Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
52 results about "Locally advanced" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Guidance and implantation of catheters
InactiveUS20100222668A1Ultrasonic/sonic/infrasonic diagnosticsSurgeryProstate cancerNavigation system
Owner:PEAK BIOSCI
Method of treating metastatic stage prostate cancer
ActiveUS20090203622A1Deterioration in survival rateImprove life expectancyPeptide/protein ingredientsPharmaceutical delivery mechanismDosing regimenRegimen
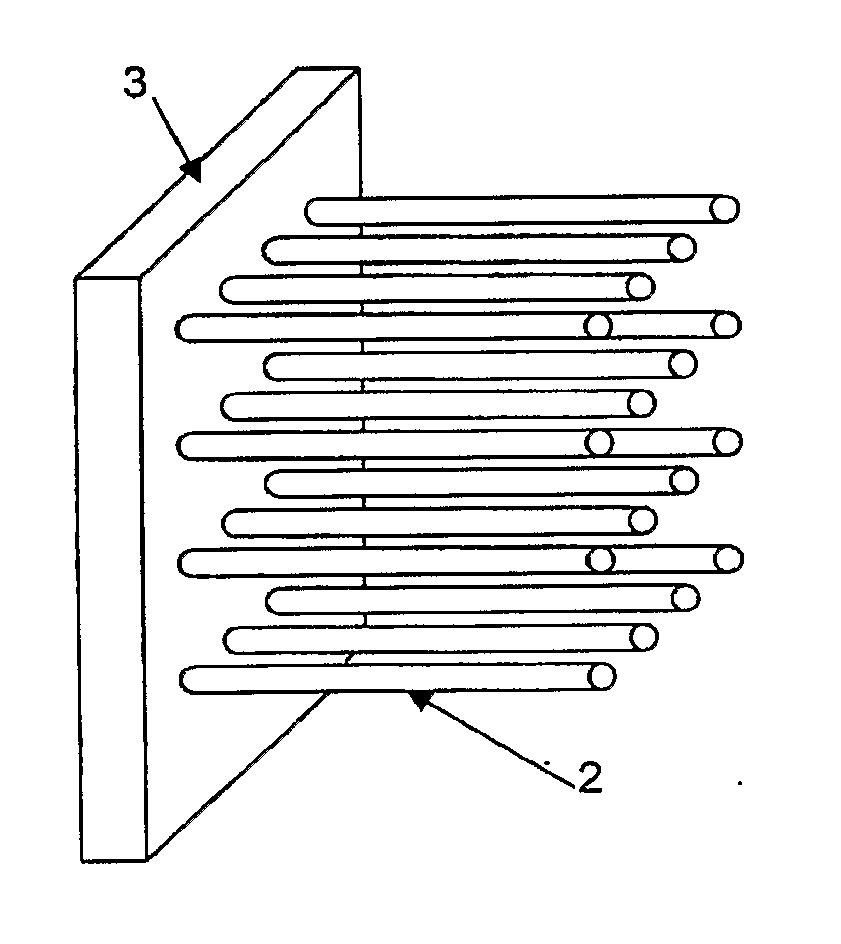
The invention provides methods and dosing regimens for treating metastatic stage prostate cancer in a subject using degarelix, as well as related methods of using degarelix in a subject identified as having metastatic stage prostate cancer, and methods of using degarelix to prevent or delay the progression of locally advanced prostate cancer.
Owner:FERRING INT CENT SA
Methods of treating metastatic breast cancer with 4-iodo-3-nitrobenzamide and irinotecan
Provided herein are methods, compositions and kits for the treatment of locally advanced or metastatic breast cancer or breast cancer brain metastases. The method comprises administration of 4-iodo-3-nitrobenzamide, a metabolite or salt thereof in combination with irinotecan. The method of treating locally advanced or metastatic breast cancer comprises at least one 21 day treatment cycle.
Owner:BIPAR SCI INC
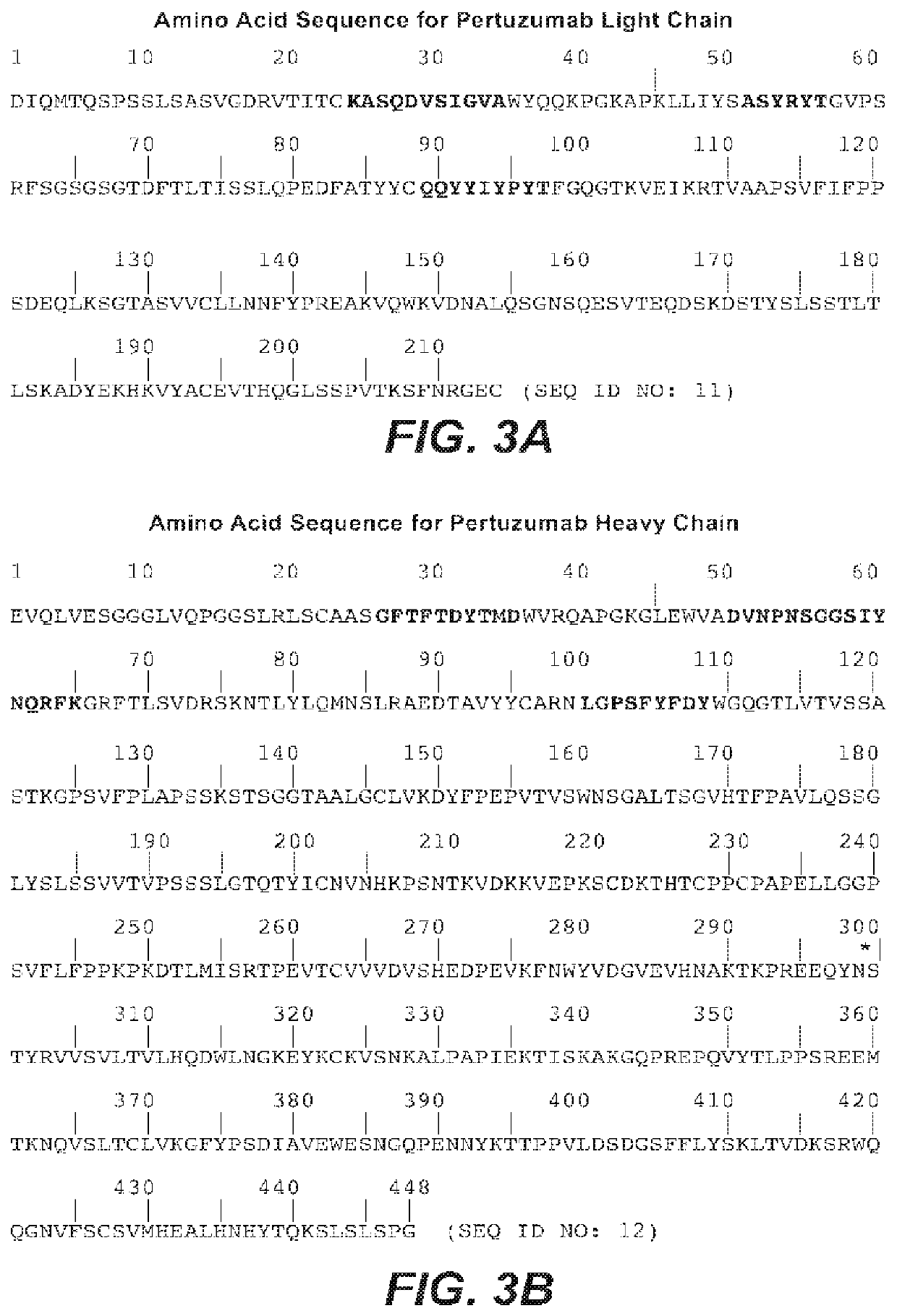
Methods of treating early breast cancer with trastuzumab-mcc-dm1 and pertuzumab
InactiveUS20170035907A1Improve responseExtension of timeOrganic active ingredientsAntibody ingredientsGynecologyEarly breast cancer
Methods of treating patients having HER2-positive, operable, locally advanced or inflammatory breast cancer with the antibody-drug conjugate Trastuzumab-MCC-DM1 and Pertuzumab are provided.
