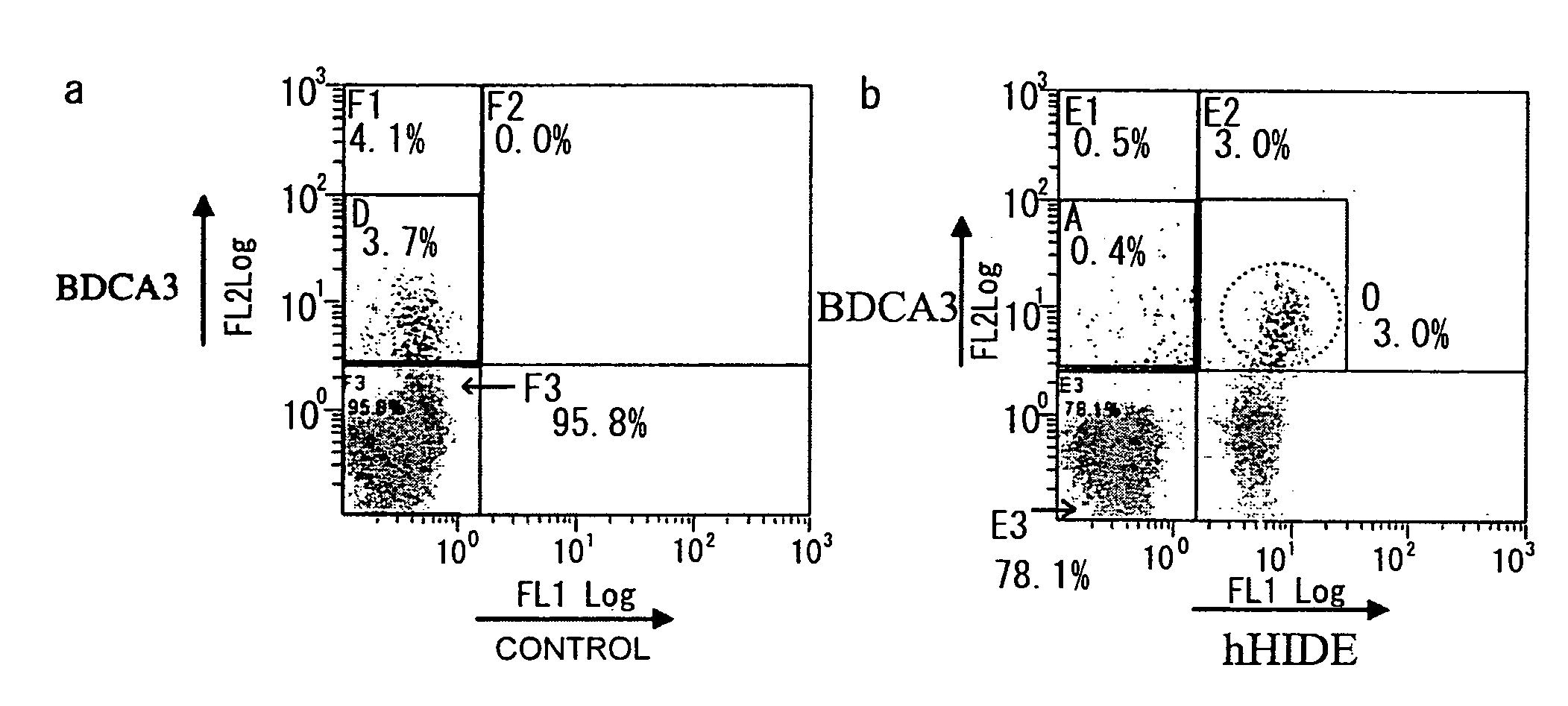
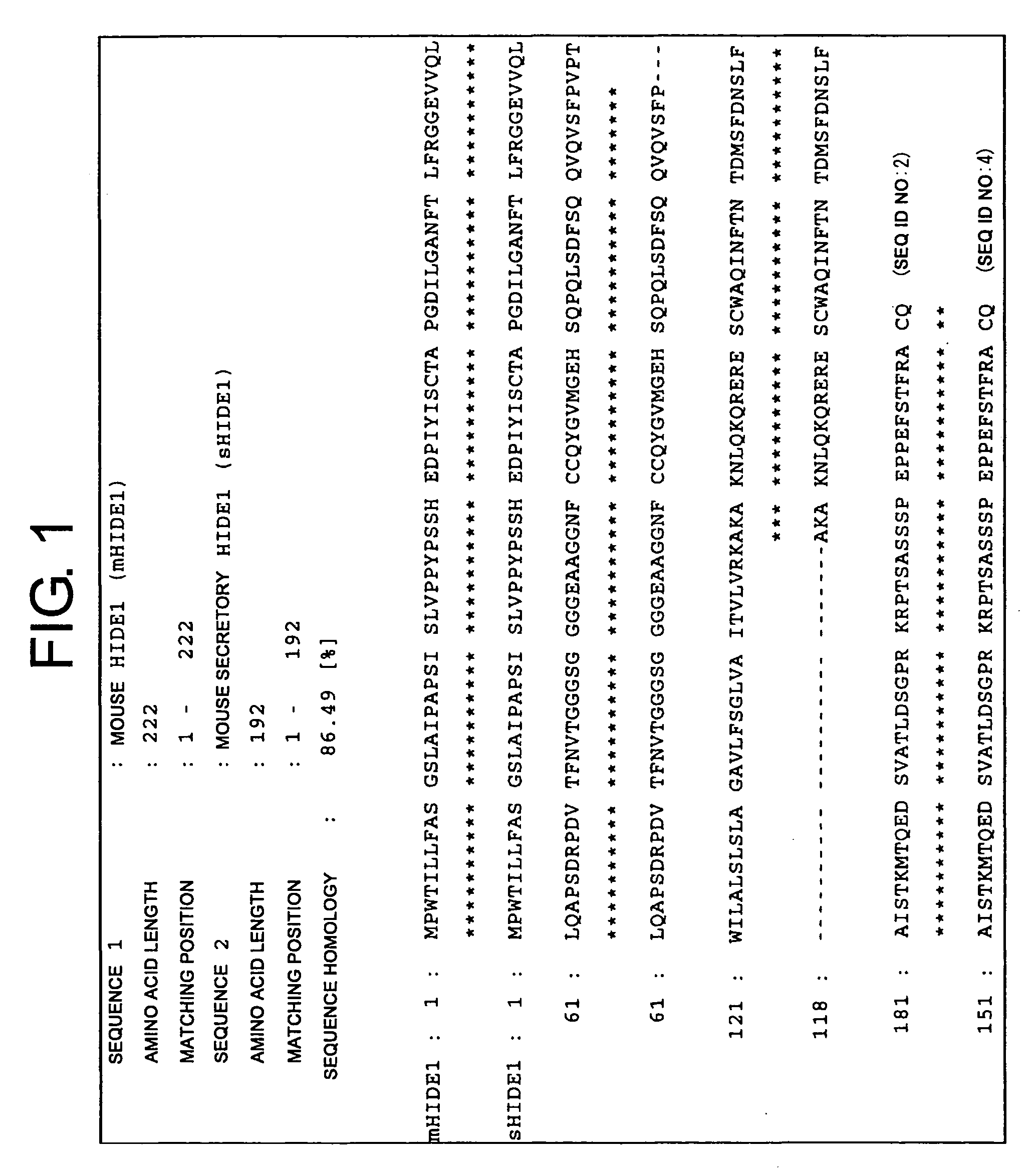
Methods for Isolating Monocytes
InactiveUS20080248011A1Increase opportunitiesBiocideNervous disorderCell sorterCell Membrane Proteins
The present invention provides HIDE1 as novel monocyte markers. Since HIDE1 are membrane proteins, monocytes can be specifically detected by using antibodies that bind to HIDE1. Further, HIDE1-positive monocytes can also be collected from peripheral blood or the like using a cell sorter, magnet, or such. Monocytes that can be prepared based on the present invention are useful in cell immunotherapy.
