Expansion of lymphocytes with a cytokine composition for active cellular immunotherapy
A technology of lymphocytes and cytokines, applied in the field of cytokine composition and clinically relevant lymphocytes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
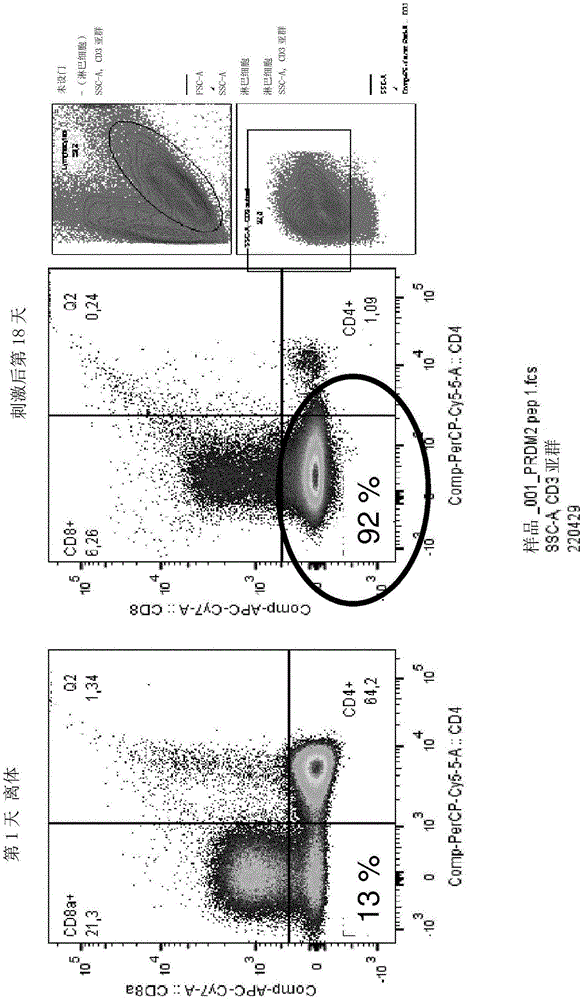
[0215] Example 1 - Lymphocyte Expansion Protocol Using Peripheral Blood Mononuclear Cells
[0216] A) Materials and Instruments
[0217] i) Instrument
[0218] 24-well plate, (Becton, Dickinson (BD), REF 353504)
[0219] sterile scalpel
[0220] sterile tweezers
[0221] Medimachine (Tissue Homogenizer), (BD, catalog number: 340588 )
[0222] Medicon, 50 μm, (BD, catalog number: 340591 )
[0223] Filcons, 200 μm (BD, catalog number: 340613 )
[0224] Laminar Flow Hood (Level 2 Biological Safety Hood)
[0225] Low temperature freezer, –80°C
[0226] refrigerator
[0227] centrifuge
[0228] ii) Supplies
[0229] gloves (latex)
[0230] lab coat
[0231] Sterile centrifuge tube, 15mL.
[0232] Pipet tips
[0233] Pipette aid
[0234] waste container
[0235] iii) Reagents
[0236] RPMI 1640, (Gibco, REF 61870-044)
[0237] Cellgro, (CellGenix, Cat: 20801-0100)
[0238] Mixed human AB serum (Innoative Research, IPLA-SerAB-13458)
[0239] Fetal bovine ser...
Embodiment 2
[0248] Example 2 Lymphocyte Expansion Protocol Using Peripheral Blood Mononuclear Cells
[0249] The procedure of Example 2 is the same as that of Example 1, except that the donor is a patient receiving NY-ESO-1 vaccination.
Embodiment 3
[0250] Example 3 - Lymphocyte Expansion Protocol Using Peripheral Blood Mononuclear Cells
[0251] Example 3 is the same as Example 1, except that the donor is a patient whose tumor already expresses TAA NY-ESO-1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com