Owner:GENENTECH INC
Methods for the treatment of locally advanced breast cancer
ActiveUS20150224104A1Prevent relapseLengthening time of remissionBiocideBiological material analysisEnantiomerInflammatory breast cancer
Provided herein are methods of treating, preventing and / or managing locally advanced breast cancer, including inflammatory breast cancer, which comprise administering to a patient one or more immunomodulatory compounds or enantiomers or mixtures of enantiomers thereof, or pharmaceutically acceptable salts, solvates, hydrates, co-crystals, clathrates, or polymorphs thereof.
Owner:CELGENE CORP
Catheter and array for anticancer therapy
InactiveUS20100280494A1High mechanical strengthMulti-lumen catheterMedical syringesGlioblastomaWilms' tumor
A catheter array system adapted for implanting a plurality of catheters within the tissue of a patient in a spatially defined array, comprising a plurality of catheters, a catheter guide template adapted to guide the implantation of catheters, and a liquid supply system including a pressurizer and a manifold, is provided. A method of treatment of a malcondition in a patient comprises implantation of a spatially definted array of catheters using the system is also provided. The bioactive agent can be a radiotherapeutic agent, a chemotherapeutic agent, a protein, an antibody, an oligonucleotide-based therapeutic agent such as siRNA, or a combination of agents. A preferred radiotherapeutic agent is 123I- or 125I-IUDR, for example in the treatment of locally advanced tumors, such as glioblastoma multiforme.
Owner:MATSUURA JAMES E +2
Crystalline forms of erlotinib base and erlotinib hcl
InactiveUS20100004449A1Organic active ingredientsOrganic chemistry methodsPharmacologyNon small cell
Owner:TEVA PHARM USA INC
Rectal cancer preoperative concurrent neoadjuvant radiotherapy and chemotherapy effect evaluation system and method based on big data analysis MRI images
InactiveCN108922602AFast evaluationLong working hoursImage enhancementImage analysisNerve networkAdjuvant chemoradiotherapy
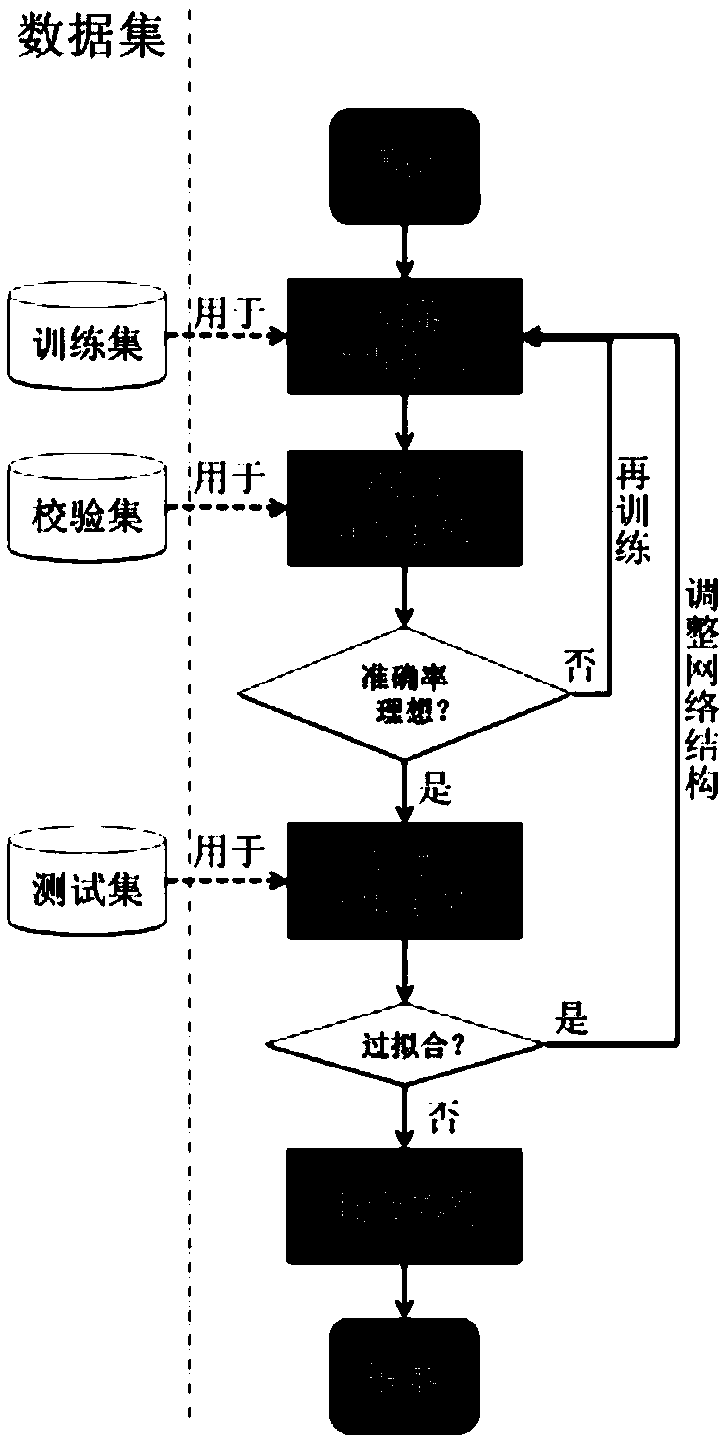
The invention relates to a rectal cancer preoperative concurrent neoadjuvant radiotherapy and chemotherapy effect evaluation system and method. The evaluation system comprises: an image acquisition unit for acquiring MRI images before neoadjuvant radiotherapy and chemotherapy for patients with the locally advanced rectal cancer, and dividing the rectal cancer patients into a training set, a checkset and a test sets as input image data; an image annotation unit for performing data annotation on MRI images of the training set, the check set and the test set; a convolutional neural network construction unit for constructing a first convolutional neural network model; and a convolutional neural network model training unit for acquiring a second convolutional neural network model for evaluating the rectal cancer preoperative concurrent neoadjuvant radiotherapy and chemotherapy effect. The rectal cancer preoperative concurrent neoadjuvant radiotherapy and chemotherapy effect evaluation system has many advantages such as high accuracy, short time-consuming, long working duration, objective and three-dimensional result.