Owner:MEDICAL & BIOLOGICAL LAB CO LTD
BCMA antibody, chimeric antigen receptor and drug
ActiveCN109265550AAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsHeavy chainBCMA Protein
The invention discloses a BCMA antibody, a chimeric antigen receptor and a drug, and relates to the technical field of cellular immunotherapy. The BCMA antibody provided by the invention has a heavy chain CDR region shown in SEQ ID NO. 6-8 and a light chain CDR region shown in SEQ ID NO. 14-16, or has a heavy chain CDR region shown in SEQ ID NO. 38-40 and a light chain CDR region shown in SEQ ID NO. 46-48. The BCMA antibody has the ability to specifically bind a BCMA protein, and a chimeric antigen receptor T cell prepared by using the BCMA antibody has a specific killing effect on a target cell positive for the BCMA protein and can be used to prepare drugs for treating or preventing tumors.
Owner:EAST CHINA NORMAL UNIV +1
Chimeric antibody and immunocyte
InactiveCN102120772ASpecific immune functionSpecific cleavageMicroorganismsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenSpecific immunity
The invention discloses a chimeric antibody and an immunocyte. The chimeric antibody provided by the invention has an amino acid sequence containing a target antibody, a transmembrane domain segment of a HLA-A2 (Human Leukocyte Antigen-A2) molecule and an intracellular domain segment of a CD3 compound protein zeta chain in sequence from a terminal N to a terminal C. The chimeric antibody can be expressed on the surfaces of any cells, when the chimeric antibody is expressed on the surface of an immunocyte, the immunocyte is endowed with a specific immunity by virtue of the target antibody on the chimeric antibody, and thus, the chimeric antibody provided by the invention can be specifically combined with a plurality of antigens along with different target antibodies in the chimeric antibody. The chimeric antibody, a single-domain antibody and an immunocyte for expressing the chimeric antibody, provided by the invention, are about to have broad application prospects in the cellular immunotherapy.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
CAR-T cytotoxicity indicating vector
ActiveCN106086078ARealize analysisDirect detection methodImmunoglobulin superfamilyMicrobiological testing/measurementInsertion sequenceLuciferases
The invention belongs to the field of cellular immunotherapy and particularly relates to a CAR-T cytotoxicity indicating vector. The cytotoxicity indicating vector is a recombined lentiviral vector and is composed of an original lentiviral vector and an insertion sequence. The insertion sequence is composed of a tumor-correlation target antigen coding gene, a connection peptide coding gene and a luciferase coding gene from the 5' terminal to the 3'terminal in sequence. After CAR-T cells and CAR-T cytotoxicity indicating cells are mixed, a tumor-correlation target antigen expressed by the indicating cells can perform target guiding on CAR-T for combination, the indicating cells are split, and luciferase is released. Due to the fact that split cells are located in supernatant and the un-split cells are located in precipitate, the number of spit cells and the number of un-split cells can be worked out according to the relation between the indicating cell number and the luciferase activity value by detecting the activity value of luciferase in the supernatant and the activity value of luciferase in the precipitate, the proportion of the split cells is calculated, and the CAR-T cytotoxicity is indicated.