Owner:THE SIXTH AFFILIATED HOSPITAL OF SUN YAT SEN UNIV
Marker for locally advanced esophageal squamous cell carcinoma prognosis and application of marker
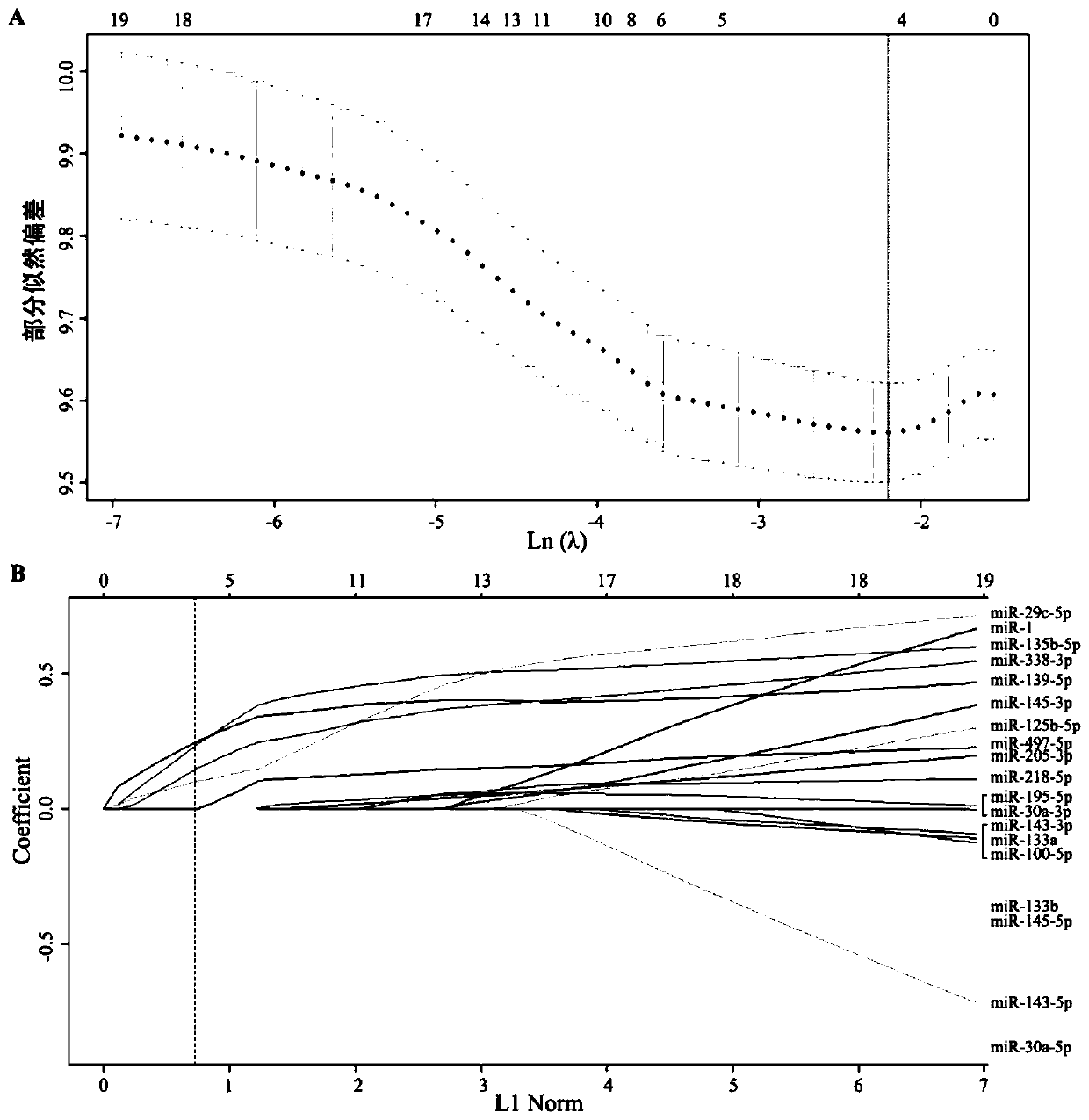
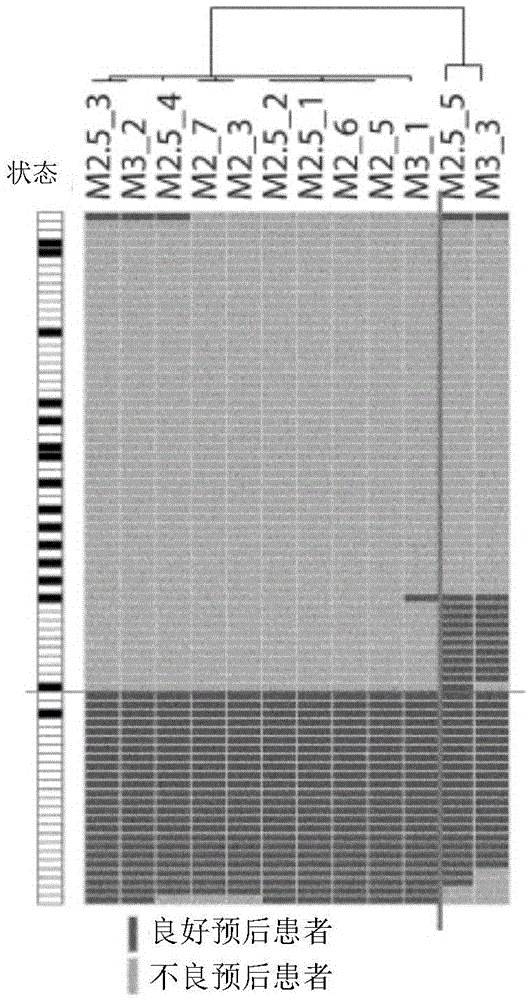
ActiveCN109897899ARealize individualized treatmentImprove treatmentHealth-index calculationMicrobiological testing/measurementLife qualitySquamous Carcinomas
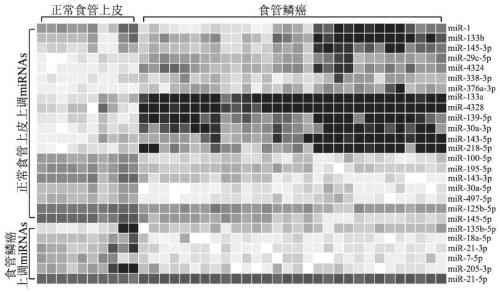
The invention relates to the field of bioengineering and tumor markers, in particular to a marker for locally advanced esophageal squamous cell carcinoma prognosis and an application of the marker. The marker is a combined marker consisting of one or more of miR-135b-5p, miR-139-5p, miR-29c-5p and miR-338-3p, and locally advanced esophageal squamous cell carcinoma prognosis is predicted by detecting expression levels of the four miRNA in tumor tissue and performing calculation according to a formula (0.4690*miR-135b-5p expression level)+(0.3839*miR-139-5p expression level)+(0.1733*miR-29c-5p expression level)+(0.3368*miR-338-3p expression level). The combined marker has the advantages of good stability and high sensitivity and specificity and can more accurately and more valuably evaluateprognosis of patients than traditional clinical pathological factors such as TNM (tumor-node-metastasis) staging and the like. On one hand, the molecular marker related to esophageal squamous cell carcinoma prognosis is provided, on the other hand, an esophageal squamous cell carcinoma prognosis prediction model is established, so that individual treatment of esophageal squamous cell carcinoma isrealized, the comprehensive treatment level of the esophageal squamous cell carcinoma is improved, the life quality of esophageal squamous cell carcinoma patients is improved, and the lifetime of theesophageal squamous cell carcinoma patients is prolonged.
Owner:SUN YAT SEN UNIV CANCER CENT
System for predicting prognosis of locally advanced gastric cancer
The present invention relates to a novel system for predicting a prognosis capable of predicting the prognosis of locally advanced gastric cancer, and more specifically, capable of predicting a clinical result after a resection during gastric cancer surgery through gene set enrichment comparative analysis.
Owner:NOVOMICS CO LTD
Treatment of her2-positive breast cancer
InactiveUS20180134803A1Improve responseSignificant increase in in adverse eventsOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsHER2 Positive Breast CarcinomaAnthracycline
Methods for the treatment of HER2-positive breast cancer are provided by neoadjuvant administration of pertuzumab and trastuzumab in combination with anthracycline-based chemotherapy. In particular, the methods concerns the treatment patients with HER2-positive, locally advanced, inflammatory, or early-stage breast cancer by neoadjuvant administration of pertuzumab and trastuzumab following anthracycline-based chemotherapy, wherein the combined administration of pertuzumab and trastuzumab increases pathological complete response (pCR) relative to administration of trastuzumab as a single agent, without significant increase in adverse events, such as cardiac toxicity, relative to neoadjuvant anthracycline-based chemotherapy.
Owner:F HOFFMANN LA ROCHE & CO AG
System for predicting prognosis of locally advanced gastric cancer
ActiveCN105431737APredict clinical outcomeMicrobiological testing/measurementBiological testingCancer surgerySurgery
The present invention relates to a novel system for predicting a prognosis capable of predicting the prognosis of locally advanced gastric cancer, and more specifically, capable of predicting a clinical result after a resection during gastric cancer surgery through gene set enrichment comparative analysis.
Owner:NOVOMICS CO LTD
Methods for the treatment of locally advanced breast cancer
Owner:CELGENE CORP
Guidance and implantation of catheters
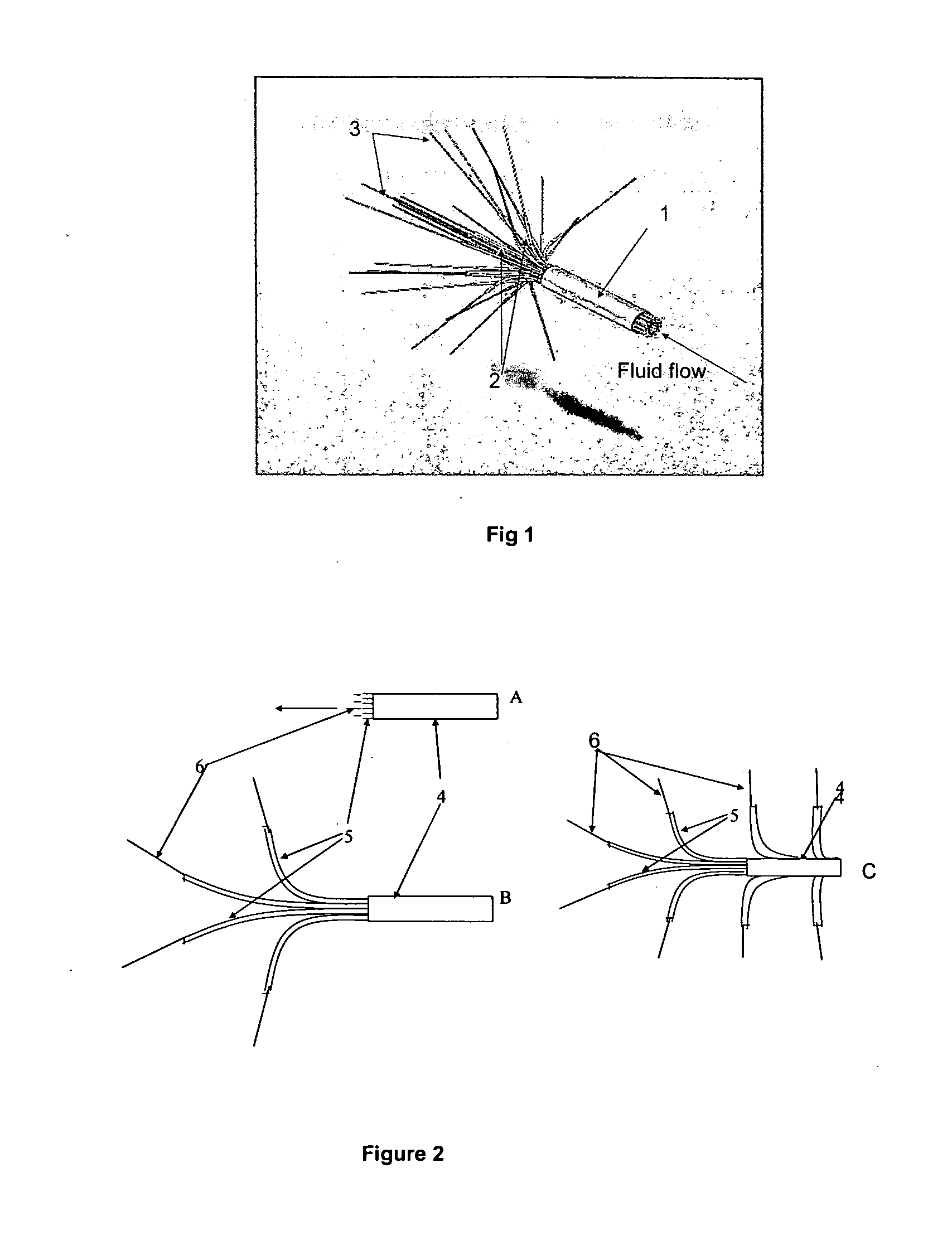
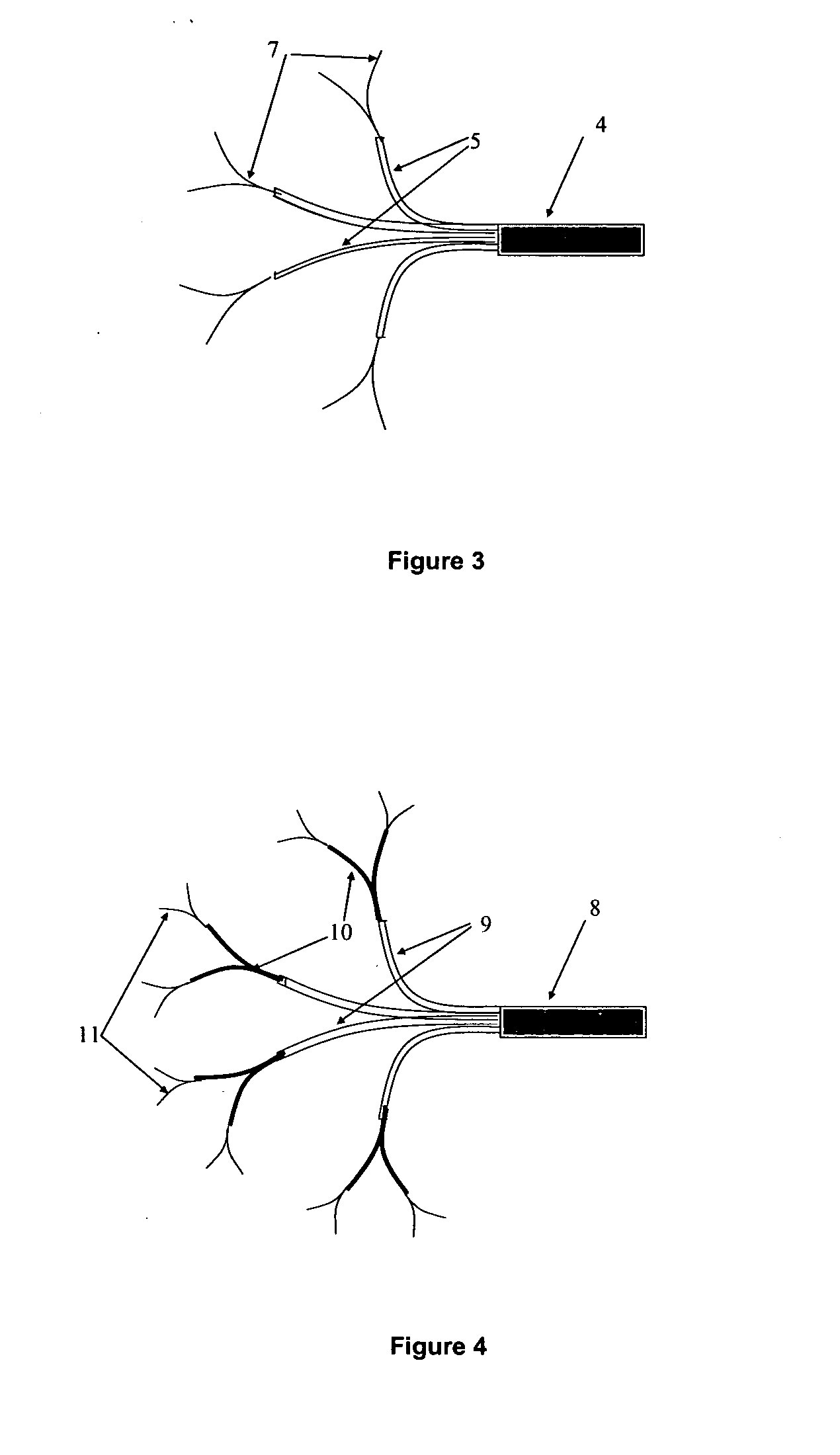