Owner:济南宜明医疗科技有限公司
Application of gold platinum thermometal nano particles on preparation of cell immunotherapy accelerant
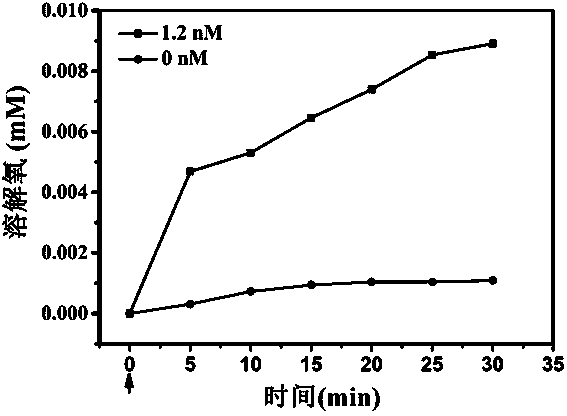
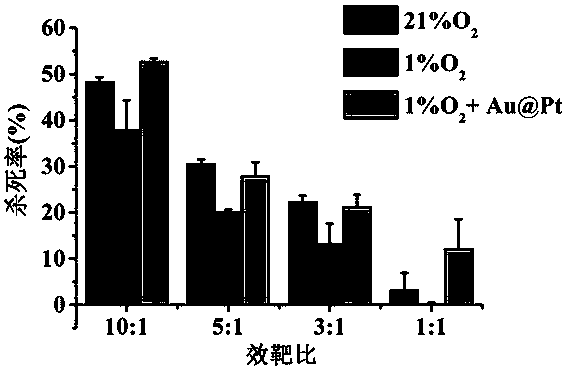
InactiveCN107648263AReduce inhibitionIncrease lethalityHeavy metal active ingredientsMammal material medical ingredientsPlatinumBiocompatibility Testing
The invention discloses an application of gold platinum thermometal nano particles on preparation of cell immunotherapy accelerant. According to the application, the hydrogen peroxide enzymatic activity of the gold platinum thermometal nano particles is used, the gold platinum thermometal nano particles can react with hydrogen peroxide in the tumour hypoxia microenvironment and generate oxygen andhave good biocompatibility, the gold platinum thermometal nano particles are combined with cell immunotherapy and used as accelerant of cell immunotherapy, so that the tumour hypoxia situation is improved, inhibition of the tumour hypoxia environment to the killing capacity of the immune cell is lowered, accordingly, the killing effect of the immune cell to the hypoxia tumour cell is remarkably improved, and the gold platinum thermometal nano particles have wide application prospects on tumour immunotherapy.
Owner:FUZHOU UNIV
PD-1 gene knockout MUC1-targeting CAR-T cell as well as preparation method and application of PD-1 gene knockout MUC1-targeting CAR-T cell
PendingCN112940137AStrong specificityImprove securityNucleic acid vectorImmunoglobulinsIntracellular signallingAntigen binding
The invention belongs to the technical field of cellular immunotherapy, and particularly relates to a PD-1 gene knockout MUC1-targeting CAR-T cell as well as a preparation method and application thereof. An MUC1-targeting chimeric antigen receptor is prepared firstly, wherein the MUC1-targeting chimeric antigen receptor comprises an antigen binding structural domain, a transmembrane structural domain, a co-stimulation structural domain and an intracellular signal transduction structural domain. Tn glycosylation MUC1 is used as a target spot, the target spot specificity is high, off-target is not likely to occur, and the safety of the CAR-T cells is improved. On the basis, the PD-1 gene knockout MUC1-targeting CAR-T cell is obtained, and after the PD-1 gene is knocked out and the CAR-T cell is transfused back into a body, the CAR-T cell failure and inactivation caused by PD-L1 expressed by tumors cannot occur, so that the efficient specific cell killing effect on tumor cells is achieved, and the curative effect of the CAR-T cell is improved.