A catheter system adapted for navigating, guiding and implanting a catheter or a plurality of catheters in a spatially-defined implantation within the tissue of a patient is provided. The system can include a tissue navigation system and a probe to inform the navigation system to guide emplacement of the catheters within a target tissue. The probe can provide images, such as fiberoptic visual images, or ultrasound images, or can provide radiolocation data, to guide the catheter emplacement. The catheters supply a pressurized liquid including a bioactive agent, such as can be used in the treatment of cancer, for example 123I- or 125I-IUDR. The system and methods provided can be used in the treatment of locally advanced tumors, such as cancers of the brain, head or neck, esophagus, prostate, ovary, liver, pancreas, bladder or rectum.
Owner:PEAK BIOSCI
Methods of assessing the effect of a gene of interest on human prostate cancer progression
The present invention provides an immune deficient mouse having a human prostate xenograft of locally advanced or metastatic prostate cancer and uses thereof.
Owner:RGT UNIV OF CALIFORNIA
Method for improved safety in externally focused microwave thermotherapy for treating breast cancer
A method and apparatus that increases safety when employing externally focused adaptive phased array microwave thermotherapy (hyperthermia) for breast cancer treatment include microwave absorbing pads and metallic shielding to prevent undesired or stray surface tissue heating and to cushion the breast from mechanical pressure during breast compression. The microwave absorbing pads are attached on the top of the microwave waveguide thermotherapy applicators and on top of the breast compression paddles. A metallic-shielding strip mounted across the top portion of the microwave applicator aperture acts to block microwave radiation from illuminating the base of the breast and chest wall area. The patient treatment table utilizes a metallic shield to shield the body from stray microwave radiation. Treatments incorporating the safety improvements include thermotherapy for early-stage breast carcinomas, locally advanced breast cancer, ductal carcinoma in-situ, and benign breast lesions.
Owner:CELSION CANADA
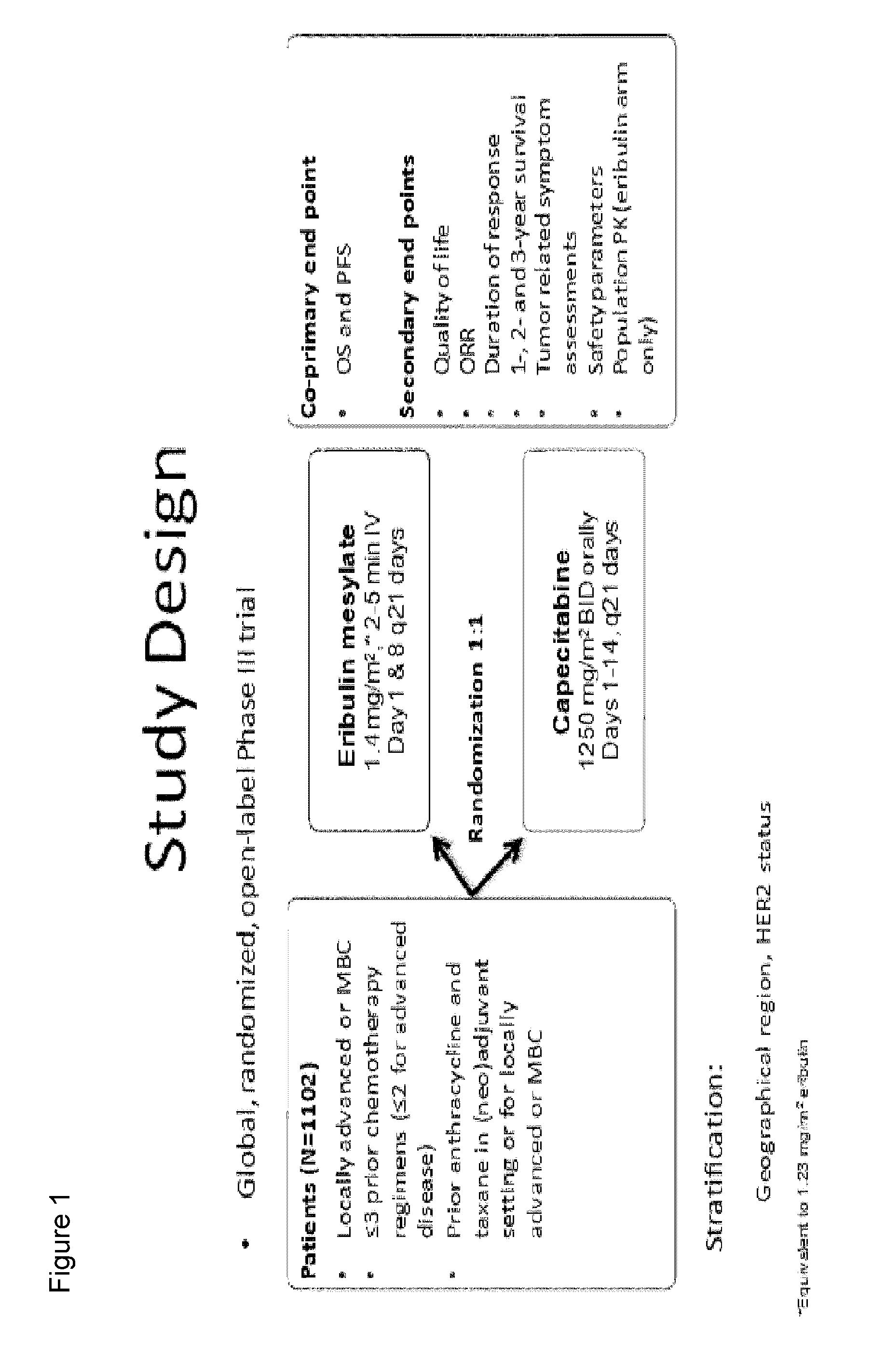
Use of eribulin in the treatment of breast cancer
The invention provides methods of treating breast cancer in subjects having locally advanced or metastatic breast cancer.
Owner:EISIA R&D MANAGEMENT CO LTD
Methods of treating early breast cancer with trastuzumab-mcc-dm1 and pertuzumab
Methods of treating patients having HER2 -positive, operable, locally advanced or inflammatory breast cancer with the antibody-drug conjugate Trastuzumab-MCC-DMl and Pertuzumab are provided.
Owner:F HOFFMANN LA ROCHE & CO AG
Preparation method for micro-molecular kinase inhibitor
ActiveCN106632260AShort synthetic routeSimple and safe operationOrganic chemistryAntineoplastic agentsEconomic benefitsKinase
The invention relates to a preparation method for a micro-molecular kinase inhibitor. Specifically, the invention discloses a novel method for preparing the micro-molecular kinase inhibitor capable of treating locally-advanced or metastatic non-small cell lung cancer (NSCLC) with positive anaplastic lymphoma kinase (ALK). The method comprises the following steps: with a compound (II) as a starting material, carrying out a substitution reaction, a reduction reaction, an oxidation reaction, a cyclization reaction and a reduction and deprotection reaction so as to form crizotinib as shown in a formula (I) in the specification. The method provided by the invention has the advantages of environment-friendly synthetic route, simple and safe operation, high overall yield, good economic benefits, and facilitation industrial production.
Owner:SHANGHAI TIANCI BIOLOGICAL VALLEY BIOLOGICAL ENG
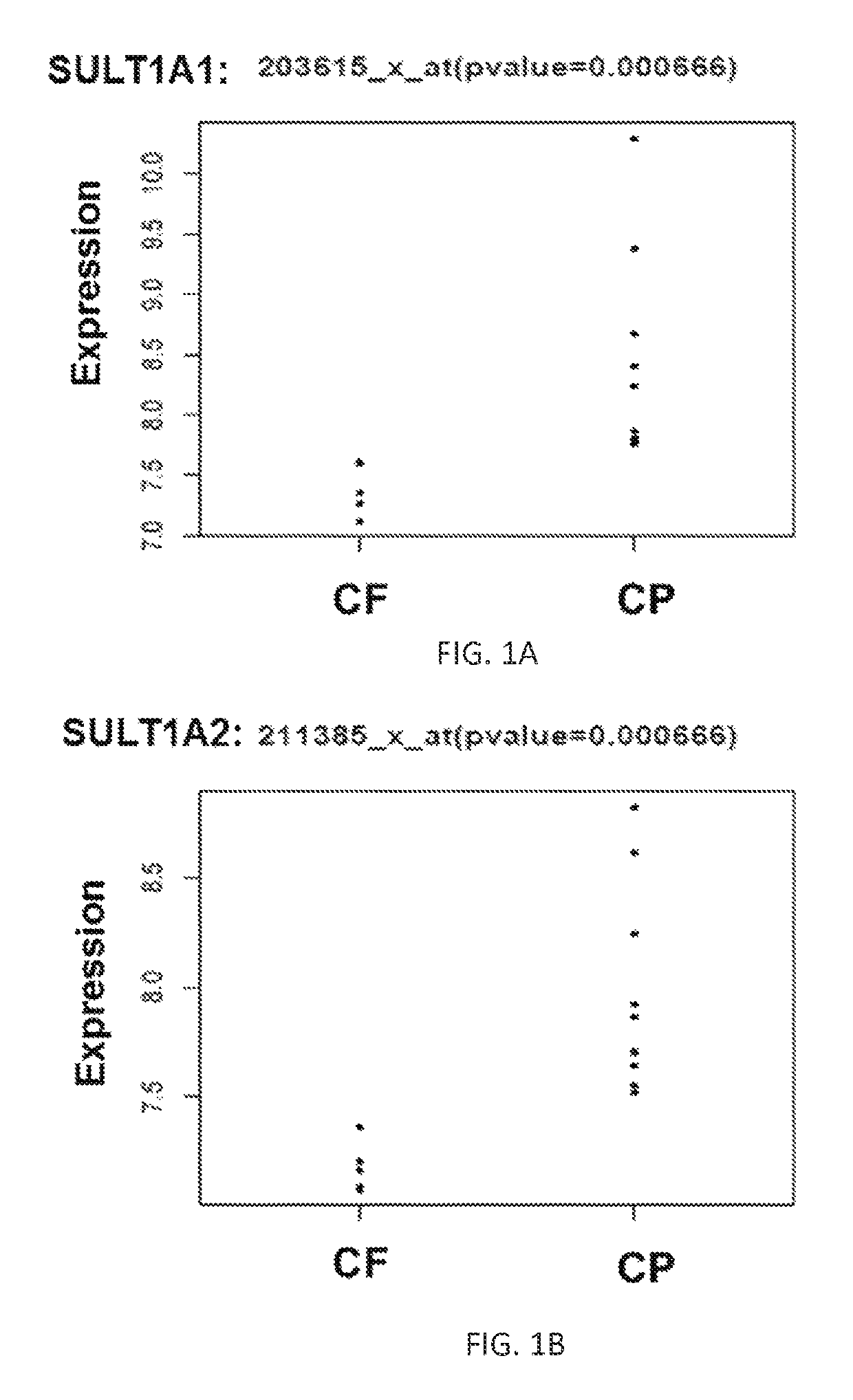
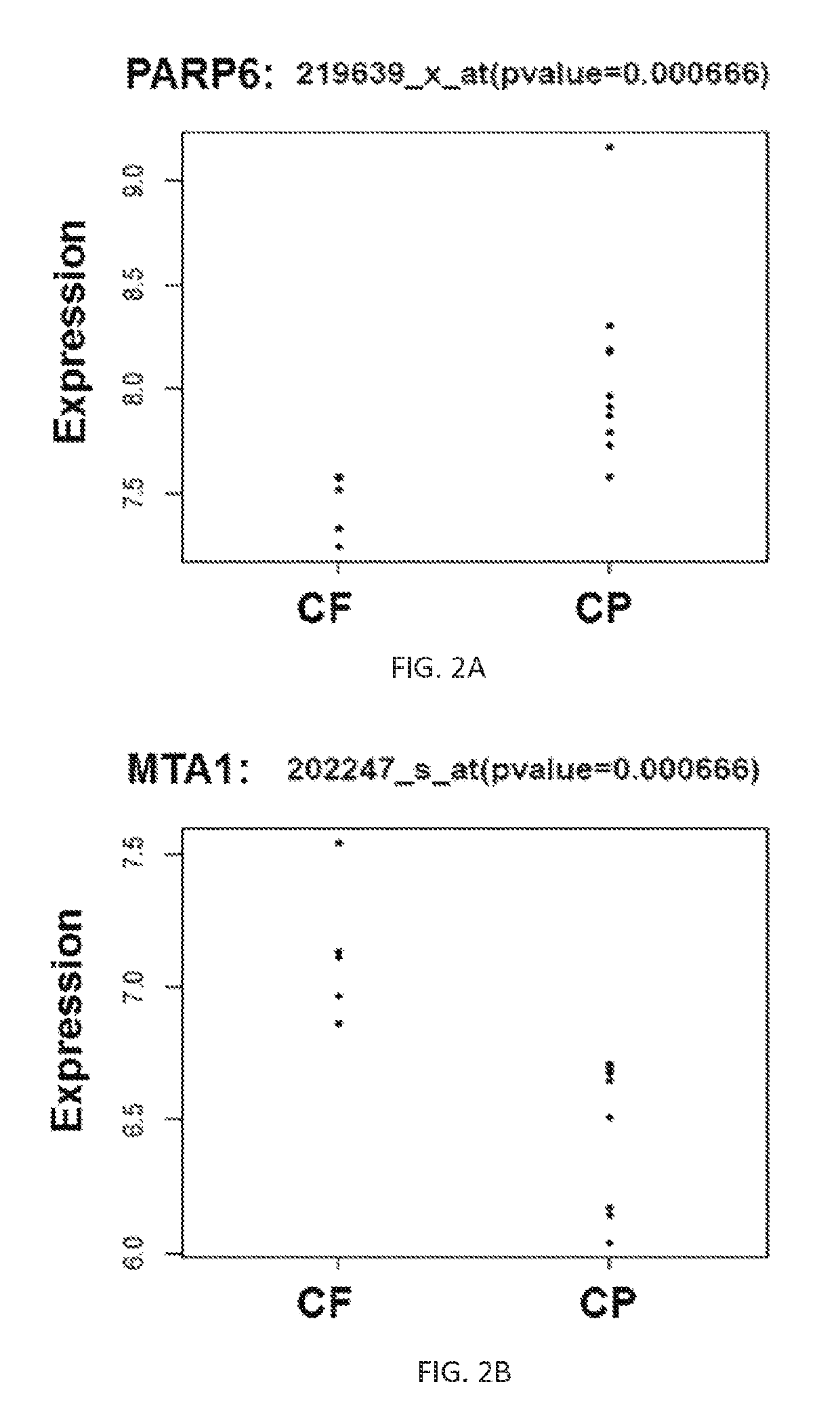
Biomarkers for locally advanced breast cancer (LABC) and inflammatory breast cancer (IBC)
ActiveUS10253367B1Increase opportunitiesReduce the amount of solutionMicrobiological testing/measurementOncologyBiomarker (petroleum)
The present disclosure provides methods and kits for using biomarkers for predicting treatment response, determining likely survival rate, and / or determining aggressiveness of conditions such as Locally Advanced Breast Cancer (LABC) and Inflammatory Breast Cancer (IBC) in a subject.
Owner:DUKE UNIV
Fibrin glue composite system loaded with cisplatin
ActiveCN112043834APrevent recurrenceInhibition of proliferative abilityInorganic active ingredientsOintment deliveryFibrin glueRadiotherapy unit
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Traditional Chinese medicine composition used in three-dimensional conformal and intensity modulated radiation therapy for treating locally advanced pancreatic carcinomas
ActiveCN104667123AImprove radiosensitivityPrevent proliferationAntipyreticAnalgesicsIntensity-modulated radiation therapyTraditional medicine
The invention provides a traditional Chinese medicine composition and an application thereof in preparation of medicines which are used in three-dimensional conformal and intensity modulated radiation therapy for treating advanced pancreatic carcinomas and have synergistic sensitizing and pain relieving effects. The traditional Chinese medicine composition comprises compound Danshen root injection and Qingyi Huaji formula granules. The radiosensitivity of pancreatic carcinomas can be obviously improved, the proliferation inhibition effects on pancreatic carcinomas can be improved and the pains of patients can be relieved by applying the traditional Chinese medicine composition seven days before radiation therapy or while radiation therapy.