Owner:GUANGZHOU ANJIE BIOMEDICAL TECH CO LTD
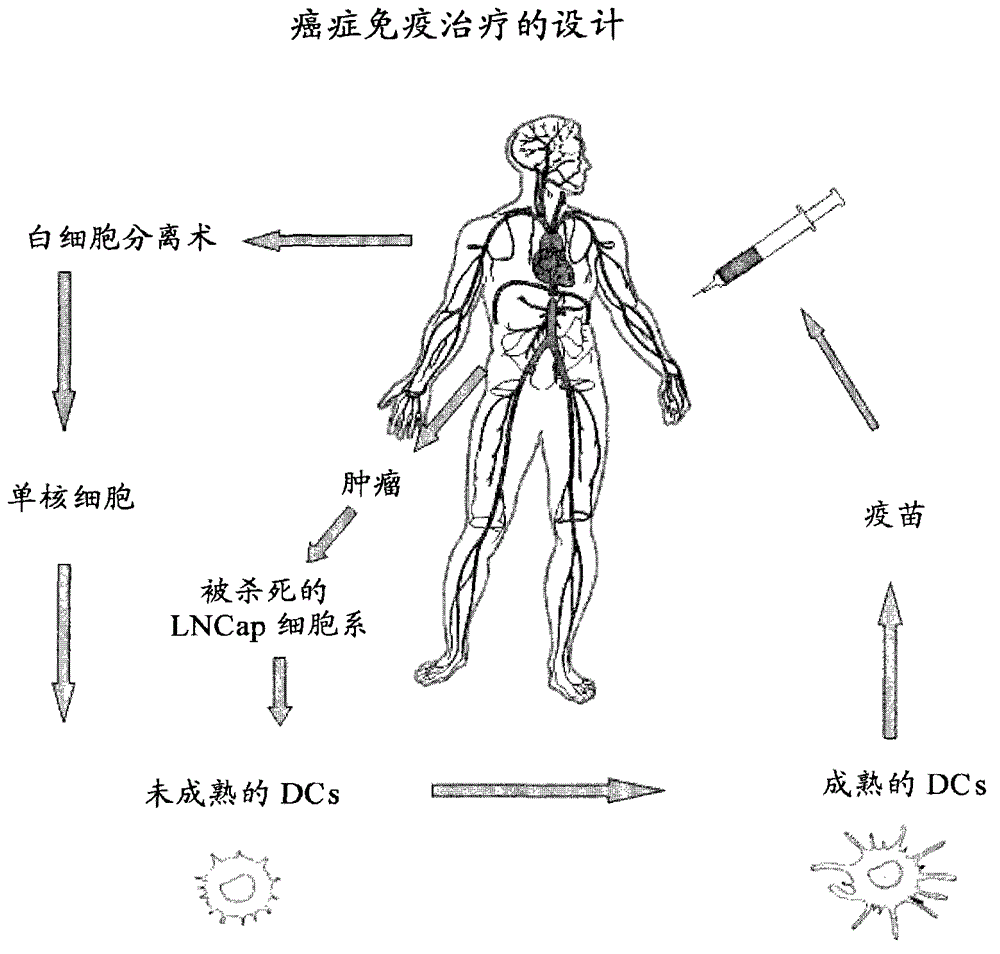
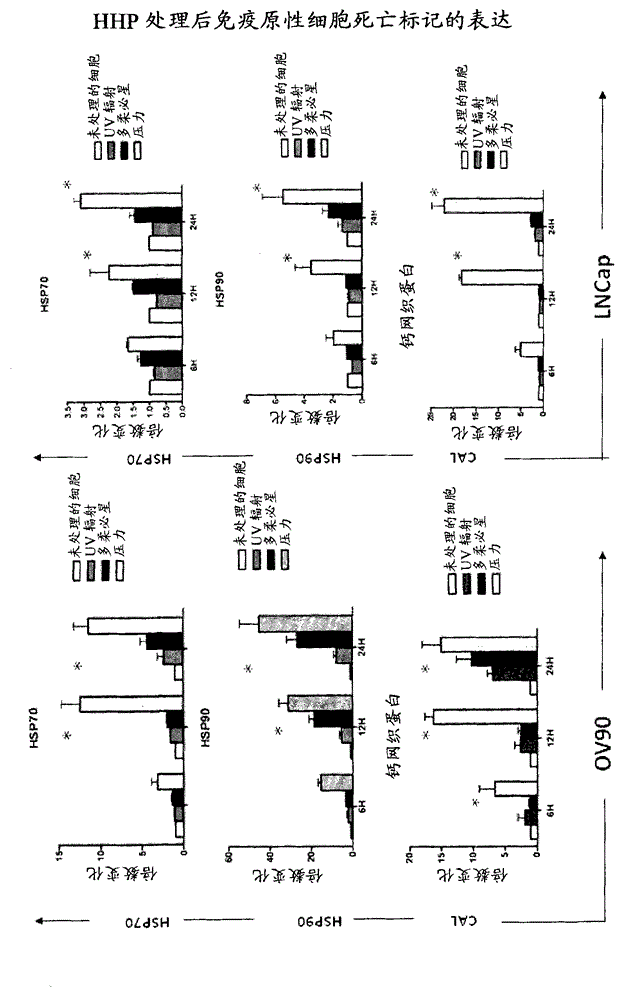
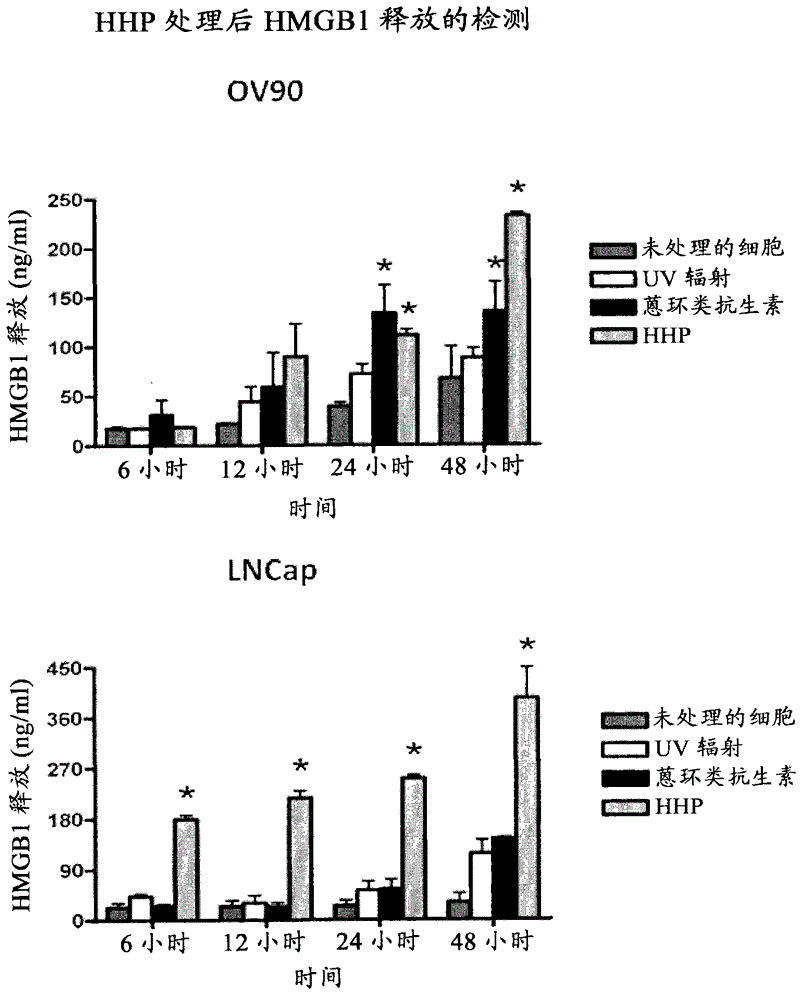
Means and methods for active cellular immunotherapy of cancer by using tumor cells killed by high hydrostatic pressure
InactiveCN102861103AEasy to controlSlow down or even stop progressMammal material medical ingredientsCancer antigen ingredientsDendritic cellHydrostatic pressure
Disclosed are pharmaceutical compositions for inducing an immune response against tumor cells comprising tumor cells which are made apoptotic by treatment with high hydrostatic pressure and dendritic cells, and methods for producing such compositions.