Owner:QINGDAO TUMOR HOSPITAL
Novel targeting antineoplastic drug and manufacture method thereof and application thereof
ActiveCN103130760AWith independent intellectual property rightsLow idiosyncratic toxicityOrganic active ingredientsOrganic chemistryChemical treatmentTyrosine-kinase inhibitor
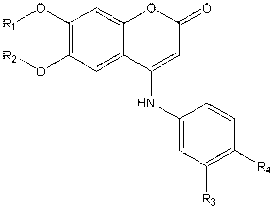
The invention provides a novel targeting antineoplastic drug and a manufacture method thereof and application thereof. The antineoplastic drug is a tyrosine kinase inhibitor, a novel core section of the drug is a catechol part and the left side is a hydroxide radical etherification part. The manufacture method of the drug is that 2, 4, 5-trihydroxy acetophenone reacts with methylclhlorofonmate; through an aldol reaction, a benzo dihydropyran structure is generated in a mode of cyclization through the aldol reaction; then the benzo dihydropyran structure reacts with interhalogen aniline or acetylene aniline to generate 4-interhalogen aniline-6, 7-dyhydroxy coumarin or 4-acetylene aniline-6, 7-dyhydroxy coumarin; methyl formate of benzene dihydroxy is removed; etherification is performed; and purification and crystallization are performed through flash chromatography to obtain the target product. The drug is used for treating people with locally advanced or metastatic non-small cell lung cancer and receiving chemical treatment before.
Owner:ZHANGJIAGANG IND TECH RES INST CO LTD DALIAN INST OF CHEM PHYSICS CHINESE ACADEMY OF SCI
Methods of treating HER2-positive metastatic breast cancer
Methods of treating patients having HER2-positive, locally advanced or previously untreated metastatic breast cancer having received prior treatment with a taxane using an anti-HER2-maytansinoid conjugate (for example trastuzumab emtansine) are provided.
Owner:GENENTECH INC
Method for predicting the response of locally advanced rectal cancer to chemoradiotherapy
The present invention relates to a method for determining or predicting the response of a patient diagnosed with locally advanced rectal cancer to chemoradiotherapy. The present invention also aims to provide methods and devices for predicting the response of patients diagnosed with rectal cancer to specific medicaments, radiotherapy and / or chemotherapy. More specifically, the present invention provides methods which measure kinase activity by studying phosphorylation levels and profiles in samples of said patients.
Owner:OSLO UNIVERSITY HOSPITAL +1
Method for predicting the response of locally advanced rectal cancer to chemoradiotherapy
The present invention relates to a method for determining or predicting the response of a patient diagnosed with locally advanced rectal cancer to chemoradiotherapy. The present invention also aims to provide methods and devices for predicting the response of patients diagnosed with rectal cancer to specific medicaments, radiotherapy and / or chemotherapy. More specifically, the present invention provides methods which measure kinase activity by studying phosphorylation levels and profiles in samples of said patients.
Owner:OSLO UNIVERSITY HOSPITAL +1
Methods of treating melanoma
ActiveUS20180280724A1Pharmaceutical active ingredientsX-ray/gamma-ray/particle-irradiation therapyMetastatic melanomaMolecular Targeted Therapies
The present disclosure is directed to methods of treating malignant melanoma by irradiating sites to which melanoma cells have become locally advanced, surgically undesirable, or have metastasized. In various embodiments of the invention, patients are treated with radiation doses in amounts ranging from about 25 to 230 cGy, preferably about 100 cGy to about 200 cGy, at least twice a day. The treatment regimen can be performed in the absence of additional treatments for the metastatic melanoma (e.g., chemotherapy / targeted therapy / immunotherapy) or in combination with additional therapies for chemotherapy / targeted therapy / immunotherapy. Various additional embodiments relate to the administration of between 20 and 100 fractions of radiation, preferably between 20 and 56 fractions of radiation.
Owner:MALIGNANT MELANOMA LLC
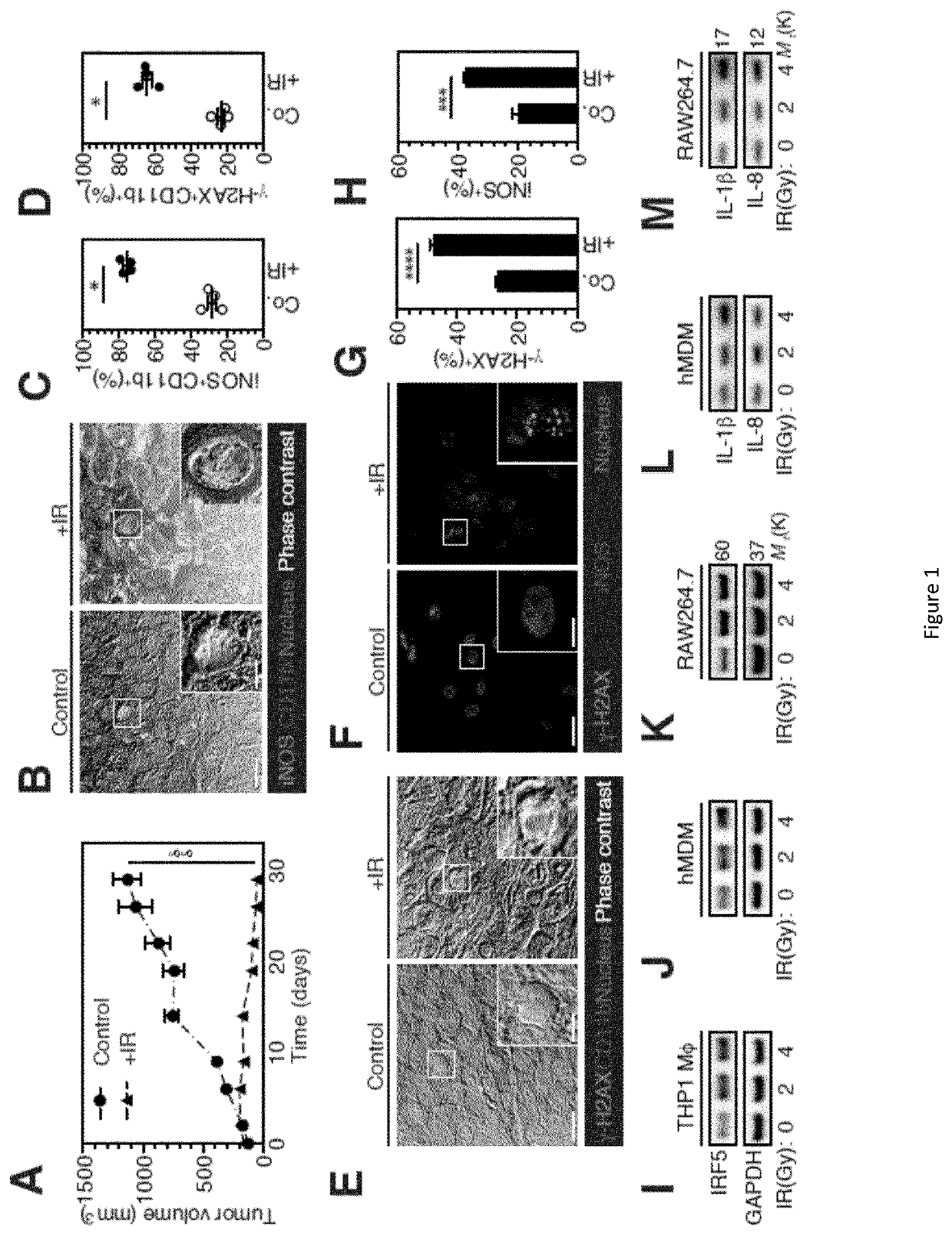
NOX2 as a Biomarker of Radiotherapy Efficiency in Cancer Patients
ActiveUS20200181714A1Improve efficiencyCytochromesMicrobiological testing/measurementAtaxia-telangiectasiaTumor response
Although tumor-associated macrophages have been extensively studied in the control of response to radiotherapy, the molecular mechanisms involved in the ionizing radiation-mediated activation of macrophages remain elusive. Here the present inventors show that ionizing radiation induces the expression of interferon-regulatory factor 5 (IRF5) promoting thus macrophage activation toward a pro-inflammatory phenotype. They reveal that the activation of the Ataxia telangiectasia mutated (ATM) kinase is required for ionizing radiation-elicited macrophage activation, but also for macrophage reprogramming after treatments with γ-interferon, lipopolysaccharide or chemotherapeutic agent (such as cis-platin), underscoring the fact that the kinase ATM plays a central role during macrophage phenotypic switching toward a proinflammatory phenotype. They further demonstrate that NADPH oxidase 2 (NOX2)-dependent ROS production is upstream to ATM activation and is essential during this process. They also report that hypoxic conditions and the inhibition of any component of this signaling pathway (NOX2, ROS and ATM) impairs pro-inflammatory activation of macrophages and predicts a poor tumor response to preoperative radiotherapy in locally advanced rectal cancer. Altogether, these results identify a novel signaling pathway involved in macrophage activation that may enhance effectiveness of radiotherapy through the re-programming of tumor infiltrating macrophages.
Owner:INSTITUT GUSTAVE ROUSSY
Liquid Dosage Forms to Treat Cancer
ActiveUS20200268737A1Dispersion deliveryPharmaceutical non-active ingredientsRenal Cell CancersPharmaceutical drug
This invention relates to a liquid pharmaceutical composition comprising cabozantinib to treat locally advanced or metastatic solid tumors, particularly advanced urothelial cancer or renal cell carcinoma in patients in need thereof.
Owner:EXELIXIS INC
Method of treating carcinoma
A method of treating patients suffering from carcinoma of intrahepatic or extra hepatic bile duct or gall bladder which is locally advanced or metastatic, by intravenously administering to the patient, paclitaxel in the form of a nanodispersion. The nanodispersion comprises particles with a mean particle size less than 300 nm and is free of polyoxyethylated castor oil and free of a protein.
Owner:SUN PHARMA INDS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com