Owner:SOTIO AS
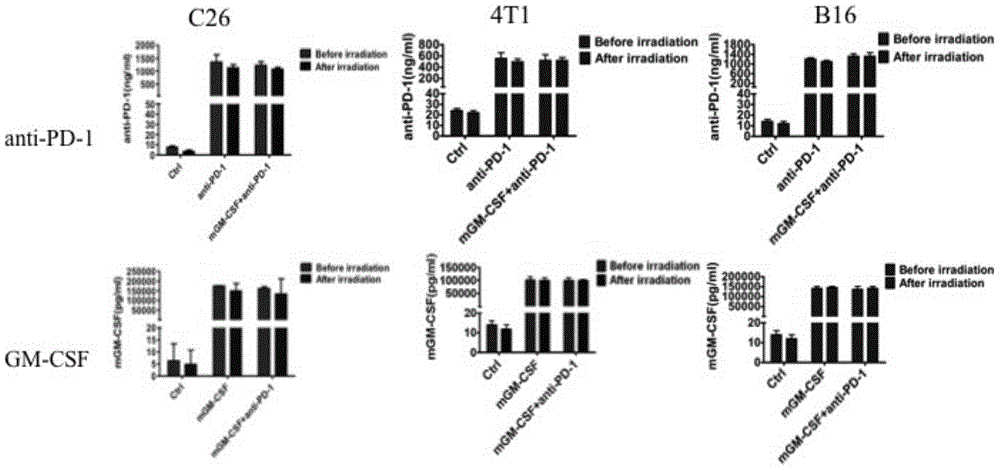
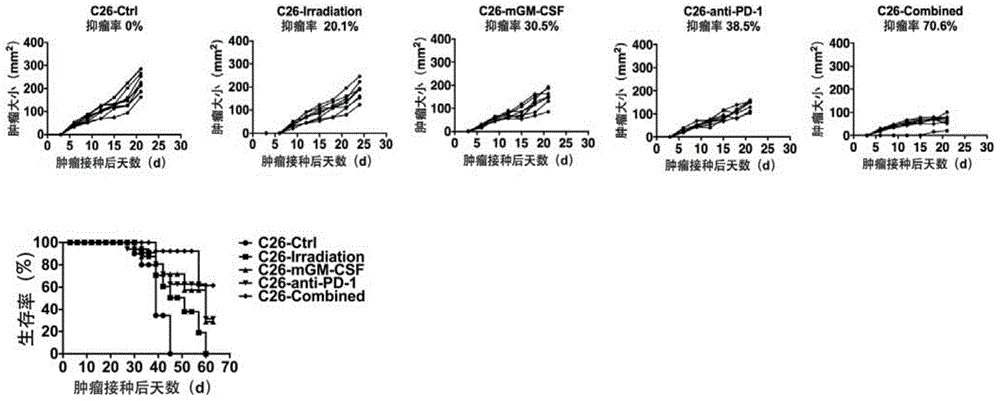
Tumor cell vaccine simultaneously secreting PD-1 neutralizing antibody and GM-CSF factor and preparation method thereof
InactiveCN105031630AGood anti-tumor effectLifting of immunosuppressionAntibody ingredientsImmunological disordersTreatment fieldTherapeutic effect
The invention discloses the field of tumor cellular immunotherapy and particularly relates to a tumor cell vaccine which is modified by a PD-1 neutralizing antibody with combination of a GM-CSF factor and a preparation method thereof. In the invention, tumor cellular vaccine therapy is creatively combined with antibody therapy which relieves tumor immune-suppression, so that the prepared tumor cell vaccine simultaneously secreting the PD-1 neutralizing antibody and the GM-CSF factor can not only relieve the immune-suppression status of a tumor micro-environment but also enhance an antitumor immune reaction. The tumor cell vaccine is good in antitumor treatment effect, is excellent in development value and provides a new approach of tumor cellular immunotherapy.
Owner:SICHUAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com





![gRNA for knockout of wild type T cell TCR [beta] strand and method gRNA for knockout of wild type T cell TCR [beta] strand and method](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6f8b222e-5a7b-41ca-b86a-4b04c7497be5/DEST_PATH_HDA0001417539750000011.png)
![gRNA for knockout of wild type T cell TCR [beta] strand and method gRNA for knockout of wild type T cell TCR [beta] strand and method](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6f8b222e-5a7b-41ca-b86a-4b04c7497be5/DEST_PATH_HDA0001417539750000012.png)
![gRNA for knockout of wild type T cell TCR [beta] strand and method gRNA for knockout of wild type T cell TCR [beta] strand and method](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6f8b222e-5a7b-41ca-b86a-4b04c7497be5/DEST_PATH_HDA0001417539750000013.png)